Abstract
Background:
Long COVID (coronavirus disease 2019) syndrome includes a group of patients who, after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), exhibit lingering mild-to-moderate symptoms and develop medical complications that can have lasting health problems. In this report, we propose a model for the pathophysiology of the long COVID presentation based on increased proinflammatory cytokine production that results from the persistence of the SARS-CoV-2 virus or one of its molecular components. Associated with this hyperproduction of inflammatory cytokines is a heightened activity of nuclear factor κ B (NF-κB) and p38 mitogen-activated protein kinase signaling pathways that regulate cytokine production.
Objective:
The purpose of the present report was to review the causes of long COVID syndrome and suggest ways that can provide a basis for a better understanding of the clinical symptomatology for the of improved diagnostic and therapeutic procedures for the condition.
Methods:
Extensive research was conducted in medical literature data bases by applying terms such as “long COVID” associated with “persistence of the SARS-CoV-2 virus” “spike protein' “COVID-19” and “biologic therapies.”
Results and Conclusions:
In this model of the long COVID syndrome, the persistence of SARS-CoV-2 is hypothesized to trigger a dysregulated immune system with subsequent heightened release of proinflammatory cytokines that lead to chronic low-grade inflammation and multiorgan symptomatology. The condition seems to have a genetic basis, which predisposes individuals to have a diminished immunologic capacity to completely clear the virus, with residual parts of the virus persisting. This persistence of virus and resultant hyperproduction of proinflammatory cytokines are proposed to form the basis of the syndrome.
Keywords: COVID-19, Cytokines, Long COVID, Long Hauler, inflammation, NF-κB and p38 MAP kinase signaling pathways
After infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the majority of patients generally show complete recovery within 3–4 weeks; however, as many as 30% continue to have at least one symptom and lingering effects after 3 months, and develop medical complications that can have lasting health problems. The condition is referred to as “long-haul COVID” (or “long COVID”) and has been designated as “our next public health disaster in the making.”1 Long COVID (coronavirus disease 2019), therefore, is a form of chronic COVID characterized by persistent after-effects of SARS-CoV-2 infection, which include fatigue, shortness of breath, cough, and joint and chest pain, in addition to other symptoms that include problems with thought processes, difficulty concentrating, forgetfulness, depression, muscle pain, headache, rapid heartbeat, and fever.2 Long COVID symptoms can last weeks and months in some patients, who are also referred to as “long haulers” or as having “chronic COVID.” The condition was originally described in adults3 but is now being identified in children.4 The purpose of the present report is to propose a hypothesis-driven model of viral persistence for the pathophysiology of long COVID as a basis for a better understanding of the clinical symptomatology and for the development of improved diagnostic and therapeutic procedures for the condition.
METHODS
This review examined the literature data published in public data bases (PubMed, MEDLINE, U.S. National Library of Medicine, Bethesda, MD) on long COVID between 2019 and 2022. The following key terms were used: “long COVID” associated with “persistence of the SARS-CoV-2 virus” “spike protein” “COVID-19” and “biologic therapies.” Original studies were selected in priority, followed by systematic reviews and meta-analyses. Results were limited to publications in English. Preference was given to articles published over the past 5 years.
CLINICAL ASPECTS
A recent systematic review and meta-analysis of long COVID in adults identified > 50 of the most long-term effects of COVID-19 infection, including fatigue, headache, dyspnea, cognitive impairment, numbness, depression, altered perception of smell and taste, poor appetite, chronic cough, joint and chest pain, postural orthostatic tachycardia, autonomic dysregulation, thermoregulation abnormalities, skin eruptions, and gastrointestinal disorders.5 In another recent publication, the epidemiology, etiopathogenesis, clinical manifestations, predictors, and management strategies in survivors of COVID-19 in their convalescent and/or recovery phase are comprehensively reviewed.6 Shown in Table 1 are the common signs and symptoms of long COVID in adults3 and in both children and adults.4,5
Table 1.
Signs and symptoms of long COVID in adults and children*
COVID = Coronavirus disease.
Reproduced with permission from Refs. 3 and 4.
These clinical manifestations are described as persistent, with a frequency that ranges from 10% to 87% and a strong female prevalence. Fatigue was the most common symptom and was consistent with its well-known association after viral infection, including the postinfectious syndromes that followed outbreaks of chikungunya and Ebola viral infection.2 Some symptoms overlap with those of myalgic encephalomyelitis/chronic fatigue syndrome, a disease that is often triggered by viral infection and immune activation,7 with dysregulation of the autonomic and the immunologic systems.8
PREVALENCE
Currently, there is an abundance of accumulating literature that discusses long COVID, primarily in adults. A recent editorial underscores the need for more research of long COVID in children.9 Preliminary data from the English healthcare system suggest that 12% and 15% of children, of primary school and secondary school age, respectively, may have symptoms that last 5 weeks after an acute COVID-19 infection.10 In a study conducted on 510 children enrolled in different European countries, higher percentages were described, ranging from 50% to 30%, 120–160 days from the onset of COVID-19. The symptoms persisted for an average of 8 months, with periods of apparent remission, followed by exacerbations in half of the patients, and only 10% of the patients were able to return to normal levels of physical activity.11
RISK FACTORS
In adults, the current literature suggests that, in addition to a female predominance,12 associated risk factors include more than five early symptoms, which consist of early dyspnea, previous psychiatric disorders, and specific biomarkers (e.g., D-dimer, C-reactive protein, and elevated lymphocyte counts), although more research will be required to substantiate the pathogenetic role of these risk factors.2 Because there is a paucity of information on predisposing factors in children, risk factors for this group are usually extrapolated from those used in adults. Other risk factors, which include increased body mass index, asthma, and advanced age, are conditions associated with persistent inflammation.2
The role of genetics on the susceptibility to COVID-19 has also been suggested from studies of polymorphisms in genes encoding angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2.13 The distribution of ACE2 variants, as well as observed patterns in gene expression, differs among ethnic populations and may contribute to the variation of COVID-19 severity and susceptibility among ethnicities. The COVID-19 Host Genetics Initiative showed that a cluster of risk alleles at locus 3p21.31 confers the most significant risk to severe disease after infection and hospitalization.14 This locus, interestingly, also comprises six major candidate genes involved in normal immunity, ACE2 function, and a LZTFL1 (leucine zipper transcription factor-like) gene.14
LONG COVID: INCREASING EVIDENCE OF PERSISTENCE OF SARS-COV-2
One of the most important considerations that supports the proposed pathogenesis model of long COVID is driven by the striking pathologic inflammation that results from persistence of the virus, virus-infected cells, or a component of the virus, e.g., the spike protein described in recent publications.15–17 Preliminary evidence, however, suggests that patients with long COVID may be helped by rehabilitation and certain therapeutic drugs repurposed from other similar conditions, such as myalgic encephalomyelitis or chronic fatigue syndrome, postural orthostatic tachycardia syndrome, and mast cell activation syndrome.18 In support of this hypothesis-driven viral persistence etiology are reports of patients with COVID-19 who remain positive for SARS-CoV-2, by using reverse transcription real-time polymerase chain reaction tests, for extended periods of time.19–21 The presence of SARS-CoV-2, e.g., has been detected in the cerebrospinal fluid19 and shedding of the virus in the feces of patients with COVID-19 for months,20 regardless of gastrointestinal symptoms.
LONG COVID: AN IMMUNOLOGIC RESPONSE TO PERSISTENCE OF SARS-CoV-2
In 1971, we put forth a hypothesis that describes immunologic phenomena as an array of potential responses of the host concerned with the recognition and elimination of foreign substances and in which we postulated that immunologic mechanisms that are stimulated are dependent on both the degree and the persistence and efficiency of elimination of the foreign agent.22 This framework has provided both a foundation for a discussion and understanding not only of several of the immunologically mediated clinical disorders, including the allergic diseases, the autoimmune disorders, cancer, and many chronic microbial infections, but also, most recently, the innate and adaptive immune responses to COVID-19. It also serves as a useful stratagem for discussions of the immunologic basis for the pathogenesis of long COVID as well as for potential strategies for diagnosis and therapeutic intervention.
The primary response to counter the effects of a foreign agent, including SARS-CoV-2, is performed by cells of the innate immune system, which carry out the functions of phagocytosis and inflammation (Fig. 1). Housed within the innate immune system are macrophages, neutrophils, mast cells and basophils, natural killer cells, innate lymphoid cells, and dendritic cells as well the biologic amplification systems of complement and the coagulation system.23 All of these components of the innate immune system are activated as part of the host's inflammatory response in COVID-19 and are responsible for many of the clinical and laboratory findings seen during the initial phases of infection (e.g., fever, anemia, thrombocytopenia, neutropenia, hyperferritinemia, hypercoagulopathy, elevated fibrinogen and D-dimer levels).
Figure 1.
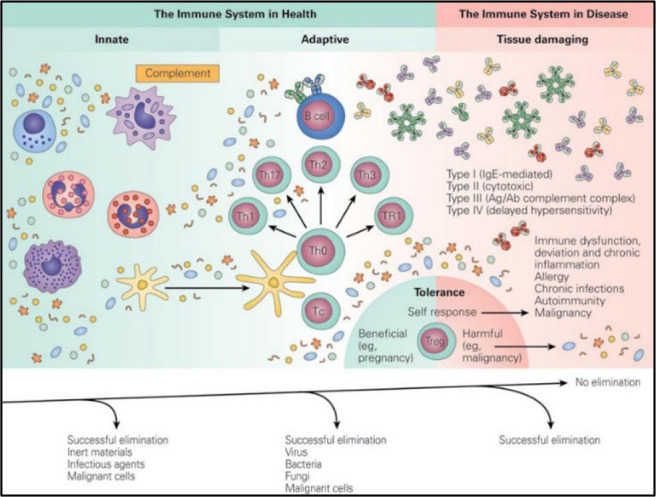
Schematic representation of the total immune capability of the host based on efficiency of elimination of foreign matter. Reproduced with permission from Ref. 23.
During this initial phase of viral entry into and attachment of the virus to ACE2 receptors of cells in the host respiratory system, destruction of lung cells triggers a local immune response, which recruits macrophages and monocytes that respond to the infection, release cytokines, and prime the immune system for the encounter with the second phase of the adaptive immune encounter with T and B cells (Fig. 1). Usually, in the majority of patients, this encounter with cells of the innate and immune system is capable of resolving the infection. However, in some patients, a dysfunctional immune response occurs, in which virus persistence leads to the third phase of the immune response, in which the encounter is no longer beneficial and is associated with excessive release of proinflammatory cytokines and local and systemic tissue injury, referred to as the cytokine storm, the prime determinant of COVID-19 activity.24,25
HOW CAN THE PERSISTENCE OF A MICROBIAL ANTIGEN LEAD TO IMMUNOPATHOLOGY?
For ease of discussion, the proposed pathogenic role of viral persistence is shown schematically in Fig. 2. It shows the immunologic response to a chronic microbial infection in which there is failure of elimination and persistence of the microbial antigen, which leads to the immunopathologic sequelae of chronic microbial infection and the proposed pathogenesis of the long COVID 19 syndrome.
Figure 2.
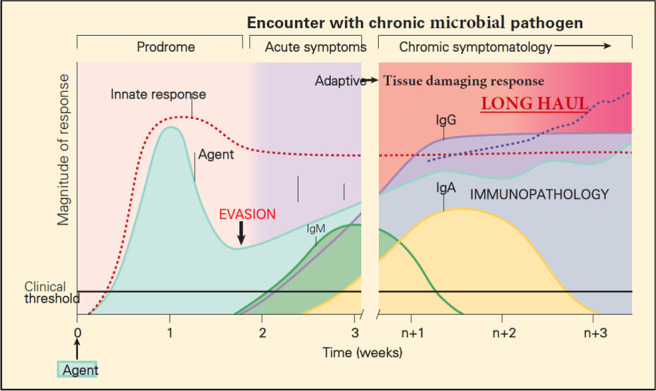
Schematic representation of the innate and adaptive immune responses after the encounter of a nonimmune host with a chronic microbial pathogen, showing evasion of the immune response and the persistence of the bacterial pathogen with immunopathology. Reproduced and modified with permission from Ref. 23.
COVID-19, LONG COVID, AND THE ROLE OF AUTOANTIBODIES
SARS-CoV-2 infection has been shown to induce the development of several autoantibodies, which can persist and explains why some patients develop persisting symptoms.8,26–28 In a recent report by Gagiannis et al.,29 22 patients with RT-PCR-confirmed SARS-CoV-2 infection and 10 patients with non-COVID-19-associated pneumonia were enrolled in a study to evaluate the role of autoantibody (AAB) in SARS-CoV-2-associated respiratory failure. Antinuclear antibody (ANA) and extractable nuclear antigen antibody (ENA) testing revealed ANA titers ≥1:320 and/or positive ENA immunoblots in 11/13 (84.6%) COVID-19 patients with ARDS, in 1/9 (11.1%) COVID-19 patients without ARDS (p = 0.002) and in 4/10 (40%) patients with non-COVID-19-associated pneumonias (p = 0.039). Detection of AABs was significantly associated with a need for intensive care treatment (83.3 vs. 10%; p = 0.002) and occurrence of severe complications (75 vs. 20%, p = 0.03). In a similar type of retrospective study from China, of 21 patients with critical SARS-CoV-2 pneumonia, the investigators showed a prevalence of between 20% and 50% of autoimmune disease–related autoantibodies, which suggests a causal relationship between SARS-CoV-2 infection and autoimmune disease.30
SUMMARY OF INNATE AND ADAPTIVE IMMUNE RESPONSES TO A CHRONIC MICROBIAL INFECTION
The initial innate and adaptive immune response events that occur after the encounter of a nonimmune and susceptible host with a chronic microbial infection are similar to those seen in the primary encounter of a host with an acute microbial pathogen, with the same sequential appearance of T cells and production of immunoglobulin antibody-associated isotypes (Fig. 2). However, in contrast to the primary encounter with an acute microbial pathogen, in this scenario, the microbial pathogen evades the host immune response and persists in the host for extended periods of time. This continued presence of microbial antigen that results from the persistent infection drives continued stimulation of both the innate and adaptive immune systems, which can result in the deleterious expressions of the immune response responsible for the development of the long COVID.
CLINICAL SEQUELAE OF LONG COVID
The consequences of the intense inflammation and immune dysregulation associated with persistence of SARS-CoV-2 or any of its structural proteins on disease manifestations for patients with long COVID are profound and are related to the diffuse endothelial damage and microthrombosis, with consequent continuing organ damage associated with this pathologic entity.2,31 The alteration of taste and smell, e.g., can be related to damage of specific cortical structures32 and arrhythmias, and other cardiac signs and symptoms may be linked to persistent myocardial inflammation.33
In children, the neuropsychological symptomatology can be associated with neuroinflammatory processes34 related to the lymphoproliferative responses known to be heightened in the pediatric population as well as the subsequent toxic buildup of inflammatory cytokines within the central nervous system.35 Also, the gender difference observed in adults is consistent with an inflammatory pathogenesis. It is known, e.g., that women respond to viral infections with greater type I interferon production,36 thought to be related to an enhanced activation of a Toll-like receptor-7 encoded mechanism encoded by genes present on the X chromosome.37 Although this heightened gender-related immunologic response could be beneficial to the female patient in viral elimination during the early stages of infection, it would also expose the female patient to a more persistent deleterious inflammatory state known to occur in long COVID. This gender difference, however, has not been observed in the pediatric population38 and, therefore, hormonal phenomena related to pubertal development could also come into play.
Intestinal dysbiosis, which, in patients with COVID-19 correlates with the severity of the disease, with the level of inflammatory biomarkers, and with the prolonged fecal excretion of SARS-CoV-239 could also play an important pathogenetic role given the close relationships of the gut-brain axis40 in the control of central nervous system inflammatory conditions.41 The reactivation of latent Epstein-Barr virus infection in more than half of the patients with long COVID42 is yet another interesting potential pathogenetic finding because Epstein-Barr virus reactivation has been associated with skin, cardiovascular, hematologic, and neurologic complications of other conditions,43 which may also occur in long COVID.
In practice, long COVID could be induced, both in adults and in children, by long-term damage in different organs, such as lung, brain, kidney, and heart, by inflammation due to viral persistence. In support of this hypothesis-driven viral persistence etiology, a recent study showed the presence of nucleic acids and SARS-CoV-2 proteins in the small intestine of 50% of patients with asymptomatic cases of COVID-19 after 4 months from the onset of the disease,44 probably in relation to an altered specific humoral and cell-mediated response,45 which can have both genetic and epigenetic implications, which need to be studied. In relation to these pathogenetic mechanisms, it becomes essential to investigate the possible deep roots of this new pandemic.
THE POSSIBLE DEEP ROOTS OF THE SYNDROME AND THEIR BIOLOGICAL PLAUSIBILITY
In addition to and supportive of the hypothesis-driven viral persistence etiology is the role of dietary supplementation and the immune response. A diet poor of anti-inflammatory and/or antioxidant substances with potential immune-modulating and antiviral activity can be a predisposing but preventable factor for more severe SARS-CoV-2 and also for the development of persistent long symptoms after the acute phase of the disease. This proposition took its origins from epidemiologic studies that demonstrated that large differences in COVID-19 death rates existed among countries and regions of the world, which seemed to be related to dietary practices.46
These large geographic variations are unlikely totally due to reporting differences among countries, and a striking finding became evident that populations in some countries with very low death rate settings were found to have an unusual common feature of eating large quantities of fermented vegetables, including members of the cruciferous and Brassicaceae family, and, in some continents, various spices.46 At the beginning of the pandemic, treatment with a wide range of existing host-directed therapies, including nutrient supplements, was suggested to be possibly beneficial in the care of SARS-CoV-2 infection.47 Furthermore, the central role of the nuclear factor kappa B (NF-κB) signaling pathway was suggested as one of the explanations concerning the much lower infection rates and mortality reported, generally in Africa.48
THE ROLE OF THE NF-κB AND p38 MITOGEN-ACTIVATED PROTEIN KINASE SIGNALING PATHWAYS IN COVID-19 INFECTION
The proinflammatory cytokine storm represents a dysregulated amplification of proinflammatory cytokines and one of the hallmarks of severe coronavirus infection (COVID-19). These uncontrolled inflammatory responses in patients with severe COVID-19 lead to the acute respiratory symptoms of infection with SARS-CoV-2 as well as many of the manifestations of the long COVID syndrome. Two of the most important signaling pathways involved in the pathogenesis of these conditions are the NF-κB49,50 and p38 mitogen-activated protein kinase signaling pathways.51,52 An understanding of these signaling mechanisms underlying marked proinflammatory mediator release is critical for the development of new diagnostic and therapeutic procedures for improved management of patients with COVID-19 as well as for the long COVID syndrome.
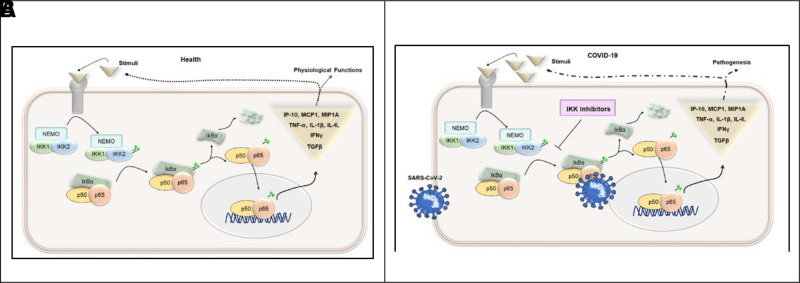
The NF-κB pathway that mediates the release of pro-inflammatory cytokines is a trimolecular complex called inhibitor of NF-κB kinase (IKK). This complex is composed of two catalytic subunits, IKK1 (also known as IKKα) and IKK2 (also known as IKKβ), and a regulatory subunit, NF-κB essential modulator (NEMO) (also known as IKK-γ). Among them, the phosphorylation of IKKβ, followed by its degradation, plays a significant role in the activation of NF-κB signaling pathways (Fig. 3).53 Thus, the prevention of phosphorylation of IKK-β is a key step to inactivate NF-κB in providing therapeutic benefit against many diseases that cause inflammation. A large number of pharmacologic inhibitors have been identified to block the phosphorylation of IKK-β, including PS-1145, SAR113945, IKK-16, TPCA-1, BAY11-7082, BAY11-7085, SC-514, TBK-1, ML-120B, BMS-345541, vinpocetine, and resveratrol.53
Figure 3.
Schematic representation of the NF-κB signaling pathway during healthy and COVID-19 conditions in association with the production of proinflammatory factors. (A) The NF-κB signaling pathway during usual conditions, showing the sequential effects of phosphorylation and the usual production of proinflammatory cytokines. (B) The NF-κB signaling pathway during COVID-19 infection, showing the effects of blockage by inhibitors that block the phosphorylation of IKK-β and inhibit production of proinflammatory cytokines. NF-κB = nuclear factor κ B; COVID-19 = coronavirus disease 2019; IKK-β = IκB kinase-β. Reproduced with permission from Ref. 53.
Therapeutically, impeding NF-κB signaling pathway during SARS-CoV-2 may help control the exaggerated activation of these immune regulatory pathways during a COVID-19 infection and thereby inhibit production of the deleterious effects of the proinflammatory cytokines. Also, NF-κB has been known to potentiate the production of reactive oxygen species, which leads to apoptosis in various tissues. Shown in Fig. 4 is a schematic representation of the two yin and yang opposing inflammatory pathways by which NF-κB can proceed: one by promoting inflammation by triggering the expression of proinflammatory cytokines, the second by dampening proinflammatory signaling by nuclear factor-erythroid factor 2-related factor 2 activation through oxidation of Kelch-like ECH-associated protein 1 (Keap1)oxidation by peroxidases.54
Figure 4.
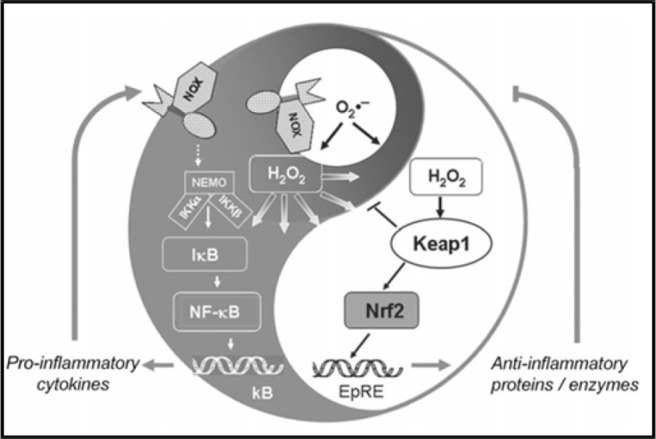
Schematic representation of the yin and yang inflammatory response carried out by the NF-κB signaling pathway. Intranuclear signal transduction can proceed via two pathways; pathway NF-κB can progress to enhance and perpetuate the inflammatory response by triggering the expression of proinflammatory cytokines, and pathway nuclear factor-erythroid factor 2–related factor 2 (Nrf2) activation through oxidation of Kelch-like ECH-associated protein 1 (Keap1) by peroxidases and other anti-inflammatory proteins can dampens proinflammatory signaling. NF-κB = nuclear factor κ B. Reproduced with permission from Ref. 54.
CONCLUSION
It is clear that some individuals, including those with mild initial symptoms of COVID-19, may have variable and debilitating symptoms for many months after the initial infection. In children, the signs and symptoms reported by parents are similar to those described in adults with long COVID. This report proposes a hypothesis-driven model of viral persistence for the pathophysiology of long COVID as a basis for an understanding of the clinical symptomatology and some guidelines for diagnosis and treatment of the condition. The symptoms of long COVID are driven by the excessive production of proinflammatory cytokines, which are potentiated by the production of reactive oxygen species through involvement of the NF-κB and p38 mitogen-activated protein kinase signaling pathways. The condition seems to have a genetic basis, which predisposes individuals to have a diminished immunologic capacity to completely clear the virus, with residual parts of the virus persisting. This persistence of virus and resultant hyperproduction of proinflammatory cytokines are the basis of the syndrome. Prevention of the condition can be achieved by comprehensive vaccination and treatment of the condition by antiviral and anti-inflammatory biologic medications, which are rapidly becoming available.
Footnotes
A. L. Boner serves as a consultant for Envicon Medical srl Italy. The remaining author have no conflicts to declare
Funding provided by a grant from the Martyn A. Vickers Sr, MD, Endowment Fund, Georgetown University Medical Center (J.A.B.)
REFERENCES
- 1. Phillips S, Williams MA. Confronting our next national health disaster - long-haul Covid. N Engl J Med. 2021; 385:577–579. [DOI] [PubMed] [Google Scholar]
- 2. Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021; 374:n1648. [DOI] [PubMed] [Google Scholar]
- 3. van Kessel SAM, Olde Hartman TC, Lucassen PLBJ, et al. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2022; 39:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021; 110:2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg M, Maralakunte M, Garg S, et al. The conundrum of 'long-COVID-19': a narrative review. Int J Gen Med. 2021; 14:2491–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006; 333:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sotzny F, Blanco J, Capelli E, et al. Myalgic encephalomyelitis/chronic fatigue syndrome - evidence for an autoimmune disease. Autoimmun Rev. 2018; 17:601–609. [DOI] [PubMed] [Google Scholar]
- 9. Long COVID and kids: more research is urgently needed. Nature. 2022; 602:183. [DOI] [PubMed] [Google Scholar]
- 10. Nafilyan V, Islam N, Ayoubkhani D, et al. Ethnicity, household composition and COVID-19 mortality: a national linked data study. J R Soc Med. 2021; 114:182–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buonsenso D, Di Gennaro L, Rose C, et al. Long-term outcomes of pediatric infections: from traditional infectious diseases to long covid. Future Microbiol. 2022. Mar (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2021; S1198-743X(21)00629–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choudhary S, Sreenivasulu K, Mitra P, et al. Role of genetic variants and gene expression in the susceptibility and severity of COVID-19. Ann Lab Med. 2021; 41:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Severe Covid-19 GWAS Group, Ellinghaus D, Degenhardt F, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020; 383:1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson BK, Francisco EB, Yogendra R, et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front Immunol. 2022; 12:746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao X-H, He Z-C, Li T-Y, et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020; 30:541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nienhold R, Ciani Y, Koelzer VH, et al. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun. 2020; 11:5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021; 53:737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viszlayová D, Sojka M, Dobrodenková S, et al. SARS-CoV-2 RNA in the cerebrospinal fluid of a patient with long COVID. Ther Adv Infect Dis. 2021; 8:20499361211048572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020; 5:434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omololu A, Ojelade B, Ajayi O, et al. Long COVID”: a case report of persistent symptoms in a patient with prolonged SARS-CoV-2 shedding for over 110 days. SAGE Open Med Case Rep. 2021; 9:2050313X211015494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellanti JA, Green RE. Immunological reactivity. Expression of efficiency in elimination of foreignness. Lancet. 1971; 2:526–529. [DOI] [PubMed] [Google Scholar]
- 23. Bellanti JA. Immunology IV: Clinical Applications in Health and Disease. Bethesda, MD: I Care Press; 2012. [DOI] [PubMed] [Google Scholar]
- 24. Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021; 13:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020; 71:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun. 2021; 4:100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richter AG, Shields AM, Karim A, et al. Establishing the prevalence of common tissue-specific autoantibodies following severe acute respiratory syndrome coronavirus 2 infection. Clin Exp Immunol. 2021; 205:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehrenfeld M, Tincani A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020; 19:102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gagiannis D, Steinestel J, Hackenbroch C, et al. Clinical, Serological, and Histopathological Similarities Between Severe COVID-19 and Acute Exacerbation of Connective Tissue Disease-Associated Interstitial Lung Disease (CTD-ILD). Front Immunol. 2020; 11:587517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020; 13:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021; 27:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020; 17:1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020; 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bostancıklıoğlu M. SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain Behav Immun. 2020; 87:122–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berghöfer B, Frommer T, Haley G, et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006; 177:2088–2096. [DOI] [PubMed] [Google Scholar]
- 37. Webb K, Peckham H, Radziszewska A, et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol. 2019; 9:3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ortona E, Buonsenso D, Carfi A, et al. Long COVID: an estrogen-associated autoimmune disease? Cell Death Discov. 2021; 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeoh YK, Zuo T, Lui GC-Y, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021; 70:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014; 157:121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yong SJ, Tong T, Chew J, et al. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020; 13:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gold JE, Okyay RA, Licht WE, et al. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. 2021; 10:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Gao F. Clinical characteristics of primary and reactivated Epstein-Barr virus infection in children. J Med Virol. 2020; 92:3709–3716. [DOI] [PubMed] [Google Scholar]
- 44. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021; 591:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo M, Tao W, Flavell RA, et al. Potential intestinal infection and fecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021; 18:269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bousquet J, Anto JM, Czarlewski W, et al. Cabbage and fermented vegetables: from death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy. 2021; 76:735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zumla A, Hui DS, Azhar EI, et al. Reducing mortality from 2019-nCoV: host directed therapies should be an option. Lancet. 2020; 395:e35–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kircheis R, Schuster M, Planz O. COVID-19: mechanistic model of the African paradox supports the central role of the NF-κB pathway. Viruses. 2021; 13:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanan T, Kanan D, Al Shardoub EJ, et al. Transcription factor NF-κB as target for SARS-CoV-2 drug discovery efforts using inflammation-based QSAR screening model. J Mol Graph Model. 2021; 108:107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012; 4:a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang L, Shi Y, Gong B, et al. Dynamic blood single-cell immune responses in patients with COVID-19. Signal Transduct Target Ther. 2021; 6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2021; 394:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brigelius-Flohé R, Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011; 15:2335–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]