Abstract
The edible straw mushroom, Volvariella volvacea, produces a multicomponent enzyme system consisting of endo-1,4-β-glucanase, cellobiohydrolase, and β-glucosidase for the conversion of cellulose to glucose. The highest levels of endoglucanase and cellobiohydrolase were recorded in cultures containing microcrystalline cellulose (Avicel) or filter paper, while lower but detectable levels of activity were also produced on carboxymethyl cellulose, cotton wool, xylitol, or salicin. Biochemical analyses of different culture fractions in cultures exhibiting peak enzyme production revealed that most of the endoglucase was present either in the culture filtrate (45.8% of the total) or associated with the insoluble pellet fraction remaining after centrifugation of homogenized mycelia (32.6%). Cellobiohydrolase exhibited a similar distribution pattern, with 58.9% of the total enzyme present in culture filtrates and 31.0% associated with the pellet fraction. Conversely, most β-glucosidase activity (63.9% of the total) was present in extracts of fungal mycelia whereas only 9.4% was detected in culture filtrates. The endoglucanase and β-glucosidase distribution patterns were confirmed by confocal laser scanning microscopy combined with immunolabelling. Endoglucanase was shown to be largely cell wall associated or located extracellularly, with the highest concentrations being present in a region 1 to 2 μm wide immediately adjacent to the outer surface of (and possibly including) the hyphal wall and extending 60 to 70 μm from the hyphal tip. Immunofluorescence patterns indicated little if any intracellular endoglucanase. Most β-glucosidase was located intracellularly in the apical area extending 60 to 70 μm below the hyphal tip, although enzyme was also evident in the extracellular region extending approximately 15 μm all around the hyphal tip and trailing back along the length of the hypha. The regions of the hypha located some distance from the apical region appeared to be devoid of intracellular β-glucosidase, and the enzyme appears to be associated almost exclusively with, or located on the outside surface of, the hyphal wall.
The edible straw mushroom, Volvariella volvacea (Bull ex Fr.) Sing., is grown on an industrial scale in many tropical and subtropical regions and currently ranks fifth among the world’s most important commercially cultivated species (14). Although rice straw has traditionally been used as a growth substrate, the mushroom has also been cultivated on a variety of lignocellulosic wastes including other cereal straws, sugar cane bagasse, oil palm pericarp, and banana leaves. However, V. volvacea appears unable to grow well on “woody” materials which have a substantial lignin content, and earlier fructification and increased growth yields have been achieved by the introduction of high-cellulose cotton waste “composts” (13).
The different abilities of an individual mushroom species to grow and fruit on a particular lignocellulosic substrate are determined by both fungus- and substrate-associated factors (7). These include the level of tolerance of the mushroom to potentially toxic phenolic monomers present in lignocellulosic residues of the type used for mushroom cultivation (9, 28) and the capacity of the mushroom to produce the hydrolytic and oxidative enzymes necessary to degrade individual components (e.g., cellulose, hemicellulose, and lignin) of the growth substrate (8). Like many cellulolytic fungi, V. volvacea produces a multicomponent enzyme system, consisting of endo-1,4-β-glucanase (EC 3.2.1.4), cellobiohydrolase (EC 3.2.1.91), and β-glucosidase (β-d-glucosidic glucohydrolase; EC 3.2.1.21), for the conversion of cellulose to glucose (10, 11). Five endoglucanase, five cellobiohydrolase, and two β-glucosidase isoforms have been identified by gel electrophoresis, and a number of individual components of the cellulolytic system have been isolated and partially characterized. Here, we describe a combined biochemical and immunocytochemical study of cellulase distribution in cultures of V. volvacea by using cell fractionation and confocal laser microscopy. This study aims to provide a better understanding of the production and secretion of lignocellulolytic enzymes in V. volvacea and is part of a broader research program directed at enhancing fungal bioconversion of the growth substrate and improving growth yields of commercially important edible mushrooms.
MATERIALS AND METHODS
Organism and cultivation.
V. volvacea V14 was obtained from the culture collection of the Centre for International Services to Mushroom Biotechnology located at The Chinese University of Hong Kong (accession no. CMB 002). The fungus was maintained on potato dextrose agar (PDA) at room temperature with periodic transfer.
Fungal inoculum was prepared by growing V. volvacea on potato dextrose broth (PDB) for 8 to 10 days at 32°C in stationary culture. The fungal mat was washed twice by decantation with sterile distilled water, transferred to a sterile Waring blender cup containing 50 ml of sterile distilled water, and homogenized at full power three times for 5 s each. To determine biomass production and enzyme levels in culture fluids following fungal growth on different carbon sources, 1-ml aliquots were transferred to 250-ml Erlenmeyer flasks containing 50 ml of basal medium plus 1% (wt/vol) carbon source as indicated. The mycelium used to determine the distribution of cellulolytic enzymes in different cell fractions was prepared by transferring aliquots (4 ml) to 2-liter Erlenmeyer flasks containing 500 ml of basal medium. For the production of sufficient quantities of mycelium to purify the cell-associated β-glucosidases, 10-ml aliquots were transferred to 2-liter flasks containing 600 ml of basal medium plus 1% (wt/vol) Sigmacell as the carbon source. The basal medium contained (in grams per liter) KH2PO4, 1.0; K2HPO4, 0.4; MgSO4 · 7H2O, 0.5; CaCl2 · 2H2O, 0.013; yeast extract (Difco), 0.1; l-asparagine, 1.5; NH4NO3, 0.5; and thiamine · HCl, 0.0025 (sterilized by filtration and added after autoclaving of other medium components); it also contained 0.2% (vol/vol) Tween 80 and 1 ml of a trace element solution consisting of (grams per liter) ferric citrate, 4.8; ZnSO4 · 7H2O, 2.64; MnCl2 · 4H2O, 2.0; CoCl2 · 6H2O, 0.4; and CuSO4 · 5H2O, 0.4. The medium was adjusted to pH 6.0 with 2 M KOH and sterilized by autoclaving (15 lb/in2 for 15 min). The cultures were incubated at 32°C for 5 days (unless stated otherwise) in an orbital incubator shaker operated at 150 rpm.
Fungal samples for immunocytochemical analysis were grown on plates containing the basal medium with either crystalline cellulose (Sigmacell) or glucose as the carbon source (1%, wt/vol). The plates were inoculated with a 0.5-cm-diameter plug of V. volvacea from 7-day-old PDA plate cultures. A sterile coverslip was inserted into the medium at approximately 10 to 20° to the agar surface and about 2 cm distant from the inoculum. Once the coverslip was overlaid with hyphal growth, it was removed and prepared for analysis.
Preparation of different fractions for enzyme distribution studies.
The four fractions assayed for cellulolytic enzyme activities were culture fluids, mycelial washings, mycelial extracts, and insoluble pellet fraction remaining after centrifugation of homogenized mycelia. The various fractions were prepared as follows. Culture fluids were obtained after the contents of two culture flasks were filtered through layers of cheesecloth to retain the fungal mycelium and further clarified by centrifugation prior to enzyme assay. Mycelial washings were obtained by washing mycelia three times with 100- to 200-ml aliquots of sterile distilled water; excess liquid was removed between each wash by gentle squeezing of the collected mycelium. Fungal mycelium was suspended in 10 mM potassium phosphate buffer (pH 6.5) (1:1 [wt/vol] ratio based on the wet weight of mycelium) and homogenized with a glass homogenizer; the cell break was then centrifuged at 12,000 × g for 30 min, and the supernatant was retained as the mycelial extract. The remaining pellet was resuspended in the same volume of buffer and centrifuged as before, and the second supernatant fraction was combined with the first. The insoluble residue, resuspended a second time in the same volume of buffer, served as the insoluble pellet fraction.
Enzyme assays.
Endoglucanase (CMCase) activity was determined by measuring the amount of glucose released from carboxymethyl cellulose (CMC) by the Somogyi-Nelson method with glucose as the standard (23, 29). Reaction mixtures contained 0.8 ml of 50 mM potassium phosphate buffer (pH 6.2), 0.1 ml of 1% (wt/vol) CMC solution, and 0.1 ml of enzyme fraction. Controls lacked either CMC or the enzyme fraction. After incubation at 50°C for 30 min, the reaction was terminated by adding 1.0 ml of Somogyi reagent. The mixture was vortexed, placed in a boiling-water bath for 15 min, and cooled to room temperature, and 1.0 ml of Nelson reagent was added. After being vortexed, the mixture was allowed to stand at room temperature for 20 min and centrifuged to remove any precipitate, and the absorbance of the supernatant was measured at 520 nm. Cellobiohydrolase (Avicelase) activity was determined in shaken reaction mixtures (in 25-ml flasks) containing 1.7 ml of 50 mM potassium phosphate buffer (pH 6.2), 0.8 ml of 1% (wt/vol) microcrystalline cellulose suspension (Sigmacell type 20), and 0.5 ml of enzyme fraction; essentially the same procedure was used. Controls lacked cellulose and enzyme fraction. At the end of the reaction period, mixtures were immediately placed in ice and centrifuged for 5 min at 4°C to remove residual cellulose before addition of Somogyi reagent. β-Glucosidase activity was determined by measuring the hydrolysis of p-nitrophenyl-β-d-glucopyranoside (pNPβG). The incubation mixture comprised 2 mM pNPβG, 50 mM potassium phosphate buffer (pH 6.5), and appropriately diluted enzyme solution in a total volume of 1 ml. The reaction was carried out at 40°C for 30 min and terminated by the addition of 3 ml 1.0 M Na2CO3. The amount of p-nitrophenol released was determined spectrophotometrically by measuring the absorbance of the solution at 400 nm. One unit of enzyme activity was defined as the amount of enzyme that produced 1 μmol of product per min under the conditions of assay. The enzyme activity in material for microscopy studies was confirmed by overlaying the hypha-coated coverslips with 1% agarose containing 40 mM pNPβG, incubating the mixture at 45°C for 10 min, and observing the appearance of a yellow color due to the release of p-nitrophenol.
Protein determination.
Protein was determined by the method of Bradford (4), with bovine serum albumin (BSA) as the standard.
Chemicals.
pNPβG, BSA, Freund’s complete adjuvant, CMC, and microcrystalline cellulose (Sigmacell) were purchased from Sigma Chemical Co. (St. Louis, Mo.). PDB and PDA were from Difco. All other chemicals were purchased from commercial sources and were of analytical grade.
Production of polyclonal antibodies.
The protein fraction used to raise antibodies to endoglucanase (endoglucanase III) was purified from spent culture fluids following growth of V. volvacea on Avicel by anion-exchange chromatography, chromatofocusing, and Mono-Q fast protein liquid chromatography. The fraction separated as a discrete peak on Mono-Q fast protein liquid chromatography and could not be resolved further by anion-exchange chromatography with Mono-Q or Mono-P columns or by hydrophobic interaction chromatography with phenyl-Sepharose. Isoelectric focusing polyacrylamide gel electrophoresis (PAGE) revealed that the fraction consisted of three endoglucanase III isoforms, with isoelectric points between 4.6 and 5.2, all of which cleaved 4-methylumbelliferylcellotrioside. Anti-endoglucanase antiserum was produced by a modification of the method of Baumgarten et al. (2). Antibodies were raised in a female mouse in response to two intramuscular injections of purified enzyme at 7-day intervals. The first injection comprised 0.1 mg of endoglucanase III in 184 μl of 20 mM sodium phosphate buffer (pH 7.3) containing 0.14 M NaCl and mixed with an equal volume of Freund’s complete adjuvant, while the second injection consisted of half this dosage. Blood was collected 4 days after the second injection, and the serum was separated by centrifugation (10,000 × g for 30 min at 4°C).
Anti-β-glucosidase antiserum was raised by using combined fractions from a Mono-P column that exhibited both BGL-I and BGL-II activity (11). Antibodies were raised in rabbits in response to two subcutaneous injections of purified β-glucosidase at 4-week intervals. For each injection, 0.18 mg of β-glucosidase in 1.0 ml of 20 mM sodium phosphate buffer (pH 7.3) containing 0.14 M NaCl was mixed thoroughly with an equal volume of Freund’s complete adjuvant. Blood was collected 7 days after the second injection, and the serum was separated by centrifugation (10,000 × g for 30 min at 4°C). This antiserum (10 ml) was applied to an Affinity HiTrap protein A column (Pharmacia) equilibrated with 20 mM sodium phosphate buffer (pH 7.0). Unbound protein was removed by washing the column with 10 ml of the same buffer, and bound protein was eluted with 100 mM citric acid–NaOH buffer (pH 3.0). The eluted fractions containing protein were combined, adjusted to pH 7.0 with 0.5 M NaOH, assessed for anti-β-glucosidase activity by the Ouchterlony double-diffusion procedure (24), freeze-dried, and stored at −20°C. The purity of the material was confirmed by sodium dodecyl sulfate-PAGE which revealed a single protein band with an apparent molecular mass of 51 kDa.
Anti-plant phytochrome antibody was the generous gift of C. S. Evans.
Specificity of antibodies.
Interaction of anti-endoglucanase serum and purified endoglucanase III was confirmed by immunodiffusion. Interaction of both anti-β-glucosidase antiserum and the purified antibody with β-glucosidase was determined by immunodiffusion and immunoblotting procedures (6). For immunodiffusion, Ouchterlony double diffusion was carried out in petri plates containing 1% (wt/vol) agar in 50 mM sodium phosphate buffer (pH 7.0) and 0.02% NaN3. Endoglucanase or β-glucosidase (200 μg) was placed in the central well, and serially diluted antiserum was placed in the peripheral wells. The plate was sealed with Parafilm and incubated for 48 h at room temperature, and the formation of arcs of precipitation was recorded.
For Western blotting, β-glucosidase was subjected to native PAGE (7.5% polyacrylamide gels) and then transferred to nitrocellulose sheets by the Bio-Rad electroblotting system. The nitrocellulose sheets were suspended for 1 h in a blocking solution containing 0.5% BSA in Tris-buffered saline (TBS) (10 mM Tris, 150 mM NaCl [pH 7.4]) to saturate additional binding sites, and then for 2 h in the same solution containing a 1:32 dilution of purified anti-β-glucosidase antibody. After three 5-min washings with 0.1% BSA in TBS, the membrane was incubated at room temperature for 4 h with TBS containing 0.1% BSA and secondary antibody, a 1:3,000 dilution of goat anti-rabbit antiserum conjugated to alkaline phosphatase. After six 10-min washes with TBS, overlaying the membranes with 1% agarose containing 0.2 M Tris-HCl (pH 8.3), 1 mM MgCl2, and 0.05% (wt/vol) 5-bromo-4-chloro-3-indolyl phosphate revealed a single blue-staining band.
Confocal laser scanning microscopy.
For localizing endoglucanase, coverslips coated with fungal hyphae from agar plate cultures containing the different carbon sources were briefly heat fixed and then chemically fixed with 2% glutaraldehyde for 30 min. The samples were then washed for 5 min each in six changes of 10 mM sodium phosphate buffer (pH 7.4) and then quenched for 2 h in a blocking solution consisting of the same buffer containing 1% (wt/vol) BSA and 0.02% sodium azide (albumin-azide buffer [AZB]) and normal rabbit serum (1:32 dilution). Hyphae were then incubated for 2 h in a solution containing anti-endoglucanase antiserum diluted 1:32 in AZB. Controls were incubated with preimmune mouse serum diluted 1:32 in AZB. After four 5-min washes with AZB, test and control samples were incubated for 15 min with rabbit anti-mouse–tetramethylrhodamine-5-isothiocyanate (TRITC)-labelled antibody diluted 1:64 in AZB containing 1% BSA, washed thoroughly with 10 mM sodium phosphate buffer and distilled water, air dried, and mounted in glycerol for confocal laser scanning microscopy (Leica microscope). The same procedure was used for localizing β-glucosidase, with the following modifications: (i) quenching was carried out with a blocking solution containing normal goat serum (1:32 dilution) in place of normal rabbit serum; (ii) hyphae were treated with purified β-glucosidase antibody diluted 1:32 in AZB in place of anti-endoglucanase antiserum (controls in this case were incubated with preimmune rabbit serum diluted 1:32 in AZB); and (iii) test and control samples were treated with goat anti-rabbit immunoglobulin G-fluorescein isothiocyanate (FITC)-labelled antibody diluted 1:960 with AZB instead of rabbit anti-mouse-TRITC-labelled antibody.
RESULTS
Effect of different carbon sources on fungal growth and on the levels of free endoglucanase and cellobiohydrolase in culture fluids.
The effects of different carbon sources on the growth of V. volvacea and on free endoglucanase and cellobiohydrolase levels in culture fluids are shown in Table 1. The highest levels of both enzymes were recorded in cultures containing Avicel or filter paper, while lower but detectable levels of activity were also produced on CMC, cotton wool, xylitol, or salicin. No activity was recorded in cultures supplemented with arabinose, cellobiose, esculin, galactose, glucose, lactose, maltose, mannose, sorbose, starch, sucrose, birch or oat spelt xylan, or xylose, even though the fungus grew well on all these carbon sources except arabinose and sorbose.
TABLE 1.
Effect of carbon source on the growth of V. volvacea V14 in submerged culture and on endoglucanase and cellobiohydrolase levels in culture fluids
| Carbon source (1%, wt/vol) | Growth (mg dry wt) | Endoglucanasea (mU/ml of culture broth) | Cellobiohydrolasea (mU/ml of culture broth) |
|---|---|---|---|
| Sigmacell | NDb | 644 | 63 |
| CMC | 64 | 57 | 6 |
| Cotton wool | ND | 5 | 5 |
| Filter paper | ND | 574 | 67 |
| Salicin | 25 | 3 | 14 |
| Xylitol | 141 | 48 | 13 |
Values shown are averages of duplicate experiments for each substrate; maximum variation was <10%. Cultures were grown in 250-ml Erlenmeyer flasks containing 50 ml of medium at 32°C for 4.5 days.
ND, not determined due to residual solid substrate.
Production and distribution of endoglucanase, cellobiohydrolase, and β-glucosidase in different culture fractions.
Endoglucanase, cellobiohydrolase, and β-glucosidase activities were detectable at low levels after 48 h in the culture fluid of V. volvacea cultures grown with microcrystalline cellulose (Avicel). The levels of all three enzymes increased sharply to reach peaks (0.68, 0.135, and 0.13 U per ml of culture fluid for endoglucanase, cellobiohydrolase, and β-glucosidase, respectively) within the next 48 to 72 h before declining.
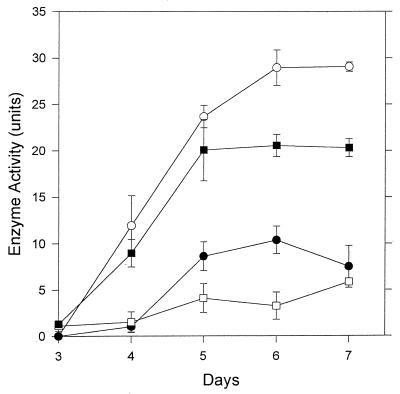
In cultures exhibiting peak enzyme production (after 6 days of incubation), 45.8% of the total endoglucase was present in the culture filtrate and 32.6% was associated with the insoluble pellet fraction remaining after centrifugation of homogenized mycelia (Fig. 1). Of the total activity, 16.4% could be removed by washing intact mycelia, and 5.2% of the enzyme was detected in mycelial extracts (Fig. 1).
FIG. 1.
Distribution of endoglucanase in different fractions of V. volvacea cultures. □, Mycelial extracts; ■, pellet; •, washings; ○, culture fluid. Values represent the mean of two replicate determinations; error bars indicate the standard deviations. When not shown, the error bars fall within the symbols.
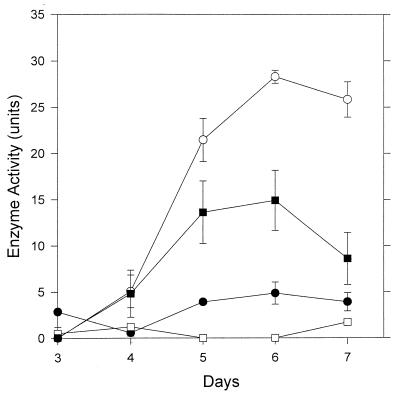
A similar pattern of distribution was found with cellobiohydrolase after 6 days of growth with 58.9% of the total enzyme present in culture filtrates, and 31.0% was associated with the pellet fraction (Fig. 2). In this case, approximately 10.0% of the total activity could be removed by washing intact mycelia but no enzyme was detected in mycelial extracts (Fig. 2).
FIG. 2.
Distribution of cellobiohydrolase in different fractions of V. volvacea cultures. □, Mycelial extracts; ■, pellet; •, washings; ○, culture fluid. Values represent the mean of two replicate determinations; error bars indicate the standard deviations. When not shown, the error bars fall within the symbols.
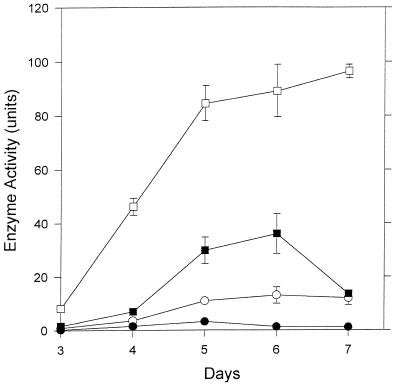
A different pattern of enzyme distribution was observed for β-glucosidase in 6-day-old cultures. Here, 63.9% of the total was present in extracts of fungal mycelia (Fig. 3) while the pellet fraction contained 25.8% of the enzymic activity. Only 9.4 and 0.9% of the total β-glucosidase was detected in culture filtrates and mycelial washings, respectively (Fig. 3).
FIG. 3.
Distribution of β-glucosidase in different fractions of V. volvacea cultures. □, Mycelial extracts; ■, pellet; •, washings; ○, culture fluid. Values represent the mean of two replicates; error bars are the standard deviations. When not shown, the error bars fall within the symbols.
Distribution of endoglucanase, cellobiohydrolase, and β-glucosidase as determined by confocal laser scanning microscopy.
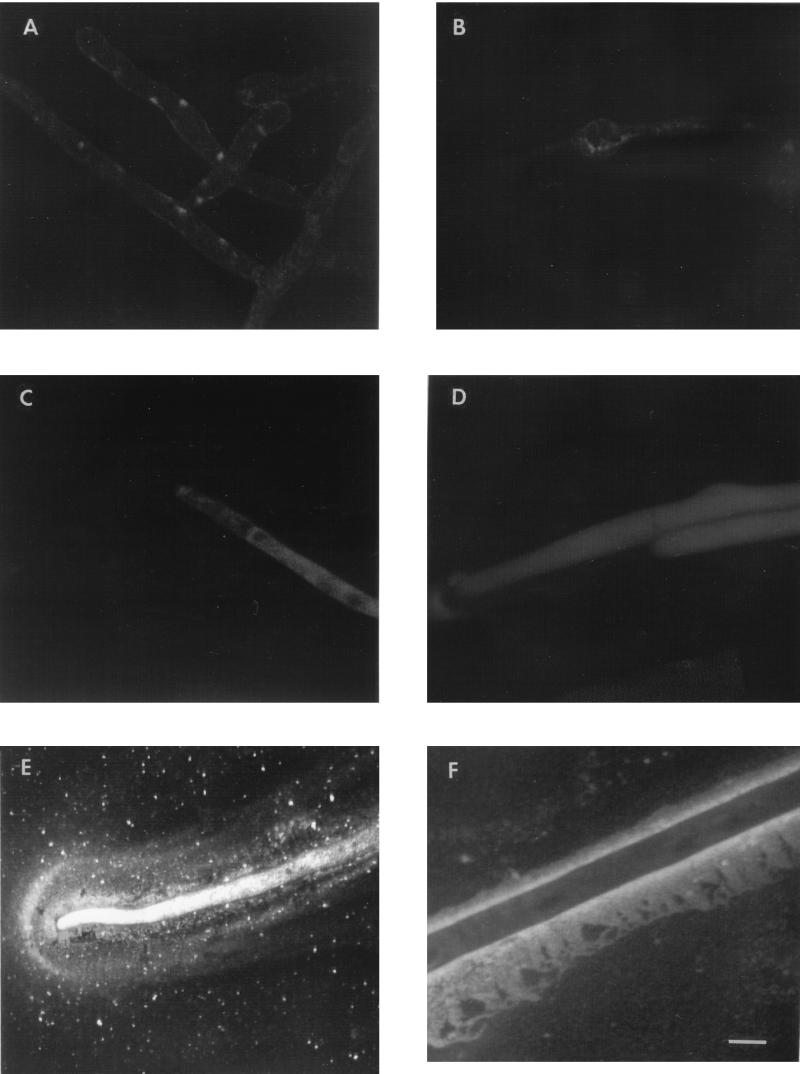
Figure 4E shows the distribution of β-glucosidase in the apical region of fungal hyphae grown on cellulose following treatment with purified anti-β-glucosidase antibody and fluorescent-dye-labelled secondary antibody combined with confocal laser scanning microscopy. The intracellular localization of the enzyme at the hyphal apex and in the apical area extending 60 to 70 μm below the hyphal tip was revealed by the presence of intense fluorescence in these regions. β-Glucosidase was also evident in the extracellular region extending approximately 15 μm all around the hyphal tip and trailing back along the length of the hypha. No significant fluorescence was observed when cellulose-grown hyphae were treated with either normal rabbit serum or immune sera raised in rabbits to plant phytochrome instead of the anti-β-glucosidase antiserum prior to exposure to the secondary antibody (Fig. 4B and D). Moreover, no significant fluorescence was seen associated with hyphae grown on glucose and treated with either normal rabbit serum (Fig. 4A) or anti-β-glucosidase antiserum (Fig. 4C). The regions of the hypha located some distance from the apical region appeared to be devoid of intracellular β-glucosidase, and the enzyme appears almost exclusively to be associated with, or located on the outside surface of, the hyphal wall. Sectioning by the moving laser beam revealed a relatively intense band of fluorescence approximately 1 to 2 μm wide in the region immediately adjacent to the hyphal wall, surrounded by a wider and more diffuse, less intensely fluorescent “sheath” (Fig. 4F).
FIG. 4.
Confocal laser scanning micrographs showing secretion and localization of β-glucosidase in V. volvacea hyphae grown on glucose (A and C) or crystalline cellulose (Sigmacell) (B, D, E, and F). Hyphae were treated with goat anti-rabbit antibody–FITC following exposure to the primary anti-β-glucosidase antibody (C, E, and F), rabbit anti-plant phytochrome antibody (D), or preimmune rabbit serum (A and B). The bar represents 10 μm. Magnification, ×800.
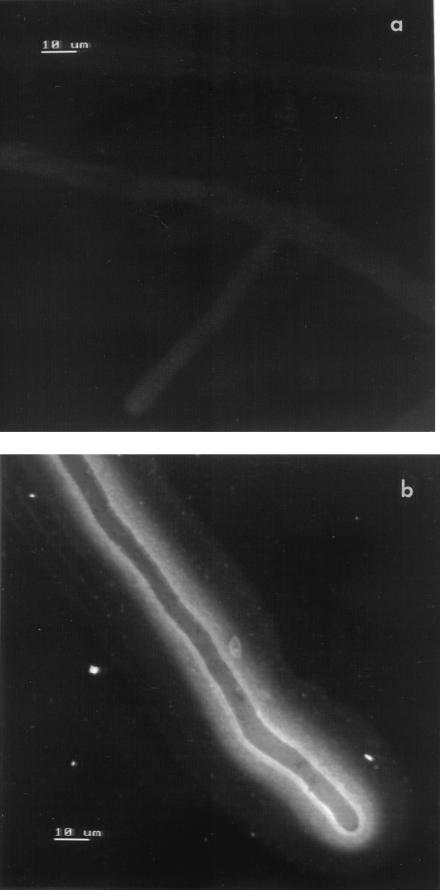
Confocal laser scanning microscopy combined with immunolabelling revealed that endoglucanase was largely cell wall associated or located extracellularly. Fluorescence patterns indicated that the highest concentration of enzyme was present in a region 1 to 2 μm wide immediately adjacent to the outer surface of (and possibly including) the hyphal wall and extending 60 to 70 μm from the hyphal tip (Fig. 5). Large amounts of enzyme also appear to be localized in a compact band 5 to 6 μm wide extending from this layer and along the length of the hyphal specimen. This, in turn, was encompassed by a broader (8- to 10-μm-wide), discrete sheath of diffuse fluorescence indicating a region of low enzyme concentration. There is a clear contrast between the fluorescence intensity of the two innermost extracellular bands and internal hyphal areas, including the hyphal apex zone, indicating that little if any intracellular endoglucanase is present (Fig. 5b). No significant fluorescence was observed when cellulose-grown hyphae were treated with normal mouse serum prior to exposure to the secondary antibody (Fig. 5a) or with hyphae grown on glucose and treated with either normal mouse serum or anti-endoglucanase antiserum.
FIG. 5.
Confocal laser scanning micrographs showing secretion and localization of endoglucanase in V. volvacea hyphae grown on crystalline cellulose (Sigmacell). Hyphae were treated with rabbit anti-mouse antibody–TRITC following exposure to the primary mouse anti-endoglucanase antiserum (b) or to normal mouse serum (a). The bars represent 10 μm. Magnification, ×760.
DISCUSSION
A separate study (19a) has revealed that cloned genes coding for cellobiohydrolases in V. volvacea (cbhI and cbhII) have similar architectures to analogous genes from other fungi with typical cellulose binding, linker, and catalytic domain regions (18). Thus, the levels of cellobiohydrolase detected in culture fluids prior to peak enzyme production (Fig. 2) are probably an underestimate of the total extracellular enzyme present, since these data do not take into account enzyme which may be bound to residual insoluble substrate which remains in the cultures during this period and then sediments as part of the pellet fraction. More representative are the distribution patterns for cellobiohydrolase shown for cultures aged 5 days or more, since no residual insoluble substrate was usually evident after this time.
Although the hyphae analyzed in this study were growing across a coverslip and therefore not in direct contact with the cellulosic substrate, they are still supported nutritionally by the parental mycelium and can be considered representative of hyphae growing, for example, in paddy straw or cotton waste. Such hyphae must forage across the lumen of the plant cell or between the spaces separating the cotton fibers and are in no danger of starvation since the essence of hyphal systems is the translocation of nutrients in appropriate directions as determined by those hyphae.
Although an earlier report concluded that β-glucosidase in V. volvacea was exclusively extracellular (12), data presented here show that large amounts of the enzyme are located either in the cytosol (in hyphal apical regions) or bound to the cell wall in the form of a discrete external sheath (in regions extant from the hyphal apex). Cai et al. (10, 11) also found that most enzyme activity was hyphal associated although some extracellular β-glucosidase was detected. A similar pattern of β-glucosidase distribution was described in Trichoderma reesei QM9414 by Acebal et al. (1), who used biochemical assays to detect β-glucosidase in different cellular fractions and demonstrated enzyme activity in the cell wall, in cell extracts, and in the extramycelial fraction. Messner et al. (22) reported that the extracellular β-glucosidase of T. reesei QM 9414 is mainly bound to the cell wall of the fungus and only partially released into the medium. The enzyme appeared to be tightly associated with a cell wall polysaccharide which functions as an “anchor glycan.” Addition of the polysaccharide to β-glucosidase in vitro also increased by twofold the activity of the enzyme against pNPβG.
Data from experiments with V. volvacea involving protoplast formation and the isolation and treatment of wall fragments with murolytic enzymes are inconclusive, and so far it has not been possible to determine the proportion of cell-bound activity which is present in the actual hyphal wall. When disrupted mycelia and mycelial protoplast preparations were used, approximately 86% of the constitutive β-glucosidase activity in Trichoderma viride was detected in a fraction containing the cytosol, plasma membrane, and periplasm and only 14% was detected in the cell wall fraction (32). Most of the β-glucosidase was on or near the cell surface, especially in the cell wall and periplasm. Sprey (30) used ferritin-antibody conjugates combined with transmission electron microscopy to demonstrate the presence of β-glucosidase in the outermost exopolysaccharide layer, in the plasma membrane region, and, to a lesser extent, within the carbohydrate middle portion of cell walls of T. reesei. This distribution pattern led to the suggestion that a sequential degradation of cellooligomers with higher to lower degree of polymerization (DP) occurs with water-insoluble cellooligomers (higher DPs) attached and degraded in the exopolysaccharide region followed by further degradation to soluble cellooligomers (lower DPs) by the centrally located β-glucosidase and removal of glucose moieties from these soluble cellooligomers by the plasma membrane-located enzyme.
The distribution patterns for β-glucosidase in the white rot basidiomycete Coriolus versicolor varied according to the growth conditions (16). Immunogold cytochemical labelling of hyphal sections revealed that β-glucosidase was localized in the extracellular mucilage, cell wall layers, and the cell interior in hyphae grown on a glucose-rich malt extract medium. When the fungus was grown with CMC as the sole carbon source, little extracellular mucilage was encountered and most labelling occurred in the cell wall layers and cell interior. Hyphae from beechwood cultures showed gold labelling of β-glucosidase in mucilage and fungal cell walls with some intracellular labelling. It has been suggested that the mucilage associated with C. versicolor hyphae serves as a matrix for immobilization of β-glucosidase (16, 17). A polysaccharide sheath-like structure composed of β-1,3–β-1,6-d-glucan was also found in Phanerochaete chrysosporium (3). The structure may play a role in retaining lignin-degrading enzymes and in establishing a material junction between the fungal hypha and the wood cell wall (27). Hyphae of V. volvacea are also surrounded by a thick mucilaginous layer through which endoglucanase, after secretion from the hyphal tip, may permeate both laterally and/or longitudinally (Fig. 5b).
Our data provide further support for earlier suggestions that protein secretion by filamentous fungi is probably restricted to the hyphal tip area (15, 25, 26, 31). Immunocytochemical techniques were used previously to demonstrate that glucoamylase secretion in Aspergillus niger occurred predominantly at the growing hyphal tips (34). The tips of newly formed hyphal branches were also shown to be associated with the secretion of lignin-degrading enzymes (33). Since the hyphal wall is known to be thinnest and most plastic at the tip, it is possible that the reduced signal relating to intracellular β-glucosidase observed in the regions of the hypha located some distance from the apex is a function of decreasing cell wall permeability. At present, there is no direct evidence from V. volvacea to eliminate this possibility, but recent confocal scanning laser microscopy studies have shown that the walls of germinating conidiospores of different filamentous fungi (Aspergillus, Penicillium, Trichoderma, and Paecilomyces) do not form an exclusion barrier for FITC-labelled dextrans of up to 150 kDa (5), compared to an apparent molecular mass of approximately 51 kDa for the anti-β-glucosidase antibody.
Transport and secretion of enzymes to and across the plasma membrane surface is thought to proceed via a highly polarized process involving intracytoplasmic vesicles, large numbers of which are evident in the hyphal tip region and which have been observed to fuse with the plasma membrane (19). Enzyme secretion involving vacuoles has also been proposed for lignin peroxidase transport in P. chrysosporium (20) and, more recently, for xylanase transport in T. reesei (21). Involvement of vesicles in β-glucosidase secretion in V. volvacea is supported by preliminary observations in which immunogold labelling of sections of fungal hyphae grown on rice straw combined with transmission electron microscopy revealed that the distribution of the enzyme was most dense in a peripheral layer extending 1 to 2 μm inward from the cell wall, which is also characterized by a high concentration of vesicles (11a).
ACKNOWLEDGMENTS
We thank David Moore for helpful discussions.
This work was supported by a grant from the Hong Kong Research Grants Council (grant CUHK 378/95M) and a Strategic Research Grant from The Chinese University of Hong Kong.
REFERENCES
- 1.Acebal C, Castillon M P, Estrada P. Endoglucanase and β-glucosidase location in Trichoderma reesei QM9414 growing on different carbon sources. Biotechnol Appl Biochem. 1988;10:1–5. [Google Scholar]
- 2.Baumgarten H, Schulze M, Hebell T. Methods of immunising mice. In: Peters J H, Baumgarten H, editors. Monoclonal antibodies. Berlin, Germany: Springer-Verlag KG; 1992. pp. 50–57. [Google Scholar]
- 3.Bes B, Pettersson B, Lennholm H, Iverson T, Eriksson K E. Synthesis, structure and enzyme degradation of an extracellular glucan produced in nitrogen-starved cultures of the white rot fungus Phanerochaete chrysosporium. Appl Biochem Biotechnol. 1987;9:310–318. [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for detecting microgram amounts of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:48–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Brul S, Nussbaum J, Dielbandhoesing S K. Fluorescent probes for wall porosity and membrane integrity in filamentous fungi. J Microbiol Methods. 1997;28:169–178. [Google Scholar]
- 6.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 7.Buswell J A, Cai Y J, Chang S T. Fungal- and substrate-associated factors affecting the ability of individual mushroom species to utilise different lignocellulosic growth substrates. In: Chang S T, Buswell J A, Chiu S W, editors. Mushroom biology and mushroom products. Hong Kong: Chinese University Press; 1993. pp. 141–150. [Google Scholar]
- 8.Buswell J A, Cai Y J, Chang S T, Peberdy J F, Fu S Y, Yu H-S. Lignocellulolytic enzyme profiles of edible mushroom fungi. World J Microbiol Biotechnol. 1996;12:537–542. doi: 10.1007/BF00419469. [DOI] [PubMed] [Google Scholar]
- 9.Cai Y-J, Buswell J A, Chang S T. Effect of lignin-related phenols and tannic acid derivatives on the growth of edible mushrooms. World J Microbiol Biotechnol. 1993;9:503–507. doi: 10.1007/BF00386283. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y J, Buswell J A, Chang S T. Cellulases and hemicellulases of Volvariella volvacea and the effect of Tween 80 on enzyme production. Mycol Res. 1994;98:1019–1024. [Google Scholar]
- 11.Cai Y J, Buswell J A, Chang S T. β-Glucosidase components of the cellulolytic system of the edible straw mushroom, Volvariella volvacea. Enzyme Microb Technol. 1998;22:122–129. doi: 10.1128/aem.65.2.553-559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Cai, Y. J., J. A. Buswell, and S. T. Chang. Unpublished results.
- 12.Chang S C, Steinkraus K H. Lignocellulolytic enzymes produced by Volvariella volvacea, the edible straw mushroom. Appl Environ Microbiol. 1982;43:440–446. doi: 10.1128/aem.43.2.440-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang S T. Production of the straw mushroom (Volvariella volvacea) from cotton wastes. Mushroom J. 1974;21:348–354. [Google Scholar]
- 14.Chang S T. Mushroom research and development—equality and mutual benefit. In: Royse D J, editor. Mushroom biology and mushroom products. University Park: Pennsylvania State University; 1996. pp. 1–10. [Google Scholar]
- 15.Chung P L Y, Trevithick J R. Biochemical and histochemical localization of invertase in Neurospora crassa during conidial germination and hyphal growth. J Bacteriol. 1970;102:423–429. doi: 10.1128/jb.102.2.423-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans C S, Dutton M V, Guillen F, Veness R G. Enzymes and small molecular mass agents involved with lignin degradation. FEMS Microbiol Rev. 1994;13:235–240. [Google Scholar]
- 17.Gallagher I M, Evans C S. Immunogold-cytochemical labelling of β-glucosidase in the white-rot fungus Coriolus versicolor. Appl Microbiol Biotechnol. 1990;32:588–593. [Google Scholar]
- 18.Gilkes N R, Henrissat B, Kilburn D G, Miller R C, Warren R A J. Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991;55:303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooday G W, Trinci A P J. Wall structure and biosynthesis in fungi. Symp Soc Gen Microbiol. 1980;30:207–251. [Google Scholar]
- 19a.Jia, J., P. S. Dyer, J. A. Buswell, and J. F. Peberdy. Unpublished results.
- 20.Kuan I C, Tien M. Phosphorylation of lignin peroxidases from Phanerochaete chrysosporium: identification of mannose-6-phosphate. J Biol Chem. 1989;264:20350–20355. [PubMed] [Google Scholar]
- 21.Kurzatkowski W, Solecka J, Filipek J, Rozbicka B, Messner R, Kubicek C P. Ultrastructural localization of cellular compartments involved in secretion of the low molecular weight, alkaline xylanase by Trichoderma reesei. Arch Microbiol. 1993;159:417–422. [Google Scholar]
- 22.Messner R, Hagspiel K, Kubicek C P. Isolation of a β-glucosidase binding and activating polysaccharide from the cell walls of Trichoderma reesei. Arch Microbiol. 1990;154:150–155. [Google Scholar]
- 23.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 24.Ouchterlony O. Antigen-antibody reactions in gels. Acta Pathol Microbiol Scand. 1949;26:507–515. doi: 10.1111/j.1699-0463.1949.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 25.Peberdy J F. Protein secretion in filamentous fungi—trying to understand a highly productive black box. Trends Biotechnol. 1994;12:50–57. doi: 10.1016/0167-7799(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 26.Pugh D, Cawson R A. The cytochemical localization of acid hydrolases in four common fungi. Cell Mol Biol. 1977;22:125–132. [PubMed] [Google Scholar]
- 27.Ruel K, Joseleau J-P. Involvement of an extracellular glucan sheath during degradation of Populus wood by Phanerochaete chrysosporium. Appl Environ Microbiol. 1991;57:374–384. doi: 10.1128/aem.57.2.374-384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuen S K, Buswell J A. Effect of lignin-derived phenols and their methylated derivatives on the growth of Lentinus spp. Lett Appl Microbiol. 1992;15:12–14. [Google Scholar]
- 29.Somogyi M J. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- 30.Sprey B. Localisation of β-glucosidase in Trichoderma reesei cell walls with immunoelectron microscopy. FEMS Microbiol Lett. 1986;36:287–292. [Google Scholar]
- 31.Sprey B. Cellular and extracellular localization of endoglucanase in Trichoderma reesei. FEMS Microbiol Lett. 1988;55:283–294. [Google Scholar]
- 32.Usami S, Kirimura K, Imura M, Morikawa S. Cellular localization of the constitutive β-glucosidase in Trichoderma viride. J Ferment Bioeng. 1990;70:185–187. [Google Scholar]
- 33.Wessels J G H. Wall growth, protein secretion and morphogenesis in fungi. New Phytol. 1993;123:397–413. doi: 10.1111/j.1469-8137.1993.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 34.Woesten H A B, Moukha S M, Sietsma J H, Wessels J G H. Localization of growth and secretion of proteins in Aspergillus niger. J Gen Microbiol. 1991;137:2017–2023. doi: 10.1099/00221287-137-8-2017. [DOI] [PubMed] [Google Scholar]