To the Editor: Major grape polyphenols include the flavanols (catechin, epicatechin, and proanthocyanidins), stilbene compounds (resveratrol), and ellagic acid. Many of these constituents have anti-inflammatory and antineoplastic effects in murine models. Resveratrol suppresses ultraviolet (UV)-induced tumorigenesis and reduces interleukin (IL)-6 and cyclooxygenase-2 expression.1 Proanthocyanidins downregulate IL-1β, tumor necrosis factor α, and prostaglandin E2 in vitro.2
A prospective single-group, open-label, intervention study was carried out to examine whether dietary grape would prevent UV-induced photo damage in humans. Healthy volunteers (age 19 and older, Fitzpatrick skin types I-III) were recruited at 2 separate timepoints (cohort 1 and cohort 2). Volunteers were given California table grape (CTG) powder (75 g daily) to ingest for 14 days. The minimal erythema dose (MED) was determined, and punch biopsies were obtained from the MED site and control site before and after treatment. Of 19 individuals who underwent MED testing, biopsy samples from 18 underwent further analysis (1 sample was damaged during tissue processing). The results of the molecular analysis were as follows.
Acute UV damage generates apoptotic keratinocytes, which may be quantified using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Following CTG treatment, the number of TUNEL1 cells in the epidermis decreased significantly (Table I; Supplementary Fig 5 available via Mendeley at https://doi.org/10.17632/njyzzrhscj.1).
Table I.
Reduction of CPDs, γ-H2AX and TUNEL+ cells after CTG treatment
| Pretreatment (% positive cells ± SEM) |
Posttreatment (% positive cells ± SEM) |
% change* | |
|---|---|---|---|
| CPDs | Cohort 1: 33.1 ± 5.0% | Cohort 1: 22.7 ± 3.9% | Cohort 1: −31.3%† |
| Cohort 2: 19.8 ± 3.8% | Cohort 2: 5.6 ± 2.1% | Cohort 2: −71.5‡ | |
| γ-H2AX | Cohort 1: 19.1 ± 4.8% | Cohort 1: 9.5 ± 1.5% | Cohort 1: −50.6%§ |
| Cohort 2: 4.6 ± 2.1% | Cohort 2: 1.4 ± 0.7% | Cohort 2: −70.1%§ | |
| TUNEL | Cohort 1: 2.1 ± 0.8% | Cohort 1: 0.4 ± 0.1% | Cohort 1: −80.7%§ |
| Cohort 2: 7.1 ± 1.7% | Cohort 2: 3.1 ± 0.5% | Cohort 2: −56.1%§ |
Analyzed with paired 1-tailed t test. Statistically significant differences considered when P < .05, and denoted as:
P < .001.
P < .01.
P < .05.
UV radiation generates cyclobutane pyrimidine dimers (CPDs) and double-strand breaks (DSBs) in DNA. Unrepaired CPDs contribute to initiating mutations of photocarcinogenesis, and DSBs induce γ-H2AX formation. Significantly lower levels of CPD and γ-H2AX were seen after CTG treatment (Supplementary Fig 5).
Nanostring analysis was carried out from cohort 2 samples to determine genes influenced by CTG ingestion. Differentially expressed genes (DEG) associated with inflammation (Fig 1) and apoptosis (Supplementary Fig 6; available via Mendeley at https://doi.org/10.17632/njyzzrhscj.1) were calculated as ratios. DEGs were examined in those whose MED increased (MED responders) after CTG treatment and in those who did not respond (MED nonresponders). For the inflammation pathway, significant downregulation of the genes encoding members of NF-κB signaling was observed. DEGs included genes encoding for a ligand of this pathway, TNFRSF10A, as well as its downstream proinflammatory cytokine products (IL8). Downregulation of IL1β, IL8, and IL22RA2, proinflammatory cytokines secreted from human skin in response to UVB3 was also observed. Expression of STAT3, a downstream signal transducer of IL-8 and IL-22 signaling, was attenuated.
Fig 1.
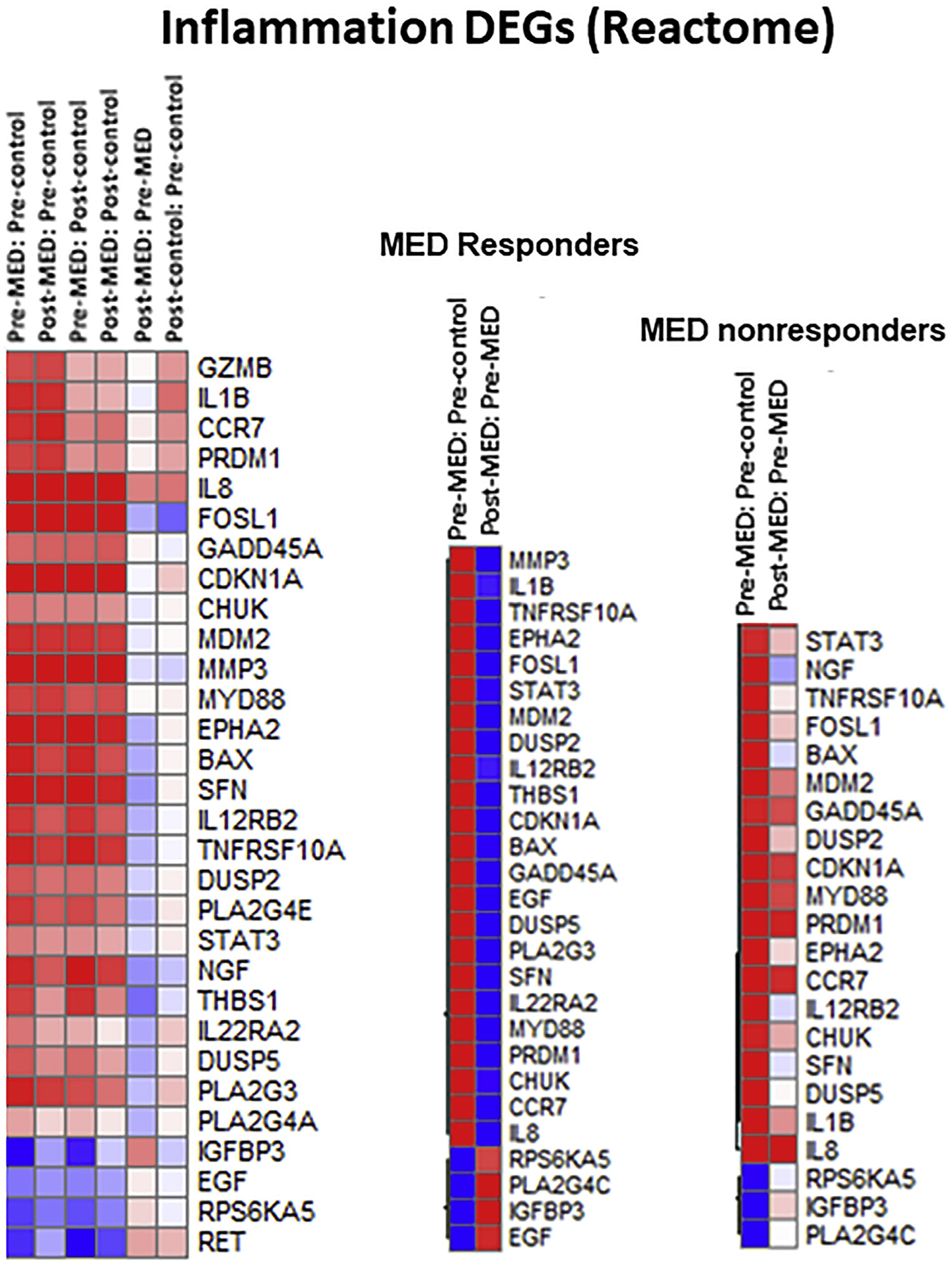
DEGs associated with inflammation. Nanostring analysis found that grape administration downregulated UV-induced proinflammatory cytokines, IL1β, IL8, and IL22RA2, as well as STAT3, a transducer of IL-8 and IL-22 signaling. These findings were more pronounced in the MED responders.
Nanostring analysis of genes associated with apoptosis (Supplementary Fig 6) found upregulation of the tumor suppressor gene GAS1 (growth arrest specific 1) and downregulation of oncogene EIF4EBP1 (eukaryotic initiation factor 4e-binding protein). Real-time polymerase chain reaction found a significant downregulation in the expression of neutrophil-activating chemokine CXCL1.
CTG ingestion reduced markers of UV-mediated DNA damage, inflammation, and apoptosis. Nanostring analysis found differential expression of UVB-induced proinflammatory cytokines, such as IL-22 that impairs skin barrier function.4,5 Differential expressions were more pronounced in MED responders. The mechanism behind the photoprotective effect of CTGs likely involves downregulation of multiple proinflammatory pathways and repair of DNA damage. However, we cannot exclude the possibility that CTG components contribute as UV absorbers. Based on prior studies, it is posited that polyphenols, including resveratrol, are key compounds responsible for photoprotection.
Funding sources:
California Table Grape Commission.
We thank D.L. Della Manna for her guidance with Nanostring analysis.
Footnotes
Conflicts of interest
None disclosed.
IRB approval status: Reviewed and approved by the University of Alabama at Birmingham IRB; approval #F140627004.
Clinicaltrials.gov listing: NCT02760160
REFERENCES
- 1.Pezzuto JM. Resveratrol: twenty years of growth, development and controversy. Biomol Ther (Seoul). 2019;27(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li WG, Zhang XY, Wu YJ, Tian X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol Sin. 2001;22(12):1117–1120. [PubMed] [Google Scholar]
- 3.Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol. 2019;19(11):688–701. [DOI] [PubMed] [Google Scholar]
- 4.Arbiser JL, Nowak R, Michaels K, et al. Evidence for biochemical barrier restoration: topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis. Sci Rep. 2017;7(1):11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbiser JL, Bonner MY, Ward N, Elsey J, Rao S. Selenium unmasks protective iron armor: a possible defense against cutaneous inflammation and cancer. Biochim Biophys Acta Gen Subj. 2018;1862(11):2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]


