Abstract
Benzene oxidation to carbon dioxide linked to nitrate reduction was observed in enrichment cultures developed from soil and groundwater microcosms. Benzene biodegradation occurred concurrently with nitrate reduction at a constant ratio of 10 mol of nitrate consumed per mol of benzene degraded. Benzene biodegradation linked to nitrate reduction was associated with cell growth; however, the yield, 8.8 g (dry weight) of cells per mol of benzene, was less than 15% of the predicted yield for benzene biodegradation linked to nitrate reduction. In experiments performed with [14C]benzene, approximately 92 to 95% of the label was recovered in 14CO2, while the remaining 5 to 8% was incorporated into the nonvolatile fraction (presumably biomass), which is consistent with the low measured yield. In benzene-degrading cultures, nitrite accumulated stoichiometrically as nitrate was reduced and then was slowly reduced to nitrogen gas. When nitrate was depleted and only nitrite remained, the rate of benzene degradation decreased to almost zero. Based on electron balances, benzene biodegradation appears to be coupled more tightly to nitrate reduction to nitrite than to further reduction of nitrite to nitrogen gas.
The BTEX (benzene, toluene, ethylbenzene, and xylenes) compounds are the most soluble components of gasoline and are common groundwater contaminants. Of all of the BTEX compounds, benzene is of most concern because it is the most toxic and a known human carcinogen. Benzene biodegradation occurs readily under aerobic conditions. However, at many contaminated sites anaerobic conditions predominate as any available oxygen is rapidly depleted. While biodegradation of alkylbenzenes, especially toluene, occurs readily under a variety of anaerobic conditions, it has proven to be much more difficult to obtain anaerobic benzene-degrading bacterial cultures. Recently, studies have linked benzene biodegradation to sulfate reduction (10), iron reduction (13), and methanogenesis (8). However, benzene biodegradation linked to nitrate reduction has not been confirmed previously (1, 3, 6–9). In this study we found that benzene biodegradation can be linked to nitrate reduction.
In 1995, we began a study to determine the potential for anaerobic benzene biodegradation in subsurface soil samples collected from six different sites. Batch microcosms were constructed with soil and groundwater from each site, and a variety of electron acceptors were evaluated. The results of this survey study confirmed what had been shown previously, namely, that benzene can be biodegraded under sulfate-reducing and iron-reducing conditions (15, 16). We also observed sustained and relatively rapid benzene biodegradation in the presence of nitrate in microcosms prepared with soil from two distinct sites, a decommissioned retail gasoline station in Toronto, Ontario, Canada (site A), and an uncontaminated freshwater swamp near Perth, Ontario, Canada (site B). Benzene biodegradation in two replicate nitrate-amended microcosms prepared with soil from each site proceeded in the presence of nitrate, ceased when nitrate became depleted, and resumed when nitrate was added. The fact that mineralization of benzene to CO2 occurred in these microcosms was confirmed by using 14C-labeled benzene (15, 16). Benzene biodegradation in these microcosms has been sustained since 1995. The purpose of this study was to confirm the link between benzene biodegradation and nitrate reduction in enrichment and transfer cultures derived from the original nitrate-amended microcosms.
MATERIALS AND METHODS
Original microcosms.
The microcosms used in the original study (16) were constructed in 250-ml screw-cap glass bottles sealed with Mininert caps (Alltech Associates, Inc., Deerfield, Ill.). Each microcosm consisted of 60 g of soil and 120 ml of groundwater or medium, which left a headspace of about 100 ml. Benzene (150 μM) was added to each microcosm with a syringe from a neat anaerobic stock. The microcosms were stored in the dark upside down in an anaerobic chamber with an atmosphere supplied with 10% H2, 10% CO2, and 80% N2. All manipulations were conducted inside the anaerobic chamber.
Transfer cultures.
Transfer cultures were prepared by inoculating 40-ml portions of ferrous sulfide-reduced, defined mineral medium with 40-ml portions of liquid, including small amounts of poorly settling solids, from microcosms that had been repeatedly refed with benzene and nitrate. The medium was prepared as described by Edwards et al. (4), except that the MgSO4 solution was replaced by MgCl2 to avoid adding sulfate to the cultures. Very little soil remained in the first-generation transfer cultures. These transfer cultures were prepared in 120-ml glass bottles sealed with Mininert caps and were amended with 5 mM NaNO3 from a 500 mM anaerobic stock solution and 150 μM benzene (ca. 1 μl per bottle) from a neat anaerobic stock. Subsequent transfer cultures were prepared in 40-ml glass vials sealed with Mininert caps by inoculating 15-ml portions of medium with 15-ml portions of liquid from first-generation transfer cultures.
End products of benzene biodegradation.
[14C]benzene was added to six active (inoculated) vials and to two uninoculated control vials. Each 17-ml vial contained 10 ml of medium and was sealed with a Mininert cap. [14C]benzene (Sigma) was diluted with neat benzene and was added to each vial to give a starting aqueous benzene concentration of about 15 mg/liter and an initial activity of about 6,000 dpm/ml. The amounts of [14C]benzene, 14CO2, and 14C-labeled nonvolatile compounds were determined by scintillation counting of the radioactivity in the acid, base, and neutral fractions of an aqueous sample by using the method of Grbic-Galic and Vogel (5). The 14C activity was determined by using UniverSol ES liquid scintillation cocktail (ICN Biomedicals, Inc., Costa Mesa, Calif.).
End products of nitrate reduction.
Four 17-ml vials containing 12 ml of inoculated nitrate-amended medium were purged with helium for 20 min. Benzene was added to two of the four vials. The Nitrogen concentrations in the headspaces of the cultures were measured before and after a known amount of benzene was consumed.
Effect of nitrate on benzene biodegradation.
Under strictly anaerobic conditions, a 50-ml aliquot from a first-generation transfer culture derived from a microcosm prepared with soil from site A was centrifuged (30 min, 4,000 rpm) in a Sorvall (Norwalk, Conn.) superspeed RC-2 B centrifuge and resuspended in 50 ml of fresh medium. The preparation was then transferred into five identical 40-ml vials (10 ml of culture per vial), and the vials were sealed with mininert caps. Three of the five vials were amended with 150 μM benzene (ca. 0.2 μl of neat benzene) but no nitrate, and the other two vials were amended with 150 μM benzene plus 5 mM nitrate.
Electron balances.
Nitrate, nitrite, sulfate, methane, Fe(II), and total iron contents were monitored as benzene was degraded. A set of cultures in an FeS-poor medium was prepared as follows: cells from second- and third-generation transfer cultures were collected by centrifugation (30 min, 4,000 rpm) and resuspended in medium supernatant (medium in which the black FeS precipitate had settled). The inoculated medium supernatant did not contain detectable levels of iron(II) or total iron. The estimated detection limit was 0.1 mM iron. Another set of cultures was prepared with molybdate (sodium salt), a specific inhibitor of sulfate-reducing bacteria, which was added to a concentration of 2 mM to two of four replicate culture vials. The four vials were amended with the same concentration of benzene, and degradation was monitored over time. Nitrate consumption and nitrite accumulation were monitored in sets of identical culture vials amended with benzene and not amended with benzene. Because the experiments were set up inside an anaerobic glove box, the headspaces of the culture vials initially contained hydrogen from the glove box atmosphere. Nitrate consumption resulting from H2 oxidation was observed in all of the culture vials; however, this nitrate consumption usually occurred before the onset of benzene biodegradation. The net nitrate consumption associated with benzene biodegradation was determined by subtracting the amount of nitrate consumed due to H2 oxidation from the total amount of nitrate consumed in the culture vials.
Analytical procedures.
Benzene and methane concentrations were monitored by removing a 300-μl sample of headspace gas from a microcosm or culture bottle with a 500-μl Pressure-Lok gas syringe (Precision Sampling Corp., Baton Rouge, La.) and injecting the sample into a gas chromatograph (Hewlett-Packard model 5890 Series II) equipped with a Supel-Q plot column (0.53 mm by 30 m; Supelco Co.) and a flame ionization detector. The injector temperature was 200°C, the oven temperature was 160°C, and the detector temperature was 250°C. The carrier gas was helium at a flow rate of 11 ml/min. Nitrogen concentrations were measured by injecting a headspace gas sample (100 μl) into a gas chromatograph (Hewlett-Packard model 5890) equipped with a Molecular Sieve (Supelco) packed column and a thermal conductivity detector. The injector temperature was 200°C, the oven temperature was 50°C, and the detector temperature was 200°C. The carrier gas was helium at a flow rate of 30 ml/min. Sulfate, nitrate, and nitrite concentrations were measured by removing a 0.5-ml liquid sample from a microcosm or culture bottle and injecting the filtered sample into a Dionex ion chromatograph equipped with a type AS4A column. The eluent was a 1.8 mM sodium carbonate–1.7 mM sodium bicarbonate solution at a flow rate of 2.0 ml/min. Ferrous iron and total extractable iron concentrations were measured by using a slurry sample from a microcosm or culture bottle as previously described (11, 12). Protein concentrations were measured by the method of Bradford (2) by using a microassay kit (Bio-Rad) and bovine serum albumin as the standard. The cell pellet from either 5 or 10 ml of culture was resuspended in 600 μl of 0.66 N NaOH and incubated for 3 h at 35°C to solubilize the protein. After centrifugation, the supernatant was removed, neutralized with 200 μl of 2 N HCl, mixed with 200 μl of dye reagent, and examined spectrophotometrically at 595 nm.
RESULTS AND DISCUSSION
Benzene biodegradation in transfer cultures.
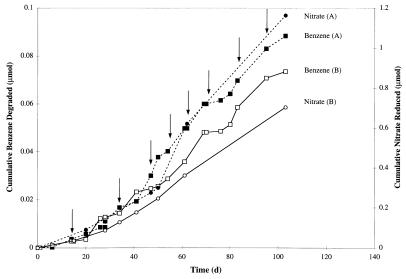
Sustained anaerobic benzene biodegradation was observed in nitrate-amended transfer cultures derived from the original microcosms. Benzene biodegradation was not observed in sterilized or uninoculated control cultures. The rates of benzene biodegradation and nitrate consumption steadily increased with time and enrichment (Table 1; Fig. 1). The ratio of the amount of nitrate consumed to the amount of benzene degraded stabilized at about 10 mol of nitrate per mol of benzene (Table 1).
TABLE 1.
Benzene biodegradation in microcosms and enriched cultures
| Microcosms or cultures | Year(s) | Rate of benzene degradation (μmol/liter/day) | Nitrate/ benzene ratio |
|---|---|---|---|
| Original microcosms | 1995 | 3.2 (0.14)a | 49.6 (15.8) |
| Enriched microcosms | 1995–1997 | 7.6 (0.10) | 12.6 (3.3) |
| First-generation transfer cultures | 1997 | 11.0 (2.5) | 11.1 (2.5) |
| Subsequent transfer cultures | 1997–1998 | 18.7 (13.0) | 10.1 (1.7) |
Values are means (standard deviations).
FIG. 1.
Cumulative amounts of benzene degraded (squares) and nitrate utilized (circles) in typical transfer cultures derived from microcosms prepared with soil from site A (dashed lines) and from site B (solid lines). The arrows indicate times when benzene (approximately 10 mg/liter) was added to the cultures. d, days.
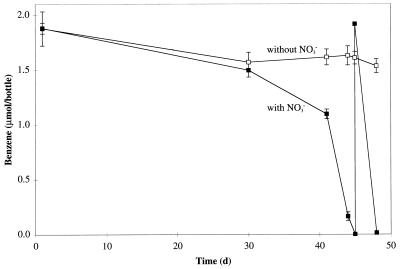
To establish if benzene biodegradation was dependent on nitrate, an inoculum was split into two sets of subcultures; one set was amended with nitrate, and the other was not. Benzene biodegradation occurred only in the cultures amended with nitrate (Fig. 2). Many similar experiments with and without nitrate were performed over the course of the enrichment of the cultures, and each time benzene biodegradation proceeded in the presence of nitrate and ceased when the nitrate was depleted.
FIG. 2.
Plot of benzene concentration versus time for transfer cultures grown in the presence of nitrate (■) and in the absence of nitrate (□). The data are means ± standard deviations from triplicate cultures (without nitrate) and means ± ranges from duplicate cultures (with nitrate). d, days.
End products of benzene biodegradation.
To confirm that benzene was mineralized to CO2, transfer cultures were incubated with [14C]benzene (Table 2). Approximately 92 to 95% of the initial benzene added was recovered as CO2, and the remaining 5 to 8% occurred in the nonvolatile fraction, presumably as biomass.
TABLE 2.
Mineralization of [14C]benzene under denitrifying conditionsa
| Culture | Fraction | % of 14C on:
|
|
|---|---|---|---|
| Day 0 | Day 14 | ||
| Active | Volatile | 95.3 (1.2)b | 0.0 |
| Nonvolatile | 2.1 (0.6) | 9.6 (1.5) | |
| Carbon dioxide | 2.5 (1.0) | 90.4 (1.5) | |
| Uninoculated | Volatile | 95.3 (1.2) | 94.2 (0.2) |
| Nonvolatile | 2.1 (0.6) | 4.2 (0.2) | |
| Carbon dioxide | 2.5 (1.0) | 1.6 (0.1) | |
The experiment included two replicate uninoculated control cultures and six replicate active cultures. The total counts recovered on day 14 ranged from 96 to 112% of the initial total counts.
Data are means (standard deviations).
End products of nitrate reduction.
During microbially mediated nitrate reduction, the first product of nitrate reduction is nitrite (NO2−). Further reduction of nitrite can proceed either directly to ammonia (NH3) or indirectly to nitrogen gas (N2) via several intermediates (17). Denitrification to nitrogen gas probably occurred in our cultures since gas bubbles (presumably of N2) developed in active cultures and the redox dye resazurin changed color from clear to pink during active nitrate reduction; NO and N2O, the intermediates of denitrification, oxidize resazurin and turn cultures pink (17). Confirmation that denitrification occurred was obtained with helium-purged cultures. Nitrogen (N2) production was significant only in the culture vials that were amended with benzene and was essentially stoichiometric to nitrate consumption (Table 3).
TABLE 3.
Nitrogen balance
| Treatment | Amt of benzene degraded (μmol) | Amt of nitrate consumed (μmol) | Initial N2 concn (%) | Final N2 concn (%) | Amt of N2 produced (μmol) |
|---|---|---|---|---|---|
| Benzene plus nitrate | 1.6 | 16.0 | 5.2 (1.2)a | 11.8 (0.6) | 13.7 (3.7) |
| Nitrate alone | 0 | 1.3 | 5.2 (1.2) | 6.5 (0.7) | 2.6 (4.0) |
| Not inoculated | 0 | 0 | 5.2 (1.2) | 5.4 | 0.4 (2.5) |
Data are means (standard deviations).
Evaluation of potential electron acceptors.
The overall energy-generating equations for benzene biodegradation linked to the four most common anaerobic electron acceptors are shown in Table 4. Methane, sulfate, total iron, ferrous iron, nitrate, and nitrite concentrations were measured over time in several experiments to determine if methanogenesis, sulfate reduction, or iron reduction occurred to any significant extent and to confirm that nitrate reduction was the dominant electron-accepting process in our cultures. Methane was not produced to a significant extent in any nitrate-amended microcosm or transfer culture, which eliminated the possibility that CO2 was a terminal electron acceptor. Sulfate was not present in the culture medium. However, when nitrate was added to inoculated culture medium, ferrous sulfide (FeS) in the medium was rapidly oxidized to stoichiometric amounts of ferric iron and small amounts of sulfate. These oxidation reactions occurred whether benzene was present or not, and the ferric iron and sulfate produced were not subsequently re-reduced (data not shown). However, to eliminate the possibility that sulfate was an electron acceptor in these cultures, the rate of benzene biodegradation was determined in the presence of molybdate, an inhibitor of sulfate reduction. Benzene biodegradation proceeded at exactly the same rate in cultures amended with 2 mM molybdate and in cultures not containing molybdate (data not shown), which eliminated the possibility that sulfate was an a possible electron acceptor. The same concentration of molybdate completely inhibited benzene degradation in confirmed sulfate-reducing cultures in our laboratory (15, 16). To eliminate the possibility that ferric iron acted as an electron acceptor, the rate of benzene biodegradation was determined in an iron-poor medium. Benzene biodegradation occurred in the iron-poor medium at the same rate that it occurred in the original medium (data not shown). Methane, sulfate, iron, and nitrate concentrations were measured initially and after degradation of 3.38 μmol of benzene (three feedings consisting of 100 μM benzene each) in the iron-poor medium (Table 5). Of the electron acceptor couples monitored, only nitrate was consumed in significant amounts. There was sufficient nitrate consumption (net consumption, about 36 μmol per vial) in benzene-amended cultures to account for the amount of benzene degraded (Table 5).
TABLE 4.
Theoretical overall energy-generating equations for benzene degradation under methanogenic, sulfate-reducing, iron-reducing, and nitrate-reducing conditions
| Electron acceptors (oxidized/reduced) | Overall energetic equation | ΔG°′ (kJ/mol)a |
|---|---|---|
| CO2/CH4 | C6H6 + 6.75 H2O→2.25 HCO3− + 3.75 CH4 + 2.25 H+ | −116 |
| SO42−/H2S | C6H6 + 3 H2O + 3.75 SO42−→6 HCO3− + 1.875 H2S + 1.875 HS− + 0.375 H+ | −200 |
| Fe3+/Fe2+ | C6H6 + 18 H2O + 30 Fe3+→6 HCO3− + 30 Fe2+ + 36 H+ | −3,070 |
| NO3−/N2 | C6H6 + 6 NO3−→6 HCO3− + 3 N2 | −2,990 |
| NO3−/NO2− | C6H6 + 15 NO3− + 3 H2O→6 HCO3− + 15 NO2− + 6 H | −2,020 |
TABLE 5.
Methane, sulfate, ferric iron, and nitrate concentrations in benzene-degrading culturesa
| Cultures | Day | Concn (μmol/vial) ofb:
|
|||
|---|---|---|---|---|---|
| Methane | Sulfate | Fe(III)c | Nitrate | ||
| Background control cultures containing nitrate but no benzene | 0 | 0.002 ± 0.00 | 0.71 ± 0.10 | <10d | 113.1 ± 0.9 |
| 66 | 0.012 ± 0.002 (+0.010) | 0.84 ± 0.00 (+0.13) | <10 (<10) | 89.5 ± 0.5 (−23.6) | |
| Active cultures containing nitrate and benzene | 0 | 0.004 ± 0.001 | 0.71 ± 0.10 | <10 | 113.1 ± 0.9 |
| 66 | 0.013 ± 0.002 (+0.011) | 0.91 ± 0.21 (+0.21) | <10 (<10) | 53.9 ± 0.03 (−59.2) | |
The theoretical values based on the amount of benzene actually degraded (3.38 μmol/vial) and the stoichiometries shown in Table 4 were as follows: methane production, 13 μmol/vial; sulfate consumption, 13 μmol/vial; Fe(III) consumption, 101 μmol/vial; and nitrate consumption, 20 μmol/vial.
Data are means ± standard deviations. The values in parentheses are the differences between the day 0 values and the day 66 values. The sulfate and nitrate values can be converted to aqueous concentrations (micromolar) by dividing by the liquid volume (0.01 liter).
Fe(III) concentrations were determined by calculating the difference between the total iron concentration and the Fe(II) concentration.
The concentration was below the detection limit (0.1 mM or 10 μmol/10-ml vial). Both the Fe(II) concentration and the total iron concentration were below the detection limit.
Nitrate reduction to nitrite.
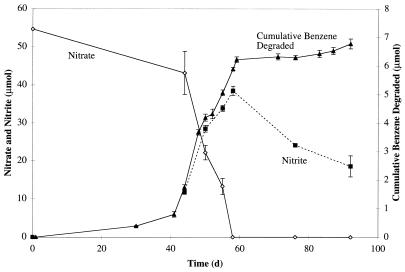
Nitrite, an intermediate in the reduction of nitrate to nitrogen gas, transiently accumulated in benzene-degrading cultures. Figure 3 is a plot showing net nitrate consumption, nitrite production, and cumulative benzene degradation in replicate cultures amended with both nitrate and benzene. As Fig. 3 shows, benzene degradation initially proceeded relatively slowly; during this period the rate of nitrate reduction was also relatively slow. On about day 40, the rates of benzene biodegradation and nitrate reduction increased significantly and simultaneously. During the period of rapid benzene degradation, days 40 to 58, nitrate was converted nearly stoichiometrically to nitrite. When nitrate became depleted on day 58, benzene degradation essentially ceased, even though nitrite was still present. These data suggest that nitrate was a much better electron acceptor for benzene oxidation than nitrite was. Nitrite may have partially inhibited the microorganisms responsible for benzene degradation, resulting in a slower rate of benzene degradation.
FIG. 3.
Net nitrate utilization (◊) and nitrite production (■) during benzene biodegradation (▴) in transfer cultures. The data are means ± ranges from duplicate cultures. d, days.
Cell yield.
To determine the cell yield, the amounts of cellular protein were measured before and after degradation of a known amount of benzene (Table 6). We determined that 8.8 g of cells was generated per mol of benzene degraded, assuming that protein comprised 50% of the dry weight of a cell. To estimate the fraction of electrons from benzene used for cell synthesis (fs), the measured yield (in grams per mole) was converted to units of electron equivalents. This calculation gave an fs value of 0.05, which meant that 5% of the electrons in benzene were recovered in biomass (Table 6). This value is consistent with the results of the [14C]benzene experiment in which approximately 5 to 8% of the carbon from benzene was recovered in the nonvolatile fraction.
TABLE 6.
Protein concentration and cell yield
| Culture | Protein concn (mg/liter)a
|
Amt of benzene degraded (μmol/vial) | Net amt of protein formed (μg/vial)b | Yield (g of cells/mol of benzenec | fsd | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| Background control culture containing nitrate but no benzene | 0.44 (0.07) | 0.84 (0.36) | ||||
| Active culture containing nitrate and benzene | 0.44 (0.07) | 2.82 (0.25) | 13.5 | 59.4 | 8.80 | 0.05 |
Data are means (standard deviations).
The culture volume was 30 ml.
Assuming that 50% of the cell dry weight was protein.
Assuming that there were 6.27 g of cells/electron equivalent of cells and 30 electron equivalents/mol of benzene.
Often, the value of fs can be reliably predicted by using the method of McCarty (14), which is based on a correlation between the amount of free energy released during oxidation of the substrate and the cell yield. When this method was used and when we assumed that a typical efficiency of electron transfer was 60%, the theoretical fs values were 0.45 for benzene oxidation coupled to nitrate reduction to nitrogen and 0.35 for benzene oxidation coupled to nitrate reduction to nitrite. These theoretical values are much greater than the value determined experimentally, 0.05. This may indicate that the actual efficiency of electron transfer in the cells examined was far lower than the average efficiency (60%), perhaps due to suboptimal growth conditions, the presence of inhibiting substances (such as nitrite), or an inefficient pathway for benzene metabolism (14).
Comparison of theoretical stoichiometry and experimental stoichiometry.
As shown in Table 4, 6 mol of nitrate is required to oxidize 1 mol of benzene if nitrate is reduced to nitrogen gas, and 15 mol of nitrate is required to oxidize 1 mol of benzene if nitrate is reduced only to nitrite. However, these ratios cannot be directly compared to experimentally measured nitrate-to-benzene ratios because the former ratios do not account for the fraction of benzene used for cell synthesis. If the cell yield is known or can be estimated, a theoretical overall nitrate-to-benzene stoichiometric ratio that does account for the fraction of benzene used for cell synthesis can be calculated by multiplying the stoichiometric coefficients for nitrate in the energy equations in Table 4 by the fraction of benzene used for energy production, (fe), where fe = 1 − fs. The ratios of amount of nitrate consumed to amount of benzene degraded in individual culture bottles at various stages of enrichment were determined (Table 1). In the original microcosms, the nitrate-to-benzene ratio was initially much greater than the theoretical value, presumably due to nitrate demand from unknown electron donors in the soil. However, as the sources of carbon were depleted, the ratio stabilized close to 10 mol of nitrate/mol of benzene. For benzene oxidation to CO2 coupled to complete reduction of nitrate to nitrogen gas, the predicted nitrate-to-benzene ratios are about 5.7 mol/mol with the experimentally measured yield of 8.8 g of cells/mol of benzene (corresponding to an fe value of 0.95) and even lower (3.3 mol/mol for an fe value of 0.55) if it is assumed that the yield is the yield predicted by the method of McCarty (14). Actually, our observed ratio, 10 mol/mol, is closer to the predicted ratios for oxidation of benzene to CO2 coupled to partial reduction of nitrate to nitrite. In this case the ratios should be about 14 mol/mol when the experimentally determined yield is used and 9.8 mol/mol if fe is 0.65 as predicted by the method of McCarty (14). The reality may be somewhere in between, with most of the cells’ energy derived during the first stage of nitrate reduction and a smaller amount derived during subsequent stages.
In conclusion, in this study we found that benzene biodegradation can be linked to nitrate reduction via nitrite to nitrogen gas. This conclusion was supported by the following experimental results: (i) benzene was mineralized to CO2 in active cultures but not in sterile or uninoculated controls; (ii) benzene biodegradation occurred concurrently with nitrate reduction, and the ratio of the amount of nitrate consumed to the amount of benzene degraded was constant; (iii) other electron acceptors [sulfate, iron(III), and CO2] were not involved in the degradation; (iv) nitrate was first reduced stoichiometrically to nitrite as benzene biodegradation proceeded, and subsequently nitrite was reduced to nitrogen; and (v) benzene degradation was accompanied by cell growth. To the best of our knowledge, this is the first report which confirms that benzene biodegradation can be linked to nitrate reduction.
ACKNOWLEDGMENTS
We thank Marit Nales for invaluable contributions to this work. We also thank Kirsten Krastel, Aled Edwards, and two anonymous reviewers for critical reviews of the manuscript.
This research was funded by the National Science and Engineering Research Council of Canada (NSERC) through an operating grant to E.A.E. and a postgraduate scholarship to S.M.B.
REFERENCES
- 1.Ball H A, Reinhard M. Monoaromatic hydrocarbon transformation under anaerobic conditions at Seal Beach, California—laboratory studies. Environ Toxicol Chem. 1996;15:114–122. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Davis J W, Klier N J, Carpenter C L. Natural biological attenuation of benzene in ground water beneath a manufacturing facility. Ground Water. 1994;32:215–226. [Google Scholar]
- 4.Edwards E A, Wills L E, Reinhard M, Grbić-Galić D. Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Appl Environ Microbiol. 1992;58:794–800. doi: 10.1128/aem.58.3.794-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grbić-Galić D, Vogel T M. Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol. 1987;53:254–260. doi: 10.1128/aem.53.2.254-260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchins S R. Biodegradation of monoaromatic hydrocarbons by aquifer microorganisms using oxygen, nitrate, or nitrous oxide as the terminal electron acceptor. Appl Environ Microbiol. 1991;57:2403–2407. doi: 10.1128/aem.57.8.2403-2407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchins S R. Optimizing BTEX biodegradation under denitrifying conditions. Environ Toxicol Chem. 1991;10:1437–1448. [Google Scholar]
- 8.Kazumi J, Caldwell M E, Suflita J M, Lovely D R, Young L Y. Anaerobic degradation of benzene in diverse anoxic environments. Environ Sci Technol. 1997;31:813–818. [Google Scholar]
- 9.Kuhn E P, Zeyer J, Eicher P, Schwarzenbach R P. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl Environ Microbiol. 1988;54:490–496. doi: 10.1128/aem.54.2.490-496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley D R, Coates J D, Woodward J C, Phillips E J P. Benzene oxidation coupled to sulfate reduction. Appl Environ Microbiol. 1995;61:953–958. doi: 10.1128/aem.61.3.953-958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovley D R, Phillips E J P. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987;53:1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovley D R, Woodward J C, Chapelle F H. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarty P L. Energetics and bacterial growth. In: Faust S D, Hunter J V, editors. Organic compounds in aquatic environments. Vol. 7. New York, N.Y: Marcel Dekker; 1971. pp. 157–172. [Google Scholar]
- 15.Nales M. M.S. thesis. Waterloo, Ontario, Canada: University of Waterloo; 1997. [Google Scholar]
- 16.Nales M, Butler B, Edwards E. Anaerobic benzene biodegradation: a microcosm survey. Bioremed J. 1998;2:125–144. [Google Scholar]
- 17.Suflita J M, Londry K L, Ulrich G A. Determination of anaerobic biodegradation activity. In: Hurst C J, editor. Manual of environmental microbiology. Washington, D.C: ASM Press; 1997. pp. 790–801. [Google Scholar]
- 18.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weast R C. Handbook of chemistry and physics. 56th ed. Cleveland, Ohio: CRC Press; 1975. [Google Scholar]