Abstract
During the past several decades, numerous trials have compared various diets for the management of overweight and obesity, assuming that a single dietary strategy would be appropriate for all individuals. These studies have failed to provide strong evidence for the efficacy of any particular diet, and it is likely that different people will have different levels of success on different diets. We identified studies investigating pretreatment glycemia or insulinemia status, or both, of the individual as prognostic markers of weight loss during periods in which the composition of a participant’s diet was known. Overall, research suggests that providing specific diets for weight management based on pretreatment glycemia and insulinemia statuses holds great promise for advancing personalized nutrition.
Keywords: personalized nutrition, overweight, glucose, insulin
INTRODUCTION
Projections indicate that by 2025 the global prevalence of obesity will reach 18% in men and surpass 21 % in women (52). This global obesity epidemic increases health and economic burdens on both the personal and societal levels, as obesity leads to a plethora of diseases, such as type 2 diabetes mellitus (T2DM), cardiovascular disease, and several cancers. As a result of population growth and aging, the number of adults with T2DM worldwide has nearly quadrupled since 1980 (53). Decades’ worth of interventions and policies have failed to curtail the rise in overweight and obesity in most countries. Providing advice to exercise more and limit calorie intake sounds reasonable and practical, but alone has not had much long-term success. During the past 30 years there has been a great deal of controversy about the composition of the optimal diet for weight loss and maintenance, yet efforts to identify an optimal diet for weight management based on macronutrient composition have not been successful. Some have defended the more conventional low-fat–high-carbohydrate diet (3,31), whereas others point at a restriction in carbohydrates as being more effective. Numerous strategies for modifying carbohydrate intake have been proposed, from ketogenic very-low-carbohydrate diets (6) to diets with increased protein and a lowered glycemic index (GI) of the carbohydrates (44). However, numerous randomized controlled trials have compared various diets for treating overweight and obesity and failed to provide strong evidence for the efficacy of any particular one. This has given rise to the notion that the content and source of specific macronutrients are of minor importance compared with the critical element of the ability of the person to adhere to a specified diet (26, 63). These conclusions are based on the notion that all people will respond similarly to a given diet. However, it is far more likely that different people will have different levels of success on different diets. The task is to identify biomarkers that can be used to match a person to a diet for effective weight management. If this is the case, our energy could be shifted from arguing that one diet is better than others to identifying how to better match a diet to a specific individual, thus pursuing the concept of personalized nutrition.
Elevated postprandial blood glucose levels are a major risk factor for prediabetes and T2DM, and attempts to identify personalized dietary recommendations have considered the impact of glycemic and insulinemic statuses. Interindividual variation in glycemic responses to a large number of foods and meals has been measured to design personalized diets to reduce postprandial glucose (78). However, it is not clear whether true interindividual variation exists because, theoretically, the variation might have been caused by intraindividual variation in measurements (76).
Numerous studies have attempted to identify the optimal diet for insulin-resistant individuals. Two systematic reviews and meta-analyses of these studies found no differences in weight loss among individuals with T2DM eating a low-carbohydrate diet, a low-GI diet, or a high-protein diet compared with a control diet (1, 67). One study found that a Mediterranean diet, which is high in fat and dietary fiber, produced greater weight loss among individuals with T2DM when compared with a control diet (1). This suggests that reducing the amount of carbohydrate while consuming a relatively higher amount of fat and protein may be particularly effective for weight loss in overweight and obese individuals with T2DM (67).
While individuals without T2DM have largely been treated as a homogeneous group, emerging data suggest that differences in pretreatment glycemic and insulinemic measurements in this group may lead to different degrees of success in weight loss on different diets.
The aim of this review is to compile studies that investigated pretreatment glycemic and insulinemic measurements as prognostic markers for weight loss and the maintenance of weight loss when participants were allocated to diets varying in macronutrient content, GI, or the consumption of fiber and whole grains, or a combination of these. Evaluating these studies may help identify the best diets for weight loss and maintenance of weight loss for patients with different glycemic and insulinemic statuses.
METHODS
We performed literature searches for studies published up to October 9, 2017, in PubMed, CAB Abstracts, and the Web of Science. Search strategies used the following three blocks: one including words and synonyms for insulin and glucose, one for weight, and one for diets. Only publications in English were considered. Reference lists of the relevant publications were cross-checked for additional publications and conference abstracts by the same authors.
Inclusion Criteria
Studies with at least one prescribed diet (energy intake >1,000 kcal/day) or the stratification of dietary intake into a minimum of two groups in observational studies were included in the review if weight loss or weight-loss maintenance (≥8 weeks) was analyzed according to pretreatment glycemic or insulinemic status, or both, for a minimum of two groups or by continuous analysis.
Exclusion Criteria
Studies that did not specify the prescribed diet or actual diet consumed were not included. Additionally, different diets for successful weight management in individuals with T2DM have been compared in previous systematic reviews and meta-analyses (1, 67), and these studies were included in this review only if people with T2DM were compared with individuals without T2DM. Finally, a recent systematic review and meta-analysis containing five studies compared weight loss among diabetic and nondiabetic individuals, all of whom consumed a low-calorie total-formula-replacement diet (300–1,000 kcal/day) for 4 to 52 weeks (46). These five studies have not been included. All studies are listed in three separate tables according to the glycemia or insulinemia analysis performed.
SUMMARY OF THE PERSONALIZED APPROACH TO HEALTH AND DISEASE
Personalized medicine is the tailoring of medical treatment to the individual characteristics of each patient, and it is not a new concept; a classic example is blood transfusions. In recent years, developments in science and technology have changed the way people are diagnosed and treated, for the prediction of both beneficial responses and adverse side effects. This personalized approach includes the consideration of a number of risk factors for adverse health and diseases, such as hypertension (10); epilepsy (74); cancer, for example, by examining tumor cells (55); and Parkinson’s disease (54). Biomarkers measured, for example, in urine, hair, blood, the human genome, and microbiota are being investigated for use in making personalized recommendations for nutrition, but despite general agreement that biomarkers hold great promise, they have not yet produced clinically relevant results. Recently, a pan-European study with more than 1,200 participants used phenotype (anthropometry and blood biomarkers) and genotype (five diet-responsive genetic variants), but failed to identify any advantages in health-related behavioral changes. The only 6-month advantage in health-related behavioral change was seen when using the baseline diet of the individual to tailor dietary recommendations (12). Even one of the genes most robustly associated with obesity, FTO, was recently shown not to change the responsiveness of individuals with obesity to various weight-management options (48).
In recent years, the potential for the gut microbiota to have a pivotal role in obesity management through the use of personalized nutrition has drawn a lot of scientific attention, and accumulating evidence links gut microbiota to obesity. Individuals with obesity show decreased bacterial diversity (69) and gene richness (16,45), and fecal transplantation suggests a possible causal relationship between the microbiome and obesity (47, 60, 73). The composition of the gut microbiota has the potential to affect the efficacy of energy harvest (70), particularly through fiber-utilization capacity (14); to influence the secretion of gastrointestinal hormones affecting appetite (40,68); and, potentially, to affect human behavior through the gut–brain axis (49). Metabolic responses to different diets have recently been shown to vary among individuals depending on the composition of their gut microbiota (42,78), and the ratio of Prevotella to Bacteroides has been identified as an important biomarker associated with the loss of both body weight and body fat in participants consuming an ad libitum diet (35) as well as an energy-restricted diet (32) rich in fiber and whole grains.
Whereas almost every weight loss diet, regardless of its composition, results in an average weight loss for the group being studied, there is always large variability among individuals. Some individuals will have dramatic weight loss of 15–20 kg during 6 months, while others will gain up to 5 kg (Figure 1). For this reason people are often designated as responders or nonresponders to a specific weight loss or weight-loss-maintenance intervention. The use of pretreatment biomarkers could potentially explain some of this variation and help identify the most appropriate diet for each individual.
Figure 1.
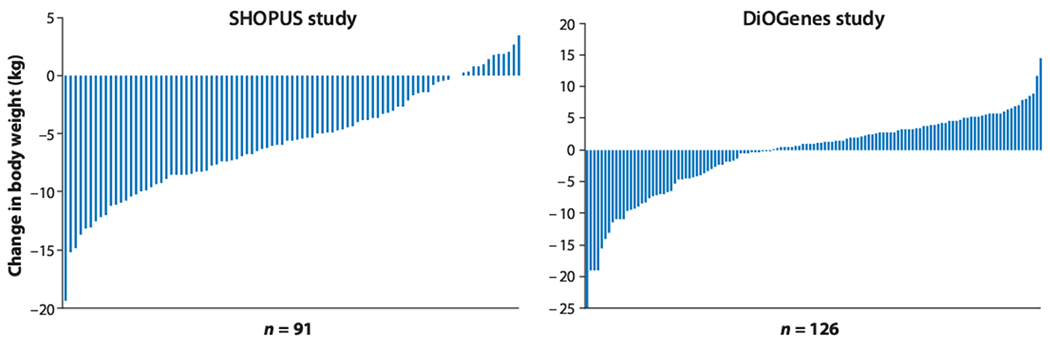
Individual 6-month diet-induced weight change on the healthy-weight-loss diet, known as the New Nordic Diet, in the Supermarket Intervention trial (SHOPUS) and a weight-loss-maintenance diet, an ad libitum low-glycemic-load diet, in the Diet, Obesity, and Genes (DiOGenes) trial (34). Data for the DiOGenes trial show weight-maintenance responses after losing 11 kg in 8 weeks.
PERSONALIZED DIETARY MANAGEMENT BASED ON GLYCEMIA STATUS
Participants with higher preoperative fasting plasma glucose (FPG) have been found to lose less weight in the 12 months following a Roux-en-Y gastric bypass (23); however, this conclusion was drawn without any specification of the diet consumed by those who had undergone the surgery. This finding was also confirmed in a study of 76 participants with overweight and obesity who consumed a hypocaloric diet (≈1,500 kcal/day) for 3 months, although the macronutrient composition of the diet was not controlled (18); in this study, participants with FPG < 109 mg/dL lost 4.3 kg and those with FPG > 110 mg/dL lost 2.8 kg (a statistically insignificant difference of 1.5 kg) (18). A study in Kuwait found similar results when 64 participants with obesity were encouraged to eat a ketogenic diet for 56 weeks, with carbohydrates gradually reintroduced. Overall, participants lost an incredible 25.8 kg, with those who had a high FPG (>6.1 mmol/L) losing a statistically insignificant 2.8 kg less (p = 0.32) than those who had a low FPG (5.1 ± 0.4 mmol/L) (17). A growing number of studies have investigated the potential interaction between categories of glycemia and different dietary compositions on weight loss and the maintenance of weight loss (Table 1) (17, 18, 21, 33, 34, 36, 38, 66, 71).
Table 1.
Studies investigating the interaction between different diets and pretreatment glycemia status and markersa
| Reference | Study design and country | Participants | Duration and number of participants | Prescribed diets | Groups | Results |
|---|---|---|---|---|---|---|
| Hussain et al. (38) | Prespecified controlled trial (diets according to preference); Kuwait | BMI > 25 kg/m2; FPG ≥ 126 mg/dL | 24 weeks; n = 363 | Low-carbohydrate ketogenic diet (initially including ≈20 g carbohydrates/day and gradually increasing) versus low-calorie diet (2,200 kcal/day) | Diabetic versus nondiabetic individuals (although with FPG ≥ 126 mg/dL) | Diabetic individuals: −12.0 kg versus −7.0 kg, p < 0.001; nondiabetic individuals: −12.4 kg versus −5.1, p < 0.001; both groups lost more weight on the low-carbohydrate ketogenic diet than on the low-calorie diet; weight loss between diabetics and nondiabetics was not tested |
| Shyam et al. (66) | Secondary analysis of an RCT; Malaysia | BMI 26.4 ± 4.6 kg/m2; women with history of gestational diabetes | 6 months; n = 77 | −500 kcal/day (women with BMI < 23 or breastfeeding a child aged <6 months were prescribed energy requirements); prescribed intake was capped at 1,800 kcal/day Conventional healthy dietary recommendations (diet low in fat and refined sugars and high in fiber) versus conventional healthy dietary recommendation plus low GI (low-glycemic-load diet) |
Normoglycemic: FPG ≥ 5.6 mmol/L and 2-h OGTT < 7.8 mmol/L Prediabetic: FPG < 5.6 mmol/L and/or 2-h OGTT ≥ 7.8 and < 11.1 mmol/L |
Normoglycemic and prediabetic individuals lost, respectively, 1.7 kg more (p = 0.45) and 1.2 kg more (p = 0.45) weight on the low-glycemic-load diet compared with the conventional healthy diet (Δ0.5 kg not tested) |
| Hjorth et al. (34) (SHOPUS) | Secondary analysis of an RCT; Denmark | 11% normal weight; 43% overweight; 46% obese | 26 weeks; n = 176 | Ad libitum New Nordic Diet: C 47% + 44 g fiber/10 MJ: P 18%: F 30% Average Danish Diet: C 46% + 28 g fiber/10 MJ: P 17%: F 34% |
FPG < 5.6 mmol/L and FPG= 5.6–6.9 mmol/L | Prediabetic individuals lost a mean of 6.0 kg more on the New Nordic Diet than on the Average Danish Diet, whereas normoglycemic individuals lost 2.2 kg more on the New Nordic Diet (Δ3.8 kg; p = 0.001) |
| Hjorth et al. (34)(NUGENOB) | Secondary analysis of an RCT; pan-European | 5% overweight; 95% obese | 10 weeks; n = 743 | −600 kcal/day Low-fat–high-carbohydrate diet: C 58%: P 17%: F 25% Low-carbohydrate–high-fat diet: C 42%: P 17%: F 41% |
FPG < 5.6 mmol/L; FPG = 5.6–6.9 mmol/L; FPG ≥ 7.0 mmol/L | Individuals with FPG ≥ 7.0 mmol/L lost 2.0 kg more (p = 0.07) on the low-carbohydrate–high-fat diet than on the low-fat–high-carbohydrate diet, whereas individuals with FPG < 5.6 mmol/L lost 0.4 kg more (p = 0.03) on the low-fat–high-carbohydrate diet (Δ2.5 kg; p = 0.03) |
| Hjorth et al. (34) (DiOGenes) | Secondary analysis of an RCT; pan-European | 19% overweight; 81% obese | 26 weeks; n = 266 | Ad libitum High-glycemic load: C 51% (GI = 61): P 17%: F 30% Low-glycemic load: C 46% (GI = 56): P 21%: F 30% |
FPG < 5.6 mmol/L and FPG = 5.6–6.9 mmol/L | Prediabetic individuals regained a mean of 5.8 kg more on a high-glycemic-load diet than on a low-glycemic-load diet, whereas normoglycemic individuals regained only 1.4 kg more on a high-glycemic-load diet (Δ4.4 kg; p = 0.001) |
| Hjorth et al. (33) (MUFObes) | Secondary analysis of an RCT; Denmark | BMI = 31 (range, 29.3–33.0) kg/m2 | 6 months; n = 104 | MUFA diet: C 43% (42 g fiber/10 MJ): P 15%: F 38% Nordic Nutrition Recommended Diet: C 58% (40 g fiber/10 MJ): P 16%: F 24% Average Danish Diet: C 50% (29 g fiber/10 MJ): P 16%: F 32% |
FPG < 5.0 mmol/L and 5.0–5.8 mmol/L | Participants with low FPG and randomized to the MUFA diet, Nordic Nutrition Recommended Diet, and Average Danish Diet regained 2.3 kg, 2.5 kg, and 2.1 kg, respectively, whereas participants with high FPG regained 2.7 kg, lost 0.05 kg, and regained 4.2 kg, respectively; participants with high compared with low FPG regained more on the Average Danish Diet compared with the Nordic Nutrition Recommended Diet (4.7 kg; p = 0.005) and the MUFA diet compared with the Nordic Nutrition Recommended Diet (3.1 kg; p = 0.037); there was no difference between the MUFA diet and the Average Danish Diet (p = 0.31) |
| Dashti et al. (17) | Prespecified trial (not randomized or controlled); Kuwait | BMI > 30 kg/m2 | 56 weeks; n = 64 | Ketogenic diet (initially <20 g carbohydrates/day + 20 g/day after 12 weeks) | Diabetic participants (FPG > 6.1 mmol/L; mean ± SD, 10.5 ± 3.0 mmol/L) and nondiabetic participants (FPG mean ± SD, 5.1 ± 0.4 mmol/L) |
Diabetic participants lost 2.8 kg less compared with those without diabetes (p = 0.32) |
| De Luis et al. (18) | Unrandomized and uncontrolled trial; Spain | BMI = 34.6 ± 5.3 kg/m2; no participants with diabetes | 3 months; n = 76 | Prescribed consumption: 1,200 kcal/day, with C 51%: P 18%: F 31%; reported consumption: ≈1,500 kcal/day | FPG < 109 mg/dL (n = 50); FPG > 110 mg/dL (n = 26); participants also stratified by tertiles of FPG | Among participants with FPG < 109 mg/dL, weight loss was 4.3 kg; among those with FPG > 110 mg/dL, weight loss was 2.8 kg (Δ1.5 kg; p > 0.05); also no difference between tertiles of FPG (p > 0.05) |
| Wan et al. (75) | RCT; China | BMI < 28 kg/m2 without traits of metabolic syndrome | 6 months; n = 245 | Low-fat–high-carbohydrate diet: C 66%: P 14%: F 20% Medium-fat–medium-carbohydrate diet: C 56%: P 14%: F 30% High-fat–low-carbohydrate diet: C 46%: P 14%: F 40% |
FPG mean 4.1 to 4.2 ± 0.5 to 0.6 mmol/L (depending on diet group); all assumed to be normoglycemic | Weight loss was 1.6 kg, 1.1 kg, and 0.9 kg, respectively, on the low-fat, medium-fat, and high-fit diets Weight loss was greater on the low-fat diet compared with the medium-fat diet (Δ0.5 kg) and the high-fat diet (Δ0.7 kg) (both p < 0.001) |
| Estruch et al. (21) (PREDIMED) | Secondary analysis of an RCT; Spain | BMI = 29.8 ± 3.7 kg/m2 | 5 years; n = 1,846 | High-fat–low-carbohydrate diet with C 40%: P 16%: F41% (two Mediterranean diets combined) | FPG < 100 mg/dL; FPG = 100–<115 mg/dL; FPG= 115–125 mg/dL; FPG ≥ 126 mg/dL | Participants with FPG ≥ 115 mg/dL (n = 771) lost significantly more weight (−1.64 kg; p < 0.001) on the high-fat–low-carbohydrate diet compared with participants with FPG < 115 mg/dL (n = 1,075) |
| Hjorth et al. (36) (CHO) | Secondary analysis of an RCT; United States | Obese, with BMI = 36.1 ± 3.5 kg/m2 | 24 months; n = 307 | Low-carbohydrate diet (20 g/day for 3 months with low-GI vegetables and unrestricted fat and protein; after 3 months carbohydrates increased by 5 g/day per week until a stable desired weight was achieved) Low-fat diet with limited energy intake (1,200 to 1,800 kcal/day; C 55%: P 15%: F 30%) |
FPG < 100 mg/dL and FPG = 100–125 mg/dL | Weight loss on the two diets was not different among participants with FPG < 100 mg/dL (Δ1.55 kg; p = 0.14) or FPG = 100–125 mg/dL (Δ0.57 kg; p = 0.78) |
| Urban et al. (71) | Secondary analysis of an RCT; United States | BMI = 33.2 ± 0.7 kg/m2 | 24 weeks; n = 70 | Targets: C 48% low GI plus 40 g fiber/day: P 25%: F 27% | FPG < 90 mg/dL and FPG = 90–125 mg/dL | Participants with high FPG lost more weight (−9.4%; n = 58) compared with those with low FPG (−4.1%; n = 12) (p = 0.038) |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; GI, glycemic index; MUFA, monounsaturated fatty acid; OGTT, oral glucose tolerance test; RCT, randomized controlled trial; SD, standard deviation.
BMI values are summarized as mean ± standard deviation, median (interquartile range), or as percentage in different weight categories. C:P:F refers to energy percentages of carbohydrate, protein, and fat in the diet.
In a study in Kuwait, 363 overweight participants with FPG ≥ 126 mg/dL were given the choice of consuming a low-carbohydrate ketogenic diet with the gradual reintroduction of carbohydrates or a low-calorie diet for 24 weeks. Both the diabetic individuals and nondiabetic individuals lost more weight on the low-carbohydrate ketogenic diet compared with the low-calorie diet (diabetic individuals, −12.0 kg on the ketogenic diet versus −7.0 kg on the low-calorie diet, p < 0.001; nondiabetic individuals, −12.4 kg on ketogenic versus −5.1 kg on low-calorie, p < 0.001). Weight loss among diabetics was not compared with weight loss among nondiabetics (38). Another study investigated an energy-restricted conventional healthy diet (aimed at reducing intake by 500 kcal/day) with and without a focus on lowering the GI of the carbohydrates (66). The 77 women, all with a history of gestational diabetes but without T2DM, were further stratified into normoglycemic and prediabetic groups, according to their FPG and glucose levels 2 h after an oral glucose tolerance test (OGTT). The normoglycemic and prediabetic individuals lost, respectively, a statistically insignificant 1.7 kg and 1.2 kg more weight on the low-glycemic-load diet compared with consuming a conventional healthy diet. This minor 0.5-kg interaction between diet and glycemic status was not tested for significance (66).
FASTING PLASMA GLUCOSE AS A PROGNOSTIC BIOMARKER FOR WEIGHT LOSS
Recently, we observed that participants with higher baseline FPG values lost more weight than those with lower baseline FPG values when consuming a fibrous hydrogel together with a hypocaloric diet for 12 weeks (5). Overall, those who consumed the fibrous hydrogel lost significantly more weight than did those in the placebo group (6.1 % of initial body weight lost versus 4.1%; p = 0.024). Interestingly, this weight loss was higher among participants with prediabetes (FPG > 100 mg/dL) who consumed the fibrous hydrogel compared with controls (10.9% of initial body weight lost versus 5.7%; p = 0.029). This hydrogel is designed to mimic the viscoelastic properties of green leafy vegetables. Because of its composition and properties, it led us to the idea that a person’s FPG value might be a prognostic marker of response to diets varying not only in macronutrient composition but also in naturally occurring dietary fiber. Following this observation, a number of large-scale randomized dietary intervention studies that originally compared diets in the general population have been reexamined, with participants stratified according to their FPG value, to investigate the effects of different weight loss diets among subgroups of the population (21, 33, 34, 36, 71). Some of these analyses have been published (33, 34) and others have been presented at international conferences (Figure 2) (21, 36, 71).
Figure 2.
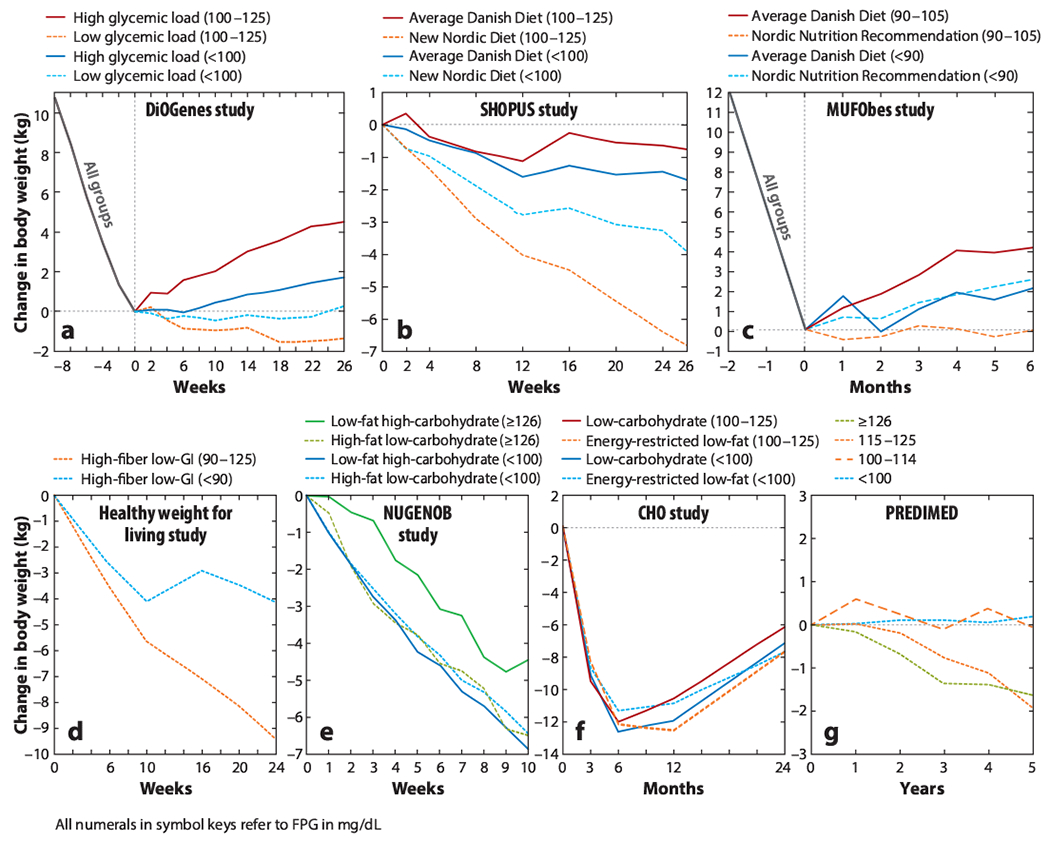
Change in body weight between groups stratified by pretreatment FPG concentrations within and between different diets. In the MUFObes study, no FPG measures exist prior to the 8-week weight-loss period. Therefore, values were used from after the 8-week weight-loss period and before randomization. The negative weeks in parts a and c indicate the weeks before intervention. The studies included are the healthy weight for living study (71), the CHO study (36), DiOGenes (34), MUFObes (33), NUGENOB (34), PREDIMED (21), and SHOPUS (34). Abbreviations: CHO, Manipulation of Carbohydrate study; DiOGenes, Diet, Obesity, and Genes trial; FPG, fasting plasma glucose; GI, glycemic index; MUFObes, Monounsaturated Fatty Acids in Obesity study; NUGENOB, Nutrient–Gene Interactions in Human Obesity trial; PREDIMED, Prevention with Mediterranean Diet study; SHOPUS, New Nordic Diet in the Supermarket Intervention trial.
In the pan-European Diet, Obesity, and Genes (DiOGenes) trial, after obese individuals achieved a diet-induced weight loss of 11 kg, they were randomized to different diets without any caloric restriction (i.e., ad libitum eating) for 6 months. Overall, individuals consuming a high-glycemic-load diet regained 1.9 kg more than those eating the low-glycemic-load diet (44). When reanalyzing the results, prediabetic individuals regained a mean of 5.8 kg more on a high-glycemic-load diet than did those on a low-glycemic-load diet, whereas normoglycemic individuals regained only 1.4 kg more on the high-glycemic-load diet (difference between glycemic groups, 4.4 kg; p = 0.001) (Figure 2a) (34).
In the Supermarket Intervention study (SHOPUS) participants in Denmark with an increased waist circumference were randomized either to the New Nordic Diet—based on Nordic foods and the consumption of fruit, vegetables, fiber, and whole grains—or to the Average Danish Diet (the Western control diet). To increase compliance, all foods were provided free of charge at a specially designed supermarket at the University of Copenhagen’s campus, and barcodes were scanned to ensure that foods were consumed according to intervention group (59). When stratifying according to FPG value, we saw that prediabetic individuals (FPG = 100–125 mg/dL) lost a mean of 6.0 kg more on the New Nordic Diet than on the Average Danish Diet, whereas normoglycemic individuals lost only 2.2 kg more on the New Nordic Diet (difference between glycemic groups, 3.8 kg; p = 0.001) (Figure 2b) (34).
In the Monounsaturated Fatty Acids in Obesity (MUFObes) study, Danes with obesity initially lost 12 kg on a low-calorie diet and were subsequently randomized to one of three different ad libitum diets that differed in macronutrient composition and fiber content (19). Again, all foods were provided free of charge at a specially designed supermarket at the University of Copenhagen’s campus. We previously reported no overall differences in weight maintenance among those on a diet high in monounsaturated fat, the Nordic Nutrition Recommended Diet—which was similar to the New Nordic Diet, being low in fat [20–30% of total energy intake (E%)], high in fiber (>30 g/10 MJ), and low in energy density—and the Average Danish Diet (19). However, when participants were stratified based on their initial FPG value, there was a more than 4 kg difference in weight regained by participants with a high FPG value (with less weight regained on the Nordic Nutrition Recommended Diet), while we saw no difference among participants with low FPG values (Figure 2c) (33).
In the healthy weight for living study employees with overweight and obesity at worksites in Boston, Massachusetts, United States, were given recommendations to consume a reduced-energy, low-glycemic-load, high-fiber diet together with behavioral change education for 24 weeks (64). When reanalyzing the results, there was a tendency for a negative association between pretreatment FPG value and weight change (r = 0.29; p = 0.07). Participants with a high FPG value (≥90 to 125 mg/dL) lost more weight (9.4% of initial body weight lost, n = 58) after 24 weeks compared to participants with a low FPG (<90 mg/dL) (4.1% of initial body weight lost, n = 12; p = 0.038) (Figure 2d) (71).
The pan-European Nutrient–Gene Interactions in Human Obesity (NUGENOB) study was a 10-week, randomized, traditional energy-restricted diet comparison of a low-fat–high-carbohydrate diet versus a low-carbohydrate–higher-fat diet, and overall there was no difference between the two diets in the amount of weight lost (mean weight loss, 7.5 kg) (56). In a reanalysis, individuals with FPG ≥ 126 mg/dL lost a mean of 2.04 kg more [95% confidence interval (CI), −0.20 to 4.28 kg; p = 0.07] on the low-carbohydrate–higher-fat diet than on the low-fat–high-carbohydrate diet, whereas normoglycemic individuals lost a mean of 0.43 kg more (95% CI, 0.03 to 0.83 kg; p = 0.03) on the low-fat–high-carbohydrate diet (difference between glycemic groups, 2.47 kg; p = 0.03) (Figure 2e) (34).
In support of recommending a low-fat–high-carbohydrate diet to normoglycemic individuals, a 6-month randomized dietary intervention study among 245 participants was recently conducted in China (4, 75). The researchers recruited participants with BMI < 28 without any elements of the metabolic syndrome and ended up with participants who had low FPG values (mean ± standard deviation ≈75 ± 10 mg/dL) (4, 75). Weight loss was 0.5 kg and 0.7 kg higher in the low-fat group compared with, respectively, the medium-fat and high-fat groups.
A multicenter trial conducted in the United States, the Manipulation of Carbohydrate (CHO) study, involved 307 individuals with obesity who were randomized to a low-carbohydrate diet (with the gradual reintroduction of carbohydrates) or a caloric-restricted low-fat diet for 24 months. In the overall population as well as between the glycemic groups (FPG < 100 mg/dL versus FPG=100–125 mg/dL) there were no differences in weight lost between the diets after 24 months (Figure 2f) (36). However, when groups were stratified by fasting insulin (FI) value, there were large differences in weight development (see the section titled Personalized Dietary Management Based on Insulinemia Status).
The Spanish Prevention with Mediterranean Diet (PREDIMED) study of individuals with overweight and obesity was designed to investigate the primary prevention of cardiovascular disease using an ad libitum Mediterranean diet without any focus on weight management (22). In a reanalysis of this study (21), after 5 years on this high-fat–low-carbohydrate diet, participants with FPG ≥ 115 mg/dL (n = 771) lost significantly more weight (− 1.64 kg; p < 0.001) than participants with FPG < 115 (n= 1,075) (Figure 2g). While the observed difference was relatively small, it should be regarded as clinically meaningful since this group of individuals tends to respond less favorably in studies of weight loss diets (27).
The research suggests that three groups of individuals—normoglycemic, prediabetic, and diabetic—may respond differently to different diets. Normoglycemic individuals (FPG < 100 mg/dL) with overweight and obesity appear to benefit the most from low-fat–high-carbohydrate diets; prediabetic individuals (FPG = 100–125 mg/dL) benefit most from increasing the amount of fiber consumed and reducing the GI of the consumed carbohydrates; and individuals with FPG ≥ 126 mg/dL (in many cases individuals with T2DM) should focus not only on the quality of the carbohydrates consumed but also on reducing their total carbohydrates and increasing their intake of dietary fat. Normoglycemic individuals with an FPG between 90 and 100 mg/dL may also benefit from increasing their fiber intake (33,71), and prediabetic individuals with an FPG between 115 and 125 mg/dL may benefit from increasing the amount of fat in their diet at the expense of total carbohydrates (21, 34).
The limited evidence about weight management and the management of blood glucose levels among patients with T2DM points toward a Mediterranean-style diet that is higher in fat and lower in carbohydrates (1, 24, 67). Higher intakes of fiber and protein also seem to be indicated. The evidence also suggests that these diets would be beneficial for those with high FPG values.
Often participants with overweight and obesity are assumed to respond similarly to all weight loss diets. As the prevalence of diabetes is expected to increase further, it is likely that more people will also have an elevated FPG value (that is, they will be prediabetic). Helping these prediabetic people lose weight would be one strategy for effectively reducing overall rates of diabetes. Weight loss is also the first-line treatment for diabetic patients. This review provides evidence that this group of people is extremely susceptible to regaining weight on a Western diet, but they may avoid regaining weight on a diet that includes carbohydrates with a lower GI, as much dietary fiber as possible, and a slightly higher protein intake. This is true even without prescribing calorie restriction.
In conclusion, these results indicate that considering a patient’s FPG value when choosing a diet for managing obesity may allow for greater satiety and greater weight loss. This fine of research is still young, and we have a lot to learn about the mechanisms of action (see the section titled Potential Mechanisms) and the best way to define groups and subgroups. We encourage researchers to reanalyze completed dietary intervention studies and stratify groups based on their pretreatment FPG values. Such studies might validate previous findings and help better define FPG cutoffs for specific dietary differences. We also encourage the development of prospective trials that characterize participants before they take part in dietary interventions.
PERSONALIZED DIETARY MANAGEMENT BASED ON INSULINEMIA STATUS
The FPG value is determined by overall insulin resistance and the ability of pancreatic β-cells to increase insulin secretion to compensate for resistance. Therefore, it is not surprising that the plasma insulin concentration also may be involved in the response to different diets. Several studies have examined how individuals with different levels of insulinemia respond in terms of weight change to diets differing in composition (Table 2). The FI level has been the most frequently tested pretreatment insulinemia predictor of dietary weight loss (7,15,30,33,34,36,37,41,50,66).
Table 2.
Studies investigating the interaction between different diets and pretreatment insulinemia status or markersa
| Reference | Study design and country | Participants | Duration and number of participants | Prescribed diets | Groups | Results |
|---|---|---|---|---|---|---|
| Ballesteros-Pomar et al. (7) | Prespecified RCT; Spain | BMI = 28–35 kg/m2; no participants with diabetes | 16 weeks; n = 36 | −1,000 kcal/day of energy needs Low-carbohydrate diet: C 40%: P 30%: F 30% High-carbohydrate diet: C 55%: P 15%: F 30% |
Insulin resistant (n = 21): FI ≥ 15 mU/L and/or HOMA-IR ≥ 3.8 and peak insulin after OGTT >100 mU/L Insulin sensitive (n = 15): remaining participants |
Insulin-resistant participants lost 2.6 kg more (p = 0.37) on the low-carbohydrate diet; insulin-sensitive participants lost 0.7 kg more (p = 0.74) on the low-carbohydrate diet |
| De Luis et al. (18) | Unrandomized, uncontrolled trial; Spain | BMI = 34.6 ± 5.3 kg/m2; no participants with diabetes | 3 months; n = 76 | Prescribed consumption: 1,200 kcal/day, with C 51%: P 18%: F 31%; reported consumption: ≈1,500 kcal/day | Groups based on tertiles of HOMA-IR | No difference between tertiles of HOMA-IR (p > 0.05) |
| Pittas et al. (57) | Prespecified RCT; United States | BMI = 25–29.9 kg/m2; FPG < 100 mg/dL | 24 weeks; n = 32 | −30% of energy needs High-glycemic-load diet: C 60%: P 20%: F 20% with 15 g fiber and 1,000 kcal, GI = 86 and glycemic load = 116 g/1,000 kcal Low-glycemic-load diet: C 40%: P 30%: F 30% with 15 g fiber/1,000 kcal, GI = 53 and glycemic load = 45 g/1,000 kcal |
Groups separated by the median insulin-30 value into low [<473 pmol/L (66 mU/L)] and high [>473 pmol/L (66 mU/L)]; also used HOMA-IR | Participants with high insulin-30 values lost ≈4 kg more (p = 0.047) on the low-glycemic-load diet than on the high-glycemic-load diet; participants with low insulin-30 values lost ≈2 kg more (p = 0.31) on the high-glycemic-load diet than on the low-glycemic-load diet (≈Δ6 kg; no p value reported); no diet by HOMA-IR interaction was found (no p value reported) |
| Chaput et al. (13) (Quebec Family Study) | Observational; Canada | BMI = 24.5 ± 4.6 kg/m2 (low-fat tertile); BMI = 27.1 ± 6.7 kg/m2 (high-fat tertile); no participants with diabetes | 6 years; n = 184 | Lowest tertile of self-reported fat intake (<30% total energy intake) versus highest tertile (>36% total energy intake) | Groups separated by tertiles of insulin-30 values into lowest (<300 pmol/L), top (300–525 pmol/L), and highest (>525 pmol/L) | Participants with high insulin-30 values in the lowest dietary fat tertile gained 1.8 kg more weight (p = 0.034) when compared with the highest dietary fat tertile; no difference was observed among those with low or medium insulin-30 values (p > 0.05) |
| Ebbeling et al. 2007 (20) | Prespecified RCT; United States | BMI ≥ 30 kg/m2; no participants with diabetes (FPG < 126 mg/dL) | 18 months; n = 56 | Ad libitum low-glycemic-load diet emphasizing low-GI sources for carbohydrates (40% carbohydrates and 35% fat) compared with a low-fat diet (55% carbohydrates and 20% fat) | Groups separated by median insulin-30 values into low (≤57.5 μIU/mL) and high (>57.5 μIU/mL) | Participants with high insulin-30 values lost 4.6 kg more p = 0.004) on the low-glycemic-load diet than on the low-fat diet; no difference in diet response was observed among those with insulin-30 values below the median p = 0.90) |
| Gardner et al. (29) | Prespecified RCT; United States | BMI = 28–40 kg/m2 | 12 months; n = 609 | ~500-kcal deficit relative to baseline Reported consumption after 12 months Low-fat diet (C 48%: P 21%: F 29%) versus low-carbohydrate diet (C 30%: P 23%: F 45%) |
Insulin-30 value used as a continuous variable (no groups) | Insulin-30 values did not modify the effect of diet on weight loss p for interaction = 0.474) |
| Gardner et al. (28) | Prespecified RCT; United States | BMI = 28–40 kg/m2; no participants with diabetes | 6 months; n = 49 | Ad libitum low-fat versus low-carbohydrate diet using a limbo-titrate-quality approach | Insulin area under the curve from OGTT: insulin resistant (below median value); insulin sensitive (above median value); also insulin area under the curve was used as a continuous measure as well as insulin-30 values, insulin-120 values, and glucose area under the curve0–30 by insulin area under the curve0–30 | Insulin-sensitive participants lost 1.8 kg more on the low-fat diet than on the low-carbohydrate diet, whereas the insulin-resistant individuals lost 2.2 kg more on the low-carbohydrate diet than on the low-fat diet (Δ4.0 kg), but no weight loss was statistically significant; no meaningful differences were found when using insulin-30 values or other indexes (p > 0.05) |
| McClain et al. (50) (A TO Z study) | Secondary analysis of an RCT; United States | BMI = 27–40 kg/m2; women; no participants with diabetes | 1 year (2 months plus 10 months); n = 81 | Low-carbohydrate Atkins diet (goal to consume ≤50 g carbohydrates/day) versus low-fat Ornish diet (goal to consume ≤10% fat as total energy intake) | Groups separated by tertiles of FI values and middle tertile omitted: low (≤6.9 μIU/mL) and high (>10.6 μIU/mL) | Participants with high FI values lost a statistically insignificant 4 kg more on the low-carbohydrate diet versus the low-fat diet (p > 0.05); the difference among those with low FI values was 0.6 kg (p > 0.05) |
| Cornier et al. (15) | Prespecified RCT; United States | BMI = 30–35 kg/m2; women without diabetes | 16 weeks; n = 21 | −400 kcal/day High-carbohydrate–low-fat diet (C 60%: P 20%: F 20%) Low-carbohydrate–high-fat diet (C 40%: P 20%: F 40%) | Groups based on FI values were low (≤ 10 μU/mL) and high (>15 μU/mL) | Participants with low FI values lost 5.2 kg more on the low-fat diet (p < 0.01), and participants with high FI values lost 3.7 kg more on the low-carbohydrate diet (p < 0.05); 8.8-kg interaction; no p value |
| Rock et al. (62) | Prespecified RCT; United States | BMI = 33.5 (range, 27–40) kg/m2; nondiabetic women | 1 year; n = 214 | −500–1,000 kcal/day on one of three diets Low fat–high carbohydrate (C 65%: P 15%: F 20%); low carbohydrate–high fat (C 45%: P 20%: F 35%); low carbohydrate–high fat with 18% energy intake from walnuts (C 45%: P 20%: F 35%) |
Groups based on HOMA-IR values were insulin sensitive (HOMA-IR ≤ 3) and insulin resistant (HOMA-IR > 3) | Participants with low HOMA-IR lost 3.2 to 3.8 kg less weight (p = 0.04 to 0.06) on the low-carbohydrate diet compared with the low-fat and walnut-rich diets, respectively; no difference between diets among participants with high HOMA-IR (p > 0.05) |
| Hron et al. (37) | Run-in phase of an RCT; United States | BMI = 34.4 ± 4.9 kg/m2 (BMI ≥ 27); no participants with diabetes | 12 weeks; n = 21 | −40% calorie-restricted diet for 12 weeks containing C 45%: P 25%: F 30% with 27.1 g fiber/day | Linear associations using insulin-30 values, insulin secretion, hepatic insulin sensitivity and HOMA-IR | Participants with lower insulin-30 values lost more fat mass (p = 0.04), corresponding to a 1.7-kg difference between the 10th and 90th percentiles of insulin-30 values; no other measures of insulinemia were associated with weight change |
| Hjorth et al. (34) (SHOPUS) | Secondary analysis of an RCT; Denmark | 11% normal; 43% overweight; 46% obese | 26 weeks; n = 176 | Ad libitum New Nordic Diet: C 47% + 44 g fiber/10 MJ: P 18%: F 30% Average Danish Diet: C 46% + 28 g fiber/10 MJ: P 17%: F 34% |
Groups based on FI values with median among prediabetic individuals used as the split | Participants with low and high FI values lost 4.1 kg more (p < 0.001) and 1.6 kg more (p = 0.02), respectively, on the New Nordic Diet than on the Average Danish Diet (Δ2.5 kg; p = 0.006) |
| Hjorth et al. (34) (NUGENOB) | Secondary analysis of an RCT; pan-European | 5% overweight; 95% obese | 10 weeks; n = 743 | −600 kcal/day on either a low-fat–high-carbohydrate diet (C 58%: P 17%: F 25%) or a low-carbohydrate–high-fat diet (C 42%: P 17%: F 41%) | Groups based on FI values with median among prediabetic individuals used as the split | Participants with low and high FI values lost 0.4 kg more (p = 0.046) and 0.1 kg more (p = 0.84), respectively, on the low-fat–high-carbohydrate diet than on the high-fat–low-carbohydrate diet (Δ0.1 kg; p = 0.33) |
| Hjorth et al. (34) (DiOGenes) | Secondary analysis of an RCT; pan-European | 19% overweight; 81% obese | 26 weeks; n = 266 | Ad libitum High-glycemic-load diet: C 51% (GI = 61): P 17%: F 30% Low-glycemic-load diet: C 46% (GI = 56): P 21%: F 30% |
Groups based on FI values; median among prediabetic individuals used as the split | Participants with low and high FI values regained 2.3 kg more (p < 0.001) and 0.9 kg more (p = 0.02), respectively, on the high-glycemic-load diet than on the low-glycemic-load diet (Δ1.4 kg; p = 0.14) |
| Hjorth et al. (33) (MUFObes) | Secondary analysis of an RCT; Denmark | BMI = 31 (range, 29.3–33.0) kg/m2 | 6 months; n = 104 | Monounsaturated fatty acid diet: C 43% (42 g fiber/10 MJ): P 15%: F 38% Nordic Nutrition Recommended Diet: C 58% (40 g fiber/10 MJ): P 16%: F 24% Average Danish Diet: C 50% (29 g fiber/10 MJ): P 16%: F 32% |
Groups based on FI values; median among high-glucose group used as the split | Participants with high FI values regained 2.5 kg, 1.5 kg, and 4.2 kg on the monounsaturated fatty acid diet, Nordic Nutrition Recommended Diet, and Average Danish Diet, respectively (p ≥ 0.061 between diets), whereas weight regain was 2.5 kg, 2.1 kg, and 2.4 kg among participants with low FI values, on the same respective diets (p ≥ 0.63 between diets); no differences were found between diets for participants with low and high FI values (all p ≥ 0.16) |
| Hjorth et al. (36) (CHO) | Secondary analysis of an RCT; United States | Obese with BMI = 36.1 ± 3.5 kg/m2 | 24 months; n = 307 | Low-carbohydrate diet (20 g/day for 3 months with low-GI vegetables and unrestricted fat and protein; after 3 months carbohydrates increased by 5 g/day per week until a stable desired weight was achieved) Low-fat diet with limited energy intake (1,200 to 1,800 kcal/day; C 55%: P 15%: F 30%) |
Groups based on FI values; median value used as the split | Participants with low and high FI values lost 0.4 kg more (p = 0.79) and 3.4 kg more (p = 0.012), respectively, on the low-fat diet than on the low-carbohydrate diet (Δ3.7 kg; p = 0.052) |
| Shyam et al. (66) and Ghani et al. (30) | Secondary analysis of an RCT; Malaysia | BMI = 26.4 ± 4.6 kg/m2; women with a history of gestational diabetes | 6 months; n = 77 | −500 kcal/day (women with BMI < 23 or breastfeeding a child aged <6 months were prescribed energy requirements); prescribed intake capped at 1,800 kcal/day Conventional healthy dietary recommendations (diet low in fat and refined sugars and high in fiber) versus conventional healthy dietary recommendation plus low GI (low-glycemic-load diet) |
Groups based on FI values were low (<2 μlU/mL; n = 44) and high (≥2 μlU/mL; n = 33) | Participants with low and high FI values lost, respectively, a statistically insignificant 1.4 kg more (p = 0.41) and 3.3 kg more (p = 0.09) on the low-GI diet (Δ1.9-kg interaction not tested) |
| Kong et al. (41) | Unrandomized trial; France | Overweight and obese individuals | 12 weeks; n = 50 | 6-week hypocaloric diet (women consumed 1,200 kcal/day and men consumed 1,500 kcal/day) rich in fiber and with 35% protein, 25% fat and 40% low-GI carbohydrates; followed by a 6-week weight maintenance diet (individually prescribed by dietitian) | FI value, HOMA-IR value, and a number of other indexes | The more insulin-sensitive participants (based on FI and HOMA-IR values) lost the most weight p ≤ 0.02) |
| McLaughlin et al. (51) | Unrandomized trial; United States | BMI = 28.0–35.0 kg/m2; nondiabetic women | 60 days; n = 31 | −1,000 kcal/day (minimum 1,200 kcal/day) Commercial liquid nutrition formula plus two high-fiber muffins and sodium supplement daily |
Insulin-mediated glucose disposal estimated by insulin and glucose concentrations during the last 30 min of a 180-min infusion of somatostatin, insulin, and glucose; total integrated insulin response across two meals | Weight loss did not vary as a function of insulin resistance or total integrated insulin response (p ≥ 0.83) |
Abbreviations: BMI, body mass index; FI, fasting insulin; FPG, fasting plasma glucose; GI, glycemic index; HOMA-IR, homeostatic model assessment-insulin resistance; OGTT, oral glucose tolerance test; RCT, randomized controlled trial.
BMI values are summarized as mean ± standard deviation, median (interquartile range), or as percentage in different weight categories. C:P:F refers to the energy percentages of carbohydrate, protein, and fat in the diet.
In a small study of 21 women with obesity who consumed an energy-restricted diet (reduced by 400 kcal/day) for 16 weeks, those with a low FI level lost 5.2 kg more while consuming a low-fat diet; individuals with a high FI level lost 3.7 kg more on a low-carbohydrate diet. Therefore, a notable 8.8-kg interaction between diets and FI level was found (15). Similar observations were seen in a more recent year-long study comparing weight loss on a low-carbohydrate diet (the Atkins diet) with weight loss on a low-fat diet (the Ornish diet) (50). Those participants with higher FI values who consumed the low-carbohydrate diet lost a statistically insignificant 4 kg more than those on the low-fat diet, whereas in those with a low FI value, there was virtually no difference between diets in the weight lost (0.6 kg).
In yet another study, 36 participants with overweight and obesity were stratified on the basis of being insulin sensitive (n = 15) or insulin resistant (n = 21) based on the results of their FI test, the homeostatic model assessment–insulin resistance (HOMA-IR), and peak insulin after OGTT; then they were given either a low- or high-carbohydrate energy-restricted diet (reduced by 1,000 kcal/day). Insulin-resistant participants lost a statistically insignificant 2.6 kg more (p = 0.37) on the low-carbohydrate diet compared with the insulin-sensitive participants, who lost a statistically insignificant 0.7 kg more (p = 0.74) on the same diet (7). Another study included 77 women with a history of gestational diabetes but not T2DM and provided them with an energy-restricted (reduced by 500 kcal/day) conventional healthy diet with or without instructions to consume carbohydrates with a lower GI. The 77 women were further stratified according to their FI values. Those categorized as having a low FI value lost a statistically insignificant 1.4 kg more (p = 0.41) on the lower-GI diet, while those with a high FI value lost 3.3 kg more (p = 0.09) on the lower-GI diet. This 1.9-kg interaction between diet and FI group was not tested for significance (30, 66).
Recently, data from the five studies reviewed earlier (with 104 to 743 participants), all conducted by the same research group, were also reanalyzed using the pretreatment FI values of the participants as a potential determinant of weight loss while on various diets (33, 34, 36). In the DiOGenes study, participants with a low FI value regained 2.3 kg more on the high-glycemic-load diet than on the low-glycemic-load diet, whereas no difference was seen for participants with a high FI value (0.9 kg). No significant difference in responses to the diets in terms of weight regained was found between participants with a high FI and those with a low FI (1.4 kg; p = 0.14) (34).
In the SHOPUS study, participants with a low FI value lost 4.1 kg more on the New Nordic Diet than on the control diet, whereas participants with a high FI value lost only 1.6 kg more. Additionally, in the NUGENOB study, a 2.5-kg difference in response to the diets was found between participants with high and low FI values (34).
In the NUGENOB study, participants with a low FI value lost 0.4 kg more on the low-fat–high-carbohydrate diet than they did on the high-fat–low-carbohydrate diet, whereas no difference in the amount of weight lost was observed for participants with a high FI value (0.1 kg). Consequently, no difference in response to the diets was found between participants with high and low FI values (34).
In the MUFObes study, participants with a high FI value who were randomized to a diet high in monounsaturated fat, a diet high in fiber, or the Average Danish Diet regained after 6 months, respectively, 2.5 2 kg, 1.49 kg, and 4.19 kg, with no statistically significant differences in weight loss between the three diets (all p ≥ 0.061). Participants with low FI values who were randomized to a diet high in monounsaturated fat, a diet high in fiber, or the Average Danish Diet regained after 6 months, respectively, 2.46 kg, 2.07 kg, and 2.35 kg, with no statistically significant differences in weight loss between the three diets (all p ≥ 0.63). Consequently, no differences in responses to the diets were found between individuals with low and high FI values (all p ≥ 0.16), although participants with high FI values regained almost 2 kg more on the Average Danish Diet compared with those with low FI values (33).
In the CHO study, participants in the low FI group responded similarly to the low-fat and low-carbohydrate diets, whereas participants in the high FI group lost 3.3 kg more body weight during the 24 months when consuming a low-fat diet (36).
Another study included 50 participants with overweight and obesity and found that the more insulin-sensitive participants (based on FI and HOMA-IR values) lost the most weight while on a 12-week energy-restricted low-glycemic-load diet that was also high in fiber (41). Finally, 214 women with overweight and obesity were randomized to a low-fat diet versus a low-carbohydrate diet with or without substantial supplementation of walnuts (18E%) for 1 year. Insulin-resistant participants did not show diet-related differential weight loss, whereas the insulin-sensitive participants lost 3.2 to 3.8 kg less weight on the low-carbohydrate diet compared with the other two diets (p = 0.04 to 0.06). As the low-carbohydrate-plus-walnut diet was relatively lower in carbohydrates compared with the low-carbohydrate diet, these results are difficult to explain from a macronutrient point of view (62).
Overall, there is not strong support for the use of pretreatment FI value alone as a predictor of weight change. Some studies find that a diet with a lower glycemic load is more important for participants with a high FI value (i.e., insulin-resistant participants) or that diets lower in fat are more important for those with low FI values (i.e., insulin-sensitive participants) (7, 15, 30, 33 34, 50, 66). However, other studies have found no evidence for this difference (34, 36, 41).
Some data suggest that insulin concentration 30 minutes after an oral glucose load (insulin-30), a measure of insulin secretion, is a good predictor of weight loss on specific diets (13,20,28,37,57). In a substudy of 32 overweight, normoglycemic participants assigned to a 30% calorie-restricted diet for 24 weeks, Pittas et al. (57) found an overall interaction between diets and insulin-30. Specifically, weight loss was found to be approximately 4 kg greater among participants with a high insulin-30 value when they consumed a low-glycemic-load diet compared with a high-glycemic-load diet. At the same time, participants with a low insulin-30 value lost a statistically insignificant 2 kg more on the high-glycemic-load compared with the low-glycemic-load diet. This difference was not found when using the HOMA-IR as a measure of insulin sensitivity (57). In a study with 73 obese participants, Ebbeling et al. (20) found that those with insulin-30 values above the median had lost 4.6 kg more weight at 18 months while consuming an ad libitum low-glycemic diet compared with a low-fat diet. No difference in diet response was observed among those with insulin-30 values below the median (20). As an extension to this, a subsequent study of 21 overweight and obese participants assigned to a 40% calorie-restricted moderate-carbohydrate high-protein diet for 12 weeks found that individuals with lower insulin-30 values lost more fat, corresponding to a 1.7-kg difference between the 10th and 90th percentiles of insulin-30 values. No other measure of insulinemia, including HOMA-IR, was found to predict weight change (37). The same result was obtained in the Quebec Family Study (13). In this study, insulin-30 values showed a higher correlation with 6-year weight change than FI or any other insulin measure during an OGTT. This was especially strong in the tertile of the population that consumed the lowest amount of dietary fat. Individuals in the lowest dietary fat tertile who had high insulin-30 values gained 1.8 kg more weight over the subsequent 6-year observation period than those with high insulin-30 values in the highest dietary fat tertile. No difference in weight change by fat intake was observed among individuals with low or medium insulin-30 values (13).
Recently, 49 participants with overweight and obesity were randomized to an ad libitum low-fat or low-carbohydrate diet for 6 months, and they were further stratified by the median of the insulin area under the curve from an OGTT. The insulin-sensitive individuals lost a statistically insignificant 1.8 kg more weight on the low-fat diet compared with the low-carbohydrate diet, whereas the insulin-resistant individuals lost a statistically insignificant 2.2 kg more weight on the low-carbohydrate compared with the low-fat diet. Therefore, an insignificant 4.0-kg interaction was found between diets and the median split of the insulin area under the curve after an OGTT. No meaningful differences were found when using insulin-30 values (28). Similarly, the insulin-30 value was not found to modify the effect of 12-month weight loss (p for interaction, 0.47) among 609 participants randomized either to a low-fat or to a low-carbohydrate diet (29). Finally, in a study of 31 nondiabetic women with overweight or obesity who were following a calorie-restricted commercial liquid diet, 60-day weight loss did not vary as a function of insulin resistance or total integrated insulin response (51).
The pretreatment insulin-30 value seems to be a better predictor than the FI value of weight change on diets that vary the glycemic load. Most published studies at least partly support the evidence that a diet lower in glycemic load is more important among participants with high insulin-30 values or that diets lower in fat are more beneficial for people with low insulin-30 values, or that both are important (13, 20, 37, 57). Only two studies mentioned that no meaningful differences in weight change were found when using insulin-30 values (28, 29).
PERSONALIZED DIETARY MANAGEMENT BASED ON COMBINED GLYCEMIA AND INSULINEMIA STATUSES
Five studies have examined whether the simultaneous stratification of FPG and FI values can predict weight loss for different diets (Table 3). These are the same studies that have been described in previous sections of this review (33, 34, 36).
Table 3.
Studies investigating the interaction between different diets and the combined effect of pretreatment glycemia and insulinemia status or markersa
| Reference | Study design and country | Participants | Duration and number of participants | Prescribed diets | Groups | Results |
|---|---|---|---|---|---|---|
| Hjorth et al. (34) (SHOPUS) | Secondary analysis of an RCT; Denmark | 11% normal weight; 43% overweight; 46% obese | 26 weeks; n = 176 | Ad libitum New Nordic Diet: C 47% + 44 g fiber/10 MJ: P 18%: F 30% Average Danish Diet: C 46% + 28 g fiber/10 MJ: P 17%: F 34% |
Groups based on combined FPG and FI values: FPG < 5.6 mmol/L and FPG = 5.6–6.9 mmol/L; median FI value among prediabetic individuals used as the split | Participants with high FPG values and low FI values lost 6.3 kg more (p < 0.001) on the New Nordic Diet than on the Average Danish Diet; no difference between diets among those with low FPG and high FI values (Δ0.10 kg; p = 0.89) |
| Hjorth et al. (34) (NUGENOB) | Secondary analysis of an RCT; pan-European | 5% overweight; 95% obese | 10 weeks; n = 743 | −600 kcal/day on either a low-fat–high-carbohydrate diet (C 58%: P 17%: F 25%) or a low-carbohydrate–high-fat diet (C 42%: P 17%: F 41%) | Groups based on combined FPG and FI values: FPG < 5.6 mmol/L, FPG = 5.6–6.9 mmol/L, and FPG ≥ 7.0 mmol/L; median FI among prediabetic individuals used as the split | Participants with high FPG and low FI values lost 3.1 kg more (p = 0.02) on the low-carbohydrate–high-fat diet than on the low-fat–high-carbohydrate diet; participants with low FPG and low FI values lost 0.5 kg more (p = 0.02) on the low-fat–high-carbohydrate diet |
| Hjorth et al. (34) (DiOGenes) | Secondary analysis of an RCT; pan-European | 19% overweight; 81% obese | 26 weeks; n = 266 | Ad libitum High-glycemic-load diet: C 51% (GI = 61):P 17%: F 30% Low-glycemic-load diet: C 46% (GI = 56): P 21%: F 30% |
Groups based on combined FPG and FI values: FPG < 5.6 mmol/L and FPG = 5.6–6.9 mmol/L; median FI among prediabetic individuals used as the split | Participants with high FPG and low FI values regained 7.8 kg more (p < 0.001) on the high-glycemic-load diet than on the low-glycemic-load diet; no difference between diets among participants with low FPG and high FI values (Δ 1.2 kg; p = 0.19) |
| Hjorth et al. (33) (MUFObes) | Secondary analysis of an RCT; Denmark | BMI = 31 (range, 29.3–33.0) kg/m2 | 6 months; n = 104 | Nordic Nutrition Recommended Diet: C 58% (40 g fiber/10 MJ): P 16%: F 24% Average Danish Diet: C 50% (29 g fiber/10 MJ): P 16%: F 32% The MUFA diet was not analyzed when combining FPG and FI as n = 1 in one of the four phenotypes |
Groups based on combined FPG and FI values: FPG < 5.0 mmol/L and FPG = 5.0–5.8 mmol/L; median FI among prediabetic individuals used as the split | Participants with high FPG and high FI values regained 7.0 kg more (p = 0.001) on the Average Danish Diet than on the Nordic Nutrition Recommended Diet; no difference was observed between diets for the other three combinations of FPG and FI (p ≥ 0.15) |
| Hjorth et al. (36) (CHO) | Secondary analysis of an RCT; United States | Obese with BMI = 36.1 ± 3.5 kg/m2 | 24 months; n = 307 | Low-carbohydrate diet (20 g/day for 3 months with low-GI vegetables and unrestricted fat and protein; after 3 months carbohydrates increased by 5 g/day per week until a stable desired weight was achieved) Low-fat diet with limited energy intake (1,200 to 1,800 kcal/day; C 55%: P 15%: F 30%) |
Groups based on combined FPG and FI values: FPG < 5.6 mmol/L and FPG = 5.6–6.9 mmol/L; median FI used as the split | Participants with high FPG and low FI values tended to lose 6.2 kg more (p = 0.088) on the low-carbohydrate ad libitum diet, whereas those with high FPG and high FI values lost 7.2 kg more (p = 0.005) on the low-fat, hypocaloric diet (Δ13.3 kg; p = 0.003); no difference between diets among participants with low FPG values regardless of their FI value (p ≥ 0.16) |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; FI, fasting insulin; GI, glycemic index; MUFA, monounsaturated fatty acid; RCT, randomized controlled trial.
BMI values are summarized as mean ± standard deviation, median (interquartile range), or as percentage in different weight categories. C:P:F refers to energy percentages of carbohydrate, protein, and fat in the diet.
In the NUGENOB study, participants with an FPG ≥ 6.4 mmol/L and low FI values lost 3.06 kg more (95% CI, 0.40 to 5.71; p = 0.02) on the high-fat–low-carbohydrate diet than on the low-fat–high-carbohydrate diet. Participants with FPG < 6.4 mmol/L and low FI values lost 0.49 kg more (95% CI, 0.08 to 0.91; p = 0.02) on the low-fat–high-carbohydrate diet (34). In the SHOPUS study, prediabetic participants with low FI values lost 6.27 kg more (95% CI, 3.51 to 9.02; p < 0.001) on the New Nordic Diet than on the control diet, whereas no difference was observed for normoglycemic participants with high FI values (0.10 kg lost; 95% CI, −1.37 to 1.57; p = 0.89) (34). In the DiOGenes study, prediabetic participants with low FI values who were on the high-glycemic-load diet regained 7.78 kg more (95% CI, 4.39 to 11.18; p < 0.001) than they did on the low-glycemic-load diet, whereas no difference was observed for normoglycemic participants with high FI values (1.17 kg regained; 95% CI, −0.59 to 2.93; p = 0.19) (34). In the MUFObes study, participants with high FPG and high FI values lost 0.04 kg on the high-fiber diet and regained 6.9 kg on the Average Danish Diet, resulting in a difference of 7.0 kg. No difference was observed between diets for the other three phenotypes (33). In the CHO study, the high- and low-carbohydrate diets elicited nearly identical changes in body weight at all time points. However, participants with prediabetes and FI values below the median responded better to a low-carbohydrate Atkins ad libitum diet, whereas participants with prediabetes and FI values above the median responded better to a low-fat hypocaloric diet. Consequently, an impressive 13.3-kg difference in 24-month diet response was observed between prediabetic participants with low and high FI values. Normoglycemic participants, regardless of their FI values, responded equally well to both diets (36).
While additional data are certainly needed, the combined use of pretreatment FPG and FI values appears to have great promise as a predictor of weight loss and maintenance of weight loss for different diets. The data were obtained from a retrospective reanalysis of dietary interventions and may have limitations, such as small sample sizes in the different FPG and FI groups, differences in study design, and a lack of control over the actual diets consumed. For example, the actual content of the randomized diets in the CHO study after 24 months could be questioned. Those in the low-carbohydrate group gradually added more vegetables, fruits, and whole grains, so that at the end of the study, that diet was probably not very low in carbohydrates but contained more fiber and whole grains and so had a somewhat lower GI or glycemic load. In this way, results among prediabetic people with low FI values are identical to three recent reanalyses of dietary intervention studies (33, 34). Although the low-fat diet in the CHO study was in theory calorie restricted throughout the 24 months, this was likely not the case, as is evidenced from weight regain occurring after 6 months. This emphasizes the need for more carefully controlled and planned studies of dietary interventions that use good markers of dietary compliance and intake.
Adding FI values as a marker of weight loss success on different diets seems to be especially important for those in the normoglycemic subgroup because weight loss and maintaining weight loss in individuals with low FPG and high FI values appear to be less influenced by specific dietary composition (that is, this subgroup does not respond differently to dietary interventions) (33, 34, 36). Furthermore, participants with FPG values between 100 and 125 mg/dL also seem to respond differently depending on their FI value. Those with the lowest pretreatment FI value responded better to diets restricting the type or amount of carbohydrates (34, 36), while those with high FI values responded better to diets that were lower in fat (33, 36). Exactly how FPG and FI values interact to impact weight change is unknown.
POTENTIAL MECHANISMS
It is useful to consider why aspects of glycemic control might impact various weight loss diets differently. One possible mechanism relates to insulin resistance in the brain. Impaired brain uptake of glucose due to insulin resistance has been linked to cognitive decline and reduced memory function in T2DM (61). Moreover, persons with T2DM have a higher risk of developing Alzheimer’s disease and other dementias, strongly suggesting that insulin resistance exerts adverse effects not only on peripheral tissues and organs but also in the brain. The glucostatic hypothesis suggests that as part of the established appetite regulatory system, the central nervous system monitors blood glucose. This hypothesis is based on the notion that glucose utilization by critical cells in the hypothalamus generates a signal to brain areas controlling appetite and food intake. When glucose utilization is decreasing or low, hunger is elicited, and eating ensues if food is available. As eating progresses, glucose in the blood increases, leading to increased hypothalamic glucose utilization, ultimately causing the individual to become satiated and to stop eating (77). In rodents, genetic deletion of the brain’s insulin receptors causes central insulin resistance and obesity (9). In humans, acute meal-induced increases in blood glucose and insulin have been found to be positively associated with satiety in normal-weight participants, but blunted in overweight participants (25). In a stepwise regression analysis, the 3-h insulin area under the curve was found to explain 67% of the variation in ad libitum energy intake in normal-weight individuals, but not in those with obesity (72). This inverse association among normal-weight individuals suggests that people with the largest postprandial insulin response achieve the greatest suppression of appetite, leading to substantially lower food and energy intake. This supports the hypothesis that meals containing carbohydrate may be satiating in insulin-sensitive overweight individuals, but less so in more insulin-resistant prediabetic individuals with obesity and even less in individuals with obesity and T2DM. Hwang et al. (39) used magnetic resonance spectroscopy scanning of the occipital lobe to measure changes in intracerebral glucose levels during a hyperglycemic clamp in healthy participants, nondiabetic participants with obesity, and participants with poorly controlled T2DM. They found that changes in intracerebral glucose levels were significantly different across groups after controlling for age and sex. Brain glucose uptake was lower among participants with obesity and T2DM when compared with lean participants. This study shows that individuals with obesity and T2DM have progressively reduced brain glucose uptake, supporting the hypothesis that the satiating and rewarding effects of carbohydrate-rich diets may diminish as insulin resistance increases. The fact that prediabetic individuals with high FI values compared with those with low FI values tend to lose more weight (possibly through better satiety) in response to carbohydrates might be explained by those with higher FI values being better able to overcome insulin resistance by increasing insulin secretion. This is supported by strong correlations between the baseline FI values and 2-h insulin area under the curve values found among prediabetic individuals (FPG = 100–125 mg/dL) in the DiOGenes study (r = 0.73; p < 0.001; n = 132; M.F. Hjorth, unpublished analysis).
In patients with T2DM, satiety signals may depend more on other satiety hormones that are released mainly in response to fats and protein [for example, cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), and peptide YY (PPY)] reaching the small intestine (8). If this is the case, high-carbohydrate (low-fat) meals would be more effective in producing weight loss in normoglycemic individuals with obesity than in prediabetic individuals with obesity. Individuals with T2DM would benefit more from diets with reduced carbohydrate content (and more fat and protein).
A ROLE FOR THE MICROBIOME IN RESPONSES TO HIGH-FIBER DIETS
The microbiome might also be involved in determining how glycemic control impacts weight change for different diets. The administration of short-chain fatty acids (SCFAs) has been reported to result in a wide range of health benefits, including improvements in blood lipid profiles, glucose homeostasis, and body composition, and in reduced body weight (11). However, studies tend to investigate all SCFAs as a whole and neglect to report the specific effects associated with individual SCFAs, the most abundant of which are acetate, propionate, and butyrate (11). Members of the phylum Bacteroidetes are known to be efficient degraders of dietary fiber and include the genera Bacteroides and Prevotella (65). In vitro, the Prevotella-driven and Bacteroides-driven microbiota have been shown to produce different amounts and profiles of SCFAs from the same carbohydrate substrates (14). Therefore, the differences in the ratio of Prevotella to Bacteroides that have been observed to affect weight-loss responses to a 6-month fiber-rich diet (32, 35) might potentially be explained by the efficacy of energy harvested primarily as SCFAs (70) or it may be that the production of SCFAs affects appetite either directly in the brain or through different signaling pathways that influence the secretion of gastrointestinal hormones (11, 68) and, thereby, also circulating glucose and insulin. Improvements in postprandial blood glucose and insulin after dietary fiber intake were recently found to be positively associated with the abundance of Prevotella (43). Therefore, gut microbiota profiles may be part of why pretreatment insulinemia or glycemia status, or both, impacts the response to different diets.
STRENGTHS AND LIMITATIONS OF THE EVIDENCE
Generating evidence to support precision medicine is challenging, but testing for interactions in randomized clinical trials provides a potentially efficient means of doing so, especially when the results are replicated in multiple studies (58). Studies in this review were largely reanalyses of completed dietary interventions, and there were no prospective randomized studies in which stratification on the basis of glycemic control had been prespecified. For this reason publication bias is likely.
There is no generally accepted way to stratify participants based on their insulinemia or glycemia status, or both. Thus, studies have analyzed data using different cutoffs before deciding which cutoff to report. To present the most transparent results, our recent publications have used the most widely accepted FPG cutoffs recommended by the American Diabetes Association (2) and the median split of FI values. This post hoc approach may be considered a strength in that the analyses can be regarded as being double-blind with respect to the insulinemia or glycemia status, or both, of the participants. Differences in responses to different diets cannot have been influenced by the knowledge of the participants or investigators.
The reasons for the differences between some of the studies are unclear. Different studies were performed in populations of different origins, with differences in habitual diets, and with different designs relative to the nature and duration of the intervention. Furthermore, compliance with the diets to which participants were randomized is likely to differ substantially between the studies and might partly explain the differences between studies. Some studies provided intensive counseling by a dietitian and ensured that dietary records were completed throughout the study, while others did neither of these. In the studies with little or no dietitian counseling and without dietary records maintained throughout, participants’ compliance after months or years with the diet to which they had been randomized should be questioned. In two of the studies providing substantial evidence for using FPG (and FI) values as pretreatment markers for selecting an appropriate diet to combat obesity, all foods were provided free of charge at a specially designed supermarket where a barcode scanner was used to ensure the correct diet composition (33, 34). We need more well-controlled dietary studies to move this line of research forward. When possible, we need to use biomarkers of dietary compliance. Finally, differences in the results, in particular within studies using FI values, might be due to extensive residual confounding; for example, FPG values and microbiota composition are likely to play vital parts.
Throughout this review, several significant and borderline significant findings have been presented. When evaluating these findings study by study, they do not necessarily imply clinical significance, nor do they present effect sizes large enough to warrant diet personalization. However, at this stage, the superior diets tailored to individual glucose levels should be designed using evidence from multiple studies. An example of this would be to include participants with fasting glucose values between 100 (or possibly as low as 90) and 125 mg/dL for whom a diet high in fiber and protein but with a low GI (Figure 2a–d) would be expected to result in a more than 4 kg greater weight loss during 6 months.
CONCLUSIONS
A relatively large number of studies have investigated pretreatment measures of glycemia and insulinemia to determine whether they would be useful predictors of weight loss success for individuals following specific diets. Promising data suggest that based on FPG values at least three groups of individuals—normoglycemic, prediabetic, and diabetic—respond differently to different diets. In particular, individuals with elevated FPG values (prediabetic individuals) respond better to diets high in fiber and with a low GI, whereas individuals with high FPG values (mostly individuals with T2DM) should also reduce their total carbohydrate and increase their fat intake. Clearly, using FPG values to predict a response to a specific diet should be explored more. Insulin-30 values, but not FI values alone, appear to be the best measure of insulinemia for predicting the response to a diet. Most studies find that those participants with high insulin-30 values benefit from low-glycemic-load diets, while those with low insulin-30 values do not seem to differ in their responses to different diets. The most promising predictor of responses to different diets is the combination of FPG and FI values.
The conclusions from this review are that pretreatment glycemia or insulinemia status, or both, may predict responses to different diets. The data presented should be seen as preliminary since most were obtained from retrospective reanalyses of studies of different dietary interventions. Despite these limitations, being able to match a person to an appropriate diet for weight management based on biomarkers would be a great leap forward for our field.
FUTURE DIRECTIONS
We call for researchers to pursue this line of research by reanalyzing completed studies of dietary interventions, intentionally classifying participants along these parameters before beginning such studies, and exploring mechanisms by which glycemia or insulinemia status, or both, might impact weight loss and its maintenance. There are other lines of research that might also be pursued. For example, weight loss interventions themselves can dramatically change glycemia and insulinemia statuses, raising the issue of whether the optimum diet for maintaining weight loss might be different from the one that produces the greatest weight loss. Additionally, we need to better understand if and how physical activity might interact with diet composition to affect weight loss in different groups because physical activity is an effective tool for reducing insulin resistance and improving glucose metabolism. Demonstrating our ability to deliver personalized nutrition (or personalized lifestyle) interventions would move our field forward and help resolve the seemingly constant argument about which diet is the best.
DISCLOSURE STATEMENT
The work reported in this manuscript was funded by grants from Gelesis, Inc. M.F.H., Y.Z., and A.A. are co-inventors on a pending provisional patent application for the use of biomarkers to predict responses to weight loss diets. A.A. is coinventor of other related patents and patent applications that are owned by UCPH, in accordance with Danish law. Y.Z. is CEO of Gelesis, Inc. A.A. and J.O.H. are consultants for Gelesis, Inc., providing scientific advice unrelated to the current review manuscript. J.O.H. has stock options in Retrofit and is a member of Shakabuku LLC. A.A. is also a consultant or member of the advisory boards of Groupe Éthique et Santé, France; Nestlé Research Center, Switzerland; Weight Watchers, United States; BioCare Copenhagen; Zaluvida, Switzerland; Basic Research, United States; Novo Nordisk, Denmark; and Saniona, Denmark. M.F.H and A.A. are coauthors of the book Spis dig slank efter dit blodsukker (Eat according to your blood sugar and be slim), published by Politikens Forlag, Denmark, and of other books about personalized nutrition for weight loss. A.A. is co-owner and member of the board of the consultancy company Dentacom ApS, Denmark, and cofounder and co-owner of the UCPH spin-off Mobile Fitness A/S and Flax-Slim ApS. M.F.H. and A.A. are cofounders and co-owners of the UCPH spin-off the Personalized Weight Management Research Consortium ApS (http://Gluco-diet.dk).
LITERATURE CITED
- 1.Ajala O, English P, Pinkney J. 2013. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr 97:505–16 [DOI] [PubMed] [Google Scholar]
- 2.Am. Diabetes Assoc. 2015. 2. Classification and diagnosis of diabetes. Diabetes Care 39(Suppl. 1):S8–16 [Google Scholar]
- 3.Astrup A, Grunwald G, Melanson E, Saris W, Hill J. 2000. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int. J. Obes 24:1545–52 [DOI] [PubMed] [Google Scholar]
- 4.Astrup A, Hjorth MF. 2017. Low-fat or low carb for weight loss? It depends on your glucose metabolism. EBioMedicine 22:20–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astrup A, Kristensen M, Gnessi L, Watanabe M, Svacina S, et al. 2014. Oral administration of Gelesis100, a novel hydrogel, significantly decreases body weight in overweight and obese subjects. Poster presented at the ICE/ENDO Meeting, Chicago, IL, June 12–14 [Google Scholar]
- 6.Astrup A, Larsen TM, Harper A 2004. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet 364:897–99 [DOI] [PubMed] [Google Scholar]
- 7.Ballesteros-Pomar MD, Calleja-Fernandez AR, Vidal-Casariego A, Urioste-Fondo AM, Cano-Rodríguez I.2010. Effectiveness of energy-restricted diets with different protein:carbohydrate ratios: the relationship to insulin sensitivity. Public Health Nutr. 13:2119–26 [DOI] [PubMed] [Google Scholar]
- 8.Belza A, Ritz C, Sorensen MQ, Holst JJ, Rehfeld JF, Astrup A. 2013. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am. J. Clin. Nutr 97:980–89 [DOI] [PubMed] [Google Scholar]
- 9.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, et al. 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–25 [DOI] [PubMed] [Google Scholar]
- 10.Byrd JB. 2016. Personalized medicine and treatment approaches in hypertension: current perspectives. Integr. Blood Press. Control 9:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne CS, Chambers ES, Morrison DJ, Frost G. 2015. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes 39:1331–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, et al. 2016. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int. J. Epidemiol 46:578–88 [DOI] [PubMed] [Google Scholar]
- 13.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. 2008. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am. J. Clin. Nutr 87:303–9 [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. 2017. Fiber-utilizing capacity varies in Prevotella-versus Bacteroides-dominated gut microbiota. Sci. Rep 7:2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornier M, Donahoo WT, Pereira R, Gurevich I, Westergren R, et al. 2005. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes. Res 13:703–9 [DOI] [PubMed] [Google Scholar]
- 16.Cotillard A, Kennedy SP, Kong LC, Prifri E, Pons N, et al. 2013. Dietary intervention impact on gut microbial gene richness. Nature 500:585–88 [DOI] [PubMed] [Google Scholar]
- 17.Dashti HM, Mathew TC, Khadada M, Al-Mousawi M, Talib H, et al. 2007. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell. Biochem 302:249–56 [DOI] [PubMed] [Google Scholar]
- 18.De Luis D, Aller R, Izaola O, Sagrado MG, Conde R. 2006. Differences in glycaemic status do not predict weight loss in response to hypocaloric diets in obese patients. Clin. Nutr 25:117–22 [DOI] [PubMed] [Google Scholar]
- 19.Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. 2008. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am. J. Clin. Nutr 88:1232–41 [DOI] [PubMed] [Google Scholar]
- 20.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. 2007. Effects of a low–glycemic load versus low-fat diet in obese young adults: a randomized trial. JAMA 297:2092–102 [DOI] [PubMed] [Google Scholar]
- 21.Estruch R, Corella D, Salas-Salvado J, Hjorth MF, Astrup A, et al. 2017. Pretreatment fasting plasma glucose determines weight loss on high-fat diets: the PREDIMED Study. Rep., CIBERobn, Madrid. http://www.gelesis.com/pdf/estruch-redimed-ada2017.pdf [Google Scholar]
- 22.Estruch R, Ros E, Salas-Salvadó J, Covas M, Corella D, et al. 2013. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med 368:1279–90 [DOI] [PubMed] [Google Scholar]
- 23.Faria G, Preto J, Almeida AB, Guimarães JT, Calhau C, Taveira-Gomes A. 2014. Fasting glycemia: a good predictor of weight loss after RYGB. Surg. Obes. Relat. Dis 10:419–24 [DOI] [PubMed] [Google Scholar]
- 24.Feinman RD, Pogozelsld WK, Astrup A, Bernstein RK, Fine EJ, et al. 2015. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31:1–13 [DOI] [PubMed] [Google Scholar]
- 25.Flint A, Gregersen NT, Gluud LL, Møller BK, Raben A, et al. 2007. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br. J. Nutr 98:17–25 [DOI] [PubMed] [Google Scholar]
- 26.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, et al. 2010. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann. Intern. Med 153:147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franz MJ. 2007. The dilemma of weight loss in diabetes. Diabetes Spectr. 20:133–36 [Google Scholar]
- 28.Gardner CD, Offringa LC, Hartle JC, Kapphahn K, Cherin R. 2016. Weight loss on low-fat vs. low-carbohydrate diets by insulin resistance status among overweight adults and adults with obesity: a randomized pilot trial. Obesity 24:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, et al. 2018. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 319:667–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghani RA, Shyam S, Arshad F, Wahab NA, Chinna K, et al. 2014. The influence of fasting insulin level in post-gestational diabetes mellitus women receiving low-glycaemic-index diets. Nutr. Diabetes 4:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall KD, Bemis T, Brychta R, Chen KY, Courville A, et al. 2015. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 22:427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hjorth MF, Blædel T, Bendtsen LQ, Lorenzen JK, Holm JB, Kiilerich P, et al. 2018. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int. J. Obes In press. 10.1038/s41366-018-0093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hjorth MF, Due A, Larsen TM, Astrup A. 2017. Pre-treatment fasting plasma glucose modifies dietary weight loss maintenance success: results from a stratified RCT. Obesity 25:2045–48 [DOI] [PubMed] [Google Scholar]
- 34.Hjorth MF, Ritz C, Blaak EE, Saris WH, Langin D, et al. 2017. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am. J. Clin. Nutr 106:499–505 [DOI] [PubMed] [Google Scholar]
- 35.Hjorth MF, Roager HM, Larsen TM, Poulsen SK, Licht TR, et al. 2018. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes 42:580–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjorth MF, Zohar Y, Astrup A, Urban LE, Sayer RD, et al. 2017. Pretreatment fasting plasma glucose and insulin determine long-term dietary weight loss success on low-carbohydrate vs. low-fat diet. Rep., Univ. Colo. Anschutz Med. Campus, Aurora. http://www.gelesis.com/pdf/hjorth-cho-ada2017.pdf
- 37.Hron BM, Ebbeling CB, Feldman HA, Ludwig DS. 2015. Relationship of insulin dynamics to body composition and resting energy expenditure following weight loss. Obesity 23:2216–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM. 2012. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 28:1016–21 [DOI] [PubMed] [Google Scholar]
- 39.Hwang JJ, Jiang L, Hamza M, Rangel ES, Dai F, et al. 2017. Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JC1 Insight 2:e95913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang C, Zhang Y, Zhu X, Liu K, Wang X, et al. 2016. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J. Clin. Endocrinol. Metab 101:4681–89 [DOI] [PubMed] [Google Scholar]
- 41.Kong LC, Wuillemin PH, Bastard JP, Sokolovska N, Gougis S, et al. 2013. Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian network approach. Am. J. Clin. Nutr 98:1385–94 [DOI] [PubMed] [Google Scholar]
- 42.Korem T, Zeevi D, Zmora N, Weissbrod O, Bar N, et al. 2017. Bread affects clinical parameters and induces gut microbiome–associated personal glycemic responses. Cell Metab. 25:1243–53 [DOI] [PubMed] [Google Scholar]
- 43.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, et al. 2015. Dietary fiber–induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22:971–82 [DOI] [PubMed] [Google Scholar]
- 44.Larsen TM, Dalskov S, van Baak M, Jebb SA, Papadaki A, et al. 2010. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med 363:2102–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, et al. 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–46 [DOI] [PubMed] [Google Scholar]
- 46.Leslie W, Taylor R, Harris L, Lean M. 2017. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta-analysis. Int. J. Obes 41:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, Hong J, Xu X, Feng Q, Zhang D, et al. 2017. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med 23:859–68 [DOI] [PubMed] [Google Scholar]
- 48.Livingstone KM, Celis-Morales C, Papandonatos GD, Erar B, Florez JC, et al. 2016. FTO genotype and weight loss: systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 354:i4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayer EA, Tillisch K, Gupta A. 2015. Gut/brain axis and the microbiota. J. Clin. Investig 125:926–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClain AD, Otten JJ, Hekler EB, Gardner CD. 2013. Adherence to a low-fat vs. low-carbohydrate diet differs by insulin resistance status. Diabetes Obes. Metab 15:87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLaughlin T, Abbasi F, Carantoni M, Schaaf P, Reaven G. 1999. Differences in insulin resistance do not predict weight loss in response to hypocaloric diets in healthy obese women. J. Clin. Endocrinol. Metab 84:578–81 [DOI] [PubMed] [Google Scholar]
- 52.NCD Risk Factor Collab. 2016. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387:1377–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NCD Risk Factor Collab. 2016. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387:1513–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okun MS. 2017. Management of Parkinson disease in 2017: personalized approaches for patient-specific needs. JAMA 318:791–92 [DOI] [PubMed] [Google Scholar]
- 55.Ophir E, Bobisse S, Coukos G, Harari A, Kandalaft LE. 2016. Personalized approaches to active immunotherapy in cancer. Biochim. Biophys. Acta 1865:72–82 [DOI] [PubMed] [Google Scholar]
- 56.Petersen M, Taylor M, Saris W, Verdich C, Toubro S, et al. 2006. Randomized, multi-center trial of two hypo-energetic diets in obese subjects: high- versus low-fat content. Int. J. Obes 30:552–60 [DOI] [PubMed] [Google Scholar]
- 57.Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, et al. 2005. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE trial. Diabetes Care 28:2939–41 [DOI] [PubMed] [Google Scholar]
- 58.Pletcher MJ, McCulloch CE. 2017. The challenges of generating evidence to support precision medicine. JAMA Intern. Med 177:561–62 [DOI] [PubMed] [Google Scholar]
- 59.Poulsen SK, Due A, Jordy AB, Kiens B, Stark KD, et al. 2014. Health effect of the new Nordic diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am. J. Clin. Nutr 99:35–45 [DOI] [PubMed] [Google Scholar]
- 60.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riederer P, Korczyn AD, Ali SS, Bajenaru O, Choi MS, et al. 2017. The diabetic brain and cognition. J. Neural Transm 124:1431–54 [DOI] [PubMed] [Google Scholar]
- 62.Rock CL, Flatt SW, Pakiz B, Quintana EL, Heath DD, et al. 2016. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1-year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism 65:1605–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, et al. 2009. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med 360:859–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salinardi TC, Batra P, Roberts SB, Urban LE, Robinson LM, et al. 2013. Lifestyle intervention reduces body weight and improves cardiometabolic risk factors in worksites. Am. J. Clin. Nutr 97:667–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schroeder BO, Bäckhed F. 2016. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med 22:1079–89 [DOI] [PubMed] [Google Scholar]
- 66.Shyam S, Arshad F, Ghani RA, Wahab NA, Safii NS, et al. 2013. Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: a six months randomized trial. Nutr. J 12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snorgaard O, Poulsen GM, Andersen HK, Astrup A. 2017. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 5:e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, et al. 2012. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes 61:364–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–31 [DOI] [PubMed] [Google Scholar]
- 71.Urban LE, Roberts SB, Zohar Y, Astrup A, Hjorth MF, et al. 2017. Pretreatment fasting glucose concentration predicts weight loss on a high-fiber, low glycemic load diet: the healthy weight for living study. Rep., Tufts Univ., Hum. Nutr. Res. Cent. Aging http://www.gelesis.com/pdf/urban_worksite_ada2017.pdf [Google Scholar]
- 72.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. 2001. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int. J. Obes. Relat. Metab. Disord 25:1206–14 [DOI] [PubMed] [Google Scholar]
- 73.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, et al. 2012. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143:913–16 [DOI] [PubMed] [Google Scholar]
- 74.Walker L, Mirza N, Yip V, Marson A, Pirmohamed M. 2015. Personalized medicine approaches in epilepsy. J. Intern. Med 277:218–34 [DOI] [PubMed] [Google Scholar]
- 75.Wan Y, Wang F, Yuan J, Li J, Jiang D, et al. 2017. Effects of macronutrient distribution on weight and related cardiometabolic profile in healthy non-obese Chinese: a 6-month, randomized controlled-feeding trial. EBioMedicine 22:200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolever T 2016. Personalized nutrition by prediction of glycaemic responses: fact or fantasy? Eur. J. Clin. Nutr 70:411–13 [DOI] [PubMed] [Google Scholar]
- 77.Woods SC. 2013. Metabolic signals and food intake: forty years of progress. Appetite 71:440–44 [DOI] [PubMed] [Google Scholar]
- 78.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, et al. 2015. Personalized nutrition by prediction of glycemic responses. Cell 163:1079–94 [DOI] [PubMed] [Google Scholar]


