Abstract
Simple Summary
Anthrax is a globally distributed, neglected, underreported, soil-borne zoonotic disease. In West Africa, the disease is hyper-endemic, severely affecting the livestock sector. Many challenges exist to control the disease in this region, particularly constraints on financial and human resources. Therefore, methods that can be utilized to improve reporting, guide and prioritize surveillance and control activities and rationalize the allocation of limited resources are crucial. In this study, we showed how to optimize the use of fragmented, heterogeneous and limited precise reporting data of anthrax in Burkina Faso, Ghana, Togo, Benin and Niger to understand risk periods as well as identify and predict risk areas. To achieve this, we used anthrax data from different databases in combination with environmental and climate variables and geospatial remote sensing techniques. Our study demonstrated that the number of anthrax outbreaks by month increase with the increasing monthly rates of change in precipitation and normalized difference vegetation index (NDVI) during the transition period from the dry to the wet season. Livestock density, precipitation, NDVI and alkaline soils were the main predictors of anthrax suitability in the region. Our findings on anthrax seasonality and ecological suitability can inform surveillance, prevention and control programs undertaken by animal and public health authorities and enhance collaborative One Health strategies.
Abstract
Anthrax is hyper-endemic in West Africa affecting wildlife, livestock and humans. Prediction is difficult due to the lack of accurate outbreak data. However, predicting the risk of infection is important for public health, wildlife conservation and livestock economies. In this study, the seasonality of anthrax outbreaks in West Africa was investigated using climate time series and ecological niche modeling to identify environmental factors related to anthrax occurrence, develop geospatial risk maps and identify seasonal patterns. Outbreak data in livestock, wildlife and humans between 2010 and 2018 were compiled from different sources and analyzed against monthly rates of change in precipitation, normalized difference vegetation index (NDVI) and land surface temperature. Maximum Entropy was used to predict and map the environmental suitability of anthrax occurrence. The findings showed that: (i) Anthrax outbreaks significantly (99%) increased with incremental changes in monthly precipitation and vegetation growth and decremental changes in monthly temperature during January–June. This explains the occurrence of the anthrax peak during the early wet season in West Africa. (ii) Livestock density, precipitation seasonality, NDVI and alkaline soils were the main predictors of anthrax suitability. (iii) Our approach optimized the use of limited and heterogeneous datasets and ecological niche modeling, demonstrating the value of integrated disease notification data and outbreak reports to generate risk maps. Our findings can inform public, animal and environmental health and enhance national and regional One Health disease control strategies.
Keywords: anthrax, climate variability, ecological niche modeling, time series analysis, seasonality, West Africa
1. Introduction
Anthrax is a neglected, underreported, soil-borne zoonotic disease caused by the Gram-positive, spore-forming bacterium Bacillus anthracis. It mainly affects wild and domestic herbivores such as cattle, sheep and goats. It occurs globally, and remains enzootic in many regions of the world, particularly sub-Saharan Africa, Central and South-western Asia, and Central and South America [1,2]. Though vaccines are available for veterinary use, they are rarely sought. The overall disease burden and economic impact of anthrax in livestock are not fully known; however, epizootics occur every year and result in the deaths of hundreds to thousands of animals, with subsequent negative socio-economic consequences and impacts on food security. Significant outbreaks in wildlife have been also reported in many regions of the world [3,4,5,6,7,8]. Humans most often contract cutaneous anthrax from contact with infected animals, their carcasses, or by handling the meat, hides, bones, and other materials from those animals, or gastrointestinal anthrax from consuming meat from infected animals. Inhalation anthrax is less common but may represent an occupational hazard in mills and tanneries processing wool/hair, skins and hides from infected animals. An estimated 2000 to 20,000 human anthrax cases occur annually worldwide [9].
The primary reservoir for anthrax is the environment—soil—and previous studies have shown that B. anthracis spores are mainly concentrated in the upper few centimeters of ‘suitable’ soils, in low lying depressions or eroded shallow and deep rich alluvial soils, particularly alkaline soils (pH = 6–8), characterized by high calcium and organic matter content [10,11,12]. The spores can persist in the soil under extreme environmental and climatic conditions for long periods of time, and be a source for re-emergence of disease when conditions become favorable, contributing to disease persistence [6,13,14]. Livestock and wildlife likely contract the disease while grazing and ingesting forage or soil contaminated with B. anthracis spores, browsing on vegetation contaminated by carrion flies, or by percutaneous exposure from biting flies, and possibly spore inhalation [1,10,15]. Carnivorous and omnivorous animals can also become infected, usually by scavenging on the carcasses of infected animals. Vegetative bacilli are shed in blood and other discharges from infected animals that are dying or dead, and those bacilli then sporulate at temperatures between 9 and 12 °C and contaminate surrounding soil and water, where they complete this cycle of infection [1]. Factors associated with the occurrence of outbreaks around these contaminated foci can be both natural (i.e., heavy rains and floods during the wet season, followed by a drought; landslides) and anthropogenic (land-use change, i.e., agricultural encroachment into pastoral areas; land excavation such as for irrigation, digging canals, roads or houses).
Temperature and precipitation patterns are considered the main constraints to anthrax sporulation and for the onset of anthrax outbreaks, which varies among locations [16]: it may be observed after prolonged periods of hot, dry weather that follow heavy rains and flooding, or with the onset of rains ending a period of drought [1,5,10,17,18,19]. Vegetation growth (spring green-up) has been associated with anthrax seasonality [5]. However, few studies have demonstrated or measured this directly [20]. Other factors favoring the occurrence and spread of anthrax outbreaks include high livestock density [21], and movement and grazing such as transhumance population movement and trade [18,22]. Nevertheless, the transmission mechanisms, as well as the environmental and climatic risk factors associated with anthrax outbreaks and its seasonality, require more investigation for better understanding disease dynamics and devising appropriate prevention and control measures.
Anthrax is a significant problem in West Africa, where many countries in the region frequently experience outbreaks. At the end of the 20th century, the region was considered the largest hyper-endemic and epidemic anthrax area in the world due to widespread civil unrest and the resultant breakdown of veterinary health care systems, and the ecological and socio-economic factors present [10]. Anthrax remains a devastating disease in the region, especially in rural areas where livestock farming is a primary occupation and where capacities of veterinary services are weak. Lack of awareness, beliefs and behaviors, such as consumption of diseased animals, slaughtering of sick animals and eating or handling meat from infected animals, and dumping of dead carcasses in the open area, are some of the compounding factors of the persistence of anthrax in many countries of the sub-region [23]. As with many countries with poorly resourced public and animal health services, the disease is likely underreported: anthrax surveillance is often limited, and frequently animal outbreaks are detected only when human cases are identified [17,18]. There are few data or literature reports that accurately describe the occurrence of anthrax in either humans or in livestock and wildlife, areas of risk, and anthrax epidemiology in these countries [17,18]. However, this information is crucial to better prevent and control disease occurrence and inform surveillance. Ecological niche modeling [24] is increasingly used for disease mapping in order to develop risk maps, and to identify temporal patterns and specific environmental factors that may be used to predict when outbreaks may occur [25]. The ecological suitability for anthrax occurrence has been predicted using a genetic algorithm for rule-set prediction (GARP) [22], random forest [26], boosted regression trees [7] and the Maximum Entropy (MaxEnt) [27,28,29,30,31] methods.
In this study, we analyzed the seasonality and modeled the ecological niche of anthrax in West Africa in order to develop risk maps that can inform surveillance, prevention and control strategies undertaken by veterinary and public health authorities. The study area included Benin, Burkina Faso, Ghana, Niger and Togo, which was the area of focus for an FAO regional workshop on anthrax prevention and control in 2015 (http://www.fao.org/africa/news/detail-news/en/c/332755/ accessed on 19 July 2019). This region is frequently affected by anthrax cases and outbreaks in livestock and humans [1,7]. The objectives of this study were to: (a) test whether the outbreaks increased with incremental changes in climate variables (precipitation, NDVI and temperature) during the early wet season as suggested by anecdotal evidence of field observations; (b) identify ecological risk factors; and (c) apply MaxEnt in combination with limited and heterogeneous datasets on anthrax occurrences to generate risk maps.
2. Materials and Methods
2.1. Study Area
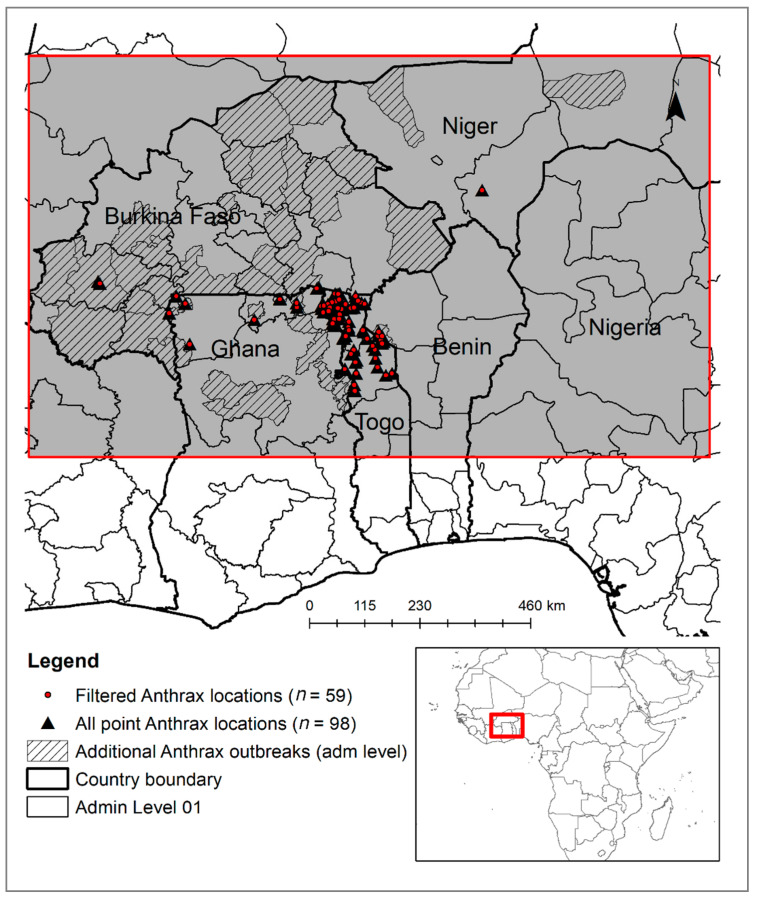
The study area was located between 8°0′ N and 15°25′ N and 5°50′ W and 7°25′ E in West Africa across the climatic and ecological transition area between the Saharan zone in the north and the Guinean zone in the south. It included northern Togo, Ghana, Benin, Burkina Faso and southern Niger (Figure 1). Three main eco-climatic and agro-ecological zones can be identified in this study region from the north to the south, based on average annual rainfall and vegetation types [32]: (1) the Sahelian zone—an arid belt characterized by average rainfall between 250 and 500 mm and the steppe/open savannah, i.e., grassland with scattered acacia trees, including the northern and southern region of Burkina Faso and Niger, respectively; (2) the Sahelo-Sudanian zone—a semi-arid belt with an average annual rainfall of 500–900 mm and open tree to wooded savannah, including mostly Burkina Faso as well as the northern region of Ghana, Togo and Benin; (3) the Sudano-Guinean zone—a sub-humid belt characterized by average annual rainfall between 900 and 1100 mm and wooded savannah to open woodlands. This zone includes the central region of Ghana, Togo and Benin.
Figure 1.
Study area and anthrax outbreak locations before (black triangle) and after (red dots) filtering spatial auto-correlated records (point location data source: EMPRES-i, GIMD and FAO workshop). The dashed polygons are anthrax-affected districts (adm02 level) for which geo-locations of outbreaks are missing (polygon data source: OIE-WHAIS).
The main livestock production systems in the study region are pastoralism and agro-pastoralism mixed with subsistence agriculture, with significant livestock movement along short and long-range migration routes. The seasonal movement of livestock herds is mainly transhumant to access the seasonal availability of water and forage resources during the dry season in the south and during the wet season in the north. Pastoralism mainly occurs in the Sahelian zone [33].
2.2. Anthrax Outbreak Occurrence Data
Data available from open information sources on anthrax cases and outbreaks in domestic livestock, wildlife and humans that occurred between January 2010 and November 2018 were collected, georeferenced and mapped. Data sources included: (a) official periodic disease incidence and outbreak data reported by countries to the World Organization for Animal Health (OIE) and available from the World Animal Health Information System (OIE-WAHIS); data are reported by year and by month or semi-annual reporting period, disease presence or specific outbreak occurrence at the first or second (regional/provincial) administrative level, and may include number of animal cases and susceptible population; (b) FAO Global Animal Disease Information System (EMPRES-i), which contains data on confirmed anthrax outbreaks in livestock, wildlife and humans collected and provided by FAO field officers and field mission reports, and additionally data on confirmed outbreaks obtained from national authorities and media reports; (c) Global Incident Map Database (GIMD; http://outbreaks.globalincidentmap.com/ accessed on 19 July 2019), which reports disease event information on: date of the event, location (country name and city), animal species infected, and a hyperlink to the source of information including geographical coordinates and the number of infected/dead people or animals (domestic or wildlife); anthrax outbreak event data for the study area in West Africa were manually entered for analysis; (d) data (presence records) acquired from reports, presentations and maps provided by the official authorities involved in the 2015 FAO regional anthrax workshop (hereafter named FAO workshop). Locations were georeferenced but information on date and animal species infected were missing. The disease data were collated in a single database and checked for duplication and inconsistency based on their spatial and temporal information. Records with the same coordinates and date were considered duplicates because they were related to the same event.
Two sets of data were generated: (1) point location data (i.e., EMPRES-i, GIMD and FAO workshop) that were used for training and validation of the prediction model and (2) polygon data (i.e., OIE-WAHIS) that were used for validation purposes as well as for analyzing and understanding the seasonality of anthrax in the region (Figure 1).
2.3. Anthrax Seasonality
The OIE data were used to estimate a monthly median number of anthrax outbreaks by administrative units and by country. The decadal median time series of precipitation (RFE2; 0.1-degree spatial resolution; years: 1995–2016), Normalized Difference Vegetation Index (eModis NDVI C6, 250 m spatial resolution; 2007–2016) and land surface temperature (Modis LST, 0.05-degree spatial resolution; years; 2002–2016) aggregated by administrative units were downloaded from the USGS FEWS NET Data Portal (https://earlywarning.usgs.gov/fews accessed on 19 July 2019) for each of the OIE administrative units reporting anthrax occurrences. Then, the median monthly precipitation (mm), NDVI and temperature (°C) were calculated at the national level and for the whole study area to obtain monthly profiles of the environmental variables. We hypothesized that anthrax outbreaks were related to incremental changes in monthly precipitation and vegetation growth and decremental changes in monthly temperature during the early wet season (i.e., the transition period between the dry and the wet season). To identify the month of key climatic and phenological transitions (rate of change), first derivative analysis was applied to the temporal profiles of precipitation, NDVI and temperature [34]. Because we calculated those changes between two subsequent months from January to June, the rate of change (first derivative) of the climate variable profiles (CV) was calculated by subtracting the median precipitation (or NDVI, temperature) value at month t + 1 from the median precipitation (or NDVI, temperature) value at month t (e.g., median precipitation for February—median precipitation for January) for each couple of subsequent months during the period of study. Large positive rates indicate the rapidity of monthly incremental change in the climate variable time series.
| FDCV = (CV(t+1) − CV(t))/Δmonth, | (1) |
where FDCI is the first derivative value of CV (precipitation, NDVI or temperature) between the month t and t + 1, CV(t) is the CV value at the month t, CV(t+1) is the CV value at the month t + 1 and Δmonth equals 1 as the interval.
We also calculated the second derivative of the climate variable time series to find the local maximum and minimum rates of change [34].
Then the median number of anthrax occurrences by month was regressed against the first and second derivatives (rates of change) of precipitation, NDVI and temperature. The monthly median anthrax occurrences, precipitation, NDVI and temperature were standardized to a mean of zero and a standard deviation of one prior to conducting the statistical analysis [26]. The statistical analysis was performed in R 3.1.0.
2.4. Ecological Niche Modelling
Predictor variables. The predictor variables selected to model the ecological niche for B. anthracis in this West African region were grouped in 4 categories (bioclimatic, vegetation, topography and livestock) and included: 19 bioclimatic variables describing temperature and precipitation obtained from the Worldclim database (http://worldclim.org accessed on 19 July 2019) [35]; 24 classes of soil obtained from the Digital Soil Map of the World [36]; 2 topographic variables (elevation and slope) from the Shuttle Radar Topography Mission [37]; 2 land surface variables (landform, Topographic Position Index) from the USGS Africa Land Surface Forms database [38]; species-specific (cattle, sheep and goat) livestock density (Gridded Livestock of the World v02, FAO 2010, [39]) and total livestock (FAO Africa Ruminants Tropical Livestock Units v1.0; 2015); a set of 16 vegetation indices (NDVI, a measure of vegetation greenness derived from multi-spectral remote sensing images) including monthly NDVI as well as the annual mean, minimum, maximum and standard deviation, and 12 NDVI maps representing the rate of change (i.e., first derivative), i.e., NDVI difference from montht+1 to montht1, as well as their descriptive statistics (e.g., maximum, minimum, median and standard deviation of the monthly rates of change). The vegetation indices were calculated from 16 years of a 10-day period of the satellite SPOT-Vegetation time series (1998–2014; http://www.vito-eodata.be/PDF/portal/Application.html#Home accessed on 19 July 2019). The predictor variables and their properties are listed in Table S1. Predictor variables were standardized to a mean of zero with a standard deviation of one, prior to performing the statistical analysis, as their values were different physical quantities and on different scales of magnitude [40]. Spatial analysis was performed in ArcGIS 10.0 (ESRI).
Modeling approach and evaluation. For the purpose of this study, a maximum entropy algorithm [27] implemented in the MaxEnt software package (version 3.3.3 k) was chosen to model anthrax suitability across the study area. MaxEnt has been widely used to model geographic suitability for many zoonotic infectious diseases, including anthrax [29,30]. It is robust and provides settings and parameters to deal with small sample sizes [41,42,43,44,45,46] and can handle categorical and continuous predictors interactively [46,47]. Briefly, MaxEnt inherently deals with, and is not sensitive to, spatial autocorrelation [48]. The probabilities of species (disease) presence are calculated without assumptions about the distribution of either species or predictors. In addition, MaxEnt generates response curves of each continuous predictor and bar graphs of categorical predictors, essential in interpreting model performance [49]. We explored different parameters and different combinations of response types [31]. In this study, the goodness-of-fit of the model was assessed by the Area under the Curve (AUC), which varies from no-better-than-random (0.5) to perfect (1). The relative contribution of a predictor to the model was assessed by the ‘heuristic estimate’ (percentage) and the Jackknife method calculated by MaxEnt. The least contributing predictors were removed stepwise, until the most parsimonious number of predictors resulting at least in an AUC > 0.80 was reached [46]. Parsimony in the number of predictors reduces the risk of overfitting and removes collinear variables [46].
We first removed highly intercorrelated (Pearson correlation coefficient computed by ArcGIS 10; absolute threshold > 0.7) variables within each group of predictors (bioclimatic, vegetation, topography and livestock) because multicollinearity may violate statistical assumptions and may alter model predictions [48].
Because presence-only methods are sensitive to sampling biases [27,50,51], the outbreak locations, which were mainly concentrated in Northern Togo, were first inspected for spatial autocorrelation using the Moran’s I statistic and then filtered using a threshold of 5 km [26,31] to improve model performance and avoid overfitting [52]. Specifically, the rarefy tool in the SDM ArcTool [52] was used to remove spatially autocorrelated occurrences by reducing multiple occurrence records (clusters of points) to a single (filtered) record within the specified distance. In addition, a bias file based on the Minimum Convex Polygons of the rarefied (filtered) outbreaks was used to reduce background sampling bias [52]. An averaged final model was then produced by entering the selected predictors into the MaxEnt program and by empirically setting the number of a random seed, and ten subsample runs (replicates). The Standard Deviations (SD) of the AUC of the ten replicates were calculated to assess the robustness of the model.
We followed Araújo and Guisan [53] to classify model accuracy: 0.6–0.7 poor, 0.7–0.8 average, 0.8–0.9 good and 0.9–1 excellent.
3. Results
3.1. Anthrax Outbreak Data
The number of reported outbreaks between 2010 and 2018 varied among the different data sources by month and year as well as in terms of different spatial units. Table S2 summarizes these data by country and data sources, while the spatial distribution is shown in Figure 1.
3.1.1. Point Location Data
Because no spatial and temporal overlap was observed among the outbreak point locations acquired from the GIMD (n = 7) and EMPRES-i (n = 13) databases, the data were considered independent (i.e., no duplicates). The point data acquired during the FAO workshop (n = 78) were also not coincident with the other point data and were pulled together in a single database (the outbreak point location dataset) to build the ecological niche model (n = 98) (Figure 1). The anthrax point data were available for Togo (83%), Ghana (11%), Benin (3%), Burkina Faso (2%) and Niger (1%) (Table S2).
3.1.2. Polygon Data
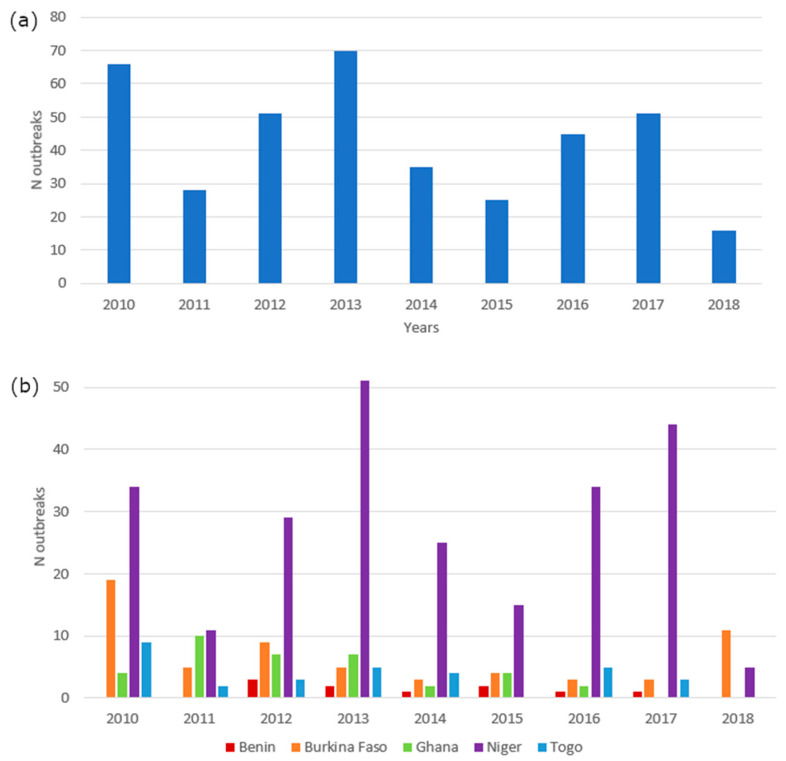
OIE-WHAIS periodical data (n = 387), which were only available at a sub-national level (i.e., regional/provincial) reported information on date, species and number of anthrax cases for each country. According to these data, the study area was most heavily affected during 2010, 2012, 2013 and 2017 (17%, 13%, 18% and 13%, respectively) (Figure 2a). Niger and Burkina Faso were highly affected (Figure 2b; Table S2), but only three geolocations were available from the outbreak point location dataset for the spatial risk modeling (Figure 1).
Figure 2.
Number of anthrax outbreaks by year across the study area (a) and by country (b) between January 2010 and November 2018 (OIE-WHAIS data).
3.2. Seasonality of Anthrax Occurrence
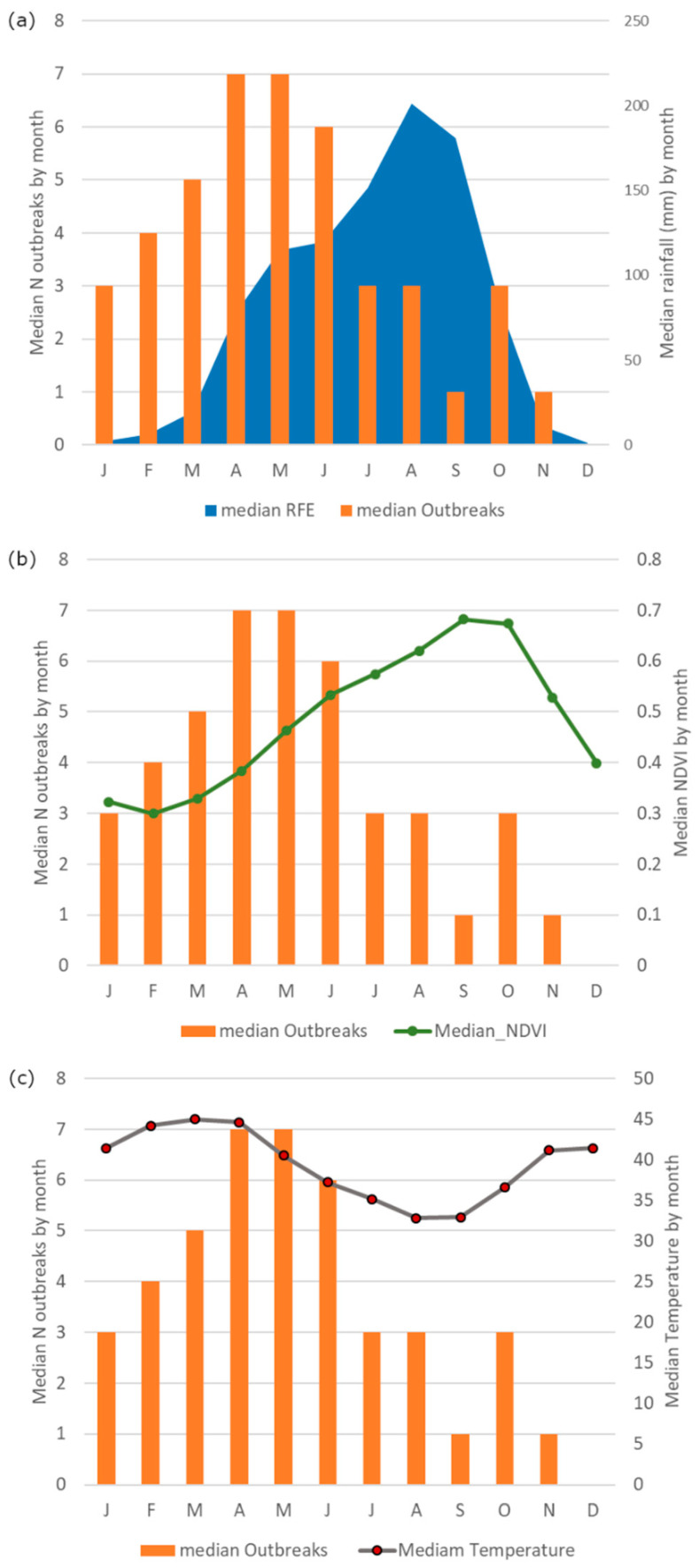
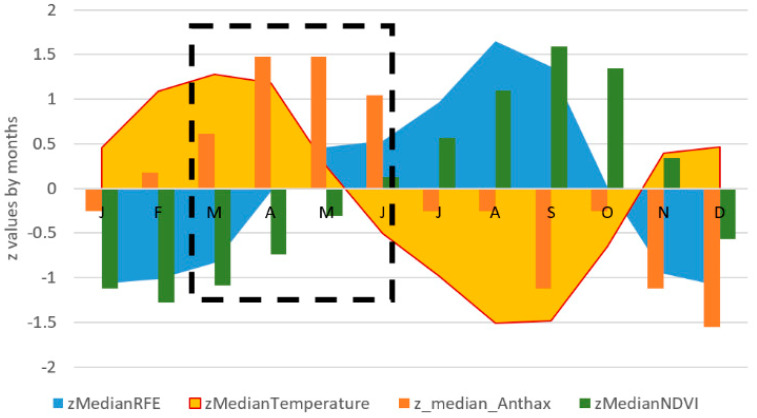
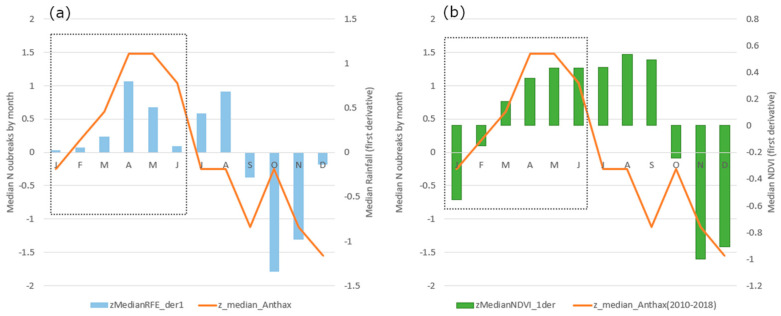
The outbreaks occurred in the study area throughout the year but were higher during the transition period from the dry to the wet season, particularly in April/May, when precipitation and vegetation increase, and the temperature starts decreasing after having reached the maximum values (45 °C) (Figure 3a–c). This pattern was observed in each country, except for Niger where the higher number of outbreaks was observed in July. Detailed charts of each country are reported in Figure S1. For the whole study area, the standardized median rainfall and NDVI were, respectively, higher in August/September and September/October, several months later than the peak of the standardized median number of anthrax outbreaks (Figure 3a,b). The standardized median temperature was higher in March, a month earlier than the anthrax peak (Figure 3c). On the contrary, the lowest numbers of outbreaks were observed during the wettest months (July to September), when the temperature was lowest, while the rainfall and vegetation reached the highest values. These patterns and periodicity are shown in Figure 4.
Figure 3.
Median number of anthrax outbreaks (orange bar) against (a) median rainfall (blue area), (b) median NDVI (green line) and (c) median temperature (grey line) by month in the study area.
Figure 4.
Periodicity of the standardized median number of anthrax outbreaks (z_median_Anthrax; orange bar), rainfall (zMedianRFE; blue area), NDVI (zMedianNDVI; green bars) and temperature (zMedianTemperature; yellow area) by month in the study area. The inset shows that the peak in anthrax outbreaks occurs between April and May, when the temperature decreases while rainfall and NDVI increase.
The standardized median number of outbreaks by month was significantly and positively related to the rate of change (first derivative) of standardized median precipitation by month during the period from January to June (linear regression, b = 1.8, t = 2.6, p = 0.05, adjusted R2 = 0.54). The peak of the standardized median number of outbreaks occurred in April–May, matching the period with the highest increment in precipitation (March–April), i.e., increased rate of change in standardized median precipitation by month (Figure 5a). During the same period (January–June), the standardized median number of outbreaks by month was positively related to the rate of change (first derivative) of standardized median NDVI by month (linear regression, b = 1.7, t = 5.5, p = 0.005, adjusted R2 = 0.85). The highest increase in vegetation growth occurred between April and May, fully matching the period with the highest number of anthrax occurrences (Figure 5b) and the maximum increase in precipitation rate. The relationship between the standardized median number of anthrax outbreaks and the second derivative of standardized median rainfall as well as the second derivative of standardized median NDVI by month was not significant (for both linear regressions, p > 0.05). There was also no significant relationship between the standardized median number of anthrax outbreaks and the standardized median temperature and its first and second derivatives by month (p > 0.05).
Figure 5.
Standardized median number of anthrax outbreaks (orange line) against (a) first derivative of standardized median precipitation (blue bars) by month and (b) first derivative of standardized median NDVI (green bars) by month. The inset of each chart shows that the number of outbreaks increases with incremental change in precipitation and NDVI during January–June.
Between the period January and June, the relation between the standardized median number of anthrax outbreaks, the first derivative of standardized median rainfall and the first derivative of standardized median NDVI by month explained 97% of the variance in anthrax outbreaks (linear regression, adjusted R2 = 0.97, p = 0.003, F = 77.26, y = 0.37 + 1.28 NDVI1st_derivative + 0.86 RFE1st_derivative) (Figure S2). The variance increased to 99% when the standardized median temperature by month was also included in the regression (Table 1). Indeed, the first derivative of standardized median NDVI was exponentially related to the first derivative of standardized median precipitation by month from January to June (adjusted R2 = 0.95, p = 0.003, F = 36.93, Figure S2).
Table 1.
Results of the linear regression between standardized median number of anthrax outbreaks: first derivative of standardized median rainfall, first derivative of standardized median NDVI and standardized median temperature by month during the period January–June.
| Variable | Estimate | Standard Error | t Value | p-Value |
|---|---|---|---|---|
| Intercept | 0.43 | 0.03 | 13.24 | 0.005 |
| zMedianNDVI (first derivative) | 1.12 | 0.08 | 13.7 | 0.005 |
| zMedianRFE (first derivative) | 1.08 | 0.11 | 10.3 | 0.009 |
| zMedianTemperature | −0.16 | 0.04 | −4.02 | 0.05 |
Residual standard error: 0.05 on 2 degrees of freedom (DF). Adjusted R-squared: 0.99. F-statistic: 316.8 on 3 and 2. DF p-value: 0.003.
3.3. MaxEnt Ecological Predictive Niche Model
The anthrax point locations were spatially autocorrelated (Moran I = 1.31, expected I= −0.012 n = 98 p = 0.04). After filtering, 59 independent and uncorrelated point locations were obtained for the ecological niche model (Figure 1). The predictors (continuous variables) that were not significantly correlated (Person correlation coefficient <0.7 and >−0.7 p < 0.05) are shown in Table 2.
Table 2.
Uncorrelated predictors based on the results of the correlation matrix. Total number of analyzed predictors in brackets. Legend and acronyms as of Table S1.
| N Uncorrelated Variable | Name | |
|---|---|---|
| Bioclimatic | 10 (out of 19) | Bio1, Bio2, Bio9, Bio11, Bio13, Bio14, Bio15, Bio18 |
| Vegetation | 17 (out of 32) | NDVI rate of change max, NDVI rate of change min, NDVI rate of change med, NDVI rate of change SD, NDVI rate of change between: June and May, Sept and Aug, Nov and Oct NDVI 03, NDVI 04, NDVI 07, NDVI 10, NDVI 11, NDVI 12, NDVI min, NDVI max, NDVI mean, NDVI SD |
| Topography | 2 (out of 2) | Elevation and slope |
| Livestock density | 1 (out of 4) | LTU |
| Soil and landform | All categories | |
| GlobCover | All categories |
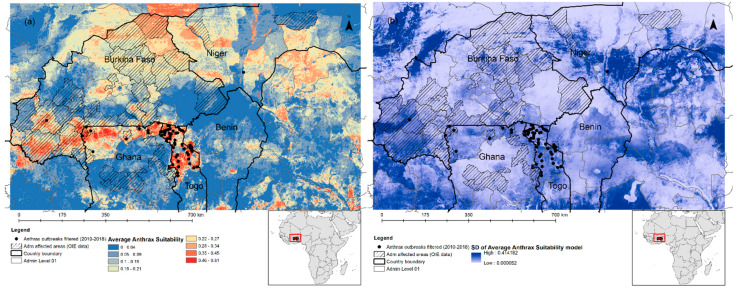
The MaxEnt modeling analysis was performed using 59 outbreaks (45 for training and 14 for testing, selected using a random seed) and the uncorrelated predictors listed in Table 2. The final MaxEnt averaged spatial prediction model of the anthrax suitability niche was based on nine uncorrelated, parsimonious predictors: bio2, bio11, bio15, bio18, LTU, soil, landform, elevation and NDVI 10. The average training AUC for the replicate runs was 0.93 and the standard deviation was 0.01, indicating a highly accurate model. The predicted anthrax suitability map and the standard deviation (measure of uncertainty) are shown in Figure 6a,b. In descending order, the most important variables selected by the model were: livestock density (LTU: 22.2% of contribution), precipitation seasonality (bio15: 19.3%), NDVI in October (NDVI 10: 18.8%), Mean Diurnal Range (Bio2: 10.7%) and mean temperature during the coldest three months of the year, i.e., August–October (bio11: 8.5%). Elevation and landform contributed to about 7% each, while soil and precipitation in the warmest quarter, i.e., February–April (bio18) contributed to about 6% together. The order of importance was also confirmed by the permutation analysis (Table 3). The environmental variable with the highest gain when used in isolation was NDVI 10 (for October). The variable that decreases the gain the most when it is omitted is livestock density, which therefore appears to have the most information that is not present in the other variables. Another important variable was the mean diurnal range (bio2), which reflects temperature fluctuations. The response curves of the anthrax ecological niche models and their statistics are shown in Figure S3. The soil categories mostly contributing to the model were: Lithosols, Dystric Nitosols, Luvic Arenosols and Solodic Planosols. Except for the nitosols (pH = 5.5), these soil types are alkaline with a pH between 6 and 7. Regarding the landform, irregular plains and breaks contributed the most. Large suitable areas for anthrax occurrence were mainly located in northern and central Togo (i.e., Savanes and Kara regions), northern Ghana (i.e., Upper East and Upper West regions), as well as in north-eastern (Atakora region) and western Benin (i.e., Borgou region), reflecting the distribution of the anthrax training data. In Burkina Faso, large suitable areas were located in the south-eastern side of the country (i.e., Sud-Ouest, Cascades and Hauts-Bassins regions) and small, fragmented areas in the central (i.e., Centre-Ouest and Centre-Sud regions) and eastern side (i.e., Est region). In Niger, anthrax suitability was limited to the south-eastern side (Dosso and Tilaberi regions), between the borders with Burkina Faso, Benin and Nigeria.
Figure 6.
(a) The predicted suitability of anthrax presence (average model) and (b) its standard deviation based on 9 selected and uncorrelated variables.
Table 3.
Contribution of the predictors to the MaxEnt model (averages over 10 replicates).
| Predictors | Percent Contribution | Permutation Importance |
|---|---|---|
| LTU | 22.2 | 29.7 |
| Bio15 | 19.3 | 22.5 |
| NDVI 10 | 18.8 | 8.5 |
| Bio2 | 10.7 | 6.9 |
| Bio11 | 8.5 | 9.8 |
| Elevation | 7.1 | 17.6 |
| Landform | 6.7 | 1.2 |
| Soil | 3.3 | 1.4 |
| Bio18 | 3.3 | 2.4 |
4. Discussion
Anthrax is a globally distributed neglected disease that is often underreported, particularly in Africa. For this reason, data on the occurrence of disease are often unavailable or limited, fragmented and incomplete, and our study was not an exception. This may be related to the limited availability of digital data collection and reporting tools, and the lack of sustainable data systems and human resources. However, accurate information on anthrax occurrences is crucial to determine, predict and forecast the spatiotemporal distribution of the disease occurrence in order to enhance prevention and control measures in animal and public health, and to guide targeted surveillance strategies.
Our study optimized the use of limited available data to provide a better understanding of the spatiotemporal dynamics of anthrax in West Africa, and to predict areas at potential risk and environmental and climatic risk factors for the occurrence of anthrax. The OIE reporting dataset should include all known anthrax events in a country but is accurate to only the first- or second subnational administrative level and so does not include accurate geolocation data on the anthrax occurrences. This dataset was used to analyze the spatiotemporal patterns of anthrax outbreaks and seasonality at the sub-national and regional levels. The other datasets (EMPRES-i, GIMD, and FAO workshop) contained geolocation information for anthrax occurrences, but were missing temporal information that was used to train and test the ecological suitability model in MaxEnt.
In particular, the spatiotemporal approach based on the analysis of incremental changes in precipitation and NDVI (first derivative) and temperature confirmed our hypothesis on anthrax seasonality and its association with environmental and climatic risk factors in West Africa, providing a scientific explanation for the evidence of anthrax during the early wet season [16]. Regional studies of climate variable profiles are necessary to fully understand the ecology of anthrax and inform activities to protect the animal, public and environmental health [20]. In previous literature reports, outbreaks in grazing herbivores occurred in seasonal or weather conditions along a spectrum from drought to hot-dry seasons often following years of unusually high rainfall, to transition periods between dry-wet or wet-dry conditions and wet season outbreaks [1,5,10,17,18,19]. Recent studies have shown an association between anthrax occurrence and vegetation green-up [5,20]. So far, two main climatic/ecological hypotheses on the seasonality of anthrax occurrence [16] were formulated and focused on: (1) heavy rainfall and very wet conditions (e.g., wet season) could unearth buried spores and/or move spores from soil to vegetation, as well as transport and concentrate spore-contaminated water in low-lying areas in the landscapes, or increase habitat suitability for arthropod-vectors [10]; (2) dry conditions could increase congregation/density of animals in high-risk areas due to reduced resources availability, as well as increase herbivores’ exposure to contaminated vegetation, soil and dust; and various stressors during droughts could also lead to reduced host resistance [16]. In Namibia, anthrax outbreaks were associated with soil ingestion by herbivores during the wet season [16], while in Tanzania outbreaks were associated with prolonged droughts or rains [54]. In our study, we demonstrated that anthrax occurrences were associated with increased monthly precipitation and vegetation growth during the early wet season, which were calculated as the rate of change (first derivative) of the precipitation and NDVI time series.
Although the region was characterized by outbreaks of anthrax throughout the year, the analysis revealed that the peak occurs in April, i.e., in the early wet season, when the incremental change in precipitation is highest. April also represents the month with the highest increase in vegetation growth. The larger the amount of precipitation increase from one month to the next during the early wet season, the higher the increase in vegetation growth and the higher the number of outbreaks during that month. This is in line with the hypothesis that anthrax spores become increasingly available with increasing rainfall and vegetation. As shown in Figure 3 and Figure 4, at the end of the dry season and during the transition period between the dry and the wet season, the vegetation is poorly available, and the grass height is very low (low NDVI values). However, as shown by the exponential relation between precipitation and NDVI in Figure S2, the vegetation response to rainfall and, therefore, water availability is extremely rapid.
The vegetation grows fast during the first period of the wet season, potentially carrying with it the spores that then become available to the grazing livestock. Because the grass height during this period is still short, the grazing animals do likely ingest soil and, hence, potentially anthrax spores. This confirms the study by [18,26] that reported the occurrence of anthrax outbreaks in northern Togo and Ghana mainly during the hot-humid period of the year. In our model, vegetation growth and rainfall increase by month explained 97% of the variance in anthrax occurrence during the period January–June, and reached 99% of variance when the temperature was included. Indeed, the peak in anthrax outbreaks occurs immediately after the hottest period of the year, when the temperature is high but lower than the maximum values and precipitation and NDVI increase. This period is also characterized by high evapotranspiration, which is an important predictor of anthrax occurrence [30,55]. In Bangladesh, high temperature and heavy rains during the wet season in areas with high Ca content (prone to anthrax) were the main contributors to anthrax occurrence [56]. More research is needed to investigate the patterns of environmental variables and their rate of change during the transition periods from the dry to the wet season and vice versa.
The anthrax ecological niche was highly accurate (AUC = 0.93) and was generated by nine parsimonious variables, confirming that the method is well suited to investigate the distribution of infectious diseases [57,58,59], including anthrax [28,29,30,31]. Livestock density was the main predictor of anthrax suitability, confirming the results of previous studies [21,30,55]. Cattle and small ruminants are the species most affected in the region [18]. This corroborates our results on livestock density and highlights the importance of accurate and timely available livestock data in spatial modeling. The data used in this study (Gridded Livestock of the World v02) are publicly available and regularly updated and can be used for the Anthrax suitability model. In line with the studies conducted in Africa [4,28,29,31,55] and elsewhere [60,61], the environmental variables that contributed the most to the predictions of anthrax occurrence were precipitation, NDVI and soil type. In particular, our study identified precipitation seasonality (bio15) as the second most important predictor after livestock density. Bio15 is a measure of the variation in monthly precipitation totals over the course of the year. Since species distributions can be strongly influenced by variability in precipitation, this index provides a percentage of precipitation variability where larger percentages represent greater variability of precipitation. The MaxEnt model revealed that the predicted probability of anthrax occurrence is negatively associated with precipitation variability, suggesting that the disease follows predictable seasonal patterns as also shown by our seasonal analysis. NDVI is a measure of vegetation greenness and was found to be a strong positive predictor of anthrax suitability in the study area, corroborating the results of other studies [4,7,26,28,29,60]. The soil types most suitable for anthrax occurrence were alkaline and those with a pH between 5.5 and 7, therefore confirming the results of previous studies in the region [26] and other areas of the world [56,60]. Soil alkalinity and high soil calcium levels in the environment promote bacterial sporulation and survival [20]. The annual mean diurnal range (bio2), which is a measure of daily temperature fluctuation, showed a positive relationship suggesting that the predicted suitability for anthrax presence may be affected by large fluctuations of daily temperature [7]. Anthrax suitability was negatively related to elevation. Low-lying areas in the landscapes may facilitate the concentration of anthrax spores [18].
A limit of his study was that the model was not able to extrapolate the suitability outside of the training data. Large suitable areas for anthrax occurrence were mainly located in south-east Burkina Faso, north and central Togo, northern Ghana and eastern Benin, thus reflecting the observed anthrax training and validation data, including the data available at the subnational level. In Ghana, the eastern corridor of the northern area, which is considered a hotspot for Anthrax [62], was correctly identified by our model as a suitable area, in line with the study by [26]; in addition, other areas in the central region were correctly identified as unsuitable [62].
However, the model failed to predict the northern side of the study area, which is also affected by the disease. This could be explained by the lack of training and testing data on the northern side of Burkina Faso and Niger, which may have limited the extrapolation and prediction power of the model in the Sahelian zone. These countries were highly affected, but only three geolocations were available for the spatial risk modeling (Table S2). The Sahelian zone is very arid as compared with the Sahelo-Sudanian and Sudano-Guinean zones, where the majority of anthrax point locations were located. Other variables, such as human practices, vaccination practices and pastoral movement, not considered in our study, may explain the anthrax occurrence in the northern region of the study area. In Zambia, the outbreaks appeared to be associated with human practices, e.g., land preparation and plowing, rather than climate variables [63]. In Togo, the outbreaks occur during the transhumance period, thus enhancing the risk of disease spread [18]. These variables should be considered in the future development of the anthrax suitability model.
Application to Animal Health Management
Many challenges exist, particularly constraints on financial and human resources, which can hamper the implementation of control programs for anthrax in countries where the disease is endemic. Therefore, methods that can be utilized to guide and prioritize control activities and the allocation of limited resources will be exceptionally useful to both the animal and human health authorities in such countries as they attempt to control anthrax. This study demonstrates the use of limited precise reporting data, in combination with anthrax reporting data at the first or second administrative level, to predict both regions at risk and identify seasonal periods of increased risk; most countries will have this latter data available both as part of their reporting to OIE, and in historical or research records. The other data elements found to contribute the most to the final model in this study are available from other sources (NASA, FAO, etc.), which may be adaptable to other national or regional settings.
The results of this study will help to inform animal health and anthrax control programs across multiple countries and eco-climatic and agro-ecological zones within the West African region and may be adaptable to other regions. The study identifies both regions at risk and temporal relationships to meteorological events, such as substantial rainfall as predictors for seasonal periods of increased risk. In particular, it identifies the rate of change in the primary environmental constraints on anthrax outbreaks, precipitation and temperature, as key predictors, and indicates that real-time analysis of data on these variables can be used to identify escalating risk for anthrax emergence or re-emergence.
This information can inform differential or risk-based disease prevention and control strategies that can be adapted to, and make the best use of, limited resources for disease prevention and control. For example, if resource restrictions limit widespread preventive anthrax vaccination in all areas with a history of, or potential for, outbreaks, vaccination-based strategies may be implemented in selected zones and in seasonal time periods identified as being of increased risk, and vaccination campaigns can be timed and targeted to optimize vaccination and ensure delivery prior to the onset of “anthrax seasons”. In areas with low to medium risk, control strategies may focus on disease awareness campaigns, improving surveillance including media surveillance and active surveillance, and the establishment and training of response teams for the rapid identification, confirmation and response to any outbreaks [64]. Additionally, the identification of at-risk areas and periods can indicate when and where to direct efforts to increase awareness in communities and among health providers, and to implement effective surveillance in animal and human populations. This can include the allocation of potentially limited laboratory diagnostic resources to ensure rapid and accurate diagnostics are available in these areas, or ensuring transport networks are in place to provide specimen transport to supporting diagnostic laboratories. These activities should be One Health in nature to ensure detection, diagnosis and reporting of suspect animal and human cases, as detection of human cases is frequently the first indicator for animal anthrax outbreaks.
The importance of transhumant populations and their livestock in anthrax surveillance and control in these areas has previously been identified [18]. Areas identified as at risk for anthrax outbreaks, which include transit corridors for pastoralist or transhumant populations, could indicate the need for outreach and provision of preventive veterinary services to these populations; the areas and times of increased risk can be identified to ensure that the animals transiting these areas are protected by vaccination in advance of their movement, or that the animal herders are warned against introducing their animals to those areas and during those periods if they are not vaccinated due to owner practices, financial or other factors.
The information from analyses such as these can also help to guide and improve the anthrax surveillance picture in the countries across the region, as education and awareness programs and training for health providers can be provided in zones that are identified as suitable environments, ecologically, for anthrax and increase the likelihood of detection and confirmation of new cases as they occur, adding to the robustness of the data for risk mapping. Novel approaches such as the use of sentinel serosurveys in domestic or peridomestic canines have been developed that could be utilized to further investigate the presence of anthrax in these regions [65,66]. Additionally, focused efforts to improve surveillance and laboratory capacity to detect and confirm anthrax cases in at-risk regions will help improve the ability to characterize circulating B. anthracis strains. This can inform anthrax vaccination strategies, including addressing the extent of potential circulation of anthrose-deficient B. anthracis strains that have been previously identified in the region, and which could persist in the face of current livestock vaccines [22,67], throughout the region.
5. Conclusions
This study optimized the use of limited available data to provide a better understanding of the spatiotemporal dynamics of anthrax in West Africa, and to predict areas at potential risk and also environmental and climatic risk factors for the occurrence of anthrax. Our findings on anthrax seasonality and ecological suitability can inform surveillance, prevention and control programs undertaken by animal and public health authorities and enhance national and regional multi-sectoral One Health collaborations.
Acknowledgments
The authors acknowledge the FAO regional workshop on anthrax prevention and control in 2015 where information on anthrax occurrences in West Africa was shared and discussed. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of FAO or USAID.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12091146/s1, Figure S1: Median number of anthrax outbreaks, median precipitation and median NDVI by month for each country; Figure S2 Left: 2-D scatter plot of the rates of change, i.e., first derivative of standardized median precipitation by month and first derivative of standardized median NDVI by month during the period from January to June. Right: 3-D scatter plot of the relation between the standardized median number of anthrax outbreaks, the first derivatives of monthly precipitation and first derivatives of monthly NDVI. Standardized values are shown; Figure S3: MaxEnt response curves of the probability of Anthrax presence to the main predictors. The charts show standardized values (mean = 0 and sd = 1). Actual mean, minimum, maximum and standard deviation (sd) values of each predictor are reported in the table below. Table S1: Climatic and environmental predictor variables for modeling the distribution of anthrax. Table S2. Number of reported outbreaks by country and data sources.
Author Contributions
Conceptualization, C.P., S.S., A.E.I. and Y.M.; methodology, C.P., S.S. and A.E.I.; software, C.P. and S.S.; validation, C.P., S.S., A.E.I., Y.M., B.S. and J.L.; formal analysis, C.P., S.S. and A.E.I.; investigation, C.P., S.S., A.E.I., Y.M., B.S. and J.L.; resources, A.E.I., Y.M., B.S. and J.L.; data curation, C.P. and S.S.; writing—original draft preparation, C.P., S.S., A.E.I., Y.M., B.S. and J.L.; writing—review and editing, C.P., S.S., A.E.I., Y.M., B.S. and J.L.; visualization, C.P. and S.S.; supervision, A.E.I. and Y.M.; project administration, A.E.I. and Y.M.; funding acquisition, A.E.I., Y.M., B.S. and J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. All data on outbreaks were collected from existing databases and reports.
Data Availability Statement
All relevant data are within the manuscript and its supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The writing of this paper was supported by the USAID-funded “Global Health Security Agenda (GHSA)—OSRO/GLO/507/USA” project.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Food and Agriculture Organization of the United Nations. World Organisation for Animal Health . Anthrax in Humans and Animals. 4th ed. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 2.Beyer W., Turnbull P.C.B. Anthrax in Animals. Mol. Asp. Med. 2009;30:481–489. doi: 10.1016/j.mam.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Cossaboom C.M., Khaiseb S., Haufiku B., Katjiuanjo P., Kannyinga A., Mbai K., Shuro T., Hausiku J., Likando A., Shikesho R., et al. Anthrax Epizootic in Wildlife, Bwabwata National Park, Namibia, 2017. Emerg. Infect. Dis. J. 2019;25:947. doi: 10.3201/eid2505.180867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obanda V., Otieno V.A., Kingori E.M., Ndeereh D., Lwande O.W., Chiyo P.I. Identifying Edaphic Factors and Normalized Difference Vegetation Index Metrics Driving Wildlife Mortality from Anthrax in Kenya’s Wildlife Areas. Front. Ecol. Evol. 2021;9:939. doi: 10.3389/fevo.2021.643334. [DOI] [Google Scholar]
- 5.Blackburn J.K., Goodin D.G. Differentiation of Springtime Vegetation Indices Associated with Summer Anthrax Epizootics in West Texas, USA, Deer. J. Wildl. Dis. 2013;49:699–703. doi: 10.7589/2012-10-253. [DOI] [PubMed] [Google Scholar]
- 6.Ezhova E., Orlov D., Suhonen E., Kaverin D., Mahura A., Gennadinik V., Kukkonen I., Drozdov D., Lappalainen H.K., Melnikov V., et al. Climatic Factors Influencing the Anthrax Outbreak of 2016 in Siberia, Russia. EcoHealth. 2021;18:217–228. doi: 10.1007/s10393-021-01549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson C.J., Kracalik I.T., Ross N., Alexander K.A., Hugh-Jones M.E., Fegan M., Elkin B.T., Epp T., Shury T.K., Zhang W., et al. The Global Distribution of Bacillus Anthracis and Associated Anthrax Risk to Humans, Livestock and Wildlife. Nat. Microbiol. 2019;4:1337–1343. doi: 10.1038/s41564-019-0435-4. [DOI] [PubMed] [Google Scholar]
- 8.Gachohi J.M., Gakuya F., Lekolool I., Osoro E., Nderitu L., Munyua P., Ngere I., Kemunto N., Bett B., Otieno F., et al. Temporal and Spatial Distribution of Anthrax Outbreaks among Kenyan Wildlife, 1999–2017. Epidemiol. Infect. 2019;147:e249. doi: 10.1017/S0950268819001304. [DOI] [Google Scholar]
- 9.Martin G., Friedlander A. Bacillus Anthracis (Anthrax) In: Mandell J., editor. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Volume 10. Churchill Livingstone; Philadelphia, CA, USA: 2010. pp. 2715–2725. [Google Scholar]
- 10.Hugh-Jones M., Blackburn J. The Ecology of Bacillus Anthracis. Mol. Asp. Med. 2009;30:356–367. doi: 10.1016/j.mam.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Van Ness G.B. Ecology of Anthrax. Science. 1971;172:1303–1307. doi: 10.1126/science.172.3990.1303. [DOI] [PubMed] [Google Scholar]
- 12.Dragon D.C., Rennie R.P. The Ecology of Anthrax Spores: Tough but Not Invincible. Can. Vet. J. 1995;36:295–301. [PMC free article] [PubMed] [Google Scholar]
- 13.Driks A. The Bacillus Anthracis Spore. Mol. Aspects Med. 2009;30:368–373. doi: 10.1016/j.mam.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Manchee R.J., Broster M.G., Melling J., Henstridge R.M., Stagg A.J. Bacillus Anthracis on Gruinard Island. Nature. 1981;294:254–255. doi: 10.1038/294254a0. [DOI] [PubMed] [Google Scholar]
- 15.Bell W.J., Laing P.W. Pulmonary Anthrax in Cattle. Vet. Rec. 1977;100:573–574. doi: 10.1136/vr.100.26.573. [DOI] [PubMed] [Google Scholar]
- 16.Turner W.C., Imologhome P., Havarua Z., Kaaya G.P., Mfune J.K.E., Mpofu I.D.T., Getz W.M. Soil Ingestion, Nutrition and the Seasonality of Anthrax in Herbivores of Etosha National Park. Ecosphere. 2013;4:art13. doi: 10.1890/ES12-00245.1. [DOI] [Google Scholar]
- 17.Patassi A.A., Saka B., Landoh D.E., Agbenoko K., Tamekloe T., Salmon-Ceron D. Detection and Management of the First Human Anthrax Outbreak in Togo. Trop. Doct. 2016;46:129–134. doi: 10.1177/0049475515622331. [DOI] [PubMed] [Google Scholar]
- 18.Kulo A.E., Kada O. Anthrax in Togo: Spatial Risk in the Savannah Region. Bull. Anim. Health Prod. Afr. 2011;59:281–288. doi: 10.4314/bahpa.v59i3. [DOI] [Google Scholar]
- 19.Gomez J.P., Nekorchuk D.M., Mao L., Ryan S.J., Ponciano J.M., Blackburn J.K. Decoupling Environmental Effects and Host Population Dynamics for Anthrax, a Classic Reservoir-Driven Disease. PLoS ONE. 2018;13:e0208621. doi: 10.1371/journal.pone.0208621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norris M.H., Blackburn J.K. Linking Geospatial and Laboratory Sciences to Define Mechanisms behind Landscape Level Drivers of Anthrax Outbreaks. Int. J. Environ. Res. Public Health. 2019;16:3747. doi: 10.3390/ijerph16193747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W.-J., Lai S.-J., Yang Y., Liu K., Li X.-L., Yao H.-W., Li Y., Zhou H., Wang L.-P., Mu D., et al. Mapping the Distribution of Anthrax in Mainland China, 2005–2013. PLoS Negl. Trop. Dis. 2016;10:e0004637. doi: 10.1371/journal.pntd.0004637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackburn J.K., Odugbo M.O., Ert M.V., O’Shea B., Mullins J., Perrenten V., Maho A., Hugh-Jones M., Hadfield T. Bacillus Anthracis Diversity and Geographic Potential across Nigeria, Cameroon and Chad: Further Support of a Novel West African Lineage. PLoS Negl. Trop. Dis. 2015;9:e0003931. doi: 10.1371/journal.pntd.0003931. Correction in PLoS Negl. Trop. Dis. 2015, 9, e0004089. https://doi.org/10.1371/journal.pntd.0004089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opare C., Nsiire A., Awumbilla B., Akanmori B.D. Human Behavioural Factors Implicated in Outbreaks of Human Anthrax in the Tamale Municipality of Northern Ghana. Acta Trop. 2000;76:49–52. doi: 10.1016/S0001-706X(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 24.Elith J., Graham C.H., Anderson R.P., Dudík M., Ferrier S., Guisan A., Hijmans R.J., Huettmann F., Leathwick J.R., Lehmann A., et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography. 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- 25.Escobar L.E., Craft M.E. Advances and Limitations of Disease Biogeography Using Ecological Niche Modeling. Front. Microbiol. 2016;7:1174. doi: 10.3389/fmicb.2016.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kracalik I.T., Kenu E., Ayamdooh E.N., Allegye-Cudjoe E., Polkuu P.N., Frimpong J.A., Nyarko K.M., Bower W.A., Traxler R., Blackburn J.K. Modeling the Environmental Suitability of Anthrax in Ghana and Estimating Populations at Risk: Implications for Vaccination and Control. PLoS Negl. Trop. Dis. 2017;11:e0005885. doi: 10.1371/journal.pntd.0005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips S.J., Anderson R.P., Schapire R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- 28.Steenkamp P.J., van Heerden H., van Schalkwyk O.L. Ecological Suitability Modeling for Anthrax in the Kruger National Park, South Africa. PLoS ONE. 2018;13:e0191704. doi: 10.1371/journal.pone.0191704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chikerema S.M., Murwira A., Matope G., Pfukenyi D.M. Spatial Modelling of Bacillus Anthracis Ecological Niche in Zimbabwe. Prev. Vet. Med. 2013;111:25–30. doi: 10.1016/j.prevetmed.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Walsh M.G., de Smalen A.W., Mor S.M. Climatic Influence on Anthrax Suitability in Warming Northern Latitudes. Sci. Rep. 2018;8:9269. doi: 10.1038/s41598-018-27604-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Alvarez D., Peterson A.T., Salzer J.S., Pittiglio C., Shadomy S., Traxler R., Vieira A.R., Bower W.A., Walke H., Campbell L.P. Potential Distributions of Bacillus Anthracis and Bacillus Cereus Biovar Anthracis Causing Anthrax in Africa. PLoS Negl. Trop. Dis. 2020;14:e0008131. doi: 10.1371/journal.pntd.0008131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FAO . FAO Animal Production and Health Paper. FAO; Rome, Italy: 1985. FAO Integrating Crops and Livestock in West Africa. [Google Scholar]
- 33.FAO . Animal Production and Health Division Atlas of Trends in Pastoral Systems in the Sahel 1970–2012: Information System on Pastoralism in the Sahel. FAO; Rome, Italy: 2012. [Google Scholar]
- 34.Zheng H., Cheng T., Yao X., Deng X., Tian Y., Cao W., Zhu Y. Detection of Rice Phenology through Time Series Analysis of Ground-Based Spectral Index Data. Field Crops Res. 2016;198:131–139. doi: 10.1016/j.fcr.2016.08.027. [DOI] [Google Scholar]
- 35.Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 36.FAO Digital Soil Map of the World (DSMW) | Land & Water | Food and Agriculture Organization of the United Nations | Land & Water | Food and Agriculture Organization of the United Nations. [(accessed on 7 March 2022)]. Available online: https://www.fao.org/land-water/land/land-governance/land-resources-planning-toolbox/category/details/en/c/1026564/
- 37.Rodríguez E., Morris C.S., Belz J.E. A Global Assessment of the SRTM Performance. Photogramm. Eng. Remote Sens. 2006;72:249–260. doi: 10.14358/PERS.72.3.249. [DOI] [Google Scholar]
- 38.Sayre R., Dangermond J., Frye C., Vaughan R., Aniello P., Breyer S., Cribbs D., Hopkins D., Nauman R., Derrenbacher W., et al. A New Map of Global Ecological Land Units—An Ecophysiographic Stratification Approach. Association of American Geographers; Washington, DC, USA: 2014. p. 25. [Google Scholar]
- 39.Robinson T.P., Wint G.R.W., Conchedda G., Boeckel T.P.V., Ercoli V., Palamara E., Cinardi G., D’Aietti L., Hay S.I., Gilbert M. Mapping the Global Distribution of Livestock. PLoS ONE. 2014;9:e96084. doi: 10.1371/journal.pone.0096084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn G.P., Keough M.J. Experimental Design and Data Analysis for Biologists. Cambridge University Press; Cambridge, UK: 2002. p. 557. [Google Scholar]
- 41.Wisz M.S., Hijmans R.J., Li J., Peterson A.T., Graham C.H., Guisan A., Group N.P.S.D.W. Effects of Sample Size on the Performance of Species Distribution Models. Divers. Distrib. 2008;14:763–773. doi: 10.1111/j.1472-4642.2008.00482.x. [DOI] [Google Scholar]
- 42.Morales N.S., Fernández I.C., Baca-González V. MaxEnt’s Parameter Configuration and Small Samples: Are We Paying Attention to Recommendations? A Systematic Review. PeerJ. 2017;5:e3093. doi: 10.7717/peerj.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galante P.J., Alade B., Muscarella R., Jansa S.A., Goodman S.M., Anderson R.P. The Challenge of Modeling Niches and Distributions for Data-Poor Species: A Comprehensive Approach to Model Complexity. Ecography. 2018;41:726–736. doi: 10.1111/ecog.02909. [DOI] [Google Scholar]
- 44.Duan R.-Y., Kong X.-Q., Huang M.-Y., Fan W.-Y., Wang Z.-G. The Predictive Performance and Stability of Six Species Distribution Models. PLoS ONE. 2014;9:e112764. doi: 10.1371/journal.pone.0112764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elith J., Phillips S.J., Hastie T., Dudík M., Chee Y.E., Yates C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- 46.van Gils H., Westinga E., Carafa M., Antonucci A., Ciaschetti G. Where the Bears Roam in Majella National Park, Italy. J. Nat. Conserv. 2014;22:23–34. doi: 10.1016/j.jnc.2013.08.001. [DOI] [Google Scholar]
- 47.Elith J., Graham C.H. Do They? How Do They? Why Do They Differ? On Finding Reasons for Differing Performances of Species Distribution Models. Ecography. 2009;32:66–77. doi: 10.1111/j.1600-0587.2008.05505.x. [DOI] [Google Scholar]
- 48.Fourcade Y., Engler J.O., Rödder D., Secondi J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE. 2014;9:e97122. doi: 10.1371/journal.pone.0097122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Gils H., Conti F., Ciaschetti G., Westinga E. Fine Resolution Distribution Modelling of Endemics in Majella National Park, Central Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012;146:276–287. doi: 10.1080/11263504.2012.685194. [DOI] [Google Scholar]
- 50.Anderson R.P., Gonzalez I. Species-Specific Tuning Increases Robustness to Sampling Bias in Models of Species Distributions: An Implementation with Maxent. Ecol. Model. 2011;222:2796–2811. doi: 10.1016/j.ecolmodel.2011.04.011. [DOI] [Google Scholar]
- 51.Bean W.T., Stafford R., Brashares J.S. The Effects of Small Sample Size and Sample Bias on Threshold Selection and Accuracy Assessment of Species Distribution Models. Ecography. 2012;35:250–258. doi: 10.1111/j.1600-0587.2011.06545.x. [DOI] [Google Scholar]
- 52.Brown J.L. SDMtoolbox: A Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. Methods Ecol. Evol. 2014;5:694–700. doi: 10.1111/2041-210X.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Araújo M.B., Guisan A. Five (or so) Challenges for Species Distribution Modelling. J. Biogeogr. 2006;33:1677–1688. doi: 10.1111/j.1365-2699.2006.01584.x. [DOI] [Google Scholar]
- 54.Hampson K., Lembo T., Bessell P., Auty H., Packer C., Halliday J., Beesley C.A., Fyumagwa R., Hoare R., Ernest E., et al. Predictability of Anthrax Infection in the Serengeti, Tanzania. J. Appl. Ecol. 2011;48:1333–1344. doi: 10.1111/j.1365-2664.2011.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otieno F.T., Gachohi J., Gikuma-Njuru P., Kariuki P., Oyas H., Canfield S.A., Bett B., Njenga M.K., Blackburn J.K. Modeling the Potential Future Distribution of Anthrax Outbreaks under Multiple Climate Change Scenarios for Kenya. Int. J. Environ. Res. Public Health. 2021;18:4176. doi: 10.3390/ijerph18084176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan J., Ahsan M., Rahman M., Chowdhury S., Parvej M., Nazir K. Factors Associated with Repeated Outbreak of Anthrax in Bangladesh: Qualitative and Quantitative Study. J. Adv. Vet. Anim. Res. 2015;2:158. doi: 10.5455/javar.2015.b72. [DOI] [Google Scholar]
- 57.Du Z., Wang Z., Liu Y., Wang H., Xue F., Liu Y. Ecological Niche Modeling for Predicting the Potential Risk Areas of Severe Fever with Thrombocytopenia Syndrome. Int. J. Infect. Dis. 2014;26:1–8. doi: 10.1016/j.ijid.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Murray K.A., Retallick R.W.R., Puschendorf R., Skerratt L.F., Rosauer D., McCallum H.I., Berger L., Speare R., VanDerWal J. Assessing Spatial Patterns of Disease Risk to Biodiversity: Implications for the Management of the Amphibian Pathogen, Batrachochytrium Dendrobatidis. J. Appl. Ecol. 2011;48:163–173. doi: 10.1111/j.1365-2664.2010.01890.x. [DOI] [Google Scholar]
- 59.Slater H., Michael E. Predicting the Current and Future Potential Distributions of Lymphatic Filariasis in Africa Using Maximum Entropy Ecological Niche Modelling. PLoS ONE. 2012;7:e32202. doi: 10.1371/journal.pone.0032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackburn J.K., McNyset K.M., Curtis A., Hugh-Jones M.E. Modeling the Geographic Distribution of Bacillus anthracis, the Causative Agent of Anthrax Disease, for the Contiguous United States Using Predictive Ecological [Corrected] Niche Modeling. Am. J. Trop. Med. Hyg. 2007;77:1103–1110. doi: 10.4269/ajtmh.2007.77.1103. [DOI] [PubMed] [Google Scholar]
- 61.Blackburn J.K., Matakarimov S., Kozhokeeva S., Tagaeva Z., Bell L.K., Kracalik I.T., Zhunushov A. Modeling the Ecological Niche of Bacillus anthracis to Map Anthrax Risk in Kyrgyzstan. Am. J. Trop. Med. Hyg. 2017;96:550–556. doi: 10.4269/ajtmh.16-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nsoh A.E., Kenu E., Forson E.K., Afari E., Sackey S., Nyarko K.M., Yebuah N. Mapping as a Tool for Predicting the Risk of Anthrax Outbreaks in Northern Region of Ghana. Pan Afr. Med. J. 2016;25:14. doi: 10.11604/pamj.supp.2016.25.1.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munang’andu H.M., Banda F., Siamudaala V.M., Munyeme M., Kasanga C.J., Hamududu B. The Effect of Seasonal Variation on Anthrax Epidemiology in the Upper Zambezi Floodplain of Western Zambia. J. Vet. Sci. 2012;13:293–298. doi: 10.4142/jvs.2012.13.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vieira A.R., Salzer J.S., Traxler R.M., Hendricks K.A., Kadzik M.E., Marston C.K., Kolton C.B., Stoddard R.A., Hoffmaster A.R., Bower W.A., et al. Enhancing Surveillance and Diagnostics in Anthrax-Endemic Countries. Emerg. Infect. Dis. 2017;23:S147. doi: 10.3201/eid2313.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lembo T., Hampson K., Auty H., Beesley C.A., Bessell P., Packer C., Halliday J., Fyumagwa R., Hoare R., Ernest E., et al. Serologic Surveillance of Anthrax in the Serengeti Ecosystem, Tanzania, 1996–2009. Emerg. Infect. Dis. 2011;17:387–394. doi: 10.3201/eid1703.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukarati N.L., Ndumnego O., van Heerden H., Ndhlovu D.N., Matope G., Caron A., de Garine-Wichatitsky M., Pfukenyi D.M. A Serological Survey of Anthrax in Domestic Dogs in Zimbabwe: A Potential Tool for Anthrax Surveillance. Epidemiol. Infect. 2018;146:1526–1532. doi: 10.1017/S0950268818001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamborrini M., Bauer M., Bolz M., Maho A., Oberli M.A., Werz D.B., Schelling E., Zinsstag J., Seeberger P.H., Frey J., et al. Identification of an African Bacillus Anthracis Lineage That Lacks Expression of the Spore Surface-Associated Anthrose-Containing Oligosaccharide. J. Bacteriol. 2011;193:3506–3511. doi: 10.1128/JB.00078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its supplementary material.