Abstract
Cyanobacteria are autotrophic prokaryotes which carry out oxygenic photosynthesis and accumulate glycogen as the major form of stored carbon. In this research, we introduced new genes into a cyanobacterium in order to create a novel pathway for fixed carbon utilization which results in the synthesis of ethanol. The coding sequences of pyruvate decarboxylase (pdc) and alcohol dehydrogenase II (adh) from the bacterium Zymomonas mobilis were cloned into the shuttle vector pCB4 and then used to transform the cyanobacterium Synechococcus sp. strain PCC 7942. Under control of the promoter from the rbcLS operon encoding the cyanobacterial ribulose-1,5-bisphosphate carboxylase/oxygenase, the pdc and adh genes were expressed at high levels, as demonstrated by Western blotting and enzyme activity analyses. The transformed cyanobacterium synthesized ethanol, which diffused from the cells into the culture medium. As cyanobacteria have simple growth requirements and use light, CO2, and inorganic elements efficiently, production of ethanol by cyanobacteria is a potential system for bioconversion of solar energy and CO2 into a valuable resource.
Cyanobacteria, also known as blue-green algae, are autotrophic prokaryotes which exhibit diversity in metabolism, structure, morphology, and habitat. However, all of these organisms perform oxygenic photosynthesis, and this photosynthesis is similar to that performed by higher plants (29, 32). As the cyanobacteria have simple growth requirements, grow to high densities, and use light, carbon dioxide, and other inorganic nutrients efficiently, they could be attractive hosts for production of valuable organic products. In fact, many cyanobacteria can be used directly as food and fodder since they are nonpathogenic and have high nutrient value (27). Some cyanobacteria also synthesize secondary metabolites which have been reported to have significant therapeutic effects (4). In addition, mass cultivation for commercial production of some cyanobacteria can be performed efficiently.
Synechococcus sp. strain PCC 7942 (previously referred to as Anacystis nidulans R2), a unicellular cyanobacterium that lives in freshwater, is one of the few cyanobacterial strains which have been relatively well-characterized in terms of physiology, biochemistry, and genetics. This organism is able to take up foreign DNA and can be transformed either by using shuttle vectors capable of replicating in both Escherichia coli and the cyanobacterium or by integrating foreign DNA into the chromosome through homologous recombination at targeted sites (14, 36). In recent years, workers have achieved limited success in expressing foreign genes in this cyanobacterium, as well as other transformable strains. For example, the human carbonic anhydrase gene caII used to investigate CO2-concentrating mechanisms (26), E. coli and human superoxide dismutase genes used to investigate oxidative stress (15, 34), E. coli pet genes used to increase salt stress resistance (25), and Bacillus thuringiensis larvicidal genes used to develop bioinsecticidal hosts (33, 35) have all been expressed in Synechococcus sp. at sufficiently high levels to generate discernible phenotypes. In this paper, we describe our attempts to transform Synechococcus sp. strain PCC 7942 with bacterial genes in order to create a novel pathway for ethanol production in cyanobacteria.
The major pathway for ethanol synthesis is catalyzed by two enzymes, pyruvate decarboxylase (PDC) (EC 4.1.1.1) and alcohol dehydrogenase (ADH) (EC 1.1.1.1). PDC catalyzes the nonoxidative decarboxylation of pyruvate, which produces acetaldehyde and CO2. Acetaldehyde is then converted to ethanol by ADH. This fermentation pathway plays a role in the regeneration of NAD+ for glycolysis under anaerobic conditions in fungi, yeasts, and higher plants. Although many bacteria can produce some ethanol, the obligately fermentative bacterium Zymomonas mobilis is one of few prokaryotes which generate ethanol as the predominant fermentative product (22). In this bacterium, PDC and ADH are very abundant; PDC alone accounts for as much as 5% of the total soluble protein in the cells (2). Zymomonas PDC is a tetramer composed of identical subunits and the monomeric molecular mass is approximately 60 kDa, while two isozymes of ADH are present and contribute to fermentation (17, 24). ADH II, the more abundant isozyme, is also a homotetramer, and the monomeric molecular mass is 40 kDa. Cloning and molecular characterization of the genes encoding Z. mobilis PDC (pdc) (6, 8, 23) and ADH II (adhII) (9) have been reported previously.
In an innovative previous study, the adh and pdc genes of Z. mobilis were used to transform E. coli in order to produce a novel ethanogenic bacterium capable of using a variety of substrates for growth (18). In the study described below, Z. mobilis pdc and adh genes were cloned into a shuttle vector and used to transform the cyanobacterium Synechococcus sp. strain PCC 7942. Under control of the promoter of the cyanobacterial rbcLS operon encoding the ribulose-1,5-bisphosphate carboxylase/oxygenase large and small subunits, the pdc and adh genes were expressed at high levels. As a result, a significant amount of ethanol accumulated in the culture medium. This is the first study in which oxygenic photoautotrophic microorganisms have been genetically engineered to produce ethanol.
MATERIALS AND METHODS
Strains and culture conditions.
E. coli DH5α cells were grown at 37°C in Luria broth (LB) or LB supplemented with 50 μg of ampicillin ml−1.
The unicellular cyanobacterium Synechococcus sp. strain PCC 7942 (29) was maintained on BG-11 medium (3, 20) solidified with 1% Bacto Agar (Difco) and was grown at 30°C under cool white fluorescent light (50 microeinsteins · m−2 · s−1). Cells were also grown in liquid batch cultures in 50-ml portions of BG-11 medium in 250-ml flasks closed with foam plugs and aluminum foil. The cultures were agitated constantly on an oscillating shaker. For rapid growth, cells were grown in 500-ml liquid batch cultures in 900-ml bottles that were aerated by forcing air through a Pasteur pipette. Cell growth was monitored by measuring the optical density at 730 nm (OD730) of each culture. As estimated by plating, an OD730 of 1.0 was equivalent to approximately 108 cells · ml−1. Transformation of Synechococcus sp. strain PCC 7942 was carried out essentially as described previously (14). Transformants were directly selected on BG-11 medium plates supplemented with 1 μg of ampicillin ml−1. During subculturing of the transformants, the concentration of ampicillin was increased to 10 μg · ml−1. Transformants were grown in liquid batch cultures supplemented with 25 μg of ampicillin ml−1.
Plasmid construction.
The immediate source of the Z. mobilis pdc (8) and adhII (9) genes used in this study was plasmid pLOI295 (18). This plasmid contained an 1.8-kb fragment of the pdc sequence, which started at position −46 (relative to the transcription start site) and continued until 27 bp after the stop codon, as well as an 1.4-kb fragment of the adh sequence from the position 31 bp upstream from the ATG initiation codon to the position 164 bp after the stop codon, including the transcription terminator. In plasmid pLOI295, pdc expression and adh expression were under control of the E. coli lac promoter.
A PCR was used to clone the promoter region of the rbcLS operon from Synechococcus sp. strain PCC 7942. The forward primer 5′-CGCGGATCCGCGGCTGAAAGTTTCGGACTCAGTAG-3′ (containing a BamHI site) and the reverse primer 5′-GCTGAATTCATGTCGTCTCTCCCTAGAGA-3′ (containing an EcoRI site) were designed by using the rbcLS sequence from the cyanobacterium A. nidulans 6301 (31), a strain that is genetically similar to Synechococcus sp. strain PCC 7942 (13, 29). The 361-bp amplified fragment included the rbcLS promoter and its 5′ untranslated sequence, starting at position −198 (relative to the transcription start site) and continuing to the ATG initiation codon. Each PCR mixture (100 μl) contained each primer at a concentration of 0.5 μM, each deoxynucleoside triphosphate at a concentration of 0.4 mM, 10 ng of genomic DNA from Synechococcus sp. strain PCC 7942, and 2 U of VentR DNA polymerase (New England Biolabs) in 1× reaction buffer [10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris-HCl (pH 8.8 at 25°C), 2 mM MgCl2, 0.1% Triton X-100]. PCR were carried out with a model PTC-100TM programmable thermal controller (MJ Research, Inc.) by using the following temperature program: 93°C for 3 min; 30 cycles consisting of 93°C for 1 min, 62°C for 1.5 min, and 72°C for 0.5 min; and 72°C for 5 min. The PCR product of the expected size was cloned into pBlueScript SK (Stratagene) between BamHI and EcoRI sites to generate a plasmid designated pRBCp. The 3.2-kb EcoRI-SalI fragment containing the pdc-adh sequence was removed from pLOI295 and ligated into the corresponding sites of pRBCp to generate plasmid pRpa. Finally, the 3.6-kb BamHI fragment containing the rbc-pdc-adh sequence was removed from pRpa and cloned into the shuttle vector pCB4 (11) at the corresponding site, which resulted in the construct pCB4-Rpa (Fig. 1). In addition, the 3.6-kb BamHI fragment of the rbc-pdc-adh sequence from pRpa was cloned at the BamHI site of pCB4-lac, a modified version of pCB4 in which a 220-bp PvuII-BamHI fragment from plasmid pBS (Stratagene) containing the E. coli lac promoter region was ligated into the modified XbaI-BamHI sites of the pCB4 multiple cloning site (33). The following two recombinant plasmids were generated during this cloning procedure: pCB4-Rpa(lac), in which the rbc-pdc-adh sequence was placed in the opposite direction with respect to the lac promoter; and pCB4-LRpa, in which expression of the pdc and adh genes was driven by a combination of the lac and rbc promoters (Fig. 1).
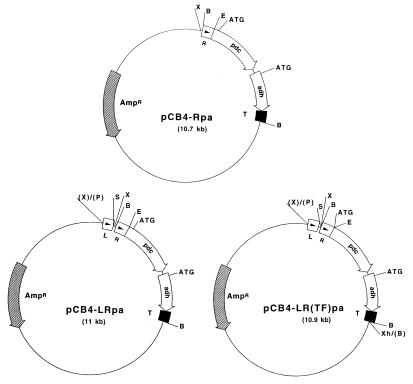
FIG. 1.
Plasmid vectors for expressing Z. mobilis PDC (pdc) and ADH II (adh) in cyanobacteria. All of the plasmids were constructed in the shuttle vector pCB4 for transforming Synechococcus sp. strain PCC 7942. In pCB4-Rpa, the pdc and adh genes are under the control of the promoter of the rbcLS operon (labelled R). In both pCB4-LRpa and pCB4-LR(TF)pa, pdc expression and adh expression are driven by a combination of the rbcLS promoter and the E. coli lac promoter (labelled L). The ribosome-binding site and the start codon of the rbcL gene were fused in frame to the second codon of the pdc gene in pCB4-LR(TF)pa. The arrows indicate the directions of transcription and translation. The position of the effective translation initiation codon (ATG) for the pdc and adh genes is indicated. The transcription terminator sequence of the adh gene is represented by a solid box (labelled T). The restriction sites used in cloning are shown (B, BamHI; P, PvuII; E, EcoRI, S, SalI; X, XbaI; Xh, XhoI). Letters in parentheses indicate restriction sites which were eliminated by blunt end ligation.
In an attempt to increase the level of pdc-adh expression, the ribosome-binding site and start codon of the pdc gene were removed and replaced with the corresponding DNA region of the rbcL sequence to generate a translation fusion. For this construct, the pdc-adh sequence in pLOI295 was amplified by PCR with forward primer 5′-GCATGAATTCTTATACTGTCGGTACCTAT-3′ (containing an EcoRI site) and reverse primer 5′-GGACTCGAGGATCCCCAAATGGCAA-3′ (containing BamHI and XhoI sites). The PCR mixture was the same as the PCR mixture described above. The following temperature program was used: 93°C for 5 min; four cycles consisting of 93°C for 1 min, 56°C for 1.5 min, and 72°C for 3.5 min; 30 cycles consisting of 93°C for 1 min, 65°C for 1.5 min, and 72°C for 3.5 min; and 72°C for 5 min. The 3.1-kb PCR product was then cloned into pRBCp between EcoRI and XhoI sites to generate plasmid pR(TF)pa (TF indicates translation fusion). Cloning for translation fusion generated an extra AAT (asparagine) codon immediately after the initiation codon, and the original second codon in the pdc open reading frame, AGT, was replaced by TCT, which encodes the same amino acid (serine). Plasmid pR(TF)pa was digested with XhoI, and the cut site was blunt ended with the Klenow fragment of bacterial DNA polymerase I and then digested with XbaI. The XbaI-XhoI fragment containing the rbc-(TF)pdc-adh sequence was then cloned into pCB4-lac which had been prepared by digestion with BamHI, blunt ended with the Klenow fragment, and redigested with XbaI. The resulting plasmid was designated pCB4-LR(TF)pa (Fig. 1).
Enzyme extraction and assays.
PDC was extracted and assayed essentially as described previously (8, 17) by monitoring the pyruvic acid-dependent reduction of NAD+ in the presence of yeast ADH as a coupling enzyme. ADH activity was assayed in the direction of ethanol oxidation as described elsewhere (9, 24). Enzyme activity is reported below in nanomoles per minute per milligram of total protein. To determine the PDC and ADH activities in E. coli cells harboring pLOI295, an overnight culture was diluted 1:100 into 50 ml of fresh LB and grown to an OD550 of 0.5 before 1 mM (final concentration) IPTG (isopropyl-β-d-thiogalactopyranoside) was added. Proteins were extracted from the cells after growth for an additional 2.5 h. The E. coli cells and cyanobacterial cells in 50-ml liquid batch cultures were harvested by centrifugation (8,000 × g, 10 min) and resuspended in 2 ml of PDC extraction buffer containing 50 mM sodium phosphate (pH 6.5), 1 mM thiamine pyrophosphate, 1 mM MgCl2, 5 mM dithiothreitol, and 1 mM benzamidine. Similarly, cells were resuspended in ADH extraction buffer consisting of 30 mM potassium phosphate (pH 8.8), 0.5 mM ferrous ammonium sulfate, 10 mM sodium ascorbate, 5 mM dithiothreitol, and 1 mM benzamidine. The resuspended cells were lysed with a prechilled French pressure cell at 20,000 lb · in−2. The lysates were centrifuged very briefly (2 min, 3,000 × g) to remove unbroken cells and were used immediately in enzyme assays. Total protein concentrations in the lysates were determined by the Coomassie Blue-G dye method (5) by using bovine serum albumin as the standard.
Western blot analysis.
The protein extracts prepared for the enzyme activity assays were used in a Western blot analysis performed by using standard procedures (30). PDC was probed with a goat antiserum directed against Z. mobilis PDC protein, and ADH was detected with a rabbit antiserum raised against Z. mobilis ADH II protein (1). Binding of the primary antibody was detected by incubating the preparation with an alkaline phosphatase-conjugated secondary immunoglobulin G antibody, followed by substrate staining.
RNA isolation and Northern blot analysis.
Total RNA was isolated from cyanobacterial cells in 50-ml liquid cultures by a previously described method (28) and was analyzed by Northern blotting by using standard methods (30). An 8-μg sample of total RNA was fractionated by formaldehyde-agarose gel electrophoresis and transferred onto a nitrocellulose membrane. The membrane was probed with 32P-labelled DNA probes. Plasmid pLOI295 was digested with SacI and SalI to generate 1.8-kb SacI fragment (pdc probe) and a 1.4 SacI-SalI fragment (adh probe), which were used to detect the pdc-adh dicistronic mRNA transcript. A 2.1-kb PstI DNA fragment containing the Synechococcus iron superoxide dismutase gene (sodB probe) was isolated from plasmid pFSB145 (20) and used to probe sodB mRNA. A 0.24- to 9.5-kb RNA ladder (GIBCO BRL, Life Technology, Inc.) was used to estimate transcript sizes.
Metabolite assay.
Ethanol was assayed with an ethanol kit (Boehringer Mannheim) by determining the amount of NADH generated after yeast ADH was added. Acetaldehyde was also assayed with the buffer and yeast ADH from the kit by monitoring the oxidation of NADH.
RESULTS
Construction of vectors for ethanol synthesis.
Using the cloning strategies described above, we generated three constructs for production of ethanol in Synechococcus sp. strain PCC 7942 (Fig. 1). In all of the constructs the pdc gene coding sequence was placed 5′ with respect to the adh gene, which included its original transcription terminator sequence. No promoter sequence or transcription terminator structure was present in the approximately 100-bp region between the stop codon of the pdc gene and the initiation codon of the adh gene. Therefore, the two genes formed a unit for cotranscription. Expression of the pdc and adh genes was under the control of the cyanobacterial rbcLS promoter in plasmid pCB4-Rpa and under the control of a combination of the rbcLS promoter and the E. coli lac promoter in both pCB4-LRpa and pCB4-LR(TF)pa. Plasmid pCB4-LR(TF)pa is a translation fusion construct in which, along with the promoter region, the ribosome-binding site and the initiation codon ATG of the rbcL gene are fused in frame to the second codon of the pdc gene.
Expression of the ethanol synthesis genes.
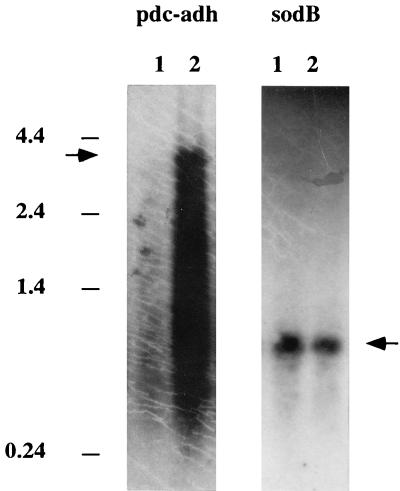
Total RNA was isolated from Synechococcus sp. strain PCC 7942 cells harboring plasmid pCB4-LRpa and was probed with the pdc sequence. A 3.2-kb band was detected on Northern blots (Fig. 2), which confirmed that the pdc and adh genes were correctly cotranscribed as a dicistronic RNA transcript. Similar results were obtained when the preparation was probed with the adh sequence (data not shown). The overall integrity of the RNA preparations was determined by probing them with sodB, the native cyanobacterial gene encoding iron superoxide dismutase. Hybridization of a 2.1-kb PstI DNA fragment containing the sodB gene to total cyanobacterial RNA produced a single 0.7-kb transcript, as expected for the gene (20). This suggests that the somewhat smeared pdc hybridization signal may have partially reflected rapid turnover of the pdc-adh transcript in the cells and was not necessarily an artifact of the RNA isolation procedure.
FIG. 2.
Northern blot analysis of cyanobacterial RNA. Samples (8 μg) of total RNA isolated from wild-type Synechococcus sp. strain PCC 7942 (lanes 1) and cyanobacterial cells transformed with the ethanol synthesis construct pCB4-LRpa (lanes 2) were fractionated by denaturing agarose gel electrophoresis and blotted onto a nitrocellulose membrane. The membrane was hybridized with the pdc probe to detect the dicistronic transcript pdc-adh and was hybridized with the sodB probe to check the overall quality of the RNA preparations. The positions of RNA molecular size markers (in kilobases) are indicated on the left. The arrows indicate the positions of the expected cotranscription products obtained from the pdc and adh genes (3.2 kb) and the sodB transcript (0.7 kb).
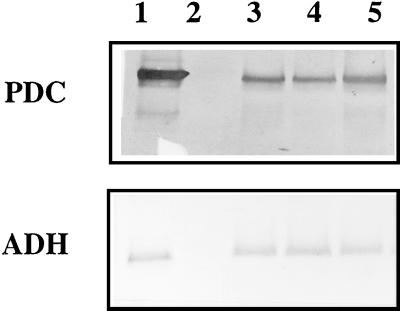
Expression of pdc and adh in cyanobacterial cells was further analyzed by performing a Western blot analysis (Fig. 3) and enzyme activity assays (Table 1). E. coli transformed with plasmid pLOI295 was used as a positive control in the Western blot analysis. In the protein extracts obtained from E. coli cells harboring pLOI295 and from cyanobacterial cells transformed with an ethanol synthesis construct, the antiserum directed against Z. mobilis PDC detected a band at 60 kDa, which was consistent with the predicted molecular mass of the deduced amino acid sequence (1). The antiserum raised against Z. mobilis ADH revealed a band at 40 kDa, as expected for ADH II polypeptide (1). High levels of PDC and ADH II polypeptides and high levels of enzyme activities were detected in the protein extracts from cyanobacterial cells transformed with the ethanol synthesis genes. No immunologically cross-reacting materials or PDC and ADH enzymatic activities were detected in the proteins extracted from the cells transformed with the vector pCB4 alone.
FIG. 3.
Western blot analysis of cyanobacterial proteins. Lanes 2 through 5 contained 8-μg portions of proteins extracted from the cyanobacterial cells harboring the shuttle vector pCB4 (lane 2) and the following vectors containing the pdc and adh genes: pCB4-Rpa (lane 3), pCB4-LRpa (lane 4), and pCB4-LR(TF)pa (lane 5). Proteins extracted from E. coli harboring pLOI295 containing the pdc and adh genes were used as a positive control (lane 1). The blots were probed with antiserum directed against Z. mobilis PDC protein and antiserum raised against Z. mobilis ADH II protein.
TABLE 1.
Enzyme activities and ethanol production in transformed Synechococcus sp. strain PCC 7942a
| Cell line | Activity (nmol · min−1 · mg of total protein−1)
|
Ethanol concn (μM) | |
|---|---|---|---|
| PDC | ADH | ||
| pCB4 (control) | NDb | ND | ND |
| pCB4-Rpa | 130 | 168 | 1,370 |
| pCB4-LRpa | 136 | 140 | 1,540 |
| pCB4-LR(TF)pa | 234 | 168 | 1,710 |
The shuttle vector pCB4 (control) and the constructs containing the pdc and adh genes were used individually to transform cyanobacterial cells. Transformed cells were grown in 50 ml of culture medium at 30°C in the presence of light for 21 days (final OD730, approximately 1.5). Cells were harvested to assay in order to determine the PDC and ADH activities in the cell lysates. The ethanol concentration in the culture medium was determined. The values are means of two or three values obtained with different samples from replicate cultures. For each replicate culture the values differed by less than 5%.
ND, not detected.
In our preliminary experiments, no pdc and adh expression or ethanol synthesis was detected in the transformed cyanobacterial cells when the genes were placed under the control of the E. coli lac promoter alone (data not shown). Consequently, the promoter region from the gene encoding ribulose bisphosphate carboxylase/oxygenase (the rbcLS operon), one of the most abundant soluble proteins synthesized in cyanobacteria, was employed. The rbcLS promoter directed a high level of expression of the Z. mobilis pdc and adh genes in Synechococcus sp. strain PCC 7942. It has been reported previously that the lac promoter enhances the expression of a foreign gene in Synechococcus sp. strain PCC 7942 when it is placed upstream of the endogenous bacterial promoter of the foreign gene (33). However, placing the lac promoter 5′ with respect to the rbcLS promoter did not enhance pdc and adh expression (construct pBC4-LRpa) compared to the expression directed by the rbcLS promoter alone (construct pCB4-Rpa).
It appears that the translation machinery of Synechococcus sp. strain PCC 7942 can recognize (and function efficiently with) the ribosome-binding sites and initiation codon of the Z. mobilis pdc and adh genes. In addition, as shown in Table 1, a twofold increase in pdc expression was observed when the ribosome-binding site and ATG initiation codon of the pdc gene were replaced with the corresponding DNA region of the rbcL gene to generate a translation fusion in plasmid pCB4-LR(TF)pa.
The abundance and enzymatic activity of PDC and ADH synthesized in transformed cyanobacterial cells were compared to the abundance and enzymatic activity of PDC and ADH produced in E. coli containing pLOI295. Different amounts of E. coli proteins and cyanobacterial proteins were analyzed on the same Western blots, and equal portions were used for enzyme activity measurements (data not shown). Based on the signal strength visualized on Western blots and catalytic activity, the PDC proteins synthesized in cyanobacterial cells and in E. coli appeared to have approximately the same specific activity. A similar relationship was observed for ADH proteins.
Production of ethanol in the cyanobacterium.
Cyanobacteria transformed with the genes encoding PDC and ADH produced ethanol, as shown by the analysis of the culture medium (Table 1). No ethanol was detected in the culture medium of the cells transformed with pCB4 alone (control), while all three preparations transformed with the pdc and adh genes produced ethanol at similar levels (about 1.4 to 1.7 mM) after 3 weeks of culture. An ethanol concentration of approximately 5 mM was obtained following 4 weeks of growth (data not shown). These values are certainly underestimates of the actual ethanol production as some ethanol was lost from the unsealed culture vessels during the growth period. Measurements of the rates of loss indicated that from 5 to 15% of the total ethanol in solution was lost; the variation was a function of the rate of culture flask shaking. Moreover, only very low levels of acetaldehyde (10 to 20 μM) were detected in the culture media of the cells transformed with the pdc and adh genes, suggesting that the ADH activity was sufficient to avoid accumulation of acetaldehyde.
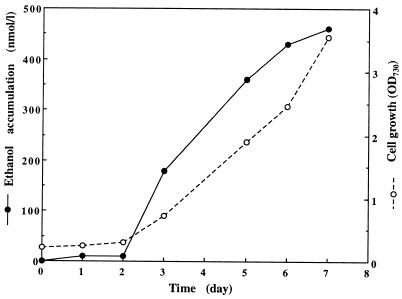
When cell cultures were aerated by bubbling, cell growth accelerated. Cell growth, the ethanol synthesis rate, and the PDC and ADH activities of the cells transformed with pCB4-LRpa were evaluated under these conditions (Fig. 4). From day 3 to 6, there was a linear increase in both cell growth (0.58 OD730 unit · day−1) and ethanol synthesis (54 nmol · OD730 unit−1 · liter−1 · day−1). The specific activities of PDC and ADH on day 5 were similar, approximately 160 nmol · min−1 · mg of total protein−1, equivalent to a potential ethanol synthesis rate of 6 mmol · OD730 unit−1 · liter · day−1. The PDC and ADH activities were, therefore, approximately 100 times higher than the actual rate of ethanol synthesis.
FIG. 4.
Cell growth and ethanol synthesis in Synechococcus sp. strain PCC 7942 transformed with pCB4-LRpa. Cells were grown at 30°C in the presence of light in a 500-ml liquid batch culture aerated by forcing air through a Pasteur pipette. Samples were taken at intervals in order to monitor cell growth (OD730) and ethanol accumulation in the culture medium. The PDC and ADH activities in cell lysates on day 5 were 320 and 170 nmol · min−1 · mg of total protein−1, respectively. The values are the means of two or three values obtained with different samples.
DISCUSSION
With the continuing depletion of known fossil fuel reserves, much research effort is being directed towards the discovery and utilization of renewable energy sources. Production of fuel ethanol through bioconversion of a variety of feedstocks is one strategy for reducing the need for fossil fuels. Traditionally, ethanol is produced by yeast fermentation of relatively expensive feedstocks, such as corn starch and cane sugar. With advances in biotechnology, new systems have been developed to use alternative carbohydrates found in biomass generated by agricultural or industrial activity. For example, addition of the Z. mobilis pdc and adh genes to E. coli generated an organism with a greatly expanded range of potential substrates for ethanol synthesis (18). In addition, Z. mobilis itself has recently been modified by adding a pentose metabolism capability, which improves the usefulness of this organism in bioconversion of lignocellulosic feedstocks (39). One improvement in these processes, which was considered in our study, would be direct coupling of ethanol biosynthesis to photosynthetic carbon fixation in autotrophic organisms, such as algae, aquatic plants, or cyanobacteria. Their high photosynthetic efficiency, limited nutrient requirements, rapid growth rates, and capacity to transport inorganic carbon suggest that cyanobacteria could be useful agents for ethanol synthesis.
We successfully engineered a pathway for ethanol synthesis in the cyanobacterium Synechococcus sp. strain PCC 7942 by expressing the Z. mobilis genes encoding PDC and ADH. Under the control of the cyanobacterial rbcLS promoter, the specific activities of these two enzymes ranged from 130 to 320 nmol · min−1 · mg of total protein−1 in cell lysates. These specific activities are comparable to the specific activities of some cyanobacterial enzymes involved in carbon metabolism, such as ribulose-1,5-bisphosphate carboxylase (100 nmol · min−1 · mg of protein−1) and hexokinase (95 nnmol · min−1 · mg of protein−1) in A. nidulans PCC 6301 (19) and 6-phosphogluconate dehydrogenase (60 nnmol · min−1 · mg of protein−1) and phosphoenolpyruvate carboxylase (77 nnmol · min−1 · mg of soluble protein−1) in Synechococcus sp. strain PCC 7942 (7, 21). The levels of PDC and ADH activities were also in the same range as the levels of the β-glucuronidase activity (75 to 155 nnmol · min−1 · mg of protein−1) encoded by the E. coli uidA gene which was integrated into the cyanobacterial chromosome and placed under the control of the E. coli trc promoter (10). The human superoxide dismutase gene has also been expressed in A. nidulans PCC 6301 under the control of the rbcLS promoter derived from the host genome (34). The level of expression of the enzyme, however, was considerably higher than the level of expression observed in our study. The difference between the levels of expression may be a function of plasmid copy number and of transcript and protein size and stability, which are important factors that affect foreign gene expression in cyanobacteria (36).
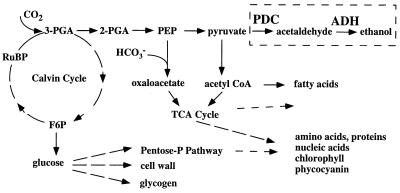
An easily assayed amount of ethanol accumulated in the culture medium of Synechococcus sp. strain PCC 7942 transformed with the ethanol synthesis genes, whereas the level of ethanol, if any, was under the limit of detection in the culture medium of the wild-type cells. Although the amount of ethanol that accumulated in the medium of the transformed Synechococcus culture was significant compared with the absence of ethanol in wild-type cultures, it was is still quite low compared with the amount produced by microbial fermentation. Certainly the rate at which ethanol is released into the medium and the final concentration can be increased by using a cyanobacterial cell density greater than the relatively low density used in this study, of 108 cells · ml−1; however, industrial microbial fermentation processes can generate levels of ethanol greater than 1 M in the culture medium. It is interesting that the rate of ethanol synthesis in the transformed cells was approximately 100 times less than the in vitro activities of PDC and ADH, suggesting that ethanol production was primarily limited by factors other than the PDC and ADH activities. It is possible that competition between different pathways for carbon metabolism, including storage carbohydrate biosynthesis, may limit ethanol production (Fig. 5). Attempts to manipulate carbon flux in these pathways and thus maximize substrate levels are now in progress.
FIG. 5.
Photosynthesis and photoassimilate metabolism in cyanobacteria. Abbreviations: 2-PGA, 2-phosphoglyceric acid; 3-PGA, 3-phosphoglyceric acid; F6P, fructose-6-phosphate; PEP, phosphoenolpyruvic acid; RuBP: ribulose 1,5-bisphosphate; TCA cycle, tricarboxylic acid cycle; acetyl CoA, acetyl coenzyme A. The pathway at the upper right is the added pathway for ethanol synthesis.
In some algae and cyanobacteria, ethanol is synthesized as one of the fermentation products under dark and anaerobic conditions (16, 37). However, the fermentation process is generally kept at a minimal level; the level of fermentation is only sufficient for the survival of the organisms. Moreover, ethanol synthesis is completely inhibited in the presence of light (12). A cloning strategy similar to that described in this paper was used to develop an ethanogenic Rhodobacter sp. recombinant in which carbon was also redirected from the Calvin cycle of this anaerobic phototroph (38). This recombinant was able to synthesize ethanol in the presence of light but required anoxic conditions, as well as a reductant, such as H2. In our study, by using genetic engineering we created a pathway for ethanol synthesis in Synechococcus sp. strain PCC 7942 which functions during oxygenic photosynthesis and requires no special conditions, such as an anaerobic environment. Optimization of such a system by manipulating the growth conditions and genetically modifying the host cell metabolism, as well as development of ethanol retrieval or sequestering technologies for the growth medium, could lead to production of ethanol by these simple photoautotrophic organisms at an industrial level.
ACKNOWLEDGMENTS
We thank L. O. Ingram, Department of Microbiology and Cell Science, University of Florida, Gainesville, for plasmid PLOI295 and antibodies raised against Z. mobilis PDC and ADH.
This research was supported by funds to J.R.C. from Enol Energy Inc. and the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Aldrich H C, McDowell L, Barbosa M de F S, Yomano L P, Scopes R K, Ingram L O. Immunocytochemical localization of glycolytic and fermentative enzymes in Zymomonas mobilis. J Bacteriol. 1992;174:4504–4508. doi: 10.1128/jb.174.13.4504-4508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algar E M, Scopes R K. Studies on cell-free metabolism: ethanol production by extracts of Zymomonas mobilis. J Biotechnol. 1985;2:275–287. [Google Scholar]
- 3.Allen M M. Simple conditions for growth of unicellular blue-green algae on plates. J Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 4.Belay A, Ota Y, Miyakawa K, Shimamatsu H. Current knowledge on potential health benefits of Spirulina. J Appl Phycol. 1993;5:235–241. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brau B, Sahm H. Cloning and expression of the structural gene for pyruvate decarboxylase of Zymomonas. Arch Microbiol. 1986;144:296–301. [Google Scholar]
- 7.Broedel S E, Wolf R E., Jr Growth-phase-dependent induction of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase in the cyanobacterium Synechococcus sp. PCC7942. Gene. 1991;109:71–79. doi: 10.1016/0378-1119(91)90590-8. [DOI] [PubMed] [Google Scholar]
- 8.Conway T, Osman Y A, Konnan J I, Hoffmann E M, Ingram L O. Promoter and nucleotide sequences of the Zymomonas mobilis pyruvate decarboxylase. J Bacteriol. 1987;169:949–954. doi: 10.1128/jb.169.3.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway T, Sewell G W, Osman Y A, Ingram L O. Cloning and sequencing the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987;169:2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geerts D, Bovy A, de Vrieze G, Borrias M, Weisbeek P. Inducible expression of heterologous genes targeted to a chromosomal platform in the cyanobacterium Synechococcus sp. PCC 7942. Microbiology. 1995;141:831–841. doi: 10.1099/13500872-141-4-831. [DOI] [PubMed] [Google Scholar]
- 11.Gendel S, Straus N, Pulleyblank D, Williams J. Shuttle cloning vectors for the cyanobacterium Anacystis nidulans. J Bacteriol. 1983;156:148–154. doi: 10.1128/jb.156.1.148-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gfeller R P, Gibbs M. Fermentation metabolism of Chlamydomonas reinhardtii: analysis of fermentation products from starch in the dark and light. Plant Physiol. 1984;75:212–218. doi: 10.1104/pp.75.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden S S, Nalty M S, Cho D C. Two functional psbD genes in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1989;171:4707–4713. doi: 10.1128/jb.171.9.4707-4713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden S S, Brussian J, Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- 15.Gruber M Y, Glick B R, Thompson J E. Cloned Mn-superoxide dismutase reduces oxidative stress in Escherichia coli and Anacystis nidulans. Proc Natl Acad Sci USA. 1990;87:2608–2612. doi: 10.1073/pnas.87.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyer H, Krumbein W E. Excretion of fermentation products in the dark and anaerobically incubated cyanobacteria. Arch Microbiol. 1991;155:284–287. [Google Scholar]
- 17.Hoppner T C, Doelle H W. Purification and kinetic characteristics of pyruvate decarboxylase and ethanol dehydrogenase from Zymomonas mobilis in relation to ethanol production. Eur J Appl Microbiol Biotechnol. 1983;17:152–157. [Google Scholar]
- 18.Ingram L O, Conway T, Clark D P, Sewell G W, Preston J F. Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol. 1987;53:2420–2425. doi: 10.1128/aem.53.10.2420-2425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karagouni A D, Slater J H. Enzymes of the Calvin cycle and intermediary metabolism in the cyanobacterium Anancystis nidulans grown in chemostat culture. J Gen Microbiol. 1979;115:369–376. [Google Scholar]
- 20.Laudenbach D E, Trick C G, Straus N A. Cloning and characterization of an Anacystis nidulans R2 superoxide dismutase gene. Mol Gen Genet. 1989;216:455–461. doi: 10.1007/BF00334390. [DOI] [PubMed] [Google Scholar]
- 21.Luinenburg I, Coleman J R. Expression of Escherichia coli phosphoenolpyruvate carboxylase in a cyanobacterium. Functional complementation of Synechococcus PCC 7942 ppc. Plant Physiol. 1993;101:121–126. doi: 10.1104/pp.101.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montenecourt B S. Zymomonas, a unique genus of bacteria. In: Demain A L, Solomon N A, editors. Biology of industrial microorganisms. Menlo Park, Calif: Benjamin-Cummings Publishing Co., Inc.; 1985. pp. 261–289. [Google Scholar]
- 23.Neale A D, Scopes R K, Wettenhall R E H, Hoogenraad N J. Nucleotide sequence of the pyruvate decarboxylase gene from Zymomonas mobilis. Nucleic Acids Res. 1987;15:1753–1761. doi: 10.1093/nar/15.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neale A D, Scopes R K, Kelly J M, Wettenhall R E H. The alcohol dehydrogenases of Zymomonas mobilis: purification by differential dye ligand chromatography, molecular characterization and physiological role. Eur J Biochem. 1986;154:119–124. doi: 10.1111/j.1432-1033.1986.tb09366.x. [DOI] [PubMed] [Google Scholar]
- 25.Nomura M, Ishitani M, Takabe T, Rai A K, Takabe T. Synechococcus sp. PCC7942 transformed with Escherichia coli bet genes produces glycine betaine from choline and acquires resistance to salt stress. Plant Physiol. 1995;107:703–708. doi: 10.1104/pp.107.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price G D, Badger M R. Expression of human carbonic anhydrase in the cyanobacterium Synechococcus PCC 7942 creates a high-CO2 requiring phenotype. Plant Physiol. 1989;91:505–513. doi: 10.1104/pp.91.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radmer R J. Algal diversity and commercial algal products. BioScience. 1996;46:263–270. [Google Scholar]
- 28.Reith M E, Laudenbach D E, Straus N A. Isolation and nucleotide sequence analysis of the ferredoxin I gene from the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1986;168:1319–1324. doi: 10.1128/jb.168.3.1319-1324.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Shinozaki K, Sugiura M. Genes for the large and small subunits of ribuloase-1,5-bisphosphate carboxylase/oxygenase constitute a single operon in the cyanobacterium Anacystis nidulans 6301. Mol Gen Genet. 1985;200:27–32. [Google Scholar]
- 32.Smith A J. Modes of cyanobacterial carbon metabolism. In: Carr N G, Whitton B A, editors. The biology of cyanobacteria. Oxford, United Kingdom: Blackwell Press; 1982. pp. 47–85. [Google Scholar]
- 33.Soltes-Rak E, Kushner D J, Williams D D, Coleman J. Effect of promoter modification on mosquitocidal cryIVB gene expression in Synechococcus sp. strain PCC 7942. Appl Environ Microbiol. 1993;59:2404–2410. doi: 10.1128/aem.59.8.2404-2410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeshima Y, Takatsugu N, Sugiura M, Hagiwara H. High level expression of human superoxide dismutase in the cyanobacterium Anacystis nidulans 6301. Proc Natl Acad Sci USA. 1994;91:9685–9689. doi: 10.1073/pnas.91.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tandeau de Marsac N, de la Torre F, Szulmajster J. Expression of the larvicidal gene of Bacillus sphaericus 1853M in the cyanobacterium Anacystis nidulans R2. Mol Gen Genet. 1987;209:396–398. doi: 10.1007/BF00329671. [DOI] [PubMed] [Google Scholar]
- 36.Thiel T. Genetic analysis of cyanobacteria. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Press; 1995. pp. 581–611. [Google Scholar]
- 37.Van der Oost J, Bulthuis B A, Feitz S, Krab K, Kraayenhof R. Fermentation metabolism of the unicellular cyanobacterium Cyanaothece PCC 7822. Arch Microbiol. 1989;152:415–419. [Google Scholar]
- 38.Wahlund T M, Conway T, Tabita F R. Bioconversion of CO2 to ethanol and other products. Am Chem Soc Div Fuel Chem. 1996;41:1403–1406. [Google Scholar]
- 39.Zhang M, Eddy C, Deanda K, Finkelstein M, Picataggio S. Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Science. 1995;267:240–243. doi: 10.1126/science.267.5195.240. [DOI] [PubMed] [Google Scholar]