Abstract
Background.
Progress in immunotherapy use for gynecologic malignancies is hampered by poor tumor antigenicity and weak T cell infiltration of the tumor microenvironment (TME). Wnt/β-catenin pathway modulation demonstrated patient benefit in clinical trials as well as enhanced immune cell recruitment in preclinical studies. The purpose of this study was to characterize the pathways by which Wnt/β-catenin modulation facilitates a more immunotherapy-favorable TME.
Methods.
Human tumor samples and in vivo patient-derived xenograft and syngeneic murine models were administered Wnt/β-catenin modulating agents DKN-01 and CGX-1321 individually or in sequence. Analytical methods included immunohistochemistry, flow cytometry, multiplex cytokine/chemokine array, and RNA sequencing.
Results.
DKK1 blockade via DKN-01 increased HLA/MHC expression in human and murine tissues, correlating with heightened expression of known MHC I regulators: NFkB, IL-1, LPS, and IFNy. PORCN inhibition via CGX-1321 increased production of T cell chemoattractant CXCL10, providing a mechanism for observed increases in intra-tumoral T cells. Diverse leukocyte recruitment was noted with elevations in B cells and macrophages, with increased tumor expression of population-specific chemokines. Sequential DKK1 blockade and PORCN inhibition decreased tumor burden as evidenced by reduced omental weights.
Conclusions.
Wnt/β-catenin pathway modulation increases MHC I expression and promotes tumor leukocytic infiltration, facilitating a pro-immune TME associated with decreased tumor burden. This intervention overcomes common tumor immune-evasion mechanisms and may render ovarian tumors susceptible to immunotherapy.
Keywords: Ovarian cancer, Immunotherapy, Wnt/β-catenin signaling, Immune checkpoint blockade therapy, Tumor microenvironment
1. Introduction
Ovarian cancer is the most deadly gynecologic malignancy in the United States [1]. Most patients present with advanced stage disease, and although primary therapy may achieve remission, the majority will experience recurrence within 2 years and die of disease. Despite extensive research, breakthroughs in changing this paradigm have been limited. The role of the tumor microenvironment (TME) and its influence on immune system function and tumor progression is increasingly gaining attention in translational research as developments in immunotherapy provide an opportunity to change the treatment landscape. However, ovarian cancer has been associated with an immune-evasive TME characterized by poor antigen presentation and cytotoxic T cell activity and low tumor mutation burden [2]. These factors contribute to tumor immune evasion [3] and have prevented practice-changing successes in immunotherapy comparable to those seen in other malignancies. Elucidating the mechanisms that drive these phenotypes in ovarian cancer could highlight potential therapeutic targets to dampen immune evasion and promote anti-tumor immune response [4].
The Wnt/β-catenin pathway is under investigation as a potential target for immunotherapy in ovarian cancer. This pathway is active in cell proliferation, epithelial-to-mesenchymal transition (EMT), and anti-PD-1 therapy resistance [5–8]. It has also been correlated with a cold TME via poor T-cell infiltration and is frequently activated in gynecologic malignancies [9]. Dickkopf-1 (DKK1) glycoprotein, produced from Wnt target genes, serves as a negative regulator of the Wnt/β-catenin dependent (canonical) pathway (Fig. 1.) Increased levels of DKK1 have been correlated to poor oncologic outcomes in cervical and ovarian cancers; human clinical data show improved outcomes following DKK1 inhibition in patients with Wnt-activating mutations in gynecologic tumors [10]. In pre-clinical ovarian and pancreatic cancer models, DKK1 inhibition has been associated with reduced frequencies of myeloid-derived suppressor cells (MDSCs), illustrating a reversal of Wnt-pathway-mediated immunosuppression and facilitating development of a host-protective TME [11,12]. However, DKK1 blockade may also prevent negative feedback of the Wnt pathway. Wnt pathway activation itself has been associated with decreased MHC I expression and c-myc-mediated downregulation of HLA, contributing to poor tumor antigen presentation and immune escape [13–15]. It may therefore be warranted to inhibit the Wnt pathway itself. Porcupine (PORCN) enzyme regulates the secretion of Wnt ligands, and PORCN inhibition has been shown to suppress Wnt signaling and enhance B cell and cytotoxic T cell presence in murine ovarian cancer models. PORCN inhibition is associated with decreased tumor burden and improved survival [16,17]. These data suggest that sequential therapy via Wnt-modulating agents may act synergistically to address immune escape by improving tumor antigenicity and enhancing immune cell infiltration and activity to the TME. Blockade of the DKK1 glycoprotein itself and the Wnt pathway via PORCN inhibition combines two inter-related components that have been associated with favorable tumor outcomes in gynecologic malignancies. In light of these findings, we investigated the potential benefits of Wnt/β-catenin modulating agents on key regulators of anti-tumor immunity in the ovarian TME.
Fig. 1. Wnt/β-catenin pathway modulation via DKK1 inhibition.
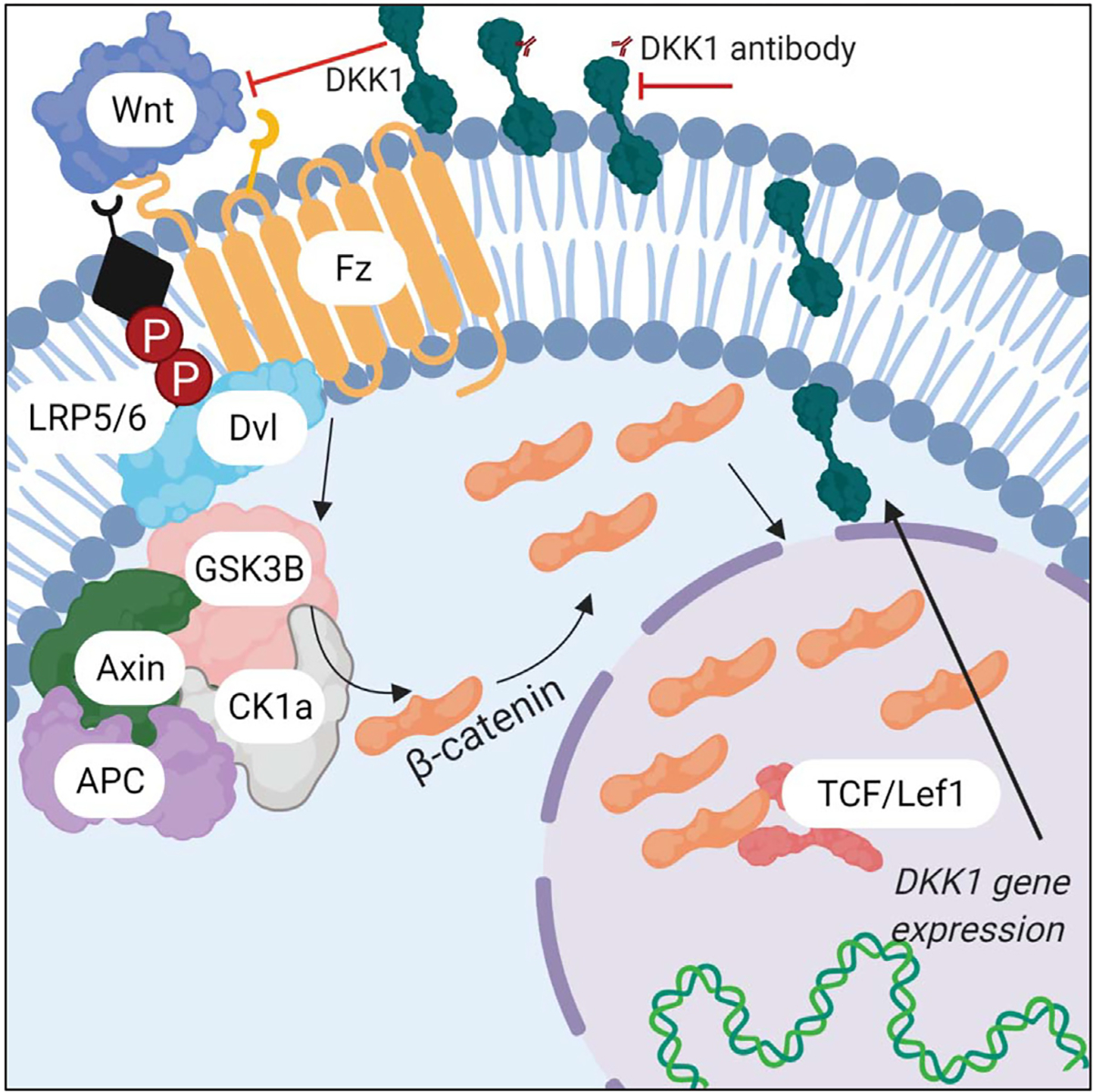
Normal Wnt signaling is characterized by 1. Wnt ligand binding to receptors and 2. activated downstream Wnt signaling causing expression of Wnt target genes such as DKK1. DKK1 is up-regulated in response to an increase in Wnt signaling and 3. serves as a negative regulator blocking Wnt ligand binding to receptor. 4. DKN-01 binds DKK1, preventing its ability to negatively regulate Wnt signaling, resulting in increased expression of Wnt target genes.
2. Materials and methods
2.1. Cell lines and culture
ID8 murine epithelial ovarian cancer cell line was provided by Dr. Yancey Gillespie (University of Alabama at Birmingham, Birmingham, Alabama). The ID8p53−/− cell line is a derivative of ID8 with CRISPR/ Cas9 gene editing for a single gene mutation (Trp53−/−) and was provided by Dr. Iain McNeish (Wolfson Wohl Cancer Research Centre, Institute of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom). ID8 cells were maintained in RPMI supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA, USA); ID8p53−/− cells were maintained in DMEM (Corning) supplemented with 4% fetal bovine serum, 1× insulin/selenium/transferrin (Invitrogen), penicillin/streptomycin and 2 mM L-glutamine. Cells were maintained in culture fewer than 10 passages from the parent stock. Experiments were performed at 70–80% confluency for all cell lines.
2.2. Murine experiments
All animal studies were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC). C57Bl/6 mice were obtained from NCI Charles River (Wilmington, MA, USA). Experiments were performed in mice at 7–8 weeks of age. Intraperitoneal tumor challenge included abdominal injection with 7 × 106 cells in 200 μL PBS on day 0. Treatment was started 7 or 14 days post-tumor challenge with DKN-01 and CGX-1321, respectively. Mice were treated with 10 mg/kg of DKK1 inhibitor mDKN-01 [18] (2× weekly intraperitoneal injection) or vehicle/control (Murine IgG2a isotype control: Bio X Cell, catalog no. BE0085, RRID:AB_1107771.) In a previous iteration of this experiment mice received 1 mg/kg CGX-1321 (5× weekly oral gavage), and experienced significant dose-related toxicities. As a result, 2× weekly dosage of 1 mg/kg CGX-1321 was performed in the current iteration. Less toxicity was observed, but not completely eliminated. Animals were weighed weekly and sacrificed according to IACUC protocols on day 21 or before if mice showed impairment or distress. At sacrifice, cardiac puncture was utilized for blood collection and omenta were harvested. Presence of peritoneal disease and ascites were noted. For the carcinosarcoma PDX experiment, SCID mice were subcutaneously administered 2 mm3 of patient-derived fresh tissue and observed until tumor size was 10 mm3, at which point 10 mg/kg DKN-01 or vehicle was administered twice weekly until the day 21 endpoint (6 total doses.)
2.3. Immunohistochemistry
Formalin-fixed, paraffin embedded (FFPE) tissue samples from human and murine specimens were cut to 4 μm and deparaffinized. Immunohistochemistry was performed using HLA-ABC (Proteintech Catalog # 15240–1-AP) antibody with appropriate primary and secondary comparison controls. Staining was assessed microscopically using chromogenic detection and graded by a board-certified pathologist. H-scores were calculated for patient samples from DKN-01 monotherapy trial [19].
2.4. RNA sequencing
RNA samples were isolated with Qiagen RNeasy Mini kit 74,104 kit. For each sample, 100 ng of RNA was used for full RNA sequencing. The PanCancer IO 360 (mouse) panel was used and included 750 cancer-related genes involved in the complex interplay between the tumor, microenvironment and immune response including 20 internal reference controls. Ingenuity Pathway Analysis (IPA) was used to perform upstream regulator analysis (URA) to identify the cascade of upstream transcriptional regulators that can explain observed gene expression changes.
2.5. Flow cytometry
Single cell suspensions of omental and blood cells were counted and stained with Zombie Aqua Fixable Viability Dye (Biolegend #423102; San Diego, CA), followed by TruStain FcX (Biolegend) to block Fc receptors. Cells were then stained with saturating concentrations of conjugated antibodies including CD45 (Biolegend #103106), CD3 (Bio-legend #100322), CD8 (Biolegend #100723), MHCII (Biolegend #107643), F4/80 (Biolegend #123130), B220 (Biolegend #103210), CD69 (Biolegend #104512), and CD103 (Biolegend #121430). Results were obtained from multiparameter flow cytometry using an Attune NxT Flow Cytometer (ThermoFisher Scientific) and analyzed with FlowJo, LLC software (Becton, Dickinson and Company, Ashland, OR: 2019.) Cells were gated via general immune cells (CD45+), lymphocytes (CD45 + CD3+), CD8 T cells (CD45 + CD3 + CD8+), CD8− T cells (CD45 + CD3 + CD8−), activated CD8 T cells (CD45 + CD3 + CD8 + CD69+ or CD103+), activated CD8− T cells (CD45 + CD3 + CD8-CD69+ or CD103+), B cells (CD45 + B220+), activated B cells (CD45 + B220 + MHCII+), and macrophages (CD45 + F4/80) + (see Supplemental Figs. 2, 3, 4).
2.6. Survival analysis
Online database Kaplan-Meier (KM) Plotter [20] was used to generate KM curves indicating effects of gene expression on ovarian cancer patient survival (n = 2190). Sources for the database include GEO, EGA, TCGA (Gene Expression Omnibus, European Genome-phenome Archive, The Cancer Genome Atlas.) The primary purpose of this online database is to be used as a tool for meta-analysis-based discovery and validation of survival biomarkers. Website: https://kmplot.com/analysis/.
2.7. Statistical analysis
One-Way ANOVA analyses were used to determine statistical significance of HLA expression between screening on on-treatment patient biopsies from DKN-01 mono-therapy clinical trial. In addition, they were used to determine statistical significance between median number of immune cells in vehicle, CGX-1321, DKN-01, and CGX-1321 + DKN-01-treated tumors, followed by post hoc analyses with studenťs t-tests to determine significance between specific treatment groups. Studenťs t-tests (two-tailed, two-sample unequal variance) were performed to determine statistical significance between median gene expression in treated murine tumors. *p < 0.05, **p < 0.01, and ***p < 0.001.
3. Results
3.1. DKK1 inhibition enhances HLA expression in tumor biopsies from human clinical trials
Gynecologic malignancies evade immune detection by downregulating HLA/MHC expression and decreasing antigen presentation, thereby ‘cloaking’ tumor cells from detection by cytotoxic T cells [21,22]. A recent basket trial (NCT03395080) assessed patients with recurrent gynecologic malignancies receiving DKN-01, an anti-DKK1 monoclonal antibody, with or without intravenous paclitaxel [10] (Fig. 2A.) Tumor tissue biopsies (n = 4) were obtained prior to treatment and after 1 cycle (28 days) of 300 mg daily oral DKN-01 monotherapy. Pre- and post-treatment tissue biopsy slides from the DKN-01 monotherapy arm underwent immunohistochemistry (IHC) of HLA (human leukocyte antigen) expression. H-scores demonstrated that HLA (MHC I) expression was significantly increased after 1 cycle of intraperitoneal DKN-01 treatment [19] (Fig. 2B, n = 4, p < 0.05). High HLA expression is associated with significantly improved overall survival in patients with ovarian cancer, as demonstrated by Kaplan-Meier curves from TCGA, GEO, EGA (The Cancer Genome Atlas, Gene Expression Omnibus, European Genome-phenome Archive) databases [20] (Fig. 2C). Increased antigenicity via HLA expression has been associated with improved immune cell recognition by cytotoxic T-cell receptors, facilitating tumor cell lysis. Decreased antigen presentation on tumor cells allows immune escape via decreased T-cell recognition, enabling tumor progression [3,23] (Fig. 2D).
Fig. 2. High HLA/MHC I expression is correlated to increased overall survival in patients with ovarian cancer; DKN-01 up-regulates HLA expression on tumor cells.

(A.) Trial schema for DKN-01 monotherapy investigating the effects of DKK1 blockade in patients with EEC (endometrioid endometrial cancer) and EOC (epithelial ovarian cancer.) (B.) HLA Class I ABC (MHC I) expression in biopsies from patient screening and post-DKN-01 treatment demonstrates increased HLA expression (n = 4, p < 0.05, each color represents an individual patient). (C.) Kaplan Meier curves show improved overall survival in ovarian cancer patients with high HLA-A, HLA—B, and HLA-C mRNA expression. (D.) Increased tumor tissue HLA expression facilitates T-cell-mediated tumor lysis via cell-cell interactions. Poor antigenicity via low HLA expression, or poor immune cell infiltration, facilitates ‘immune escape’ and cancer progression.
3.2. Upstream regulation of tumor antigenicity is influenced by DKK1 inhibition
HLA expression by tumor cells is a complex process regulated by multiple pathways [24,25]. To assess factors contributing to the observed increase of HLA expression in tumor biopsies from clinical trials, a carcinosarcoma patient-derived xenograft (PDX) model was established by subcutaneous tissue administration (see Materials and Methods.) PDX models received 10 mg/kg DKN-01 monotherapy vs. vehicle twice weekly for a total of 6 doses (Fig. 3A, n = 8 per arm.) RNAseq upstream regulator analysis demonstrated significant upregulation of NFkB complex, lipopolysaccharide (LPS), and IL-1 (interleu-kin-1) expression in the DKN-01 PDX compared to vehicle (Fig. 3B, p < 0.05, Z-score > 2.) LPS and IL-1 are associated with phosphorylation activation of the NFkB complex [26], which promotes increased transcription of HLA genes [25] (Fig. 3C.)
Fig. 3. DKN-01 up-regulates expression of multiple genes that stimulate HLA in a carcinosarcoma patient-derived xenograft.

(A.) Schematic of treatment with vehicle or DKN-01 monotherapy (n = 8 per treatment arm). (B.) RNAseq results demonstrate increased gene expression based on Z-score fold change ≥2 and p-value <0.05 (C.) IL-1 and LPS activate NFkB signaling, thereby stimulating HLA-ABC transcription.
A model of ID8p53−/− epithelial ovarian carcinoma in fully immune-competent C57Bl/6 mice demonstrated concordant results: in this model, DKN-01 10 mg/kg mono-therapy or vehicle was intraperitoneally administered twice weekly until a day 45 end-point (Fig. 4A, n = 5). Nanostring mRNA analysis of tumors revealed a significant increase in TNF (tumor necrosis factor, p < 0.05) after DKN-01 monotherapy, accompanied by trending increases in IL-1α and IFNy (interferon-gamma.) All three factors are upstream stimulators of NFkB [27] (Fig. 4B.) NFkB mRNA expression was concordantly upregulated in the DKN-01 monotherapy arm. IFNy is a well-recognized potent activator of HLA expression, operating via the Jak-Stat signaling pathway, and is being researched for its role in immunotherapy [28,29] (Fig. 4C.) Kaplan-Meier analyses of overall survival in ovarian cancer patients demonstrated significantly improved outcomes with high expression of TNF, IL-1A, and IFNy as well as NFkB complex [20,30] (Fig. 4D.)
Fig. 4. DKN-01 up-regulates signaling pathways related to HLA stabilization in a syngeneic ID8p53−/− murine model.

(A.) Schematic of treatment timeline with either vehicle (n = 2) or DKN-01 (n = 3) monotherapy. (B.) RNAseq demonstrates increased TNF (* = p < 0.05) and a trending increase in IL-1a, IFNy, NFkB, and RELA mRNA expression with DKN-01 therapy. (C.) TNF, IL-1, and IFNy signaling activation of NFkB increases HLA transcription. (D.) Kaplan-Meier curves show improved overall survival in ovarian cancer patients with high expression of the indicated factors.
3.3. Sequential Wnt modulation promotes synergistic tumor antigen expression and TME immune cell infiltration, resulting in decreased tumor burden
Tumor antigenicity influences T cell infiltration and movement within the TME, and allows immune targeting of cancer cells [3,31]. We hypothesized that increased tumor antigenicity, followed by enhanced TME immune cell infiltration, would have a synergistic impact on tumor control. We therefore utilized a syngeneic murine model of ID8 epithelial ovarian carcinoma in fully immune-competent C57Bl/6 mice to test the efficacy of a novel combination therapy comprised of intraperitoneally injected DKN-01 mono-therapy followed by oral gavage of the PORCN inhibitor CGX-1321 (Fig. 5A.) Controls included untreated mice and mice receiving only one of the two Wnt modulators. At day 21, mice were sacrificed for analysis. IHC for MHC I (major histocompatibility complex I) demonstrated increased MHC I expression in omental tumor tissue from mice treated with DKN-01 and sequential DKN-01/ CGX-1321 therapy, relative to vehicle controls (Fig. 5B.) The T cell chemoattractant CXCL10 [32–34] was significantly increased in treatment arms containing CGX-1321 (p < 0.01, Fig. 5C). This provides a potential mechanism behind previously observed CD8+ T-cell infiltration to the TME [16,17]. In patients with ovarian cancer, high expression of CXCL10 is associated with improved overall survival [20] (Fig. 5D.)
Fig. 5. DKN-01 and CGX-1321 sequential treatment upregulates MHC I expression and the T cell chemotactic factor CXCL10 in a syngeneic ID8 murine model.

(A.) Schema of sequential treatment with DKN-01 (i.p.; 1 mg/kg; 2×/week) and CGX-1321 (oral gavage; 10 mg/kg; 5×/week), n = 8 per treatment arm. (B.) Immunohistochemistry (IHC) demonstrates increased MHCI expression on ID8 cells with DKN-01 administration (*p < 0.05.) (C.) Tumor expression of chemotactic factor CXCL10 stimulates T cell infiltration to TME. (D.) RNAseq data demonstrate that DKN-01 + CGX-1321 therapy increases CXCL10 (*p < 0.05, **p = 0.01) mRNA expression in tumors. (E.) Kaplan Meier curve shows increased overall survival in ovarian cancer patients with high expression of CXCL10.
Flow cytometry was performed on excised omental tissue. Compared to vehicle, sequential therapy demonstrated increased leukocytic (CD45+) infiltration (Fig. 6A) with an increased number of activated cells compared to vehicle (Fig. 6B.) Sequential therapy was associated with increased activation of CD8+ and CD8− T cells, as evidenced by elevated numbers of CD69+ and CD103+ T cells, which have been correlated to improved patient survival when noted on histopathologic evaluation of ovarian cancer [35,36] (Fig. 6C.) Sequential therapy also enhanced activated B cell and macrophage infiltration (Supplemental Fig. 1). Notably, our novel sequential therapy significantly decreased tumor burdens via omental weight assessment when compared to both vehicle/control and DKN-01 monotherapy (Fig. 6D.) The above findings reveal new downstream targets of Wnt/β-catenin modulation in the ovarian cancer TME.
Fig. 6. Sequential Wnt/β-catenin modulation via DKN-01 and CGX-1321 significantly increases leukocyte infiltration to the tumor micro-environment while decreasing tumor burden.
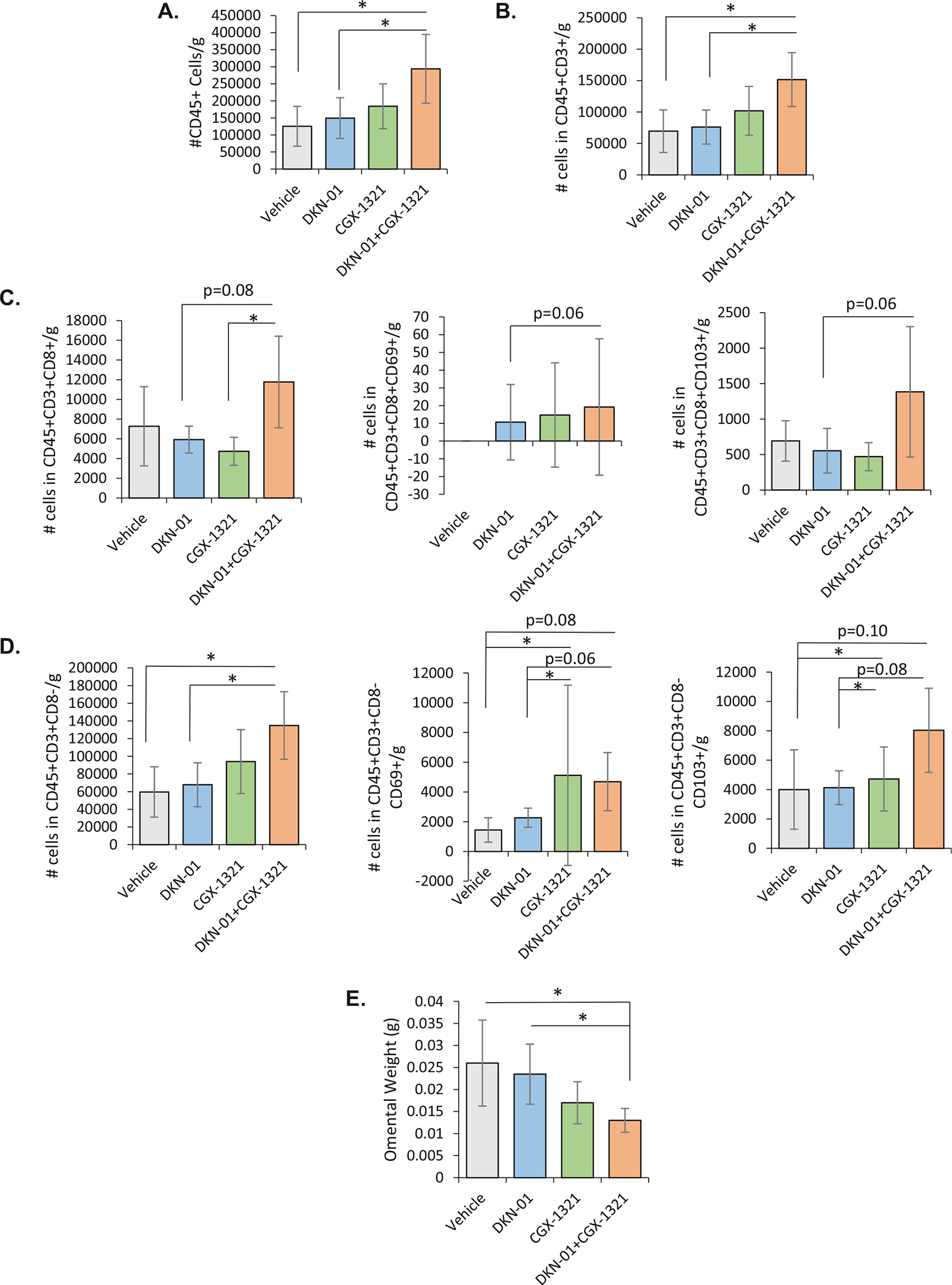
(A.) Leukocyte populations (CD45+) are significantly increased with sequential therapy, consistent with increased antigenicity and immune cell recruitment. (B.) Total T cell (CD45 + CD3 +) presence in the omenta of mice in the sequential therapy arm is significantly increased compared to vehicle. (C.) CD8+ T cells (CD3 + CD8+) and activated CD8+ T cells (CD8 + CD69+ or CD103+) demonstrate a trend towards increase in the sequential therapy arm. (D.) Presumptive CD4+ T cells (CD3 + CD8−) in the ID8 TME are significantly increased with sequential therapy arm compared to vehicle, and activated CD8− T cells (CD8-CD69+ or CD103+) demonstrate a trend towards increase with sequential therapy arm compared to vehicle. (E.) Sequential therapy significantly decreases omental weight, a surrogate for tumor burden, in ID8 tumors. (* = p < 0.05, n = 8 per treatment arm).
4. Discussion
The TME and tumor-immune interactions are of increasing interest in ovarian cancer treatment. Understanding these complex molecular and paracrine processes is crucial to the development of more efficacious immune-based therapies. Overcoming ‘immune escape’ via enhanced tumor antigenicity and immune cell infiltration has been correlated with improved patient survival and has been highlighted as essential for effective immunotherapy [3,37].
The Wnt/β-catenin pathway impacts diverse aspects of cell and tumor proliferation and metastasis. In ovarian cancer models, we have previously demonstrated that PORCN inhibition via CGX-1321 increases CD8+ T cell infiltration to the TME [16], which in turn promotes a decrease in tumor burden correlated with improved survival [17]. Clinical trials of DKK1 inhibition via DKN-01 monotherapy in patients with advanced or recurrent gynecologic malignancies were associated with improved patient survival [10]. However, concurrent administration of PORCN inhibitors with DKK1 inhibitors did not demonstrate synergy in preclinical models [11]. We therefore utilized sequential administration of these Wnt modulators in our current study to capitalize on enhanced antigenicity followed by an influx of activated immune cells to synergistically decrease tumor burden.
Our results suggest that DKN-01 increases tissue HLA/MHC I expression in multiple gynecologic malignancy tissue types in both human and murine models and provide a potential mechanism for this phenomenon. Cellular production of TNF, IFNy, LPS, and IL-1 proteins stimulates the transcription factor NFkB, which increases HLA/MHC I expression [25,27,30]. Loss of antigenicity via the loss of HLA/MHC I represents a major mechanism of immune escape for many solid tumor types and is common in ovarian malignancies [23,38–40]. Conversely, increased tumor tissue antigen presentation on HLA/MHC I molecules allows for improved recognition by CD8+ T cells. Clinical trials demonstrate that tumor antigen-specific T cells can be developed in vitro and generate a durable patient response with increased survival [41,42]. Identifying mechanisms to enhance antigenicity is therefore critical to achieving optimal anti-tumor immunity and immunotherapeutic efficacy [25,43].
We also provide a potential mechanism for increased T cell infiltration into the TME following PORCN inhibition [32]. Increased CXCL10 has been associated with improved survival in triple-negative breast cancer and lung adenocarcinoma, and has been associated with favorable prognostic factors in colorectal cancer [44–46]. Analyses have identified a significant survival benefit for patients with ovarian cancer who had high CXCL10 expression [20,47]. Therapeutics such as IL-12 plasmid therapy are currently in clinical trials in ovarian cancer [32]: identifying key cytokines and chemokines may allow further development of this approach.
The demonstrated increases in activated CD8+ and CD8− T cells with sequential therapy suggest that this treatment strategy also stimulates immune cell function. As originally shown by Zhang et al. and confirmed by subsequent groups, activated T cells improve patient survival and are influenced by tumor chemokines such as TNF and IFNy [35,48]. Our experiments suggest that this mechanism can be manipulated to improve both activation and infiltration using sequential Wnt/β-catenin modulation, shifting the conversation from observation to direct intervention using this axis. Further investigation into Wnt/β-catenin modulation for immune cell migration and activation in ovarian cancer is warranted.
The majority of ovarian cancer TME research has centered on T-cell presence and function [35,49]. However, a growing body of literature supports the importance of diverse cell populations and interactions, such as the role of tumor-associated macrophages (TAMs) in intrinsic cancer biology and T cell regulation and the co-stimulatory function of B cells [50,51]. Our findings suggest that DKN-01/CGX-1321 sequential treatment in-creases activated B cell influx to the omental TME. Assessment of human serous ovarian cancer metastases demonstrated a strong activated B cell response to tumor antigens: B cells recruit a diverse immune cell population and co-stimulate antigen-presenting cells via their own cytokine production [51]. Activated B cells have been associated with an anti-tumor immune response, improved overall survival, and may have synergistic impact with CD8+ T cells in ovarian tumors [52–54]. Patients with soft-tissue sarcoma and increased B-cell tumor infiltration who received anti-PD-1 agent pembrolizumab had improved treatment response and survival [55]. Identifying anti-tumor B cells and harnessing these co-stimulatory and synergistic pathways may similarly enhance immuno-therapy outcomes in ovarian cancer. Broadening the scope of TME cell components and interactions may provide insight into targetable pathways. For example, targeting TAMs, which have been suggested to exert a protumor response in ovarian cancers [50,56], may have also synergistic response with regulatory T cells via PD-1 and CTLA-4 axes. DKK1 inhibition decreases MDSC populations and activity, including in ovarian cancers [11,57]. Our exploratory analysis of diverse immune cells populations advocates for increased research into the role of these cell populations to enhance understanding of the complexity of the TME and immune cell interactions in ovarian cancer.
Interactions between the Wnt/β-catenin and PI3K/AKT/mTOR pathways, also often hyper-activated in ovarian cancer, merit further investigation [58,59]. The mTOR pathway is a robust area of research in gynecologic oncology, with numerous phase I/II clinical trials. Preliminary results in endometrial cancer suggest a relationship between DKK-1 inhibition and PIK3CA mutation status [10], and synergy between inhibition of PORCN and PI3K has been demonstrated in pancreatic cancers [60]. This is concordant with our results demonstrating the impact of Wnt/β-catenin modulation on NFkB, which is interconnected with the PI3K/AKT/mTOR pathway. Better characterization of this synergy in gynecologic malignancies may further the goal of personalized medicine by enabling targeted therapy based on individual patient mutation status.
Strengths of our current study include the utilization of multiple tumor types and model systems to support our findings. Clinical trial NCT03395080 enrolled patients with recurrent gynecologic malignancies of multiple tumor origins: as a result, we used a similar diversity of tumor types and models to confirm molecular pathways. For example, NFkB complex upregulation and increased expression of upstream regulators was confirmed in a syngeneic ID8p53−/− murine epithelial ovarian cancer model as well as carcinosarcoma PDX. Increased antigenicity with DKN-01 treatment, initially noted in human tissue samples, was confirmed in a syngeneic ID8 murine model. Correlation of murine and human experiments demonstrated consistent cytokine and gene expression profiles that were concordant with existing observational research in human tumor models. Increased gene expression also correlated with overall survival in human datasets. These internally and externally valid findings support the scientific and mechanistic results; furthermore, they indicate the translational value of these markers and their potential as treatment targets.
Limitations of this study were the limited number of mice per treatment group in our in vivo models. In future experiments, it would be pertinent to expand the size of our treatment groups to ensure a power of 0.80 and alpha <0.05. The dose-related toxicity of CGX1321 when administered 5× weekly merits further investigation; this was decreased when dosage administration was decreased to 2× weekly, but not completely eliminated. Furthermore, only a small subset of immune cell populations was analyzed with limited data on their activity. Moving forward, we would aim to provide a more thorough analysis of additional immune cell populations (e.g. natural killer cells) and their activity in order to gain further understanding of tumor microenvironment. Future studies assessing the impact of sequential modulation and these changes in immune cell response and the TME should also incorporate survival outcomes; this next step in research would provide useful information regarding the potential clinical impact of Wnt/β-catenin modulation.
Wnt/β-catenin modulation as exemplified in our experiments may provide an avenue to prime the immune response for checkpoint inhibition via a stepwise process: 1) increased tumor antigenicity to address immune escape followed by 2) enhanced leukocyte infiltration of tumors by a diverse array of beneficial cell types. Further research should investigate subsequent incorporation of checkpoint inhibition to capitalize on the favorable tumor micro-environment generated by Wnt modulation. The Wnt pathway has been identified as a prognostic biomarker, but our data support its role as a targetable axis [61,62]. For example, combinations of DKK1 inhibition with anti-PD-1 agent pembrolizumab in patients with advanced gastroesophageal adenocarcinoma showed improved responses in patients with high tumoral DKK1 expression [63].
5. Conclusions
Facilitating a pro-immune tumor micro-environment and preventing immune escape are critical to the development of immunotherapy for gynecologic malignancies. Ovarian malignancies in particular are characterized by poor tumor antigenicity and relative immune cell deficiency compared to many other solid tumor types. Here, we found that sequential Wnt/β-catenin modulation enhances tumor MHC I expression and T cell recruitment and activation, important components towards enhanced tumor antigenicity. Increasing tumor antigenicity is predicted to facilitate tumor recognition and killing via activated T-cells and may enable more effective treatment with immunotherapy agents. Our experiments also identified contributory cytokine/chemokine pathways and transcriptional regulation processes, deepening our understanding of molecular processes within the micro-environment and immune-tumor interactions.
Our findings support further research into Wnt/β-catenin modulation impact on diverse immune cell types in the tumor microenvironment of gynecologic malignancies and their potential as targetable axes in translational immune-oncology. Broadening the perspective from a narrow CD8+ T-cell focus and incorporating antigenicity, immune cell activation, and diverse cell populations allows the development of a more diverse treatment armamentarium that may facilitate personalized immune-oncology for gynecologic malignancies, matching the expansion of genetic and genomic testing. Future experiments will address these goals and the role of the Wnt/β-catenin pathway in modulating these factors towards a more anti-tumor micro-environment.
Supplementary Material
HIGHLIGHTS.
Wnt/β-catenin modulation via DKK1 blockade increases tumor MHC I expression.
PORCN inhibition increases CXCL10 production, providing a mechanism for leukocyte infiltration.
Wnt/β-catenin modulation stimulates diverse leukocyte recruitment (B cells and macrophages) to the tumor micro-environment.
Acknowledgements
The authors would like to acknowledge Dr. Jaydev Dholakia for his assistance and support.
Funding/support
Arend - W81XWH-18-1-0231 DOD.
Footnotes
Conflict of interest statement
Dr. Arend participates in the advisory boards for Leap Therapeutics, Clovis Oncology, AstraZeneca, GSK, Merck, VBL Therapeutics, Caris Life Sciences, Sutro Biopharma, and Seagen. All other authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2021.09.026.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J. Clin. 70 (1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Galon J, Bruni D, Approaches to treat immune hot, altered and cold tumours with combination immunotherapies, Nat. Rev. Drug Discov. 18 (3) (2019) 197–218. [DOI] [PubMed] [Google Scholar]
- [3].Beatty GL, Gladney WL, Immune escape mechanisms as a guide for cancer immunotherapy, Clin. Cancer Res. 21 (4) (2015) 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yigit R, et al. , Ovarian cancer creates a suppressive microenvironment to escape immune elimination, Gynecol. Oncol. 117 (2) (2010) 366–372. [DOI] [PubMed] [Google Scholar]
- [5].Luke JJ, et al. , WNT/β-catenin pathway activation correlates with immune exclusion across human cancers, Clin. Cancer Res. 25 (10) (2019) 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li X, et al. , WNT/β-catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment, Front. Immunol. 10 (2019) 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Teeuwssen M, Fodde R, Wnt signaling in ovarian cancer stemness, EMT, and therapy resistance, J. Clin. Med. 8 (10) (2019) 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sheng X, Gong F, Bai Y, 742P activation of the WNT signaling pathway correlates with innate resistance to immune checkpoint therapies in clear cell renal cell carcinoma, Ann. Oncol. 31 (2020) S577. [Google Scholar]
- [9].Betella I, et al. , Wnt signaling modulator DKK1 as an immunotherapeutic target in ovarian cancer, Gynecol. Oncol. 157 (3) (2020) 765–774. [DOI] [PubMed] [Google Scholar]
- [10].Arend R, et al. , Patients with recurrent epithelial endometrial cancers (EEC) and Wnt signaling alterations demonstrated greater clinical benefit when treated with DKN-01 monotherapy, Clinical Cancer Research, 2021. [Google Scholar]
- [11].Wall J, et al. , Manipulating the Wnt/β-catenin signaling pathway to promote antitumor immune infiltration into the TME to sensitize ovarian cancer to ICB therapy, Gynecol. Oncol. 160 (1) (2021) 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kagey MH, He X, Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology, Br. J. Pharmacol. 174 (24) (2017) 4637–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang W, et al. , MHC class I dysfunction of glioma stem cells escapes from CTL-mediated immune response via activation of Wnt/β-catenin signaling pathway, Oncogene 39 (5) (2020) 1098–1111. [DOI] [PubMed] [Google Scholar]
- [14].Li Y, et al. , WNT/β-catenin-signaling pathway stimulates the proliferation of cultured adult human Sertoli cells via upregulation of C-myc expression, Reprod. Sci. 19 (11) (2012) 1232–1240. [DOI] [PubMed] [Google Scholar]
- [15].Versteeg R, et al. , C-myc down-regulates class I HLA expression in human melanomas, EMBO J. 7 (4) (1988) 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Doo DW, et al. , Inhibition of the Wnt/β-catenin pathway enhances antitumor immunity in ovarian cancer, Ther. Adv. Med. Oncol. 12 (2020) p. 1758835920913798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goldsberry WN, et al. , Inhibiting wnt ligand production for improved immune recognition in the ovarian tumor microenvironment, Cancers 12 (3) (2020) 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haas MS, et al. , mDKN-01, a novel anti-DKK1 mAb, enhances innate immune responses in the tumor microenvironment, Mol. Cancer Res. 19 (4) (2021) 717–725. [DOI] [PubMed] [Google Scholar]
- [19].Stenger M, Calculating H-Score, [cited 2021 Jan 1]; Available from https://ascopost.com/issues/april-10-2015/calculating-h-score/ April 10, 2015. [Google Scholar]
- [20].Győrffy B, Lánczky A, Szállási Z, Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients, Endocr. Relat. Cancer 19 (2) (2012) 197–208. [DOI] [PubMed] [Google Scholar]
- [21].Rolland P, et al. , Human leukocyte antigen class I antigen expression is an independent prognostic factor in ovarian cancer, Clin. Cancer Res. 13 (12) (2007) 3591. [DOI] [PubMed] [Google Scholar]
- [22].Stelloo E, et al. , Microsatellite instability derived JAK1 frameshift mutations are associated with tumor immune evasion in endometrioid endometrial cancer, Oncotarget 7 (26) (2016) 39885–39893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Campoli M, Chang C-C, Ferrone S, HLA class I antigen loss, tumor immune escape and immune selection, Vaccine 20 (2002) A40–A45. [DOI] [PubMed] [Google Scholar]
- [24].Campoli M, Ferrone S, HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance, Oncogene 27 (45) (2008) 5869–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cornel AM, Mimpen IL, Nierkens S, MHC Class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy, Cancers (2020) 12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weber A, Wasiliew P, Kracht M, Interleukin-1 (IL-1) pathway, Sci. Signal 3 (105) (2010) p. cm1. [DOI] [PubMed] [Google Scholar]
- [27].Javitt A, et al. , Pro-inflammatory cytokines alter the immunopeptidome landscape by modulation of HLA-B expression, Front. Immunol. 10 (141) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rodríguez T, et al. , Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines, BMC Cancer 7 (1) (2007) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tarhini AA, Gogas H, Kirkwood JM, IFN-α in the treatment of melanoma, J. Immunol. 189 (8) (2012) 3789–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marth C, et al. , Interferon-γ expression is an independent prognostic factor in ovarian cancer, Am. J. Obstet. Gynecol 191 (5) (2004) 1598–1605. [DOI] [PubMed] [Google Scholar]
- [31].Boissonnas A, et al. , In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor, J. Exp. Med. 204 (2) (2007) 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu M, Guo S, Stiles JK, The emerging role of CXCL10 in cancer (review), Oncol. Lett. 2 (4) (2011) 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moran CJ, et al. , RANTES expression is a predictor of survival in stage I lung adenocarcinoma, Clin. Cancer Res. 8 (12) (2002) 3803. [PubMed] [Google Scholar]
- [34].Araujo JM, et al. , Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer, Sci. Rep. 8 (1) (2018) 4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang L, et al. , Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer, N. Engl. J. Med. 348 (3) (2003) 203–213. [DOI] [PubMed] [Google Scholar]
- [36].Duhen T, et al. , Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors, Nat. Commun. 9 (1) (2018) 2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Han LY, et al. , HLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinoma, Clin. Cancer Res. 14 (11) (2008) 3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garcia-Lora A, Algarra I, Garrido F, MHC class I antigens, immune surveillance, and tumor immune escape, J. Cell. Physiol. 195 (3) (2003) 346–355. [DOI] [PubMed] [Google Scholar]
- [39].Norell H, et al. , Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu specific immunity, Cancer Res. 66 (12) (2006) 6387. [DOI] [PubMed] [Google Scholar]
- [40].Dhatchinamoorthy K, Colbert JD, Rock KL, Cancer immune evasion through loss of MHC Class I antigen presentation, Front. Immunol. 12 (469) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Morse MA, et al. , MHC class I–presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T-cell responses against breast and ovarian cancer, Clin. Cancer Res. 17 (10) (2011) 3408. [DOI] [PubMed] [Google Scholar]
- [42].Stevanović S, et al. , A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus–associated epithelial cancers, Clin. Cancer Res. 25 (5) (2019) 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Turner TB, et al. , Epigenetic modifiers upregulate MHC II and impede ovarian cancer tumor growth, Oncotarget 8 (27) (2017) 44159–44170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zumwalt TJ, et al. , Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration, Oncotarget 6 (5) (2015) 2981–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chuan T, Li T, Yi C, Identification of CXCR4 and CXCL10 as potential predictive biomarkers in triple negative breast cancer (TNBC), Med. Sci. Monit. 26 (2020) p. e918281–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Arenberg DA, et al. , Improved survival in tumor-bearing SCID mice treated with interferon-γ-inducible protein 10 (IP-10/CXCL10), Cancer Immunol. Immunother. 50 (10) (2001) 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bronger H, et al. , CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer, Br. J. Cancer 115 (5) (2016) 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nelson BH, The impact of T-cell immunity on ovarian cancer outcomes, Immunol. Rev. 222 (1) (2008) 101–116. [DOI] [PubMed] [Google Scholar]
- [49].Sato E, et al. , Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8 +/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer, Proc. Natl. Acad. Sci. U. S. A. 102 (51) (2005) 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Colvin EK, Tumor-associated macrophages contribute to tumor progression in ovarian cancer, Front. Oncol. 4 (2014) 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Montfort A, et al. , A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases, Clin. Cancer Res. 23 (1) (2017) 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Flynn NJ, et al. , The multifaceted roles of B cells in solid tumors: emerging treatment opportunities, Target. Oncol. 12 (2) (2017) 139–152. [DOI] [PubMed] [Google Scholar]
- [53].Wei X, et al. , Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients, Tumor Biol. 37 (5) (2016) 6581–6588. [DOI] [PubMed] [Google Scholar]
- [54].Nielsen JS, et al. , CD20+ tumor-infiltrating lymphocytes have an atypical CD27-memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer, Clin. Cancer Res. 18 (12) (2012) 3281. [DOI] [PubMed] [Google Scholar]
- [55].Petitprez F, et al. , B cells are associated with survival and immunotherapy response in sarcoma, Nature 577 (7791) (2020) 556–560. [DOI] [PubMed] [Google Scholar]
- [56].Raghavan S, et al. , Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments, J. ImmunoTher. Cancer 7 (1) (2019) 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].D’Amico L, et al. , Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer, J. Exp. Med. 213 (5) (2016) 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kimura H, et al. , CKAP4 is a Dickkopf1 receptor and is involved in tumor progression, J. Clin. Invest. 126 (7) (2016) 2689–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mabuchi S, et al. , The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer, Gynecol. Oncol. 137 (1) (2015) 173–179. [DOI] [PubMed] [Google Scholar]
- [60].Zhong Z, et al. , PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers, Oncogene 38 (40) (2019) 6662–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bodnar L, et al. , Wnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer, J. Ovarian Res. 7 (1) (2014) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sui Q, et al. , Dickkopf 1 impairs the tumor response to PD-1 blockade by inactivating CD8+ T cells in deficient mismatch repair colorectal cancer, J. Immunother. Cancer (2021) 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Klempner SJ, et al. , DKN-01 in combination with pembrolizumab in patients with advanced gastroesophageal adenocarcinoma (GEA): tumoral DKK1 expression as a predictor of response and survival, J. Clin. Oncol. 38 (4_suppl) (2020) 357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


