Abstract
A systematic review of clinical trials investigating the safety and efficacy of left gastric artery (LGA) embolization as a bariatric procedure was performed. The Methodological Index for Nonrandomized Studies (MINORS) instrument was used for quality assessment. Patient characteristics, weight loss after embolization, and complications were reviewed. Meta-regression was performed to assess associations of age, sex, body mass index, and ghrelin and leptin levels with weight change after LGA embolization. The final meta-analysis included 6 nonrandomized prospective trials. Findings of 3 additional studies reporting weight changes after LGA embolization for control of gastrointestinal bleeding were also reviewed. Pooled analysis of 47 subjects with overweight/obesity showed mean ± SD weight loss after embolization of 8.1% ± 1.5% and 8.85 kg ± 1.24 kg (both P < .001) after a mean 12-month follow-up. Male sex (β = 11.36 ± 5.79, P = .049) was associated with greater weight loss. Transient superficial mucosal ulcers were common after LGA embolization. One major adverse event comprising severe pancreatitis, splenic infarct, and gastric perforation was reported; treatment was supportive care. LGA embolization was associated with statistically significant weight loss and limited complications during short-term follow-up. Given that LGA embolization is an investigative method, it is important for researchers to follow standardized protocols and techniques to avoid complications.
Obesity is a global epidemic among adults and children in developed and developing countries (1). Initial treatment strategies include diet modification and exercise, which can be coupled with behavioral interventions and pharmacotherapy, but the outcomes of these interventions vary according to patient compliance. Surgical approaches to weight loss have been developed, including Roux-en-Y gastric bypass, sleeve gastrectomy, and gastric banding (2). Bariatric surgery causes weight loss by restricting meal size, inhibiting macronutrient absorption and, importantly, by altering the hormones involved in metabolic homeostasis (2-8). Although effective, surgical approaches carry high risks of patient morbidity and mortality (2,6).
Left gastric artery (LGA) embolization is a minimally invasive, image-guided procedure most commonly used to treat upper gastrointestinal bleeding. Recently, LGA embolization has also been proposed as an alternative method to treat obesity by targeting the appetite-stimulating endocrine functions of the gastric fundus (9,10). Specifically, embolic materials are delivered via a transcatheter approach into the LGA, the primary vascular supply to the gastric fundus, with the goal of inducing ischemia (10). The resultant ischemia depresses the production of ghrelin, the only known appetite-stimulating hormone, and suppresses the sensation of hunger (7,8,11,12). Several studies have evaluated the effects of LGA embolization on weight and ghrelin production in both animal models (11-13) and humans (14-18). A systematic review and meta-analysis of the available studies were performed to (i) summarize patient characteristics, embolization techniques, and complications associated with LGA embolization and (ii) determine the amount of weight loss and identify clinical predictors of weight loss associated with LGA embolization.
MATERIALS AND METHODS
This study used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (19).
Search Strategy and Study Selection
Institutional review board approval was not required for this study. A systematic literature search was performed to identify studies of LGA embolization that reported weight changes after the procedure (Appendix A [available online on the article’s Supplemental Material page at www.jvir.org]). PubMed, Embase, Scopus, and Web of Science were searched using the terms “gastric artery,” “bariatric,” and “embolization.” Titles and abstracts of the identified records were screened using the terms “weight” and “BMI.” A total of 47 studies were selected for further evaluation against predetermined inclusion and exclusion criteria.
Original, peer-reviewed studies of human subjects that reported LGA embolization or bariatric embolization as the main procedure of interest were included. To be included, studies had to report weight change (percentages or absolute values) after the procedure. Studies performed for the primary purpose of treating gastrointestinal bleeding or malignancy as well as duplicate studies were excluded. Animal studies, expert opinions, and case reports were also excluded. Of 19 eligible studies, 6 were review articles (no original data), 2 were commentaries, 1 was a case report, and 3 were retrospective reports of patients with gastrointestinal bleeding. This left 7 reports from 6 individual studies for the meta-analysis (Fig 1).
Figure 1.
Flow chart showing study selection. RSNA = Radiological Society of North America.
Data Collection and Data Items
Study data were extracted, including author names, year of publication, study design (prospective vs retrospective), number of patients, indications for embolization, duration of follow-up, and institution where the study was performed. Next, the study populations were analyzed to determine mean patient age, proportion of women versus men, mean baseline weight (kg) and body mass index (BMI) (kg/m2), dietary considerations after the procedure, inclusion of patients with cancer, availability of computed tomography (CT) angiography before the procedure, and treatment setting (inpatient vs emergency department). Baseline levels and changes in ghrelin and leptin were also recorded. Finally, information regarding embolization techniques and complications after the procedure was extracted. Attempts were made to contact the authors for additional information whenever the published report was deemed insufficient.
Study Characteristics
Included studies were published between January 2014 and April 2019. Six nonrandomized prospective trials comprising 47 patients investigating the safety and/or efficacy of LGA embolization for weight loss were included in the meta-analysis (14,16-18,20,21). Two reports were available from the Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) Trial, and data from the most recent report were included in the meta-analysis. Significant variation was observed in the duration of follow-up, which ranged from 3 months to 20–24 months (Table 1).
Table 1.
Characteristics of LGA Embolization Studies
| First Author, Year (Reference) |
Design | No. Patients |
Indication | Follow-up (range), mo |
Institution, Country |
|---|---|---|---|---|---|
| Obesity clinical trials | |||||
| Weiss, 2019 (21) | Prospective | 15 | Severe obesity (BMI 40–60) | 15 (NA) | The Johns Hopkins Hospital, USA |
| Elens, 2019 (20) | Prospective | 11 | Overweight (BMI 25–30) | 6 (NA) | Hospital Erasme, Belgium |
| Pirlet, 2019 (23) | Prospective | 7 | Obesity (BMI > 40) | 12 (NA) | Bács-Kiskun County Hospital, Hungary |
| Bai, 2018 (14) | Prospective | 5 | Obesity (BMI > 30) | 9 (NA) | Southeast University, China |
| Syed, 2016 (17) | Prospective | 4 | Morbid obesity (BMI > 40) | 6 (NA) | Wright State University, USA |
| Kipshidze, 2015 (16) | Prospective | 5 | Obesity† | NA (20-24) | New York Cardiovascular Research, USA |
| GI bleeding studies | |||||
| Takahashi, 2019 (22) | Retrospective | 16 | GI bleeding | 1.5 | Mayo Clinic, USA |
| Kim, 2018 (15) | Retrospective* | 21 | GI bleeding | 12 (2–72) | Mallinckrodt Institute of Radiology, USA |
| Gunn, 2014 (9) | Retrospective | 19 | GI bleeding | 13.6 (NA) | Massachusetts General Hospital, USA |
BMI = body mass index; GI = gastrointestinal; LGA = left gastric artery; NA = not available.
Embolization of additional vessels was performed per individual study protocols.
BMI criteria was not specified, although mean (± SD) BMI was 42 ± 6.8.
Three additional studies were retrospective evaluations of patients who underwent LGA embolization for gastrointestinal bleeding. Findings from these 3 gastrointestinal bleeding studies (9,15,22) were reviewed but were not included in the meta-analysis. The other 5 studies were included, and each reported on 4–7 eligible patients (14,16-18).
Patient Characteristics
No studies included patients < 18 years of age (Table 2). Mean patient ages ranged from 38 years (20) to 48 years (23). Mean patient weight before the procedure ranged from 79 kg (20) to 160 kg (23). Studies varied according to whether they used dietary recommendations, excluded patients with cancer, and used CT angiography to rule out vascular anomalies before embolization. All studies reported a brief hospitalization period after the procedure (1–3 days) except the study by Syed et al (17), in which patients were not admitted to the hospital for observation (Table El [available online on the article’s Supplemental Material page at www.jvir.org]).
Table 2.
Baseline Characteristics of Study Populations and Treatment Variables
| First Author (Reference) |
Age, y | Female, % |
Baseline Weight, kg |
Baseline BMI |
Dietary Consideration |
Cancer Diagnosis |
CT Angiography for Vascular Anomalies |
Setting |
|---|---|---|---|---|---|---|---|---|
| Obesity clinical trials (n = 47 patients included in meta-analysis) | ||||||||
| Weiss (21) | 44 ± 11 | 80 | 139 ± 20 | 45 ± 4.1 | Intake assessment and dietician consultation | None; excluded | Yes | Inpatient (for ≤ 48 h) |
| Elens (20) | 38 ± 10 | 87 | 79 ± 10 | 28.9 ± 2.5 | No | NA | NA | Inpatient (for ≤ 48 h) |
| Pirlet (23) | 48 ± 7 | 0 | 160 ± 27 | 52 ± 8 | No | None; excluded | NA | NA |
| Bai (14) | 42.8 ± 13.9 | 40 | 102 ± 16 | 38.12 ± 3.8 | All followed by dietician | None; excluded | NA | Inpatient (for 3 d) |
| Syed (17) | 41.0 ± 9.2 | 75 | 118 ± NA | 42.4 ± NA | All followed by dietician | None; excluded | Yes | No hospital admission |
| Kipshidze (16) | 44.7 ± 7.4 | 20 | 128 ± 24 | 42.2 ± 6.8 | NA | NA | NA | Inpatient (for 1 d) |
| GI bleeding studies (not included in meta-analysis) | ||||||||
| Takahashi (22) | 57.6 ± 12.9 | 44 | 87.9 ± 12.5 | 30.0 ± 4.3 | NA | None; excluded | Some patients* | NA |
| Kim (15) | 56.3 ± 15.6 | 43 | 93 ± NA | 29.9 ± NA | NA | None; excluded | NA | Inpatient or ED |
| Gunn (9) | 64.6 (46–92)† | 37 | NA | 30.3 (20.3–58.9)† | NA | Yes (11 of 19 patients) | NA | Inpatient |
Note–Age, baseline weight, and baseline BMI are reported as mean ± SD.
BMI = body mass index; ED = emergency department; NA = not available.
Of the 16 patients, 14 had portal/venous phase CT, 1 had CT angiography, and 1 had non-contrast CT.
Expressed as mean (range).
Embolization Procedures
Technical details of the embolization procedure are presented in Table 3. The procedure typically started with obtaining femoral or radial artery access, engaging the celiac trunk, obtaining angiographic imaging of the downstream branches, locating the LGA, inserting a microcatheter into the LGA, and performing embolization of the LGA. After the procedure, patients were monitored for complications. Proton pump inhibitor therapy (for up to 6 weeks after the procedure) with or without endoscopy (immediately after the procedure and 3 days and 3 months after the procedure) was used to monitor for mucosal ulcers in some studies.
Table 3.
Techniques, Follow-up Variables, and Complications of LGA Embolization
| First Author (Reference) |
Embolization Techniques | Follow-up Evaluations and Treatments | Complications |
|---|---|---|---|
| Obesity clinical trials | |||
| Weiss (18,21) | Femoral artery approach, celiac artery digital subtraction angiography, cone-beam CT protocol with modified liver parenchymal blood volume protocol 5-F SOS catheter (AngioDynamics, Inc, Latham, NY) and 2.9-F high-flow microcatheter (Maestro; Merit Medical Systems, Inc, South Jordan, UT) LGA identification followed by nitroglycerin, verapamil, and heparin injection to improve distal penetration of embolic particles (300–500 μm Embosphere microspheres; Merit Medical Systems, Inc) |
Upper endoscopy 2 wk and 3 mo after procedure; gastric motility study at 1 mo; oral omeprazole (40 mg twice daily) and sucralfate (1 g 4 times daily) from 2 wk before to 6 wk after procedure | No major adverse events; 11 minor adverse events in 8 patients, including superficial mucosal ulcers; 1 case of mild gastritis (gastric body) that resolved 3 mo after procedure; delayed gastric emptying study in 1 patient (repeat study was normal at 6-mo follow-up); 1 patient developed transient subclinical pancreatitis (evident by elevated lipase during hospital stay), received supportive care, and was discharged after 48 h (remained asymptomatic during follow-up) |
| Elens (20) | Femoral artery access, insertion of 5-F sheath (Cordis Corp, Miami Lakes, Florida) Cannulation of celiac trunk using 5-F Cobra catheter (Cook, Inc, Bloomington, Indiana) Selective catheterization of LGA using 5-F multipurpose catheter or 5-F Van Schie catheter (Cook, Inc) Coaxial Progreat 2.7-F (Terumo Corp, Tokyo, Japan) microcatheter with guide wire advanced 3–4 cm distal to LGA origin Embosphere microspheres (Merit Medical Systems, Inc) 500–700 μm (300–500 μm in 2 cases) Repeated injections (alternated with contrast agent) followed by Gelfoam (Pfizer, New York, New York) to prevent reflux |
Upper endoscopy after 1–2 mo in 10 patients; pantoprazole 40 mg daily for 1 mo before procedure | One major adverse event: severe pancreatitis with splenic infarct and late gastric perforation (1-mo hospital stay, including intensive care); 1 case of superficial gastric ulceration treated with 40 mg pantoprazole daily for 6 wk |
| Pirlet (23) | Right radial access, Seldinger technique, insertion of 5- to 6FF sheath Cannulation of celiac artery using 5-F right Judkins catheter; 3-F Renegade microcatheter (Boston Scientific, Marlborough, Massachusetts) was used in a difficult case Selective catheterization of LGA over Terumo 0.035-inch guide wire (Terumo Corp) Angiography, infusion of 300–500 μm PVA particles, and confirmation of absence of distal flow |
PPI therapy for 24 h | Transient abdominal pain in 6 patients |
| Bai (14) | Right femoral artery access, Seldinger technique 5-F sheath and 5-F angiographic catheter to engage celiac artery 2.7-F microcatheter Progreat (Terumo Corp) and PVA (500–710 μm), dosed based on flow, for embolization |
Gastric endoscopy on day of procedure and day 3 and day 30 after procedure in case of positive findings; omeprazole prophylaxis (IV for 3 d, oral for 2 wk after procedure) | No severe adverse events (CTCAE grade III or worse); fullness, decreased appetite, mild epigastric discomfort in 4 patients; puncture site hematoma in 1 patient resolved after 22 d; small superficial gastric ulcer in 1 patient at cardia |
| Syed (17) | Ultrasound-guided right femoral or left arterial access followed by angiography of abdominal aorta Celiac artery access using 4-F or 5-F Simmons 1 catheter Coaxial microcatheter in LGA and Bead Block microspheres 300–500 μm (Biocompatibles, Farnham, United Kingdom) injection until complete cessation of flow |
Upper endoscopy before procedure and on day 3 and day 30 after procedure in case of positive findings; PPI 1 wk before to 1 mo after procedure | Transient mild nausea, occasional vomiting, and mild epigastric discomfort immediately after procedure in 3 patients; superficial gastric ulcers on day 3 in 3 patients |
| Kipshidze (16) | Femoral artery access using 6-F catheter and celiac trunk engagement using 6-F Heartrail II JR-4.0 guide catheter (Terumo Europe N.V., Leuven, Belgium) LGA wiring with 0.014-inch Runthrough NS-PTCA guide wire (Terumo Europe N.V.) Excelsior 1018 Microcatheter (Boston Scientific Corp, Cork, Ireland) in mid-LGA and Bead Block microspheres 300–500 μm embolization until distal branches of LGA are no longer visible |
Gastroscopy 1 d and 1 wk after procedure | Mild transient epigastric discomfort after procedure in 3 patients |
| GI bleeding studies | |||
| Takahashi (22) | NA | NA | NA |
| Kim (15) | Femoral artery access, followed by Bentson wire and insertion of 6-F sheath 5-F SOS catheter to engage celiac artery followed by Progreat catheter and gastrostomy wire insertion Gelfoam slurry in 19 patients followed by coil embolization in 6 patients, PVA particle embolization in 5 patients, or combination embolics in 9 patients |
Upper endoscopy examination (in 6 patients); assessed up to 30 d after procedure | Worsening ulcer in 2 patients; jejunal ischemia likely caused by holding anticoagulation in 1 patient; death in 1 patient within 60 d of procedure related to complex medical history, heart failure |
| Gunn (9) | Arterial embolization using coils in 9 patients, Gelfoam in 5 patients, and PVA particles in 5 patients | NA | NA |
CTCAE = Common Terminology Criteria for Adverse Events; IV = intravenous; LGA = left gastric artery; NA = not available; PVA = polyvinyl alcohol; PPI = proton pump inhibitor.
Complications after Procedure
Data were extracted on complications after the procedure, including immediate adverse events and complications that were detected during follow-up examinations. When applicable, complications were classified as major or minor according to the American Society for Metabolic and Bariatric Surgery (24). Follow-up evaluations and treatments were also reviewed.
Summary Measures and Results Synthesis
The 2 primary outcomes of interest were absolute weight change and percentage weight change. Data on weights before and after the procedure were used to calculate mean (and 95% confidence interval) weight change (in kg and %). When only 1 of the 2 primary outcomes was reported, the other outcome was calculated using the baseline weight (assuming similar dispersion and mean ± SD ratios). For studies with follow-up from multiple time points, data from the latest follow-up visit were used for analysis.
Pooled results were calculated by using random-effects models. Per the methods of Borenstein et al (25), random-effects models were used because the authors could not assume that all included studies were identical. There were considerable variations in patient age, sex distribution, and baseline characteristics among the studies. I2 values were calculated and used as a relative measure of true statistical heterogeneity to the observed variation. The standard error of the mean was calculated for each study. Standardized mean differences were calculated and pooled. Because baseline values and values following the procedure may not be independent of each other, consistency of pooled estimates was confirmed with multiple pre-post correlation values, following Follmann et al (26). Mean difference in weight loss after the procedure was used as the main outcome of interest.
Sensitivity Analysis.
To assess the robustness of the findings, sensitivity analysis was conducted by excluding each study from the pooled results and recalculating the pooled results. Similarly, the analysis was repeated by excluding studies with < 12 months of follow-up. Finally, multiple meta-regression analyses were used to investigate age, sex, weight, BMI, and ghrelin and leptin levels as predictors of weight loss. Because the purpose of the meta-regression was to identify potential determinants of weight loss after LGA embolization, only obesity-related clinical trials were used for meta-regression. Data regarding ghrelin and leptin were available for 4 and 3 of the obesity-related trials, respectively.
Risk of Bias in Individual Studies
Risk of bias in included studies was assessed by using the Methodological Index for Non-Randomized Studies instrument (27). Two authors (N.H.N., C.R.B.) assessed the potential risk of bias, scoring each item as 0 if not reported, 1 if reported but inadequate, and 2 if reported and adequate. Discrepancies were discussed, and consensus scores were used. Methodological Index for Non-Randomized Studies consists of 8 items: (a) having a clearly stated aim; (b) including consecutive patients; (c) collecting data prospectively; (d) having appropriate endpoints with regard to the aim of the study; (e) using unbiased assessment of the endpoints; (f) having an appropriate follow-up period; (g) having < 5% loss to follow-up; and (h) calculating the sample size prospectively. The global ideal score was 16, and studies with total scores of ≤ 12 were considered to have a high risk of bias.
Publication Bias.
Potential publication bias was assessed using a quantitative approach to calculate the number of nonsignificant studies that could nullify the observed effect (i.e., classic fail-safe number). Next, effect sizes of the included studies were reviewed and plotted against their standard errors, and Duval and Tweedie’s trim-and-fill method was used to identify significant changes in the pooled results (28).
All analyses were performed using Stata 13.0 (StataCorp LLC, College Station, Texas) or CMA Version 2.0 (Biostat, Englewood, New Jersey). P values < .05 were considered significant.
RESULTS
Weight Loss after Embolization
Pooled absolute mean (SE) weight loss was 8.85 kg ± 1.24 kg, ranging from 7.62 kg (21) to 22.00 kg (16) (Table 4). Pooled percentage of mean weight loss was 8.11% ± 1.46%, ranging from 4.77% (23) to 17.19% (16) (Fig 2). The mean duration of follow-up for the participants included in the final analysis was 12 months (Table 1). No significant statistical heterogeneity was detected (I2 = 12.35) among pooled studies.
Table 4.
Pooled Analysis of Weight Loss after Bariatric LGA Embolization
| First Author (Reference) |
Absolute Weight Loss |
Percentage Weight Loss |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean, kg | 95% CI Lower Limit |
95% CI Upper Limit |
SE | Z | P | Mean, % | 95% CI Lower Limit |
95% CI Upper Limit |
SE | Z | P | |
| Weiss (21) | 7.62 | 4.16 | 11.08 | 1.76 | 4.32 | < .001 | 5.96 | 3.30 | 8.62 | 1.36 | 4.40 | < .001 |
| Elens (20) | 8.00 | 4.94 | 11.03 | 1.54 | 5.18 | < .001 | 10.00 | 6.22 | 13.78 | 1.93 | 5.18 | < .001 |
| Pirlet (23) | 13.00 | 0.41 | 25.60 | 6.42 | 2.02 | .043 | 4.77 | 0.15 | 9.39 | 2.36 | 2.02 | .043 |
| Bai (14) | 12.90 | 0.05 | 25.75 | 6.56 | 1.97 | .049 | 12.64 | 0.04 | 25.24 | 6.43 | 1.97 | .049 |
| Syed (17) | 9.19 | 2.38 | 16.04 | 3.47 | 2.64 | .008 | 8.52 | 0.93 | 16.10 | 3.87 | 2.20 | .028 |
| Kipshidze (16) | 22.00 | 9.36 | 34.64 | 6.45 | 3.41 | .001 | 17.19 | 7.31 | 27.07 | 5.04 | 3.41 | .001 |
| Pooled result | 8.85 | 6.43 | 11.28 | 1.24 | 7.15 | < .001 | 8.11 | 5.23 | 10.98 | 1.46 | 5.53 | < .001 |
CI = confidence interval; LGA = left gastric artery.
Figure 2.
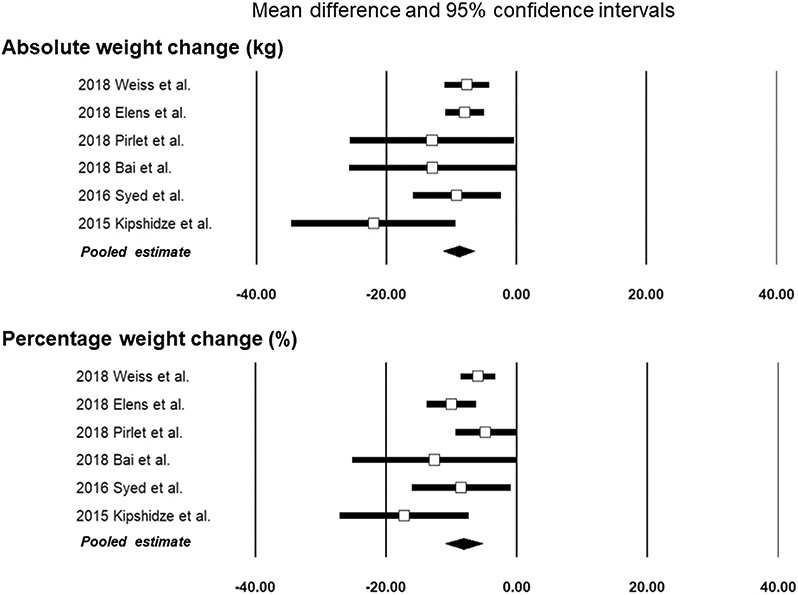
Forest plots showing effect sizes and the pooled results.
Clinical Variables Associated with Weight Loss
Male sex was associated with absolute weight loss (β = 11.36 ± 5.79; P = .049). Other clinical variables, including age, weight, and BMI, were not significantly associated with absolute weight loss (Table 5). Likewise, no statistically significant association was found between baseline ghrelin (β = 0.01 ± 0.01; P = .297) or leptin (β = 0.14 ± 0.12; P = .229) and weight loss after procedure.
Table 5.
Associations of Weight Loss with Patient Factors
| Patient Factor |
β (Regression Slope) |
95% CI Lower Limit |
95% CI Upper Limit |
P Value |
|---|---|---|---|---|
| Age, y | 0.23 ± 0.35 | −0.45 | 0.92 | .502 |
| Male sex, % | 11.36 ± 5.79 | 0.01 | 22.72 | .049 |
| Weight, kg | 0.01 ± 0.04 | −0.06 | 0.08 | .731 |
| BMI, kg/m2 | 0.06 ± 0.13 | −0.20 | 0.32 | .421 |
BMI = body mass index; CI = confidence interval.
Embolization Techniques and Complications
Embolization techniques, follow-up evaluations and treatments, and reported complications are summarized in Table 3. Despite variations, major and minor events were used in similar fashion across the included studies. Elens et al (20) defined major complications as adverse events leading to longer hospital stay or intensive care unit admission. Minor complications were complications that were medically managed after discharge. Bai et al (14) used the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0), with grade III or above considered major complications (14). Weiss et al (18) used the American Society for Metabolic and Bariatric Surgery recommendations. Syed et al (17) used the Society of Interventional Radiology (SIR) guidelines to categorize procedure-related complications (29). Per the SIR Standards of Practice Committee recommendation for classification of complications, major complications included events requiring therapy with hospitalization or resulting in permanent sequelae or death (29).
The most common adverse events were transient abdominal pain and superficial gastric ulcers. Because of variations in the endoscopy protocols, availability of the results, and reports of superficial gastritis, no pooled analysis of adverse events was performed. Superficial gastritis was observed in 8 of 20 patients in the study by Weiss et al (21), 1 of 16 patients in the study by Elens et al (20), 1 of 5 patients in the study by Bai et al (14), and 3 of 4 patients in the study by Syed et al (17). In addition to superficial gastric ulcers, other minor complications included transient subclinical pancreatitis (18,21) and hematoma at the puncture site (14).
Major adverse events were reported in 1 of the patients in the series by Elens et al (20), including severe pancreatitis, splenic infarction, and late gastric perforation. The patient had a total length of hospital stay, including intensive care unit stay, of 1 month. Other obesity clinical trials reported no major complications. Kim et al (15) reported 1 death within 60 days after the procedure, which was unrelated to the procedure.
Sensitivity Analysis
The effects on the pooled weight change data were evaluated by excluding each study in turn. The greatest change (8.24 kg ± 1.07 kg) occurred when excluding the study by Kipshidze et al (16), but this was not significantly different from the pooled result. Exclusion of studies with < 12 months of follow-up produced no statistically significant differences in the pooled results (Fig 3 and Table E2 [available online on the article’s Supplemental Material page at www.jvir.org]).
Figure 3.
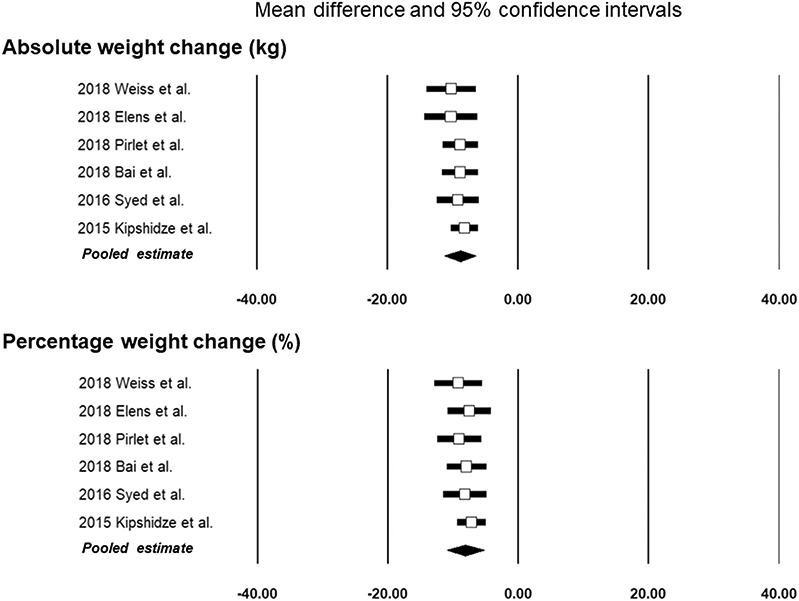
Forest plots of the pooled results for absolute and percentage weight change after excluding each individual study.
Risk of Bias in Individual Studies
The individual scores using the Methodological Index for Non-Randomized Studies tool are summarized in Table E3 (available online on the article’s Supplemental Material page at www.jvir.org). Only 2 of the 6 included studies had total scores > 12 and were considered to be at low risk of bias. Absence of prospective sample size calculation and loss to follow-up of > 5% were the most common sources of bias.
Publication Bias
The vulnerability of results to publication bias was investigated by calculating the classic fail-safe number of studies that could potentially nullify this study’s findings. Given the Z values of the included studies, number of included studies (n = 6), and type I error of .05, 94 additional studies (with a reported effect size of 0) would be required to nullify the findings. Thus, the pooled results are unlikely to be nullified by several unpublished studies with nonsignificant findings. When using the fill-and-trim method to confirm the robustness of the pooled results, the addition of presumed missing studies to the right of the calculated average effect did not produce a statistically significant change in the pooled result (adjusted pooled estimate of 8.05 kg [95% confidence interval 4.99–11.11]).
DISCUSSION
Data on weight change after LGA embolization in 47 patients from 6 studies were collected, and mean weight loss was 8.68 kg ± 1.24 kg (P < .001) after a mean 12 months of follow-up. Men were more likely than women to lose weight after the procedure. This is the first systematic review and meta-analysis elucidating patient characteristics, techniques, and complications related to LGA embolization and pooling the available evidence from bariatric trials to quantify the amount of weight loss that can be expected after LGA embolization.
Weight loss of approximately 8–9 kg (approximately 8% of baseline total body weight) was achieved after a mean follow-up of 12 months (range, 3–24 months), which is superior to the weight loss expected from diet and exercise programs but comparable to other, more invasive interventions. Diet and exercise programs frequently aim for weight loss of 5%–10% after 12 months (30,31). However, evidence suggests that most patients in diet and exercise programs lose approximately ≤ 5% (5 kg) of their baseline body weight (32-36). Significantly less of the initial weight loss is maintained during longer follow-up periods (37-39). In a meta-analysis by Franz et al (38), 80 weight loss–focused randomized trials were reviewed and showed that, on average, 3%–6% (approximately 3–6 kg) of weight loss is maintained after 48 months. From a procediual standpoint, the intragastric balloon device can help patients achieve weight loss of approximately 10%–20% of their baseline weight during short-term follow-up of 3–6 months (40-42). However, up to 50% of patients may be unable to maintain their weight at 12- to 18-month follow-up (40), and approximately 63% may be unsatisfied with the procedure and unwilling to undergo it again (41).
Bariatric surgery is typically reserved for patients with morbid obesity (2,6) and is associated with substantial risk of perioperative death. In a meta-analysis, Buchwald et al (43) pooled the results of 22,094 patients and showed excess body weight loss of 62%–70% after various bariatric surgical procedures. The 30-day mortality rate was 1.1% after biliopancreatic diversion. From a clinical standpoint, weight loss was associated with complete resolution of diabetes, hypertension, hyperlipidemia, and obstructive sleep apnea in 62%–86% of patients (43). The current study shows that bariatric LGA embolization is an effective treatment that is associated with statistically significant weight loss in studies with mean 12-month follow-up. Only 1 case of major complications (severe pancreatitis, splenic infarct, and gastric perforation) after the procedure was reported by Elens et al (20), and the patient was treated with supportive care (20). Given that LGA embolization is an investigative method and is not yet proven to be effective for the management of obesity, it is important for researchers to follow standardized protocols and techniques to avoid unwanted complications. These include detailed technical considerations and stringent inclusion and exclusion criteria. No additional detailed history regarding the patient who developed severe pancreatitis was available. Of note, Elens et al (20) included patients with BMI of 25–35 kg/m2. Of their 16 patients, 11 had a BMI < 30 kg/m2 (overweight category) (20). In contrast, all US clinical trials of LGA embolization were designed for patients with obesity (BMI > 30 kg/m2; most with BMI > 40 kg/m2), resulting in a different risk-benefit balance (16,17,21). All patients with a history of pancreatitis were excluded from the included trials. Risk of gastric perforation, prior gastrointestinal bleeding, and related complications were assessed before inclusion (14,18,20,21,23). From a technical standpoint, the volume of injected microspheres should be closely monitored; Elens et al (20) restricted volume to < 2 mL. Efforts should be made to prevent reflux and avoid potentially related complications, including pancreatitis and splenic infarct. An antireflux device (Surefire Medical, Inc, West-minster, Colorado) was used in select cases by Weiss et al (18,21). Elens et al (20) used Gelfoam (Pfizer, New York, New York) as a liquid suspension mixed with contrast agent in a 3-mL syringe. Finally, prospectively designed randomized clinical trials are needed to determine the efficacy of such considerations in preventing adverse events, including pancreatitis and gastric perforations.
The studies reviewed in this meta-analysis were similar in many ways. All studies included patients who were ≥ 18 years of age who underwent gastric artery embolization with or without embolization of additional branches of the celiac trank. The reviewed clinical trials used additional criteria to select appropriate patients. Patients with a history of gastrointestinal surgery, peptic ulcer disease, nonsteroidal anti-inflammatory drag use, major cardiovascular events, psychiatric disorders, or other comorbid conditions (eg, liver or kidney dysfunction) were excluded. Although heterogeneity statistics can be inherently biased in small meta-analyses (44), I2 values did not show significant heterogeneity among the studies included in this report. Before calculating the pooled results, potential underlying causes of heterogeneity were examined, and the initial objectives of the LGA embolization studies were identified. Among the evaluated studies, embolization was performed for weight loss (6 studies) (14,16-18,20,21) or control of gastrointestinal bleeding (3 studies) (9,15,22). Patient characteristics, embolization techniques, and complications of all eligible reports were systematically reviewed. However, because of considerable risk of bias, only clinical trials of bariatric LGA embolizations were included in the meta-analysis. Variations in study design, duration of follow-up, and population characteristics are further explained in Tables E2 and E3 (available online on the article’s Supplemental Material page at www.jvir.org). Significant between-subject variations in the amount of weight loss with regard to follow-up intervals were reported. For example, Weiss et al (21) plotted the amount of excess weight loss over time. Although most of their participants had an upward trend of weight loss over time, significant variations existed, and some patients had partial weight gains (Fig 3). A fitted cubic spline was converged, yielding an approximate excess weight loss of 10% at 12 months of follow-up (R2 = .69). Regarding the size of embolic particles, of the 6 included clinical trials, 4 used 300–500 μm microspheres, and 2 used 500–700 μm microspheres. In the current study, there was no statistically significant difference in weight loss after embolization based on microsphere size. Moreover, in the study by Elens et al (20), 2 patients received 300–500 μm microspheres. In the current study, there was no statistically significant difference in pooled results based on microsphere size. However, this analysis may be underpowered to detect variations among subgroups, and larger studies with patient-level data based on microsphere characteristics are needed for an accurate comparison.
Several technical and statistical considerations with regard to heterogeneity were deliberated while pooling the available data. Availability of weights before and after the procedure was 1 inclusion criterion. In addition to weight change after the procedure, some of the included studies provided more detailed assessment and reporting of their outcomes. For example, Kipshidze et al (16) included assessments of serum ghrelin changes as one of the primary purposes of their study. Bai et al (14) reported ghrelin and leptin measurements immediately before and 3 months after the procedure. They also measured changes in waist circumference and further assessed the amount of abdominal fat and its distribution by using magnetic resonance imaging both before and after embolization. Weiss et al (18) included an extensive series of assessments, including hormonal panels, appetite/satiety assessments, quality-of-life assessments, hemoglobin A1c levels, and lipid panels. They also reported their results as the percentage of excess weight loss by using the Devine formula for ideal body weight calculation (21,45). The studies by Weiss et al (18,21) and Syed et al (17) also evaluated the technical feasibility of LGA embolization as a bariatric procedure (defined as the ability to perform embolization of the gastric fundus in a severely obese patient). Relevant parameters, including fluoroscopy time, radiation dose, procedure time, and contrast agent dose, were reported. Syed et al (17) also measured changes in satiety hormones and quality of life and reported their results as percentage of excess body weight loss. Because of limitations in the consistency and availability of detailed data on patient outcomes, the outcome of interest in the current study was limited to weight change after the procedure. Likewise, despite the well-known roles of ghrelin and leptin in modulation of appetite, weight loss, and response to bariatric surgery (3,4,8,36), no statistically significant conclusion could be drawn with regard to LGA embolization because of limitations in the number of participants and number of studies with available reports of baseline ghrelin and leptin values. Ghrelin acts as an appetite-stimulating hormone (4,7). In contrast, leptin secretion is associated with decreased appetite, increased energy expenditure, and body weight modulation (46). Increasing leptin levels may act as a signal that orchestrates resistance to obesity (47).
This study is subject to the limitations of the included studies, which should be considered when interpreting the results. These include variations in the indications for LGA embolization, study designs, embolization techniques, follow-up plans, dietary assessments, patient comorbidities, and availability of control subjects. For example, the presence of superficial gastric ulcers may act as a modulator of weight loss following the procedure. Nevertheless, individual-level data regarding the incidence of superficial ulcers (and most complications) were unavailable in the literature. Although pooled results were retrievable from the included trials, individual patient-level data would be needed to compare the amount of weight loss in patients with versus without a complication (e.g., superficial gastriculcer). Larger prospective reports with detailed patient-level data describing clinical characteristics, exposures, and complications are needed to expand these findings and determine other correlates of weight loss after LGA embolization. Finally, because the amount of weight loss after bariatric embolization has been shown to vary over time, static measures of mean weight loss may be less accurate than the mean weight loss at particular time intervals. However, because of limitations in the number of available studies and participants, no detailed time-varying analysis was performed.
CONCLUSIONS
LGA embolization was associated with statistically significant weight loss during short-term follow-up. Male sex was associated with greater weight loss after embolization. Transient superficial mucosal ulcers were common after LGA embolization. Major complications were uncommon and included severe pancreatitis, splenic infarct, and gastric perforation.
Supplementary Material
ABBREVIATIONS
- BMI
body mass index
- LGA
left gastric artery
Footnotes
C.R.W. receives research grants from Siemens Healthcare (Erlangen, Germany), Merit Medical Systems, Inc (South Jordan, Utah), Medtronic (Minneapolis, Minnesota), and BTG (London, United Kingdom) and is a paid consultant for BTG and Medtronic. None of the other authors have identified a conflict of interest.
Contributor Information
Nima Hafezi-Nejad, Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, 1800 Orleans Street, Zayed Tower 7203, Baltimore, MD 21287.
Christopher R. Bailey, Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, 1800 Orleans Street, Zayed Tower 7203, Baltimore, MD 21287.
Andrew J. Gunn, Division of Vascular and Interventional Radiology, Department of Radiology, University of Alabama at Birmingham, Birmingham, Alabama.
Clifford R. Weiss, Division of Interventional Radiology, The Johns Hopkins University School of Medicine, 1800 Orleans Street, Zayed Tower 7203, Baltimore, MD 21287.
REFERENCES
- 1.Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology 2007; 132:2253–2271. [DOI] [PubMed] [Google Scholar]
- 3.Beckman LM, Beckman TR, Sibley SD, et al. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr 2011; 35:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346:1623–1630. [DOI] [PubMed] [Google Scholar]
- 5.Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab 2010; 21:337–344. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke RW, Andrus J, Diggs BS, Scholz M, McConnell DB, Deveney CW. Perioperative morbidity associated with bariatric surgery: an academic center experience. Arch Surg 2006; 141:262–268. [DOI] [PubMed] [Google Scholar]
- 7.Paxton BE, Kim CY, Alley CL, et al. Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology 2013; 266:471–479. [DOI] [PubMed] [Google Scholar]
- 8.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365:1597–1604. [DOI] [PubMed] [Google Scholar]
- 9.Gunn AJ, Oklu R. A preliminary observation of weight loss following left gastric artery embolization in humans. J Obes 2014; 2014:185349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madoff DC. Science to practice: can transarterial embolotherapy be used as a viable alternative to treat obesity? Radiology 2013; 266:369–371. [DOI] [PubMed] [Google Scholar]
- 11.Arepally A, Barnett BP, Montgomery E, Patel TH. Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: initial experience. Radiology 2007; 244:138–143. [DOI] [PubMed] [Google Scholar]
- 12.Arepally A, Barnett BP, Patel TH, et al. Catheter-directed gastric artery chemical embolization suppresses systemic ghrelin levels in porcine model. Radiology 2008; 249:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bawudun D, Xing Y, Liu WY, et al. Ghrelin suppression and fat loss after left gastric artery embolization in canine model. Cardiovasc Intervent Radiol 2012; 35:1460–1466. [DOI] [PubMed] [Google Scholar]
- 14.Bai ZB, Qin YL, Deng G, Zhao GF, Zhong BY, Teng GJ. Bariatric embolization of the left gastric arteries for the treatment of obesity: 9-month data in 5 patients. Obes Surg 2018; 28:907–915. [DOI] [PubMed] [Google Scholar]
- 15.Kim DJ, Raman HS, Salter A, et al. Analysis of weight changes after left gastric artery embolization in a cancer-naive population. Diagn Interv Radiol 2018; 24:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kipshidze N, Archvadze A, Bertog S, Leon MB, Sievert H. Endovascular bariatrics: first in humans study of gastric artery embolization for weight loss. JACC Cardiovasc Interv 2015; 8:1641–1644. [DOI] [PubMed] [Google Scholar]
- 17.MI Syed, Morar K, Shaikh A, et al. Gastric artery embolization trial for the lessening of appetite nonsurgically (GET LEAN): six-month preliminary data. J Vase Interv Radiol 2016; 27:1502–1508. [DOI] [PubMed] [Google Scholar]
- 18.Weiss CR, Akinwande O, Paudel K, et al. Clinical safety of bariatric arterial embolization: preliminary results of the BEAT obesity trial. Radiology 2017; 283:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elens S, Roger T, Elens M, et al. Gastric embolization as treatment for overweight patients: efficacy and safety. Cardiovasc Intervent Radiol 2019; 42:513–519. [DOI] [PubMed] [Google Scholar]
- 21.Weiss CR, Abiola GO, Fischman AM, et al. Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) Trial: results at 1 year. Radiology 2019; 291:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi EA, Takahashi N, Reisenauer CJ, Moynagh MR, Misra S. Body composition changes after left gastric artery embolization in overweight and obese individuals. Abdom Radiol (NY) 2019; 44:2627–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirlet C, Ruzsa Z, Costerousse O, et al. Transradial left gastric artery embolization to treat severe obesity: a pilot study. Catheter Cardiovasc Interv 2019; 93:365–370. [DOI] [PubMed] [Google Scholar]
- 24.Bayham BE, Bellanger DE, Hargroder AG, Johnson WD, Greenway FL. Racial differences in weight loss, payment method, and complications following Roux-en-Y gastric bypass and sleeve gastrectomy. Adv Ther 2012; 29:970–978. [DOI] [PubMed] [Google Scholar]
- 25.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1:97–111. [DOI] [PubMed] [Google Scholar]
- 26.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992; 45:769–773. [DOI] [PubMed] [Google Scholar]
- 27.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003; 73:712–716. [DOI] [PubMed] [Google Scholar]
- 28.Weinhandl ED, Duval S. Generalization of trim and fill for application in meta-regression. Res Synth Methods 2012; 3:51–67. [DOI] [PubMed] [Google Scholar]
- 29.Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vase Interv Radiol 2003; 14:S293–S295. [DOI] [PubMed] [Google Scholar]
- 30.Evans EM, Mojtahedi MC, Thorpe MP, Valentine RJ, Kris-Etherton PM, Layman DK. Effects of protein intake and gender on body composition changes: a randomized clinical weight loss trial. Nutr Metab 2012; 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas DM, Ivanescu AE, Martin CK, et al. Predicting successful long-term weight loss from short-term weight-loss outcomes: new insights from a dynamic energy balance model (the POUNDS Lost study). Am J Clin Nutr 2015; 101:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astrup A, Ryan L, Grunwald GK, et al. The role of dietary fat in body fatness: evidence from a preliminary meta-analysis of ad libitum low-fat dietary intervention studies. Br J Nutr 2000; 83(Suppl 1):S25–S32. [DOI] [PubMed] [Google Scholar]
- 33.Batch BC, Goldstein K, Yancy WS Jr, et al. Outcome by gender in the Veterans Health Administration Motivating Overweight/Obese Veterans Everywhere weight management program. J Womens Health (Larchmt) 2018; 27:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courie R, Gaillard M, Lainas P, et al. Weight outcome after 2 years of a diet that excludes six processed foods: exploratory study of the “1,2,3 diet” in a moderately obese population. Diabetes Metab Syndr Obes 2018; 11:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickson M, Macqueen C, Frost G. Evaluation of attendance and weight loss in an intensive weight management clinic compared to standard dietetic care. J Hum Nutr Diet 2009; 22:72–76. [DOI] [PubMed] [Google Scholar]
- 36.Messier V, Hayek J, Karelis AD, et al. Anthropometric, metabolic, psychosocial and dietary factors associated with dropout in overweight and obese postmenopausal women engaged in a 6-month weight loss programme: a MONET study. Br J Nutr 2010; 103:1230–1235. [DOI] [PubMed] [Google Scholar]
- 37.Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol 2013; 28(Suppl 4):59–63. [DOI] [PubMed] [Google Scholar]
- 38.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007; 107:1755–1767. [DOI] [PubMed] [Google Scholar]
- 39.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obesity Relat Metab Disord 1997; 21:941–947. [DOI] [PubMed] [Google Scholar]
- 40.Dogan UB, Gumurdulu Y, Akin MS, Yalaki S. Five percent weight lost in the first month of intragastric balloon treatment may be a predictor for long-term weight maintenance. Obes Surg 2013; 23:892–896. [DOI] [PubMed] [Google Scholar]
- 41.Palmisano S, Silvestri M, Melchioretto B, et al. Intragastric balloon device: weight loss and satisfaction degree. Obes Surg 2016; 26:2131–2137. [DOI] [PubMed] [Google Scholar]
- 42.Peker Y, Durak E, Ozgurbuz U. Intragastric balloon treatment for obesity: prospective single-center study findings. Obes Facts 2010; 3:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 44.von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol 2015; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm 1974; 8:650–655. [Google Scholar]
- 46.Mechanick JI, Zhao S, Garvey WT. Leptin, an adipokine with central importance in the global obesity problem. Global Heart 2018; 13:113–127. [DOI] [PubMed] [Google Scholar]
- 47.Flier JS, Maratos-Flier E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab 2017; 26:24–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.