Abstract
We report here that the naturally occurring choline ester choline-O-sulfate serves as an effective compatible solute for Bacillus subtilis, and we have identified a high-affinity ATP-binding cassette (ABC) transport system responsible for its uptake. The osmoprotective effect of this trimethylammonium compound closely matches that of the potent and widely employed osmoprotectant glycine betaine. Growth experiments with a set of B. subtilis strains carrying defined mutations in the glycine betaine uptake systems OpuA, OpuC, and OpuD and in the high-affinity choline transporter OpuB revealed that choline-O-sulfate was specifically acquired from the environment via OpuC. Competition experiments demonstrated that choline-O-sulfate functioned as an effective competitive inhibitor for OpuC-mediated glycine betaine uptake, with a Ki of approximately 4 μM. Uptake studies with [1,2-dimethyl-14C]choline-O-sulfate showed that its transport was stimulated by high osmolality, and kinetic analysis revealed that OpuC has high affinity for choline-O-sulfate, with a Km value of 4 ± 1 μM and a maximum rate of transport (Vmax) of 54 ± 3 nmol/min · mg of protein in cells grown in minimal medium with 0.4 M NaCl. Growth studies utilizing a B. subtilis mutant defective in the choline to glycine betaine synthesis pathway and natural abundance 13C nuclear magnetic resonance spectroscopy of whole-cell extracts from the wild-type strain demonstrated that choline-O-sulfate was accumulated in the cytoplasm and was not hydrolyzed to choline by B. subtilis. In contrast, the osmoprotective effect of acetylcholine for B. subtilis is dependent on its biotransformation into glycine betaine. Choline-O-sulfate was not used as the sole carbon, nitrogen, or sulfur source, and our findings thus characterize this choline ester as an effective compatible solute and metabolically inert stress compound for B. subtilis. OpuC mediates the efficient transport not only of glycine betaine and choline-O-sulfate but also of carnitine, crotonobetaine, and γ-butyrobetaine (R. Kappes and E. Bremer, Microbiology 144:83–90, 1998). Thus, our data underscore its crucial role in the acquisition of a variety of osmoprotectants from the environment by B. subtilis.
Rapid fluxes of water along the osmotic gradient are triggered by the exposure of microorganisms to high osmolality environments, which in turn imposes considerable strain on all the physiological processes of the cell (57). Microorganisms actively respond to such increases in the osmolality of their habitat to prevent dehydration of the cytoplasm and to maintain turgor within acceptable boundaries. Since bacteria do not possess active transport mechanisms for water, turgor is adjusted by controlling the pool of osmotically active solutes within the cell (12, 15, 34, 45, 54).
Most nonhalophilic bacteria do not use ions to permanently increase the osmotic potential of their cytoplasm when they are faced by high osmotic growth conditions (15, 67). Instead, large amounts of organic osmolytes, the so-called compatible solutes, are accumulated. These compounds are well tolerated by enzymes and cell components and are thus highly congruous with the entire cellular physiology, even when they are amassed at molar concentrations (72). The intracellular accumulation of compatible solutes as a strategy of adaption to high osmolality has been widely adopted not only by Bacteria and Archaea but also by fungal, plant, animal, and even human cells (4, 8, 39, 42, 58) and is thus a remarkable example of convergent evolution. Moreover, the nature of the organic osmolytes that are amassed during water stress is maintained across the kingdoms, reflecting fundamental constraints on the kinds of solutes that are compatible with macromolecular and cellular functions (39, 72).
One of the most effective and widely used osmoprotectants in both the prokaryotic and eukaryotic world is the trimethylammonium compound glycine betaine (12, 15, 58). It can be amassed by microorganisms through either synthesis or uptake, and its intracellular accumulation confers a considerable degree of osmotic tolerance. A few bacteria produce this compatible solute by a stepwise methylation of glycine (15, 60). However, in most bacterial species glycine betaine synthesis proceeds via a two-step enzymatic oxidation of choline with glycine betaine aldehyde as the intermediate (6, 38, 61). Direct glycine betaine uptake from the environment has been observed in a wide variety of gram-negative and gram-positive Bacteria (12, 34, 45, 54) and a few Archaea (56). Molecular analysis of glycine betaine uptake systems initially focused on the gram-negative enterobacteria Escherichia coli and Salmonella typhimurium, for which two osmoregulated high-affinity transporters have been characterized in detail. These are the secondary transporter ProP and the binding-protein-dependent ATP-binding cassette (ABC) transporter ProU (11).
Recently, the genetics and physiology of the osmostress response in various gram-positive microorganisms have attracted wider attention (3, 16, 29, 52, 55, 64, 68). Bacillus subtilis is a ubiquitous inhabitant of the soil and is exposed to frequent osmotic changes due to flooding and desiccation of the upper layers of its habitat (45). Upon exposure to high-osmolality conditions, this bacterium produces large amounts of proline via de novo synthesis as an endogenous osmostress response (70). In addition, B. subtilis makes considerable effort to acquire compatible solutes or their precursors from the environment, and five osmoregulated transport systems have so far been characterized at the molecular level (34). OpuE (Opu denotes osmoprotectant uptake), a member of the sodium/solute symporter family, is used to efficiently scavenge proline from the habitat for osmoprotective purposes (69). In addition, three systems serve as effective glycine betaine transporters: OpuA, OpuC, and OpuD (31, 33, 35). OpuA and OpuC are multicomponent systems and are members of the ABC superfamily of transporters; both are related to the ProU transporter from E. coli. The secondary transporter OpuD is a representative of a new class of glycine betaine uptake systems (31) which also comprises BetP from the gram-positive soil bacterium Corynebacterium glutamicum (52). Acquisition of choline for the synthesis of glycine betaine (5, 6) is accomplished by both OpuC and the structurally closely related, substrate-specific ABC transporter OpuB (32). The OpuC system also serves for the high-affinity uptake of the betaines l-carnitine, crotonobetaine, and γ-butyrobetaine, all highly effective osmoprotectants for B. subtilis (30), and with low affinity (28), for the uptake of the frequently used osmoprotectant ectoine (15, 67). Hence, like other microorganisms, B. subtilis can rely on a spectrum of exogenously provided compatible solutes for osmoprotective purposes.
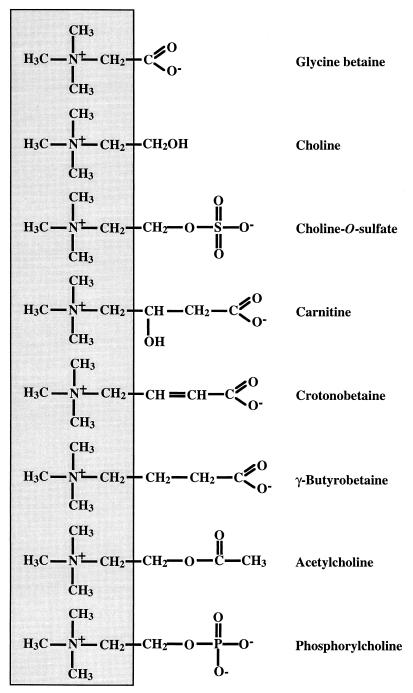
The choline ester choline-O-sulfate (Fig. 1) is widespread in nature and plays an important role in the microbial transformation of sulfur in the soil (1, 41). It is synthesized by a variety of plants, lichens, algae, and fungi and by several bacterial species (10, 14, 20–24, 26). Its formation is catalyzed by sulfur transferases that use 3′-phosphoadenosine-5′-phosphosulfate and choline as their substrates (59). Production of choline-O-sulfate probably serves in the detoxification of SO42−, and this ester can also function as a source of choline and sulfur after hydrolysis by choline sulfatases (41). An additional role for choline-O-sulfate as an osmoprotective compound was suggested by the observation that the intracellular pool of this betaine increased in various taxa of halophytic plants subjected to salt stress (21, 22, 59). Indeed, in bioassays choline-O-sulfate proved to be osmoprotective both for several gram-negative bacterial species (9, 19, 21, 44) and for the osmotolerant and xerotolerant fungus Penicillium fellutanum (51). However, the wider function of this choline ester as an effective osmoprotectant for the microbial world and its mechanisms for uptake from the environment and its intracellular accumulation either as a compatible solute or as a precursor for glycine betaine synthesis (50) have remained largely unexplored. Here, we report on the identification of a high-affinity choline-O-sulfate transport system at the molecular level in B. subtilis and on the role of this betaine as an effective compatible solute for this soil bacterium.
FIG. 1.
Osmoprotective compounds for B. subtilis and chemically related substances.
MATERIALS AND METHODS
Bacterial strains.
Strain JH642 (trpC2 pheA1; BGSC 1A96; gift from J. Hoch) is a derivative of the wild-type B. subtilis strain 168 and is the parent of all mutants used in the study. The construction and properties of strains RMKB20 [Δ(opuA::erm)4 opuC-20::Tn10(spc) Δ(opuD::neo)2], RMKB22 [Δ(opuA::erm)4 opuB-20::Tn10(spc) Δ(opuD::neo)2], RMKB25 [opuC-20::Tn10(spc)], RMKB33 [Δ(opuA::erm)4 Δ(opuB::tet)23 opuC-20::Tn10(spc)], and RMKB34 [Δ(opuB::tet)23 opuC-20::Tn10(spc) Δ(opuD::neo)2] have been described (30). Strain JBB5 [Δ(gbsAB::neo)2] is defective in glycine betaine synthesis since it lacks the GbsA and GbsB enzymes (6).
Growth conditions, media, and chemicals.
The B. subtilis strains were grown in Spizizen’s minimal medium (SMM) with 0.5% (wt/vol) glucose as the carbon source and supplemented with l-tryptophan (20 mg/liter), l-phenylalanine (18 mg/liter), and a solution of trace elements (25). When it was used as the sole carbon source, choline-O-sulfate was added to SMM to a final concentration of 30 mM and the growth yield of the cultures was monitored spectrophotometrically (optical density at 578 nm [OD578]) after 24 h. Use by B. subtilis of choline-O-sulfate as the sole sulfur source was tested by substituting (NH4)2SO4 with NH4Cl and substituting MgSO4 with MgCl2 in the SMM salts. The (NH4)2SO4 present in SMM was substituted with 30 mM K2SO4 to test the use of choline-O-sulfate as the sole nitrogen source by B. subtilis JH642. The cultures used to probe the function of choline-O-sulfate as the sole carbon, nitrogen, or sulfur source were inoculated to an OD578 of approximately 0.05 from SMM-grown overnight cultures which had been carefully washed with the modified SMM to remove residual traces of (NH4)2SO4, MgSO4, and glucose from the inoculum. The osmotic strength of SMM was increased by the addition of NaCl from stock solutions. The osmolality values of these media were determined with a vapor pressure osmometer (model 5500; Wescor); the osmolality values of SMM, SMM with 0.4 M NaCl, and SMM with 1.2 M NaCl were 340, 1,100, and 2,700 mosmol/kg of water, respectively. Growth conditions for B. subtilis cells cultured in SMM and SMM with increased osmolality (1.2 M NaCl) and testing for the osmoprotective effects of various compounds were as described previously (30). [1-methyl-14C]glycine betaine (2.03 GBq/mmol) was custom synthesized by American Radiolabeled Chemicals Inc. (St. Louis, Mo.). [1,2-dimethyl-14C]choline-O-sulfate was prepared from [1,2-dimethyl-14C]choline (259 MBq/mmol) (New England Nuclear, Boston, Mass.) by the protocol of Stevens and Vohra (63) and was a kind gift from D. Le Rudulier and M. Østerås (Université de Nice, France) (50). [methyl-3H]acetylcholine (3.15 TBq/mmol) was obtained from American Radiolabeled Chemicals Inc. Choline, glycine betaine, acetylcholine, and phosphorylcholine were purchased from Sigma (Steinheim, Germany). 2H2O containing D4-3-(trimethylsilyl)propionate was obtained from Aldrich (Deisenhofen, Germany).
Synthesis of choline-O-sulfate.
Nonradiolabeled choline-O-sulfate was synthesized by heating 2 moles of choline chloride with an excess (5.2 mol) of concentrated H2SO4 at 95°C overnight. The ester produced was then precipitated by adding the reaction mixture (500 ml) to four liters of 95% ethanol. The precipitated choline-O-sulfate was collected by filtration and was recrystallized from 60% ethanol as described by Bellenger et al. (2). Production of choline-O-sulfate was confirmed by 1H nuclear magnetic resonance (NMR) spectroscopy (10) by using a Bruker AC300 spectrometer operating at 300 MHz. The purity of choline-O-sulfate was checked by elementary analysis in a CHN-Rapid apparatus from Heraeus (Osterode, Germany) by the analytical service laboratory of the Chemistry Department of the Philipps University of Marburg, Germany.
Transport assays.
The uptake of [1,2-dimethyl-14C]choline-O-sulfate was measured at 37°C at various final substrate concentrations in bacterial cultures grown to log phase (OD578 = 0.3 to 0.5) in minimal media of high osmolality (SMM with 0.4 M NaCl) with glucose as the carbon source, as detailed previously for the uptake of radiolabeled glycine betaine (31, 33). The choline-O-sulfate concentration in the uptake assay was varied from 1 to 40 μM for kinetic studies. To determine the degree of inhibition of glycine betaine transport via the OpuC system by choline-O-sulfate, the concentration of the unlabeled choline-O-sulfate was kept at 50 μM and the substrate concentration of the radiolabeled [1-methyl-14C]glycine betaine was varied from 1 to 40 μM.
Preparation of cell extracts for 13C-NMR spectroscopy.
Cultures (300 ml) of different strains (JH642 and JBB5) were grown overnight in 1-liter Erlenmeyer flasks at 37°C on a rotary shaker (220 rpm) in SMM or SMM with 1.2 M NaCl and 0.5% (wt/vol) glucose as the carbon source in the absence or presence of choline-O-sulfates; these stationary-phase cultures reached OD578 between 2.5 and 3. The cells were harvested by centrifugation and washed once with 150 ml of SMM containing 1.2 M NaCl but no glucose, and the cell pellet was extracted with 10 ml of 80% (vol/vol) ethanol. The cellular debris was removed by centrifugation, and the supernatant was evaporated to dryness; ethanolic cell extracts from two 300-ml cultures were then dissolved in 1 ml of 2H2O supplemented with 1.2 mg of D4-3-(trimethylsilyl)propionate as a standard. 13C-NMR spectra were recorded with a Bruker AC300 spectrometer operating at 75 MHz (65). 13C-NMR tracings from choline-O-sulfate, glycine betaine, choline, and acetylcholine relative to D4-3-(trimethylsilyl)-propionate were recorded to identify unambiguously resonances from these compounds present in the 13C-NMR spectra from the ethanolic cell extracts.
RESULTS
Choline-O-sulfate functions as an osmoprotectant.
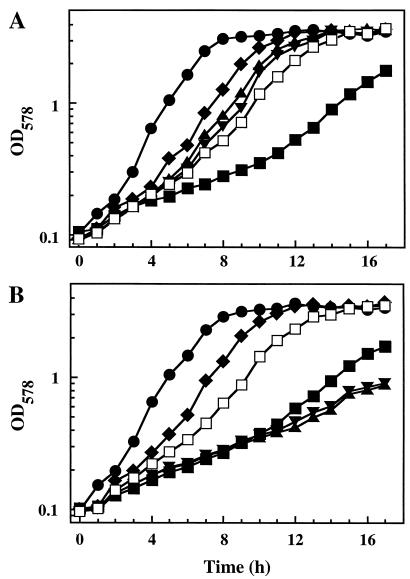
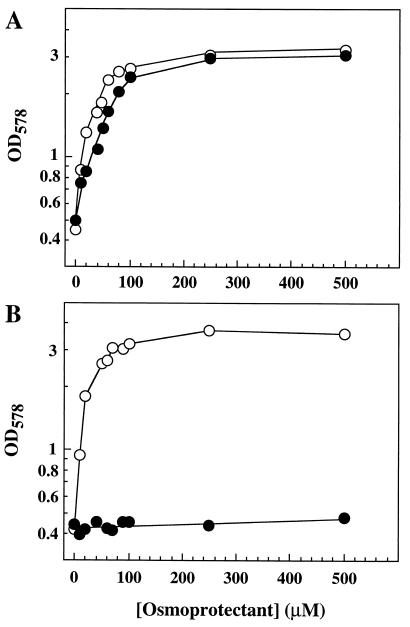
To examine a possible osmoprotective effect of choline-O-sulfate (Fig. 1) for the proliferation of osmotically stressed B. subtilis cells, we grew the wild-type strain JH642 in SMM (340 mosmol/kg) and high-osmolality minimal medium (SMM with 1.2 M NaCl; 2,700 mosmol/kg) in the absence or presence of 1 mM choline-O-sulfate. High osmolality strongly impaired the growth of the cultures, and choline-O-sulfate largely reversed this growth inhibition (Fig. 2A). A concentration as low as 20 μM of choline-O-sulfate was sufficient to exert a notable osmoprotective effect on the growth of B. subtilis in the high-osmolality medium, and approximately 100 to 200 μM provided full osmoprotection (Fig. 3A). The osmoprotective effects of choline-O-sulfate thus closely match those of the potent osmoprotectant glycine betaine and its biosynthetic precursor choline (Fig. 2 and 3) (5, 31). These data establish that choline-O-sulfate can serve as an effective osmoprotectant for B. subtilis.
FIG. 2.
Osmoprotective effects of choline-O-sulfate and acetylcholine for B. subtilis. The wild-type strain JH642 (A) and its Δ(gbsAB::neo)2 derivative JBB5 (B) were grown in SMM (•) and SMM with 1.2 M NaCl in the absence (■) or presence of 1 mM glycine betaine (⧫), choline (▴), acetylcholine (▾), or choline-O-sulfate (□). Cultures (75 ml) were inoculated to an OD578 of 0.1 from overnight cultures pregrown in SMM and were propagated in 500-ml Erlenmeyer flasks in a shaking water bath (220 rpm) at 37°C. Cell growth was monitored over time by measuring the OD578.
FIG. 3.
Uptake of choline-O-sulfate is mediated by the ABC transport system OpuC. Strains RMKB22 (OpuA− OpuB− OpuC+ OpuD−) (A) and RMKB25 (OpuA+ OpuB+ OpuC− OpuD+) (B) were grown in high osmolality minimal medium (SMM with 1.2 M NaCl) in the presence of various concentrations of glycine betaine (○) and choline-O-sulfate (•). The cells were inoculated to an OD578 of 0.05 from an overnight SMM culture and were grown in 20 ml of medium in a 100-ml Erlenmeyer flask on an orbital shaker (220 rpm) at 37°C. The growth yield of each culture was determined spectrophotometrically by measuring the OD578 after 22-h incubation. Under these circumstances, there is essentially no cell growth of strains inoculated in SMM with 1.2 M NaCl in the absence of an osmoprotectant, in contrast to cells grown in 75-ml cultures in 500-ml Erlenmeyer flasks, conditions that provide a much better aeration for the cells (30).
Identification of the choline-O-sulfate transport system.
Bacteria frequently employ multiple transport systems with different substrate specificities to acquire compatible solutes from the environment (11, 34). We therefore investigated the involvement of the known B. subtilis transporters for glycine betaine (OpuA, OpuC, and OpuD) (30, 33) or its biosynthetic precursor choline (OpuB and OpuC) (32) in the uptake of choline-O-sulfate from the environment. In a preliminary growth experiment, we used an isogenic set of mutants expressing only one of these uptake systems (30). This survey revealed that only B. subtilis strains synthesizing the OpuC transporter (31) could use choline-O-sulfate as an osmoprotectant. OpuC exhibits the characteristic features of a bacterial binding-protein-dependent transport system (7) and is a member of the ABC superfamily of transporters. It consists of an evolutionarily highly conserved ATPase (OpuCA), two sequence-related integral cytoplasmic membrane proteins (OpuCB and OpuCD), and a hydrophilic lipoprotein (OpuCC). This latter polypeptide is likely to serve as an extracellular substrate-binding protein tethered by its N-terminal lipid modification to the cytoplasmic membrane (32).
Disruption of the OpuC transporter in an otherwise wild-type background completely abolished osmoprotection by choline-O-sulfate (Fig. 3B). In contrast, this mutant strain was still fully protected by glycine betaine (Fig. 3B), which can be accumulated not only by OpuC but also via the high-affinity ABC transporter OpuA (33) and the secondary uptake system OpuD (31). These growth experiments unequivocally demonstrate that B. subtilis possesses only one choline-O-sulfate uptake system and establish that the multicomponent ABC transporter OpuC mediates the uptake of this betaine from exogenous sources.
Competitive inhibition of glycine betaine transport by choline-O-sulfate.
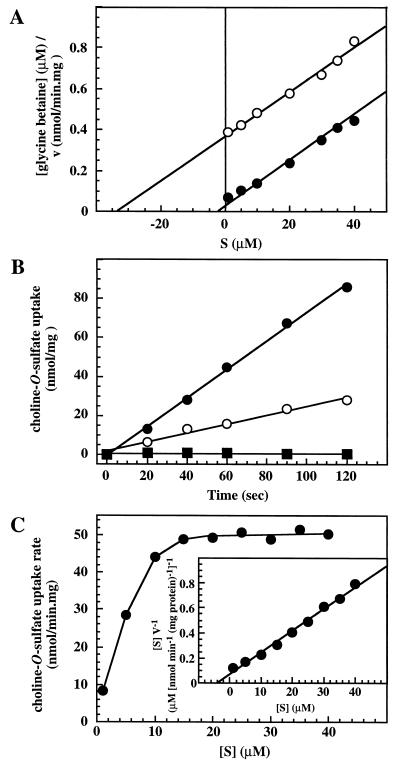
Transport of radiolabeled glycine betaine via OpuC is moderately osmotically stimulated and exhibits a Km of 6 μM and a Vmax of 65 nmol/min · mg of protein in cultures grown in SMM in the presence of 0.4 M NaCl (31). To analyze the entry of choline-O-sulfate into the B. subtilis cells through the OpuC system, we performed competition experiments with [1-methyl-14C]glycine betaine and unlabeled choline-O-sulfate. We grew strain RMKB22 (OpuA− OpuB− OpuC+ OpuD−) in SMM with 0.4 M NaCl to mid-exponential phase and measured the uptake of [1-methyl-14C]glycine betaine over a range (1 to 40 μM) of substrate concentrations in either the absence or the presence of a fixed concentration (50 μM) of the competitor choline-O-sulfate. In close agreement with the previously reported data (31) we found a Km of 2.8 μM for glycine betaine uptake in the absence of choline-O-sulfate, and an apparent Km of 34 μM in its presence (Fig. 4A). From these kinetic data we calculated a Ki value of 4.5 ± 1 μM. The lines in the Hanes plot were linear and parallel and yielded very similar Vmax values for [1-methyl-14C]glycine betaine uptake in the absence (Vmax was 89 nmol/min · mg of protein) or presence (Vmax was 92 nmol/min · mg of protein) (Fig. 4A) of the inhibitor. These transport characteristics indicated that choline-O-sulfate behaves as a competitive inhibitor (71) for glycine betaine uptake via OpuC and that this ABC transporter serves as a high-affinity uptake system for this osmoprotectant.
FIG. 4.
Transport of choline-O-sulfate in B. subtilis. (A) Inhibition of radiolabeled glycine betaine by choline-O-sulfate in strain RMKB22 (OpuA− OpuB− OpuC+ OpuD−). [1-methyl-14C]glycine betaine uptake was measured at various substrate concentrations in cells grown in SMM with 0.4 M NaCl in the absence (•) or the presence (○) of 50 μM unlabeled choline-O-sulfate. In the absence of the inhibitor the apparent Km of glycine betaine transport via OpuC is 2.8 μM and the Vmax is 89 nmol/min · mg of protein. In the presence of choline-O-sulfate the apparent Km of glycine betaine transport is 34 μM, the Vmax is 92 nmol/min · mg of protein, and the Ki is 4.5 μM. (B) Osmotically stimulated choline-O-sulfate transport. Cells of the wild-type strain JH642 (OpuA+ OpuB+ OpuC+ OpuD+) (○, •) and its OpuC− derivative RMKB25 (■) were grown in SMM (○) or SMM with 0.4 M NaCl (•, ■) and [1,2-dimethyl-14C]choline-O-sulfate uptake was assayed at a final substrate concentration of 10 μM. (C) Kinetics of choline-O-sulfate transport. Strain JH642 was grown in SMM with 0.4 M NaCl, and the initial velocities of [1,2-dimethyl-14C]choline-O-sulfate uptake were measured at various substrate concentrations (1 to 40 μM).
Kinetic parameters for OpuC-mediated choline-O-sulfate transport.
To determine the parameters for choline-O-sulfate transport directly, we measured the initial uptake of [1,2-dimethyl-14C]choline-O-sulfate at a final substrate concentration of 10 μM in the wild-type strain JH642 in cultures grown in SMM or SMM with elevated osmolality (SMM with 0.4 M NaCl). Like the OpuC-mediated transport of glycine betaine and l-carnitine (30, 31), choline-O-sulfate uptake was moderately stimulated (three- to fourfold) by increases in the osmolality of the growth medium (Fig. 4B). Disruption of the OpuC system in an otherwise wild-type background by the opuC-20::Tn10(spc) mutation (strain RMKB25) completely abolished radiolabeled choline-O-sulfate transport at both low and high osmolality (Fig. 4B). This observation corroborates the finding that osmoprotection by choline-O-sulfate is eliminated in an OpuC-lacking strain that still possesses the OpuA, OpuB, OpuD, and OpuE osmoprotectant uptake systems (Fig. 3B).
We then determined the initial velocities of [1,2-dimethyl-14C]choline-O-sulfate uptake over a range of substrate concentrations (1 to 40 μM) in cells of strain JH642 grown in SMM with 0.4 M NaCl. Choline-O-sulfate uptake was saturable and showed typical Michaelis-Menten kinetics. Transformation of the transport data according to the Lineweaver-Burk method suggested that there is a single, high-affinity choline-O-sulfate transport activity in B. subtilis. This uptake system exhibits a Km of 4 ± 1 μM and a Vmax of 54 ± 3 nmol/min · mg of protein (Fig. 4C). These kinetic parameters for choline-O-sulfate transport are comparable to those for the OpuC-mediated glycine betaine (Km = 6 μM; Vmax = 65 nmol/min · mg of protein) and l-carnitine (Km = 5.8 μM; Vmax = 71.5 nmol/min · mg of protein) transport (30, 31).
Osmoprotective effects of choline-O-sulfate are not dependent on its enzymatic conversion into glycine betaine.
Choline-O-sulfate is a derivative of choline (Fig. 1), and hydrolysis of the ester bond would liberate the precursor for glycine betaine biosynthesis (5). Consequently, the osmoprotective effects of choline-O-sulfate for B. subtilis (Fig. 2A and 3A) could be indirect and dependent on its enzymatic conversion to glycine betaine. To determine whether choline-O-sulfate acted as a compatible solute per se or whether its osmoprotective effects resulted from its biotransformation into glycine betaine, we tested its osmoprotective capacity in strain JBB5 [Δ(gbsAB::neo)2]. This mutant cannot synthesize glycine betaine from choline because it lacks the choline dehydrogenase (GbsB) and the glycine betaine aldehyde dehydrogenase (GbsA) (6). Unlike choline, both choline-O-sulfate and glycine betaine were fully osmoprotective for strain JBB5 (Fig. 2B). Hence, osmoprotection of B. subtilis by choline-O-sulfate clearly does not depend on its conversion into glycine betaine.
Osmoprotection by acetylcholine requires its conversion into glycine betaine.
We also observed that the choline ester acetylcholine (Fig. 1) protected B. subtilis cells from the detrimental effects of high osmolality with an efficiency similar to those of choline and choline-O-sulfate (Fig. 2A). Acetylcholine is found widely in plants and thus could reach soil bacteria through decaying plant material. However, its concentration in plants is rather low compared with that of choline (46, 66). In contrast to choline-O-sulfate, acetylcholine could not serve as an osmoprotectant for strain JBB5 [Δ(gbsAB::neo)2] (Fig. 2B). Hence, for the gbsAB+ B. subtilis strain JH642 (Fig. 2A), osmoprotection by acetylcholine was entirely dependent on its hydrolysis to choline and acetate and the subsequent enzymatic conversion of the choline moiety to glycine betaine. As observed previously for choline, the presence of acetylcholine was detrimental to cell growth for strain JBB5 (Fig. 2B), an effect possibly connected with the accumulation of a large number of positively charged choline molecules inside the cells (6). Thus, the mechanism of osmoprotection by acetylcholine in B. subtilis is like that of Pseudomonas aeruginosa, where acetylcholine serves as a precursor for glycine betaine synthesis (40), but it differs from that of the gram-positive bacterium Lactobacillus plantarum, where both acetylcholine and choline are accumulated as moderately effective cationic osmolytes (37).
We also conducted preliminary experiments on the uptake of acetylcholine in B. subtilis by measuring the transport of [methyl-3H]acetylcholine at different substrate concentrations in cultures grown in SMM with 0.4 M NaCl. However, its uptake was very low and variable (data not shown), suggesting either that acetylcholine transport is very inefficient or that the hydrolysis of the ester bond and the subsequent transport of [methyl-3H]choline into the cell is rather slow. We previously observed the cleavage of the β-substituted acylesters acetylcarnitine and octanoylcarnitine by B. subtilis and their intracellular accumulation in the form of l-carnitine in osmotically stressed cells (30). Although it is not clear where the hydrolysis of acetylcholine and the acylesters of l-carnitine occurs, in its natural habitats B. subtilis might use such compounds as additional sources for generating compatible solutes.
In addition, we also investigated a possible osmoprotective effect of the choline ester phosphorylcholine (Fig. 1) for the growth of B. subtilis in high-osmolality media, since this trimethylammonium compound can serve as a precursor for glycine betaine synthesis in P. aeruginosa under hyperosmotic conditions (13, 40). However, this negatively charged choline ester did not display any osmoprotection for the B. subtilis wild-type strain JH642. Hence, not all choline esters serve as a source of precursor for glycine betaine synthesis in B. subtilis.
Choline-O-sulfate is accumulated in the cytoplasmic solute pool.
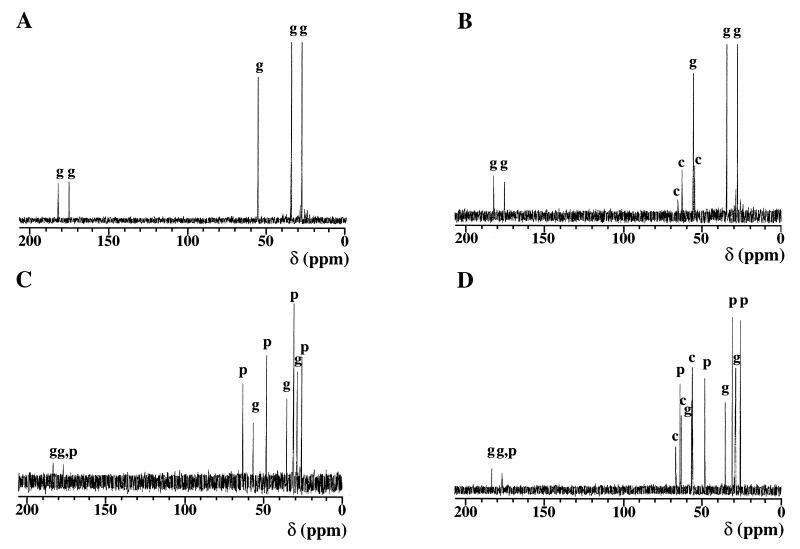
To further substantiate the result that choline-O-sulfate serves as a compatible solute per se for B. subtilis, we monitored the fate of this compound in cultures grown at high osmolality by natural abundance 13C-NMR spectroscopy, a technique that is frequently used to assess the spectrum of compatible solutes inside bacterial cells (15, 30, 65, 70). Crude ethanol extracts were prepared from cells of strain JH642 grown in SMM or in SMM with 1.2 M NaCl in the presence or absence of choline-O-sulfate and were analyzed by 13C-NMR spectroscopy. As observed previously (28, 30, 70), glutamate and proline were the predominant osmolytes in salt-stressed cells grown in the absence of an osmoprotectant. The presence of 1 mM choline-O-sulfate in the growth medium resulted in the accumulation of this trimethylammonium compound in the cytoplasmic solute pool at both low and high osmolality (Fig. 5). Resonances from glycine betaine could not be detected in these spectra, although signals from this compound were readily found when it was added (1 mM) to the growth medium (data not shown).
FIG. 5.
13C-NMR spectra of ethanol cell extracts. Strain JH642 was grown in SMM (A), in SMM with 1 mM choline-O-sulfate (B), in SMM with 1.2 M NaCl (C), and in SMM with 1.2 M NaCl in the presence of 1 mM choline-O-sulfate (D). Resonances originating from glutamate (g), proline (p), and choline-O-sulfate (c) are indicated. Chemical shifts for glycine betaine [given in ppm relative to the standard D4-3-(trimethylsilyl)propionate] are 56.1, 69.0, and 171.8.
The addition of 1 mM choline or acetylcholine, in contrast to choline-O-sulfate, to cultures of strain JH642 resulted in their complete conversion into glycine betaine as assessed by 13C-NMR spectroscopy. When the production of glycine betaine from either choline or acetylcholine was blocked in the gbsAB mutant strain JBB5, choline (but no glycine betaine) was detected in the cytoplasmic solute pools (data not shown). Thus, the 13C-NMR spectroscopy analysis confirmed the results of our growth experiments (Fig. 2) and revealed that choline-O-sulfate serves as a compatible solute per se in B. subtilis and that the osmoprotective effect of the structurally related compound acetylcholine depends on its enzymatic conversion into glycine betaine.
Choline-O-sulfate is metabolically inert in B. subtilis.
We tested whether B. subtilis degrades choline-O-sulfate for use as a carbon, nitrogen, or sulfur source. Choline-O-sulfate did not support the growth of the B. subtilis wild-type strain JH642 when supplied at a concentration of 30 mM as the sole carbon source. Likewise, it did not serve as a nitrogen source (provided at a concentration of 30 mM) in a modified SMM medium in which (NH4)2SO4 had been replaced by K2SO4 at the equivalent concentration, whereas proline (30 mM) served as an effective nitrogen source in this (NH4)2SO4-depleted medium. In an SMM medium where (NH4)2SO4 and MgSO4 were replaced by NH4Cl and MgCl2 at equivalent concentrations, choline-O-sulfate (15 mM) did not support the growth of strain JH642, but methionine at a concentration of 15 mM readily served as a sole sulfur source. Hence, choline-O-sulfate is not used by B. subtilis for anabolic purposes.
DISCUSSION
The use of a mixture of osmoprotectants by a given microorganism is frequently observed. It enhances the ability of bacteria to adapt flexibly to different ecological niches and to varying conditions in their habitats (12, 15, 34, 45). The data presented here show that choline-O-sulfate, which is produced by a wide variety of plants, lichens, algae, fungi, and bacteria, serves as an effective and metabolically inert compatible solute for B. subtilis. By using a set of strains with mutations in previously defined systems for uptake of osmoprotectants (34), we identified the osmotically stimulated ABC transporter OpuC as the uptake system for choline-O-sulfate. To our knowledge this is the first report in which the kinetic parameters and molecular details of a bacterial choline-O-sulfate uptake system have been characterized.
Compatible solutes are highly soluble molecules; they do not carry a net charge at physiological pH, and often they also stabilize proteins in vitro and protect against denaturation in solutions of high ionic strength (72). Choline-O-sulfate fulfills the criteria for a compatible solute. This zwitterion (Fig. 1) is amassed in osmotically stressed E. coli and S. typhimurium cells to levels exceeding 0.5 M (21). Such high concentrations do not inhibit the enzymatic function of purified barley leaf malate dehydrogenase, and they protect the enzyme against inhibition by NaCl (53). Depending on the degree of osmotic stress, osmoregulating B. subtilis cells amass glycine betaine from exogenous sources at intracellular levels ranging between 0.5 and 1 M (47, 70). Since choline-O-sulfate and glycine betaine have similar osmoprotective effects for B. subtilis when accumulated via OpuC (Fig. 3), one can expect that both compounds are amassed to comparable intracellular concentrations.
In addition to the chemical and biophysical properties of compatible solutes, the kinetic parameters of their transport systems greatly determine their effectiveness as osmoprotectants for bacteria. A telling case is the uptake of the cyclic amino acid derivative ectoine, an effective compatible solute for a wide range of bacterial species (15, 67). An exogenous supply (1 mM) of ectoine is only moderately osmoprotective for B. subtilis due to its inefficient import by OpuC (Ki = 1.5 mM) (28). However, this tetrahydropyrimidine has an osmoprotective capacity similar to that of glycine betaine for E. coli (27) and the plant pathogen Erwinia chrysanthemi (17), bacteria which possess high-affinity ectoine and glycine betaine uptake systems. Although OpuC shows only low affinity for ectoine, this ABC transporter exhibits high-affinity uptake for the compatible solutes choline-O-sulfate, glycine betaine, l-carnitine, crotonobetaine, and γ-butyrobetaine, with Km and Ki values in the low micromolar range (4 to 7 μM) and a substantial transport capacity (Vmax of 54 to 71 nmol/min · mg of protein) (30, 31). With the exception of glycine betaine (31, 33), these compounds are taken up only via OpuC. Thus, this transporter plays a pivotal role in supplying B. subtilis with a variety of structurally related osmoprotectants.
Choline-O-sulfate, l-carnitine, crotonobetaine, and γ-butyrobetaine each serve as effective inhibitors for glycine betaine uptake via OpuC (Fig. 4A) (30), suggesting that these trimethylammonium compounds compete for the same binding site(s). The substrate-binding proteins of binding-protein-dependent ABC transporters serve as the primary site for high-affinity substrate recognition (7). The OpuC system possesses a lipoprotein (OpuCC) that is likely to function as an extracellular substrate-binding protein which is tethered by its N-terminal lipid tail to the cytoplasmic membrane (32). Unfortunately, it has not yet been possible to isolate the OpuCC protein in a functional form (36), which precludes a direct biochemical analysis of substrate binding. We have successfully performed such experiments with purified OpuAC, which is the lipid-modified substrate-binding protein from the glycine betaine ABC transporter OpuA (35). Consideration of the chemical nature of the substrates transported with high affinity via OpuC reveals that all of them possess a fully methylated ammonium group and that the main carbon chains exhibit considerable variation with respect to length, chemical bonds, and branching (Fig. 1). Each of these substrates is a zwitterion at physiological pH, whereas uptake of the positively charged choline via OpuC proceeds with a somewhat reduced affinity (Km = 38 μM) (32).
Choline-O-sulfate is a major product of sulfate metabolism in a variety of plant, fungal, and bacterial species (14, 22, 24, 26) and is thus likely to be released from decaying cells of these primary producers into the soil. Several species of the genus Pseudomonas are even known to excrete choline-O-sulfate into the medium (14). Like other osmoprotectants, this betaine is probably present only at low and variable concentrations in the natural habitats of B. subtilis. Therefore, the ability to scavenge compatible solutes efficiently from the environment and to accumulate them to very high intracellular levels against a steep concentration gradient is a distinctive requirement for a transport system involved in the acquisition of osmoprotectants. The ABC transporter OpuC meets these physiological demands, and the osmoprotective effects of choline-O-sulfate (Fig. 3A), glycine betaine, l-carnitine, crotonobetaine, and γ-butyrobetaine were clearly noticeable when these compounds were supplied at concentrations as low as 20 μM to an OpuC-positive B. subtilis strain (30). The presence of choline-O-sulfate in physiologically relevant concentrations in the soil is substantiated by reports demonstrating the presence of transport systems for this ester in barley roots (49), in soil fungi (2, 24), and in strains of Corynebacterium and of the rhizosphere bacteria Pseudomonas, Agrobacterium, and Rhizobium (48). Although the details on the type(s) of transport system involved and the kinetic parameters for choline-O-sulfate uptake by most of these organisms have not been documented, in barley roots (49), Neurospora crassa (43), and Penicillium notatum (2) choline-O-sulfate transport activities with Km values of 7.7, 25, and 300 μM, respectively, have been reported. Choline-O-sulfate from the environment is accumulated in each of these cells as a source of sulfur which becomes available after intracellular hydrolysis of choline-O-sulfate by choline sulfatases. The OpuC-mediated choline-O-sulfate transport in B. subtilis for osmoprotective purposes reported here compares favorably (Km value of 4 μM) with that described for anabolic functions.
Choline sulfatases are present in various microbial species (18, 23, 43, 62). They may play a role in providing the precursor for glycine betaine synthesis. However, biotransformation of choline-O-sulfate into glycine betaine is clearly not the cause for its osmoprotective effects in B. subtilis. As we have demonstrated here, choline-O-sulfate was fully osmoprotective for a B. subtilis mutant defective in the glycine betaine biosynthetic pathway (Fig. 2B) and was readily detected as an osmolyte in the cytoplasmic solute pool of osmotically stressed cells (Fig. 5). These findings contrast with the observations made with acetylcholine, whose osmoprotective effects for B. subtilis were entirely dependent on its enzymatic conversion into glycine betaine (Fig. 2). The accumulation of choline-O-sulfate as a metabolically inert stress compound is common to the bacteria B. subtilis, E. coli, and S. typhimurium (21), and its amassing at high intracellular levels is also observed in the fungus P. fellutanum (51). However, the osmoprotective effect of choline-O-sulfate does not always depend on its accumulation in an unmodified form. Recently, Østerås et al. (50) reported the presence of a choline sulfatase activity (BetC) in the rhizosphere bacterium Sinorhizobium meliloti that catalyzed the hydrolysis of choline-O-sulfate into choline and sulfate; the produced choline was then further oxidized into glycine betaine by the BetA and BetB enzymes. The structural gene (betC) for this choline sulfatase is part of the S. meliloti betICBA operon. The fact that both choline-O-sulfate and choline serve as inducers for this gene cluster thus further underscores the physiological relevance of the choline-O-sulfate to glycine betaine biosynthetic pathway. In contrast to B. subtilis (5), S. meliloti can use both glycine betaine and choline-O-sulfate not only for osmoprotection but also as sole carbon and nitrogen (sulfur) sources for growth (50).
The previously reported osmoprotective effects of choline-O-sulfate for several plant, fungal, and bacterial species (9, 19, 21, 44, 51) and the function of this choline ester as a precursor for glycine betaine synthesis in S. meliloti (50) coupled with our discovery of a high-affinity choline-O-sulfate transport system in B. subtilis for osmoprotective purposes all suggest that this betaine plays a much wider and physiologically more important role for osmoprotection than has previously been assumed.
ACKNOWLEDGMENTS
We are grateful to Daniel Le Rudulier and Magne Østerås for generously providing us with a sample of radiolabeled choline-O-sulfate and for communicating data prior to publication. The help of V. Koogle in editing the manuscript is greatly appreciated.
Financial support for this study was provided by the Deutsche Forschungsgemeinschaft through SFB-395 and the Graduiertenkolleg Enzymchemie and the Fonds der Chemischen Industrie. J. A. Le Good participated in an exchange program between the University of Oxford and the University of Marburg, and her stay in the Laboratory for Microbiology (Marburg) was supported by a grant through the ERASMUS program from the EC.
REFERENCES
- 1.Alexander M. Introduction to soil microbiology. New York, N.Y: John Wiley & Sons; 1977. Microbial transformation of sulfur; pp. 350–367. [Google Scholar]
- 2.Bellenger N, Nissen P, Wood T C, Segel I H. Specificity and control of choline-O-sulfate transport in filamentous fungi. J Bacteriol. 1968;96:1574–1585. doi: 10.1128/jb.96.5.1574-1585.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard T, Jebbar M, Himidi-Kabbab S, Hamelin J, Blanco C. Ectoine accumulation and osmotic regulation in Brevibacterium linens. J Gen Microbiol. 1993;139:129–136. [Google Scholar]
- 4.Blomberg A. Osmoresponsive proteins and functional assessment strategies in Saccharomyces cerevisiae. Electrophoresis. 1997;18:1429–1440. doi: 10.1002/elps.1150180818. [DOI] [PubMed] [Google Scholar]
- 5.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boch J, Kempf B, Schmid R, Bremer E. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB genes. J Bacteriol. 1996;178:5121–5129. doi: 10.1128/jb.178.17.5121-5129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1175–1209. [Google Scholar]
- 8.Burg M, Kwon E, Kültz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 9.Cánovas D, Vargas C, Csonka L N, Ventosa A, Nieto J J. Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J Bacteriol. 1996;178:7221–7226. doi: 10.1128/jb.178.24.7221-7226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalfomo P, Block J H, Constantine G H, Kirk P W. Choline sulfate (ester) in marine higher fungi. Mar Chem. 1972;1:157–162. [Google Scholar]
- 11.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 12.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza-Ault M R, Smith L T, Smith G M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl Environ Microbiol. 1993;59:473–478. doi: 10.1128/aem.59.2.473-478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald J W, Luschinski P C. Further studies on the formation of choline sulfate by bacteria. Can J Microbiol. 1977;23:483–490. doi: 10.1139/m77-072. [DOI] [PubMed] [Google Scholar]
- 15.Galinski E A, Trüper H G. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev. 1994;15:95–108. [Google Scholar]
- 16.Glaasker E, Konings W N, Poolman B. Glycine betaine fluxes in Lactobacillus plantarum during osmostasis and hyper- and hypo-osmotic shock. J Biol Chem. 1996;271:10060–10065. doi: 10.1074/jbc.271.17.10060. [DOI] [PubMed] [Google Scholar]
- 17.Gouesbet G, Trautwetter A, Bonnassie S, Wu L F, Blanco C. Characterization of the Erwinia chrysanthemi osmoprotectant transporter gene ousA. J Bacteriol. 1996;178:447–455. doi: 10.1128/jb.178.2.447-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravel R A. Choline-O-sulphate utilization in Aspergillus nidulans. Genet Res. 1976;28:261–276. doi: 10.1017/s0016672300016955. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez J A, Csonka L N. Isolation and characterization of adenylate kinase (adk) mutations in Salmonella typhimurium which block the ability of glycine betaine to function as an osmoprotectant. J Bacteriol. 1995;177:390–400. doi: 10.1128/jb.177.2.390-400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson A D, Gage D A. Identification and determination by fast atom bombardment mass spectrometry of the compatible solute choline-O-sulfate in Limonium species and other halophytes. Aust J Plant Physiol. 1991;18:317–327. [Google Scholar]
- 21.Hanson A D, Rathinasabapathi B, Chamberlin B, Gage D A. Comparative physiological evidence that β-alanine betaine and choline-O-sulfate act as compatible osmolytes in halophytic Limonium species. Plant Physiol (Rockville) 1991;97:1199–1205. doi: 10.1104/pp.97.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson A D, Rathinasabapathi B, Rivoal J, Burnet M, Dillon M O, Gage D A. Osmoprotective compounds in the plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci USA. 1994;91:306–310. doi: 10.1073/pnas.91.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada T. The formation of sulphatases by Pseudomonas aeruginosa. Biochim Biophys Acta. 1964;81:193–196. [Google Scholar]
- 24.Harada T, Spencer B. Choline sulphate in fungi. J Gen Microbiol. 1960;22:520–527. doi: 10.1099/00221287-22-2-520. [DOI] [PubMed] [Google Scholar]
- 25.Harwood C R, Archibald A R. Growth, maintenance and general techniques. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons, Inc.; 1990. pp. 1–26. [Google Scholar]
- 26.Ikawa M, Chakrvarti A, Taylor R F. Occurrence of choline in Lactobacillus plantarum. Can J Microbiol. 1972;18:1241–1245. doi: 10.1139/m72-192. [DOI] [PubMed] [Google Scholar]
- 27.Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C. Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol. 1992;174:5027–5035. doi: 10.1128/jb.174.15.5027-5035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jebbar M, von Blohn C, Bremer E. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol Lett. 1997;154:325–330. [Google Scholar]
- 29.Jewell J B, Kashket E R. Osmotically regulated transport of proline by Lactobacillus acidophilus IFO 3532. Appl Environ Microbiol. 1991;57:2829–2833. doi: 10.1128/aem.57.10.2829-2833.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kappes R, Bremer E. Response of Bacillus subtilis to high osmolarity: uptake of carnitine, crotonobetaine and γ-butyrobetaine via the ABC transport system OpuC. Microbiology. 1998;144:83–90. doi: 10.1099/00221287-144-1-83. [DOI] [PubMed] [Google Scholar]
- 31.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappes, R. M., B. Kempf, S. Kneip, J. Boch, J. Gade, J. Meier-Wagner, and E. Bremer. Two evolutionarily closely related ABC-transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol., in press. [DOI] [PubMed]
- 33.Kempf B, Bremer E. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J Biol Chem. 1995;270:16701–16713. doi: 10.1074/jbc.270.28.16701. [DOI] [PubMed] [Google Scholar]
- 34.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 35.Kempf B, Gade J, Bremer E. Lipoprotein from the osmoregulated ABC transport system OpuA of Bacillus subtilis: purification of the glycine betaine binding protein and characterization of a functional lipidless mutant. J Bacteriol. 1997;179:6213–6220. doi: 10.1128/jb.179.20.6213-6220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempf, B., J. Gade, and E. Bremer. 1998. Unpublished data.
- 37.Kets E P W, Groot M N, Gallinski E A, De Bont J A M. Choline and acetylcholine: novel cationic osmolytes in Lactobacillus plantarum. Appl Microbiol Biotechnol. 1997;48:94–98. [Google Scholar]
- 38.Lamark T, Kaasen I, Eshoo M W, Falkenberg P, McDougall J, Strøm A R. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol Microbiol. 1991;5:1049–1064. doi: 10.1111/j.1365-2958.1991.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 39.Le Rudulier D, Strøm A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 40.Lisa T A, Casale C H, Domenech C E. Cholinesterase, acid phosphatase, and phospholipase C of Pseudomonas aeruginosa under hyperosmotic conditions in a high-phosphate medium. Curr Microbiol. 1994;28:71–76. [Google Scholar]
- 41.Markham P, Robson G D, Bainbridge B W, Trinci A P. Choline: its role in the growth of filamentous fungi and the regulation of mycelial morphology. FEMS Microbiol Rev. 1993;10:287–300. doi: 10.1111/j.1574-6968.1993.tb05872.x. [DOI] [PubMed] [Google Scholar]
- 42.Martins L, Huber R, Huber H, Stetter K, Da Costa M, Santos H. Organic solutes in hyperthermophilic Archaea. Appl Environ Microbiol. 1997;63:896–902. doi: 10.1128/aem.63.3.896-902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marzluf G A. Genetic and metabolic control of sulfate metabolism in Neurospora crassa: a specific permease for choline-O-sulfate. Biochem Genet. 1972;7:219–233. doi: 10.1007/BF00484820. [DOI] [PubMed] [Google Scholar]
- 44.Mason T, Blunden G. Quaternary ammonium and tertiary sulphonium compounds of algal origin as alleviators of osmotic stress. Bot Mar. 1989;32:313–316. [Google Scholar]
- 45.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 46.Miura G A, Shih T-M. Cholinergic constituents in plants: characterization and distribution of acetylcholine and choline. Physiol Plant. 1984;61:417–421. [Google Scholar]
- 47.Moses, S., C. von Blohn, E. Bakker, and E. Bremer. 1998. Unpublished data.
- 48.Nissen P. Choline sulfate permease: transfer of information from bacteria to higher plants? Biochem Biophys Res Commun. 1968;32:696–703. doi: 10.1016/0006-291x(68)90295-7. [DOI] [PubMed] [Google Scholar]
- 49.Nissen P, Benson A A. Active transport of choline sulfate by barley roots. Plant Physiol. 1964;39:586–589. doi: 10.1104/pp.39.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Østerås M, Boncompagni E, Vincent N, Poggi M-C, Le Rudulier D. Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: choline-O-sulfate is metabolized into glycine betaine. Proc Natl Acad Sci USA. 1998;95:11394–11399. doi: 10.1073/pnas.95.19.11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park Y-I, Gander J E. Choline derivatives involved in osmotolerance of Penicillium fellutanum. Appl Environ Microbiol. 1998;64:273–278. doi: 10.1128/aem.64.1.273-278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peter H, Burkovski A, Krämer R. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J Bacteriol. 1996;178:5229–5234. doi: 10.1128/jb.178.17.5229-5234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollard A, Wyn Jones R G. Enzyme activities in concentrated solutions of glycine betaine and other solutes. Planta. 1979;144:291–298. doi: 10.1007/BF00388772. [DOI] [PubMed] [Google Scholar]
- 54.Poolman B, Glaasker E. Regulation of compatible solute accumulation in bacteria. Mol Microbiol. 1998;29:397–407. doi: 10.1046/j.1365-2958.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 55.Pourkomailian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for feedback regulation of the activity of the two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- 56.Proctor L M, Lai R, Gunsalus R P. The methanogenic archaeon Methanosarcina thermophila TM-1 possesses a high-affinity glycine betaine transporter involved in osmotic adaptation. Appl Environ Microbiol. 1997;63:2252–2257. doi: 10.1128/aem.63.6.2252-2257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Record M T, Jr, Courtenay E S, Cayley D S, Guttman H J. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem Sci. 1998;23:143–148. doi: 10.1016/s0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- 58.Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- 59.Rioval J, Hanson A D. Choline-O-sulfate biosynthesis in plants. Identification and partial characterization of a salinity-inducible choline sulfotransferase from species of Limonium (Plumbaginaceae) Plant Physiol (Rockville) 1994;106:1187–1193. doi: 10.1104/pp.106.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson P M, Roberts M F. Effects of osmolyte precursors on the distribution of compatible solutes in Methanohalophilus portucalensis. Appl Environ Microbiol. 1997;63:4032–4038. doi: 10.1128/aem.63.10.4032-4038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rozwadowski K L, Khachatourians G G, Selvaraj G. Choline oxidase, a catabolic enzyme in Arthrobacter pascens, facilitates adaptation to osmotic stress in Escherichia coli. J Bacteriol. 1991;173:472–478. doi: 10.1128/jb.173.2.472-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spencer B, Hussey E C, Orsi B A, Scott J M. Mechanism of choline-O-sulphate utilization in fungi. Biochem J. 1968;106:461–469. doi: 10.1042/bj1060461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens C M, Vohra P. Occurrence of choline sulfate in Penicillium chrysogenum. J Am Chem Soc. 1955;77:4935–4936. [Google Scholar]
- 64.Stimeling K W, Graham J E, Kaenjak A, Wilkinson B J. Evidence for feedback (trans) regulation of, and two systems for, glycine betaine transport by Staphylococcus aureus. Microbiology. 1994;140:3139–3144. doi: 10.1099/13500872-140-11-3139. [DOI] [PubMed] [Google Scholar]
- 65.Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wróblewski H, Blanco C, Bernard T. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tretyn A, Kendrick R E. Acetylcholine in plants: presence, metabolism and mechanism of action. Bot Rev. 1991;57:33–73. [Google Scholar]
- 67.Ventosa A, Nieto J J, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Blohn C, Kempf B, Kappes R M, Bremer E. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 70.Whatmore A M, Chudek J A, Reed R H. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J Gen Microbiol. 1990;136:2527–2535. doi: 10.1099/00221287-136-12-2527. [DOI] [PubMed] [Google Scholar]
- 71.Xavier K B, Martins L O, Peist R, Kossmann M, Boos W, Santos H. High-affinity maltose/trehalose transport system in the hyperthermophilic archaeon Thermococcus litoralis. J Bacteriol. 1996;178:4773–4777. doi: 10.1128/jb.178.16.4773-4777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yancey P H. Compatible and counteracting solutes. In: Strange K, editor. Cellular and molecular physiology of cell volume regulation. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 81–109. [Google Scholar]