Abstract
Background:
Saliva contains a large array of metabolites, many of which can be informative for the detection of diseases. Gas chromatography-mass spectrometry (GC-MS) is a system that has long been used for metabolite profiling owing to its sensitivity, specificity, reproducibility and synchronized analysis; it has relatively broad coverage of compound classes including sugars, sugar alcohols, glycosides and lipophilic compounds.
Aim and Objectives:
The present study was conducted to explore the use of GC-MS in assessing variation in salivary metabolites and to recognize the metabolites which can be used as disease diagnostic tools and metabolite markers for detection of oral squamous cell carcinoma.
Materials and Methods:
The present study included clinically and histopathologically confirmed oral squamous cell carcinoma (OSCC) and oral leukoplakia patients (OLK) and the control group. Patients were divided into three groups: OSCC (n = 30), OLK (n = 30) and healthy individuals as controls (n = 30). Patients were refrained from eating, drinking, smoking or oral hygiene procedures for at least 1.5 h before the collection. Saliva was collected between 9.00 and 10.00 am. Samples were stored at −80°C. Filtered samples were used for GC-MS.
Results:
Fifteen compounds differed significantly between control, OLK and OSCC. These metabolites were decanedioic acid, 2-methyloctacosane, eicosane, octane, 3,5-dimethyl, pentadecane, hentriacontane, 5, 5-diethylpentadecane, nonadecane, oxalic acid, 6-phenylundecanea, l-proline, 2-furancarboxamide, 2-isopropyl-5-methyl-1-heptanol, pentanoic acid, Docosane.
Conclusion:
The findings of the study suggest the application of salivary metabolomics as a promising tool in the identification of tumor-specific biomarkers in early diagnosis and prediction of OSCC and oral leukoplakia. In future, standardizing the protocol for salivary analysis and overcoming some of the limitations will be helpful to establish salivary metabolomics as a reliable, the highly sensitive and specific method for clinical use as an independent diagnostic aid.
Keywords: Gas chromatography-mass spectrometry, oral cancers, salivary metabolomics
INTRODUCTION
Oral cancer (OC) is the sixth-most common head-and-neck cancer (HNC) in the world. It has an overall 5-year survival rate of <50%.[1] This accounts for an estimated 550,000 new cancer cases and 300,000 cancer-related mortality worldwide every year.[1] In general, oral squamous cell carcinoma (OSCC) has been thought to arise from preexisting oral lesions or de novo. Such lesions collectively come under the category of oral potentially malignant disorders.[2]
Oral leukoplakia (OLK) is one of the most frequently occurring oral precancerous lesions with a malignant transformation range of 1.58%–27.27%,[3,4]
Despite therapeutic and technological advances, the prognosis for HNC has not improved in decades due to its malignant and recurrent properties. The most widely accepted risk factors for HNC include tobacco (smoked or chewed), alcohol use and human papillomavirus infection. However, these risk factors alone cannot explain the observed incidence and pathogenesis of HNC, since some patients are not in these risk categories. Thus, it is likely that other unknown factors play important roles in tumorigenesis, tumor progression and metastasis of HNC.[5,6] Early detection of OSCC and OLK, as well as screening the highly prone populations, are promising strategies for reducing the incidence of these lesions. Scientists expect to find high output, more efficient, low-cost and rapid diagnostic and screening approaches. Currently, the most definitive procedure for OC diagnosis and screening is physician's office visit, a scalpel biopsy if needed, and its histopathological evaluation.[7,8] Hence, to diagnose OSCC at its early stage, such novel diagnostic technologies are urgently required. Currently, drawing more attention, are molecular-based biomarkers that are used to diagnose OSCC drawing more and more attention. Saliva as a screening medium offers an easy, inexpensive, safe and noninvasive approach.[8,9] Human saliva is a mixture of secretions from multiple salivary glands, including the parotid, submandibular, sublingual and other minor glands lying beneath the oral mucosa. It is increasingly being viewed as a way to screen for diseases and was recently referred to as “the mirror of the body” in the sense that it is the good biological medium for health and disease surveillance. Saliva may contain specific biomarkers associated with certain diseases, it has been used in diagnostics for >2000 years by many traditional medical systems such as traditional Chinese medicine.[3,10] The salivary metabolic profile is also referred to as the “mirror of the body.” This is because it can capture the onco-metabolites that originate from the metabolic rewiring. Furthermore, it provides an outlook on metabolites with significant aberrant enzymatic regulation and focuses on the altered pathways during metabolic reprogramming.[11] Over the past decade, various chromatographic techniques and spectrophotometric assays were employed for the measurement of metabolites. These techniques include liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis mass spectrometry Fourier transform ion cyclotron resonance and mass spectrometry and gas chromatography-mass spectrometry (GC-MS).[12] GC-MS is a system that has long been used for metabolite profiling owing to its sensitivity, specificity, reproducibility and synchronized analysis, It has a relatively broad coverage of compound classes including sugars, sugar alcohols, glycosides and lipophilic compounds. Due to its high separation efficiency, which can resolve very complex biological mixtures, this technology identifies and quantifies a few hundred metabolites in a single sample.[9,13]
The present study uses GC-MS for the very first time for metabolite profiling in OSCC and oral leukoplakia.
MATERIALS AND METHODS
The present study was approved by Institutional Ethical Committee and Review Board. All of the subjects consented to the Ethical Committee form and agreed to donate saliva for the experiment.
Patient selection
It was carried out over a period, from June 2020 to January 2021. The study was conducted in the Department of Oral and Maxillofacial Pathology and Microbiology, Santosh Dental College and Hospital, Santosh Deemed to be University, Ghaziabad. Saliva samples were collected from a group of 30 OSCC patients aged 34–77 years, 30 OLK patients aged ranging from 34 to 80 years. These patients were analyzed and compared to a control group of 30 healthy individuals aged 21–73 years. The study participants of the OLK group were included based on the clinical criteria for its diagnosis and the study participants of the OSCC group were included based on the clinical as well as histopathological criteria. The patients with a known history of systemic illness and medications, history of therapy for OLK and OSCC and with recurrent oral lesions were excluded from the study.
Sample collection and sample preparation
The subjects were asked to refrain from eating, drinking, smoking or using oral hygiene products for at least 1.5 h before the collection of saliva. Unstimulated whole saliva was collected between 9.00 and 10.00 am under aseptic conditions by the drooling method in a collecting jar. The collected saliva was then immediately centrifuged and stored at −80°C before analysis. Then, these samples were centrifuged at 10,000 rpm for 10 min (1 ml). Supernatent, taken was lypolized in lyophilizer. Lypholised samples were dissolved in HEXANE (1 ml). Samples were again centrifuged at 10,000 rpm for 10 min. Supernatent taken was filtered by 0.45 micro molar filter (Merck) Filtered samples were used for GC-MS analysis.
Salivary metabolomics analysis
The study used GC-MS-QP2010 Ultra (Shimadzu Co., Kyoto, Japan) system with a fused silica capillary column (CP-SIL 8 CB low bleed/MS; 30 m × 0·25 mm inner diameter; 0·25 μm film thickness; Agilent Co., Palo Alto, CA, USA). The front inlet temperature was set at 230°C. The helium gas flow rate via the column was maintained at 39.0 cm/s. The column temperature was maintained at 80°C for 2 min which was increased incrementally (15°C/min) up to 330°C and held at the same temperature for 6 min. The samples (2-μl injection volume) were injected in split mode holding a split ratio of 1:25. Transfer line and ion-source temperatures were maintained at 250°C and 200°C, respectively. A minimum of 20 scans/s were recorded over a mass range of 85–500 m/z with the help of Advanced Scanning Speed Protocol (ASSP; Shimadzu Co.). MS data were exported in the CDF format, and the peaks were recorded and organized using MetAlign software (Wageningen UR, the Netherlands). The conclusive data were exported as a CSV format file and aligned using analytical software (AI output), using which peaks were recognized and quantified using an in-house metabolite library.
The final data made for the multiple classification analysis was assembled from the metabolite profiling results, and principal component analysis (PCA) was done using the Metaboanalyst v 5.0.
Statistical analysis
Statistical analysis between three groups (OSCC, Oral leukoplakia, and control was done by using MetaboAnalyst 5.0. is a freely available online statistical analyser for identifying the metabolites. It is a comprehensive platform dedicated for metabolomics data analysis via user-friendly, web-based interface.
The current MetaboAnalyst (V5.0) supports raw MS spectra processing, comprehensive data normalization, statistical analysis, functional analysis, meta-analysis as well as integrative analysis with other omics data.
Multivariate analysis in the form of PCA was performed using 3D PCA grouping to a trend of intergroup separation on the scores plot. Further, one-way analysis of variance was employed to identify the metabolites that were either significantly upregulated or downregulated in the diseased groups (P < 0.05).
RESULTS
A total of 60 patients were enrolled and were divided into a group of OSCC (n = 30) with a mean age range of 56 ± 11 years, OLK (n = 30) with a mean age range of 60 ± 13 years. These were then compared to a control group (n = 30) of healthy individuals with a mean age range of 43 ± 14 years. None of the individuals showed any history of receiving medication or treatment with topical or systemic steroids. Diagnosis of all cases was based on clinical and histopathologic criteria. In the present study, 90 salivary metabolites were detected and among these 90 metabolites [Table 1 and Figure 1], 15 compounds differed significantly between control, oral leukoplakia, and OSCC. PCA score plot [Figure 2] showing clear cut separation among different metabolites in control, OLK and OSCC [Figure 3]. These metabolites were Decanedioic acid, 2-methyloctacosane, Eicosane, Octane, 3,5-dimethyl, pentadecane, hentriacontane, 5,5-diethylpentadecane, nonadecane, oxalic acid, 6-phenylundecanea, L-proline, 2-furancarboxamide, 2-isopropyl-5-methyl-1-heptanol, pentanoic acid, docosane. Whereas in OLK Eicosane was detected in higher concentration as compared OSCC and control group [Figures 3, 4 and 5].
Table 1.
The details of these features
| Compounds | f | P | -log10(p) | FDR | Fisher’s LSD |
|---|---|---|---|---|---|
| Pentadecane | 539.400 | 4.9087e-76 | 75.309 | 4.4178e-74 | OSCC - Control; OSCC - OLK |
| Nonadecane | 487.330 | 1.0432e-72 | 71.982 | 4.6943e-71 | OSCC - Control; OSCC - OLK |
| 5,5-Diethylpentadecane | 465.320 | 3.2827e-71 | 70.484 | 9.8482e-70 | OSCC - Control; OSCC - OLK |
| L-Proline, TMS derivative | 422.000 | 4.4362e-68 | 67.353 | 9.9813e-67 | OSCC - Control; OSCC - OLK |
| 2-Isopropyl-5-methyl-1-heptanol | 408.160 | 5.0454e-67 | 66.297 | 9.0818e-66 | OSCC - Control; OSCC - OLK |
| Oxalic acid, 6-ethyloct-3-yl ethyl ester | 360.120 | 4.1074e-63 | 62.386 | 6.1611e-62 | OSCC - Control; OSCC - OLK |
| 2-Furancarboxamide, N-methyl- | 333.070 | 1.0083e-60 | 59.996 | 1.2964e-59 | OSCC - Control; OSCC - OLK |
| 6-phenylundecanea | 310.670 | 1.2635e-58 | 57.898 | 1.4214e-57 | OSCC - Control; OSCC - OLK |
| Heptadecane | 309.430 | 1.6661e-58 | 57.778 | 1.6661e-57 | OSCC - Control; OSCC - OLK |
| Octyl tetracosyl ether | 302.480 | 7.9206e-58 | 57.101 | 7.1286e-57 | OSCC - Control; OSCC - OLK |
| Decanedioic acid, didecyl ester | 247.610 | 5.1331e-52 | 51.290 | 4.1998e-51 | OLK - Control; OSCC - Control |
| Pentanethioic acid | 236.760 | 9.3656e-51 | 50.028 | 7.0242e-50 | OSCC - control; OSCC - OLK |
| Name | 223.770 | 3.4520e-49 | 48.462 | 2.3899e-48 | Control - OLK; Control - OSCC |
| 2-Methylhexacosane | 189.860 | 9.0515e-45 | 44.043 | 5.8188e-44 | OSCC - Control; OSCC - OLK |
| Hexacosane, 1-iodo- | 188.180 | 1.5459e-44 | 43.811 | 9.2755e-44 | OLK - Control; OSCC - Control |
| Tetradecane, 4-methyl- | 182.480 | 9.7693e-44 | 43.010 | 5.4952e-43 | OSCC - Control; OSCC - OLK |
| Hentriacontane | 182.290 | 1.0384e-43 | 42.984 | 5.4975e-43 | OSCC - Control; OSCC - OLK |
| 1-Docosanol, acetate | 179.430 | 2.6561e-43 | 42.576 | 1.3280e-42 | OSCC - Control; OSCC - OLK |
| Sulfurous acid, | 176.620 | 6.7687e-43 | 42.169 | 3.2062e-42 | OLK - Control; OSCC - Control |
| 2-methyloctacosane | 169.170 | 8.4115e-42 | 41.075 | 3.7852e-41 | OLK - Control; OSCC - Control |
| Eicosane | 138.070 | 7.4003e-37 | 36.131 | 3.1716e-36 | OLK - Control; OSCC - Control |
| 1-Hexacosanol | 115.530 | 7.8642e-33 | 32.104 | 3.2172e-32 | OLK - Control; OSCC - Control |
| Octane, 3,5-dimethyl- | 95.628 | 6.9405e-29 | 28.159 | 2.7158e-28 | OLK - Control; OSCC - Control |
| Palmitic Acid, TMS derivative | 77.210 | 7.8005e-25 | 24.108 | 2.9252e-24 | Control - OLK; Control - OSCC |
| Cyclohexane, eicosyl- | 60.086 | 1.2143e-20 | 19.916 | 4.3714e-20 | OLK - Control; OSCC - Control |
| 2-Hexanol | 59.274 | 1.9723e-20 | 19.705 | 6.5742e-20 | OLK - Control; OSCC - Control |
| Docosane, 1-iodo- | 59.274 | 1.9723e-20 | 19.705 | 6.5742e-20 | OLK - Control; OSCC - Control |
| Pentatriacontane | 53.620 | 6.2316e-19 | 18.205 | 2.0030e-18 | OSCC - Control; OSCC - OLK |
| Heptyl octacosyl ether | 52.853 | 1.0059e-18 | 17.997 | 3.1217e-18 | OSCC - Control; OSCC - OLK |
| 3-Methyldotriacontane | 51.028 | 3.1767e-18 | 17.498 | 9.5302e-18 | OSCC - Control; OSCC - OLK |
| Tritriacontane | 50.422 | 4.6694e-18 | 17.331 | 1.3556e-17 | OSCC - Control; OSCC - OLK |
| 1-Heptacosanol | 50.308 | 5.0220e-18 | 17.299 | 1.4124e-17 | OSCC - Control; OSCC - OLK |
| Dotriacontane | 50.151 | 5.5514e-18 | 17.256 | 1.5140e-17 | OSCC - Control; OSCC - OLK |
| Cyclohexane, 1-1,5-dimethylhexyl-4-methy | 49.672 | 7.5392e-18 | 17.123 | 1.9668e-17 | OSCC - Control; OSCC - OLK |
| 9-Methyltritriacontane | 49.607 | 7.8590e-18 | 17.105 | 1.9668e-17 | OSCC - Control; OSCC - OLK |
| 1-Docosanol | 49.605 | 7.8674e-18 | 17.104 | 1.9668e-17 | OSCC - Control; OSCC - OLK |
| Tetrapentacontane | 49.351 | 9.2607e-18 | 17.033 | 2.1006e-17 | OSCC - Control; OSCC - OLK |
| p-anisaldehydea | 49.351 | 9.2607e-18 | 17.033 | 2.1006e-17 | OSCC - Control; OSCC - OLK |
| Decane, 2,3,8-trimethyl- | 49.349 | 9.2753e-18 | 17.033 | 2.1006e-17 | OLK - Control; OLK - OSCC |
| Heptacosyl tri uoroacetate | 49.339 | 9.3360e-18 | 17.030 | 2.1006e-17 | OSCC - Control; OSCC - OLK |
| Succinic Acid | 49.011 | 1.1524e-17 | 16.938 | 2.5296e-17 | OSCC - Control; OSCC - OLK |
| 1,2-Hexadecanediol | 48.922 | 1.2203e-17 | 16.914 | 2.6149e-17 | OSCC - Control; OSCC - OLK |
| 1-Hentetracontanol | 48.793 | 1.3260e-17 | 16.877 | 2.7753e-17 | OSCC - Control; OSCC - OLK |
| n-Tridecylcyclohexane | 48.744 | 1.3684e-17 | 16.864 | 2.7989e-17 | OSCC - Control; OSCC - OLK |
| Octatriacontyl tri uoroacetate | 48.519 | 1.5827e-17 | 16.801 | 3.1654e-17 | OSCC - Control; OSCC - OLK |
| Tetratriacontyl penta uoropropio0te | 48.434 | 1.6724e-17 | 16.777 | 3.2720e-17 | OSCC - Control; OSCC - OLK |
| Hexadecane | 48.232 | 1.9050e-17 | 16.720 | 3.6480e-17 | Control - OLK; OSCC - OLK |
| Sebacic acid | 47.821 | 2.4867e-17 | 16.604 | 4.6503e-17 | OSCC - Control; OSCC - OLK |
| Heptane, 3,3,5-trimethyl- | 47.793 | 2.5318e-17 | 16.597 | 4.6503e-17 | OLK - Control; OLK - OSCC |
| Propyl triacontyl ether | 47.519 | 3.0270e-17 | 16.519 | 5.4487e-17 | OSCC - Control; OSCC - OLK |
The post-hoc Sig. Comparison column shows the comparisons between different levels that are significant given the P value threshold. OSCC: Oral squamous cell carcinoma, OLK: Oral leucoplakia patients, LSD: Least Significant Difference, FDR: False discovery rate
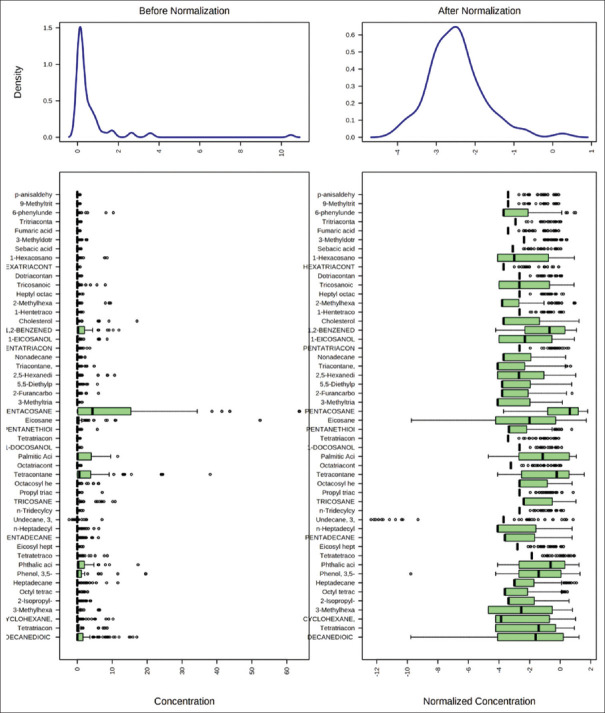
Figure 1.
Box plots and kernel density plots before and after normalization
Figure 2.
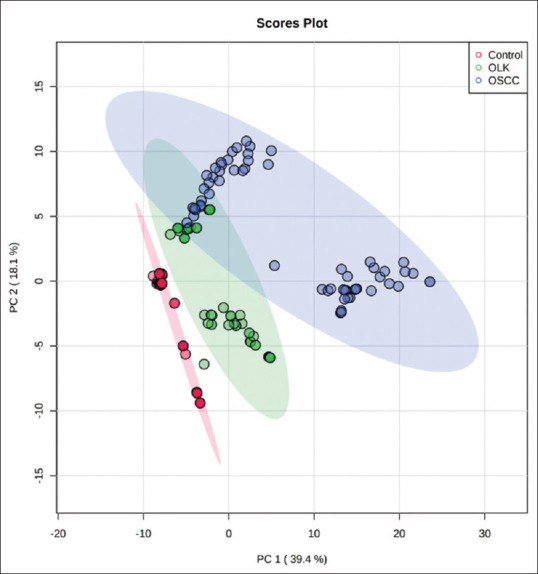
Principal component analysis (PCA) score plot showing clear cut separation among different metabolites in Control,OLK,and OSCC
Figure 3.
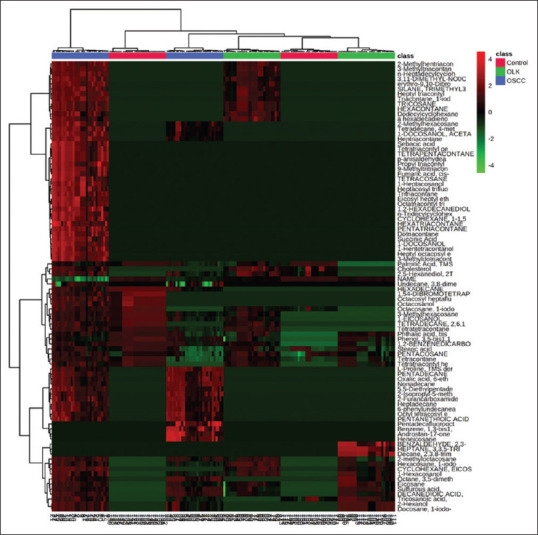
Hierarchically-clustered Heatmap showingSpearman correlation values among metabolites control ,oral leukoplakia and oral squamous cell carcinoma
Figure 4.
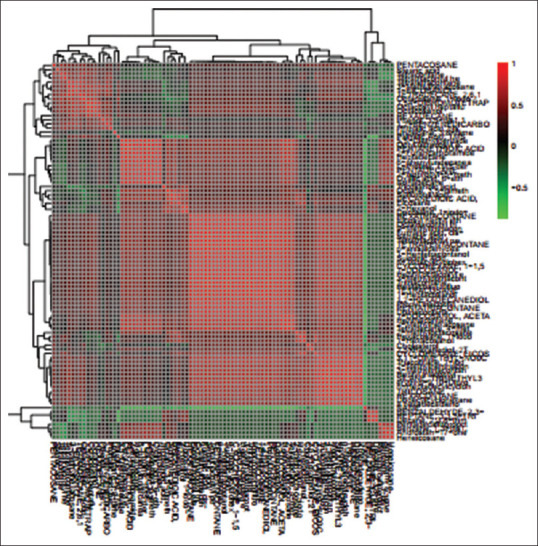
Correlation analysis of the 90 differential metabolites, marked on the hierarchical clustering plot to understand the potential relationships among metabolites
Figure 5.
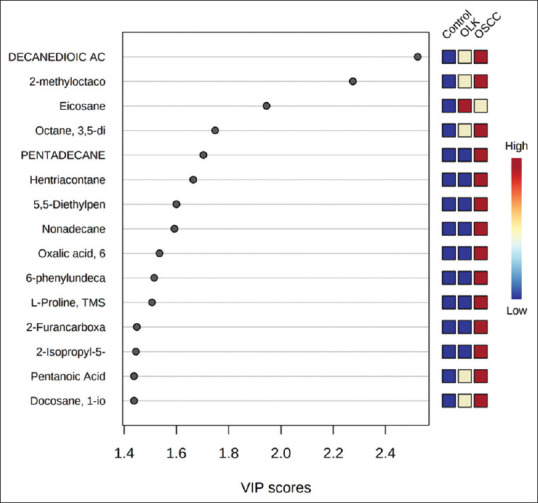
Variable importance in projection score showing top 15 most important metabolite changes significantly between control. Oral leukoplakia and oral squamous cell carcinoma
DISCUSSION
Salivary metabolomics is a significant advancement in oral carcinogenesis. It is an emerging field in oral leukoplakia and OSCC, and it is an advantage to analyze such molecules that may get transferred into saliva by various cells including the tumor cells. In the present study, salivary metabolomic profiling was done in the study participants belonging to apparently normal controls, oral leukoplakia and OSCC by GC-MS.[14] In this study, GCMS has been used for the very first time for metabolite profiling in OSCC and oral leukoplakia. A total of 90 salivary metabolites were identified and among these 90 metabolites, 15 compounds differed significantly between control, OLK and OSCC. In OSCC patients Decanedioic acid, 2-methyloctacosane, octane, 3,5-dimethyl, pentadecane, eicosane, hentriacontane, 5, 5-diethylpentadecane, nonadecane, oxalic acid, 6-phenylundecanea, l-proline, 2-furancarboxamide, 2-isopropyl-5-methyl-1-heptanol, pentanoic acid, and docosane were upregulated compared to oral leukoplakia and control group. Whereas in OLK Eicosane was detected in higher concentration as compared OSCC and control group [Figures 5 and 6]. These biomarkers were identified using the salivary metabolomics approach. However, these identified candidate biomarkers need to be extensively validated before they can be translated into real-world for screening and diagnostic application. If appropriately validated in large patient cohorts, the discovered candidates will be measured and verified with multiple complementary analytical technologies (e.g., a combined LC-MS and GC-MS approach). At present, OSCC is not detected until it reaches an advanced stage. This could result in a poor prognosis and survival rate. Therefore, early detection of OSCC as well as the screening of high risk populations with precancerous lesions remains to be an unmet need. The integration of various types of biomarkers including salivary metabolite signatures, coupled with a conventional oral examination (e.g., a scalpel biopsy) may become an applicable strategy for early detection of oral precancerous lesions and cancer. The study signifies that different salivary metabolites are present/or reveal at different stages of pathology and by detecting/quantifying various salivary metabolites, early detection of pathology (premalignant/malignant) is possible.
Figure 6.
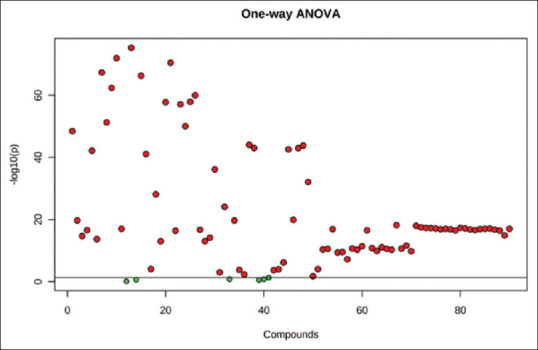
The important features identified by ANOVA analysis. Table 1 shows the details of these features. The post-hoc significantly comparison column shows the comparisons between different levels that are significant given the P value threshold
CONCLUSION
Upregulated salivary metabolites such as decanedioic acid, 2-methyloctacosane, eicosane, octane, 3,5-dimethyl, pentadecane, hentriacontane, 5,5-diethylpentadecane, nonadecane, oxalic acid, 6-phenylundecanea, l-proline, 2-furancarboxamide, 2-isopropyl-5-methyl-1-heptanol, pentanoic acid, docosanemetabolites could possess clinical utility in oral leukoplakia and OSCC. The findings of our study suggest the application of salivary metabolomics as a promising tool in the identification of tumor-specific markers in early diagnosis and prediction of OSCC and oral leukoplakia. Further to this, it is necessary to evaluate the diagnostic, therapeutic and prognostic utility of the individual metabolites in oral leukoplakia and OSCC with a two-pronged benefit of preventing the malignant transformation of oral leukoplakia and to decrease the morbidity and mortality of OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tantray S, Sharma S, Jhamb P. Assessement of lipid profile in oral submucous fibrosis and oral squamous cell carcinoma. IJRA. 2020;7:908–25. [Google Scholar]
- 2.Sridharan G, Ramani P, Patankar S, Vijayaraghavan R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2019;48:299–306. doi: 10.1111/jop.12835. [DOI] [PubMed] [Google Scholar]
- 3.Wei J, Xie G, Zhou Z, Shi P, Qiu Y, Zheng X, et al. Salivary metabolite signatures of oral cancer and leukoplakia. Int J Cancer. 2011;129:2207–17. doi: 10.1002/ijc.25881. [DOI] [PubMed] [Google Scholar]
- 4.Yonezawa K, Nishiumi S, Kitamoto-Matsuda J, Fujita T, Morimoto K, Yamashita D, et al. Serum and tissue metabolomics of head and neck cancer. Cancer Genomics Proteomics. 2013;10:233–8. [PubMed] [Google Scholar]
- 5.Shin JM, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Metabolomics of head and neck cancer: A mini-review. Front Physiol. 2016;7:526. doi: 10.3389/fphys.2016.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan W, Wistuba II, Emmert-Buck MR, Erickson HS. Squamous cell carcinoma – Similarities and differences among anatomical sites. Am J Cancer Res. 2011;1:275–300. [PMC free article] [PubMed] [Google Scholar]
- 7.Wong DT. Towards a simple, saliva-based test for the detection of oral cancer ‘oral fluid (saliva), which is the mirror of the body, is a perfect medium to be explored for health and disease surveillance’. Expert Rev Mol Diagn. 2006;6:267–72. doi: 10.1586/14737159.6.3.267. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Gao P, Wang X, Duan Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci Rep. 2014;4:6802. doi: 10.1038/srep06802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner A, Carpenter G, So PW. Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites. 2020;10:47. doi: 10.3390/metabo10020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavrier C, Couble ML, Magloire H, Grimaud JA. Connective tissue organization of healthy human gingiva. Ultrastructural localization of collagen types I-III-IV. J Periodontal Res. 1984;19:221–9. doi: 10.1111/j.1600-0765.1984.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 11.Song X, Yang X, Narayanan R, Shankar V, Ethiraj S, Wang X, et al. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc Natl Acad Sci U S A. 2020;117:16167–73. doi: 10.1073/pnas.2001395117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry based metabolomics. Mass Spectrom Rev. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa S, Sugimoto M, Kitabatake K, Sugano A, Nakamura M, Kaneko M, et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci Rep. 2016;6:31520. doi: 10.1038/srep31520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guneri P, Epstein J.B. Late stage diagnosis of oral cancer: Components and possible solutions. Oral Oncol. 2014;50:1131–6. doi: 10.1016/j.oraloncology.2014.09.005. [DOI] [PubMed] [Google Scholar]