Abstract
Cardiovascular and muscle responses to L-glutamic acid (Glut) and cholinergic agonists injected into the dorsolateral pontine tegmentum and medial medullary reticular formation (MMRF) were examined in unanesthetized, decerebrated cats. Glut, or cholinergic agonists acetylcholine (ACh) or carbachol (Carb), were injected into pons and MMRF at sites from which electrical stimulation produced bilateral suppression of muscle tone. Glut injection in MMRF produced hypotension without change in heart rate at doses as low as 1 mM. At higher doses (0.1—0.4 M), Glut induced hypotension with bradycardia in 23 out of 40 injections in both pons and MMRF. High concentrations of microinjected Glut decreased muscle tone or produced complete atonia in pons and rostral MMRF. Both N-methyl-D-aspartic acid (NMDA) and non-NMDA receptor blockers attenuated or completely blocked the cardiovascular response, while only non-NMDA antagonists blocked muscle inhibition to Glut injection. Microinjection of cholinergic agonists produced consistent hypotension in all of the injections in pons and MMRF, however, the heart rate response was variable with increase (27/42), decrease (2/42), or no change (13/42) in rate seen. Cholinergic injection produced muscle atonia in pons and caudal MMRF but not in rostral MMRF. Both muscle and cardiovascular responses were blocked by atropine but not by hexamethonium. The time course of muscle atonia and cardiovascular change differed in most of the experiments. We conclude that muscle tone suppression and cardiovascular response to Glut or cholinergic agonists use different receptor mechanisms and possibly different neurons. However, the co-localization of these mechanisms suggests that neuronal networks in the medial medulla and dorsolateral pons coordinate motor and cardiovascular responses.
Keywords: Muscle tone, Blood pressure, Heart rate, Decerebration, Cataplexy, Narcolepsy, Rapid eye movement sleep
INTRODUCTION
Electrical stimulation studies have produced the concept of a ‘mediocaudal depressor center’ and a ‘laterocranial pressor center’ in the medulla. The gross localization of the cardiovascular center to the reticular formation (RF) was first described by Wang and Ranson41, who found that stimulation of the periventricular gray and the lateral portion of the reticular formation increases, while stimulation of the midline and ventral portion of the RF lowers blood pressure. Combining electrical stimulation and transection techniques, Alexander2 identified a depressor area in the caudomedial and a pressor area in the rostrolateral portion of the medullary RF. Similar results were also found in the dog13,29, sheep4, and rat28
The medial medullary reticular formation (MMRF) has also been found to play an important role in controlling muscle activity. Electrical stimulation of MMRF produces global inhibition of muscle tone31. In the intact animal, a similar inhibition of muscle tone occurs in REM sleep. Cataplectic attacks in narcolepsy represent an abnormal activation of this atonia mechanism in waking33,35
In previous studies we have found that blood pressure determines the motor response to medullary stimulation; reductions in blood pressure caused medullary stimuli, previously suppressed muscle tone, to increase tone26 Cataplectic attacks in narcoleptics are preceded by a marked increase in heart rate and can be triggered by blood pressure increases36. Thus, the anatomical and physiological relations of brainstem mechanisms mediating cardiovascular and motor control are of considerable interest.
In a prior series of microinjection studies, we reported that the medial medulla contains two distinct zones producing muscle atonia25. A rostral zone corresponding to the nucleus magnocellularis (NMC) is sensitive to L-glutamate (Glut) but not to acetylcholine (ACh), while a caudal zone corresponding to the nucleus paramedianus (NPM) is sensitive to ACh but not Glut. We also reported that both glutamatergic and cholinergic stimulation produced a suppression of muscle tone when applied to the dorsolateral pontine region. The present study was designed to investigate the role of Glut and ACh in the control of blood pressure in the medial medulla and pons, and to determine the relation of the cardiovascular and motor responses elicited by these chemicals.
MATERIALS AND METHODS
Experiments were performed on 41 unanesthetized, decerebrated cats, of either sex, weighing 2.0–4.5 kg, The animals were anesthetized with a halothane–oxygen mixture. The trachea and the femoral artery and vein were cannulated and both common carotid arteries were ligated. The parietal bone and tentorium were removed. Decerebration was made at postmammillary, precollicular level. The anesthesia was discontinued after decerebration. Neck muscles were dissected and implanted with bipolar electrodes for electromyogram (EMG) recording. The blood pressure (BP) was recorded from the femoral artery through a Gould Statham pressure transducer. Rectal temperature was maintained at 38 ± 1 oc with a thermostatically controlled heating pad.
A 26-gauge I-μl Hamilton microsyringe was used for injections. The injection sites were between P 2‒4, L 2‒3, and H from ‒4 to ‒6 in the pontine, and P 8‒15, L 0‒2.5, H from ‒6 to ‒9 in the medullary RF according to the Berman7 atlas. Except for 3 injections in rostral dorsolateral medulla, all injection sites were points at which electrical stimulation (a 500 ms train with 100 Hz, 0.2 ms and 20‒70 μA rectangular cathodal pulses delivered through a stainless steel monopolar microelectrode with a tip size of 0.25 mm) had been found to produce bilateral inhibition of the muscle tone. After location of a point at which electrical stimulation produced suppression of muscle tone, the stimulation electrode was withdrawn and a microsyringe lowered to the same point. One half μl of fluid was injected over 1 min and the syringe was retained in place for an additional 5 min after injection. EMG, BP, and cardiotachometer outputs were recorded polygraphically. For the receptor mechanism studies, antagonist was microinjected into the same brainstem area 5 min before the agonist. In some cases, atropine was injected systemically 20 min before the agonist. The interval between any two studies was at least 90 min. The location of the injection sites was marked with iron by passing 50 μA for 20 s through the microelectrode after the injection studies. At the end of the experiments, cats were perfused transcardially with saline followed by 10% formalin solution. The brains were removed and stored in 30% sucrose-formalin solution. The brains were sectioned at 40 μm in the frontal plane, stained with Neutral red and counterstained with potassium ferrocyanide. The locations of injection sites were reconstructed according to Berman7
Chemicals were dissolved in 0.9% Ringer’s saline solution and adjusted to a pH of 7.0. The following concentrations were used: 1 mM‒0.4 M of Glut, 10 mM of τ-D-glutamylglycine (DGG), 0.2 M of L-glutamic acid diethyl ester (GDEE), 50 mM of DL-2-amino5-phosphonovaleric acid (APV), 0.01‒1.0 M of ACh, 7.0 mM‒0.7 M of atropine for local microinjection, and 1 mg/kg for intravenous injection, and 6.2 mM‒0.62 M of hexamethonium (HMT).
The change of BP and HR induced by Glut or cholinergic agonist injection was calculated as a percentage of baseline values. We used a 5% change as our response criterion. The latency corresponds to the time it took from the start of the injection until the magnitude of the HR or BP change reached 5% of baseline. The average initial mean arterial pressure (MAP) in the decerebrate cats was 134.6 ± 17.9 mm Hg (n = 41, range from 105‒169 mm Hg), Average initial heart rate was 202.8 ± 32.0 g/min (n = 41, range from 144‒252 b/min).
RESULTS
Cardiovascular response to Glut injection in the medulla
Glut produced hypotension at most injection sites within the area between 0.5 and 1.5 mm from the midline (Fig. 1, right). However, the interval between two injections in the same site was a critical factor determining the cardiovascular response to Glut injection. We found that a second injection had no effect if administered within 20 min. The response completely recovered after 50 min had elapsed. Therefore, at least a 90 min interval between agonist injections was used in the present study.
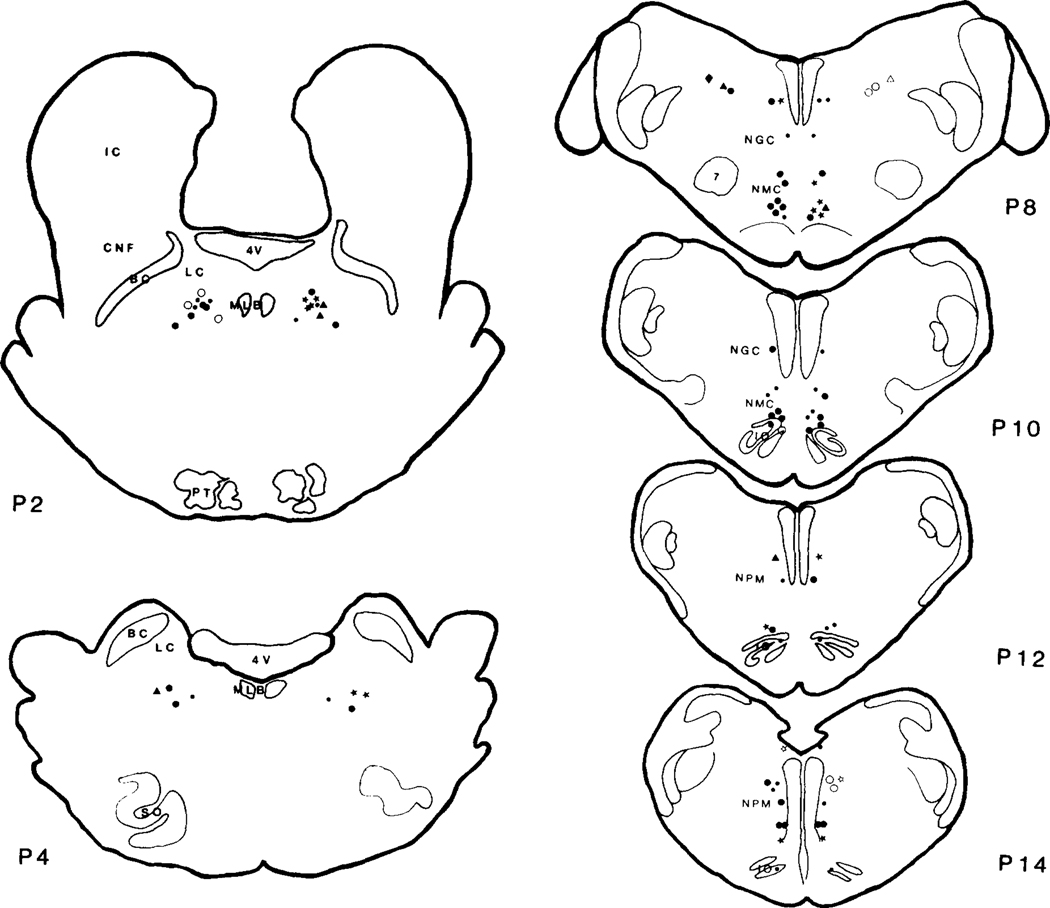
Fig. 1.
Sites of L-glutamic acid (Glut) injection in the pons (P2‒P4) and medulla (P8‒P14). 0.2 M/0.5 μl of Glut injection in the brainstem produced either cardiovascular change (≥5%) or no effect (<5%). The symbols in the left and right side represent cardiac and blood pressure responses to Glut, respectively. The open and closed symbols represent increase and decrease of cardiovascular variables, respectively. For details see text. •: <5%, ●,○: ≥5%; ★,☆: ≥15%; ▲,△: ≥25%; ◼,◻: ≥35%; ◆,◇: ≥45%; 4V: fourth ventricle; 7: facial nucleus; BC: brachium conjunctivum; CNF: nucleus cuneiformis; IC: nucleus inferior colliculus; IO: inferior olive; LC: nucleus locus coeruleus; MLB: medial longitudinal bundle; NGC: nucleus gigantocellularis; NMC: nucleus magnocellularis; NPM: nucleus paramedianus; PT: pyramidal tract; SO: superior olive.
The magnitude of the BP response to Glut was greater in the rostral than in the caudal medulla. The transition zone between the rostral gigantocellularis-magnocellularis and the caudal NPM was the least effective area. Throughout the medial medulla, the ventral regions were more effective than the dorsal regions. In rostro-ventral medulla, corresponding to the rostral part of NMC, Glut at dosages as low as 1 mM produced decreases of MAP.
At a dose of 1 mM, the average decrease was 8.3 ± 1.2% (10.0 ± 5.6 mm Hg, P < 0.001, Student t-test) and ranged from 7 to 10% (6‒15 mm Hg, n = 4). HR did not significantly change at this dose (range from 96 to 104% of baseline, n = 4). Control Ringer’s injection did not elicit any BP or HR response (n = 24, 96–103% of baseline readings). However, BP response to high Glut doses (0.1–0.4 M) was not as consistent as the response to low-dose injection. At high dose levels, decreased BP was found in 14 out of 27 experiments (10.0 ± 2.2%, 12.6 ± 3.6 mm Hg) in rostral NMC and caudal-ventral NPM. Of the remaining 13 sites, 10 sites were found not to produce a change and 3 sites in dorsomedial NPM produced an increase of BP (12.3 ± 4.8%, 13.0 ± 5.4 mm Hg). HR response to high-dose injections of Glut was also variable, with decrease in 16 out of 27 experiments (13.2 ± 10.2%, 27.3 ± 23.8 b/min), increase in one injection (30%, 49 b/min), and no change in the remaining 10 injections.
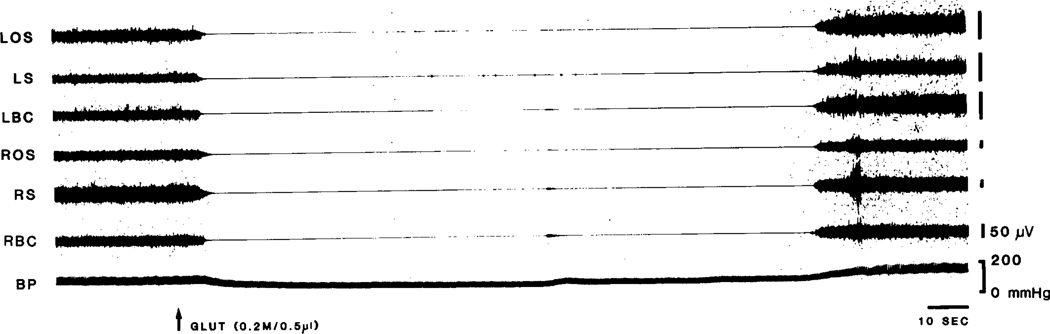
While this study was aimed at determining the cardiovascular responses at brainstem sites at which electrical stimulation produced muscle tone suppression, in order to compare our technique to previous studies of the pressor region in the dorsolateral portions of the rostral medulla, we performed 3 injections in this zone. Glut injection produced hypertension (12.3 ± 4.8%, P < 0.05, Student t-test), bradycardia (26.3 ± 8.2%, P < 0.01, Student t-test) and unilateral increase of muscle tone (Fig. 2).
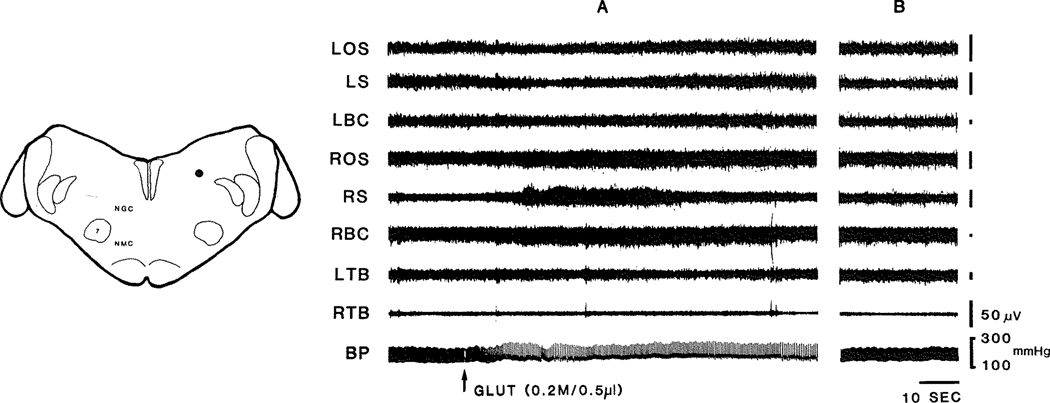
Fig. 2.
Glut (0.2 M/O.5 μl) injection into rostral dorsolateral medulla produced hypertension with bradycardia. The latency of cardiovascular response to Glut is short (8 s). Facilitation of muscle tone was also found after the injection. The dot in the left figure represents the injection site. A: cardiovascular and muscle responses to Glut injection; B: cardiovascular and muscle changes return to baseline after the injection (B is 12 min after A). BP: blood pressure; Glut: L-glutamic acid; LOS: left occipitoscapularis; LS: left splenius; LBC: left biventer cervicis; ROS: right occipitoscapularis; RS: right scapularis; RBC: right biventer cervicis; LTB, left triceps brachii; RTB, right triceps brachii.
The effect of Glut (O. 1‒0.2 M) microinjection in the medial medulla on HR slowing was a function of baseline HR but not BP. The higher the baseline HR level, the lower the percentage and the absolute magnitude of the decrease. For baselines below and above 230 b/min, the percentage of decrease of HR was 14.9 ± 7.0% (25.3 ± 16.2 b/min, n = 15) and 2.6 ± 3.5% (6.5 ± 8.9 b/min, n = 12), respectively. This difference was significant (P < 0.001, Student t-test).
Cardiovascular response to Glut injection in dorsolateral pons
Glut injection into the dorsolateral pons (0.1‒0.2 M), corresponding to dorsolateral regions of nucleus pontis centralis oralis or the region including peri-locus coeruleus alpha (peri-LCα33), produced hypotension in 11 of 14 experiments (P < 0.03 — sign test, Fig. 1). The remaining 3 injections were found to have no effect on blood pressure (range, 98–101% of baseline). The average reduction was 23.1 ± 10.6 mm Hg (18 ± 7.1%) and ranged from 9 to 41 mm Hg (8–28%). However, the heart rate response was not consistent, with bradycardia (7/14, decrease of 11.4 ± 8.5%, 17.4 ± 12.7 b/min), tachycardia (3/14, increase of 9.7 ± 3.1%; 20.3 ± 7.8 b/min) or no change (4/14) observed.
Effect of Glut antagonists on the cardiovascular response to Glut injection
Glut antagonists, GDD, GDEE, and AP V were microinjected 5 min before Glut injection. All of these 3 antagonists attenuated or completely blocked the Glut-induced hypotension and bradycardia in both pons and medulla (Table I). However, antagonists by themselves produced no significant HR or BP change. These results indicate that Glut-induced hypotension and bradycardia was mediated by both NMDA and non-NMDA receptors.
TABLE I.
Percentage change in cardiovascular variables in response to local injection of Glut and its antagonists in pons and medulla
Percent change is based on control values taken immediately prior to injections. Agonists were injected 5 min after antagonists
| n | Control |
Glut |
Antagonists |
Glut |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MAP | HR | MAP | HR | MAP | HR | MAP | HR | ||
| GDEE | |||||||||
| Pons | 5 | 100 | 100 | 83.4 ± 2.7 | 97.4 ± 5.5 | 102.8 + 2.6 | 101.2 ± 1.3 | 102.2 ± 4.1*** | 102.0 ± 1.3 |
| Medulla | 5 | 100 | 100 | 89.4 ± 4.1 | 94.4 ± 3.4 | 98.8 ± 1.3 | 101.0 ± 1.7 | 99.6 ± 3.9** | 101.8 ± 3.5** |
| DGG | |||||||||
| Pons | 5 | 100 | 100 | 82.2 ± 7.4 | 98.8 + 6.1 | 102.0 + 2.4 | 100.4 ± 2.1 | 100.0 ± 3.6** | 100.2 ± 2.5 |
| Medulla | 6 | 100 | 100 | 90.2 ± 2.4 | 96.2 ± 4.2 | 100.5 + 2.1 | 101.8 ± 2.9 | 99.7 ± 2.2*** | 101.3 ± 2.9* |
| APV | |||||||||
| Pons | 5 | 100 | 100 | 79.6 ± 6.2 | 96.2 ± 4.9 | 101.6+1.9 | 102.2 ± 0.7 | 100.8 + 2.4*** | 102.4 ± 2.2* |
| Medulla | 5 | 100 | 100 | 88.0 ± 3.6 | 94.2 ± 3.7 | 98.6 + 1.4 | 98.6 ± 2.0 | 98.0 ± 3.3** | 97.6 ± 2.4 |
P < 0.05,
P < 0.01,
P < 0.001 comparing cardiovascular changes induced by Glut injection alone with that produced by Glut injection after antagonists.
Cardiovascular response to ACh injection in the medial medulla
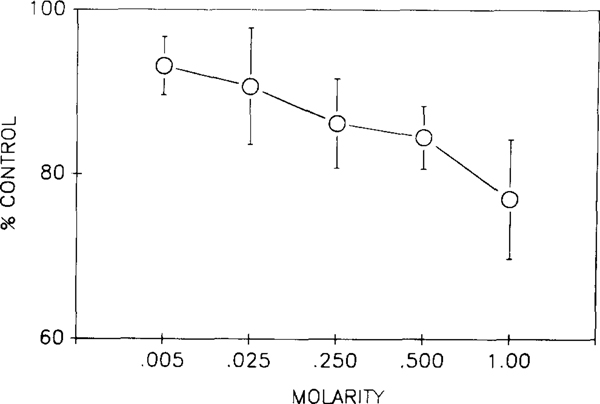
ACh injection into MMRF produced a dose-dependent blood pressure response (Fig. 3). However, the cardiovascular response to low doses (5—25 mM) of ACh injected into the medial medulla was site-dependent. In the rostral ventral medulla corresponding to NMC, ACh produced a decrease of BP (12.7 ± 3.3 mm Hg) without a change of HR in 4/4 of the experiments. These same injections in caudal medulla decreased both BP (16.2 ± 2.9 mm Hg) and HR (26.6 ± 12.7 b/min) in 3/3 of the studies. When the concentration of ACh was increased to 0.3–1.0 M, microinjection in both rostral and caudal MMRF produced a larger decrease in BP than produced by low-dose injections (Fig. 3).
Fig. 3.
Dose-dependent blood pressure response to ACh injection in the medulla. Four experiments were done at each concentration. 0.5 μl ACh solution at each dose was injected into rostro-ventral medulla (NMC). The magnitude of the control baseline was the average of 6 sample points at 30 s interval during the 3 min recording prior to the first injection.
This ACh-induced hypotension was accompanied by an increase (23/34, increase of 17.2 ± 13.0%, 30.7 ± 25.9 b/min), decrease (2/34, decrease of 6.5 ± 0.5%, 17.5 ± 1.5 b/min), or no change (9/34) in HR (Fig. 4). Hypotension in the rostromedial medulla (nucleus gigantocellularis and magnocellularis) was always accompanied by tachycardia after high-dose injection (17/18, P < 0.01 — sign test); however, the caudomedial medulla (NPM) showed hypotension with variable change of HR, with increase (6/16), decrease (2/16), and no change (8/16). As was the case with Glut injection, the initial level of the HR determined the magnitude of the HR change elicited by rostromedial medulla ACh injection. However, the most common HR response to medullary Glut microinjection was bradycardia, while the most common HR response to ACh microinjection was tachycardia. Microinjections occurring when heart rate was relatively high produced a relatively small percentage increase in heart rate. For baselines below and above 230 b/min, the increase of HR was 23.1 ± 14.0% (39.3 ± 23.8 b/min, n = 21) and 7.2 ± 4.5% (18 ± 11.8 b/min, n = 13), respectively (P < 0.001, Student t-test).
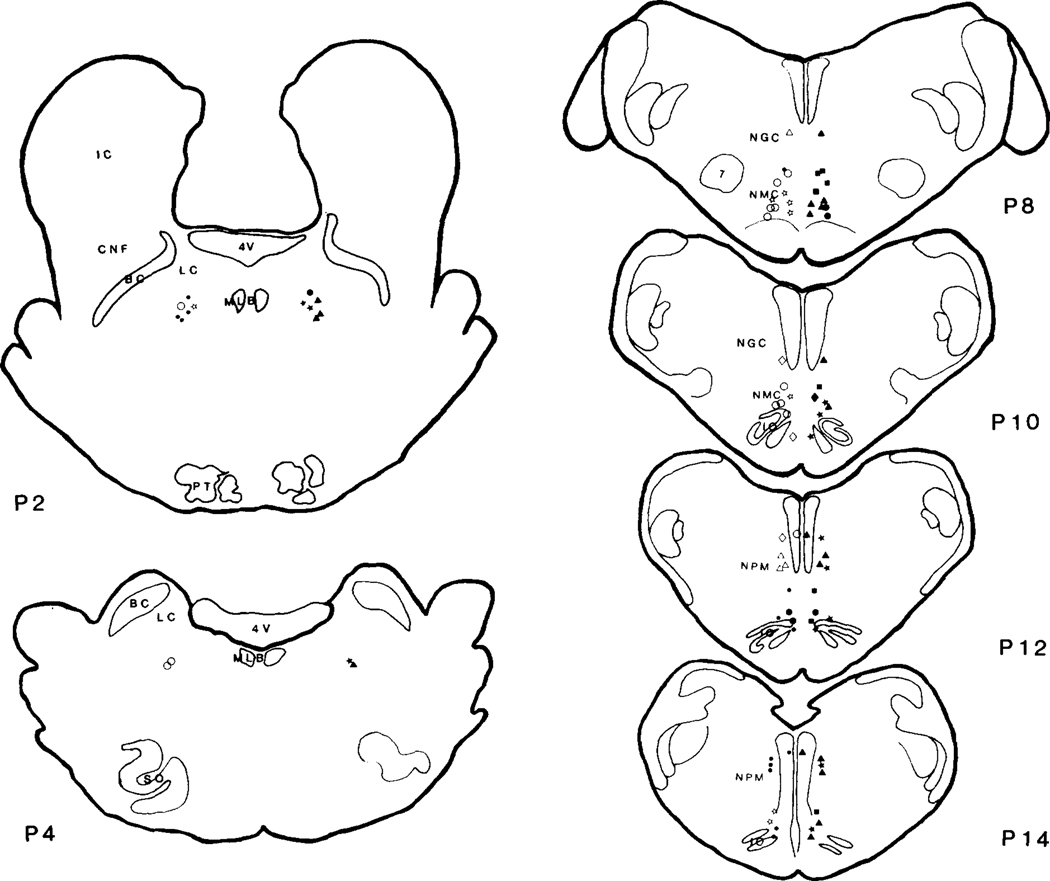
Fig. 4.
Sites of cholinergic agonist injections in the brainstem. The symbols in the left and right side represent cardiac and blood pressure responses to cholinergic injection, respectively. Carbachol was injected in pons only and produced prolonged hypotension with no change of heart rate. ACh injection produced hypotension with variable change of heart rate in most of the experiments. Tachycardia was always found in the pons and the rostral medulla, while tachycardia, bradycardia, or no change were seen in the caudal medulla. See Fig. 1 for the symbols and abbreviations.
Cardiovascular response to ACh injection in the pons
Microinjection of cholinergic agonists, ACh and Carb, into peri-LCα decreased BP in all of the experiments (Fig. 4). However, the response was slightly different to ACh and Carb injection. ACh injection produced consistent hypotension (n = 8, P < 0.004, sign test) with no change (n = 4) or increase (n = 4) in heart rate with a latency of 31.7 ± 18.4 s (range from 10 to 55 s) and duration of 16.6 ± 17.6 min (range from 1.8 to 40 min). On the other hand, hypotension without change of cardiac response could be found after both low (0.4 μg/0.5 μl, n = 5) and high (4 μg/0.5 μl, n = 4) doses of Carb injection. Carb produced prolonged hypotension with a latency of 76 ± 6.0 s (range from 50 to 82 s). Blood pressure gradually decreased and stabilized at 2 h (reaching 62.2 ± 5.3% of the baseline control) after injection and never recovered during the subsequent recording time (≥12 h).
Effect of ACh antagonists on cardiovascular response to ACh injection
Local microinjection or systemic intravenous injection of atropine, a muscarinic antagonist, attenuated or completely blocked hypotension induced by ACh microinjection in both pons and medulla (Table II). Atropine produced a non-significant attenuation of ACh-induced tachycardia (Table II). The antagonist effect of local application of atropine was found to be dose-dependent (Table III). Atropine injection by itself produced hypertension without change of HR at a dose of 0.7 M (Table III). This atropine-induced hypertension lasted for 3 min and was followed by a return to baseline. However, microinjection of nicotinic antagonist, MHT, did not block ACh-induced cardiovascular response even with doses as high as 0.62 M (Tables II and IV). These results indicate that the cholinergic-induced cardiovascular change is mediated by muscarinic receptors.
TABLE II.
Percentage change in cardiovascular variables in response to A Ch and to A Ch after antagonist injections
Percent change is based on control values taken immediately prior to injections.
| n | Control |
ACh |
Antagonists |
ACh |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MAP | HR | MAP | HR | MAP | HR | MAP | HR | ||
| Atropine | |||||||||
| Pons | 4 | 100 | 100 | 78.0 ± 5.9 | 117.5 + 8.8 | 101.3 ±2.2 | 96.8 ± 1.7 | 90.8 ±2.2** | 106.3 ± 4.3 |
| Medulla | |||||||||
| local (14 mM) | 8 | 100 | 100 | 68.4 ± 13.1 | 111.8 ± 12.8 | 101.4 ±2.7 | 99.1 ±2.0 | 85.5 + 10.2* | 104.1 ±5.2 |
| IV (1 mg/kg) | 3 | 100 | 100 | 74.3 + 6.5 | 114.0 ±5.3 | 101.3 ±3.1 | 98.7 ±1.5 | 92.0 ± 4.4* | 102.3 ± 6.4 |
| HMT (12.4 mM) | |||||||||
| Pons | 4 | 100 | 100 | 83.0 ±5.4 | 112.0 ± 10.2 | 101.0 ±2’.2 | 98.0 ±0.8 | 83.8 + 6.9 | 110.8 ±9.4 |
| Medulla | 3 | 100 | 100 | 61.3 ±3.2 | 111.0 ±12.1 | 100.0 ±4.6 | 101.0 ±3.6 | 64.0 ±3.0 | 109.3 ±10.1 |
P < 0.05,
P < 0.01, comparing cardiovascular changes induced by ACh injection alone with that produced by ACh injection after antagonists.
TABLE III.
Percentage change in cardiovascular variables in response to A Ch and to A Ch after microinjection of different doses of atropine
| Concentration of atropine | n | Control |
ACh |
Atropine |
ACh |
||||
|---|---|---|---|---|---|---|---|---|---|
| MAP | HR | MAP | HR | MAP | HR | MAP | HR | ||
| 0.7 M | 4 | 100 | 100 | 71.3 ± 7.4 | 127.0 ± 23.3 | 109.3 ± 3.3+ | 102.5 ± 6.1 | 98.8 ± 3.2*** | 101.3 ± 1.1 |
| 70.0 mM | 4 | 100 | 100 | 66.5 ± 3.2 | 137.8 ± 15.3 | 101.5 ± 2.3 | 99.8 ± 3.0 | 86.0 ± 6.2** | 113.8 ± 4.5* |
| 14.0 mM | 8 | 100 | 100 | 68.4 ± 13.1 | 111.8 ± 12.8 | 101.4 ± 2.7 | 99.1 ± 2.0 | 85.5 ± 10.2* | 104.1 ± 5.2 |
| 7.0 mM | 4 | 100 | 100 | 77.7 ± 3.8 | 114.0 ± 14.7 | 99.6 ± 1.1 | 100.1 ± 1.3 | 80.0 ± 4.2 | 108.5 ± 6.6 |
P < 0.01 compared with control, Student t-test;
P < 0.05,
P < 0.01,
P < 0.001 comparing cardiovascular changes induced by Ach injection alone with that produced by ACh injection after atropine.
TABLE IV.
Percentage change in cardiovascular variables in response to A Ch and to A Ch after microinjection of different doses of HMT
| Concentration of HMT | n | Control |
A Ch |
HMT |
A Ch |
||||
|---|---|---|---|---|---|---|---|---|---|
| MAP | HR | MAP | HR | MAP | HR | MAP | HR | ||
| 0.62 62.0 mM 12.4mM 6.2 mM |
5 5 3 1 |
100 100 100 100 |
100 100 100 100 |
66.8 ± 2.9 67.0 ± 2.8 63.3 ± 3.1 71.0 |
120.8 ± 15.1 128.4 ± 13.7 111.0 ± 9.9 114.0 |
100.4 ± 3.4 98.0 ± 4.2 100.0 ± 3.7 101.0 |
105.2 ± 6.1 104.0 ± 5.4 101.0 ± 2.9 100.0 |
64.0 ± 5.5 63.8 ± 2.4 64.0 ± 2.4 69.0 |
116.2 ± 13.4 121.8 ± 9.9 109.3 ± 8.3 110.0 |
Relationship between cardiovascular change and muscle activity
As we reported in a previous study25, microinjection of Glut into rostral medulla decreased muscle tone or produced atonia. In the present study, we found that there was no simple relationship between these muscle responses and cardiovascular changes. For example, in the Glut-induced muscle atonia area (NMC), the threshold for EMG change was higher than that for cardiovascular change. At a dose of 0.05 M, only a small change of muscle tone could be seen25. However, the blood pressure was changed even at doses as low as 1 mM. On the other hand, a relatively high dose of Glut (0.1 M) produced a decrease of muscle tone or atonia in the entire area of NMC, while the cardiovascular responses at this dose were variable (Table V) with either no changes (less than 5% change) in HR and BP, decrease in HR but no change in BP, decrease in BP but no change in HR, decrease in BP and increase in HR, or decrease in both HR and BP.
TABLE V.
Pattern ofcardiovascular and muscle response to 0.2 M Glut injection into NMC
| EMG | BP |
HR |
n | ||||
|---|---|---|---|---|---|---|---|
| In- crease |
De- crease |
No change |
In- crease |
De- crease |
No change |
||
| Decreased | X | X | 1 | ||||
| muscle | X | X | 2 | ||||
| activity or | X | X | 3 | ||||
| atonia after | X | X | 1 | ||||
| all injections | X | X | 7 | ||||
| Total | 14 | ||||||
Furthermore, there was no consistent relationship between the latency of cardiovascular and muscle responses. Cardiovascular response (latency = 19.3 ± 12.9 s) changed prior to the muscle tone (latency = 35 ± 20.9 s) in 6 of 13 experiments, while muscle tone changed (latency 12.2 ± 7.4 s) prior to the cardiovascular response (latency = 28.4 ± 10.4 s) in 5 of 13 experiments. The changes were apparently simultaneous in the 2 remaining experiments. Fig. 5 shows one of these two experiments. In Fig. 5, muscle tone gradually decreased at 2 s after starting the injection, then, complete atonia and cardiovascular change occurred simultaneously (7 s after injection).
Fig. 5.
Simultaneous change of blood pressure and muscle activity induced by Glut injection in the rostral medulla (NMC).
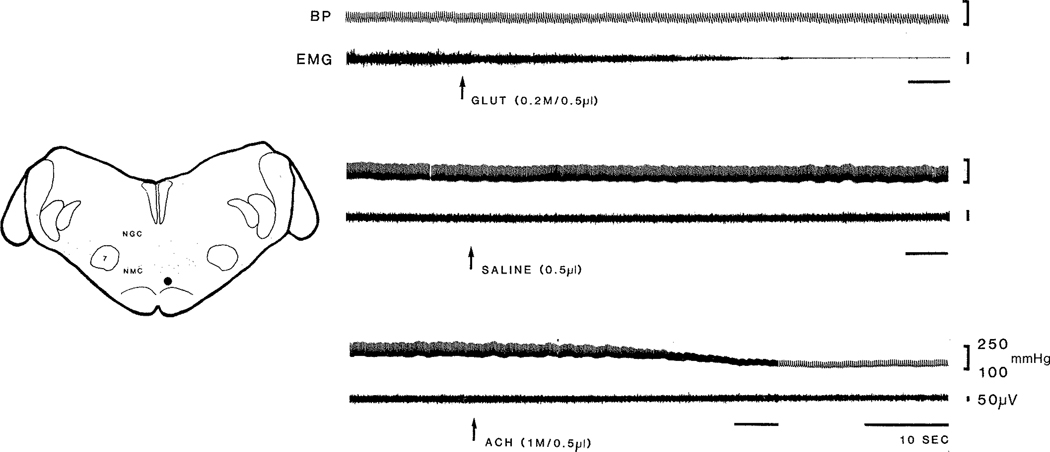
Finally, hypotension was readily produced by ACh injection in NMC, while atonia was never produced. On the other hand, Glut injection in NMC produced atonia without change of cardiovascular activity in one case. Fig. 6 shows a point at which Glut injection in NMC produced atonia without change of cardiovascular activities, but ACh injection in the same site produced hypotension, tachycardia, and no change of muscle activity.
Fig. 6.
Cardiovascular and muscle responses to Glut and ACh injection in NMC. Glut injection produced muscle atonia without change of blood pressure and heart rate. However, ACh injection produced decreased blood pressure and increased heart rate, but no change in muscle activity. Saline vehicle injection did not produce either cardiovascular or muscle change. Note change in paper speed and difference in EMG gain in ACh injection.
In the NPM, high doses of ACh decreased both muscle tone and BP in all of the experiments. The HR response was not consistent (Table VI), with increased, decreased, or unchanged HR accompanying the reduction in muscle tone and BP. The average latency of the cardiovascular response (40.1 ± 16.3 s, 3/16) was slightly longer than that of the muscle tone response (34.0 ± 21.4 s, 13/16).
TABLE VI.
Pattern of cardiovascular and muscle response to I M A Ch injection into NPM
| EMG | BP |
HR |
n | ||||
|---|---|---|---|---|---|---|---|
| In- crease |
De- crease |
No change |
In- crease |
De- crease |
No change |
||
| Decreased | X | X | 6 | ||||
| muscle | X | X | 2 | ||||
| activity or | X | X | 8 | ||||
| atonia after | |||||||
| injections | |||||||
| Total | 16 | ||||||
DISCUSSION
The present studies demonstrate anatomically and neurochemically specific cardiovascular control mechanisms in the medial medullary reticular formation. The predominant response to microinjection of Glut into ventromedial medulla corresponding to NMC and NPM was hypotension associated with bradycardia, while injection into dorsolateral pons corresponding to peri-LCα produced hypotension with variable change of heart rate. DGG, GDEE, and APV attenuated Glut induced cardiovascular change, indicating that both NMDA and non-NMDA receptors mediate the glutamate effect.
ACh injection produced a profound hypotension, with tachycardia in the rostral medulla and without substantial change in HR in caudal medulla. In the peri-LCα region, ACh injection produced hypotension associated with tachycardia, while Carb injection produced prolonged hypotension without change of heart rate. Atropine, but not HMT, abolished the cholinergic-induced cardiovascular responses. Thus, the cholinergic effects are mediated by muscarinic receptors.
Electrical stimulation studies had indicated that the lateral medulla is a pressor area and the medial medulla is a depressor area2,41. However, electrical stimulation activated both fibers of passage and cell bodies. The greater anatomical specificity of effects seen in the present chemical stimulation study can be attributed to a lack of action on fibers of passage. We have used a very wide range of dosages to minimize artifactual effects, such as depolarization blockade or non-specific receptor activation. The major effects reported here occurred in the physiological dosage range, were constant in direction from threshold levels to concentrations one order of magnitude above threshold, and could be blocked by specific antagonists. The 3 comparison Glut injections in rostral dorsolateral medulla in the present study produced hypertension, which confirmed the result of electrical stimulation in cat 12 and Glut microinjection in rabbit 17. Glut injection in NPM produced hypotension, which also confirmed the result of Chai et al11.
Electrical stimulation studies have demonstrated that medial medullary activation produces blood pressure depression and cardiac deceleration in the cat1,12,38 and rat28,30. In the present study, we found that ACh or Glut injection in the medial medulla can produce changes in heart rate and also in blood pressure. We hypothesize that the wide range of the latency and magnitude of cardiovascular responses to Glut or ACh injection is probably due to a clustered distribution of the neurons mediating the cardiovascular effects within the medial medulla. The greater the distance from the injection area to the mediating neurons, the more time is needed to activate the neurons and the lower the magnitude of the effect. Since muscle tone suppression effects are uniform within regions with widely varying magnitudes of HR responses, we hypothesize that the neurons mediating muscle tone suppression have relatively homogeneous distribution within these same regions.
Previous studies of cholinergic effects on cardiovascular response have been contradictory, with pressor, depressor, or biphasic responses being reported. Injection of cholinergic agonists (and cholinesterase inhibitors) into cerebro-ventricular system produces pressor and tachycardiac responses in dogs27,37,39, rats9,22,24,and cats8,16,34. However, carbachol injected intracerebroventricularly or applied to the ventral surface of the medulla produced a reduction of blood pressure in anesthetized cats5,6,18. In the present study, we found that microinjection of ACh directly into the medial medulla of the unanesthetized, decerebrated cat decreased BP. This difference in responses to cholinergic administration may be a function of the route of administrations and anesthetic state of the animals. Previous studies in intact unanesthetized cats have shown similar initial decreases followed by an increase of BP after carbachol injection in the dorsolateral pons, and pressor responses in the dorsal medulla34. However, systemic or ICV injection of cholinergic agonists or cholinesterase inhibitors can be expected to activate both the pressor and the depressor sites outlined above, with the net BP change representing the balance of their actions.
Overall, cardiovascular responses to high-dose Glut or ACh microinjections into MMRF were not as consistent as those to low-dose injections. The effective area for low-dose Glut injection was concentrated in the rostroventral medulla at P8 where injections produced consistent hypotension, with bradycardia at high doses. Similarly, responses to low-dose ACh injections were concentrated in the ventral medulla at P8 and P14, where hypotension with tachycardia at P8 and hypotension with tachycardia or no change of HR at P14 were observed.
The effect of chemical activation of the medio-ventral medulla on cardiovascular variables may be mediated by preganglionic autonomic neurons. Descending projections from nuclei in the medial medulla to the intermediolateral (IML) preganglionic neurons have been demonstrated by neuroanatomical and electrophysiological studies. By using autoradiographic21 or fluorescence20 techniques, neurons in the NMC, NGC, and NPM have been found to project to the IML nuclear complex. Combining both autoradiographic and HRP studies, Zemlan et al. 42 found that the neurons in NMC projected to the ipsilateral IML column in the thoracic and lumbosacral cord in the rat. Similar results were also found in the opossum32, cat3 and pigeon 10 Electrophysiological studies have also demonstrated sympatho- and cardioinhibitory effects derived from medulla stimulation. Electrical stimulation of depressor sites in the medulla reduced the activity of spinal sympathetic neurons23 Electrical stimulation of the medial medulla inhibited sympathetic postganglionic spontaneous discharge10 responses evoked from pressor areas38 and reflex discharge14,15,23 Stimulating the IML at the T2 level, the location of the largest number of cardioacceleratory neurons, and recording field potentials and single unit activity in the medulla, Henry and Calaresu 19 found that projections to the IML derived mainly from NPM, raphe nucleus and nucleus medullae oblongatae centralis subnucleus ventralis.
Although stimulation of many sites within the MMRF produced bilateral inhibition of muscle tone, as well as reduction in BP, our evidence indicates that the depressor response elicited at most sites in the medulla was not secondary to the change in EMG activity. There was no consistent relationship between cardiovascular and EMG change produced by chemical injections, i.e., at many sites only BP or only EMG changes occurred. Furthermore, the muscle atonia area in the medulla is segregated into two distinctive regions, a rostral Glut and a caudal ACh-sensitive area25. However, cardiovacular responses to Glut and ACh injections are intermixed throughout the rostrocaudal medial medulla. Distinct receptor mechanisms are involved in cardiovascular and muscle tone responses. Glut-induced cardiovascular response is mediated through both NMDA and non-NMDA receptors. However only non-NMDA receptors mediate the EMG suppression to glutamate25. Since both EMG and BP responses were elicited from the same sites in certain cases, the neuronal populations mediating these effects must be intermixed and may receive common inputs.
In summary, microinjection of Glut and ACh into regions of medial medulla corresponding to the NMC, NPM, and ventromedial part of NGC, and dorsolateral pons corresponding to peri-LCα, produced hypotension with varying changes in heart rate. Microinjection of Glut and ACh also produced decrease in muscle activity or atonia in peri-LCα as well as rostral and caudal medulla, respectively. Muscle and cardiovascular responses can be elicited independently. However, the co-mingling of neuronal groups mediating cardiovascular and muscle tone changes would provide a basis for the coordination of these effects during postural adjustments and changes in sleep—waking states.
Acknowledgements.
Supported by the Medical Research service of the Veterans Administration and PHS Grants HL41370, NS1640 and MH43811.
REFERENCES
- 1.Achari NK, Downman CBB and Weber WV, A cardioinhibitory pathway in the brain stem of the cat, J. Physiol. (Lond.), 197 (1968) 35 p. [PubMed] [Google Scholar]
- 2.Alexander RS, Tonic and reflex functions of medullary sympathetic cardiovascular centers, J. Neurophysiol, 9 (1946) 205–217. [DOI] [PubMed] [Google Scholar]
- 3.Amendt K, Czachurski J, Dembowsky K. and Seller H, Bulbospinal projections to the intermediolateral cell columns; a neuroanatomical study, J. Auton. Nerv. Syst, 1 (1979) 103–117. [DOI] [PubMed] [Google Scholar]
- 4.Amoroso EC, Bell FR and Rosenberg H, The relationship of the vasomotor and respiratory regions in the medulla oblongata of the sheep, J. Physiol. (Lond.), 126 (1954) 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage AK and Hall GH, Effect of nicotine on the sympathetic blood pressure when injected into the cerebral ventricles of cats, Int. J. Neuropharmacol, 6 (1967) 143–149. [Google Scholar]
- 6.Armitage AK and Hall GH, Further evidence relating to the mode of action of nicotine in the central nervous system, Nature (Lond.), 214 (1967) 977–979. [DOI] [PubMed] [Google Scholar]
- 7.Berman AL, The Brain Stem of the Cat, Univ. Wisconsin Press, Madison, 1968. [Google Scholar]
- 8.Bhargava KP, Jain IP, Saxena AK, Sinha JN and Tangri KK, Central adrenoceptors and cholinoceptors in cardiovascular control, Br. J. Pharmacol, 63 (1978) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brezenoff HE and Jenden DJ, Changes in arterial blood pressure after microinjections of carbachol into the medulla and IVth ventricle of the rat brain, Neuropharmacology, 9 (1970) 341–348. [DOI] [PubMed] [Google Scholar]
- 10.Cabot JB, Wild JM. and Cohen DH, Raphe inhibition of sympathetic preganglionic neurons, Science, 203 (1979) 184–186. [DOI] [PubMed] [Google Scholar]
- 11.Chai c.Y., Lin YF, Lin AMY, Pan c.M., Lee EHY and Kuo JS, Existence of a powerful inhibitory mechanism in the medial region of caudal medulla — with special reference to the paramedian reticular nucleus, Brain Res. Bull, 20 (1988) 515–528. [DOI] [PubMed] [Google Scholar]
- 12.Chai CY and Wang SC, Localization of central cardiovascular control mechanism in lower brain stem of the cat, Am. J. Physiol, 202 (1962) 25–30. [DOI] [PubMed] [Google Scholar]
- 13.Chen MP, Lim RKS, Wang SC and Yi c.L., On the question of myelencephalic sympathetic center. I. The effect of stimulation of the pressor area on visceral function, Chinese J. Physiol, 10 (1936) 445–472. [Google Scholar]
- 14.Coote JH and Downman CBB, Supraspinal control of reflex activity in renal nerves, J. Physiol. (Lond.), 202 (1969) 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coote JH, Downman CBB and Weber WV, Reflex discharges into thoracic white rami elicited by somatic and visceral afferent excitation, J. Physiol. (Lond.), 202 (1969) 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day MD and Roach AG, Cardiovascular effects of carbachol and other cholinomimetics administered into the cerebral ventricles of conscious cats, Clin. Exp. Pharmacol. Physiol, 4 (1977) 431–442. [DOI] [PubMed] [Google Scholar]
- 17.Goodchild AK and Dampney RAL, A vasopressor cell group in the rostral dorsomedial medulla of the rabbit, Brain Research, 360 (1985) 24–32. [DOI] [PubMed] [Google Scholar]
- 18.Guertzenstein PG, Blood pressure effects obtained by drugs applied to the ventral surface of the brain stem, J. Physiol. (Lond.), 229 (1973) 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry JL and Calaresu FR, Excitatory and inhibitory inputs from medullary nuclei projecting to spinal cardioacceleratory neurons in the cat, Exp. Brain Res, 20 (1974) 485–504. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch MD and Helke C,J., Bulbospinal thyrotropin-releasing hormone projections to the intermediolateral cell column: a double fluorescence immunohistochemical-retrograde tracing study in the rat, Neuroscience, 25 (1988) 625–637. [DOI] [PubMed] [Google Scholar]
- 21.Holstege G, Kuypers HGJM and Boer RC, Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord, Brain Research, 171 (1979) 329–333. [DOI] [PubMed] [Google Scholar]
- 22.Inoue IA., Takahashi H, Okajima H, Yoneda S, Sasaki S. Takada K, Yoneda S, Sasaki S, Takeda K, Yoshimura M,Nakagawa M. and Ijichi H, Centrally induced vasopressor and sympathetic nerve responses to carbachol in rats, Jap. Circ. J, 48 (1984) 144–149. [DOI] [PubMed] [Google Scholar]
- 23.Kirchner F, Wyszogrodski I. and Polosa C, Sympathetic inhibition by medulla and spinal cord stimulation, Can. Physiol, 4 (1973) 182. [Google Scholar]
- 24.Krstic MK and Djurkovic D, Cardiovascular response to intracerebroventricular administration of acetylcholine in rats, Neuropharmacology, 17 (1978) 341–347. [DOI] [PubMed] [Google Scholar]
- 25.Lai YY and Siegel JM, Medullary regions mediating atonia, J. Neurosci, 8 (1988) 4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai YY, Siegel JM and Wilson w.J., Effect of blood pressure on medial medulla-induced muscle atonia, Am. J. Physiol, 252 (1987) H1249–H1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang WJ and Rush ML, Cardiovascular responses to injections of cholinomimetic drugs into the cerebral ventricles of unanesthetized dogs, Br. J. Pharmacol, 47 (1973) 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HK, Chai c.Y., Cheng CT, Kao LC and Chung PM, Augmentation of cardiovascular reactivity in the lower brain stem of spontaneously hypertensive rats. In Wang HH, Blumenthal MR and Ngai SH (Eds.), ‘Central control mechanisms and related topics’, Futura Pub., New York, 1980, pp. 137–150. [Google Scholar]
- 29.Lim RKS, Wang SC and Yi CL, On the question of a myelencephalic sympathetic center. VIl. The depressor area as a sympathoinhibitory center, Chinese J. Physiol, 13 (1938) 61–78. [Google Scholar]
- 30.Lin AMY, Lue HM, Lin RH, Wang Y, Pan c.M., Kuo JS and Chai CY, Paramedian reticular nucleus — sympathetic inhibition in spontaneously hypertensive rats, Brain Res. Bull, 21 (1988) 651–657. [DOI] [PubMed] [Google Scholar]
- 31.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 32.Martin GF, Humbertson AO, Laxson C. and Panneton WM, Evidence for direct bulbospinal projections to laminae IX, X and the intermediolateral cell column. Studies using axonal transport techniques in the North American Opossum, Brain Research, 170 (1979) 165–171. [DOI] [PubMed] [Google Scholar]
- 33.Sakai K, Some anatomical and physiological properties of pontomesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In Hobson JA and Brazier MAB (Eds.), ‘The Reticular Formation Revisited’, Raven, New York, 1980, pp. 427–447. [Google Scholar]
- 34.Shiromani P, Siegel JM, Tomaszewski KS and McGinty DJ, Alterations in blood pressure and REM sleep after pontine carbachol microinfusion, Exp. Neurol, 91 (1986) 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel JM, Brainstem mechanisms generating REM sleep. In Kryger MK, Roth T. and Dement WC (Eds.), ‘Principles and Practice of Sleep Medicine’, Saunders, New York, 1989, pp. 104–121. [Google Scholar]
- 36.Siegel JM, Tomaszewski KS, Fahringer H, Cave G, Kilduff T. and Dement WC, Heart rate and blood pressure changes during sleep—waking cycles and cataplexy in the narcoleptic dog, Am. J. Physiol, 256 (1989) Hill-HI 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha JN, Dhawan KN, Chandra O. and Gupta GP, Role of acetylcholine in central vasomotor regulation, Cam J. Physiol. Pharmacol, 45 (1967) 503–507. [DOI] [PubMed] [Google Scholar]
- 38.Snyder DW and Gebber GL, Relationships between medullary depressor region and central vasopressor pathways, Am. J. Physiol, 225 (1973) 1129–1137. [DOI] [PubMed] [Google Scholar]
- 39.Suh TH, Wang CH and Lim RKS, Effect of intracisternal injections of acetylcholine, Proc. Soc. Exp. Biol. Med, 32 (1935) 1410. [Google Scholar]
- 40.Taber E, The cytoarchitecture of the brain stem of the cat, J. Comp. Neurol, 116 (1961) 27–70. [DOI] [PubMed] [Google Scholar]
- 41. Wang SC and Ranson SW, Autonomic responses to electrical stimulation of the lower brain stem, J. Comp. Neurol, 71 (1939) 437–455. [Google Scholar]
- 42.Zemlan FP, Behbehani MM and Beckstead RM, Ascending and descending projections from nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat, Brain Research, 292 (1984) 207–220. [DOI] [PubMed] [Google Scholar]