Abstract
Helicobacter pylori infection has been reported to be positively associated with hypertension, although with conflicting results. In this study, the relationship between H. pylori infection and hypertension, as well as atherosclerotic carotid lesions, was analyzed. Methods. Clinical records of patients referred to undergo upper endoscopy and gastric biopsy were retrieved. Information regarding the presence of H. pylori infection with atrophy/metaplasia/dysplasia (interpreted as a long-lasting infection), and current or past H. pylori infection was collected, as well as demographic variables, smoking habits, body mass index (BMI), dyslipidemia, diabetes, hypertension, presence of carotid lesions, and current treatment, and analyzed by multivariable regression models. Results. A total of 7152 clinical records from patients older than 30 years (63.4% women) were available for the study. Hypertension was present in 2039 (28.5%) patients and the risk was significantly increased in those with long-lasting H. pylori infection after adjusting for age decades, sex, BMI, cigarette smoking, diabetes, and dyslipidemia (OR 1.17, 95% CI 1.02–1.35). In addition, the long-lasting H. pylori infection was an independent risk for carotid plaques (OR 2.15, 95% CI 1.14–4.09). Conclusions. Our retrospective study demonstrated that long-lasting H. pylori infection is an independent risk factor for hypertension and the presence of carotid lesions after adjusting for potential confounders, although further validation our findings is needed from prospective studies.
Keywords: hypertension, Helicobacter pylori infection, Sardinia
1. Introduction
The incidence of cardiovascular disease (CVD) is increasing worldwide and represents a serious concern both in affluent and underdeveloped countries [1]. The number of deaths from CVD in 2020 was estimated to be 17.9 million, equivalent to 32% of all deaths globally [2]. The basic pathogenic process underlying CVD is atherosclerosis, a mechanism promoted by hypertension and other known risk factors such as age, male sex, cigarette smoking, obesity, dyslipidemia, diabetes, salt intake, and familial propensity [3]. Apart from these conventional risk factors, new potential cardiovascular risk factors are increasingly recognized [4], including infectious diseases [5,6,7,8,9,10]. Among microorganisms, Helicobacter pylori (H. pylori) is one of the most studied pathogens [11]. Helicobacter pylori is still responsible for widespread chronic bacterial infections [12,13], and its relevance promoting atheroma development is believed to be mediated by a chronic low-grade inflammation, triggered by the release of pro-inflammatory molecules, increased fibrinogen, C reactive protein, triglycerides, and low-density lipoprotein, which participate in the atherosclerotic process and promote a prothrombotic state [11,14,15,16].
Since 1988, Sonnenberg has suggested that gastric diseases and hypertension-related organ damage may share a common etiologic factor based on the results of an epidemiological study [17]. Similarly, an old study reported a statistically high significant relationship between H. pylori gastritis and hypertension in patients from an urban area undergoing a long-term follow-up [18]. Moreover, in a large European study involving autoptic material from over 50,000 patients, Sternby [19] observed that the obstruction of the left coronary artery was fivefold more frequently associated with prior duodenal or gastric ulcers than in those without peptic ulcer disease in subjects aged 40 to 59 years. Accordingly, a significantly higher prevalence of H. pylori infection has been reported in patients with coronary heart disease than among controls [20,21]. Furthermore, it has been demonstrated that H. pylori infection may contribute to plaque instability through anti-H. pylori CagA and/or VacA antibodies able to cross-react with antigens of both normal and atherosclerotic blood vessels [22,23]. In a recent meta-analysis including 40 studies for a total of 19,691 patients, H. pylori infection increased the risk of cardiovascular events (odds ratio [OR], 1.51), and the risk was even greater for H. pylori CagA positive strains (OR, 1.73). Among cardiovascular events, the risk was highest for myocardial infarction (OR, 1.80) and cerebrovascular disease (OR, 1.54) [24]. Interestingly, an additional meta-analysis showed that subjects younger than 60 years old without cardiovascular risk factors were more prone to develop atherosclerotic lesions if infected with H. pylori [25].
It has been hypothesized that one mechanism by which H. pylori infection may predispose to CVD is by increasing blood pressure values.
In a cross-sectional study performed in 5168 healthy adult Chinese subjects, it was observed that H. pylori infection was associated with an increased prevalence of hypertension (OR, 1.23), after adjustment for potential confounders [26]. Similar results were confirmed in different countries including Iran [27], England [28], and Japan [29], among others. More importantly, in a prospective study, H. pylori eradication was followed by a significant decrease in blood pressure values in hypertensive patients, especially for diastolic blood pressure [30]. These results highlight a cause-effect relationship between H. pylori infection and the occurrence of hypertension. However, other studies did not find evidence of increased risk after adjusting for potential confounders [31,32].
Based on these results, in this study we explore the relationship between H. pylori infection, blood hypertension, and the presence of atherosclerotic carotid lesions in a large cohort of patients undergoing upper endoscopy to provide further evidence about this issue.
2. Materials and Methods
2.1. Study Design
This was a retrospective, cross-sectional study aimed to evaluate the association between blood hypertension, carotid lesions, and H. pylori infection. Traditional risk factors for hypertension and atherosclerosis, including sex, age, excess of weight, smoking, diabetes, and dyslipidemia were considered as potential confounders.
2.2. Study Participants
The clinical records of adult patients who underwent upper endoscopy for any reason from January 2002 to September 2019 at the Gastroenterology Unit of the University of Sassari, Italy, were retrieved. Each patient had been evaluated by a trained gastroenterologist to collect a comprehensive medical history including a previous diagnosis of hypertension and all medications currently taken. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg in at least three measurements and matched with antihypertensive treatment. Only subjects previously assessed by a cardiologist who confirmed hypertension diagnosis were included in the analysis, while subjects for which no reliable evidence of established hypertension was available were considered as controls from the analysis.
2.3. Exclusion Criteria
Patients younger than 18 years were excluded from the study. Unknown H. pylori status and/or absence of gastric biopsies were considered additional exclusion criteria. For patients who underwent multiple EGDs within the study period, only data from the index endoscopy were recorded.
2.4. Helicobacter pylori Status
All patients underwent an upper endoscopy. Patients were referred to the endoscopy service by family physicians and/or specialists for a number of reasons, including dyspeptic or reflux symptoms, screening for celiac disease, presence of alarm signs and/or symptoms, varices detection, assessment of hemorrhage risk, and follow-up, among others. During the exam, a minimum of four non-targeted gastric biopsy specimens were taken, when not contraindicated, as previously described [33]. Briefly, gastric specimens were collected from the antrum, angulus, and two from the corpus. Helicobacter pylori infection was defined by the presence of the bacteria on histological examination. Gastric mucosa replaced by intestinal epithelium was classified as intestinal metaplasia and loss of glands as atrophy [33]. Morphology was assessed by a dedicated GI-pathologist. In the case of a chronic-active gastritis and absence of the bacteria, the infection was confirmed by the stool antigen test or ¹³C-Urea breath test (¹³C-UBT) as recommended [34].
2.5. Carotid Ultrasonography and Atherosclerosis Detection
Carotid ultrasonography was performed in a subgroup of 333 patients. All examinations were performed using linear-array transducers, with at least a frequency of emission of 7.5 MHz. Common carotid artery, bifurcation, and internal and external carotid arteries were bilaterally interrogated with the patient lying in the supine position. Intima-media thickness (IMT) and diameter were measured on both sides in the common carotid artery (about 1 cm proximally from carotid bifurcation) with the leading-edge method [35] and averaged. In order to reduce the confounding impact of differences in blood pressure on carotid IMT 2 mean, the carotid cross-sectional area was calculated from the average diameter and intima-media thickness as the difference between the area that encompasses the arterial diameter and the arterial luminal area [36], according to the formula:
| (1) |
where CCSA is the carotid cross-sectional area, CA is the carotid artery, and IMT is the intima-media thickness.
Carotid plaques were defined as focal structures encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value or demonstrating a thickness ≥ 1.5 mm as measured from the intima-lumen interface to the media-adventitia interface [35]. The atherosclerotic burden was assessed by the carotid plaque score, calculated as the number of carotid segments (common, bifurcation, internal carotid artery) bilaterally presenting atherosclerotic plaques (range 0 to 6) [37].
2.6. Ethical Considerations
An Institutional Review Board approval was obtained from the local ethics committee: Comitato di Bioetica, Azienda Ospedaliero-Universitaria di Sassari, Italy (Protocol code 2099/CE, 2014).
2.7. Statistical Analysis
Continuous variables were expressed as means ± standard deviation (SD) and compared using the Student’s t-test. For categorical variables, expressed as frequencies, the chi-square test was used. The BMI was calculated by using the formula weight (kg)/height (m)²; overweight and obesity were defined as a BMI of 25–29 and ≥30 kg/m², respectively. Helicobacter pylori status was stratified into (i) current infection (when a chronic-active gastritis was present in addition to the bacteria on gastric samples); (ii) when H. pylori infection with atrophy/metaplasia/dysplasia was observed it was interpreted as a long-lasting infection; and (iii) previous H. pylori infection, in the case of a positive clinical history of H. pylori infection, successfully eradicated, without evidence of the bacteria and/or chronic-active gastritis on histological specimens. The sample size of patients undergoing carotid ultrasonography examination was estimated to be at least of n = 141 per group required to provide 80% power to detect a 1 mm difference in the mean carotid cross-sectional area around 17 ± 3 mm with a significance of 0.05 (two-sided α). To assess the risk of hypertension and the presence of carotid lesions associated with H. pylori infection, multivariable logistic regression models were used by calculating the ORs and their 95% confidence interval (CI). Since hypertension was sporadic before age 30 years, in the regression models the risk of hypertension was calculated only in subjects older than the third decade both unadjusted and adjusted for age, BMI, sex, smoking status, diabetes, and dyslipidemia. ORs were calculated according to H. pylori status and more specifically for long-lasting and current infection.
All statistical analyses were performed using SPSS Statistics version 22.0 (Chicago, IL, USA). A two-sided p < 0.05 was considered to be statistically significant.
3. Results
Descriptive Statistics
Data from a total of 7152 clinical records (female 4532; 63.4%) were available for the analysis (Table 1). All patients were from Northern Sardinia, sharing a similar genetic background. An established diagnosis of blood hypertension was available in 2039 (28.5%) patients. Older age, overweight and obesity, hypercholesterolemia, diabetes, and former or current smokers were significantly more frequent in patients with hypertension (Table 1). Interestingly, in patients with long-lasting H. pylori infection, hypertension was detected more frequently, and the difference with patients without infection was statistically significant (p < 0.01) (Table 1).
Table 1.
Descriptive statistics in 7152 study participants according to blood hypertension.
| Variables | No Hypertension (n = 5113) | Hypertension (n = 2039) |
|---|---|---|
| Sex, n (%) | ||
| Male | 1891 (37.0) | 729 (35.8) |
| Female | 3222 (63.0) | 1310 (64.2) |
| Age, n (%) | ||
| 30–39 | 1165 (22.8) | 26 (1.3) |
| 40–49 | 1231 (24.1) | 142 (7.0) ** |
| 50–59 | 1119 (21.9) | 392 (19.2) ** |
| 60–69 | 932 (18.2) | 699 (34.3) ** |
| 70–79 | 525 (10.3) | 613 (30.1) ** |
| ≥80 | 141 (2.8) | 167 (8.2) ** |
| Body mass index, n (%) | ||
| <25 kg/m² | 2973 (58.1) | 874 (42.9) |
| 25–29 kg/m² | 1583 (31.0) | 804 (39.4) ** |
| ≥30 kg/m² | 557 (10.9) | 361 (17.7) ** |
| Smoke, n (%) | ||
| Never smoker | 2683 (52.5) | 1094 (53.7) |
| Former smoker | 218 (4.3) | 111 (5.4) * |
| Current smoker | 2212 (43.3) | 834 (40.9) |
| Dyslipidemia, n (%) | ||
| No | 4758 (93.1) | 1576 (77.3) |
| Yes | 355 (6.9) | 463 (22.7) ** |
| Diabetes, n (%) | ||
| No | 4871 (95.3) | 1649 (80.9) |
| Yes | 242 (4.7) | 390 (19.1) ** |
| History of H. pylori infection #, n (%) | ||
| No | 4972 (97.2) | 1977 (97.0) |
| Yes | 141 (2.8) | 62 (3.0) |
| H. pylori status, n (%) | ||
| No infection | 2296 (44.9) | 837 (41.0) |
| Long-lasting H. pylori infection § | 1254 (24.5) | 607 (29.8) ** |
| Current H. pylori infection ‡ | 1563 (30.6) | 595 (29.2) |
* p < 0.05; ** p < 0.01; # A positive clinical history of H. pylori infection, successfully eradicated, without evidence of the bacteria and/or chronic-active gastritis on histological specimens. § Presence of H. pylori infection associated with atrophy/metaplasia/dysplasia was interpreted as a long-lasting infection. ‡ Presence of chronic-active gastritis in addition to the bacteria on gastric specimens.
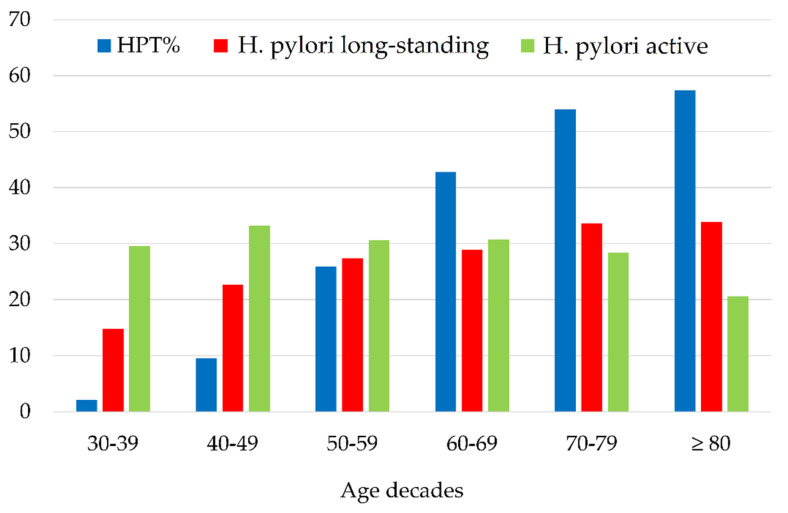
As expected, blood pressure tended to rise in oldest decades showing a near relationship with the percentage of H. pylori infection represented in the graph of Figure 1.
Figure 1.
Distribution of blood hypertension and Helicobacter pylori infection according to age decades. HTP: hypertension.
Table 2 lists the unadjusted and adjusted ORs and their 95% CIs for all variables analyzed by the logistic regression. Long-lasting H. pylori infection was confirmed as a risk factor for developing blood hypertension in addition to a previous H. pylori infection with ORs of 1.31 and 1.17, respectively (Table 2). In contrast, a current H. pylori infection—characterized only by the presence of chronic-active gastritis—was not associated with hypertension. After adjusting for all study covariates, i.e., oldest age, overweight, obesity, dyslipidemia, diabetes, and cigarette smoking, long-lasting infection remained a strong risk factor (Table 2).
Table 2.
Logistic regression analysis for blood hypertension in 7152 study participants.
| Variables | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Ref. | Ref. |
| Female | 1.05 (0.95–1.17) | 1.13 (1.00–1.28) * |
| Age | ||
| 30–49 years | Ref. | Ref. |
| 50–59 years | 4.99 (4.11–6.07) ** | 4.36 (3.58–5.31) ** |
| 60–69 years | 10.70 (8.89–12.86) ** | 8.54 (7.07–10.31) ** |
| 70–79 years | 16.65 (13.70–20.24) ** | 12.98 (10.62–15.86) ** |
| ≥80 years | 16.89 (12.85–22.20) ** | 13.48 (10.17–17.87) ** |
| Body mass index | ||
| <25 kg/m² | Ref. | Ref. |
| 25–29 kg/m² | 1.72 (1.54–1.93) ** | 1.33 (1.17–1.52) ** |
| ≥30 kg/m² | 2.20 (1.89–2.57) ** | 1.92 (1.61–2.28) ** |
| Smoke | ||
| Never smoker | Ref. | Ref. |
| Former smoker | 0.92 (0.83–1.03) | 0.98 (0.75–1.28) |
| Current smoker | 1.25 (1.02–1.59) * | 1.13 (0.98–1.94) |
| Dyslipidemia | ||
| No | Ref. | Ref. |
| Yes | 3.94 (3.39–4.57) ** | 2.38 (2.02–2.81) ** |
| Diabetes | ||
| No | Ref. | Ref. |
| Yes | 4.76 (4.02–5.64) ** | 3.94 (2.17–4.36) ** |
| History of H. pylori infection | ||
| No | Ref. | Ref. |
| Yes | 1.19 (1.07–1.32) * | 1.13 (1.01–1.26) * |
| H. pylori status | ||
| No infection | Ref. | Ref. |
| Long-lasting infection § | 1.31 (1.16–1.46) ** | 1.17 (1.02–1.35) * |
| Current infection ‡ | 0.93 (0.84–1.05) | 0.99 (0.86–1.14) |
* p < 0.05; ** p < 0.01, § Presence of H. pylori infection associated with atrophy/metaplasia/dysplasia was interpreted as a long-lasting infection. ‡ Presence of chronic-active gastritis in addition to the bacteria on gastric specimens.
After adjusting for all covariates, long-lasting H. pylori infection remained a strong risk factor for blood hypertension in both sexes (Supplementary Materials, Table S1).
Table 3 shows carotid parameters according to H. pylori status in a subgroup of 333 patients undergoing carotid ultrasonography. This subgroup did not differ from the general cohort by sex but included older subjects (62.1 ± 14.2 vs. 52.0 ± 17.3, p < 0.0001). The overall prevalence of H. pylori infection was 49.8%. The analysis detected a statistically higher prevalence of right, left and any carotid plaque in patients with a long-lasting H. pylori infection, but not for current infection when compared to H. pylori negative patients (Table 3).
Table 3.
Mean and standard deviation of carotid parameters in 333 study participants according to the long-lasting (H. pylori with atrophy/metaplasia/dysplasia), and current H. pylori (Hp) infection.
| Variables | Long-Lasting Hp Infection (n = 91) | Current Hp Infection (n = 75) | Hp Negative (n = 167) |
|---|---|---|---|
| Mean carotid cross-sectional area (mm²) | 17.4 ± 5.6 * | 15.1 ± 4.5 | 16.1 ± 5.1 |
| Mean Intima-media thickness (mm) | 0.77 ± 0.16 * | 0.72 ± 0.14 | 0.73 ± 0.14 |
| Right carotid plaques | |||
| No | 33 (36.2) | 79 (52.0) | 90 (53.9) |
| Yes | 58 (63.8) * | 36 (48.0) | 77 (46.1) |
| Left carotid plaques | |||
| No | 39 (42.9) | 43 (57.3) | 98 (58.7) |
| Yes | 52 (57.1) * | 32 (42.7) | 69 (41.3) |
| Any carotid plaque | |||
| 0 | 24 (26.4) | 35 (46.7) | 79 (47.3) |
| 1 | 23 (25.3) * | 15 (20.0) | 32 (19.2) |
| 2 | 44 (48.4) ** | 25 (33.3) | 56 (33.5) |
* p < 0.05; ** p < 0.01.
Results of the logistic regression analysis with the long-lasting H. pylori infection as exposure with hypertension and carotid atherosclerosis lesions as outcomes, adjusted for age as a continuous variable BMI, smoke, dyslipidemia, and diabetes are listed in Table 4 in the subgroup of patients undergoing carotid ultrasonography. The long-lasting H. pylori infection was significantly associated with the risk of developing atherosclerotic carotid lesions, independently from the traditional CVD risk factors.
Table 4.
Logistic regression analysis with H. pylori infection as exposure and carotid parameters as the outcome.
| Variables | Patients with Carotid Parameters (n = 333) | |
|---|---|---|
| Long-Standing H. pylori Infection | Current H. pylori Infection | |
| Hypertension § | 1.85 (1.04–3.30) * | 1.05 (0.57–1.90) |
| Carotid cross-sectional area (>90%) # | 1.51 (0.79–2.88) | 1.45 (0.75–2.81) |
| Right carotid plaque # | 1.70 (0.92–3.14) | 1.25 (0.66–2.35) |
| Left carotid plaque # | 1.69 (0.90–3.13) | 1.81 (0.95–3.45) |
| Any carotid plaque # | 2.15 (1.14–4.09) * | 1.52 (0.81–2.85) |
* p < 0.05; § based on the cardiologist diagnosis and current use of anti-hypertensive medications. # adjusted for age as a continuous variable, sex, body mass index, cigarette smoke, dyslipidemia, and diabetes.
4. Discussion
It has been reported that, beside traditional risk factors, infectious diseases may also have a role in the pathogenesis of atherosclerosis. Acute infections may increase temporarily the risk of cardiovascular events [6,7]. For example, the influenza virus and coronavirus 2 have been associated with an increased risk of acute myocardial infarction within a few days from onset in infected patients [6,7]. In addition, chronic infection may induce a systemic low-grade, persistent inflammatory process and endothelial dysfunction responsible for cardiac events. Moreover, exposure to persistent high bacterial endotoxin burden is associated with an increased risk of atherosclerosis [10].
Among infectious agents, the most studied organisms related to atherosclerosis are Chlamydia pneumoniae [9], cytomegalovirus [5] and H. pylori [8]. Helicobacter pylori is still a common infection across countries especially in less affluent populations [12]. The pathogen is etiologically related to gastritis, peptic ulcer disease and gastric cancer, although the infection has been progressively associated with several extra-GI disorders comprising blood hypertension, metabolic syndrome, atherosclerosis and, more generally, CVD [38,39].
Studies involving H. pylori infection in the rise of blood pressure are numerous [27,40,41,42,43]. Accordingly, in our study we found a statistically higher prevalence of blood hypertension, confirmed by the antihypertensive treatment, in patients with H. pylori infection, compared with patients without, after adjusting for covariates such as age, smoke, overweight/obesity, hypercholesterolemia, and diabetes, traditionally considered major risk factors for blood hypertension.
Katayoun Vahdat et al. observed that H. pylori seropositivity was significantly and independently associated with essential hypertension, with a OR similar to that found in our study (1.37), and the risk of hypertension was even higher in the presence of coinfection of H. pylori with C. pneumoniae (OR, 1.68) after adjusting for age, sex, chronic low-grade inflammation, and CVD risk factors [27]. A significantly higher frequency of seropositivity against H. pylori infection in patients with hypertension, in respect to controls, was also detected in England (p = 0.007) and Japan (p = 0.014), respectively [28,29]. In a cross-sectional study conducted in a large population of 37,263 healthy subjects, after controlling for confounders by multiple linear regression analysis, seropositivity for H. pylori was significantly and positively associated with diastolic hypertension [44]. A higher prevalence of H. pylori infection, detected by ¹³C-UBT, was also reported in healthy subjects with blood hypertension undergoing a routine checkup [45]. In a community-based cross-sectional study, H. pylori infection assessed by ¹³C-UBT showed a small effect on systolic pressure and, although it was statistically significant, the authors concluded it was not clinically relevant [43]. On the contrary, in a Czech cohort, blood pressure was not influenced by H. pylori infection [46].
Contrasting results among the different studies may be due by several reasons, including adjustment for potential confounders and assessment of H. pylori status.
Helicobacter pylori infection is generally acquired in childhood and causes a typical gastritis characterized by a morphological pattern of acute-on-chronic inflammation, and once established, the infection is typically lifelong if otherwise not diagnosed and treated. Long-lasting infection is characterized by replacement of parietal cells with gastric atrophy, reduction of glands and acid secretion, and eventually, a substitution with intestinal-type epithelium [47]. For this reason, it is commonly and universally accepted that histological signs of atrophy, intestinal metaplasia/dysplasia invariably denote a long-lasting H. pylori infection. This scenario is not inevitable and occurs in a subpopulation of infected adults at a rate of 1% to 2% per year [48]. The presence of the bacteria in the stomach enhances a serum immune response, characterized by anti–H. pylori IgG antibodies. However, serologic tests remain positive for a long time after H. pylori eradication, thus the presence of IgG in the serum is not a reliable marker to delineate between an active or past infection [34]. Instead, noninvasive tests such as ¹³C-UBT and the stool antigen test could effectively detect an active infection. Although both tests have a robust accuracy, they are unable to assess the gastric mucosa status [34]. The sequence of events leading from H. pylori gastritis to gastric premalignant lesions (e.g., atrophy, metaplasia, and/or dysplasia) is a long process associated with a systemic, low-grade proinflammatory state. In the majority of studies analyzing the association between H. pylori infection and blood hypertension, the H. pylori status was tested by serology or ¹³C-UBT, making it possible for the two tests to bias the results: serology could have captured several subjects already eradicated, and the ¹³C-UBT does not discriminate between patients with a long-lasting (associated with a persistent inflammatory state) or recent infection. In order to minimize these problems in our study, for the first time we stratified patients into those having a previous, successfully treated H. pylori infection, those having a current infection (chronic active gastritis), and those having a long-lasting infection, based on the presence of metaplasia/atrophy/dysplasia on histological examination. Consistently, in patients harboring the infection for long time, the association with blood hypertension and atherosclerotic carotid lesions, including plaques, was statistically significant whereas in the group displaying current active infection was not, after adjusting for covariates in both sexes.
Limitations
This study has a number of limitations that need to be mentioned. First, this was a retrospective cross-sectional study not suitable to assess a causal effect. Moreover, in retrospective studies there is a poor control over the exposure factor. However, in our study H. pylori status was defined by histology on gastric specimens and the duration of gastritis by the type of gastric lesions detected on morphological examination. In addition, in the absence of the bacteria in gastric specimens strongly suspected for H. pylori gastritis, the infection was further verified by ¹³C-UBT or stool antigen test. For these reasons, we are confident that in our study very few H. pylori positive patients have been missed from the analysis. Second, although we controlled for the most known risk factors for blood hypertension through the logistic regression model, additional risk factors such as markers of systemic inflammation, familiarity, genetics, diet, and especially salt intake, were not available. Third, a number of subjects with an unknown blood hypertension may have been lost from the group of H. pylori positive or negative patients with a consequent underestimation of the population with blood hypertension. However, we can assume that the effect was minimal and affected both subgroups in a similar fashion. Finally, only a small number of patients undergoing upper endoscopy underwent a carotid ultrasound examination, potentially decreasing the generalizability of our findings to the entire population.
5. Conclusions
In summary, according to the majority of studies on this topic, our result suggests that H. pylori infection, especially the long-lasting infection, is an independent risk factor for blood hypertension and for the presence of carotid plaques after adjusting for potential confounders. However, the results obtained in our study should be critically evaluated given its retro-spective design, and should be further validated in additional prospective studies before claiming a cause-effect relationship. Confirmation of H. pylori infection as an additional CV risk factor may allow to better stratify patient risk, and the bacteria eradication an ad-ditional tool in the management of blood hypertension.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm11092282/s1, Table S1: Logistic regression for blood hypertension in male and female participants.
Author Contributions
Conceptualization, M.P.D. and G.M.P.; methodology, M.P.D. and G.M.P.; software, G.M.P.; validation, M.P.D., P.S.S. and G.M.P.; formal analysis, G.M.P.; investigation, M.P.D., P.S.S., C.N., G.T. and M.M.; resources, M.P.D.; data curation, M.P.D., G.T., C.N. and M.M.; writing—original draft preparation, M.P.D. and P.S.S. and G.M.P.; writing—review and editing, M.P.D. and G.M.P.; visualization, G.T. and C.N.; supervision, M.P.D.; project administration, M.P.D.; funding acquisition, M.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Comitato di Bioetica, Azienda Ospedaliero-Universitaria di Sassari, Italy (protocol code No. 2099/CE, 2014) for studies involving humans.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pencina M.J., Navar A.M., Wojdyla D., Sanchez R.J., Khan I., Elassal J., D’Agostino R.B., Sr., Peterson E.D., Sniderman A.D. Quantifying Importance of Major Risk Factors for Coronary Heart Disease. Circulation. 2019;139:1603–1611. doi: 10.1161/CIRCULATIONAHA.117.031855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel S.A., Winkel M., Ali M.K., Narayan K.M., Mehta N.K. Cardiovascular mortality associated with 5 leading risk factors: National and state preventable fractions estimated from survey data. Ann. Intern. Med. 2015;163:245–253. doi: 10.7326/M14-1753. [DOI] [PubMed] [Google Scholar]
- 4.Dore M.P., Portoghese M., Pes G.M. The Elderly with Glucose-6-Phosphate Dehydrogenase Deficiency are More Susceptible to Cardiovascular Disease. J. Atheroscler. Thromb. 2021;28:604–610. doi: 10.5551/jat.56531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum A., Giladi M., Weinberg M., Kaplan G., Pasternack H., Laniado S., Miller H. High ant i-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am. J. Cardiol. 1998;81:866–868. doi: 10.1016/S0002-9149(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 6.Chow E.J., Rolfes M.A., O’Halloran A., Anderson E.J., Bennett N.M., Billing L., Chai S., Dufort E., Herlihy R., Kim S., et al. Acute Cardiovascular Events Associated With Influenza in Hospitalized Adults: A Cross-sectional Study. Ann. Intern. Med. 2020;173:605–613. doi: 10.7326/M20-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunn M., Stephens J.C., Thompson J.R., Rathbone B.J., Samani N.J. Significant association of cagA positive Helicobacter pylori strains with risk of premature myocardial infarction. Heart. 2000;84:267–271. doi: 10.1136/heart.84.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi R., Khandelwal B., Joshi D., Gupta O.P. Chlamydophila pneumoniae infection and cardiovascular disease. N. Am. J. Med. Sci. 2013;5:169–181. doi: 10.4103/1947-2714.109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiedermann C.J., Kiechl S., Dunzendorfer S., Schratzberger P., Egger G., Oberhollenzer F., Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the Bruneck Study. J. Am. Coll. Cardiol. 1999;34:1975–1981. doi: 10.1016/S0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 11.Mladenova I. Helicobacter pylori and cardiovascular disease: Update 2019. Minerva Cardioangiol. 2019;67:425–432. doi: 10.23736/S0026-4725.19.04986-7. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi S., Dore M.P. Epidemiology of Helicobacter pylori Infection. Helicobacter. 2005;10((Suppl. 1)):1–4. doi: 10.1111/j.1523-5378.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 13.Dore M.P., Marras G., Rocchi C., Soro S., Loria M.F., Bassotti G., Graham D.Y., Malaty H.M., Pes G.M. Changing prevalence of Helicobacter pylori infection and peptic ulcer among dyspeptic Sardinian patients. Int. Emerg. Med. 2015;10:787–794. doi: 10.1007/s11739-015-1218-4. [DOI] [PubMed] [Google Scholar]
- 14.Fichtlscherer S., Rosenberger G., Walter D.H., Breuer S., Dimmeler S., Zeiher A.M. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.CIR.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani A.D., Cross J., Kharbanda R.K., Mullen M.J., Bhagat K., Taylor M., Donald A.E., Palacios M., Griffin G.E., Deanfield J.E., et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.CIR.102.9.994. [DOI] [PubMed] [Google Scholar]
- 16.Szwed P., Gasecka A., Zawadka M., Eyileten C., Postula M., Mazurek T., Szarpak L., Filipiak K.J. Infections as Novel Risk Factors of Atherosclerotic Cardiovascular Diseases: Pathophysiological Links and Therapeutic Implications. J. Clin. Med. 2021;10:2539. doi: 10.3390/jcm10122539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg A. Concordant occurrence of gastric and hypertensive diseases. Gastroenterology. 1988;95:42–48. doi: 10.1016/0016-5085(88)90288-0. [DOI] [PubMed] [Google Scholar]
- 18.Barnes R.J., Uff J.S., Dent J.C., Gear M.W., Wilkinson S.P. Long-term follow up of patients with gastritis associated with Helicobacter pylori infection. Br. J. Gen. Pract. 1991;41:286–288. [PMC free article] [PubMed] [Google Scholar]
- 19.Sternby N.H. Atherosclerosis and peptic ulcer. Bull World Health Organ. 1976;53:571–577. [PMC free article] [PubMed] [Google Scholar]
- 20.Danesh J., Youngman L., Clark S., Parish S., Peto R., Collins R. Helicobacter pylori infection and early onset myocardial infarction: Case-control and sibling pairs study. BMJ. 1999;319:1157–1162. doi: 10.1136/bmj.319.7218.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasceri V., Cammarota G., Patti G., Cuoco L., Gasbarrini A., Grillo R.L., Fedeli G., Gasbarrini G., Maseri A. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation. 1998;97:1675–1679. doi: 10.1161/01.CIR.97.17.1675. [DOI] [PubMed] [Google Scholar]
- 22.Dore M.P., Sepulveda A.R., Bacciu P.P., Blasi F., Simula L., Marras L., Piccolo D., Cherchi G.B., Graham D.Y., Realdi G. Detection of Chlamydiae pneumoniae but not Helicobacter pylori DNA in atherosclerosis plaques. Dig. Dis. Sci. 2003;48:945–951. doi: 10.1023/A:1023059815117. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi F., Sepulveda A.R., Gasbarrini A., Pola P., Silveri N.G., Gasbarrini G., Graham D.Y., Genta R.M. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: Possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 2002;106:430–434. doi: 10.1161/01.CIR.0000024100.90140.19. [DOI] [PubMed] [Google Scholar]
- 24.Wang B., Yu M., Zhang R., Chen S., Xi Y., Duan G. A meta-analysis of the association between Helicobacter pylori infection and risk of atherosclerotic cardiovascular disease. Helicobacter. 2020;25:e12761. doi: 10.1111/hel.12761. [DOI] [PubMed] [Google Scholar]
- 25.Shi H., Li Y., Dong C., Si G., Xu Y., Peng M., Li Y. Helicobacter pylori infection and the progression of atherosclerosis: A systematic review and meta-analysis. Helicobacter. 2022;27:e12865. doi: 10.1111/hel.12865. [DOI] [PubMed] [Google Scholar]
- 26.Wan Z., Hu L., Hu M., Lei X., Huang Y., Lv Y. Helicobacter pylori infection and prevalence of high blood pressure among Chinese adults. J. Hum. Hypertens. 2018;32:158–164. doi: 10.1038/s41371-017-0028-8. [DOI] [PubMed] [Google Scholar]
- 27.Vahdat K., Pourbehi M.R., Ostovar A., Hadavand F., Bolkheir A., Assadi M., Farrokhnia M., Nabipour I. Association of pathogen burden and hypertension: The Persian Gulf Healthy Heart Study. Am. J. Hypertens. 2013;26:1140–1147. doi: 10.1093/ajh/hpt083. [DOI] [PubMed] [Google Scholar]
- 28.Lip G.H., Wise R., Beevers G. Association of Helicobacter pylori infection with coronary heart disease. Study shows association between H pylori infection and hypertension. BMJ. 1996;312:250–251. doi: 10.1136/bmj.312.7025.250b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunji T., Matsuhashi N., Sato H., Fujibayashi K., Okumura M., Sasabe N., Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am. J. Gastroenterol. 2008;103:3005–3010. doi: 10.1111/j.1572-0241.2008.02151.x. [DOI] [PubMed] [Google Scholar]
- 30.Migneco A., Ojetti V., Specchia L., Franceschi F., Candelli M., Mettimano M., Montebelli R., Savi L., Gasbarrini G. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter. 2003;8:585–589. doi: 10.1111/j.1523-5378.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu L., Liu Y., Tong W., Ye H., Zhang X., Cao W., Zhang Y. Pathogen burden in essential hypertension. Circ. J. 2007;71:1761–1764. doi: 10.1253/circj.71.1761. [DOI] [PubMed] [Google Scholar]
- 32.Tang H., Wang A., Bai S., Liu L., Tong W., Zhang Y. Relationship between pathogenic infection and hypertension in Mongolian. J. Chin. J. Public Health. 2010;26:295–296. [Google Scholar]
- 33.Dore M.P., Cipolli A., Ruggiu M.W., Manca A., Bassotti G., Pes G.M. Helicobacter pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions: A retrospective study. Medicine. 2018;97:e9734. doi: 10.1097/MD.0000000000009734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dore M.P., Pes G.M. What Is New in Helicobacter pylori Diagnosis. An Overview. J. Clin. Med. 2021;10:2091. doi: 10.3390/jcm10102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touboul P.J., Hennerici M.G., Meairs S., Adams H., Amarenco P., Bornstein N., Csiba L., Desvarieux M., Ebrahim S., Hernandez Hernandez R., et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012;34:290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eigenbrodt M.L., Bursac Z., Eigenbrodt E.P., Couper D.J., Tracy R.E., Mehta J.L. Mathematical estimation of the potential effect of vascular remodelling/dilatation on B-mode ultrasound intima-medial thickness. QJM. 2004;97:729–737. doi: 10.1093/qjmed/hch120. [DOI] [PubMed] [Google Scholar]
- 37.Hollander M., Bots M.L., Del Sol A.I., Koudstaal P.J., Witteman J.C., Grobbee D.E., Hofman A., Breteler M.M. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: The Rotterdam study. Circulation. 2002;105:2872–2877. doi: 10.1161/01.CIR.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 38.Realdi G., Dore M.P., Fastame L. Extradigestive manifestations of Helicobacter pylori infection: Fact and fiction. Dig. Dis. Sci. 1999;44:229–236. doi: 10.1023/A:1026677728175. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi F., Covino M., Roubaud Baudron C. Review: Helicobacter pylori and extragastric diseases. Helicobacter. 2019;24((Suppl. 1)):e12636. doi: 10.1111/hel.12636. [DOI] [PubMed] [Google Scholar]
- 40.Kountouras J., Papaefthymiou A., Polyzos S.A., Deretzi G., Vardaka E., Soteriades E.S., Tzitiridou-Chatzopoulou M., Gkolfakis P., Karafyllidou K., Doulberis M. Impact of Helicobacter pylori-Related Metabolic Syndrome Parameters on Arterial Hypertension. Microorganisms. 2021;9:2351. doi: 10.3390/microorganisms9112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang M., Zhu L., Jin Y., Fang Z., Chen Y., Yao Y. Association between Helicobacter Pylori Infection and Systemic Arterial Hypertension: A Meta-Analysis. Arq. Bras. Cardiol. 2021;117:626–636. doi: 10.36660/abc.20200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong X., Chen J., He M., Wu T., Yang H. Helicobacter pylori infection and the prevalence of hypertension in Chinese adults: The Dongfeng-Tongji cohort. J. Clin. Hypertens. 2020;22:1389–1395. doi: 10.1111/jch.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvey R., Lane A., Murray L., Harvey I., Nair P., Donovan J. Effect of Helicobacter pylori infection on blood pressure: A community based cross sectional study. BMJ. 2001;323:264–265. doi: 10.1136/bmj.323.7307.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim T.J., Lee H., Kang M., Kim J.E., Choi Y.H., Min Y.W., Min B.H., Lee J.H., Son H.J., Rhee P.L., et al. Helicobacter pylori is associated with dyslipidemia but not with other risk factors of cardiovascular disease. Sci. Rep. 2016;6:38015. doi: 10.1038/srep38015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu C., Yan M., Sun Y., Joo J., Wan X., Yu C., Wang Q., Shen C., Chen P., Li Y., et al. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter. 2014;19:437–442. doi: 10.1111/hel.12153. [DOI] [PubMed] [Google Scholar]
- 46.Kopacova M., Koupil I., Seifert B., Fendrichova M.S., Spirkova J., Vorisek V., Rejchrt S., Douda T., Tacheci I., Bures J. Blood pressure and stature in Helicobacter pylori positive and negative persons. World J. Gastroenterol. 2014;20:5625–5631. doi: 10.3748/wjg.v20.i19.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W., Lu H., Graham D.Y. An Update on Helicobacter pylori as the Cause of Gastric Cancer. Gastrointest. Tumors. 2014;1:155–165. doi: 10.1159/000365310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valle J., Kekki M., Sipponen P., Ihamaki T., Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand. J. Gastroenterol. 1996;31:546–550. doi: 10.3109/00365529609009126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.