Abstract
Simple Summary
Serine and glycine have an important role in the folate-dependent one-carbon metabolism. No prior epidemiologic study has evaluated the associations for serum levels of serine and glycine with pancreatic cancer risk. We performed a nested case-control study of 129 incident pancreatic cancer cases and 258 individually matched controls within the Shanghai Cohort Study, a prospective cohort study involving 18,244 male residents in Shanghai, China. We found that the risk of pancreatic cancer was reduced by more than 70% in individuals with elevated levels of serine and glycine in serum collected, on average, more than 10 years prior to cancer diagnosis. These novel findings support a protective role of serine and glycine against the development of pancreatic cancer in humans that might have an implication for pancreatic cancer prevention.
Abstract
Background. Serine and glycine play an important role in the folate-dependent one-carbon metabolism. The metabolism of serine and glycine has been shown to be associated with cancer cell proliferation. No prior epidemiologic study has investigated the associations for serum levels of serine and glycine with pancreatic cancer risk. Methods. We conducted a nested case-control study involved 129 incident pancreatic cancer cases and 258 individually matched controls within a prospective cohort study of 18,244 male residents in Shanghai, China. Glycine and serine and related metabolites in pre-diagnostic serum were quantified using gas chromatography-tandem mass spectrometry. A conditional logistic regression method was used to evaluate the associations for serine, glycine, and related metabolites with pancreatic cancer risk with adjustment for potential confounders. Results: Odds ratios (95% confidence intervals) of pancreatic cancer for the highest quartile of serine and glycine were 0.33 (0.14–0.75) and 0.25 (0.11–0.58), respectively, compared with their respective lowest quartiles (both p’s < 0.01). No significant association with risk of pancreatic cancer was observed for other serine- or glycine related metabolites including cystathionine, cysteine, and sarcosine. Conclusion. The risk of pancreatic cancer was reduced by more than 70% in individuals with elevated levels of glycine and serine in serum collected, on average, more than 10 years prior to cancer diagnosis in a prospectively designed case-control study. These novel findings support a protective role of serine and glycine against the development of pancreatic cancer in humans that might have an implication for cancer prevention.
Keywords: pancreatic cancer, risk factors, serine, glycine
1. Introduction
Pancreatic cancer is ranked as the 12th most common cancer in men and 11th most common cancer in women, with an estimated 460,000 new cases in 2018 worldwide [1]. In the United States, pancreatic cancer is the third leading cause of cancer death with an estimated 47,050 deaths due to pancreatic cancer in 2020 [2]. The prognosis of pancreatic cancer is poor; the five-year survival rate is only 8% of patients after diagnosis [3]. In addition, the incidence and mortality rates of pancreatic cancer have been increasing during past four decades [4]. In China, between 1990 and 2019, there were 1,817,952 incident cases and 1,854,761 deaths of pancreatic cancer [5]. Overall, the incidence rates of pancreatic cancer in China increased annually among those aged 25 years old and older. The annual percentage change of pancreatic cancer incidence between 1990 and 2019 in China was 2.3 (95% confidence interval-CI: 2.1–2.5) [6]. Established risk factors for pancreatic cancer are cigarette smoking, alcohol consumption, obesity, chronic pancreatitis, and type 2 diabetes [4,7]. Collectively, less than half of the pancreatic cancer burden is attributable to these identified risk factors [8]. The underlying causes are still controversial and unknown for majority of pancreatic cancer cases. Furthermore, the mechanistic networks implicated in pancreatic carcinogenesis are not fully understood. Therefore, it is an urgent need to identify novel etiological factors, especially modifiable factors that would help develop an evidence-based strategy for primary prevention of pancreatic cancer.
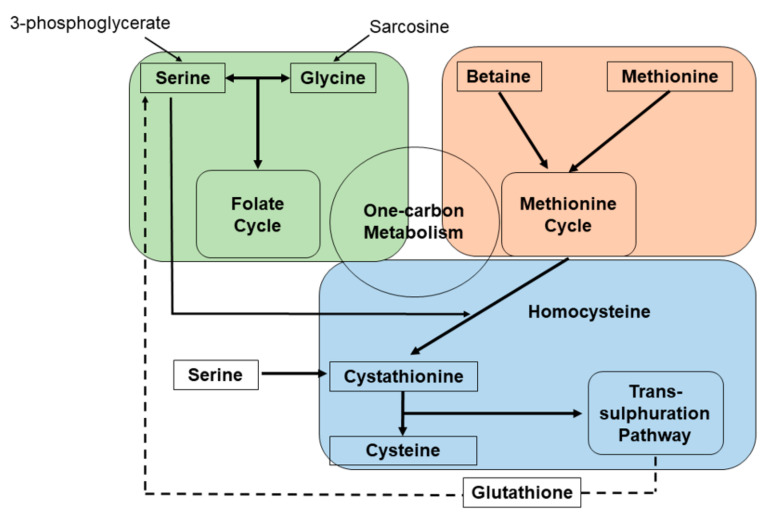
One-carbon metabolism involves the folate cycle and the methionine cycle. These cycles are responsible for the integration of different nutritional sources (i.e., amino acids, glucose and vitamins) and for the generation of diverse physiologically functional compounds (i.e., nucleotides and proteins). These one-carbon metabolic cycles also provide the substrates for methylation reactions and maintenance of redox status [9]. The two one-carbon cycles are interconnected. 3-Phosphoglycerate (3PG), which is an intermediate metabolite involved in glycolysis, can be converted into serine, a major donor of carbon to the folate cycle [10,11]. The carbon donation of serine is linked to the conversion of tetrahydrofolate (THF) to methylene-tetrahydrofolate (Methylene-THF) [12,13]. Glycolysis, a determinant for this pathway, is known to be associated with cancer initiation and progression in rat carcinoma models [12,14,15]. Another modular unit of one-carbon metabolism is the trans-sulfuration pathway, which is connected to the methionine cycle through the intermediate homocysteine [9]. Accordingly, cystathionine is generated from serine, which can condense enzymatically with homocysteine catalyzed by cystathionine beta-synthase (CBS) and plays an essential role for redox buffering (Figure 1). Both homocysteine and serine are precursors for cysteine synthesis (Review in Yang et al. [16]).
Figure 1.
Schematic diagram of serine and glycine metabolism pathways.
Evidence from prior experimental studies has shown that the serine-glycine metabolism has been associated with cancer cell proliferation and survival [17,18,19]. For example, Jain et al. [19] found the mitochondrial glycine biosynthetic pathway is strongly associated with proliferation rates of multiple cancer cells whereas serine deprivation was associated with slowed cancer cell proliferation, which may be due to the depletion of purine synthesis and decreased adenosine triphosphate (ATP) production [20,21],. Serine starvation triggers de novo synthesis of this amino acid which by utilizing glycolytic intermediates, impedes energy production [22].
Prior studies have found elevated levels of serum serine and glycine in cases of various sites of cancers including the lung [23], bladder [24], prostate [25], kidney [26], and colon [27] whereas other studies produced null results in rectal cancer [27] and pancreatic cancer [28]. For example, Jiao et al. [28] found no significant difference in serum concentrations of serine and glycine between pancreatic cancer cases and controls within the Women’s Health Initiative Study, probably due to a modest sample size (only 30 pancreatic cancer cases vs. 30 matched controls). On the contrary, in a study of 136 female non-smoking subjects (i.e., 65 patients with non-small cell lung carcinoma, 6 with benign lung tumor and 65 healthy controls), Mu et al. [23] found that the serum concentrations of both serine and glycine in lung cancer patients were significantly lower than in healthy controls. Similarly, a large case-control study involving 3000 prostate cancer patients and 3000 controls within the JANUS Cohort in Norway reported a significantly lower risk of prostate cancer (odds ratio-OR = 0.83, 95% confident interval-CI: 0.70–1.00) associated with higher level of glycine [25]. Furthermore, in a study of 58 cases of renal cell carcinoma versus 59 controls, Mustafa et al. [26] reported that serum levels of both serine and glycine in renal cell carcinoma patients were significantly lower than those in controls. In another study of 59 colon cancer patients and 58 controls, Leichtle et al. [29] found that serum glycine level was significantly higher in controls than colon cancer patients while there was no significant different between colon cancer cases vs. control in serum serine level.
One-carbon metabolism cycles are complex metabolic network with several chemical catalysts involved. Better understanding of this milieu provides ample opportunities for risk prediction and potential prevention. The objective of the current analysis was, therefore, to comprehensively examine these associations in a prospective cohort study of more than 18,000 study participants.
2. Methods
2.1. Study Population
The present study was based on the Shanghai Cohort Study that has been previously described [30]. The Shanghai Cohort Study is an on-going prospective cohort study that enrolled 18,244 male residents at 45–64 years of age who resided within four communities in the city of Shanghai, China, from January 1986 to September 1989. At the time of enrolment, a trained nurse interviewed each study participant in-person. Information on use of tobacco and alcohol consumption, usual dietary pattern and past medical history were collected via an interview. At the end of the interview, the nurse also collected a 10-mL non-fasting blood sample and a single-void urine sample from the study participant. Serum and urine samples were stored at −72 °C. The study protocol has been approved by the Institutional Review Boards (IRBs) of the University of Pittsburgh, and Shanghai Cancer Institute. The present study was also approved by the IRB of the University of Pittsburgh.
2.2. Case Ascertainment of Pancreatic Cancer
The incident cases of cancer and death among participants of the Shanghai Cohort Study were identified through annual follow-up interviews on all surviving study participants or next of kin for those deceased in the preceding 12 months. In addition, an annual record linkage analyses were conducted with the databases of the population-based Shanghai Cancer Registry and the Shanghai Municipal Vital Statistics Office to identify and confirm new cases of cancer and deaths, respectively. Pancreatic cancer was defined per the International Classification of Diseases-Oncology, 9th edition (ICD-9) code 157. In the most recent follow-up data in 2015, 3.7% of original cohort participants were lost to the follow-up and 3.3% declined to participate in the continued follow-up interview. Since their cancer incidence and/or vital status had been updated using the linkage analyses described above, the follow-up for occurrence of cancer and death among all cohort participants was virtually complete.
2.3. Nested Case-Control Study
The current analysis was a nested case-control study within the Shanghai Cohort Study in which 129 incident pancreatic cancer cases were detected as of 31 December 2009. For each pancreatic cancer case, two control subjects were randomly selected among the eligible participants of the Shanghai Cohort Study who were free of cancer and alive during the time period from the phlebotomy to the timepoint of diagnosis of pancreatic cancer of the index case. The selected controls were individually matched to the index case by age (±2 years), date of blood draw (±one month), and the neighborhood of residence at the time of enrolment.
2.4. Assessment of Serum Biomarkers
Serum specimens of cases and their matched controls were processed, aliquoted, shipped in frozen state and assayed together at Bevital A/S, Bergen, Norway (www.bevital.no, Accessed date: 15 December 2015). The serum samples of each matched case-control set (1 case and 2 controls) were placed next to each other in a random order and tested simultaneously. The personnel were blinded to the case/control status of the test samples.
Gas chromatography-tandem mass spectrometry (LC-MS/MS) [31] was used to quantify serum serine, glycine, sarcosine, glutamine, total cysteine, cystathionine and methionine, while liquid chromatography-tandem mass spectrometry was applied to measure creatinine, choline, betaine [32], cotinine [33], and pyridoxal 5′-phosphate (PLP) [33]. Serum creatinine was used for the calculation of glomerular filtration rate (eGFR), an indicator of renal function [34]. Serum cotinine is a metabolite of nicotine, and is utilized as an indicator of recent exposure to tobacco smoking and/or use of other nicotine-containing products [35]. For quality control purposes, 14 duplicated samples derived from a pool of serum samples collected from cohort participants at the same period of the study sample collection were dispersed in seven batches of test sample (two per batch). The within-batch coefficients of variation (CV) for all biomarkers tested ranged between 0.7% and 5.0% while the between-batch CV ranged between 1.3% and 2.6% (Table S1).
2.5. Statistical Analysis
We used natural logarithmic transformation of original values in order to reduce their skewness and also improve the normality of their distributions. To examine the difference in concentrations between cases and controls according to demographic characteristics we used the analysis of covariance (ANCOVA).
The conditional logistic regression method was used to calculate odds ratios (ORs) and the 95% confidence intervals (CIs) for pancreatic cancer according to quartiles of serum biomarkers studied, the cut-offs of which were determined by their distributions among control subjects. To adjust for the potential confounding effects, multivariable logistic models included level of education (no formal schooling, primary school, secondary school or above), body mass index (BMI) (<18.5, 18.5–<23, or ≥23 kg/m2), smoking status (never, former, or current smokers), serum cotinine (nmol/L), alcohol consumption (based on number of drinks per week), history of diabetes (yes, no), serum PLP levels (nmol/L) [36], eGFR [36] and total methyl donor (i.e., sum of choline, betaine and methionine) [37]. The linear trend test was based on the ordinal values of quartile (i.e., 1, 2, 3, and 4) for each of the studied biomarkers with the risk of developing pancreatic cancer. We conducted sensitivity analysis stratified by median follow-up time (<13 versus ≥13 years).
Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). All p values reported are two-sided. p less than 0.05 was considered as statistically significant.
3. Results
The mean (standard deviation (SD)) age at blood draw for pancreatic cancer cases and controls were 56.4 (5.5) years and 56.5 (5.5) years, respectively. Among cases, the average (range) time interval from blood draw to cancer diagnosis was 12.5 years (3 months-23.2 years).
The baseline characteristics and selected risk factors of study participants are presented in Table 1. Compared with controls, pancreatic cancer cases had significantly higher percentage of current smokers and higher levels of serum cotinine but lower levels of total methyl donors. There were no statistically significant differences between cases and controls in age, BMI, eGFR, PLP, education level, alcohol intake and history of diabetes.
Table 1.
Baseline demographic characteristics and lifestyle factors of pancreatic cancer cases and control subjects in the Shanghai Cohort Study.
| Baseline Characteristics | Controls | Cases | p |
|---|---|---|---|
| Number of subjects | 258 | 129 | |
| Age (years) (Mean ± SD) | 56.4 ± 5.5 | 56.5 ± 5.5 | 0.74 |
| Body mass index (kg/m2) (Mean ± SD) | 21.9 ± 2.8 | 22.5 ± 3.0 | 0.08 |
| Level of education, n (%) | 0.36 | ||
| No formal schooling | 13 (5.0) | 3 (2.3) | |
| Primary school | 74 (28.7) | 34 (26.4) | |
| Secondary school or above | 171 (66.3) | 92 (71.3) | |
| Smoking status, n (%) | 0.003 | ||
| Never | 113 (43.8) | 35 (27.1) | |
| Former | 16 (6.2) | 6 (4.7) | |
| Current | 129 (50.0) | 88 (68.2) | |
| Cotinine (nmol/L) (Geometric mean ± SD) | 440.0 ± 573.8 | 576.4 ± 573.0 | 0.02 |
| Level of alcohol intake (drinks/week), n (%) | 0.74 | ||
| 0 | 146 (56.6) | 70 (54.3) | |
| <7 | 29 (11.2) | 18 (14.0) | |
| ≥7 | 83 (32.2) | 41 (31.8) | |
| History of diabetes, n (%) | 0.52 | ||
| No | 254 (98.5) | 128 (99.2) | |
| Yes | 4 (1.55) | 1 (0.78) | |
| eGFR (mL/min/1.73 m2) (Geometric mean ± SD) | 89.7 ± 12.4 | 91.5 ± 11.3 | 0.16 |
| PLP (nmol/L) (Geometric mean ± SD) | 35.6 ± 46.9 | 33.9 ± 60.9 | 0.76 |
| Total methyl donors µmol/L(Geometric mean ± SD) a | 114.7 ± 114.2 | 109.4 ± 112.0 | 0.02 |
Abbreviations: eGFR, estimated glomerular filtration rate; PLP, pyridoxal 5′-phosphate; SD: standard deviation. a Total methyl donors: sum of choline, betaine, and methionine. The significant findings are demonstrated in bold fonts.
The serum concentrations of serine and glycine were significantly lower in pancreatic cancer cases than in controls (both p’s < 0.02; Table 2). There was no statistically significant difference in serum concentrations of sarcosine, cysteine, and cystathionine between cases and controls.
Table 2.
Geometric means of serum concentrations of biomarkers studied in pancreatic cancer cases and control subjects in the Shanghai Cohort Study.
| Biomarkers * (µmol/L) | Controls, n = 258 Geometric Mean (95% CI) |
Cases, n = 129 Geometric Mean (95% CI) |
p a |
|---|---|---|---|
| Serine | 186.37 (182.98–189.80) |
179.01 (174.33–183.80) |
0.017 |
| Glycine | 363.05 (356.96–369.24) |
345.52 (337.18–354.00) |
0.002 |
| Cystathionine | 0.28 (0.27–0.30) |
0.29 (0.27–0.31) |
0.70 |
| Cysteine | 272.00 (268.45–275.60) |
273.15 (268.01–278.30) |
0.73 |
| Sarcosine | 1.98 (1.92–2.06) |
2.03 (1.93–2.14) |
0.49 |
a p-value to compare geometric means adjusted for age and gender, level of education (no formal schooling, primary school, and secondary school or above), body mass index (<18.5, 18.5–<23.0, ≥23.0 kg/m2), smoking status (never, former, and current smokers), number of alcoholic drinkers per week (continuous), history of diabetes (no, yes), serum cotinine concentration (nmol/L), serum pyridoxal 5′-phosphate concentration (nmol/L), estimated glomerular filtration rate ((mL/min/1.73 m2) and total methyl donors. * Mean serum concentrations. The significant findings are demonstrated in bold fonts.
Higher levels of serine and glycine were significantly associated with decreased risk of pancreatic cancer (Table 3). Compared with the lowest quartile, the ORs (95% CIs) of pancreatic cancer for the highest quartile of serine and glycine were 0.33 (0.14–0.75) and 0.25 (0.11–0.58), respectively (both ptrend < 0.005). No statistically significant association was observed for risk of pancreatic cancer with any other biomarkers tested including cystathionine, cysteine, or sarcosine (Table 3).
Table 3.
Associations between serum concentrations of biomarkers studied and pancreatic cancer risk in the Shanghai Cohort Study.
| Biomarkers in Quartile | Controls | Cases | OR (95% CI) a |
|---|---|---|---|
| Serine | |||
| Q1 | 65 | 49 | 1.00 |
| Q2 | 64 | 29 | 0.56 (0.30–1.10) |
| Q3 | 65 | 28 | 0.43 (0.22–0.83) |
| Q4 | 64 | 23 | 0.33 (0.14–0.75) |
| ptrend | 0.003 | ||
| Continuous (log2) | 258 | 129 | 0.28 (0.09–0.85) |
| Glycine | |||
| Q1 | 65 | 51 | 1.00 |
| Q2 | 64 | 34 | 0.68 (0.36–1.27) |
| Q3 | 65 | 23 | 0.39 (0.19–0.79) |
| Q4 | 64 | 21 | 0.25 (0.11–0.58) |
| ptrend | 0.001 | ||
| Continuous (log2) | 258 | 129 | 0.14 (0.04–0.51) |
| Cystathionine | |||
| Q1 | 65 | 34 | 1.00 |
| Q2 | 64 | 33 | 0.94 (0.48–1.83) |
| Q3 | 65 | 30 | 0.91 (0.46–1.83) |
| Q4 | 64 | 32 | 1.46 (0.72–2.93) |
| ptrend | 0.77 | ||
| Continuous (log2) | 258 | 129 | 1.09 (0.75–1.59) |
| Cysteine | |||
| Q1 | 65 | 32 | 1.00 |
| Q2 | 64 | 28 | 1.05 (0.54–2.06) |
| Q3 | 65 | 38 | 1.52 (0.78–2.99) |
| Q4 | 64 | 31 | 1.41 (0.69–2.88) |
| ptrend | 0.26 | ||
| Continuous (log2) | 258 | 129 | 1.37 (0.31–6.01) |
| Sarcosine | |||
| Q1 | 65 | 34 | 1.00 |
| Q2 | 64 | 29 | 0.91 (0.47–1.78) |
| Q3 | 65 | 34 | 1.18 (0.62–2.24) |
| Q4 | 64 | 32 | 1.27 (0.65–2.47) |
| ptrend | 0.39 | ||
| Continuous (log2) | 258 | 129 | 1.26 (0.72–2.19) |
a Derived from multivariable logistic regression models adjusting for level of education (no formal schooling, primary school, and secondary school or above), body mass index (<18.5, 18.5–<23.0, ≥23.0 kg/m2), smoking status (never, former, and current smokers), number of alcoholic drinkers per week (continuous), history of diabetes (no, yes), serum cotinine concentration (nmol/L), serum pyridoxal 5′-phosphate concentration (nmol/L), estimated glomerular filtration rate ((mL/min/1.73 m2) and total methyl donors; OR with 95% CI excluding one or ptrend < 0.05 are in bold.
We also examined the joint effect of serine and glycine on risk of pancreatic cancer. Compared with lower levels (below median) of both glycine and serine, individuals with higher levels (above median) of serine or glycine or both serine and glycine were at reduced risk of pancreatic cancer. The respective odds ratios and 95% Cis were 0.52 (0.31–0.87), 0.38 (0.21–0.70), and 0.24 (0.11–0.63) (Table 4).
Table 4.
Joint effect of serine and glycine on the risk of pancreatic cancer in the Shanghai Cohort Study a.
| Glycine | Serine | |||||
|---|---|---|---|---|---|---|
| Low (<184.2) | High (≥184.2) | Total | ||||
| Cases/Control | OR (95% CI) | Cases/Control | OR (95% CI) | Cases/Control | OR (95% CI) | |
| Low (<353.3) | 61/90 | 1.00 | 24/39 | 0.77 (0.41–1.46) | 85/129 | 1.00 |
| High (≥353.3) | 17/39 | 0.56 (0.26–1.22) | 27/90 | 0.24 (0.11–0.63) | 44/129 | 0.38 (0.21–0.70) |
| Total | 78/129 | 1.00 | 51/129 | 0.52 (0.31–0.87) | ||
a Derived from multivariable logistic regression models adjusting for level of education (no formal schooling, primary school, and secondary school or above), body mass index (<18.5, 18.5–<23.0, ≥23.0 kg/m2), smoking status (never, former, and current smokers), number of alcoholic drinkers per week (continuous), history of diabetes (no, yes), serum cotinine concentration (nmol/L), serum pyridoxal 5′-phosphate concentration (nmol/L), estimated glomerular filtration rate (mL/min/1.73 m2) and total methyl donors; ORs with 95% CIs excluding one are in bold. Low and high are defined as individuals with concentrations below and above the median, respectively.
We investigated the correlation of serum serine, glycine, and other biomarkers measured with selected demographics, lifestyle factors, and other risk markers among control subjects. Age was positively correlated with serum levels of cysteine and cystathionine whereas BMI, cigarette smoking and alcohol intake were inversely correlated with cysteine and cystathionine (Supplementary Table S2). Smokers had elevated level of glycine (r = 0.14, p < 0.05). eGFR was inversely correlated with cysteine (r = −0.37, p < 0.001), cystathionine (r = −0.29, p < 0.001) and sarcosine (r = −0.17, p < 0.001). Serum PLP was inversely correlated with serine (r = −0.21, p < 0.001) and glycine (r = −0.26, p < 0.001) but positively with cysteine (r = 0.26, p < 0.001). Among one-carbon biomarkers, the highest correlation was between glycine and serine (r = 0.59, p < 0.001), followed by cystathionine and sarcosine (r = 0.27, p < 0.001). All other pairwise correlation coefficients were modest to null (r’s < 0.15); Supplementary Table S3).
In the full model, we found that smoking status and education levels were independent risk factors while alcohol drinking per week appeared to be protective factor for pancreatic cancer (Supplementary Table S4). We also performed additional analysis for the association for dietary intakes of protein, energy, fat, and carbohydrates, which were derived from baseline dietary survey at the same time of blood draw, with circulating levels of serine and glycine. No statistically significant association was observed (Table S5).
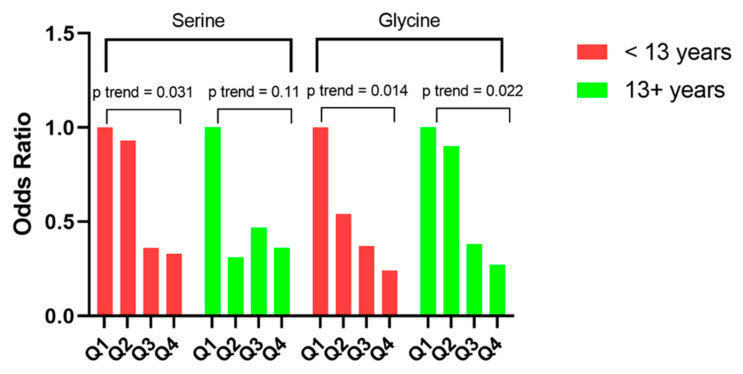
In stratified analysis by median follow-up time (i.e., <13 versus ≥13 years), the significant inverse trends for serine and glycine with risk of pancreatic cancer were present for both shorter and longer periods of follow-up. Accordingly, the ORs (95% CIs) for the highest relative to the lowest quartiles of serine and glycine were 0.33 (0.09–1.26) and 0.24 (0.07–0.78), respectively, in participants with less than 13 years of follow-up (both ptrend < 0.05). The corresponding figures in participants with 13 or more years of follow-up were 0.36 (0.11–1.14, ptrend = 0.11) and 0.27 (0.07–1.00, ptrend = 0.02), respectively (Figure 2).
Figure 2.
Risk of pancreatic cancer for serum serine and glycine in the Shanghai Cohort Study, stratified by follow-up time.
4. Discussion
In a nested case-control study of 129 pancreatic cancer and 258 free of cancer subjects within the Shanghai Cohort Study of more than 18,000 men living in Shanghai, we found that the lowest risk of pancreatic cancer was observed among individuals with high levels of both serine and glycine. Risk of pancreatic cancer was reduced by more than 70% in individuals with elevated levels of glycine and serine in serum collected, on average, 13 years prior to cancer diagnosis. These strong novel findings, together with our recent results on the inverse association for PLP [36] and methionine [37] with pancreatic cancer risk, suggest that one-carbon metabolism involving serine and glycine may play an important role in the development of pancreatic cancer.
While our result on the inverse association between serine and glycine with risk of pancreatic cancer was inconsistent with a recent study in the Women’s Health Initiative [28], our findings are in line with other studies on lung cancer [23], bladder cancer [24], prostate cancer [25], renal cell carcinoma [26], and colon cancer [29]. The different findings between our study and prior study in the Women’s Health Initiative (WHI) might be due to the difference in the studied populations (Asians vs. Caucasians) or sample size (129 incident pancreatic cancer cases vs. 259 matched controls in our study vs. 30 pairs matched cases-controls in the WHI study [28]).
Methyl donors exert a pivotal effect in genetics, epigenetics and metabolic pathways, and are considered a promising prospect for prevention and risk modification of various diseases including cancer [38]. A number of molecules in methyl-cycles are implicated in tumor formation and progression. The core of this network comprises of interconnected methionine and folate cycles. While nucleotide synthesis is the most relevant end-product of one-carbon cycles and the cornerstone of tumor cells transformation, the other products are playing context-specific roles in tumor transformation [39].
Serine, the major donor of carbon units, is synthesized by phosphoglycerate dehydrogenase (PHGDH) and feeds into the core of one-carbon cycle. Serine is further converted to glycine by serine hydroxymethyltransferase (SHMT) via transferring one carbon to tetrahydrofolate (THF). Recently, Yu et al. [40] reported that an accumulation of upstream glycolytic intermediates may cause increased flux through the serine biosynthesis pathway. Decreased pyruvate production via knocking out the PHGDH gene leads to diminished serine biosynthesis. On the other hand, they [40] revealed serine contributes to pyruvate synthesis in cell lines with knockdown pyruvate kinase M2 (PKM2), an isoform of enzyme highly expressed in pancreatic ductal adenocarcinoma (PDAC) cell [40]. Decreased serine biosynthesis pathway by knocking out the PHGDH gene decreased pyruvate production (i.e., reduced levels of 13C-glucose labelled pyruvate), which is highly expressed in PDAC cells, suggesting a protective effect of serine against pancreatic cancer development. Another study by Mohammad et al. [41] in vitro and in vivo preclinical models of pancreatic cancer showed that the treatment with the combination of TEPP-46 (an activator of PKM2) and FX-11 (an inhibitor of lactate dehydrogenase A or LDHA) resulted in increased pyrurate kinase (PK) and reduced LDHA enzyme activity in plasma and tumor tissues, and decreased PKM2 and LDHA expression in tumors and consequently decreased tumor volume and proliferation.
A study from the Vousden’s lab [22], on the other hand, questioned the protective impact of exogenous serine or glycine on tumor growth in autochthonous mouse models of Kras-driven pancreatic cancer. They [22], however, acknowledged that even though the dietary restriction of serine and glycine may be effective in suppressing the development of such cancers, whether the effect of dietary limitation of serine and glycine would change serum or tumor levels of these amino acids in human remains unknown. Further evidence from Amelio et al. indicated the upregulation of the serine/glycine biosynthesis pathway drives oncogenesis [42]. The interaction between the serine/glycine metabolism and tumorigenesis is complicated and a number of genetic and epigenetic factors may interfere with this pathway [43]. The current study might be the first human study to reveal this gap of knowledge by showing that high serum concentrations of serine and glycine many years prior to the clinical diagnosis decreased the risk of pancreatic cancer.
The findings of our study may indicate the specific impact of serine and glycine at the early stages of tumor development. While serine helps tumor progression via activating PMK2 and support metabolic reprogramming of PDAC cells, at the early stages physiologic levels of serine can inhibit tumor development. Hwang et al. [44] reported that serine biosynthesis can induce cancer-protective by-products. A serine biosynthesis by-product such as α-ketoglutarate, a tricarboxylic acid intermediate, functions as a cofactor for dioxygenases involved in regulation of gene expression. Previous study by Morris et al. [45] has shown the effector role of α-ketoglutarate in p-53 tumor suppressive function in pancreatic cancer. Another pathway that might explain the protective impact of glycine on pancreatic cancer may be through the glycine-conjugation of bile acids [46]. Secondary bile acid ursodeoxycholic acid (UCDA), formed by intestinal microbiota, is predominantly conjugated with glycine. Glycine conjugation increases the hydrophilicity with consequent reduced cytotoxicity and membranolytic properties of bile acid [47]. UDCA has antioxidant, anti-inflammatory and cytoprotective properties [48], inhibits cell proliferation by blockade of cell cycle at G1 phase [49]. In addition, Khaire et al. [50] reported that UDCA significantly inhibited Ras mutations and Cox-2 protein and mRNA levels in tumors with normal Ras activity in colon cancer suggesting its protective effect in the development of colon cancer. Studies in pancreatic cancer has shown the inhibitory effect of UDCA on cancer stem-like cells [51].
Serum concentrations of serine and glycine are reflective of their dietary intake and de novo synthesis. Changes in serine metabolism are influenced by dietary isocaloric protein restriction (i.e., protein malnutrition). Kalhan et al. [52] observed that there was a doubling serine concentration in the blood and livers of the rats fed the protein-restricted diet and 50% increase in the de novo synthesis of this amino acid while there was no change in the concentration of serine in the kidneys of rats fed with protein-restricted diet. Different studies demonstrated that a restricted-protein diet led to increase the concentration of serine in the blood, though the exact mechanism remains to be elucidated [53,54,55]. Furthermore, the consumption of glycine along biosynthesis of glycine have been associated with high-rate proliferating cancer cell lines.
We found that smokers had elevated levels of glycine. Since smoking is a well-known risk factor for pancreatic cancer [8,56,57], we adjusted smoking status in the multivariable regression model so the observed association would be less likely to be confounded by smoking. The association between smoking and glycine might be explained by different pathways. For instance, extensive evidence shows that smokers eat more meat, thus contributing to the evaluated level of glycine. In the COSMOS (Continuous Observation of Smoking Subjects) prospective cohort study of 5203 asymptomatic participants, aged ≥50 years, Gnagnarella et al. [58] found that smoking was positively and statistically significant correlated with red meat (r = 0.048, p < 0.05). In a case-control study of 187 lung cancer men and 252 controls in Poland, Hawrysz et al. [59] reported that smokers was positively associated with red meat and processed meat intakes among healthy controls (OR = 2.0, 95% CI: 1.8–2.9 among moderate smokers and OR = 2.0, 95% CI: 1.7–2.2 among heavy smokers). In our own Singapore Chinese Health Study (SCHS) [60], a prospective cohort study of more than 63,000 study participants aged 40–75 years old who are Chinese Singaporeans, we also found that smoking is positively and statistically significant correlated with red meat (r = 0.13, p < 0.0001).
The strengths of our study include prospective study design, long-term follow-up, and comprehensive assessment of biomarkers involved in the serine-glycine metabolic pathway using validated state-of-the-art GC-MS/MS and LC-MS/MC assays. In addition, we adjusted for different potential confounders, including eGFR, alcohol consumption, smoking, history of diabetes, PLP, and total methyl donors (i.e., sum of choline, betaine and methionine).
The main limitations of our study are a modest sample size and lack of female subjects, thus limit our ability to generalize our findings to other populations. Given a plausible biological mechanism of one-carbon metabolism in the development of pancreatic cancer, our study with 129 cases and 258 controls would have at least 80% power to detect a minimal odd ratio (OR) of 2 or 0.5 for the two extreme quartiles of biomarker concentrations (https://dceg.cancer.gov/tools/design/power, Accessed date: 15 January 2022). Thus, the present study provided a reasonable effect size of the biomarkers studied on the risk of developing pancreatic cancer. Another limitation is that the examined biomarkers were measured at baseline only. Serum levels of serine and glycine might change over time and these changes would affect to the risk of pancreatic cancer. Further studies are thus warranted to evaluate this association. Furthermore, while we tried to control for potential confounding factors and/or established risk factors for pancreatic cancer in the multivariable model, residual confounding might occur due to the present of unknown factor(s). Finally, our findings are from the nested case-control study within a prospective cohort study of the Chinese Singaporean population, it is thus would not generalizable to other populations.
Our finding on serum serine and glycine in relation to pancreatic cancer risk has several implications and warrants future mechanistic studies. Considering the protective impact of serine and glycine, the crosstalk between genetic profile, nutritional status, and dietary patterns may provide an evidence-based approach for the development of personalized nutrition strategy. The interconnection of one-carbon cycles and the metabolism of glycine and serine prompts a wide variety of targetable enzymes or mediators that can be further investigated for potential chemoprevention of pancreatic cancer.
5. Conclusions
In summary, our study found novel and strong, inverse associations between serine and glycine and pancreatic cancer risk in men, supporting a protective role of these two amino acids against the development of pancreatic cancer in humans that might have an implication for cancer prevention.
Acknowledgments
We thank Xue-Li Wang of the Shanghai Cancer Institute for assistance with data collection and management and the staff of the Shanghai Cancer Registry for their assistance in verifying cancer diagnoses in study participants.
Abbreviations
| BMI | body mass index |
| CI | confidence interval |
| CV | coefficient of variation |
| eGFR | estimated glomerular filtration rate |
| ICD | International Classification of Diseases-Oncology |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells or uclear factor kappa B |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| OR | odds ratio |
| PLP | pyridoxal 5′-phosphate |
| ROS | reactive oxygen species |
| SCH | Shanghai Cohort Study |
| SD | standard deviation |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14092199/s1, Table S1: Within-batch and Between-batch Coefficients of Variations (CV) of Biomarkers (N = 14); Table S2: title; Spearman Correlation Coefficients Between Serum Biomarkers and Selected Sociodemographic Characteristics among all Control Subjects, Shanghai Cohort Study (n = 258); Table S3: Spearman Correlation Coefficients of Serum Biomarkers among all Control Subjects of the Shanghai Cohort Study (n = 258); Table S4: Full Models between Serine, Glycine and other Co-variates in relation to Pancreatic Cancer Risk; Table S5: Spearman Correlation Coefficients between Energy, Fat, Protein and Carbohydrate Intakes with Serine and Glycine among Controls, Shanghai Cohort Study (n = 258).
Author Contributions
Conceptualization, J.Y.H., H.N.L., and J.-M.Y.; data curation, J.Y.H., J.A.-H., R.W., and J.-M.Y.; formal analysis, H.N.L., R.W., Ø.M., A.U., and P.M.U.; funding acquisition, J.-M.Y.; investigation, H.N.L., P.P., R.W., and J.-M.Y.; project administration, R.W. and J.-M.Y.; resources, H.N.L. and J.-M.Y.; supervision, H.N.L. and J.-M.Y.; visualization, H.N.L. and R.W.; writing—original draft, H.N.L. and P.P.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Shanghai Cohort Study has been approved by the Institutional Review Boards (IRBs) of the Shanghai Cancer Institute and the University of Pittsburgh (CR19080153-002). The present study was also approved by the IRB of the University of Pittsburgh (CR19080153-002).
Informed Consent Statement
Written informed consent was obtained from all study participants of the Shanghai Cohort Study.
Data Availability Statement
De-identified data relevant to the report can be shared and is available upon request through the University of Pittsburgh for researchers who meet the criteria for access to confidential data. Data is accessible to the corresponding author and also is available from the University of Pittsburgh Institutional Data Access/Ethics Committee with the following contact information: 3500 Fifth Avenue, Hieber Building Main Office, Suite 106 Pittsburgh, PA 15213. Main Phone: (412) 383-1480. Main Fax: (412) 383-1508. Email: askirb@pitt.edu.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The current study was supported by the National Institutes of Health (NIH) of the United States (grants # R01 CA144034 and UM1 CA182876 to J.-M. Yuan). P.P. is supported by the NIH T32CA186873 (PI: Yuan) training grant in cancer epidemiology and prevention. H.N.L. was partially supported by the University of Pittsburgh Medical Center, Hillman Cancer Center start-up grant.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Noone A., Howlader N., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D., et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD. [(accessed on 1 July 2021)]; Available online: https://seer.cancer.gov/csr/1975_2015/
- 4.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu B., Wu X., Guo T., Guan N., Liu Y. Epidemiological Characteristics of Pancreatic Cancer in China From 1990 to 2019. Cancer Control. 2021;28:10732748211051536. doi: 10.1177/10732748211051536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J., Lok V., Ngai C.H., Zhang L., Yuan J., Lao X.Q., Ng K., Chong C., Zheng Z.-J., Wong M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology. 2021;160:744–754. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund . Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018; World Cancer Research Fund; London, UK: 2018. [Google Scholar]
- 8.Maisonneuve P., Lowenfels A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiol. 2015;44:186–198. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 9.Locasale J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen R.W., Moskowitz M. Arrest of cell growth in the G1 phase of the cell cycle by serine deprivation. Exp. Cell Res. 1978;116:127–137. doi: 10.1016/0014-4827(78)90070-8. [DOI] [PubMed] [Google Scholar]
- 11.Davis S.R., Stacpoole P.W., Williamson J., Kick L.S., Quinlivan E.P., Coats B.S., Shane B., Bailey L.B., Gregory J.F. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am. J. Physiol. Endocrinol. Metab. 2004;286:E272–E279. doi: 10.1152/ajpendo.00351.2003. [DOI] [PubMed] [Google Scholar]
- 12.Fell D.A., Snell K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem. J. 1988;256:97–101. doi: 10.1042/bj2560097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzym. Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 14.Snell K., Weber G. Enzymic imbalance in serine metabolism in rat hepatomas. Biochem. J. 1986;233:617–620. doi: 10.1042/bj2330617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snell K., Natsumeda Y., Weber G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem. J. 1987;245:609–612. doi: 10.1042/bj2450609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 17.Locasale J.W., Grassian A.R., Melman T., Lyssiotis C.A., Mattaini K.R., Bass A.J., Heffron G., Metallo C.M., Muranen T., Sharfi H., et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaneton B., Hillmann P., Zheng L., Martin A.C.L., Maddocks O.D.K., Chokkathukalam A., Coyle J.E., Jankevics A., Holding F.P., Vousden K.H., et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain M., Nilsson R., Sharma S., Madhusudhan N., Kitami T., Souza A.L., Kafri R., Kirschner M.W., Clish C.B., Mootha V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labuschagne C.F., Van Den Broek N.J.F., Mackay G.M., Vousden K.H., Maddocks O.D.K. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014;7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Maddocks O.D.K., Labuschagne C.F., Adams P.D., Vousden K.H. Serine Metabolism Supports the Methionine Cycle and DNA/RNA Methylation through De Novo ATP Synthesis in Cancer Cells. Mol. Cell. 2016;61:210–221. doi: 10.1016/j.molcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddocks O.D.K., Athineos D., Cheung E.C., Lee P., Zhang T., Van Den Broek N.J.F., Mackay G.M., Labuschagne C.F., Gay D., Kruiswijk F., et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- 23.Mu Y., Zhou Y., Wang Y., Li W., Zhou L., Lu X., Gao P., Gao M., Zhao Y., Wang Q., et al. Serum Metabolomics Study of Nonsmoking Female Patients with Non-Small Cell Lung Cancer Using Gas Chromatography–Mass Spectrometry. J. Proteome Res. 2019;18:2175–2184. doi: 10.1021/acs.jproteome.9b00069. [DOI] [PubMed] [Google Scholar]
- 24.Amara C.S., Ambati C.R., Vantaku V., Badrajee Piyarathna D.W., Donepudi S.R., Ravi S.S., Arnold J.M., Putluri V., Chatta G., Guru K.A., et al. Serum Metabolic Profiling Identified a Distinct Metabolic Signature in Bladder Cancer Smokers: A Key Metabolic Enzyme Associated with Patient Survival. Cancer Epidemiol. Prev. Biomark. 2019;28:770–781. doi: 10.1158/1055-9965.EPI-18-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vogel S., Ulvik A., Meyer K., Ueland P.M., Nygård O., Vollset S.E., Tell G.S., Gregory J.F., Tretli S., Bjørge T. Sarcosine and other metabolites along the choline oxidation pathway in relation to prostate cancer--a large nested case-control study within the JANUS cohort in Norway. Int. J. Cancer. 2014;134:197–206. doi: 10.1002/ijc.28347. [DOI] [PubMed] [Google Scholar]
- 26.Mustafa A., Gupta S., Hudes G.R., Egleston B.L., Uzzo R.G., Kruger W.D. Serum Amino Acid Levels as a Biomarker for Renal Cell Carcinoma. J. Urol. 2011;186:1206–1212. doi: 10.1016/j.juro.2011.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu J., Xiao Y., Shu D., Liang X., Hu X., Xie Y., Lin D., Li H. Metabolomics Analysis in Serum from Patients with Colorectal Polyp and Colorectal Cancer by 1H-NMR Spectrometry. Dis. Markers. 2019:2019. doi: 10.1155/2019/3491852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao L., Maity S., Coarfa C., Rajapakshe K., Chen L., Jin F., Putluri V., Tinker L.F., Mo Q., Chen F., et al. A Prospective Targeted Serum Metabolomics Study of Pancreatic Cancer in Postmenopausal Women. Cancer Prev. Res. 2019;12:237–246. doi: 10.1158/1940-6207.CAPR-18-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leichtle A.B., Nuoffer J.-M., Ceglarek U., Kase J., Conrad T., Witzigmann H., Thiery J., Fiedler G.M. Serum amino acid profiles and their alterations in colorectal cancer. Metabolomics. 2012;8:643–653. doi: 10.1007/s11306-011-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan J.M., Ross R.K., Wang X.L., Gao Y.T., Henderson B.E., Yu M.C. Morbidity and mortality in relation to cigarette smoking in Shanghai, China. A prospective male cohort study. JAMA. 1996;275:1646–1650. doi: 10.1001/jama.1996.03530450036029. [DOI] [PubMed] [Google Scholar]
- 31.Midttun Ø., McCann A., Aarseth O., Krokeide M., Kvalheim G., Meyer K., Ueland P.M. Combined Measurement of 6 Fat-Soluble Vitamins and 26 Water-Soluble Functional Vitamin Markers and Amino Acids in 50 μL of Serum or Plasma by High-Throughput Mass Spectrometry. Anal. Chem. 2016;88:10427–10436. doi: 10.1021/acs.analchem.6b02325. [DOI] [PubMed] [Google Scholar]
- 32.Midttun Ø., Kvalheim G., Ueland P.M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal. Bioanal. Chem. 2013;405:2009–2017. doi: 10.1007/s00216-012-6602-6. [DOI] [PubMed] [Google Scholar]
- 33.Midttun Ø., Hustad S., Ueland P.M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:1371–1379. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 34.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benowitz N.L., Hukkanen J., Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J.Y., Butler L.M., Midttun Ø., Koh W.-P., Ueland P.M., Wang R., Jin A., Gao Y.-T., Yuan J.-M. Serum B6 vitamers (pyridoxal 5′-phosphate, pyridoxal, and 4-pyridoxic acid) and pancreatic cancer risk: Two nested case-control studies in Asian populations. Cancer Causes Control. 2016;27:1447–1456. doi: 10.1007/s10552-016-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J.Y., Luu H.N., Butler L.M., Midttun Ø., Ulvik A., Wang R., Jin A., Gao Y.-T., Tan Y., Ueland P.M., et al. A prospective evaluation of serum methionine-related metabolites in relation to pancreatic cancer risk in two prospective cohort studies. Int. J. Cancer. 2020;147:1917–1927. doi: 10.1002/ijc.32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obeid R. The Metabolic Burden of Methyl Donor Deficiency with Focus on the Betaine Homocysteine Methyltransferase Pathway. Nutrients. 2013;5:3481–3495. doi: 10.3390/nu5093481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng J.-D., Wu W.K.K., Wang H.-Y., Li X.-X. Serine and one-carbon metabolism, a bridge that links mTOR signaling and DNA methylation in cancer. Pharmacol. Res. 2019;149:104352. doi: 10.1016/j.phrs.2019.104352. [DOI] [PubMed] [Google Scholar]
- 40.Yu L., Teoh S.T., Ensink E., Ogrodzinski M.P., Yang C., Vazquez A.I., Lunt S.Y. Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells. Cancer Metab. 2019;7:13. doi: 10.1186/s40170-019-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammad G.H., Vassileva V., Acedo P., Olde Damink S.W.M., Malago M., Dhar D.K., Pereira S.P. Targeting Pyruvate Kinase M2 and Lactate Dehydrogenase A Is an Effective Combination Strategy for the Treatment of Pancreatic Cancer. [(accessed on 25 November 2020)];Cancers. 2019 11:1372. doi: 10.3390/cancers11091372. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6770573/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amelio I., Cutruzzolá F., Antonov A., Agostini M., Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maddocks O.D.K., Berkers C.R., Mason S.M., Zheng L., Blyth K., Gottlieb E., Vousden K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang I.-Y., Kwak S., Lee S., Kim H., Lee S.E., Kim J.-H., Kim Y.A., Jeon Y.K., Chung D.H., Jin X., et al. Psat1-Dependent Fluctuations in α-Ketoglutarate Affect the Timing of ESC Differentiation. Cell Metab. 2016;24:494–501. doi: 10.1016/j.cmet.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Morris J.P., Yashinskie J.J., Koche R., Chandwani R., Tian S., Chen C.-C., Baslan T., Marinkovic Z.S., Sánchez-Rivera F.J., Leach S.D., et al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573:595–599. doi: 10.1038/s41586-019-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Šarenac T.M., Mikov M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. [(accessed on 6 September 2020)];Front. Pharmacol. 2018 9:939. doi: 10.3389/fphar.2018.00939. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2018.00939/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kevresan S., Kuhajda K., Kandrac J., Fawcett J.P., Mikov M. Biosynthesis of bile acids in mammalian liver. Eur. J. Drug Metab. Pharmacokinet. 2006;31:145–156. doi: 10.1007/BF03190711. [DOI] [PubMed] [Google Scholar]
- 48.Goossens J.-F., Bailly C. Ursodeoxycholic acid and cancer: From chemoprevention to chemotherapy. Pharmacol. Ther. 2019;203:107396. doi: 10.1016/j.pharmthera.2019.107396. [DOI] [PubMed] [Google Scholar]
- 49.Choi Y.H., Im E.O., Suh H., Jin Y., Yoo Y.H., Kim N.D. Apoptosis and modulation of cell cycle control by synthetic derivatives of ursodeoxycholic acid and chenodeoxycholic acid in human prostate cancer cells. Cancer Lett. 2003;199:157–167. doi: 10.1016/S0304-3835(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 50.Khare S., Cerda S., Wali R.K., Von Lintig F.C., Tretiakova M., Joseph L., Stoiber D., Cohen G., Nimmagadda K., Hart J., et al. Ursodeoxycholic acid inhibits Ras mutations, wild-type Ras activation, and cyclooxygenase-2 expression in colon cancer. Cancer Res. 2003;63:3517–3523. doi: 10.1016/S0016-5085(03)83066-4. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.J., Jeong S.H., Kim E.-K., Kim E.J., Cho J.H. Ursodeoxycholic acid suppresses epithelial-mesenchymal transition and cancer stem cell formation by reducing the levels of peroxiredoxin II and reactive oxygen species in pancreatic cancer cells. Oncol. Rep. 2017;38:3632–3638. doi: 10.3892/or.2017.6045. [DOI] [PubMed] [Google Scholar]
- 52.Kalhan S.C., Uppal S.O., Moorman J.L., Bennett C., Gruca L.L., Parimi P.S., Dasarathy S., Serre D., Hanson R.W. Metabolic and Genomic Response to Dietary Isocaloric Protein Restriction in the Rat. J. Biol. Chem. 2011;286:5266–5277. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filho J.C., Hazel S.J., Anderstam B., Bergström J., Lewitt M., Hall K. Effect of protein intake on plasma and erythrocyte free amino acids and serum IGF-I and IGFBP-1 levels in rats. Am. J. Physiol. 1999;277:E693–E701. doi: 10.1152/ajpendo.1999.277.4.E693. [DOI] [PubMed] [Google Scholar]
- 54.Antflick J.E., Baker G.B., Hampson D.R. The effects of a low protein diet on amino acids and enzymes in the serine synthesis pathway in mice. Amino Acids. 2010;39:145–153. doi: 10.1007/s00726-009-0387-8. [DOI] [PubMed] [Google Scholar]
- 55.Nagao K., Bannai M., Seki S., Mori M., Takahashi M. Adaptational modification of serine and threonine metabolism in the liver to essential amino acid deficiency in rats. Amino Acids. 2009;36:555–562. doi: 10.1007/s00726-008-0117-7. [DOI] [PubMed] [Google Scholar]
- 56.Bosetti C., Lucenteforte E., Silverman D.T., Petersen G., Bracci P.M., Ji B.T., Negri E., Li D., Risch H.A., Olson S., et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann. Oncol. 2012;23:1880–1888. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch S.M., Vrieling A., Lubin J.H., Kraft P., Mendelsohn J.B., Hartge P., Canzian F., Steplowski E., Arslan A.A., Gross M., et al. Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am. J. Epidemiol. 2009;170:403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gnagnarella P., Maisonneuve P., Bellomi M., Rampinelli C., Bertolotti R., Spaggiari L., Palli D., Veronesi G. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann. Oncol. 2013;24:2606–2611. doi: 10.1093/annonc/mdt302. [DOI] [PubMed] [Google Scholar]
- 59.Hawrysz I., Wadolowska L., Slowinska M.A., Czerwinska A., Golota J.J. Adherence to Prudent and Mediterranean Dietary Patterns is Inversely Associated with Lung Cancer in Moderate but not Heavy Male Polish Smokers: A Case-Control Study. Nutrients. 2020;12:E3788. doi: 10.3390/nu12123788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan J.-M., Stram D.O., Arakawa K., Lee H.-P., Yu M.C. Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2003;12:890–898. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data relevant to the report can be shared and is available upon request through the University of Pittsburgh for researchers who meet the criteria for access to confidential data. Data is accessible to the corresponding author and also is available from the University of Pittsburgh Institutional Data Access/Ethics Committee with the following contact information: 3500 Fifth Avenue, Hieber Building Main Office, Suite 106 Pittsburgh, PA 15213. Main Phone: (412) 383-1480. Main Fax: (412) 383-1508. Email: askirb@pitt.edu.