Abstract
Metabolic diseases are common worldwide and include diseases of overnutrition, such as obesity, or undernutrition, such as kwashiorkor. Both the immune system and the microbiota contribute to a variety of metabolic diseases; however, these two processes have largely been studied independently of one another in this context. The gastrointestinal system houses the greatest density of microbes but also houses one of the largest collections of immune molecules, especially Abs. The IgA isotype dominates the Ab landscape at mucosal sites, and a number of studies have demonstrated the importance of this Ab to the stability of the microbiota. In this article, we review the literature that demonstrates how homeostatic Ab responses control microbiota composition and function to influence metabolic disease. We propose that many metabolic diseases may arise from disruptions to homeostatic immune control of gut commensals and that further understanding this interaction can offer a novel opportunity for therapeutic interventions.
Many factors regulate metabolism, and disturbances to the balance of energy input versus expenditures lead to metabolic disease. Malnutrition results from insufficient nutritional intake and is responsible for 45% of all deaths (3.1 million) in children under five years old (1). Malnourished children are faced with many health complications, from growth stunting to poor immunity, that leave them vulnerable to infection (2). Conversely, overnutrition is associated with obesity and type 2 diabetes (T2D), which are growing at overwhelming rates in younger populations and underserved communities (3). Obesity and T2D increase the risk of developing many comorbidities, including cardiovascular disease and cancer (4, 5). Given the increasing prevalence of these diseases across the globe, identification of factors that contribute to their development has been a focus of a myriad of recent investigations. Many of these studies have focused on individual factors, such as genetics, diet, immunity, and the gut microbiota (6, 7). However, it is becoming increasingly clear that many of these factors interact with one another. Although metabolic disease is not classically thought to involve microbial infections, it is now well appreciated that resident gut microorganisms can profoundly shape nutrient acquisition, energy expenditure, and severity of metabolic disease (8). As the microbiota is controlled by the immune system, yet also influences the development of immunity, we propose that immune–microbiota interactions are key elements underlying metabolic dysfunction.
Abs are one of the mechanisms employed by the host to directly target microbes in an Ag-dependent manner. At mucosal sites, the isotype IgA dominates the Ab repertoire; however, IgG and IgM are also present. Within the gut, IgA is made by plasma cells as a dimer, where it binds to the polymeric IgR on the basolateral surface of intestinal epithelia and is delivered to the intestinal lumen in its secreted form. IgA regulates and protects mucosal surfaces in a variety of ways that protect the host from invading pathogens and neutralizes toxins (9). More recently, however, a growing body of research has revealed that IgA is essential to regulating the composition and function of the resident commensal microbiota (9, 10). Through regulation of the microbiota, IgA can have broad effects on host health. Although chronic inflammation has been extensively studied in the context of metabolic disease (11), we focus, in this article, on homeostatic immune responses within the gut, primarily Ab mediated, that directly impact microbiota composition and function. We will begin with a review of the literature that first supports a role for the microbiota during development of metabolic disease, followed by studies that demonstrate how immune-mediated effects on the microbiota can drive metabolic defects. This area offers a unique opportunity for therapeutic intervention to treat or prevent a variety of metabolic ailments that plague the globe.
Composition of the microbiota shapes metabolic health
The gut microbiota metagenome encodes an expansive, dynamic range of functions that interact with host genes to influence metabolism. Preclinical studies comparing germ-free (GF) and specific pathogen–free mice have highlighted how important microbes are to their host’s metabolism. Seminal studies have demonstrated that obese humans possess a distinct microbiota when compared with lean individuals (12). Importantly, microbiota transplanted from obese mice or humans into GF animals results in significantly greater weight gain when compared with mice that received a microbiota from a lean individual (12, 13). Additionally, GF mice gain significantly less weight on a high-fat diet (14). Similar studies have been carried out for diseases of undernutrition. Strikingly, when mice were colonized with fecal samples from individuals with malnutrition, versus a healthy sibling control, and fed the same diet, mice with the malnutrition microbiota gained significantly less weight (15). The timing of changes in the gut microbiome may also be critical to the pathogenesis of obesity and T2D. Additionally, exposure to antibiotics, which reduces the microbiota, early in life increases the risk of developing obesity (16–18). These studies demonstrate that changes to the microbiota composition alone can influence the host’s ability to gain or lose weight.
What are the microbial differences that drive these changes in metabolic health? Initial studies in humans reported that individuals with obesity and T2D had an overall decreased bacterial diversity with an increase in the representation of organisms from the phylum Firmicutes and a concomitant decrease in the Bacteroidetes (19–26). This observed change in the ratio of Firmicutes to Bacteroidetes was reported in multiple studies and became a common measurement associated with obesity. However, more recent investigations have been able to identify more specific taxonomic changes in the gut microbiota of individuals with metabolic disease. In the case of kwashiorkor, an enrichment of the family Enterobacteriaceae was associated with disease (15). These enriched Enterobacteriaceae were isolated and used to colonize animals that had an otherwise healthy microbiota. Animals receiving these organisms failed to thrive and experienced a 50% mortality rate (27). Interestingly, in this same study, two protective organisms were also identified. Akkermansia muciniphilia and Clostridia scindens were enriched in healthy microbiotas. Provision of these two organisms to animals that possessed a microbiota from an individual with kwashiorkor rescued enhanced mortality associated with the kwashiorkor microbiota. Alternative studies on obese animals identified that obesity was associated with a loss of diversity, specifically within the class Clostridia and enrichment in the genus Desulfovibrio (28). Animals placed on a high-fat diet and orally fed Clostridia were protected from diet-induced obesity and metabolic syndrome. More studies are necessary to continue to identify the specific microbial changes that drive the differences in microbiota-dependent weight gain, as these might prove useful therapeutic interventions.
In addition to finding taxonomic changes in individuals with obesity and T2D, metagenomic sequencing has revealed differences in the functional ability of the microbiota that correlate with obesity and T2D (29). Common among both obesity and T2D is an overall decrease in the functional capacity of the microbiota, meaning a decrease in the number of overall genes that are represented in the microbiota of an individual with metabolic disease versus a healthy individual (23). Although it is still unclear exactly what pathways are lost, there has been a consistent observation that a reduction in the production of short-chain fatty acids (SCFAs) are associated with metabolic disease (30). SCFAs have a myriad of functions within the gut, including anti-inflammatory effects and regulation of intestinal epithelial cell metabolism, thus loss of bacteria that produce this metabolite could have profound effects on the proper functioning of the intestine (31). Moreover, enrichment in genes associated with sulfate reduction and oxidative stress resistance are found in the microbiomes of individuals with metabolic disease (23). Taken together, these observational studies support that disruptions to both microbiota composition and function are associated with metabolic disease.
Microbial mechanisms to influence host metabolism
The coevolution of host and microbes has resulted in essential detection of and defense from pathogens, as well as the ability to sense signals from the microbiome that direct host metabolism. Bacteria produce a plethora of different metabolites derived from the host’s diet. What metabolites are produced is dependent on the composition and function of the microbiota. Bacterial metabolites and products can then be sensed directly at the interface of the host and microbe in the epithelium or can be taken up and circulated to have a greater systemic effect. One of the most well-studied mechanisms by which bacterial metabolites influence the host are SCFAs, which, as discussed above, are decreased in individuals with metabolic disease. The SCFAs acetate, propionate, and butyrate are produced through bacterial fermentation of dietary fibers using genes that mammals do not possess. SCFAs can bind to many different receptors in the host, such as G-protein–coupled receptors, which have been redesignated as free fatty acid receptors. Binding to free fatty acid receptors can trigger inhibition of histone deacetylases that initiates a myriad of downstream signals (32). SCFAs have profound effects on immune cell function, which are detailed in other reviews, but also have important influences on metabolism (33). Indeed, mice supplemented with butyrate and succinate, another bacterial metabolite, have improved glucose sensitivity (25, 34). Accordingly, when mice were placed on a high-fiber diet, succinate produced by the microbiota increased and improved glucose and insulin tolerance (34). In the gut, acetate produced by certain bacteria regulates lipid absorption and metabolism in the enterocyte (35); thus, loss of these SCFAs can alter how fats are absorbed within the intestine. Supporting this, multiple studies have identified that the microbiota can influence how intestinal enterocytes absorb fatty acids (28, 36–38). Demonstrating the relevance of these findings in mice, humans with increased butyrate have improved glucose tolerance, and in a clinical trial, people on a high-fiber diet had increased butyrate and lower hemoglobin A1c, a measure of blood glucose control (23, 30, 39). Obesity and T2D often manifest during the aging process, as do decreases in butyrate (40, 41). Interestingly, one study has found that in aged mice and nonhuman primates, supplementation with butyrate or the butyrate-producing bacteria A. muciniphila restored a normal insulin response (42). However, not all SCFAs act equally. In mice, increased acetate production by the microbiota or supplementation of propionate may promote features of metabolic syndrome through interactions with the parasympathetic and sympathetic nervous system (43, 44). Thus, further studies are required to fully understand the role of these bacterial metabolites on metabolic disease.
Bile acids represent another class of microbial metabolites that can influence host metabolism. Primary bile acids are produced by the liver, stored in the gallbladder, and released into the small intestine upon fat consumption. They emulsify the fat and aid in lipid absorption. The terminal ileum of the small intestine reabsorbs the majority of the primary bile. Still, some reaches the colon, where bacteria metabolize them to produce diverse secondary bile acids with different signaling properties (45). Bile acids bind to a range of receptors, such as farnesoid X receptor (FXR), Takeda G-protein–coupled receptor-5 (TGR5), and the vitamin D receptor, to mediate their effects (46). Mice lacking FXR and, specifically, intestinal FXR are protected from diet-induced obesity and the development of insulin resistance, and experiments in GF and specific pathogen–free FXR−/− mice demonstrate that microbes can negatively impact host metabolism in an FXR-dependent manner (47–49). Paradoxically, FXR activation is also thought to improve insulin sensitivity and non-alcoholic fatty liver disease. Agonist activation of FXR induces glucagon-like peptide-1 (GLP-1), which can decrease glucose levels, in a microbiome-dependent manner (49–51). Studies to refine which bacterially modified bile acids act as antagonists and agonists to different bile acid receptors are ongoing and are beginning to elucidate how they impact host health (45, 52).
Diet-derived amino acids, such as tryptophan and histidine, become metabolized by gut bacteria into a variety of metabolites that bind to the nuclear aryl hydrocarbon receptor (AhR) that impacts a wide range of host processes, including metabolism (53–55). Individuals with metabolic syndrome have reduced fecal production of AhR agonists by the gut microbiota, and restoring AhR signaling through supplementation with an AhR agonist, or a tryptophan-metabolizing Lactobacillus improves insulin resistance and hyperglycemia caused by a high-fat diet (56). Alternatively, the microbial-produced histidine metabolite imidazole propionate is increased in T2D, which causes impaired glucose tolerance and insulin signaling (57). Another metabolically pathogenic microbial metabolite is derived from the amino acid l-carnitine, which is found within red meat. Gut microbes convert l-carnitine into trimethylamine (TMA), which is absorbed and further processed by the liver into the atherogenic metabolite TMA N-oxide (58, 59). l-carnitine’s atherogenic potential depends on the microbiota, and therapies that inhibit the microbial enzymes that generate TMA are being investigated (60). Research continues to discover new bacterial metabolites that influence metabolism and decipher the molecular mechanisms through which they act.
Abs influence the composition and function of the microbiota
Direct effects of inflammatory pathways on metabolic disease have been described in several reviews (11). However, there is now emerging evidence that homeostatic control of the microbiota by the immune system can prevent metabolic diseases (9, 10). This suggests that even small defects in intestinal immunity may have large consequences on host health. Supporting this, individuals with obesity and/or metabolic syndrome have reduced responses to vaccination and decreased mucosal IgA. Although this observation has largely been attributed as being a consequence of metabolic disease itself, it is very possible that these defects in the immune response actually play a causal role in induction of the metabolic disease. This section will review the basic science research that supports how the interaction between the microbiota and the immune response can influence metabolic diseases.
Seminal studies looking at TLR5 knockout mice demonstrated how immune defects are associated with metabolic disease (61). TLR5 is a TLR that recognizes bacterial flagella. Whole body knockouts of TLR5 develop many of the hallmark features of metabolic syndrome, including hyperlipidemia, hypertension, and insulin resistance. This initial study demonstrated that the microbiota composition within a TLR5-deficient animal was responsible for the development of metabolic syndrome (61). Indeed, transfer of the microbiota from a TLR5−/− animal into a GF TLR5-sufficient recipient conferred many features of the metabolic disease identified in TLR5−/− mice. Follow-up studies from this group looked at how the defect in TLR5 leads to changes in the microbial community (48, 62). An overabundance of Proteobacteria is one of the features of the microbiota that forms within a TLR5−/− animal that is commonly associated with inflammation (63). However, using metatranscriptomics, a dramatic increase in the expression of bacterial flagella was observed in the microbiota from TLR5−/− animals (48). In vitro experiments demonstrated that flagella-specific IgA, normally elevated in wild-type (WT) mice, reduced expression of flagella and motility in bacteria. Thus, TLR5 deficiency leads to reduced anti-flagellin Abs, which in turn reduce the ability to control invasive commensal bacteria leading to inflammation and subsequent metabolic disease.
Metabolic syndrome and obesity were similarly observed in animals that were engineered to lack MyD88, specifically within T cells (called T-MyD88−/−) (64). MyD88 is the adaptor molecule that lies downstream of most TLRs and a few innate immune cytokines, including IL-1 and IL-18. Animals that lack T cell–intrinsic MyD88 develop age-associated spontaneous obesity and metabolic syndrome that is microbiota dependent (28). The key immunological deficiency in these mutant animals is a lack of T follicular helper (Tfh) cells that reside within the Peyer patch of the small intestine. Tfh cells provide signals to germinal center B cells that allow for class switching and somatic hypermutation, resulting in maturation of high-affinity IgA-secreting plasma B cells (65). Thus, a lack of Tfh cells in animals that lack T cell–intrinsic MyD88, also possess reductions in germinal center B cells and IgA that target that the microbiota. To demonstrate that Tfh cell function is key to the metabolic syndrome that develops within these animals, T-MyD88−/− animals were given a T cell transplant from either WT or BCL6−/− animals. BCL6 is a transcription factor important in the development of Tfh cells; thus, whereas BCL6−/− T cells can become all other T cell subtypes, Tfh cells fail to develop in these (65). Although T-MyD88−/− animals given WT T cells gained weight normally, BCL6−/− T cells failed to rescue the obesity phenotype. Inappropriate IgA targeting of the microbiota in these animals allowed the expansion of Desulfovibro that was shown to lead to reductions in colonization of beneficial Clostridia. Thus, appropriate T cell–dependent Ab responses are important in maintaining the composition of the microbiota that prevents obesity and metabolic syndrome.
IgA responses were also shown to be important in metabolic diseases associated with undernutrition. A severe form of undernutrition is kwashiorkor, which is caused by sufficient calorie intake but a lack of protein, leading to edema, and an enlarged liver with fatty infiltrates. To study how the microbiota and immunity might interact to influence this disease, GF mice were colonized with the microbiota isolated from Malawian twins discordant for kwashiorkor (27). Animals were subsequently placed on a Malawian diet that is typically macro- and micronutrient deficient or a nutrient-sufficient diet as a control. Animals that possessed the kwashiorkor microbiota and were on the Malawian diet developed an IgA response against several Enterobacteriaceae. These IgA-coated Enterobacteriaceae were sorted and transferred to GF mice placed on a Malawian diet. Although GF mice receiving IgA-coated bacteria from a healthy individual displayed no overt symptoms, animals receiving IgA-coated bacteria from those isolated from the kwashiorkor microbiota had a mortality rate of 50%. Analysis of IgA in a large human cohort demonstrated that IgA targeting of Enterobacteriaceae was indicative of a pathogenic community and could predict disease. Thus, IgA responses target organisms associated with diet-dependent malnutrition and can be used as a biomarker for disease associated with undernutrition.
A more recent study analyzed the effects of B cell deficiency on microbiota composition and identified that a reduction in B cells was associated with gluten-sensitive enteropathy (66). CD19−/− animals have many similarities to individuals with common variable immunodeficiency. Individuals with common variable immunodeficiency have an increased risk for allergy and infections, low Ab titers, and defects in B cell development. Loss of Ab responses in the gut of these animals was reported to be associated with an expansion of anaerobic bacteria. Interestingly, CD19−/− animals develop malabsorption and crypt blunting that mimics what is observed in humans with celiac disease. Treatment with metronidazole, an antibiotic that kills anaerobic bacteria, abolished malabsorption in CD19−/− knockout mice, indicating that the outgrowth of bacteria in this model was driving disease. Thus, these studies demonstrate that Ab-producing B cells function to control the microbial community to prevent metabolic disease.
Immune–microbiota interactions as therapies for metabolic disease
Treatment of metabolic disease using the microbiota has begun to be tested in humans. Fecal microbial transplants (FMTs), in which a whole microbiota community is transplanted into a new host, have been tried with varying degrees of success. In two randomized clinical trials from the Netherlands, FMTs from lean male donors were transplanted into males with obesity via small intestinal infusions. Both studies found a transient improvement in insulin sensitivity at six weeks; however, these results were not maintained at later time points (67, 68). In comparison, a randomized controlled trial conducted in the United States using encapsulated FMT material from lean donors found that, whereas the donor microbiota appeared to engraft into the new host, they only saw a modest improvement in HbA1c levels at 12 wk (69). In their study of both male and female subjects, they reported that metabolic and microbiome responses to the FMT were highly variable. Picking the appropriate donor for the proper recipient is likely key to the therapeutic effects of an FMT in treating metabolic disease, and this could include considering potential immune system effects. We and others have demonstrated that the composition of the microbiota is dependent on the type of MHC molecules expressed (70, 71). In virtually all genome-wide association studies of autoimmune diseases, specific MHC haplotypes are associated with either protection from or susceptibility to disease. Ag presentation by MHC to T cells is key to initiating Ag-specific T and B cells responses. Using MHC congenic mice, which are genetically identical yet express distinct MHC molecules, we demonstrated that unique microbes are able to colonize animals based on their specific MHC haplotypes (70). Specific MHC molecules were associated with different IgA targeting of the microbial community, consistent with the notion that T cell–dependent IgA is an important driver of microbiota community structure. Similarly, a study identified that the type 1 diabetes–protective MHC allele operated via modulation of the intestinal microbiota (71). Indeed, transplant of the protective microbiota could confer resistance to the development of type 1 diabetes in genetically susceptible hosts. Much like bone marrow transplants must be MHC matched, these studies suggest that future successful use of FMT might have to include a consideration of recipient-to-donor MHC matching.
Immune responses against the microbiota might be harnessed therapeutically to prevent metabolic diseases. As described above, Ab responses against bacterial flagella within the gut control expression of flagella in resident bacterial populations. Taking advantage of this natural process, Tran et al. (72) repeatedly injected mice with purified bacterial flagellin to elicit a robust anti-flagellin immune response. This was associated with reduced flagella expression within the microbiota, which prevented mucosal association of these organisms. Importantly, this immunization strategy ameliorated diet-induced obesity in animals. This provides the proof-of-principle experiment that diseases that are not commonly thought to be driven by an “infectious” agent, may be prevented through vaccination approaches. Thus, it will continue to be important to identify epitopes from the microbiota that are associated with inflammatory diseases.
Alternatively, the use of Ab responses against specific organisms may serve as biomarkers for disease or response to therapeutic intervention. Analysis of Ab titers against specific Ags have emerged as common clinical tests for a variety of diseases. Although most of these clinical tests assay for proteins associated with pathogenic infection or self-antigens associated with autoimmunity, there is an example of an assay that detects the presence of Ab reactivity to common gut fungi. A panel of Abs (IgA and IgG) has been commonly used in the clinic to differentiate between the two types of inflammatory bowel diseases: Crohn disease and ulcerative colitis (73). This assay detects the presence of anti–Saccharomyces cerevisiae Abs in the blood, which are present in individuals with Crohn disease but not ulcerative colitis (74). Similarly, kwashiorkor disease was associated with a strong IgA response against Enterobacteriaceae in humans (27), and metabolic disease in mice was associated with anti-flagellin IgA responses. As anti-commensal Ab responses are now appreciated to be common in the blood (75), these data thus suggest that anti-commensal Ab detection might be a novel diagnostic assay that could be developed to track severity of disease or response to treatment.
Conclusions
Collectively, these studies allow us to propose a model whereby homeostatic interactions between Abs and the microbiota dictate microbial composition and function that regulate how the host absorbs nutrients, the inflammatory state, or provides bacterial metabolites to the rest of the body that can alter metabolism (Fig. 1). These interactions offer novel opportunities for therapeutic intervention. These include provision of beneficial organisms to prevent or reverse metabolic disease, vaccination to elicit Ab control of deleterious microorganisms, or use of Ab reactivity of the microbiota as biomarkers of disease. Based on this, it will be important to continue to study how the homeostatic immune system functions to control the microbiota, as disruption of these interactions may be causative agents in metabolic disease.
FIGURE 1.
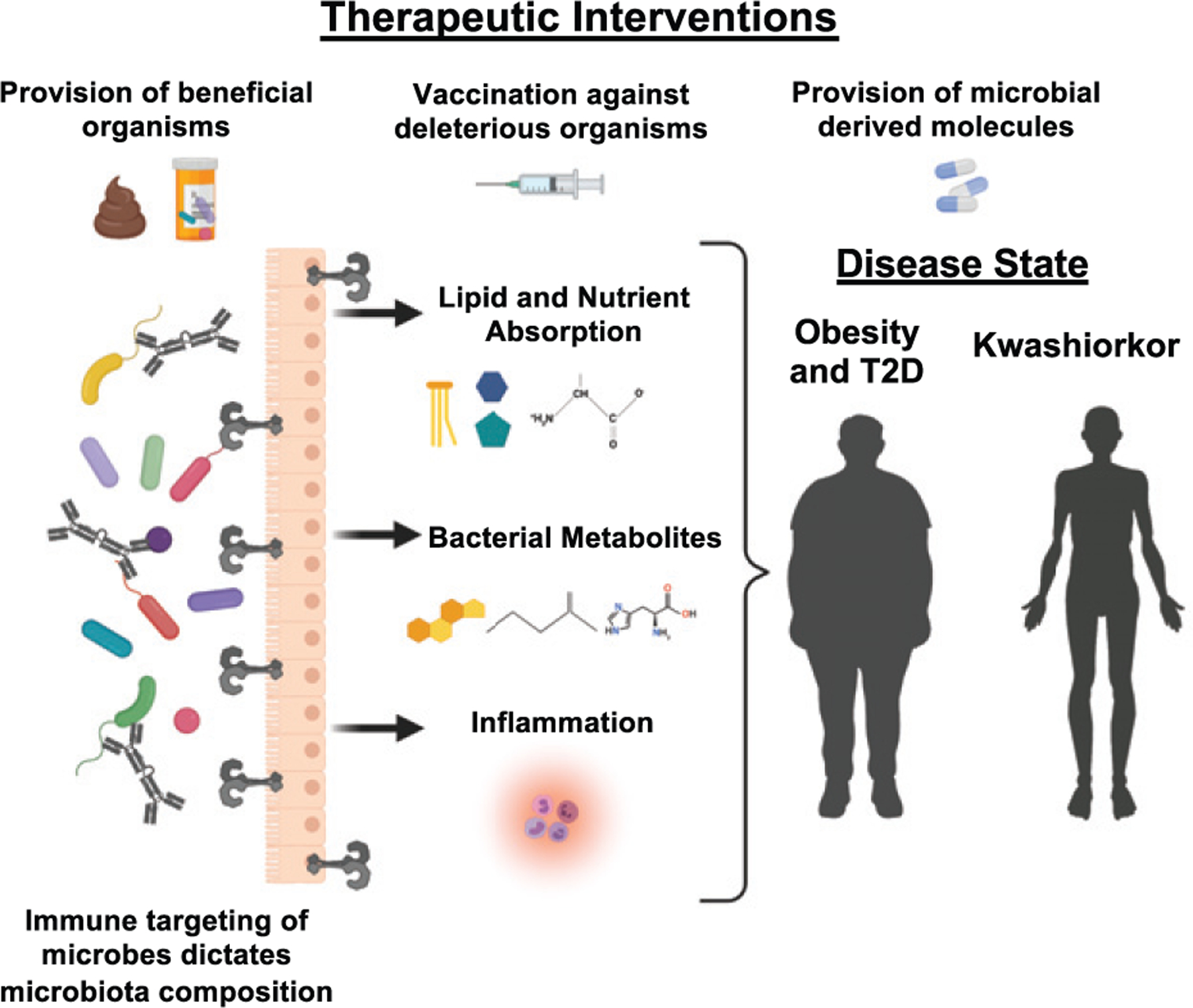
The immune system shapes the function and composition of the microbiota. Alterations in the composition of the microbiota change lipid and nutrient absorption, modify what bacterial metabolites the host receives, and influence the inflammatory state of the host. All of these changes in the microbiota form and function contribute to the pathogenesis of metabolic diseases, such as kwashiorkor, obesity, and T2D. Strategies to address the microbiota basis of metabolic disease include supplementing with beneficial organisms through FMTs or probiotics, supplementing with the beneficial products produced by microbes, such as SCFAs, or vaccinating against deleterious organisms. Deepening our understanding of which patients need what specific microbes and how the host’s unique immune landscape sculpts the microbiome will be vital to developing effective therapies. Created with Biorender.com.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive Diseases (R01DK124336, R01DK124317, and R01AT011423) and the W. M. Keck Foundation.
Abbreviations used in this article:
- AhR
aryl hydrocarbon receptor
- FXR
farnesoid X receptor
- FMT
fecal microbial transplant
- GF
germ-free
- SCFA
short-chain fatty acid
- T2D
type 2 diabetes
- Tfh
T follicular helper
- TMA
trimethylamine
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, and Uauy R; Maternal and Child Nutrition Study Group. 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 2.Kane AV, Dinh DM, and Ward HD 2015. Childhood malnutrition and the intestinal microbiome. Pediatr. Res 77: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreira GC, Cipullo JP, Ciorlia LA, Cesarino CB, and Vilela-Martin JF 2014. Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS One 9: e105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun S, Bitton-Worms K, and LeRoith D 2011. The link between the metabolic syndrome and cancer. Int. J. Biol. Sci 7: 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lusis AJ, Attie AD, and Reue K 2008. Metabolic syndrome: from epidemiology to systems biology. Nat. Rev. Genet 9: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruvada P, Leone V, Kaplan LM, and Chang EB 2017. The human microbiome and obesity: moving beyond associations. Cell Host Microbe 22: 589–599. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RC, Manges AR, Finlay BB, and Prendergast AJ 2019. The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol 27: 131–147. [DOI] [PubMed] [Google Scholar]
- 8.Pascale A, Marchesi N, Marelli C, Coppola A, Luzi L, Govoni S, Giustina A, and Gazzaruso C 2018. Microbiota and metabolic diseases. Endocrine 61: 357–371. [DOI] [PubMed] [Google Scholar]
- 9.Weis AM, and Round JL 2021. Microbiota-antibody interactions that regulate gut homeostasis. Cell Host Microbe 29: 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubinak JL, and Round JL 2016. Do antibodies select a healthy microbiota? Nat. Rev. Immunol 16: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YS, and Olefsky J 2021. Chronic tissue inflammation and metabolic disease. Genes Dev 35: 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, and Gordon JI 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 13.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bäckhed F, Manchester JK, Semenkovich CF, and Gordon JI 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. 2013. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. 2014. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158: 705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, and DeRusso PA 2014. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr 168: 1063–1069. [DOI] [PubMed] [Google Scholar]
- 18.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, and Blaser MJ 2013. Infant antibiotic exposures and early-life body mass. Int. J. Obes 37: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE, Turnbaugh PJ, Klein S, and Gordon JI 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 20.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. ; MetaHIT Consortium. 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546. [DOI] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. 2009. A core gut microbiome in obese and lean twins. Nature 457: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, and Bäckhed F 2013. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498: 99–103. [DOI] [PubMed] [Google Scholar]
- 23.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60. [DOI] [PubMed] [Google Scholar]
- 24.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, and Gordon JI 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, and Ye J 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crovesy L, Masterson D, and Rosado EL 2020. Profile of the gut microbiota of adults with obesity: a systematic review. Eur. J. Clin. Nutr 74: 1251–1262. [DOI] [PubMed] [Google Scholar]
- 27.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, Manary MJ, Liu TC, Stappenbeck TS, Maleta KM, et al. 2015. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med 7: 276ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, et al. 2019. T cell-mediated regulation of the microbiota protects against obesity. Science 365: eaat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thingholm LB, Rühlemann MC, Koch M, Fuqua B, Laucke G, Boehm R, Bang C, Franzosa EA, Hübenthal M, Rahnavard A, et al. 2019. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe 26: 252–264.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, et al. 2019. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet 51: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, and Garrett WS 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander M, and Turnbaugh PJ 2020. Deconstructing mechanisms of dietmicrobiome-immune interactions. Immunity 53: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, and Mithieux G 2016. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 24: 151–157. [DOI] [PubMed] [Google Scholar]
- 35.Araújo JR, Tazi A, Burlen-Defranoux O, Vichier-Guerre S, Nigro G, Licandro H, Demignot S, and Sansonetti PJ 2020. Fermentation products of commensal bacteria alter enterocyte lipid metabolism. Cell Host Microbe 27: 358–375.e7. [DOI] [PubMed] [Google Scholar]
- 36.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, and Gordon JI 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, and Hooper LV 2017. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 357: 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Guryn K, Hubert N, Frazier K, Urlass S, Musch MW, Ojeda P, Pierre JF, Miyoshi J, Sontag TJ, Cham CM, et al. 2018. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 23: 458–469.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, et al. 2018. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 40.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. 2011. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 108(Suppl 1): 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, and Van Remmen H 2015. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 14: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodogai M, O’Connell J, Kim K, Kim Y, Moritoh K, Chen C, Gusev F, Vaughan K, Shulzhenko N, Mattison JA, et al. 2018. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med 10: eaat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, and Shulman GI 2016. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tirosh A, Calay ES, Tuncman G, Claiborn KC, Inouye KE, Eguchi K, Alcala M, Rathaus M, Hollander KS, Ron I, et al. 2019. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med 11: eaav0120. [DOI] [PubMed] [Google Scholar]
- 45.Wahlström A, Sayin SI, Marschall HU, and Bäckhed F 2016. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24: 41–50. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad TR, and Haeusler RA 2019. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat. Rev. Endocrinol 15: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A, et al. 2011. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60: 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, Angenent LT, Bell ME, Hay AG, Peterson DA, et al. 2013. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe 14: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, et al. 2015. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest 125: 386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sattar N, Forrest E, and Preiss D 2014. Non-alcoholic fatty liver disease. BMJ 349(sep19 15): g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dyson J, and Day C 2014. Treatment of non-alcoholic fatty liver disease. Dig. Dis 32: 597–604. [DOI] [PubMed] [Google Scholar]
- 52.Yao L, Seaton SC, Ndousse-Fetter S, Adhikari AA, DiBenedetto N, Mina AI, Banks AS, Bry L, and Devlin AS 2018. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7: e37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hubbard TD, Murray IA, and Perdew GH 2015. Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab. Dispos 43: 1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonowal R, Swimm A, Sahoo A, Luo L, Matsunaga Y, Wu Z, Bhingarde JA, Ejzak EA, Ranawade A, Qadota H, et al. 2017. Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA 114: E7506–E7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neis EP, Dejong CH, and Rensen SS 2015. The role of microbial amino acid metabolism in host metabolism. Nutrients 7: 2930–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, Michel ML, Chong-Nguyen C, Roussel R, Straube M, et al. 2018. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab 28: 737–749.e4. [DOI] [PubMed] [Google Scholar]
- 57.Koh A, Molinaro A, Ståhlman M, Khan MT, Schmidt C, Mannerås-Holm L, Wu H, Carreras A, Jeong H, Olofsson LE, et al. 2018. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 175: 947–961.e17. [DOI] [PubMed] [Google Scholar]
- 58.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. 2013. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, et al. 2019. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J 40: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, et al. 2018. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med 24: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, and Gewirtz AT 2010. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chassaing B, Ley RE, and Gewirtz AT 2014. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147: 1363–77.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, and Finlay BB 2007. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2: 204. [DOI] [PubMed] [Google Scholar]
- 64.Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, and Round JL 2015. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crotty S 2019. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50: 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammed AD, Khan MAW, Chatzistamou I, Chamseddine D, Williams-Kang K, Perry M, Enos R, Murphy A, Gomez G, Aladhami A, et al. 2019. Gut antibody deficiency in a mouse model of CVID results in spontaneous development of a gluten-sensitive enteropathy. Front. Immunol 10: 2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. 2017. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 26: 611–619.e6. [DOI] [PubMed] [Google Scholar]
- 68.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. 2012. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143: 913–6.e7. [DOI] [PubMed] [Google Scholar]
- 69.Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, Ford CB, Bryant JA, Henn MR, and Hohmann EL 2020. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 17: e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kubinak JL, Stephens WZ, Soto R, Petersen C, Chiaro T, Gogokhia L, Bell R, Ajami NJ, Petrosino JF, Morrison L, et al. 2015. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nat. Commun 6: 8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silverman M, Kua L, Tanca A, Pala M, Palomba A, Tanes C, Bittinger K, Uzzau S, Benoist C, and Mathis D 2017. Protective major histocompatibility complex allele prevents type 1 diabetes by shaping the intestinal microbiota early in ontogeny. Proc. Natl. Acad. Sci. USA 114: 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran HQ, Ley RE, Gewirtz AT, and Chassaing B 2019. Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat. Commun 10: 5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitsuyama K, Niwa M, Takedatsu H, Yamasaki H, Kuwaki K, Yoshioka S, Yamauchi R, Fukunaga S, and Torimura T 2016. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol 22: 1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaffer T, Flogerzi B, Schoepfer AM, Seibold F, and Müller S 2013. Increased titers of anti-Saccharomyces cerevisiae antibodies in Crohn’s disease patients with reduced H-ficolin levels but normal MASP-2 activity. J. Crohn’s Colitis 7: e1–e10. [DOI] [PubMed] [Google Scholar]
- 75.Kamada N, Sakamoto K, Seo SU, Zeng MY, Kim YG, Cascalho M, Vallance BA, Puente JL, and Núñez G 2015. Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe 17: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]


