Abstract
Simple Summary
Rhinosinusitis and smell alterations are common side effects during and after radiotherapy and chemotherapy for head and neck cancer. The assessment of sinonasal complaints is important to increase patients’ quality of life. The aim of this review is to summarize and analyze our current knowledge of the sinonasal side effects of chemotherapy and/or radiation therapy for head and neck cancer, with a specific focus on mucosal and olfactory disorders.
Abstract
Radiotherapy and chemotherapy represent important treatment modalities for head and neck cancer. Rhinosinusitis and smell alterations are common side effects in the sinonasal region. This review will summarize and analyze our current knowledge of the sinonasal side effects of chemotherapy and/or radiation therapy for head and neck cancer (HNC), with a specific focus on mucosal and olfactory disorders. A review of the English literature was performed using several databases (PubMed, Embase, Cochrane, Scopus). Fifty-six articles were included in qualitative synthesis: 28 assessed mucosal disorders (rhinitis or rhinosinusitis), 26 evaluated olfactory alterations, and 2 articles addressed both topics. The incidence and severity of olfactory dysfunction and chronic rhinosinusitis were highest at the end of radiotherapy and at three months after treatment and decreased gradually over time. Smell acuity deterioration and chronic rhinosinusitis seemed to be related to radiation dose on olfactory area and nasal cavities, but different degrees of recovery were observed. In conclusion, it is important to establish the severity of chronic rhinosinusitis and olfactory dysfunction in order to find strategies to support patients and improve their quality of life.
Keywords: head and neck cancer, radiotherapy, rhinitis, rhinosinusitis, chemotherapy, smell, olfactory disorders, mucociliary clearance
1. Introduction
Head and neck cancer (HNC) is the sixth most common type of cancer worldwide and consists of a heterogeneous group of malignancies that may determine important morbidity to affected patients [1,2]. Smoking and alcohol consumption represent the major risk factors for the development of squamous cell carcinoma (SCC) in the head and neck district. Human papillomavirus (HPV) infection plays a role as an etiologic factor of oropharyngeal SCC, especially in tonsils and the tongue base [3].
HNC treatment includes surgery, radiotherapy (RT), and chemotherapy (CT), which are employed according to the tumor stage and primary site involved [4]. The management of early-stage cancer is usually a single modality, either surgery or radiotherapy. On the contrary, the locally advanced tumor has a multimodal treatment: either surgery followed by adjuvant radiotherapy or chemo-radiotherapy (CT-RT), or definitive CT-RT. For recurrent and metastatic diseases that are not susceptible to surgical approaches, chemotherapy or immunotherapy is suggested. Finally, in the last decade, electrochemotherapy (ECT) emerged as a curative or palliative treatment for selected cases of recurrent oral and oropharyngeal cancer [5,6].
Surgery remains the main treatment modality for most of HNC. However, radiotherapy provides an important contribution to HNC management. The radiation dose usually ranges from 60 to 70 Gy, mainly depending on adjuvant or definitive initial intent [4]. The risk of long-term toxicity from RT is dose-dependent with organ-specific susceptibility. Indeed, organs at risk (OARs) protection should be adequately planned before starting RT. The introduction of intensity-modulated radiotherapy (IMRT) allowed us to reduce side effects, providing better balance between target coverage and the sparing of adjacent organs (OARs) [7]. In particular, IMRT guaranteed fewer xerostomia and dysphagia [8].
Platinum-based compounds represent the standard radiosensitizer regimen in the treatment of HNC. Chemotherapeutic drugs such as cisplatin, 5-fluorouracil, and docetaxel may be administered as inductive chemotherapy in the selected case of locally advanced HNC or in recurrent/metastatic tumors [4]. Reducing RT and CT side effects is important to avoid treatment breaks that may negatively impact clinical outcomes [9]. For example, platinum-based compound ototoxicity should be taken into consideration during treatments [4].
Smell and taste alterations are common side effects in patients undergoing CT and/or RT for HNC [10,11]. Olfactory and gustatory dysfunction negatively affects appetite driving to an inadequate food intake, and consequent weight loss. Finally, smell and taste changes may cause a deterioration in quality of life [12].
Radiation therapy determines the damages of irradiated mucosa. This can sometimes lead to severe complaints during and immediately after RT for HNC. Moreover, concomitant CT can induce more toxicity at a cumulative dose, due to a cytotoxic effect on rapidly growing non-cancer cells, such as mucosal cells [13]. In the sinonasal district, CT-RT determines the destruction of mucosal cilia of epithelial cells, leading to the impairment of mucociliary clearance, which increases the risk of developing chronic rhinosinusitis [14]. Moreover, an inflammatory infiltrate can be observed at nasal cytology [15].
This literature review will summarize and analyze our current knowledge of the sinonasal side effects of chemotherapy and/or radiation therapy for HNC, with a specific focus on mucosal and smell disorders.
2. Materials and Methods
A review of the English literature was performed using several databases (PubMed, Embase, Cochrane, Scopus, accessed on 31 December 2021) in order to identify articles published before 31 December 2021.
A primary search was performed using the terms “(head and neck cancer) AND (chemotherapy OR radiotherapy OR radiation therapy) AND (nasal OR sinus OR rhinitis OR rhinosinusitis OR smell OR olfactory)”. Search strategies were adapted for each database.
The inclusion criteria were clinical trials, cohort studies, case-control studies, and case series, regarding olfactory dysfunction and chronic rhinosinusitis as side effects of CT and/or RT in HNC patients. Exclusion criteria were as follows: non-human studies, non-English literature, mucosal and olfactory disorders not related to CT and/or RT for HNC.
The abstracts of all suitable articles were examined using the inclusion criteria for applicability. The references of the selected publications were reviewed, in order to identify further reports that were not found by database searching. Two independent reviewers (GR, EC), working separately extracted the data from all the eligible studies, which were subsequently cross-checked. All retrieved full-texts articles were included in the review by a consensus of all the authors. The review included prospective, cross-sectional, and retrospective studies. No studies concerning the sinonasal side effects of target therapy (cetuximab) and immunotherapy were found.
3. Results
3.1. Literature Review
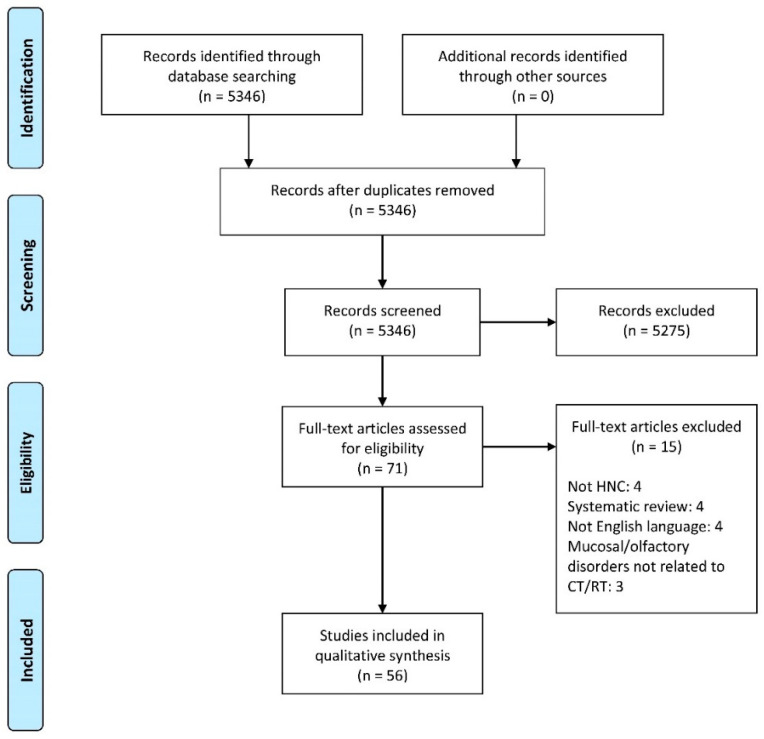
A total of 5346 published papers were identified using database searches (Figure 1). After abstract screening for eligibility, 71 articles were considered eligible. Among these, we included 56 articles in qualitative synthesis after a full-text assessment. The other 15 papers were excluded because they were systematic reviews (4), they were not in English (4), they did not include HNC patients (4), or because mucosal and/or olfactory disorders were not related to CT and/or RT for HNC (3).
Figure 1.
Flow diagram of the included studies.
The included studies were published between 1975 and 2021 and conducted in several countries worldwide. Among the 56 selected studies, 28 assessed mucosal disorders (rhinitis or rhinosinusitis), 26 evaluated olfactory alterations, and 2 articles addressed both topics [15,16]. Table 1 highlights the main results concerning sinonasal mucosa disorders, while Table 2 reports publications on olfactory dysfunction.
Table 1.
Sinonasal mucosa disorders: studies included in the review.
| Author, Year, Country |
Study Design |
Number of Patients |
Sex | Age, Mean and Range/ Standard Deviation (Years) |
Tumor (Site and Stage) | Treatments | Measurements | Time of Assessment |
Results |
|---|---|---|---|---|---|---|---|---|---|
| Stringer et al., 1995, USA [17] | Cross-sectional | Study group: n = 9 Control group: n = 9 |
Study group M: 6 (77%) F: 3 (33%) Control group: NR |
Study group 80 (36–81) Control group: NR |
Nasal vestibule or ala (n = 7), nasal cavity (n = 2), nasopharynx (n = 1) (stage NR) |
RT (63.8–74.8 Gy) Dose to nasal cavities: NR |
|
Before RT (subjective symptoms) and 20–117 months after RT (saccharine test and subjective symptoms) |
|
| Lou et al., 1999, Taiwan [18] | Cross-sectional | Study group: n = 10 (all with sinusitis) Control group: n = 6 (3 patients with sinusitis and 3 without) |
Study group M: 7 (70%) F: 3 (30%) Control group: NR |
Study group 45 (28–70) Control group: NR |
Nasopharynx (stage NR) |
RT (70–80 Gy) Dose to nasal cavities: Mean dose to infundibulum 21 Gy (17.5–25 Gy) |
Biopsy of infundibulum mucosa (light and electron microscope views) | 5.9 (0.8–23) years after RT |
|
| Kamel et al., 2004, Egypt [19] | Retrospective | n = 32 | M: 19 (59%) F: 13 (39%) |
36 (7–65) | Nasopharynx (stage NR) |
RT (doses NR) Dose to nasal cavities: NR |
|
Group I (n = 23): Saccharine test and nasal endoscopy before RT and at 2–6 weeks, 3 and 6 months, 1 and 2 years after RT; Computed Tomography scan 6–12 months after RT Group II (n = 9): 4–12 years after RT |
|
| Gupta et al., 2006, India [20] | Prospective | Study group: n = 50 Control group: n = 20 |
Study group M: 35 (70%) F: 15 (30%) Control group: NR |
Study group 54.7 (35–78) Control group: NR |
Larynx (n = 19), oropharynx (n = 15), oral cavity (n = 10), hypopharynx (n = 6) (stage I–IV) |
RT (n = 14) (14–70 Gy) CT-RT (cisplatin, 5-fluorouracil, methotrexate) (n = 33) Surgery + RT (n = 3) Dose to nasal cavities: NR |
MCC (saccharine test) | Before RT and 6 months after RT |
|
| Hsin et al., 2007, Taiwan [21] |
Cross-sectional | n = 20 | M: 12 (60%) F: 8 (40%) |
47.5 (22–69) | Nasopharynx (stage NR) |
RT (70–76 Gy):
NR |
|
4.9 (0.5–21) years after RT |
|
| Huang et al., 2007, Taiwan [22] | Retrospective | n = 112 | M: 77 (69%) F: 35 (31%) |
47.9 (18.9–76.2) | Nasopharynx (stage I–IV) |
RT (64–76 Gy):
Dose to nasal cavities: NR |
MRI scan (Lund-Mackay score) | Before RT, and at 3 months, 9 months, 2 years, 3 years, 4 years, and 5 years after RT |
|
| Hu et al., 2008, Taiwan [23] | Prospective | Study group: n = 21 Control group: n = 10 |
Study group M: 13 (62%) F: 8 (28%) Control group: NR |
Study group 49.5 (43–58) Control group: NR |
Nasopharynx (stage NR) |
RT (70–80 Gy) Dose to nasal cavities: NR |
|
Before and 1 year after FESS 2.1 (1.2–4.0) years between RT and FESS |
|
| Liang et al., 2008, Taiwan [24] |
Cross-sectional | Non irrigation group: n = 63 Irrigation group: n = 44 |
Non irrigation group M: 49 (78%) F: 14 (12%) Irrigation group M: 35 (79%) F: 9 (21%) |
47.7 (17–81) Non irrigation group: 49.13 ± 1.81 Irrigation group: 45.61 ± 1.68 |
Nasopharynx (stage I–IV) |
IMRT (56–76.8 Gy):
NR |
|
Before, at mid-course, and at the end of RT, 1, 2, 3, 6, and 12 months after RT (Computed tomography before RT and 3, 6 and 12 months after RT) |
|
| Deng et al., 2009, China [25] |
Cross-sectional |
n = 60 Post-RT CRS group: n = 30 CRS group: n = 30 |
Post-RT CRS group: M: 24 (80%) F: 6 (20%) CRS group: M: 23 (77%) F: 7 (23%) |
Post-RT CRS group: 42.7 (23–70) CRS group: 33.8 (21–59) |
Nasopharynx (stage NR) |
RT (66–74 Gy) Adjuvant CT in 11 patients (5-fluorouracil and cisplatin) Dose to nasal cavities: NR |
Cultures (maxillary sinus specimens) | 2.92 (0.5–8.5) years after RT |
|
| Lee et al., 2012, Taiwan [26] | Retrospective | n = 188 | M: 132 (70%) F: 56 (30%) |
49.49 (17–78) | Nasopharynx (stage I–IV) |
2D-RT, 3D-CRT or IMRT (69.91±3.87 Gy):
NR |
|
7.34 (3.30–26.54) years after RT |
|
| Xiang et al., 2013, China [27] | Retrospective | n = 40 | M: 22 (55%) F: 18 (45%) |
46 (23–65) | Nasopharynx (stage NR) |
RT (68–72 Gy) Dose to nasal cavities: NR |
|
3.4 (0–9) months after RT |
|
| Su et al., 2014, China [28] | Retrospective | n = 283 | M: 215 (76%) F: 68 (24%) |
48 (11–77) | Nasopharynx (stage II–IV) |
IMRT (70.4–74.8 Gy):
NR |
|
Before and 1, 3, 6, 9, 12, and 18 months after RT |
|
| Kılıç et al., 2014, Turkey [29] | Cross-sectional | n = 44 | NPC group: M: 32 (45%) F: 12 (55%) Laryngeal cancer group: M: 22 (100%) |
NPC group: 36 (18–63) Laryngeal cancer group: 56 (44–72) |
Nasopharynx (n = 22), larynx (n = 22) (stage II–IV) |
RT (70 Gy) Total laryncetomy and adjuvant RT in laryngeal cancer group Dose to nasal cavities: NR |
MCC (saccharine test) | Before and 3 and 6 months after RT |
|
| Lou et al., 2014, China [30] | Cross-sectional |
n = 1134 Group A (nasal irrigator): n = 378 Group B (homemade nasal irrigation connector combined with enemator): n = 378 Group C (nasal sprayer): n = 378 |
M: 826 (73%) F: 308 (27%) Group A: M: 268 (71%) F: 110 (29%) Group B: M: 273 (72%) F: 105 (28%) Group C: M: 285 (75%) F: 93 (25%) |
48 (12–84) Group A: 43 (13–82) Group B: 51 (12–81) Group C: 49 (13–84) |
Nasopharynx (stage I–IV) |
RT (66–70 Gy):
Dose to nasal cavities: NR |
|
Before, and 6 months, 1, 2 and 3 years after RT |
|
| Alon et al., 2014, Israel [31] | Retrospective | n = 62 | M: 42 (68%) F: 20 (32%) |
42 (11–74) | Nasopharynx (stage I–IV) |
2D-RT/3D-CRT (n = 40 or) IMRT (n = 22) (66–72.4 Gy):
NR |
|
7 (3–16) years after RT |
|
| Hsin et al., 2015, Taiwan [32] | Retrospective | n = 102 | M: 74 (73%) F: 28 (27%) |
43.5 (19–74) | Nasopharynx (stage I–IV) |
IMRT (68–81 Gy):
NR |
MRI scan (Lund-Mackay score) | Before and 5 years after RT |
|
| Riva et al., 2015, Italy [33] | Cross-sectional | Study group: n = 30 Control group: n = 30 |
Study group M: 24 (80%) F: 6 (20%) Control group M: 20 (67%) F: 10 (33%) |
Study group 53.53 (37–75) Control group 52.35 (42–76) |
Nasopharynx (stage I–IV) |
2D-RT (n = 5), 3D-CRT (n = 5), IMRT (n = 20) RT dose: 69.34 ± 1.17 Gy
NR |
|
59 (21–124) months after RT |
|
| Wang et al., 2015, Taiwan [16] |
Prospective | n = 41 | M: 31 (76%) F: 10 (24%) |
45 (29–77) | Nasopharynx (stage I–IV) |
IMRT (70–76.8 Gy):
NR |
|
Before and 12 months after RT |
|
| Hsin et al., 2016, Taiwan [34] | Retrospective | n = 94 | M: 67 (71%) F: 27 (29%) |
42.7 (20–74) | Nasopharynx (stage I–IV) |
IMRT (68–81 Gy):
NR |
MRI scan (Lund-Mackay score) | Before and 3 months, 1, 3, and 5 years after RT |
|
| Feng et al., 2016, China [35] | Prospective | Intranasal steroid group (fluticasone propionate): n = 32 Nasal irrigation group: n = 31 |
Intranasal steroid group M: 13 (41%) F: 19 (59%) Nasal irrigation group M: 14 (45%) F: 17 (55%) |
Intranasal steroid group: 38.86 ± 9.26 Nasal irrigation group: 39.36 ± 7.28 |
Nasopharynx (stage I–IV) |
RT:
NR |
|
Before, and 3 and 6 months after RT |
|
| Riva et al., 2017, Italy [36] | Cross-sectional | Study group: n = 25 Control group: n = 25 |
Study group M: 22 (88%) F: 3 (12%) Control group M: 19 (76%) F: 6 (24%) |
Study group: 68.76 (50–83) Control group: 62.64 (48–76) |
Larynx (stage II–IV) |
Total laryngectomy:
Dose to nasal cavities: NR |
|
52 (26–97) months after treatment |
|
| Kuhar et al., 2017, USA [37] | Retrospective |
n = 114 CRSr: n = 15 CRSsNP: n = 43 CRSwNP: n = 56 |
CRSr: M: 6 (41%) F: 9 (59%) CRSsNP: M: 21 (49%) F: 22 (51%) CRSwNP: M: 25 (45%) F: 31 (55%) |
CRSr: 58.1 (range NR) CRSsNP: 50.3 (range NR) CRSwNP: 50.9 (range NR) |
Nasal cavity and paranasal sinuses (n = 12), nasopharynx (n = 1), skull base (n = 1), oral cavity (n = 1) (stage I–IV) |
RT (30.75–129 Gy) Dose to nasal cavities: NR |
|
5.73 ± 7.2 years after RT |
|
| Park et al., 2018, South Korea [14] | Retrospective |
n = 186 RT group: n = 143 Non-RT group: n = 43 |
M: 162 (87%) F: 24 (13%) RT group: M: 124 (87%) F: 19 (13%) Non-RT group: M: 38 (88%) F: 5 (12%) |
60.4 (47–83) RT group: 59.09 ± 11.64 Non-RT group: 64.70 ± 8.28 |
Nasopharynx (n = 24), oral cavity (n = 31), oropharynx (n = 46), hypopharynx (n = 23), larynx (n = 62) (stage I–IV) |
RT group (60–70.4 Gy):
Dose to nasal cavities: NR |
|
Every 3 months for 3 years after RT |
|
| Shemesh et al., 2018, Israel [38] | Prospective | n = 9 | M: 5 (55%) F: 4 (45%) |
44.2 (15–74) | Nasopharynx (stage I–IV) |
RT (66–70 Gy):
NR |
|
Before and 6 months after surgery |
|
| Hamilton et al., 2019, Canada [39] | Retrospective | n = 162 | M: 80 (49%) F: 82 (51%) |
31 (15–35) | Nasopharynx (n = 48), nasal cavity and paranasal sinuses (n = 9), oral cavity (n = 21), tonsil (n = 4), larynx (n = 11), salivary glands (n = 36), thyroid (n = 30), other (n = 3) (stage I–IV) |
RT (40–70 Gy):
CT (platinum-based) in 17 patients Dose to nasal cavities: NR |
Clinical examination | Median follow-up: 6.4 years |
|
| Stoddard et al., 2019, USA [40] | Retrospective | n = 22 | M: 14 (67%) F: 8 (43%) |
68.8 (50–88) | Nasal cavity, paranasal sinuses | RT (14.4–184.8 Gy):
NR |
Sinonasal swab specimens (routine culture and next-generation molecular gene pyrosequencing) | 81.2 (1–156) weeks after RT |
|
| Riva et al., 2019, Italy [15] |
Prospective | n = 10 | M: 10 (100%) | 56.90 (39–72) | Nasopharynx (n = 3), oral cavity (n = 3), parotid gland (n = 3), primary unknown (n = 1) (stage I–IV) |
Surgery (n = 8) Concurrent CT-RT (54–70 Gy) (n = 5) Induction CT + concurrent CT-RT (n = 1) Dose to o nasal cavities:
|
|
Before (T0), at mid-course (T1), and at the end (T2) of RT, 1 and 3 months after RT (T3 and T4) |
|
| Huang et al., 2019, Taiwan [41] | Retrospective | n = 230 | M: 177 (77%) F: 53 (23%) |
48.5 (18–80) | Nasopharynx (stage I–IV) |
IMRT (54.45–70 Gy):
NR |
Computed Tomography/MRI scan | Before and more than 6 months after RT |
|
| Lu et al., 2020, Taiwan [42] | Retrospective | n = 701 | M: 625 (89%) F: 76 (11%) RT alone: M: 41 (82%) F: 9 (18%) Any-RT: M: 262 (89%) F: 33 (11%) No RT: M: 322 (90%) F: 37 (10%) |
NR (>20) | Oral cavity (n = 479), nasopharynx (n = 97), hypopharynx (n = 59), oropharynx (n = 43), larynx (n = 32) |
(RT doses NR) Dose to nasal cavities: NR |
Clinical examination | More than 3 months after treatment |
|
| Yin et al., 2020, China [43] | Cross-sectional | n = 66 | M: 46 (70%) F: 20 (30%) |
38.76 (25–45) | Nasopharynx (stage I–IV) |
IMRT (RT dose NR) CT (cisplatin and 5-fluorouracil) in 43 patients Dose to nasal cavities: 36.46 (23.14–56.38) Gy |
|
Before RT, and at the end of RT, and 3, 6, and 12 months after RT |
|
Abbreviations: 2D-RT, Two-dimensional Radiotherapy; 3D-CRT, Three-Dimensional Conformal Radiotherapy; CRS, Chronic Rhinosinusitis; CRSr, radiation-induced Chronic Rhinosinusitis; CRSsNP, Chronic Rhinosinusitis without Nasal Polyps, CRSwNP, Chronic Rhinosinusitis with Nasal Polyps; CT, Chemotherapy; CT-RT, Chemoradiotherapy; F, Female; FESS, Functional Endoscopic Sinus Surgery; Gy, Gray; IMRT, Intensity Modulated Radiation Therapy; M, Male; MCC, Mucociliary Clearance; MRI, Magnetic Resonance Imaging; NOSE, Nasal Obstruction Symptom Evaluation; NPC, Nasopharyngeal carcinoma; NR, Not reported; OI, Odor identification; RT, Radiotherapy; SNOT, Sino-Nasal Outcome Test; UPSIT, University of Pennsylvania Smell Identification Test; VAS, Visual Analog Scale.
Table 2.
Olfactory dysfunction: studies included in the review.
| Author, Year, Country | Study Design |
Number of Patients |
Sex | Age, Mean and Range/ Standard Deviation (Years) |
Tumor (Site and Stage) |
Treatments |
Measurements |
Time of Assessment |
Results |
|---|---|---|---|---|---|---|---|---|---|
| Ophir et al., 1988, Israel [44] | Prospective | n = 12 | M: 9 (75%) F: 3 (25%) |
54.8 (38–76) | Nasopharynx (n = 9), pituitary gland (n = 7) (stage NR) |
2D-RT (66 Gy) No CT Dose to olfactory area: 25–28 Gy (nasopharyngeal carcinoma), 18–22 Gy (pituitary adenoma) |
ODT (amyl acetate and eugenol) | Before RT, within a week after RT end, 1, 3 and 6 months later |
|
| Sagar et al., 1991, UK [45] | Retrospective | Study group: n = 25 Control group: n = 40 |
NR | NR | Nasopharynx, pituitary fossa, maxillary sinus (n = 25) (stage NR) |
2D-RT (doses NR) No CT Dose to olfactory area: 50–75 Gy (study group) |
Self-reported smell (ad hoc questionnaire) | During RT |
|
| Hua et al., 1999, China [46] |
Prospective | Study group (n = 49):
n = 36 |
Group 1 M: 16 (67%) F: 8 (33%) Group 2 M: 23 (92%) F: 2 (8%) Control group M: 26 (72%) F: 10 (28%) |
Group 1 40.9 (27–59) Group 2 45.2 (28–60) Control group 43.6 (28–67) |
Nasopharynx (T1-T3) |
2D-RT (68–72 Gy) CT: NR Dose to olfactory area: NR |
ODT (N-butyl alcohol), Odour Quality Discrimination test (5 odorants), Odour Recognition Memory Test, Odour-Visual Matching test, Odour-Tactile Matching test, OI (10 odorants), Odour Function test (edibility, function and identity) | Before RT (n = 24 NPC, group 1), after RT (n = 25 NPC, group 2) | NPC patients with RT had olfactory impairments including ODT, odour-tactile cross-modality matching, verbal identification of odours, recall and recognition of identity of odours |
| Ho et al., 2002, China [47] |
Prospective | n = 48 | M: 23 (48%) F: 25 (52%) |
46 (22–71) | Nasopharynx (stage I–IV) |
RT (n = 43) (doses NR) CT-RT (n = 15 Dose to olfactory area: NR |
|
Before RT, end of RT, 3, 6 and 12 months after RT |
|
| Hölscher et al., 2005, Germany [48] | Prospective | n = 44 | M: 28 (64%) F: 16 (36%) |
55 (11–81) | Maxillary sinus (n = 10), oropharynx (n = 10), oral cavity (n = 5), paranasal sinus (n = 5), nasopharynx (n = 6), hypopharynx (n = 2), nasal cavity (n = 1), brain (n = 1), skin (n = 1), unknown primary (n = 1), other (n = 2) (stage NR) |
3D-CRT (30–76 Gy) (n = 30) CT-RT (n = 14) Dose to olfactory area:
|
ODT, OI, and OD (Sniffin’ Sticks) |
|
|
| Sandow et al., 2006, USA [49] | Prospective | Study group: n = 13 Control group: n = 5 |
Study group M: 10 (77%) F: 3 (23%) Control group M: 3 (60%) F: 2 (40%) |
Study group 51.6 (40–75) Control group 47.9 (27–70) |
Oropharynx (stage NR) |
3D-CRT (63–76 Gy) CT-RT (cisplatin, n = 3) Dose to olfactory area: NR |
OI (UPSIT) | Before RT, 1, and 12 months after RT | OI was unaffected by RT |
| Bindewald et al., 2007, Germany [50] |
Cross-sectional | n = 205 | M: 190 (93%) F: 15 (7%) |
64 (32–84) | Larynx (stage I–IV) |
Total laryngectomy (n = 20) Total laryngectomy + RT (n = 72) Partial laryngectomy (n = 77) Partial laryngectomy + RT (n = 36) (doses NR) CT: NR Dose to olfactory area: NR |
Self-reported smell (EORTC QLQ-H&N35) |
|
No differences in olfactory alterations between irradiated and non-irradiated patients |
| Rhemrev et al., 2007, The Netherlands [51] |
Cross-sectional | n = 72 | M: 44 (61%) F: 28 (39%) |
57 (33–79) | Oral cavity, oropharynx (stage I–IV) |
Surgery (n = 15) Surgery + RT (n = 57) (66–70 Gy) CT: NR Dose to olfactory area: NR |
Self-reported smell (EORTC QLQ-H&N35) | 43 (2–120) months after treatment | Higher olfactory alterations in irradiated patients |
| Brämerson et al., 2013, Sweden [52] | Prospective | n = 71 | M: 51 (72%) F: 20 (28%) |
60.9 (35–86) | Paranasal sinuses (n = 10), parotid gland/ear/facial skin (n = 8), oral cavity (n = 12), nasopharynx/larynx (n = 15), oropharynx (n = 26) (stage NR) |
RT (n = 39) (doses NR) CT-RT (platinum compounds, pyrimidine compounds and taxanes, n = 32) Dose to olfactory area:
|
|
Before RT and 20 (12–35) months after RT |
|
| Momeni et al., 2013, USA [53] |
Cross-sectional | n = 21 | M: 15 (71%) F: 6 (29%) |
57.9 (24–87) | Oral cavity (n = 15), esophagus (n = 2), scalp (n = 2), pharynx (n = 1), paranasal sinus (n = 1) (stage NR) |
Surgery (n = 8) Surgery + RT (n = 13) (doses NR) CT: NR Dose to olfactory area: NR |
Self-reported smell (EORTC QLQ-H&N35) | 24 (18–48) months after treatment | No differences in olfactory alterations between irradiated and non-irradiated patients |
| Oskam et al., 2013, The Netherlands [54] | Prospective | n = 80 | M: 47 (59%) F: 33 (41%) |
58 (23–74) | Oropharynx (n = 42), oral cavity (n = 38) (stage II–IV) |
Surgery + RT (doses NR) CT: NR Dose to olfactory area: NR |
Self-reported smell (EORTC QLQ-H&N35) |
|
No statistically significant difference in taste/smell score among evaluations over time, but a deterioration was present after treatment |
| Jalali et al., 2014, Iran [55] |
Prospective | n = 54 | M: 26 (48%) F: 28 (52%) |
49 (22–86) | Nasopharynx (n = 24), oropharynx (n = 6), paranasal sinus (n = 12), brain (n = 9), skin (n = 3) (stage NR) |
RT (n = 30) CT-RT (n = 24) 12 patients with previous surgery Total RT dose: 50.1 Gy (range: 30–66 Gy) Dose to olfactory area: 334 μC (IQR 162–2068 μC) |
ODT (N-butanol) | Before RT, during RT (2,4, 6 weeks), and after RT (3 and 6 months) |
|
| Veyseller et al., 2014, Turkey [56] |
Cross-sectional | Study group: n = 24 Control group: n = 14 |
Study group M: 14 (56%) F: 10 (44%) Control group M: 5 (36%) F: 9 (64%) |
Study group 48.7 ± 11.4 Control group 48.8 ± 7.0 |
Nasopharynx (stage I–IV) |
CT-RT (68–72 Gy):
|
|
66 (14–218) months after RT |
|
| Riva et al., 2015, Italy [57] |
Cross-sectional | Study group: n = 30 Control group: n = 30 |
Study group M: 24 (80%) F: 6 (20%) Control group M: 20 (67%) F: 10 (33%) |
Study group 53.5 (37–75) Control group 52.3 (42–76) |
Nasopharynx (stage I–IV) |
CT-RT (cisplatin-based regimens):
|
|
59 (24–124) months after RT |
|
| Landström et al., 2015, Sweden [58] | Prospective | n = 19 | M: 12 (63%) F: 7 (37%) |
56.6 (20–78) | Oral cavity (n = 18), oropharynx (n = 1) (stage I–IV) |
ECT (bleomycin) (n = 6) ECT (bleomycin) + RT (57.8 Gy) (n = 13) Dose to olfactory area: NR |
Self-reported smell (EORTC QLQ-H&N35) | Before treatment, and 12 months after treatment | No differences in problems with senses from baseline to 12 months after treatment |
| Haxel et al., 2015, Germany [59] | Prospective | n = 33 | M: 25 (76%) F: 8 (24%) |
61.6 (44–85) | Oropharynx (n = 20), larynx (n = 8), hypopharynx (n = 5) (stage NR) |
CT (cisplatin, 5-fluorouracil and docetaxel) No RT Dose to olfactory area: NA |
ODT, OI, OD (Sniffin’sticks) | Before and immediately after first, second and third CT cycle |
|
| Wang et al., 2015, Taiwan [16] |
Prospective | n = 41 | M: 31 (76%) F: 10 (24%) |
45 (29–77) | Nasopharynx (stage I–IV) |
IMRT (70–76.8 Gy):
|
|
Before and 12 months after RT |
|
|
Alvarez-Camacho et al., 2016, Canada [60] |
Prospective | n = 160 | M: 126 (79%) F: 34 (21%) |
58.9 ± 11.9 | Pharynx (n = 88), larynx (n = 36), oral cavity (n = 18), salivary glands (n = 11), nasal cavity and paranasal sinuses (n = 6), soft tissue (n = 1) (stage I–IV) |
Surgery (n = 7) Surgery + RT (60 Gy) (n = 59) Surgery + CT-RT (cisplatin or carboplatin) (n = 86) Surgery + RT + cetuximab (n = 8) Dose to olfactory area: NR |
Self-reported smell (CCS) | Before treatment, end of treatment and at 2.5 months follow-up |
|
| Galletti et al., 2016, Italy [61] |
Cross-sectional | Study group: n = 9 Control group: n = 9 |
Study group M: 9 (100%) Control group M: 9 (100%) |
Study group 55 ± 9.96 Control group 52.56 ± 8.56 |
Nasopharynx (stage III–IV) |
Induction CT (cisplatin and fluorouracil) + concurrent CT-RT (cisplatin, 60–69 Gy) Dose to olfactory area: NR |
|
44.77 ± 25.93 months after treatment |
|
| Badr et al., 2017, USA [62] | Cross-sectional | n = 93 | M: 73 (78%) F: 20 (22%) |
61.5 (39–88) | Oral cavity (n = 26), oropharynx (n = 67) (stage I–IV) |
RT (n = 3) (doses NR) CT-RT (n = 22) Surgery + RT (n = 32) Surgery + CT-RT (n = 36) Dose to olfactory area: NR |
Self-reported smell (Vanderbilt Head and Neck Symptom Survey version 2.0) | Within 3 months of RT end (7% of participants), within 3–6 months (23%), within 6–9 months (24%) and within 9–12 months (46%) |
|
| Riva et al., 2017, Italy [63] |
Cross-sectional | Study group: n = 50 Control group: n = 50 |
Study group M: 43 (86%) F: 7 (14%) Control group M: 40 (80%) F: 10 (20%) |
Study group 68.76 (50–83) Control group 67.54 (53–76) |
Larynx (stage II–IV) |
Total laryngectomy + RT (n = 16) Total laryngectomy + CT-RT (n = 4) Dose to olfactory area: NR |
ODT, OI, OD (Sniffin’sticks) | 61.96 (24–132) Months after treatment |
|
| Lilja et al., 2018, Finland [64] |
Prospective | n = 44 | M: 29 (66%) F: 15 (34%) |
56.2 (38–80) | Oral cavity (n = 28), oropharynx (n = 13), hypopharynx (n = 3) (stage II–IV) |
Surgery (n = 5) Surgery + RT (n = 35) Surgery + CT-RT (n = 4) Dose to olfactory area: NR |
|
Before treatment, and 6 weeks, 3, 6 and 12 months after treatment |
|
| Riva et al., 2019, Italy [15] |
Prospective | n = 10 | M: 10 (100%) | 56.90 (39–72) | Nasopharynx (n = 3), oral cavity (n = 3), parotid gland (n = 3), primary unknown (n = 1) (stage I–IV) |
Surgery (n = 8) Concurrent CT-RT (54–70 Gy) (n = 5) Induction CT + concurrent CT-RT (n = 1) Dose to olfactory area:
|
|
Before (T0), at mid-course (T1), and at the end (T2) of RT, 1 and 3 months after RT (T3 and T4) |
|
| Epstein et al., 2020, USA [65] | Prospective | n = 10 | M: 7 (70%) F: 3 (30%) |
59.9 ± 7.0 | Oropharynx (n = 9), oral cavity (n = 1) (stage I–III) |
IMRT:
|
OI (UPSIT) | 4–6 weeks after starting of treatment (n = 6) and up to 2 years after treatment (n = 8) | Decreased OI in 3 patients (33%) during treatment with smell recovery after treatment |
| Tyler et al., 2020, USA [66] |
Cross-sectional | n = 114 | M: 68 (60%) F: 46 (40%) |
55 (18–78) | Nasopharynx (n = 61), paranasal sinuses (n = 29), nasal cavity (n = 24) (stage I–IV) |
IMRT (n = 110) (66.6 ± 5.1 Gy) 3D-CRT (n = 4) Surgery (n = 38) CT:
|
Self-reported smell (EQ-5D VAS, MDASI-HN, ASBQ) | 65 (12–154) months after treatment |
|
| Gurushekar et al., 2020, India [67] | Prospective | n = 21 | M: 16 (76%) F: 5 (24%) |
42.62 (16–75) | Nasopharynx (n = 13) oropharynx (n = 4), oral cavity (n = 2), paranasal sinuses (n = 2) (stage NR) |
CT-RT (n = 15) Surgery + RT (n = 4) RT (n = 2) (doses NR) Dose to olfactory area: NR |
|
Before RT, at mid-course of RT (n = 21), at the end of RT (n = 18), 3 months after RT (n = 13) |
|
| Sharma et al., 2020, Denmark [68] |
Cross-sectional | n = 27 | M: 17 (63%) F: 10 (37%) |
67 (47–83) | Nasal cavity (n = 19), paranasal sinuses (n = 8 (stage I–IV) |
IMRT (60–68 Gy):
Dose to olfactory area: NR |
|
6.4 (1.6–11.1) years after RT |
|
| Alfaro et al., 2021, USA [69] | Cross-sectional | Study group: n = 40 Control group: n = 20 |
Study group M: 24 (40%) F: 16 (60%) Control group: M: 11 (55%) F: 9 (45%) |
Study group 63 ± 12 Control group 58 ± 14 |
Oral cavity (n = 19), pharynx (n = 18), larynx (n = 3) (stage I–IV) |
RT (alone or combined with surgery or CT, doses NR) (n = 40) Concurrent CT (n = 24) Dose to olfactory area: NR |
|
Between 6 months and 10 years after RT |
|
Abbreviations: 2D-RT, Two-dimensional Radiotherapy; 3D-CRT, Three-Dimensional Conformal Radiotherapy; ASBQ, Anterior Skull Base Inevntory; AHSP, Appetite, Hunger and Sensory Perception; CCCRC, The Connecticut Chemosensory Clinical Research Center test; CCS, Chemosensory Complaint Score; CT, Chemotherapy; CTCAE, Common Terminology Criteria for Adverse Events; CT-RT, Chemoradiotherapy; ECT, Electrochemotherapy; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life CoreQuestionnaire; EORTC QLQ-H&N35, EORTC Quality of Life Head and Neck Module 35; EQ-5D VAS, EuroQol Group-5 Dimension Visual Analogue Scale; F, Female; Gy, Gray; IMRT, Intensity Modulated Radiation Therapy; IQR, Inter-Quartile Range; M, Male; MDASI-HN, MD Anderson Symptom Inventory–Head and Neck; MRI, Magnetic Resonance Imaging; NA, Not Applicable; NOSE, Nasal Obstruction Symptom Evaluation; NPC, Nasopharyngeal carcinoma; NR, Not reported; OI, Odor identification; OD, Odor discrimination; ODT, Odor detection threshold; RT, Radiotherapy; SNOT, Sino-Nasal Outcome Test; SOIT, Scandinavian Odor Identification test; TDI, Threshold, discrimination and identification total score; UPSIT, University of Pennsylvania Smell Identification Test; UW-QoL, University of Washington Quality of Life Questionnaire; VAS, Visual Analog Scale.
3.2. Sinonasal Mucosa Disorders
Among 30 publications concerning sinonasal mucosa disorders as sides effects of CT and/or RT in HNC patients, six studies were prospective [15,16,20,23,35,38], 10 cross-sectional [17,18,21,24,25,29,30,33,36,43], and 14 retrospective [14,19,22,26,27,28,31,32,34,37,39,40,41,42] (Table 1). Sample sizes ranged from 9 to 1134 patients. Men were predominant in all the publications, except for three papers [35,37,39]. Age ranged from 7 to 84 years. A control group was present in six studies [17,18,20,23,33,36].
The most frequent cancer sites were nasopharynx, nasal cavity, and paranasal sinuses, followed by oropharynx, oral cavity, larynx, hypopharynx, skull base, and parotid gland. Nasopharynx was the only tumor site in all the patients in twenty articles [16,18,19,21,22,23,24,25,26,27,28,30,31,32,33,34,35,38,41,43], whereas one article focused on nasopharynx and nasal cavity [17], one on nasal cavity and paranasal sinuses [40], and another one on patients with nasopharynx and larynx tumors [29]. Moreover, one study included only laryngeal cancer [36], and in one paper, the tumor sites were larynx, oropharynx, oral cavity, and hypopharynx [20]. Tumor stage was reported by 21 articles and ranged from I to IV according to TNM classification [14,15,16,20,22,24,26,28,29,30,31,32,33,34,35,36,37,38,39,41,43].
Treatments included: RT alone in 27 papers [14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,34,35,37,38,39,40,41,42,43], surgery and adjuvant RT in 5 papers [15,20,29,36,42], CT-RT in 20 papers [14,15,16,20,21,22,24,25,26,28,30,31,32,33,34,39,40,41,42,43], and trimodal therapy in 3 papers [15,36,42]. Only fourteen studies described the type of RT that was used. In particular, four studies included two-dimensional radiation therapy (2D-RT) [26,31,33,38], six papers included three-dimensional conformal radiation therapy (3D-CRT) [14,26,30,31,33,39], and fourteen papers included intensity-modulated radiation therapy (IMRT) [14,16,24,26,28,30,31,32,33,34,38,39,41,43]. Two studies evaluated brachytherapy [22,39]. RT dose to nasal cavities was detected only in three studies, and ranged from 13.59 to 56.38 Gy [15,18,43].
The main chemotherapeutic drugs administered in the studies were platinum compounds, pyrimidine compounds, and taxanes. No study evaluated CT alone.
Diagnosis and/or complaints of chronic rhinosinusitis (CRS) were performed using subjective and objective measurements: questionnaires (e.g., Sino-Nasal Outcome Test–SNOT), mucociliary clearance (saccharine test), clinical examination by nasal endoscopy, cultures, nasal biopsy, nasal cytology, computed tomography and magnetic resonance imaging (MRI). No study used only subjective measurements. Thirteen papers included both subjective and objective tools [15,16,17,24,27,30,31,33,35,36,37,38,43].
Nasal endoscopy was performed in 10 studies [15,19,24,26,27,31,33,35,36,43]; two of these used the Lund Endoscopic Staging System, assessing the appearance of nasal endoscopy findings (polyps, oedema, discharge, scarring and crusting), with a total score ranging from 0 to 20 [24,35]. A general clinical examination was included in three articles [14,39,42]. A saccharine test was employed in six studies in order to investigate mucociliary clearance rates [17,19,20,23,29,43]. It consisted of the placement of saccharin on the floor of nasal cavity behind the anterior end of the inferior turbinate. Then, the subjects were asked to swallow every 30 s and to report the first change in taste sensation. The time from the placement of saccharin to the perception of sweetness was noted as the mucociliary clearance time.
The patients underwent a radiological assessment in seventeen studies: computed tomography in six articles [16,19,24,35,37,38], MRI in five articles [22,30,32,34,43], and both computed tomography and MRI in five papers [14,26,27,28,41]. In only one study did the authors use radiographs [21]. The extent of rhinosinusitis was graded using the Lund-Mackay staging system, based on computed tomography findings in six studies [16,19,24,35,37,38], and on MRI findings in three papers [22,32,34]. The Lund-Mackay staging system was based on the opacification of each sinus (maxillary, frontal, anterior ethmoidal, posterior ethmoidal, sphenoidal, and the ostiomeatal complexes), assigning a score between 0 and 2 (0, no abnormality; 1, partial opacification; 2, total opacification) for a total score ranging between 0 and 24. The computed tomography is generally used for rhinosinusitis staging. However, some studies used MRI [22,32,34].
Three studies included nasal cytological examination [15,33,36], and four papers analyzed histopathologic findings based on biopsies [18,23,36,37]. The bacteriology of RT-induced rhinosinusitis, detected by cultures of maxillary sinus specimens, was reported in three studies [21,25,40].
Patients were evaluated before and after RT in fifteen studies [15,16,17,19,20,22,24,28,29,30,32,34,35,41,43]. The minimum time of assessment after treatment was three months [15], and the maximum was 117 months [17]. Fourteen articles evaluated patients only after RT [14,18,21,23,25,26,27,31,33,36,37,39,40,42], with a minimum time of assessment of three months [42] and a maximum of 26–54 years [26].
The percentage of patients with rhinosinusitis after RT ranged from 7% [42] to 86.1% [30]. The most common isolates in the post-RT CRS group were Staphylococcus aureus, followed by Streptocuccus viridans and Pseudomonas aeruginosa [21,25,40].
In four articles, a higher saccharin perception time was found after RT [19,20,23,29]. Kılıç et al. described a higher saccharin perception time in patients receiving RT dose >60 Gy and in patients receiving CT concurrent to RT [29]. On the contrary, Kamel et al. did not find any correlation between RT dose and mucociliary clearance delay time, endoscopic findings, and Lund-Mackay score [19]. Hu et al. found a decreased saccharin transit time after RT [23].
Maxillary sinuses were the most involved, followed by the anterior ethmoid [14,19,22,28,34]. Park et al. described a higher bilateral CRS percentage in non-RT group (85.7% vs. 60%), and, although RT itself was not associated with sinus surgery, concurrent CT was significantly associated with the need for surgery [14]. Advanced T stage (but not RT dose) was positively associated with the incidence of sinus abnormality in the fifth year after RT [22,28]. In the study by Lu et al., patients in the RT-alone and any-RT groups exhibited an increased risk of CRS compared to patients in the no-RT group (hazard ratio: 6.76 and 2.91, respectively) [42].
The incidence of sinusitis peaked at 3–9 months after RT and showed a trend toward stabilization after 1 year [28,34]. Nasal irrigation reduced CRS post-RT in patients with nasopharyngeal carcinoma [24]. Moreover, higher incidence of CRS after RT was observed in patients who used a nasal sprayer instead of a nasal irrigator [30]. Fewer nasal complaints (overall symptoms, blocked nose, and headache), better quality of life, and less severe endoscopic findings were found in the steroid group at 3 and 6 months after RT [35].
Two articles did not find any significant influence of the RT delivery method for any type of complication [31,33]. Choanal stenosis was found in 4.3% to 23% of cases after RT [23,26,31,38] and negatively affected quality of life [31].
Histologically, areas of ciliary loss, intercellular and intracellular vacuolation, and ciliary dysmorphism, and a decreased number of submucosal gland openings and ciliary areas were found after RT [18,23]. Stoddard et al. found a higher percentage of neutrophilic inflammation and squamous or mucous cell metaplasia in the study group without cytological atypia; furthermore, there was no correlation between cytological changes and symptoms, endoscopic findings, age, smoking, and tumor stage [40].
Nasal cytology showed a radiation-induced rhinitis with neutrophils [15,33]. Furthermore, mucous cell metaplasia appeared in patients during RT [15,33,37]. Mucous cell metaplasia was also found in 20% of laryngectomized patients [36]. Increased squamous metaplasia and subepithelial edema and a higher Lund-Mackay score were observed in radiation-induced CRS compared to CRS without nasal polyps [37]. Riva et al. did not find any correlation between cytological changes and symptoms, endoscopic findings (turbinate hypertrophy, mucosal hyperemia, nasal secretions), age, smoking, tumor stage, and adjuvant RT after total laryngectomy [36]. Yin et al. showed that the patients who received IMRT at a dose less than the threshold had the least damaged nasal mucosa morphology, and functional impairment scores were highest 3 months after RT, with a significant relationship between the turbinate thickness ratio and the radiation dose [43].
3.3. Olfactory Dysfunction
Among 28 publications concerning olfactory dysfunction as a side effect of CT and/or RT in HNC patients, 16 studies were prospective [15,16,44,46,47,48,49,52,54,55,58,59,60,64,65,67], 11 cross-sectional [50,51,53,56,57,61,62,63,66,68,69], and only one was retrospective [45] (Table 2). Sample size ranged from 10, in two prospective studies [15,65], to 205 patients, in a cross-sectional study [50]. Most patients were men. Only two papers reported a preponderance of women in their sample [57,58]. Age ranged from 11 to 88 years. Seven articles included a control group [46,49,56,57,61,63,69], five were cross-sectional, and two were prospective.
The most frequent cancer sites were nasopharynx, nasal cavity, and paranasal sinuses, followed by oropharynx, oral cavity, larynx, hypopharynx, skull base, and parotid gland. Nasopharynx was the only tumor site in all the patients in six articles [16,46,47,56,57,61], while one article focused on nasopharynx and nasal cavity [66]. Thirteen papers did not include nasopharynx as a tumor site [49,50,51,53,54,58,59,60,62,63,64,65,68,69]. Tumor stage was reported by nineteen articles and ranged from I to IV according to TNM classification [15,16,33,36,46,47,50,51,54,56,58,60,61,62,64,65,66,68,69].
Treatments included: RT alone in three papers [44,45,46], surgery and adjuvant RT in four papers [50,51,52,53], CT-RT in ten papers [16,47,48,49,52,55,56,57,61,65], trimodal therapy in nine papers [15,60,62,63,64,66,67,68,69], and ECT and RT in one paper [58]. Five studies included two-dimensional radiation therapy (2D-RT) [44,45,46,56,57], four studies included three-dimensional conformal radiation therapy (3D-CRT) [48,49,57,66], and five included intensity-modulated radiation therapy (IMRT) [16,57,65,66,68]. The other 14 articles did not specify the type of RT employed. RT dose to the olfactory area was reported in six articles and ranged from <10 to 75 Gy [15,44,45,48,52,55].
The evaluation of CT alone was present in only one paper [59]. The main chemotherapeutic agents administered in the studies were: platinum compounds, pyrimidine compounds, and taxanes. The agent administered for ECT was Bleomycin.
Time of assessment included evaluations before and after RT in fifteen studies [15,16,44,46,47,48,49,52,54,55,58,59,60,64,67], while it was only after treatment in twelve articles [50,51,53,56,57,61,62,63,65,66,68,69]. In one study, patients were evaluated during RT [45]. The longest follow up time was 10 years [69], while the shortest was 2.5 months after the end of RT [60]. The times of assessment in the publication that evaluated only CT were before and immediately after the first, second, and third cycle of therapy [59].
Olfactory function was evaluated by means of psychological tests in eleven papers [44,46,48,49,55,56,59,63,64,65,69], self-report instruments in eight papers [45,50,51,53,54,58,60,62], and through a combination of both tools in nine articles [15,16,47,52,57,61,66,67,68].
The psychological tests measured the three main olfactory abilities concurrently in eight studies [15,46,47,48,57,59,63,64]. They included odor detection threshold (ODT), odor discrimination (OD), and odor identification (OI). ODT was assessed with the use of amyl acetate and eugenol, n-butyl alcohol, or n-butanol. A total score (TDI—Threshold Discirmination Identification) was calculated in the studies that used Sniffin’ sticks for olfactory assessement [15,47,48,57,59,63]. Other objective measurements were olfactory event-related potential testing [61] and olfactory bulb volume in MRI [56]. Olfactory event-related potential testing consisted of a selective stimulation with 40 randomized olfactory stimuli, using phenyl ethyl alcohol and hydrogen sulphide in nitrogen as odorants, presented through a Teflon nasal outlet that was placed into the nasal vestibule [61].
Subjective measurements were obtained through self-report instruments, like Visual Analogue Scale (VAS, 0–100 or 0–10), 6-item Hyposmia Rating Scale, or self-reported smell (EORTC QLQ-H&N35, CCS, SNOT-22, Vanderbilt Head and Neck Symptom Survey version 2.0, AHSP, EQ-5D VAS, MDASI-HN, ASBQ).
Twenty articles reported substantial smell deterioration, with specific differences in odor identification and odor discrimination. ODT significantly decreased during RT, and its baseline levels had still not recovered at different months after the treatment [44,46,47,52,55,56,57,63,67], such as OD in two papers [48,63]. In two cases, ODT did not show variations during and after the radiotherapy treatment [48,64]; similarly, OD did not change in the other two papers [47,57]. OI decreased in three papers [52,56,63], while in two papers, it did not show substantial differences before and after treatment [47,57]. A significant reduction of OI was observed in one study during RT, but showed a partial recovery at 3 months follow-up [67]. On the contrary, in another publication, OI was stable at 2–6 weeks after the beginning of radiotherapy, but showed a decrease after treatment at a long term evaluation [48].
Eight studies did not demonstrate significant differences in olfactory alterations between irradiated and non-irradiated patients [49,50,53,54,58,63,65,69].
Four studies reported an impairment of quality of life [60,61,62,67]. Smell disorders were predictors of depression and anxiety [62], and had a positive correlation with the VAS scale [61].
4. Discussion
Head and neck cancer treatment is often challenging and multimodal, including radiation therapy and chemotherapy [4]. In recent decades, the development of new radiotherapy techniques has guaranteed a reduction in OARs complications. In particular, RT technology has developed from 3D-conformal planning to IMRT, and the amount of radiation applied to the surrounding tissue has been minimized [7]. However, since the percentage of long-term HNC survivors has been increasing [1], higher attention should be paid to late side effects.
The aim of this review was to summarize the current knowledge of sinonasal side effects of chemotherapy and/or radiation therapy for HNC, with a specific focus on mucosal and smell disorders. Indeed, these complications are often overlooked by physicians, but may negatively affect patients’ quality of life.
The prevalence of sinonasal mucosa disorders after RT for HNC is very heterogeneous among studies, ranging from 8% to 86.1%. The 5-year incidence of post-irradiation CRS reached 16.7% in the study by Hsin et al. conducted on 102 patients with nasopharyngeal carcinoma (NPC) [32]. However, the applicability of this information is limited because this study investigated only patients with NPC, and not with the HNC of other sites. Furthermore, potential confounders such as CT or previous sinonasal disorders have not been properly considered or controlled. Radiation-induced choanal stenosis is a rare late toxicity, observed in 4.3% to 23% of cases after RT [23,26,31,38]. It leads to serious difficulty in breathing through, and discharge from, the nose. When it comes to improving these symptoms, transnasal endoscopic surgery is often needed [31].
The results obtained by Lu et al. revealed that the risk of CRS was significantly higher in the RT groups compared to the no-RT group. In particular, the 5-year cumulative incidence rates of CRS in the RT-alone, any-RT, and no-RT groups were estimated as 12%, 9.3%, and 4.5%, respectively. The hazard ratios for CRS were 6.76 and 2.91 in the RT-alone and any-RT groups, respectively, compared to the no-RT group. Moreover, the HNC site is another factor associated with the risk of post-treatment CRS: the patients with NPC had the highest incidence of CRS (HR 4.54), while patients with oral cancer had the lowest (HR 0.29) [42].
The incidence of rhinosinusitis peaked at 3–9 months after RT and showed a trend toward stabilization after one year [27,34]. The highest incidence and severity of sinus mucosa disease were found at 3 months after RT (about 67% of cases), which decreased gradually over time. Advanced tumor stage and smoking habit were predisposing factors for sinus mucosa disease, while age, sex, RT dose, and nodal status were not. On the contrary, no factors could predict sinus mucosa disease improvement after RT [22]. In the study by Su et al., a lot of patients with CRS before treatment suffered aggravated symptoms after RT, but 75.3% of patients without CRS before RT developed it after RT. Advanced tumor stage, invasion of the nasal cavity, and nasal irrigation, but not CT or RT dose, were positively associated with the incidence of rhinosinusitis after RT [28]. In the paper by Hamilton et al., nasal crusting, CRS, and epistaxis were quite common (nasal crusting in 16% of patients, epistaxis in 16%, and chronic sinusitis in 8% of cases), attributable to the higher proportion of subjects treated with high dose RT to the nasopharynx in their study [39].
The histopathological features of radiation-induced CRS are different from non-post-RT CRS. Kuhar et al. observed that patients with RT-induced CRS exhibited greater squamous metaplasia and subepithelial edema compared to patients with CRSsNP, and decreased eosinophilia and basement membrane thickening compared to patients with CRSwNP [37]. Riva et al. showed that a radiation-induced rhinitis with neutrophils and sometimes bacteria occurred in 70% of cases and persisted after 1 month; mucous cell metaplasia appeared in 10% of patients during RT and disappeared after 3 months, and squamous cell metaplasia was observed in 10% of cases, only after the end of RT [15]. These results are in agreement with their previous study on the late effects of RT for nasopharyngeal cancer that showed 40% of neutrophilic rhinitis, 20% squamous cell metaplasia, and 13% mucous cell metaplasia, after a median follow-up of 59 months [33]. The most common isolates in the post-RT CRS group were Staphylococcus aureus, followed by Streptocuccus viridans and Pseudomonas aeruginosa [21,25,40].
Few studies have investigated the relationship between RT dose and radiation-induced damage to the nasal mucosa. Indeed, RT dose to nasal cavities was detected only in three studies, and ranged from 13.59 to 56.38 Gy [15,18,43]. Therefore, there is insufficient information on the radiation tolerance of the nasal mucosa. Yin et al. investigated 66 patients and assessed the radiation tolerance of the nasal mucosa by performing the modified saccharin test, endoscopy test, MRI, and a SNOT-20 survey. The results showed that there was a threshold radiation dose, and that above such an RT dose, nasal tissues may not recover from radiation-induced damage. The threshold doses of IMRT ranged from 37 to 40 Gy. A low dose of IMRT (inferior to the threshold dose) was associated with higher mucocilia transport rate, better endoscopy test score, and improved SNOT-20 score [43]. Moreover, an association between turbinate thickness ratio and radiation dose was observed. Riva et al. noticed a significant correlation between mean dose (Dmean) and near maximum dose (D2%) to inferior turbinates and neutrophilic rhinitis, and between D2% to inferior turbinates and mucous cell metaplasia at the end of RT [15].
Treatment for radiation-induced CRS was reported by four studies, and included functional endoscopic sinus surgery [14,23,27,37]. An algorithm for radiation-induced CRS therapy, including medical and surgical options, has never been proposed.
Smell perception plays an important role in life experiences and influences every aspect. Olfactory dysfunction can limit daily life activities and have an adverse effect on nutritional status. Concerning HNC, olfactory disorders may be secondary to direct damage caused by tumors, or to treatments, including surgery, RT, and CT. In particular, RT and CT can alter smell perception by damaging the olfactory epithelium and/or nerves.
The percentage of patients with olfactory impairment after RT ranged from 7% to 76%. Smell disorders appeared during RT and decreased after treatment, remaining higher than non-irradiated patients [45,51,56,57,61,62,63,65,66,68]. Studies that did not find any differences in smell perception between irradiated and non-irradiated patients mainly included subjects affected by non-nasopharyngeal tumors [50,53,69]. Therefore, this lack of difference could be explained by the fact that olfactory epithelium was outside the radiation fields in these patients.
A number of studies have investigated different aspects of olfactory impairment during and after RT. In particular, odor detection threshold (ODT), odor discrimination (OD), and odor identification (OI) have been analyzed. ODT was measured in thirteen studies and worsened during RT [15,33,36,44,46,47,48,52,55,56,59,64,67]. Only three studies did not find ODT alterations after RT, assessing smell at least 12 months after completing the treatment [15,48,64]. OD was measured in seven publications [15,33,36,47,48,59,64]. Among these, four studies did not report OD alterations at a long-term evaluation [15,33,47,48]. Finally, OI was measured in sixteen studies and an alteration was found in 22–63% of patients [15,33,36,46,47,48,49,52,56,59,64,65,67,68,69]. The reason for better OD and OI results compared to ODT may be the supraliminary and centrally integrated nature of OD and OI functions, and thus theoretically outside the field of irradiation. Globally, threshold discrimination identification score (TDI) was lower during RT and totally or partially recovered after treatment [15,57].
Only few studies investigated the role of different RT techniques on smell disorders. Concerning studies that included 2D-RT and 3D-CRT, there was a substantial smell deterioration [44,45,46,48,49,56,57,66], even though Riva et al. did not observe significant long-term differences for subjective hyposmia, ODT, OD, and OI between different radiation techniques for NPC (2D-RT/3D-CRT vs. IMRT) [57]. An impaired olfactory function was also found in five articles that included IMRT [16,57,65,66,68]. Nevertheless, Epstein et al. reported decreased OI in 3 patients (33%) during treatment with smell recovery after RT [65].
A worse olfactory function was observed in patients receiving ‘high dose’ compared to ‘low dose’ to the olfactory epithelium. After therapy, 40% and 7% reported subjective olfactory decline in high and low RT dose groups, respectively [52].
A small part of cited studies was derived from 2019–2022, which coincides with the SARS-CoV-2 pandemic. Since dysfunction of smell and taste could be an effect of SARS-CoV-2 infection, it may partially modulate results in the most recent studies.
Only two studies described both sinonasal mucosal disorders and olfactory disfunction after RT treatment [15,16]. Wang et al. performed patients’ evaluations before and 12 months after RT, observing an increase in sinonasal mucosa and smell disorders after treatment. Moreover, olfactory alterations correlated with total and ethmoid Lund-Mckay scores [16]. Riva et al. evaluated the patients before, during, and after RT (3 months). In agreement with Wang et al., a concurrent increase in mucosal and olfactory disorders was observed during RT. Furthermore, Riva et al. found that nasal symptoms and endoscopic findings peaked at the end of RT [15]. Further studies are necessary to identify where OAR should be set in order to reduce the incidence of sinonasal side effects.
The impact of CT on nasal side effects has been poorly analyzed. Only one study evaluated olfactory complaints during CT for HNC. The TDI score decreased during the second CT cycle, especially in older patients (>55 years), and reached almost its initial levels after 3 weeks of recovery time [59]. On the other hand, the impact of CT alone on sinonasal mucosa has never been investigated.
5. Conclusions
Sinonasal mucosa and smell disorders are a common post-treatment side effect of CT and/or RT in HNC patients. The incidence and severity of olfactory dysfunction and chronic rhinosinusitis were highest at the end of RT and at 3 months after treatment and decreased gradually with time. Smell acuity deterioration and chronic rhinosinusitis after RT seemed related to radiation dose on olfactory area and nasal cavities, but different degrees of recovery were observed. Therefore, it is important to establish the severity of chronic rhinosinusitis and olfactory dysfunction in order to find strategies to support patients and improve their quality of life. Further studies are necessary to better assess the role of medical and surgical treatments of sinonasal side effects of CT and/or RT for HNC. Finally, the role of CT should not be overlooked, and future studies are mandatory to assess its effect on nasal cavities and paranasal sinuses.
Author Contributions
Conceptualization, G.R. and G.P.; methodology, G.R.; formal analysis, G.R.; investigation, G.R., E.C., C.P., M.B.; data curation, G.R.; writing—original draft preparation, G.R., E.C., C.P., M.B.; writing—review and editing, G.C.I., C.C., O.O., M.A., U.R., G.P.; visualization, G.R.; supervision, G.P.; project administration, G.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Cohen N., Fedewa S., Chen A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. N. Am. 2018;30:381–395. doi: 10.1016/j.coms.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Riva G., Albano C., Gugliesi F., Pasquero S., Pacheco S.F.C., Pecorari G., Landolfo S., Biolatti M., Dell’Oste V. HPV Meets APOBEC: New Players in Head and Neck Cancer. Int. J. Mol. Sci. 2021;22:1402. doi: 10.3390/ijms22031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfister D.G., Spencer S., Adelstein D., Adkins D., Anzai Y., Brizel D.M., Bruce J.Y., Busse P.M., Caudell J.J., Cmelak A.J., et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020;18:873–898. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 5.Strojan P., Grošelj A., Serša G., Plaschke C.C., Vermorken J.B., Nuyts S., de Bree R., Eisbruch A., Mendenhall W.M., Smee R., et al. Electrochemotherapy in Mucosal Cancer of the Head and Neck: A Systematic Review. Cancers. 2021;13:1254. doi: 10.3390/cancers13061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riva G., Salonia L., Fassone E., Sapino S., Piano F., Pecorari G. Quality of Life in Electrochemotherapy for Cutaneous and Mucosal Head and Neck Tumors. J. Clin. Med. 2021;10:4366. doi: 10.3390/jcm10194366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alterio D., Marvaso G., Ferrari A., Volpe S., Orecchia R., Jereczek-Fossa B.A. Modern radiotherapy for head and neck cancer. Semin. Oncol. 2019;46:233–245. doi: 10.1053/j.seminoncol.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Eisbruch A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016;57:i69–i75. doi: 10.1093/jrr/rrw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco P., Potenza I., Schena M., Riva G., Pecorari G., Demo P.G., Fasolis M., Moretto F., Garzaro M., Di Muzio J., et al. Induction chemotherapy and sequential concomitant chemo-radiation in locally advanced head and neck cancers: How induction-phase intensity and treatment breaks may impact on clinical outcomes. Anticancer Res. 2015;35:6247–6254. [PubMed] [Google Scholar]
- 10.Álvarez-Camacho M., Gonella S., Campbell S., Scrimger R.A., Wismer W.V. A systematic review of smell alterations after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017;54:110–121. doi: 10.1016/j.ctrv.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande T.S., Blanchard P., Wang L., Foote R.L., Zhang X., Frank S.J. Radiation-Related Alterations of Taste Function in Patients With Head and Neck Cancer: A Systematic Review. Curr. Treat. Options Oncol. 2018;19:72. doi: 10.1007/s11864-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drareni K., Dougkas A., Giboreau A., Laville M., Souquet P.J., Bensafi M. Relationship between food behavior and taste and smell alterations in cancer patients undergoing chemotherapy: A structured review. Semin. Oncol. 2019;46:160–172. doi: 10.1053/j.seminoncol.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Brook I. Early side effects of radiation treatment for head and neck cancer. Cancer Radiother. 2021;25:507–513. doi: 10.1016/j.canrad.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Park Y.M., Cho J.G., Woo J.S. Chronic sinusitis in head and neck cancer patients who received radiotherapy or chemoradiotherapy. Eur. Arch. Otorhinolaryngol. 2018;275:2805–2811. doi: 10.1007/s00405-018-5114-1. [DOI] [PubMed] [Google Scholar]
- 15.Riva G., Franco P., Provenzano E., Arcadipane F., Bartoli C., Lava P., Ricardi U., Pecorari G. Radiation-Induced Rhinitis: Cytological and Olfactory Changes. Am. J. Rhinol. Allergy. 2019;33:153–161. doi: 10.1177/1945892418822448. [DOI] [PubMed] [Google Scholar]
- 16.Wang J.J., Liang K.L., Twu C.W., Lin J.C., Jiang R.S. Olfactory change after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int. Forum Allergy Rhinol. 2015;5:1059–1062. doi: 10.1002/alr.21575. [DOI] [PubMed] [Google Scholar]
- 17.Stringer S.P., Stiles W., Slattery W.H., Krumerman J., Parsons J.T., Mendenhall W.M., Cassisi N.J. Nasal mucociliary clearance after radiation therapy. Laryngoscope. 1995;105:380–382. doi: 10.1288/00005537-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Chen W.P., Lou P.J., Tai C.C. Delayed irradiation effects on nasal epithelium in patients with nasopharyngeal carcinoma. An ultrastructural study. Ann. Otol. Rhinol. Laryngol. 1999;108:474–480. doi: 10.1177/000348949910800510. [DOI] [PubMed] [Google Scholar]
- 19.Kamel R., Al-Badawy S., Khairy A., Kandil T., Sabry A. Nasal and paranasal sinus changes after radiotherapy for nasopharyngeal carcinoma. Acta Otolaryngol. 2004;124:532–535. doi: 10.1080/00016480410018106. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S., Chandra S., Singh M. Effects of irradiation on nasal mucociliary clearance in head and neck cancer patients. Indian J. Otolaryngol. Head Neck Surg. 2006;58:46–50. doi: 10.1007/BF02907740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsin C.H., Tsao C.H., Su M.C., Chou M.C., Liu C.M. Bacteriology of acute rhinosinusitis in nasopharyngeal carcinoma survivors: A result of maxillary sinus punctures. Eur. Arch. Otorhinolaryngol. 2007;264:1157–1162. doi: 10.1007/s00405-007-0334-9. [DOI] [PubMed] [Google Scholar]
- 22.Huang C.C., Huang S.F., Lee T.J., Ng S.H., Chang J.T.C. Postirradiation sinus mucosa disease in nasopharyngeal carcinoma patients. Laryngoscope. 2007;117:737–742. doi: 10.1097/MLG.0b013e3180325b6c. [DOI] [PubMed] [Google Scholar]
- 23.Hu K.H., Tan C.T., Lin K.N., Cheng Y.J., Huang H.M. Effect of endoscopic sinus surgery on irradiation-induced rhinosinusitis in patients with nasopharyngeal carcinoma. Otolaryngol. Head Neck Surg. 2008;139:575–579. doi: 10.1016/j.otohns.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Liang K.L., Kao T.C., Lin J.C., Tseng H.C., Su M.C., Hsin C.H., Shiao J.Y., Jiang R.S. Nasal irrigation reduces postirradiation rhinosinusitis in patients with nasopharyngeal carcinoma. Am. J. Rhinol. 2008;22:258–262. doi: 10.2500/ajr.2008.22.3166. [DOI] [PubMed] [Google Scholar]
- 25.Deng Z.Y., Tang A.Z. Bacteriology of postradiotherapy chronic rhinosinusitis in nasopharyngeal carcinoma patients and chronic rhinosinusitis. Eur. Arch. Otorhinolaryngol. 2009;266:1403–1407. doi: 10.1007/s00405-009-0915-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee C.C., Ho C.Y. Post-treatment late complications of nasopharyngeal carcinoma. Eur. Arch. Otorhinolaryngol. 2012;269:2401–2409. doi: 10.1007/s00405-011-1922-2. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Liang F.Y., Han P., Zou H., Chen Q.J., Jiang X.Y., Li R.C., Huang X.M. Management of radiation-induced early nasal adhesion after radiotherapy for nasopharyngeal carcinoma. Am. J. Rhinol. Allergy. 2013;27:3915. doi: 10.2500/AJRA.2013.27.3915. [DOI] [PubMed] [Google Scholar]
- 28.Su Y., Liu L.P., Li L., Li X., Cao X.J., Dong W., Yang X.H., Xu J., Yu S., Hao J.F. Factors influencing the incidence of sinusitis in nasopharyngeal carcinoma patients after intensity-modulated radiation therapy. Eur. Arch. Otorhinolaryngol. 2014;271:3195–3201. doi: 10.1007/s00405-014-3004-8. [DOI] [PubMed] [Google Scholar]
- 29.Kılıç C., Tunçel Ü., Cömert E., Kaya B.V. The effect of radiotherapy on mucociliary clearance in patients with laryngeal and nasopharyngeal cancer. Eur. Arch. Otorhinolaryngol. 2015;272:1517–1520. doi: 10.1007/s00405-014-3082-7. [DOI] [PubMed] [Google Scholar]
- 30.Luo H.H., Fu Z.C., Cheng H.H., Liao S.G., Li D.S., Cheng L.P. Clinical observation and quality of life in terms of nasal sinusitis after radiotherapy for nasopharyngeal carcinoma: Long-term results from different nasal irrigation techniques. Br. J. Radiol. 2014;87:20140043. doi: 10.1259/bjr.20140043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alon E.E., Lipschitz N., Bedrin L., Gluck I., Talmi Y., Wolf M., Yakirevitch A. Delayed Sino-nasal Complications of Radiotherapy for Nasopharyngeal Carcinoma. Otolaryngol. Head. Neck Surg. 2014;151:354–358. doi: 10.1177/0194599814530858. [DOI] [PubMed] [Google Scholar]
- 32.Hsin C.H., Tseng H.C., Lin H.P., Chen T.H. Post-irradiation otitis media, rhinosinusitis, and their interrelationship in nasopharyngeal carcinoma patients treated by IMRT. Eur. Arch. Otorhinolaryngol. 2016;273:471–477. doi: 10.1007/s00405-015-3518-8. [DOI] [PubMed] [Google Scholar]
- 33.Riva G., Boita M., Ravera M., Moretto F., Badellino S., Rampino M., Ricardi U., Pecorari G., Garzaro M. Nasal cytological changes as late effects of radiotherapy for nasopharyngeal cancer. Am. J. Rhinol. Allergy. 2015;29:e41–e45. doi: 10.2500/ajra.2015.29.4156. [DOI] [PubMed] [Google Scholar]
- 34.Hsin C.H., Tseng H.C., Lin H.P., Chen T.H. Sinus mucosa status in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: A 5-year follow-up. Head Neck. 2016;38:29–35. doi: 10.1002/hed.23849. [DOI] [PubMed] [Google Scholar]
- 35.Feng S., Fan Y., Liang Z., Yang G., Liao Z., Guo L. Effect of intranasal steroids on rhinosinusitis after radiotherapy for nasopharyngeal carcinoma: Clinical study. J. Laryngol. Otol. 2016;130:265–271. doi: 10.1017/S0022215115003448. [DOI] [PubMed] [Google Scholar]
- 36.Riva G., Boita M., Corvino A., Sensini M., Peruzzetto D., Chiusa L., Pecorari G., Garzaro M. Nasal and Tracheal Cytological Changes After Total Laryngectomy in Long-Term Survivors. Ann. Otol. Rhinol. Laryngol. 2017;126:124–131. doi: 10.1177/0003489416676500. [DOI] [PubMed] [Google Scholar]
- 37.Kuhar H.N., Tajudeen B.A., Heilingoetter A., Mahdavinia M., Gattuso P., Ghai R., Gunawan F., Diaz A.Z., Tolekidis G., Batra P.S. Distinct histopathologic features of radiation-induced chronic sinusitis. Int. Forum Allergy Rhinol. 2017;7:990–998. doi: 10.1002/alr.21989. [DOI] [PubMed] [Google Scholar]
- 38.Shemesh R., Alon E.E., Gluck I., Yakirevitch A. Endoscopic Surgery for Delayed Sinonasal Complications of Radiation Therapy for Nasopharyngeal Carcinoma: A Subjective Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:1222–1227. doi: 10.1016/j.ijrobp.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton S.N., Arshad O., Kwok J., Tran E., Fuchsia Howard A., Serrano I., Goddard K. Documentation and incidence of late effects and screening recommendations for adolescent and young adult head and neck cancer survivors treated with radiotherapy. Support. Care Cancer. 2019;27:2609–2616. doi: 10.1007/s00520-018-4559-5. [DOI] [PubMed] [Google Scholar]
- 40.Stoddard T.J., Varadarajan V.V., Dziegielewski P.T., Boyce B.J., Justice J.M. Detection of Microbiota in Post Radiation Sinusitis. Ann. Otol. Rhinol. Laryngol. 2019;128:1116–1121. doi: 10.1177/0003489419862583. [DOI] [PubMed] [Google Scholar]
- 41.Huang C.J., Huang M.Y., Shih M.C.P., Cheng K.Y., Lee K.W., Lu T.Y., Yuan S.S., Fang P.T. Post-radiation sinusitis is associated with recurrence in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Radiat. Oncol. 2019;14:61. doi: 10.1186/s13014-019-1261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y.T., Lu Y.C., Cheng H.C., Hsin C.H., Yang S.F., Wang P.H., Yeh H.J. Chronic rhinosinusitis after radiotherapy in patients with head and neck cancer: A population-based cohort study in Taiwan. Int. Forum Allergy Rhinol. 2020;10:692–697. doi: 10.1002/alr.22526. [DOI] [PubMed] [Google Scholar]
- 43.Yin G., Tu B., Ye L. Correlation of intensity-modulated radiation therapy at a specific radiation dose with the prognosis of nasal mucous damage after radiotherapy. Radiat. Environ. Biophys. 2020;59:245–255. doi: 10.1007/s00411-020-00830-5. [DOI] [PubMed] [Google Scholar]
- 44.Ophir D., Guterman A., Gross-Isseroff R. Changes in smell acuity induced by radiation exposure of the olfactory mucosa. Arch. Otolaryngol. Head. Neck Surg. 1988;114:853–855. doi: 10.1001/archotol.1988.01860200037011. [DOI] [PubMed] [Google Scholar]
- 45.Sagar S.M., Thomas R.J., Loverock L.T., Spittle M.F. Olfactory sensations produced by high-energy photon irradiation of the olfactory receptor mucosa in humans. Int. J. Radiat. Oncol. Biol. Phys. 1991;20:771–776. doi: 10.1016/0360-3016(91)90021-U. [DOI] [PubMed] [Google Scholar]
- 46.Hua M.S., Chen S.T., Tang L.M., Leung W.M. Olfactory function in patients with nasopharyngeal carcinoma following radiotherapy. Brain Inj. 1999;13:905–915. doi: 10.1080/026990599121106. [DOI] [PubMed] [Google Scholar]
- 47.Ho W., Kwong D.L.W., Wei W.I., Sham J.S.T. Change in olfaction after radiotherapy for nasopharyngeal cancer—A prospective study. Am. J. Otolaryngol. 2002;23:209–214. doi: 10.1053/ajot.2002.123436. [DOI] [PubMed] [Google Scholar]
- 48.Hölscher T., Seibt A., Appold S., Dörr W., Herrmann T., Hüttenbrink K.B., Hummel T. Effects of radiotherapy on olfactory function. Radiother. Oncol. 2005;77:157–163. doi: 10.1016/j.radonc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Sandow P.L., Hejrat-Yazdi M., Heft M.W. Taste loss and recovery following radiation therapy. J. Dent. Res. 2006;85:608–611. doi: 10.1177/154405910608500705. [DOI] [PubMed] [Google Scholar]
- 50.Bindewald J., Oeken J., Wollbrueck D., Wulke C., Dietz A., Herrmann E., Schwarz R., Singer S. Quality of life correlates after surgery for laryngeal carcinoma. Laryngoscope. 2007;117:1770–1776. doi: 10.1097/MLG.0b013e3180caa18c. [DOI] [PubMed] [Google Scholar]
- 51.Rhemrev R., Rakhorst H.A., Zuidam J.M., Mureau M.A.M., Hovius S.E.R., Hofer S.O.P. Long-term functional outcome and satisfaction after radial forearm free flap reconstructions of intraoral malignancy resections. J. Plast. Reconstr. Aesthetic Surg. 2007;60:588–592. doi: 10.1016/j.bjps.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Brämerson A., Nyman J., Nordin S., Bende M. Olfactory loss after head and neck cancer radiation therapy. Rhinol. J. 2013;51:206–209. doi: 10.4193/Rhino12.120. [DOI] [PubMed] [Google Scholar]
- 53.Momeni A., Kim R.Y., Kattan A., Lee G.K. Microsurgical head and neck reconstruction after oncologic ablation: A study analyzing health-related quality of life. Ann. Plast. Surg. 2013;70:462–469. doi: 10.1097/SAP.0b013e31827737a5. [DOI] [PubMed] [Google Scholar]
- 54.Oskam I.M., Verdonck-De Leeuw I.M., Aaronson N.K., Witte B.I., De Bree R., Doornaert P., Langendijk J.A., René Leemans C. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013;49:443–448. doi: 10.1016/j.oraloncology.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Jalali M.M., Gerami H., Rahimi A., Jafari M. Assessment of olfactory threshold in patients undergoing radiotherapy for head and neck malignancies. Iran. J. Otorhinolaryngol. 2014;26:211–217. doi: 10.22038/ijorl.2014.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veyseller B., Ozucer B., Degirmenci N., Gurbuz D., Tambas M., Altun M., Aksoy F., Ozturan O. Olfactory bulb volume and olfactory function after radiotherapy in patients with nasopharyngeal cancer. Auris. Nasus. Larynx. 2014;41:436–440. doi: 10.1016/j.anl.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Riva G., Raimondo L., Ravera M., Moretto F., Boita M., Potenza I., Rampino M., Ricardi U., Garzaro M. Late sensorial alterations in different radiotherapy techniques for nasopharyngeal cancer. Chem. Senses. 2015;40:285–292. doi: 10.1093/chemse/bjv011. [DOI] [PubMed] [Google Scholar]
- 58.Landström F.J., Reizenstein J., Adamsson G.B., Von Beckerath M., Möller C. Long-term follow-up in patients treated with curative electrochemotherapy for cancer in the oral cavity and oropharynx. Acta Oto-Laryngol. 2015;135:1070–1078. doi: 10.3109/00016489.2015.1049663. [DOI] [PubMed] [Google Scholar]
- 59.Haxel B.R., Berg S., Boessert P., Mann W.J., Fruth K. Olfaction in chemotherapy for head and neck malignancies. Auris. Nasus. Larynx. 2016;43:74–78. doi: 10.1016/j.anl.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez-Camacho M., Gonella S., Ghosh S., Kubrak C., Scrimger R.A., Chu K.P., Wismer W.V. The impact of taste and smell alterations on quality of life in head and neck cancer patients. Qual. Life Res. 2016;25:1495–1504. doi: 10.1007/s11136-015-1185-2. [DOI] [PubMed] [Google Scholar]
- 61.Galletti B., Santoro R.R., Mannella V.K., Caminiti F., Bonanno L., De Salvo S., Cammaroto G., Galletti F. Olfactory event-related potentials: A new approach for the evaluation of olfaction in nasopharyngeal carcinoma patients treated with chemo-radiotherapy. J. Laryngol. Otol. 2016;130:453–461. doi: 10.1017/S0022215116000761. [DOI] [PubMed] [Google Scholar]
- 62.Badr H., Lipnick D., Gupta V., Miles B. Survivorship Challenges and Information Needs after Radiotherapy for Oral Cancer. J. Cancer Educ. 2017;32:799–807. doi: 10.1007/s13187-016-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riva G., Sensini M., Corvino A., Pecorari G., Garzaro M. Smell and Taste Impairment After Total Laryngectomy. Ann. Otol. Rhinol. Laryngol. 2017;126:548–554. doi: 10.1177/0003489417709794. [DOI] [PubMed] [Google Scholar]
- 64.Lilja M., Markkanen-Leppänen M., Viitasalo S., Saarilahti K., Lindford A., Lassus P., Mäkitie A. Olfactory and gustatory functions after free flap reconstruction and radiotherapy for oral and pharyngeal cancer: A prospective follow-up study. Eur. Arch. Otorhinolaryngol. 2018;275:959–966. doi: 10.1007/s00405-018-4883-x. [DOI] [PubMed] [Google Scholar]
- 65.Epstein J.B., Villines D., Epstein G.L., Smutzer G. Oral examination findings, taste and smell testing during and following head and neck cancer therapy. Support. Care Cancer. 2020;28:4305–4311. doi: 10.1007/s00520-019-05232-y. [DOI] [PubMed] [Google Scholar]
- 66.Tyler M.A., Mohamed A.S.R., Smith J.B., Aymard J.M., Fuller C.D., Phan J., Frank S.J., Ferrarotto R., Kupferman M.E., Hanna E.Y., et al. Long-term quality of life after definitive treatment of sinonasal and nasopharyngeal malignancies. Laryngoscope. 2020;130:86–93. doi: 10.1002/lary.27849. [DOI] [PubMed] [Google Scholar]
- 67.Gurushekar P.R., Isiah R., John S., Sebastian T., Varghese L. Effects of radiotherapy on olfaction and nasal function in head and neck cancer patients. Am. J. Otolaryngol. 2020;41:102537. doi: 10.1016/j.amjoto.2020.102537. [DOI] [PubMed] [Google Scholar]
- 68.Sharma M.B., Jensen K., Urbak S.F., Funding M., Johansen J., Bechtold D., Amidi A., Eskildsen S.F., Jørgensen J.O.L., Grau C. A multidimensional cohort study of late toxicity after intensity modulated radiotherapy for sinonasal cancer. Radiother. Oncol. 2020;151:58–65. doi: 10.1016/j.radonc.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 69.Alfaro R., Crowder S., Sarma K.P., Arthur A.E., Pepino M.Y. Taste and Smell Function in Head and Neck Cancer Survivors. Chem. Sens. 2021;46:bjab026. doi: 10.1093/chemse/bjab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.