Abstract
The topology effects of cyclization on thermal phase transition behaviors were investigated for a series of amphiphilic Pluronic copolymers of both hydrophilic–hydrophobic–hydrophilic and hydrophobic–hydrophilic–hydrophobic block sequences. The dye solubilization measurements revealed the lowered critical micelle temperatures (TCMT) along with the decreased micellization enthalpy (ΔHmic) and entropy (ΔSmic) for the cyclized species. Furthermore, the transmittance and dynamic light scattering (DLS) measurements indicated a block sequence-dependent effect on the clouding phenomena, where a profound decrease in cloud point (Tc) was only found for the copolymers with a hydrophilic–hydrophobic–hydrophilic block sequence. Thus, the effect of cyclization on these critical temperatures was manifested differently depending on its block sequence. Finally, a comparison of the linear hydroxy-terminated, methoxy-terminated, and cyclized species indicated the effect of cyclization to be unique from a simple elimination of the terminal hydrophilic moieties.
Keywords: cyclic polymer, block copolymer, Pluronic, micellization, cloud point, phase transition
1. Introduction
The investigation into the properties of polymers with non-linear architecture, namely, topological polymers, has revealed various unique structure–property relationships [1]. Amongst them, polymers having a cyclic topology have especially been the subject of attention from both synthetic and physical property viewpoints, resulting in extensive reviews, highlights [2,3,4,5,6,7,8,9], and more recently, application-oriented researches [10,11,12,13,14]. Naturally, the effects of the cyclic topology have been investigated beyond simple homopolymers to self-assembling block copolymer systems, where the thermodynamics and structural properties of self-assembly phenomena were found to be affected by the geometrical constraints induced from the cyclic topology [15,16,17,18]. In general, cyclic copolymer amphiphiles were found to assemble micelles having reduced dimensions and higher densities compared to their corresponding linear counterparts [16,17,19]. In other examples, the morphology of the assembled micelles differed depending on the topology of the polymers [20,21]. Moreover, enhancement in thermal and salt stability was found for cyclized copolymer micelles compared to their corresponding linear species, resulting from the inhibition of agglomeration induced by chain-bridging of micelles [22].
The effects of polymer topology are, in theory, not bound by its chemical structure. However, physical properties exhibited by actual polymer systems are affected by a variety of factors of physical and/or chemical origin, thus complicating the elucidation of the topology effects. For this reason, polymers with a wide understanding of the relations between physical properties and structural parameters such as molecular weight, block ratio, and block sequence are attractive candidates for an investigation into the effects of polymer topology. In this context, symmetrical triblock copolymers comprised of hydrophilic poly(ethylene glycol) (PEG) and hydrophobic poly(propylene glycol) (PPG) blocks, commercially registered under trademark names of Pluronic [23] are ideal due to their well-studied nature [24,25]. Pluronic copolymers can be classified into two types based on their block sequence. Copolymers with the PEG–PPG–PEG sequence are widely known as ABA-type Pluronic, with the hydrophobic PPG block sandwiched between two hydrophilic PEG blocks. In contrast, copolymers with a PPG–PEG–PPG sequence are known as “reverse” or BAB-type Pluronic, consisting of a middle hydrophilic PEG block with two terminal hydrophobic PPG blocks. Attention has been focused on the property–structure relationships regarding parameters, such as molecular weight and block ratios [26,27,28,29,30], where, for example, micellization conditions were found to be more significantly influenced by the length of the PPG blocks compared to the PEG blocks [30]. While fewer studies have focused on the block sequence, direct comparisons between ABA-type and BAB-type Pluronic copolymer solutions revealed reduced self-assembling tendencies for the latter, owing to the looped geometry of the copolymers required upon micellization [31,32].
To date, only a single investigation conducted by Booth and coworkers has focused on the physical properties of cyclized Pluronic copolymers [33], apart from our recent report [34]. In their work, cyclization was achieved for a single relatively large Pluronic, EG52–PG34–EG52, via acetalization. The cyclization for this Pluronic species, however, did not confer a significant effect upon its micellization conditions or micelle hydrodynamic radii, while a more notable increase in the aggregation number (Nagg) was found. Our more recent study reported the synthesis and interfacial properties of cyclized Pluronic copolymers of diverse chemical compositions, revealing an enhancement in interfacial activity via cyclization to be a prevalent phenomenon [34]. Furthermore, the degree of enhancement in interfacial activity upon cyclization was found to be influenced by the block ratio and sequence of the linear species, with a prominent difference in the linear and cyclic species arising in surfactants having a large PEG block composition. Since our previous study focused on the effect of cyclization at the air–water interface, its micellization and phase transition properties were not investigated. However, the obtained results suggest the cyclic topology affects the interfacial properties of the solutions, albeit with different magnitudes depending on their structure and composition. It is, therefore, of importance to expand the work and conduct a comprehensive study into the bulk solution properties of various cyclized Pluronic copolymers in order to elucidate the effects of the cyclic topology in relation to other chemical parameters.
This work presents a comprehensive investigation into the effects of cyclization on the temperature-induced aggregation phenomena of various Pluronic copolymers. The determination of critical micellization temperatures (TCMT) through dye solubilization and cloud point (Tc) through light transmittance revealed distinct effects of cyclization. For example, cyclized species were found to display generally lower TCMT compared to their linear counterparts. Furthermore, thermodynamic studies revealed reduced micellization enthalpy (ΔHmic) and entropy (ΔSmic) for the cyclized species. Both TCMT and the thermodynamic parameters of micellization were more heavily affected upon cyclization for the copolymers with a BAB-type block sequence. The Tc, on the other hand, was more significantly affected upon cyclization for the ABA-type copolymers, resulting in prominently reduced values. The obtained results suggest that cyclization influences various phase stability of Pluronic copolymers in relation to their block sequence.
2. Materials and Methods
2.1. Materials
CH3I (>99.5%), KOH (>85.5%), NaCl (>99.0%), Na2SO4 (>99.0%), CH2Cl2 (>99.0%), MeOH (>99.5%), and CHCl3 (>99.0%) were purchased from Kanto Chemical Co., Inc.,Tokyo, Japan, while TsCl (>98.0%) and chlorobenzene (>99.0%) were purchased from Junsei Chemical Co., Ltd.,Tokyo, Japan, and Nacalai Tesque, Inc., Kyoto, Japan, respectively. All reagents of the above-mentioned were used as received. n-Hexane (>99.0%) was purchased from Kanto Chemical Co., Inc., Tokyo, Japan, and purified by distillation before use. Dehydrated tetrahydrofuran (THF), stabilizer free (>99.5%), and dehydrated dichloromethane (CH2Cl2) (>99.5%) for the reactions were purchased from Kanto Chemical Co., Inc., Tokyo, Japan, and purified using a solvent purification system (MB-SPS-Compact, MBRAUN, Garching, Germany). Poly(ethylene glycol)–block–poly(propylene glycol)–block–poly(ethylene glycol) (Pluronic L35, Mn~1900), poly(ethylene glycol)–block–poly(propylene glycol)–block–poly(ethylene glycol) (Pluronic L64, Mn~2900), poly(propylene glycol)–block–poly(ethylene glycol)–block–poly(propylene glycol) (Pluronic 10R5, Mn~1700), poly(propylene glycol)–block–poly(ethylene glycol)–block–poly(propylene glycol) (Pluronic 17R4, Mn~2700) were purchased from Sigma–Aldrich (Merck KGaA, Darmstadt, Germany) and poly(ethylene glycol) (PEG), (Mn~2000) was obtained from Tokyo Chemical Industry, Co., Inc., Tokyo, Japan. The linear polymers were purified using preparative SEC. Pluronic L35 and L64 were further purified by a previously reported method [35,36]. Thus, 1.0 g of the copolymers was vigorously stirred in 100 mL of n-hexane at room temperature for 15 min, and the supernatant n-hexane phase was removed. While the majority of the copolymer remained undissolved, hydrophobic polymeric impurities were extracted into the n-hexane phase and thus removed through this procedure. This purification procedure was repeated three times, and the copolymer was dried under reduced pressure overnight.
2.2. Nuclear Magnetic Resonance Spectroscopy
A JNM-ESC400 instrument (JEOL Ltd., Tokyo, Japan) was used to measure proton (1H, 400 MHz) and carbon (13C, 100 MHz) nuclear magnetic resonance (NMR) spectra using CDCl3 at room temperature.
2.3. Size Exclusion Chromatography
Size exclusion chromatography (SEC) measurement was carried out using a PU-980 Plus pump (JASCO Co., Tokyo, Japan) equipped with KF-804L columns (8.0 mm × 300 mm × 2, Shodex) and a KF-G guard column (Shodex, Tokyo, Japan) inside a CO-2065 Plus column oven (JASCO Co., Tokyo, Japan) set at a temperature of 40 °C. An RI-2031 Plus differential refractometer (JASCO Co., Tokyo, Japan) was used as a detector, and THF as an eluent was set at a flow rate of 1.0 mL/min.
2.4. Preparative SEC
Fractionation using preparative SEC was carried out using a Japan Analytical Industry LC-908 recycling preparative HPLC equipped with a RI detector RI-5 (JAI. Co., Ltd., Tokyo, Japan) and L-7110 pump (Hitachi, Ltd., Tokyo, Japan) and JAIGEL columns (2H, 3H, and a pre-column, JAI. Co., Ltd., Tokyo, Japan) connected in series. Filtered CHCl3 as eluent was set at a flow rate of 3.5 mL/min.
2.5. Synthesis of Cyclized Polymers
The intramolecular cyclization of PEG homopolymer and Pluronic copolymers having hydroxy end groups was performed in accordance with previous reports [33,34,37]. For PEG and ABA-type copolymers, cyclization was carried out by the Williamson-ether synthesis. Typically, the reaction was conducted by gradually adding 50 mL of a THF solution of Pluronic L35 (5.0 g, 1 equiv. mol) and TsCl (1.3 equiv. mol) at a rate of 20 μL/min into a THF/n-hexane (100 mL, 70/30 v/v) suspension of KOH (5.0 g) at 40 °C under an Ar gas atmosphere. The mixture was additionally stirred for 2 d at the same temperature. After filtration, the reaction mixture was concentrated under reduced pressure overnight. The obtained residue was dissolved in CH2Cl2 and washed thrice using saturated NaCl aqueous solution. Residual water in the organic phase was removed using Na2SO4 and was further dried overnight under reduced pressure. Silica gel column chromatography of the residue was carried out using a CHCl3/MeOH (90/10 v/v) mixture, to give 2.4 g of a crude product. Finally, 900 mg of the crude product was injected in a preparative recycling SEC to fractionate cyclized species from intermolecularly reacted byproducts and the linear precursors, to give 360 mg of pure cyclized Pluronic L35 (c-L35). Meanwhile, cyclization for BAB-type Pluronic copolymers was carried out through the acetalization reaction. Thus, typically, 5.0 g of Pluronic 10R5 was dissolved in 50 mL of CH2Cl2, and the solution was added into a KOH (5.0 g) dispersion of a CH2Cl2/n-hexane mixture (100 mL, 65/35 v/v) at a rate of 12.5 μL/min. The mixture was stirred at 40 °C for an additional 3 d. After filtration, the reaction mixture was concentrated under reduced pressure. Redissolution in CH2Cl2, three times washing of the residue using brine, and silica gel column chromatography (eluent; CHCl3/MeOH, 90/10 v/v) was used to isolate 1.7 g of a crude product. Finally, 500 mg of the crude product was injected into a preparative recycling SEC to give 170 mg of pure cyclized Pluronic 10R5 (c-10R5).
2.6. Synthesis of Chain-End Methylated Polymers
Methylation of chain-ends for ABA and BAB-type Pluronic copolymers was carried out following previous reports [10,34]. Typically, 50 mL of a chlorobenzene solution of Pluronic L35 (2.5 g, 1.0 equiv.) was added (70 μL/min) to a 100 mL chlorobenzene suspension of KOH (5.0 g) and CH3I (0.56 g, 3.0 equiv.) under an Ar gas atmosphere at room temperature. The mixture was stirred additionally for 24 h and purified through filtration and silica gel column chromatography in CHCl3/MeOH (90/10 v/v) to give 1.9 g of methoxy-terminated Pluronic L35 (l-L35(OMe)).
2.7. Preparation of Pluronic Copolymer Solutions
Required amounts of copolymer were dissolved in Milli-Q water and stirred at room temperature for over 12 h. The samples were kept in a refrigerator in tightly closed glass vials for over 48 h for complete dissolution and filtered through a 0.45 μM Millipore filter prior to measurement.
2.8. Critical Micelle Temperature (TCMT) by Dye Solubilization
A dye solubilization technique using 1,6-diphenyl-1,3,5-hexatriene (DPH) was used to determine TCMT for the Pluronic copolymer solutions [30]. First, copolymers were dissolved in Milli-Q water and stirred at room temperature for over 12 h. 25 μL of a 0.4 mM DPH/methanol solution was added into 2.5 mL of the copolymer aqueous solution. The final sample solution contained 1 vol% of methanol and 4.0 μM DPH, at four copolymer concentrations (c) of 0.30, 1.0, 3.0, and 10 g/L. The sample solutions were kept in the dark for at least 3 h prior to measurement, to ensure complete mixing equilibrium of the system. Each sample solution was heated at a rate of 0.1 °C min−1, and absorption spectrum (340–400 nm) was measured in 2 °C increments after an equilibration time of 10 min at each temperature. The absorption intensity changes upon temperature elevation at the maximum absorption wavelength of DPH (λmax, DPH = 356 nm) were used in the determination of its TCMT.
2.9. Cloud Point (Tc) Measurement
Transmission of the solution (%T) was measured at 600 nm on a V-670 UV–Visible spectrophotometer (JASCO Co., Tokyo, Japan) using an M25-UV-2 micro quartz cell (GL Science Inc., Tokyo, Japan). Aqueous solutions of the Pluronic copolymers were stirred at 60 rpm inside the spectrophotometer and heated at a rate of 1 °C/min. %T was measured in 1 °C increments. The lowest temperature at which %T became 90% or less was determined as Tc. For the PEG samples, aqueous solutions of NaH2PO4 at a concentration of 250 g/L were used for dissolution of the polymer to induce phase separation below the boiling point of water [38,39].
2.10. Dynamic Light Scattering (DLS)
DLS measurements were performed on a Malvern Zetasizer Nano instrument equipped with a 50 mW frequency-doubled DPSS Nd:YAG laser (λ = 532 nm) (Malvern Panalytical, Ltd., Malvern, UK). The light scattering signal was obtained at a fixed angle of 173°. Aqueous solutions of Pluronic L64 and its derivatives (10 g/L) were measured at various temperatures after an equilibration time of 10 min. Non-negative least squares analyses [40,41] provided in software built into the instrument were used to determine the number distribution of the apparent hydrodynamic diameter at finite concentration.
3. Results and Discussions
3.1. Preparation of Sample Polymers and Their Solutions
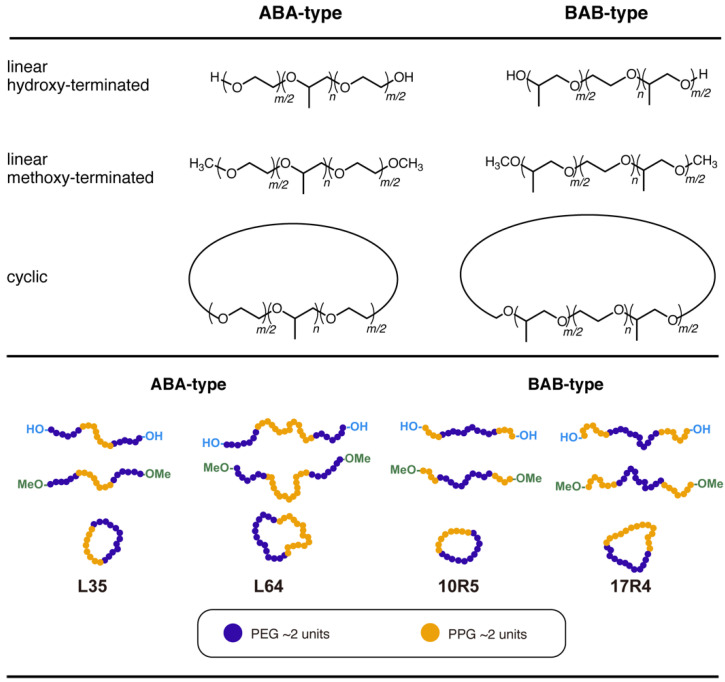
The cyclized PEG homopolymer and the Pluronic copolymers L35, L64, 10R5, and 17R4 were synthesized and purified according to a reported method [33,34,37], through intramolecular cyclization of the corresponding linear prepolymers. In the case of Pluronic L35, the starting prepolymer with hydroxy chain ends and its products are named l-L35(OH) and c-L35, respectively, in this paper. The other polymers are expressed accordingly. For the PEG homopolymers and PEG–PPG–PEG (ABA-type) Pluronic L35 and L64, the Williamson-ether synthesis was undertaken for the reaction between the chain-end hydroxy groups. For PPG–PEG–PPG (BAB-type) Pluronic 10R5 and 17R4, on the other hand, an acetalization reaction was carried out instead, due to reduced reactivity of the secondary alcohol at the chain ends. Linear polymers with methylated chain ends were also prepared via a reaction with iodomethane in order to clarify the extent of the effect of the cyclic topology from chemically induced changes due to the elimination of the chain-end hydroxy groups. In the case of L35, the methylated copolymer is named l-L35(OMe), and the others are named accordingly. The successful cyclization and dimethylation were confirmed through size-exclusion chromatography (SEC) (Figure S1) and NMR (Figures S2 and S3). The detailed characterization of the synthesized polymers has been reported elsewhere [34].
In the case of L64, the commercial product (linear prepolymer) is known to contain a certain number of polymeric impurities of a stronger hydrophobic nature. These polymeric impurities are known to substantially affect their solution properties at conditions close to micellization, with a number of studies reporting their removal [35,36]. Accordingly, the repeated washing of the prepolymer using n-hexane successfully purified the prepolymer, as confirmed through the temperature-dependent transmission of the polymer solution (Figure S4). A spike in %T that was observed at around 40 °C before purification due to the aggregation of the impurities clearly disappeared for a solution prepared from purified l-L64(OH). The cyclization and methylation reactions were both carried out using the purified copolymer. The same procedure was carried out for the purification of l-L35(OH).
Among the various types of Pluronic copolymers commercially available, L35, L64, 10R5, and 17R4 were selected since their total molecular weight and block composition cause aggregation at relatively mild conditions (Figure 1). In addition, a copious amount of research has been conducted for ABA-type Pluronic copolymers, especially L64, revealing detailed aspects of their aggregation behavior [35,42,43,44,45,46]. Reverse-type or BAB-type Pluronic copolymers, on the other hand, have received less attention, with fewer reports on their physical properties [47,48]. However, the block sequential distinction between the hydrophilic–hydrophobic–hydrophilic ABA-type and the hydrophobic–hydrophilic–hydrophobic BAB-type results in surprisingly contrasting properties, such as substantially higher critical micellization concentrations for the latter [32], even for copolymers with a similar molecular weight and hydrophilic/hydrophobic block composition. Therefore, a comparison of the changes in solution properties between ABA-type and BAB-type Pluronic copolymers allows a clarified understanding of the effect of cyclization in relation to the block sequence.
Figure 1.
Chemical structures (top) and schematic illustrations (bottom) of various Pluronic copolymers used in this study. Both ABA- and BAB-type copolymers were compared to clarify the effect of cyclization in relation to their block sequence.
The aqueous solutions of the linear polymers with either hydroxy chain ends (l-L35(OH), l-L64(OH), l-10R5(OH), l-17R4(OH), and l-PEG(OH)) or methoxy chain ends (l-L35(OMe), l-L64(OMe), l-10R5(OMe), and l-17R4(OMe)) along with their cyclized products (c-L35, c-L64, c-10R5, c-17R4, and c-PEG) were prepared by dissolving an appropriate amount of the copolymers in Milli-Q water. The copolymer solutions were stirred for over 12 h at room temperature and kept in a refrigerator for over 48 h for complete dissolution. Each solution was filtered immediately prior to measurement to remove any macroscopic impurities. For Tc measurements of the PEG homopolymers, a salting-out effect was utilized to induce phase separation below the boiling point of water [38,39]. Thus, l-PEG(OH) and c-PEG were dissolved in an aqueous solution of NaH2PO4 at a concentration of 250 g/L.
3.2. Critical Micellization Temperature (TCMT) by Dye Solubilization
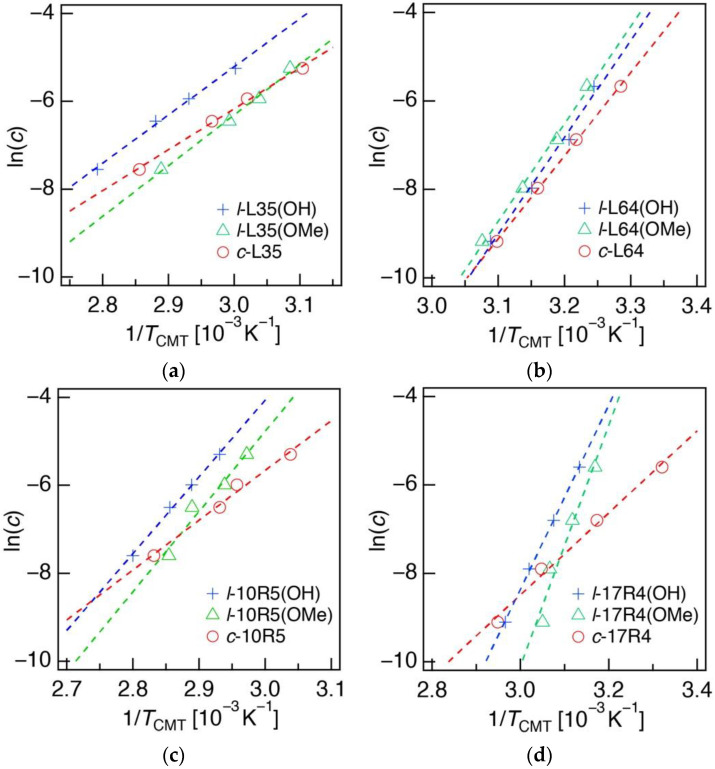
In order to evaluate the thermal response behavior of the synthesized cyclic Pluronic copolymers, a TCMT measurement through the hydrophobic dye solubilization technique, using 1,6-diphenyl-1,3,5-hexatriene (DPH) was performed [30]. TCMT was obtained from the intersection temperature value at which a change in the slope of the absorption intensity of DPH (λmax, DPH = 356 nm) was observed, indicating micelle formation and dye solubilization within the hydrophobic core (Figure S5). Furthermore, the enthalpy of micellization (ΔHmic) was calculated from the slope of the linear fitting of the ln(c) versus 1/TCMT plots obtained at four polymer molar concentrations (c), in accordance with the following Equation (1) (Figure 2). Using the ΔHmic values, ΔGmic at c = 10 g/L and at TCMT, as well as ΔSmic, were calculated by the following Equations (2) and (3) [30]:
| ΔHmic = R[∂ln (c)/∂(1/TCMT)] | (1) |
| ΔGmic = RTCMT ln (X) | (2) |
| ΔSmic = (ΔHmic − ΔGmic)/TCMT | (3) |
where R is the gas constant, and X is the copolymer concentration in mole fraction at the micellization condition.
Figure 2.
(a) ln(c) vs. 1/TCMT plot for aqueous solutions of linear hydroxy-terminated (blue), methoxy-terminated (green), and cyclized (red) L35, indicated as l-L35(OH), l-L35(OMe), and c-L35, respectively. Those of (b) L64, (c) 10R5, and (d) 17R4 are also shown. Enthalpy of micellization (ΔHmic) was calculated from the slope of the linear fitting of the plots.
For 10 g/L ABA-type copolymer solutions, TCMT of l-L35(OH) was 58 °C, and that of c-L35 decreased to 45 °C. The same trend was observed for L64, where cyclized species displayed comparably lower TCMT than their corresponding linear counterpart with hydroxy chain-end groups (l-L64(OH), 35 °C; c-L64, 31 °C in Table 1). The methylation of the chain ends also resulted in lowered TCMT for L35, but no significant effect was observed for L64 (l-L35(OMe), 51 °C; l-L64(OMe), 36 °C in Table 1). The difference in the critical micellization conditions between cyclized and linear ABA-type Pluronic was rather pronounced in comparison to the report by Booth and coworkers, where they were unable to define a clear effect of cyclization on their critical micellization concentrations for both PEG–PPG–PEG and PEG–poly(butylene glycol)–PEG triblock copolymers [33,49]. A similar decrease in TCMT was observed for the BAB-type copolymers; from 69 to 62 °C for 10R5 and from 46 to 28 °C for 17R4 upon cyclization. TCMT of 10R5 and 17R4 also decreased upon methylation of the chain ends (l-10R5(OMe), 65 °C; l-17R4(OMe), 42 °C in Table 1), suggesting the elimination of the terminal hydroxy groups of PPG to also lower their TCMT. In any case, the cyclized copolymers displayed the lowest TCMT, indicating the effect of topology to be more significant compared to the consequence of the simple elimination of hydrophilic terminal moieties.
Table 1.
Properties of Hydroxy- and Methoxy-Terminated Linear and Cyclized PEG and Pluronic Copolymers and Their Solutions’ Thermodynamic Parameters.
| Topology-Pluronic (Chain-End) | Composition |
Mn (g/mol) |
TCMT at 10 g/L (°C) |
ΔHmic (kJ/mol)a |
ΔGmic (kJ/mol) b |
ΔSmic (J/(mol K)) b |
Tc at 10 g/L (°C) |
|---|---|---|---|---|---|---|---|
| l-L35(OH) | (EG)11–(PG)16–(EG)11 | 1900 | 58 | 91 ± 4 | –26.1 | 352 | 82 |
| l-L35(OMe) | 51 | 96 ± 5 | –25.4 | 374 | 81 | ||
| c-L35 | 45 | 77 ± 3 | –25.2 | 319 | 64 | ||
| l-L64(OH) | (EG)13–(PG)30–(EG)13 | 2900 | 35 | 182 ± 10 | –24.6 | 670 | 64 |
| l-L64(OMe) | 36 | 183 ± 9 | –24.7 | 672 | 68 | ||
| c-L64 | 31 | 156 ± 2 | –24.3 | 593 | 56 | ||
| l-10R5(OH) | (PG)8–(EG)22–(PG)8 | 2000 | 69 | 145 ± 6 | –26.9 | 505 | 72 |
| l-10R5(OMe) | 65 | 152 ± 20 | –26.5 | 530 | 66 | ||
| c-10R5 | 62 | 94 ± 7 | –25.9 | 365 | 70 | ||
| l-17R4(OH) | (PG)14–(EG)24–(PG)14 | 2700 | 46 | 173 ± 3 | –26.4 | 625 | 49 |
| l-17R4(OMe) | 42 | 230 ± 30 | –26.1 | 804 | 44 | ||
| c-17R4 | 28 | 77 ± 5 | –24.9 | 339 | 53 | ||
| l-PEG(OH) | (EG)45 | 2000 | - | - | - | - | 65 |
| c-PEG | - | - | - | - | 59 |
a Average molecular weights reported from the manufacturer were used for the calculation of thermodynamic parameters of micellization. b Thermodynamic parameters obtained for 10 g/L solutions at TCMT.
When ΔHmic was determined from the slope of the ln(c) versus 1/TCMT plots, the smallest values were found for the cyclic species (Figure 2 and Table 1), suggesting decreased enthalpic inhibition against micellization. According to previous reports, smaller ΔHmic values for cyclized ABA-type copolymers arise from the reduced exposure of the hydrophobic B segment to water in the unimer state [33,49,50]. Thus, the hydrophilic A segment in the cyclized form likely more effectively shielded the hydrophobic B segment from contact with water. Furthermore, when the ABA- and BAB-type copolymers are compared, a more significant effect on ΔHmic was observed for the latter. For example, ΔHmic of c-10R5 and c-17R4 were found to be drastically reduced to 94 and 77 kJ/mol, respectively, from that of their linear hydroxy-terminated counterparts (l-10R5(OH), 145 kJ/mol; l-17R4(OH), 173 kJ mol−1). This was likely caused by the hydrophobic chain length to double upon cyclization for the BAB-type copolymers, where the ΔHmic value per hydrophobic repeating unit is known to be smaller as the segment becomes longer due to the formation of tight coils, minimizing contact with water [51].
Similarly, smaller values for the entropy of micellization (ΔSmic) were found for the cyclized species. This can also be attributed to the effect of the cyclic topology on the conformations in the unimer state and its relation to the hydrophobic effect. The hydrophobic effect is an entropic driving force towards micellization, arising from the release of water molecules from the lowered entropic states due to contact with the hydrophobic segments of the amphiphiles in the unimeric state [52]. This entropically driven process is known to be responsible for the micellization of many amphiphilic molecules, including Pluronic copolymers [32], and the positive ΔHmic and ΔSmic values obtained for the copolymers in this work also indicate micellization of the linear and cyclized Pluronic to be entropically driven. However, as mentioned above, cyclization of the copolymers leading to an efficient shielding of the hydrophobic PPG blocks from the surrounding water environment is also expected to reduce the hydrophobic effect, thus resulting in a lower ΔSmic. In addition, the presence of “dangling chains” in the less structured micelles of the BAB-type Pluronic copolymers may contribute to the more drastic decrease in ΔSmic upon cyclization (e.g., l-10R5(OH), 505 J/mol K; c-10R5, 365 J/mol K) compared to the ABA-type copolymers (e.g., l-L35(OH), 352 J/mol K; c-L35, 319 J/mol K) [50]. Monte Carlo simulations of the micellization of linear ABA- and BAB-type copolymers performed by Kim and Jo revealed the latter copolymers to possess larger ΔSmic due to their less structured micelles and dangling chains [53,54]. Therefore, since cyclization of both ABA- and BAB-type copolymers result in an AB-type diblock copolymer, the ΔSmic differences can rationally be expected to be more prominent for the copolymers with the BAB-type block sequence.
Interestingly, while the ABA-type Pluronic copolymers displayed comparable ΔHmic and ΔSmic values for the linear methoxy-terminated species to that of the linear hydroxy-terminated species, an evident increase in both the thermodynamic parameter values were found for 17R4, a copolymer with a BAB-type sequence and relatively long PPG blocks. This was indicative of a more significant influence of the hydroxy groups for sufficiently long hydrophobic PPG segments on the micellization phenomena compared to the hydroxy groups of the hydrophilic PEG segments or short PPG segments. The larger ΔSmic values for the methylated species are hypothesized to result from the stronger hydrophobicity, and the larger ΔHmic values may possibly result from relatively reduced hydrogen bonding interaction within the micelle core. Thus, the fraction of water known to be contained within the Pluronic micelle core [55,56] may have decreased by methylation of the chain-ends due to their stronger hydrophobic nature, resulting in a larger number of hydrogen bonding severances and a larger enthalpic change upon micelle formation.
3.3. Cloud Point (Tc)
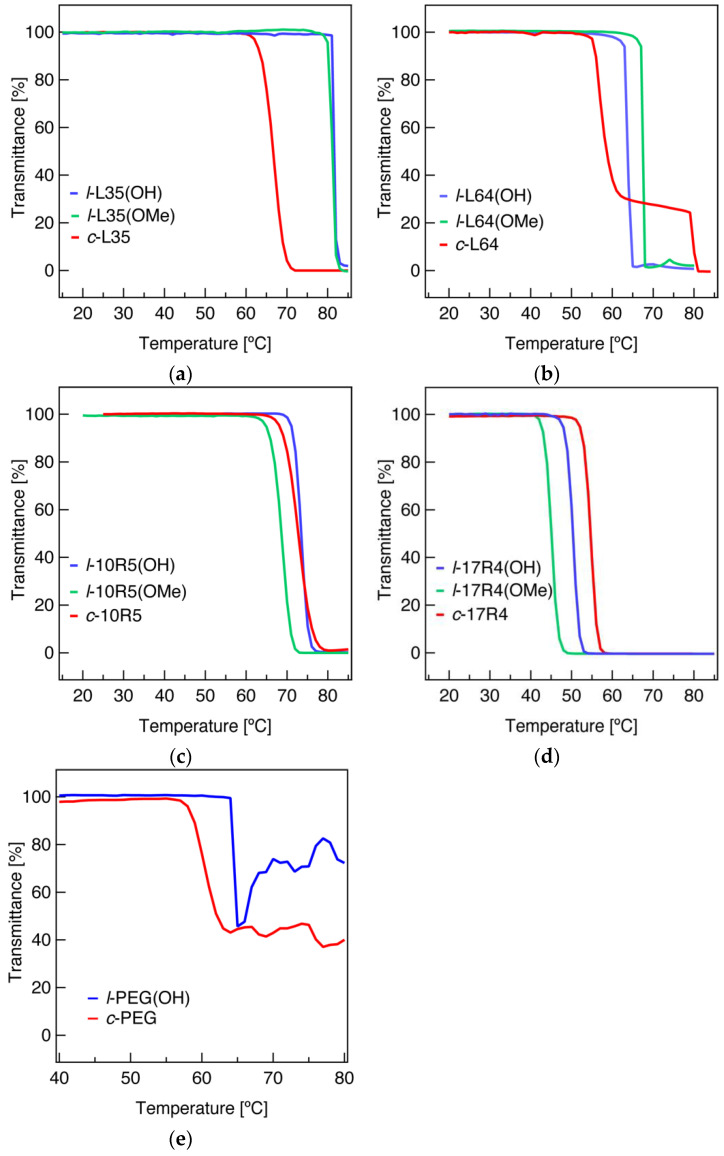
The temperature-dependent transparency (%T) of Pluronic copolymer solutions at a concentration of 10 g/L was measured to determine the aggregation and phase separation behavior. Here, Tc was defined by the lowest temperature at which %T at the wavelength of 600 nm became 90% or less. In order to separate the consequences of cyclization on the heat-induced dehydration and coil-globule transition through (i) the elimination of the strongly hydrophilic hydroxy end groups and (ii) conformational restriction of the cyclic topology, Tc for the linear hydroxy-terminated, methoxy-terminated, and cyclized species of the following four Pluronic copolymers: L35, L64, 10R5, and 17R4 were investigated (Figure 3, Table 1). For the ABA-type Pluronic, a significant decrease in Tc was observed for the cyclic species. For example, the Tc of c-L35 and c-L64 at 64 and 56 °C, respectively, were comparably lower than their linear hydroxy-terminated counterparts (l-L35(OH), 82 °C; l-L64(OH), 64 °C). In contrast, cyclization of the BAB-type copolymers resulted in comparable Tc to their corresponding linear hydroxy-terminated species. For instance, the Tc of l-10R5(OH) and c-10R5 were 72 and 70 °C, respectively, while that of l-17R4(OH) and c-17R4 were 49 and 53 °C, respectively. Interestingly, the linear methoxy-terminated species (l-L35(OMe), 81 °C; l-L64(OMe), 68 °C; l-10R5(OMe), 66 °C; l-17R4(OMe), 44 °C, Table 1) gave completely different Tc values and tendencies to those of cyclized species, where comparable or increased Tc were obtained for the ABA-type copolymers, in contrast to significant decreases in Tc for the BAB-type copolymer solutions. The obtained results suggest a clear distinction in the topology effect of cyclization to that of chemical modification of the chain ends on the clouding phenomena.
Figure 3.
(a) Temperature-dependent transmittance (%T) at 600 nm for 10 g/L aqueous solutions of linear hydroxy-terminated (blue), methoxy-terminated (green), and cyclized (red) L35, indicated as l-L35(OH), l-L35(OMe), and c-L35, respectively. Those of (b) L64, (c) 10R5 and (d) 17R4 are also shown. (e) %T at 600 nm for 10 g/L linear hydroxy-terminated (blue) and cyclized (red) PEG homopolymers in aqueous NaH2PO4 solutions at the concentration of 250 g/L.
To rationally explain the differences in transmittance changes upon temperature elevation of the ABA-type Pluronic copolymer solutions, an interpretation of the clouding mechanism is required. First, both linear hydroxy- and methoxy-terminated species of L35 and L64 (l-L35(OH), l-L35(OMe), l-L64(OH), and l-L64(OMe)) displayed similar %T profiles, where the transmittance drastically drops at the Tc. Clouding behavior of Pluronic copolymers to arise from phase transition induced through the dehydration and conformational change of the PEG segment is widely known [24,31], and our results suggest methylation of the chain-ends does not affect this mechanism. In contrast, the %T profiles of the cyclized species of the ABA-type copolymer solutions indicate a distinct aggregation upon temperature elevation; the transition of the cyclized species took place over a wider temperature range compared to their linear counterparts (Figure 3a,b), especially for c-L64. A similar phenomenon was reported on the phase transition of cyclic poly(N-isopropylacrylamide) (PNIPAM), explained to be caused by the disturbed packing of the polymer chains upon a coil-to-globule transformation due to the lack of chain ends [57,58], suggesting an analogous behavior to be exhibited in our system.
BAB-type copolymers are rationally expected to form flower-like micelles in water, where a fraction of the copolymer chains are expected to exist as or become “dangling chains” upon temperature elevation. The presence of “dangling chains” in these flower-like micelles is explained to act as inter-micellar bridging agents, which cause macroscopic aggregation, where the cyclization results in the elimination of this agglomeration mechanism [22,59]. In the case of the present Pluronic copolymers, the contribution from inhibition of the inter-micellar bridging was possibly observed for c-17R4, resulting in a slight increase in its Tc. On the other hand, the decreased Tc value for the linear methoxy-terminated species can be interpreted as a consequence of reduced solvation of the hydrophobic segment of the polymer, resulting in enhanced micellar bridging agglomeration to precede the phase transition.
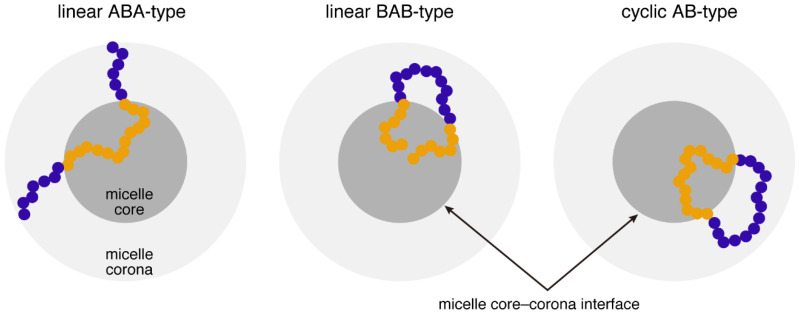
When the chain conformations and the freedom of each block in the micellar state are taken into account, linear triblock ABA-, BAB-type, and cyclic AB-type species are all expected to exhibit different characteristics (Figure 4). For example, the two PEG segments of linear ABA-type copolymers are only attached to the core–corona interface at one end of each block, in contrast to the looped PEG corona of linear BAB-type and cyclized species, with both PEG block-ends attached to the core. This is expected to produce significant differences in the conformational freedom of the PEG blocks, which may influence the hydration and Tc. Thus, the significant decrease in Tc upon cyclization observed only for the ABA-type copolymers can be explained as the result of the fixture of the free block ends at the core–corona interface, restricting their conformation.
Figure 4.
Schematic illustration of the expected conformations of linear ABA-type (left), linear BAB-type (center), and cyclized (right) copolymers in the micellar state. Cyclization results in the fixture of the PEG segment ends at the core–corona interface, and thus, is expected to affect the phase transition phenomena more prominently for the ABA-type copolymer.
To test this hypothesis of the restricted chain conformation of PEG being a factor behind the reduction in Tc, temperature-dependent transmittance measurements were carried out for the linear hydroxy-terminated and cyclized species of PEG homopolymers (l-PEG(OH) and c-PEG, respectively) (Table 1, Figure 3e). PEG solution samples were prepared at polymer concentrations of 10 g/L in a NaH2PO4 aqueous solution (NaH2PO4 concentration of 250 g/L) to induce phase transition under the boiling point of water [38,39]. As hypothesized, the cyclized species displayed lowered Tc compared to its linear counterparts (l-PEG(OH), 65 °C; c-PEG, 59 °C), indicating conformational restriction via cyclization to influence its solvation. This result bears a resemblance to thermal phase transition studies carried out for linear and cyclic species of PNIPAM [57,58,60]. Although the Tc of the cyclic PNIPAM samples was found to have concentration dependency and, therefore, not always be lower than that of their linear counterparts, reduced enthalpy changes during the clouding process were prevalent. These results, indicative of weakened hydrogen bonding interactions between the polymer and solvent water molecules for the cyclized species, have been explained as a consequence of either restrictions on the backbone conformation and/or steric constraints. A similar case could be assumed for the cyclized species of PEG and Pluronic copolymers investigated in this study.
3.4. Dynamic Light Scattering (DLS)
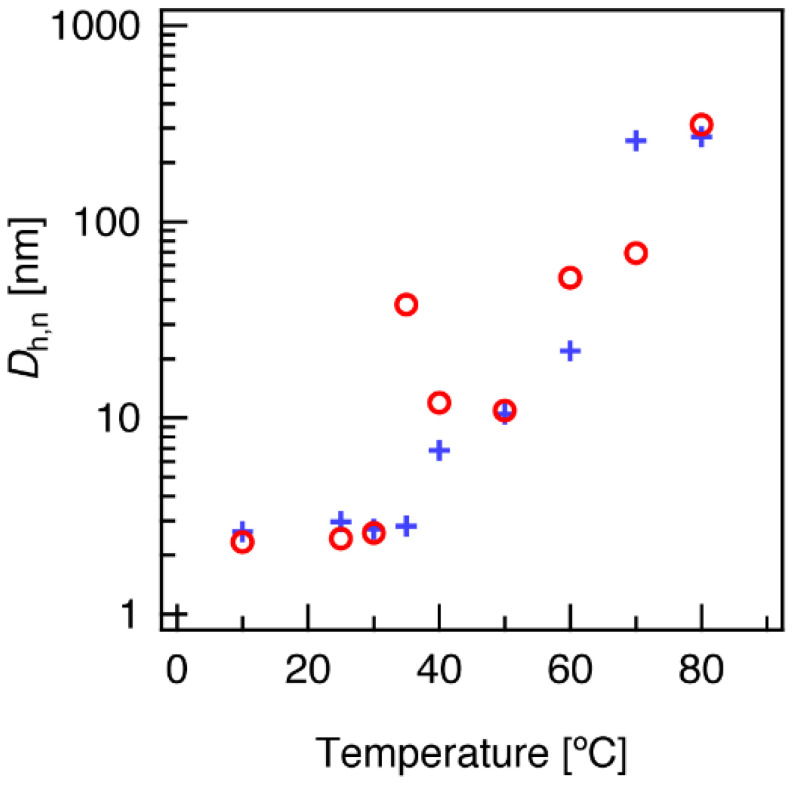
In the temperature-dependent %T profiles of c-L64 (Figure 3b), a plateau of around 30 %T was reached after the initial gradual transmission drop, from approximately 60 to 80 °C. A second sharp drop in %T was found over 80 °C, after which the solution became a 0 %T value. This implies a distinction in the thermally induced aggregation and/or phase separation phenomena of Pluronic L64, originating from its cyclic topology. In light of the anomalous association observed for c-L64, a structural investigation into the micellar aggregate size was conducted for L64 using DLS. The number-average hydrodynamic diameter (Dh,n) at finite concentration for 10 g/L solutions of l-L64(OH) and c-L64 at various temperatures is shown in Figure 5, while their number and intensity distribution profiles are shown in Figure S6. In consistency with the dye solubilization measurements, Dh,n of both l-L64(OH) and c-L64 smaller than 5 nm indicate the copolymers to be mostly molecularly dissolved as the unimer state below TCMT, with some large aggregates as indicated from multiple peaks in the intensity distribution (Figure S6). Following temperature elevation, an evident size increase and the unification of multiple peaks in the intensity distribution were observed at 40 °C for l-L64(OH) and at 35 °C for c-L64. While these suggest micellization around the corresponding temperatures, the apparent size for c-L64 at 35 °C indicates the cyclized species initially form larger aggregates around its TCMT, which break down into smaller micelles upon further heating. At 40 and 50 °C, both linear and cyclized copolymer systems displayed similar Dh,n of around 10–20 nm (Dh,n at 40 °C: l-L64(OH), 7 nm; c-L64, 12 nm), indicating the formation of well-defined micellar aggregates.
Figure 5.
Number-average hydrodynamic diameter (Dh,n) obtained from dynamic light scattering (DLS) at various temperatures for 10 g/L aqueous solutions of l-L64(OH) (blue cross) and c-L64 (red circle).
Temperature elevation for l-L64(OH) revealed a gradual increase in micelle size from 40 to 60 °C, followed by a sudden increase in the mean value and distribution of Dh,n from 22 nm at 60 °C to 260 nm at 70 °C, which underwent no further significant change upon temperature elevation to 80 °C, where Dh,n of 280 nm, was observed. The gradual increase in Dh,n between 40 and 60 °C is in coincidence with previous studies, where aggregation number and dimensions of Pluronic micelles are known to slightly increase upon temperature elevation [24,44]. Furthermore, the sudden size increase from 60 °C to 70 °C is indicative of the clouding phenomena and is consistent with the temperature-dependent transmittance results, where a drop in light transmittance occurred for l-L64(OH) between 60 and 70 °C (Figure 3b).
In contrast to the results for l-L64(OH), the c-L64 system underwent a more complex change upon heating. Between 40 and 50 °C, its Dh,n remained constant (11–12 nm), and thus, the majority of the copolymer micelles were found to retain their particle size. On the other hand, the intensity distribution indicated the formation of larger aggregates around 50–100 nm at 50 °C (Figure S6d). Upon further temperature elevation to 60 °C, these aggregates became the major component of the system as seen in Dh,n (52 nm). However, at 60 °C, the presence of even larger aggregates (ca. 500 nm) was indicated in the intensity distribution. These aggregates are expected to be the cause of the first drop in light transmittance (Figure 3b). Moreover, the second transmittance drop at 80 °C likely arose from these species’ becoming the major component of the system. The above results suggest the cyclic topology influences the thermally induced aggregation of c-L64. Nevertheless, multi-step %T changes were not observed for the other Pluronic copolymer systems (c-L35, c-10R5, and c-17R4), and thus, the effect of cyclization on the aggregation phenomena is also expected to be in relation to the block composition and sequence of the copolymer.
3.5. The Effect of Cyclization on the Critical Temperatures
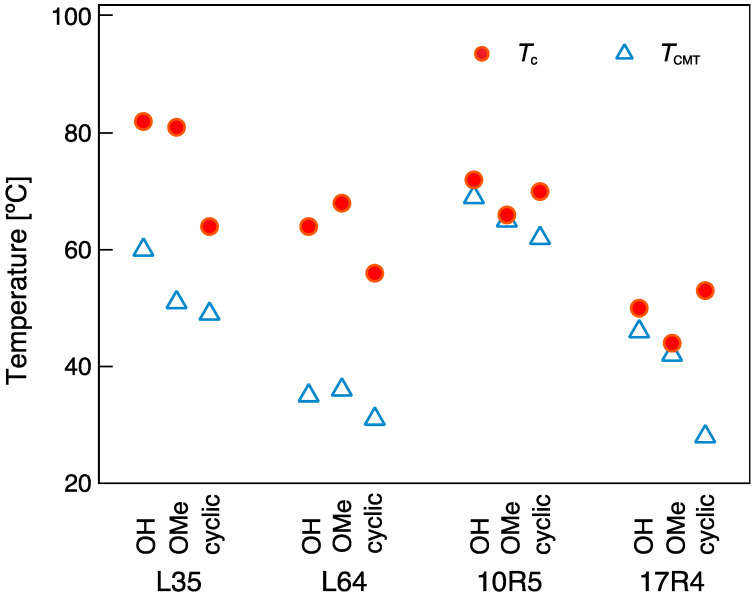
From dye solubilization, transmittance, and DLS measurements, cyclization was found to both affect the micellization and clouding behavior of Pluronic, each to a different degree for the four copolymer species explored in this study. A comparison of the critical temperatures for the linear hydroxy-terminated, linear methoxy-terminated, and cyclized species of the various Pluronic copolymers is shown in Figure 6. The micellar region, i.e., the temperature region above TCMT and below Tc, differs depending on the block sequence of the Pluronic copolymers. The micellar region of the ABA-type Pluronic L35 and L64 is relatively large, and while both methylation of the chain-ends and cyclization result in a general downward shift of TCMT, Tc was significantly affected only by cyclization. For the linear BAB-type copolymers, TCMT and Tc of the linear hydroxy-terminated and methoxy-terminated species are almost overlapping; thus, their micellar region is extremely small, or rather, they form a randomly cross-linked micellar network at this concentration [61]. The cyclized species, however, displayed prominently decreased TCMT and increased Tc compared to the linear methoxy-terminated species, resulting in the expansion of the micellar region. Summarizing the above, the topology and the chain-ends considerably affect their critical temperatures in relation to the block sequence of the linear species.
Figure 6.
Critical micellization temperature (TCMT) and cloud point (Tc) for 10 g/L solutions of Pluronic L35, L64, 10R5 and 17R4. Linear hydroxy-terminated species, linear methoxy-terminated and cyclized species are represented as OH, OMe, and cyclic, respectively.
4. Conclusions
The effects of cyclization were observed for Pluronic copolymers in their bulk solution properties. Investigation into the thermodynamic properties of micellization revealed a downward shift in TCMT with decreased ΔHmic and ΔSmic for the cyclized species. This is believed to be due to a more effective shielding of the hydrophobic PPG segments by the hydrophilic PEG segments in the cyclized species in the unimer state. Moreover, %T and DLS measurements revealed a contrasting effect on the clouding phenomena of the cyclized Pluronic micelles depending on their block sequence, where a pronounced decrease in Tc was observed for the ABA-type copolymers. A comparison with the methoxy-terminated linear species indicated the effect of cyclization to differ from the contribution from the elimination of the hydrophilic chain-end groups. Thus, the interpretation of the effect of cyclization on its phase transition behavior was discussed as arising from conformational restrictions and/or steric constraints of the polymer chains induced from the cyclic topology. A comprehensive understanding of the effects of cyclization allows the utilization of polymer topology to be a viable option in the rational design of polymeric materials and thus contributes to the fabrication of novel functional materials and their applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14091823/s1, Figure S1: SEC traces of Pluronic and PEG samples, Figure S2: 1H NMR spectra of Pluronic and PEG samples, Figure S3: 13C NMR spectra of Pluronic and PEG samples, Figure S4: Temperature-dependent transmittance of l-L64(OH), Figure S5: Temperature-dependent absorption intensity of DPH in the presence of Pluronic samples, Figure S6: Number and intensity distribution obtained from DLS.
Author Contributions
Conceptualization, T.W., S.C. and T.Y.; methodology, T.W.; formal analysis, T.W.; investigation, T.W., Y.W. and T.O.; writing—original draft preparation, T.W.; writing—review and editing, T.W., D.I. and T.Y.; visualization, T.W.; supervision, T.I., K.T., T.S., S.S., D.I. and T.Y.; project administration, T.Y.; funding acquisition, T.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Grant-in-Aid for Scientific Research (B) (21H01991, T.Y.), TERUMO LIFE SCIENCE FOUNDATION (T.Y.), NAKATANI Foundation (T.Y.), Iketani Science and Technology Foundation (T.Y.), Tanaka Kikinzoku Memorial Foundation (T.Y.), and Ogasawara Toshiaki Memorial Foundation (T.Y.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roover J. Overview on physical properties of cyclic polymers. In: Tezuka Y., editor. Topological Polymer Chemistry: Progress of Cyclic Polymers in Syntheses, Properties and Functions. World Scientific; Singapore: 2013. pp. 137–156. [Google Scholar]
- 2.Haque F.M., Grayson S.M. The synthesis, properties and potential applications of cyclic polymers. Nat. Chem. 2020;12:433–444. doi: 10.1038/s41557-020-0440-5. [DOI] [PubMed] [Google Scholar]
- 3.Lienard R., De Winter J., Coulembier O. Cyclic polymers: Advances in their synthesis, properties, and biomedical applications. J. Polym. Sci. 2020;58:1481–1502. doi: 10.1002/pol.20200236. [DOI] [Google Scholar]
- 4.Benetti E.M., Divandari M., Ramakrishna S.N., Morgese G., Yan W., Trachsel L. Loops and cycles at surfaces: The unique properties of topological polymer brushes. Chem. Eur. J. 2017;23:12433–12442. doi: 10.1002/chem.201701940. [DOI] [PubMed] [Google Scholar]
- 5.Kricheldorf H.R. Cyclic polymers: Synthetic strategies and physical properties. J. Polym. Sci. Part. A Polym. Chem. 2010;48:251–284. doi: 10.1002/pola.23755. [DOI] [Google Scholar]
- 6.Edwards J.P., Wolf W.J., Grubbs R.H. The synthesis of cyclic polymers by olefin metathesis: Achievements and challenges. J. Polym. Sci. Part. A Polym. Chem. 2018;57:228–242. doi: 10.1002/pola.29253. [DOI] [Google Scholar]
- 7.Jia Z., Monteiro M.J. Cyclic polymers: Methods and strategies. J. Polym. Sci. Part. A Polym. Chem. 2012;50:2085–2097. doi: 10.1002/pola.25999. [DOI] [Google Scholar]
- 8.Chang Y.A., Waymouth R.M. Recent progress on the synthesis of cyclic polymers via ring-expansion strategies. J. Polym. Sci. Part. A Polym. Chem. 2017;55:2892–2902. doi: 10.1002/pola.28635. [DOI] [Google Scholar]
- 9.Aoki D. A rational entry to cyclic polymers via spontaneous and selective cyclization reactions. Polym. J. 2021;53:257–269. doi: 10.1038/s41428-020-00422-6. [DOI] [Google Scholar]
- 10.Wang Y., Quinsaat J.E.Q., Ono T., Maeki M., Tokeshi M., Isono T., Tajima K., Satoh T., Sato S., Miura Y., et al. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol) Nat. Commun. 2020;11:6089. doi: 10.1038/s41467-020-19947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgese G., Cavalli E., Rosenboom J.-G., Zenobi-Wong M., Benetti E.M. Cyclic polymer grafts that lubricate and protect damaged cartilage. Angew. Chem. Int. Ed. 2018;57:1621–1626. doi: 10.1002/anie.201712534. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K., Lackey M.A., Cui J., Tew G.N. Gels based on cyclic polymers. J. Am. Chem. Soc. 2011;133:4140–4148. doi: 10.1021/ja111391z. [DOI] [PubMed] [Google Scholar]
- 13.Oziri O.J., Wang Y., Watanabe T., Uno S., Maeki M., Tokeshi M., Isono T., Tajima K., Satoh T., Sato S., et al. PEGylation of silver nanoparticles by physisorption of cyclic poly(ethylene glycol) for enhanced dispersion stability, antimicrobial activity, and cytotoxicity. Nanoscale Adv. 2022;4:532–545. doi: 10.1039/D1NA00720C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romio M., Trachsel L., Morgese G., Ramakrishna S.N., Spencer N.D., Benetti E.M. Topological polymer chemistry enters materials science: Expanding the applicability of cyclic polymers. ACS Macro. Lett. 2020;9:1024–1033. doi: 10.1021/acsmacrolett.0c00358. [DOI] [PubMed] [Google Scholar]
- 15.Williams R.J., Dove A.P., O’Reilly R.K. Self-assembly of cyclic polymers. Polym. Chem. 2015;6:2998–3008. doi: 10.1039/C5PY00081E. [DOI] [Google Scholar]
- 16.Zhang B., Zhang H., Li Y., Hoskins J.N., Grayson S.M. Exploring the effect of amphiphilic polymer architecture: Synthesis, characterization, and self-assembly of both cyclic and linear Poly(ethylene gylcol)-b-polycaprolactone. ACS Macro. Lett. 2013;2:845–848. doi: 10.1021/mz4003936. [DOI] [PubMed] [Google Scholar]
- 17.Heo K., Kim Y.Y., Kitazawa Y., Kim M., Jin K.S., Yamamoto T., Ree M. Structural characteristics of amphiphilic cyclic and linear block copolymer micelles in aqueous solutions. ACS Macro. Lett. 2014;3:233–239. doi: 10.1021/mz5000224. [DOI] [PubMed] [Google Scholar]
- 18.Qian H.-J., Lu Z.-Y., Chen L.-J., Li Z.-S., Sun C.-C. Computer simulation of cyclic block copolymer microphase separation. Macromolecules. 2005;38:1395–1401. doi: 10.1021/ma0478658. [DOI] [Google Scholar]
- 19.Ree B.J., Satoh T., Yamamoto T. Micelle structure details and stabilities of cyclic block copolymer amphiphile and its linear analogues. Polymers. 2019;11:163. doi: 10.3390/polym11010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouarti N., Viville P., Lazzaroni R., Minatti E., Schappacher M., Deffieux A., Putaux J.-L., Borsali R. Micellar aggregation in blends of linear and cyclic poly(styrene-b-isoprene) diblock copolymers. Langmuir. 2005;21:9085–9090. doi: 10.1021/la050935z. [DOI] [PubMed] [Google Scholar]
- 21.Minatti E., Borsali R., Schappacher M., Deffieux A., Soldi V., Narayanan T., Putaux J.-L. Effect of cyclization of polystyrene/polyisoprene block copolymers on their micellar morphology. Macromol. Rapid Commun. 2002;23:978–982. doi: 10.1002/1521-3927(200211)23:16<978::AID-MARC978>3.0.CO;2-K. [DOI] [Google Scholar]
- 22.Honda S., Yamamoto T., Tezuka Y. Topology-directed control on thermal stability: Micelles formed from linear and cyclized amphiphilic block copolymers. J. Am. Chem. Soc. 2010;132:10251–10253. doi: 10.1021/ja104691j. [DOI] [PubMed] [Google Scholar]
- 23.Vaughn T.H., Suter H.R., Lundsted L.G., Kramer M.G. Properties of some newly developed nonionic detergents. JAOCS J. Am. Oil Chem. Soc. 1951;28:294–299. doi: 10.1007/BF02648649. [DOI] [Google Scholar]
- 24.Almgren M., Brown W., Hvidt S. Self-aggregation and phase behavior of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymers in aqueous solution. Colloid Polym. Sci. 1995;273:2–15. doi: 10.1007/BF00655668. [DOI] [Google Scholar]
- 25.Lazzari M., Torneiro M. A Global View on Block Copolymers. Polymers. 2020;12:869. doi: 10.3390/polym12040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Errico G., Paduano L., Khan A. Temperature and concentration effects on supramolecular aggregation and phase behavior for poly(propylene oxide)-b-poly(ethylene oxide)-b-poly(propylene oxide) copolymers of different composition in aqueous mixtures, 1. J. Colloid Interface Sci. 2004;279:379–390. doi: 10.1016/j.jcis.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 27.Caragheorgheopol A., Schlick S. Hydration in the various phases of the triblock copolymers EO13PO30EO13 (Pluronic L64) and EO6PO34EO6 (Pluronic L62), based on electron spin resonance spectra of cationic spin probes. Macromolecules. 1998;31:7736–7745. doi: 10.1021/ma980982g. [DOI] [Google Scholar]
- 28.Kozlov M.Y., Melik-Nubarov N.S., Batrakova E.V., Kabanov A.V. Relationship between pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3305–3313. doi: 10.1021/ma991634x. [DOI] [Google Scholar]
- 29.Landazuri G., Fernandez V.V.A., Soltero J.F.A., Rharbi Y. Length of the core forming block effect on fusion and fission dynamics at equilibrium in PEO–PPO–PEO triblock copolymer micelles in the spherical regime. Macromolecules. 2021;54:2494–2505. doi: 10.1021/acs.macromol.0c01520. [DOI] [Google Scholar]
- 30.Alexandridis P., Holzwarth J.F., Hatton T.A. Micellization of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules. 1994;27:2414–2425. doi: 10.1021/ma00087a009. [DOI] [Google Scholar]
- 31.Naskar B., Ghosh S., Moulik S.P. Solution behavior of normal and reverse triblock copolymers (Pluronic L44 and 10R5) individually and in binary mixture. Langmuir. 2012;28:7134–7146. doi: 10.1021/la3000729. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z., Chu B. Phase behavior and association properties of poly(oxypropylene)–poly(oxyethylene)–poly(oxypropylene) triblock copolymer in aqueous solution. Macromolecules. 1994;27:2025–2033. doi: 10.1021/ma00086a008. [DOI] [Google Scholar]
- 33.Yu G.-E., Garrett C.A., Mai S.-M., Altinok H., Attwood D., Price C., Booth C. Effect of cyclization on the association behavior of block copolymers in aqueous solution. Comparison of oxyethylene/oxypropylene block copolymers cyclo-P34E104 and E52P34E52. Langmuir. 1998;14:2278–2285. doi: 10.1021/la971050t. [DOI] [Google Scholar]
- 34.Watanabe T., Chimura S., Wang Y., Ono T., Isono T., Tajima K., Satoh T., Sato S., Ida D., Yamamoto T. Cyclization of PEG and Pluronic surfactants and the effects of the topology on their interfacial activity. Langmuir. 2021;37:6974–6984. doi: 10.1021/acs.langmuir.1c00513. [DOI] [PubMed] [Google Scholar]
- 35.Jebari M.M., Ghaouar N., Aschi A., Gharbi A. Aggregation behaviour of Pluronic® L64 surfactant at various temperatures and concentrations examined by dynamic light scattering and viscosity measurements. Polym. Int. 2006;55:176–183. doi: 10.1002/pi.1935. [DOI] [Google Scholar]
- 36.Kositza M.J., Bohne C., Alexandridis P., Hatton T.A., Holzwarth J.F. Micellization dynamics and impurity solubilization of the block-copolymer L64 in an aqueous solution. Langmuir. 1999;15:322–325. doi: 10.1021/la9812368. [DOI] [Google Scholar]
- 37.Cooke J., Viras K., Yu G.-E., Sun T., Yonemitsu T., Ryan A.J., Price C., Booth C. Large cyclic poly(oxyethylene)s: Chain folding in the crystalline state studied by Raman spectroscopy, X-ray scattering, and differential scanning calorimetry. Macromolecules. 1998;31:3030–3039. doi: 10.1021/ma9717909. [DOI] [Google Scholar]
- 38.Nozary S., Modarress H., Eliassi A. Cloud-point measurements for salt + poly(ethylene glycol) + water systems by viscometry and laser beam scattering methods. J. Appl. Polym. Sci. 2003;89:1983–1990. doi: 10.1002/app.12450. [DOI] [Google Scholar]
- 39.Mohsen-Nia M., Rasa H., Modarress H. Cloud-point measurements for (water + poly(ethylene glycol) + salt) ternary mixtures by refractometry method. J. Chem. Eng. Data. 2006;51:1316–1320. doi: 10.1021/je060061y. [DOI] [Google Scholar]
- 40.Morrison I.D., Grabowski E.F., Herb C.A. Improved techniques for particle size determination by quasi-elastic light scattering. Langmuir. 1985;1:496–501. doi: 10.1021/la00064a016. [DOI] [Google Scholar]
- 41.Bryant G., Thomas J.C. Improved particle size distribution measurements using multiangle dynamic light scattering. Langmuir. 1995;11:2480–2485. doi: 10.1021/la00007a028. [DOI] [Google Scholar]
- 42.Mata J.P., Majhi P.R., Guo C., Liu H.Z., Bahadur P. Concentration, temperature, and salt-induced micellization of a triblock copolymer Pluronic L64 in aqueous media. J. Colloid Interface Sci. 2005;292:548–556. doi: 10.1016/j.jcis.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Sharma R.K., Shah U. Aggregation Behavior of PEO-PPO-PEO Tri-Block Copolymer (Pluronic® L64) in Nonionic Surfactant Additives Environment. Tenside Surfactants Deterg. 2014;51:274–281. doi: 10.3139/113.110308. [DOI] [Google Scholar]
- 44.Ganguly R., Choudhury N., Aswal V.K., Hassan P.A. Pluronic L64 micelles near cloud point: Investigating the role of micellar growth and interaction in critical concentration fluctuation and percolation. J. Phys. Chem. B. 2009;113:668–675. doi: 10.1021/jp808304w. [DOI] [PubMed] [Google Scholar]
- 45.Liao C.Y., Choi S.-M., Mallamace F., Chen S.-H. SANS study of the structure and interaction of L64 triblock copolymer micellar solution in the critical region. J. Appl. Cryst. 2000;33:677–681. doi: 10.1107/S0021889899013205. [DOI] [Google Scholar]
- 46.Yang L., Alexandridis P., Steytler D.C., Kositza M.J., Holzwarth J.F. Small-angle neutron scattering investigation of the temperature-dependent aggregation behavior of the block copolymer pluronic L64 in aqueous solution. Langmuir. 2000;16:8555–8561. doi: 10.1021/la000008m. [DOI] [Google Scholar]
- 47.Huff A., Patton K., Odhner H., Jacobs D.T., Clover B.C., Greer S.C. Micellization and phase separation for triblock copolymer 17R4 in H2O and in D2O. Langmuir. 2011;27:1707–1712. doi: 10.1021/la104350g. [DOI] [PubMed] [Google Scholar]
- 48.Kumi B.C., Hammouda B., Greer S.C. Self-assembly of the triblock copolymer 17R4 poly(propylene oxide)14–poly(ethylene oxide)24–poly(propylene oxide)14 in D2O. J. Colloid Interface Sci. 2014;434:201–207. doi: 10.1016/j.jcis.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 49.Yu G.-E., Yang Z., Attwood D., Price C., Booth C. Association and surface properties of a cyclic block copolymer of ethylene oxide and butylene oxide (Cyclo-B8E42) in water. Macromolecules. 1996;29:8479–8486. doi: 10.1021/ma9607022. [DOI] [Google Scholar]
- 50.Yamamoto T., Masuda Y., Tezuka Y., Korchagina E., Winnik F.M. Comparative thermodynamic studies of the micellization of amphiphilic block copolymers before and after cyclization. Langmuir. 2022 doi: 10.1021/acs.langmuir.0c03358. in press. [DOI] [PubMed] [Google Scholar]
- 51.Kelarakis A., Havredaki V., Rekatas C.J., Booth C. Thermodynamics of micellisation of a diblock copolymer of ethylene oxide and styrene oxide in water. Phys. Chem. Chem. Phys. 2001;3:5550–5552. doi: 10.1039/b107374e. [DOI] [Google Scholar]
- 52.Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437:640–647. doi: 10.1038/nature04162. [DOI] [PubMed] [Google Scholar]
- 53.Kim S.H., Jo W.H. A Monte Carlo simulation for the micellization of ABA- and BAB-type triblock copolymers in a selective solvent. Macromolecules. 2001;34:7210–7218. doi: 10.1021/ma0105136. [DOI] [Google Scholar]
- 54.Kim S.H., Jo W.H. A Monte Carlo simulation for the micellization of ABA- and BAB-type triblock copolymers in a selective solvent. II. Effects of the block composition. J. Chem. Phys. 2002;117:8565–8572. doi: 10.1063/1.1512646. [DOI] [Google Scholar]
- 55.Manet S., Lécchi A., Impéror-Clerc M., Zholobenko V., Durand D., Oliveira C.L.P., Pedersen J.S., Grillo I., Meneau F., Rochas C. Structure of micelles of a nonionic block copolymer determined by SANS and SAXS. J. Phys. Chem. B. 2011;115:11318–11329. doi: 10.1021/jp200212g. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen J.S., Gerstenberg M.C. The structure of P85 Pluronic block copolymer micelles determined by small-angle neutron scattering. Colloids Surf. A Physicochem. Eng. Asp. 2003;213:175–187. doi: 10.1016/S0927-7757(02)00511-3. [DOI] [Google Scholar]
- 57.Qiu X.-P., Tanaka F., Winnik F.M. Temperature-induced phase transition of well-defined cyclic poly(N-isopropylacrylamide)s in aqueous solution. Macromolecules. 2007;40:7069–7071. doi: 10.1021/ma071359b. [DOI] [Google Scholar]
- 58.Xu J., Ye J., Liu S. Synthesis of well-defined cyclic poly(N-isopropylacrylamide) via click chemistry and its unique thermal phase transition behavior. Macromolecules. 2007;40:9103–9110. doi: 10.1021/ma0717183. [DOI] [Google Scholar]
- 59.Honda S., Yamamoto T., Tezuka Y. Tuneable enhancement of the salt and thermal stability of polymeric micelles by cyclized amphiphiles. Nat. Commun. 2013;4:1574. doi: 10.1038/ncomms2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye J., Xu J., Hu J., Wang X., Zhang G., Liu S., Wu C. Comparative study of temperature-induced association of cyclic and linear poly(N-isopropylacrylamide) chains in dilute solutions by laser light scattering and stopped-flow temperature jump. Macromolecules. 2008;41:4416–4422. doi: 10.1021/ma702196g. [DOI] [Google Scholar]
- 61.Mortensen K., Brown W., Jørgensen E. Phase behavior of poly(propylene oxide)–poly(ethylene oxide)–poly(propylene oxide) triblock copolymer melt and aqueous solutions. Macromolecules. 1994;27:5654–5666. doi: 10.1021/ma00098a020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.