Abstract
IgA nephropathy (IgAN) cases histopathologically showing glomerular capillary IgA deposition represent a rare subtype of primary IgAN. Patients with IgAN categorized to this subtype often exhibit heavy proteinuria, advanced histological findings, and are resistant to therapies. Here, we report three cases of biopsy-proven IgAN with glomerular capillary IgA deposition who presented acute deterioration of urinalysis findings following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccinations. Case 1 was recurrent IgAN. Case 2 and Case 3 were newly diagnosed cases with subclinical microhematuria and proteinuria history. All three cases showed gross hematuria and acute exacerbations of proteinuria following SARS-CoV-2 mRNA vaccinations. In all three cases, kidney biopsy findings showed IgA deposition in glomerular capillary walls in addition to mesangial and para-mesangial areas; acute glomerular lesions, such as intra- and extracapillary proliferations were identified, indicating the possibility of a potentially severe type of IgAN. Therefore, attention should be paid to patients with de novo or relapsing IgAN showing marked capillary IgA deposition following SARS-CoV-2 vaccination.
Keywords: SARS-CoV-2, mRNA vaccine, COVID-19, IgA nephropathy
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine has been an effective public health intervention during the coronavirus disease 2019 (COVID-19) pandemic. However, with the spread of the SARS-CoV-2 vaccine programs worldwide, cases showing de novo or relapsing IgA nephropathy (IgAN) following SARS-CoV-2 vaccination have been reported [1–3]. Majority of the reported cases of post-vaccination IgAN show gross hematuria, low to moderate proteinuria, and focal or diffuse glomerulonephritis with mesangial IgA deposits in the biopsy, which are typical histopathological findings in IgAN.
IgAN histopathologically characterized by glomerular capillary IgA deposition is a rare subtype of the primary IgAN [4–6]. Patients with this subtype often exhibit heavy proteinuria and resistance to therapies, which are associated with poor clinical courses and disease outcomes. Herein, we report three cases of IgAN showing capillary wall IgA deposition that clinically presented with acute deterioration of urinalysis findings following SARS-CoV-2 mRNA vaccinations.
Case report
Case 1
A 48 year-old woman with biopsy-proven IgAN (Oxford classification: M0E1S1T0C1) developed nephrotic syndrome with gross hematuria 14 days after receiving the second dose of the Pfizer BNT162b2 SARS-CoV-2 vaccine. She was reported to have a medical history of tonsillectomy and corticosteroid regimen, followed by losartan (50 mg daily). Routine urinalysis obtained a few days prior to vaccination was notable for urinary protein excretion of 0.91 g/day in the absence of microhematuria.
At the time of presentation (35 days after the second vaccination), the serum creatinine (SCr) level was 0.94 mg/dl, and the serum albumin level was 2.2 g/dl. Urinalysis revealed uncountable red blood cells (RBCs) per high-power field (HPF) and urinary protein excretion of 19.05 g/gCr (urine protein-to-creatinine ratio). A kidney biopsy was performed 41 days after the second vaccination.
Kidney biopsy findings
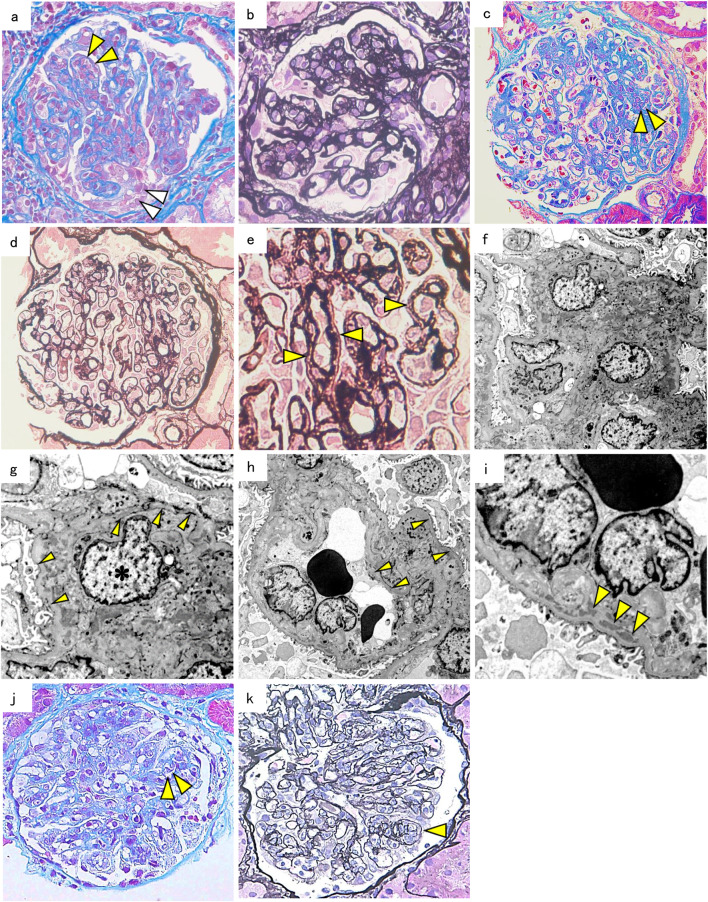
The biopsy specimen contained 51 glomeruli, 12 of which were globally sclerotic, and some of the non-sclerotic glomeruli showed lobular patterns with severe endocapillary hypercellularity containing five cellular crescents (Fig. 1a, b). The capillary walls were thickened and showed a segmental double contour of the glomerular basement membrane (GBM). Immunofluorescence (IF) staining showed predominant IgA and C3 deposits in the mesangium and glomerular capillary walls (Fig. 2a, b).
Fig. 1.
Kidney biopsy findings. a Light microscopy findings in case 1 show that the glomeruli have diffuse mesangial proliferation with endocapillary hypercellularity (yellow arrowheads) and cellular crescent (white arrowheads). Masson’s trichrome stain (× 400 original magnification). b Some glomeruli in case 2 show lobular patterns with the capillary wall thickening. Periodic acid methenamine silver (PAM) stain (× 400 original magnification). c Light microscopy findings in case 2 show glomeruli with endocapillary hypercellularity (yellow arrowheads). Elastica––Masson stain (× 400 original magnification). d, e The glomerular capillary walls in case 2 show duplication (yellow arrowheads). PAM stain (× 400, × 600 original magnification). f Electron microscopy findings in case 2 showing mesangial and paramesangial deposits (× 1200 original magnification) and g a double contour of the glomerular basement membrane (yellow arrowheads) with mesangial interposition (asterisk) (× 3000 original magnification) or h without mesangial interposition (× 1200 original magnification). i Subendothelial deposits (yellow arrowheads) (× 3000 original magnification) in case 2. j Light microscopy findings in case 3 show glomeruli with endocapillary hypercellularity (yellow arrowheads). Masson’s trichrome stain (× 400 original magnification). k The glomerular capillary walls in case 3 show duplication (yellow arrowhead). PAM stain (× 400 original magnification)
Fig. 2.
Immunofluorescence shows strongly positive deposits on IgA and C3 along the capillary wall as well as mesangial and paramesangial deposits in case 1 (a, b), case 2 (c, d), and case 3 (e, f)
Therapies and clinical course
The patient was treated with a pulse dose of methylprednisolone (500 mg daily for 3 days), followed by oral prednisolone (40 mg daily). Four weeks after the administration of corticosteroids, her proteinuria decreased to 6.0 g/gCr.
Case 2
A 19-year-old man with a history of microhematuria over the past 5 years presented with gross hematuria 18 h after receiving a second dose of the BNT162b2 vaccine. The patient had no family history of kidney disease. Laboratory assessments were notable with SCr of 0.97 mg/dl. Urinalysis revealed microhematuria with an RBC count of 50–99 per HPF and urinary protein excretion of 1.5 g/gCr. A kidney biopsy was performed 30 days after the second dose of vaccination.
Kidney biopsy findings
Under a light microscope, 13 of 17 glomeruli showed moderate mesangial and/or endocapillary proliferation (Fig. 1c), and one showed fibrocellular crescents. The glomerular capillary walls were thickened and focally showed a double contour of the GBM (Fig. 1d, e). IF staining showed predominant IgA and C3 depositions in the mesangium and capillary walls (Fig. 2c, d). Electron microscopy showed electron-dense deposits (EDDs) in the mesangial, paramesangial, and subendothelial spaces with mesangial interpositions (Fig. 1f–i).
Therapies and clinical course
The patient was treated with losartan (100 mg daily), and proteinuria gradually improved to less than 1 g/gCr until 12 weeks after the initial diagnosis.
Case 3
A 36 year-old woman with a medical history of subclinical microhematuria, proteinuria, and rheumatoid arthritis treated with certolizumab pegol developed gross hematuria 11 days after her first dose of the BNT162b2 vaccination. Laboratory findings revealed nephrotic syndrome with marked hypoalbuminemia (1.9 g/dl) and slightly elevated SCr levels (0.90 mg/dl). Urinalysis showed urinary protein excretion of 15.6 g/gCr and > 100 RBCs/HPF. A kidney biopsy was performed 25 days after the first vaccination.
Kidney biopsy findings
Renal biopsy revealed six glomeruli, all of which showed marked mesangial expansion due to increased matrices and mesangial and endocapillary hypercellularity (Fig. 1j). Additionally, segmental duplication of the glomerular capillary walls was noted (Fig. 1k). IF staining showed marked intensity for IgA and C3 located both in the mesangium and subendothelial spaces along the capillary walls (Fig. 2e, f).
Therapies and clinical course
The patient was treated with a pulse dose of methylprednisolone (500 mg daily for 3 days), followed by oral prednisolone (40 mg daily). Four weeks after the administration of corticosteroids, his proteinuria improved to 2.9 g/gCr, and an oral cyclosporine (100 mg daily) therapy was started.
Table 1 summarizes the laboratory findings, renal histopathological findings, treatment strategies, and clinical courses for the three cases.
Table 1.
Laboratory findings, renal histopathological findings, and treatment strategies of the three cases
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Laboratory findings | |||
| White blood cell count (cells/μl) | 8100 | 5800 | 8900 |
| Blood urea nitrogen (mg/dl) | 22 | 15 | 8.6 |
| Creatinine (mg/dl) | 0.94 | 0.97 | 0.90 |
| Total protein (g/dl) | 5.0 | 6.8 | 4.9 |
| Albumin (g/dl) | 2.2 | 4.5 | 1.9 |
| IgG (mg/dl) | 583 | 916 | 503 |
| IgA (mg/dl) | 244 | 183 | 424 |
| C3 (U/l) | 80 | 107 | 99 |
| Anti-nuclear antibody | < 40 | < 40 | < 40 |
| MPO-ANCA | N.D | N.D | N.D |
| PR3-ANCA | N.D | N.D | N.D |
| Anti-GBM antibody | N.D | N.D | N.D |
| Cryoglobulin | N.D | N.D | N.D |
| Proteinuria (g/gCr) | 19.05 | 1.5 | 15.6 |
| Hematuria (RBCs/HPF) | many | 50–99 | many |
| Kidney biopsy findings | |||
| Timing of biopsy | 41 days after the 2nd vaccination | 30 days after the 2nd vaccination | 25 days after the 1st vaccination |
| Light microscopy | |||
| Renal histopathological diagnosis | DPGN; IgAN | DPGN; IgAN | DPGN; IgAN |
| Oxford classification | M0E1S1T0C1 | M1E1S1T0C1 | M1E1S1T0C0 |
| IF staining | |||
| Mesangial and paramesangia l area | IgA + + + , C3 + | IgA + + + , C3 + | IgA + + , C3 + |
| Glomerular capillary spaces | IgA + + + , C3 + | IgA + + + , C3 + | IgA + , C3 + |
| Electron microscopy | |||
| EDDs | |||
| Mesangial and paramesangial area | + | + + + | + + + |
| Glomerular capillary spaces | + + | + + | + + |
| Mesangial interposition | – | + | – |
| Foot process effacement (%) | 30 | 10 | 30 |
| Therapies | mPSL + PSL | ARB | mPSL + PSL, CyA |
| Follow-up | |||
| Creatinine (mg/dl) | 0.99 | 0.95 | 0.96 |
| Albumin (g/dl) | 2.2 | 4.2 | 3.2 |
| Proteinuria (g/gCr) | 6.04 | 1.0 | 2.79 |
| Hematuria (RBCs/HPF) | many | 50–99 | 30–49 |
| Follow-up period (week) | 3 | 11 | 8 |
Anti-GBM antibody anti-glomerular basement membrane antibody, ARB angiotensin II receptor blocker, CyA cyclosporine A, DPGN diffuse proliferative glomerulonephritis, HPF high-power field, IgA immunoglobulin A, IgAN IgA nephropathy, IF immunofluorescence, IgG immunoglobulin G, MPO-ANCA myeloperoxidase anti-neutrophil cytoplasmic antibody, mPSL + PSL pulse dose of methylprednisolone (500 mg daily for 3 days); followed by oral prednisolone (40 mg daily), N.D not detected, PR3-ANCA proteinase3 anti-neutrophil cytoplasmic antibody, RBC red blood cells
Discussion
To the best of our knowledge, this study is the first to report cases of biopsy-proven IgAN with glomerular capillary IgA deposition upon SARS-CoV-2 vaccination. In the kidney biopsy findings, mesangial and endothelial hypercellularity and a focal double contour of capillary walls were noted under a light microscope. IF staining showed predominant IgA deposition with less intensive C3 in the glomerular capillary walls, along with mesangial and paramesangial areas. Typical IgAN is histopathologically characterized by the deposition of IgA in the glomerular mesangium, whereas cases showing glomerular capillary IgA deposition are rarely identified in daily clinical practice. Importantly, this subtype of IgAN with glomerular capillary IgA deposition potentially exhibits more severe clinical presentations [5, 6] and a poorer response to therapies than that in IgAN patients with typical IgA mesangial-dominant deposition [5, 7]. Thus, our cases indicate that this potentially severe subtype of IgAN may be induced or exacerbated by SARS-CoV-2 mRNA vaccination. In addition, case 3 may be an exacerbation case of IgAN secondary to rheumatoid arthritis because of her medical history.
Our cases required differential diagnosis from IgA-dominant membranoproliferative glomerulonephritis [8], IgA-dominant post-infection acute glomerulonephritis [9], or IgAN complicated with minimal change disease; however, the diagnosis of our patients was unlikely to be one of these disorders because they showed no clinical episodes suggesting systemic lupus erythematosus, hepatic virus infections, liver cirrhosis, preceding upper respiratory infections, or hypocomplementemia. Histopathologically, IgA deposition was more predominant than C3 on IF staining and hump or extensive foot process effacement under an electron microscope were not noted.
The presence of glomerular capillary IgA deposition is associated with more serious proteinuria [5, 7], more advanced histological findings [5, 6], and poor kidney disease outcomes [5, 7]. Shima et al. [5] reported that the proteinuria remission rate was lower in IgAN with glomerular capillary IgA deposition than that in typical IgAN with mesangial-dominant IgA deposition. They showed that the population of patients with subendothelial EDDs or GBM lysis was higher in IgAN with glomerular capillary IgA deposition than that in typical IgAN. Bellur et al. [6] reported that the presence of glomerular capillary IgA deposition was associated with marked endocapillary hypercellularity. Kusano et al. [10] have reported that the severity of endocapillary hypercellularity is significantly correlated with hematuria and proteinuria. Consistent with these observations, our IgAN patients with glomerular capillary IgA deposition showed endocapillary hypercellularity, prominent double contour, and/or mesangial interposition, indicative of severe glomerular capillary injuries, together with clinically severe manifestations such as gross hematuria and heavy proteinuria.
Following SARS-CoV-2 vaccination, the serum titers of both IgG and IgA antibodies specific to the SARS-CoV-2 spike antigen are higher than those in naturally infected patients with SARS-CoV-2 [11, 12]. Therefore, SARS-CoV-2 vaccination might excessively activate humoral immunity in patients with IgAN, leading to increased production of galactose-deficient IgA1 (Gd-IgA1), a characteristic abnormality of the IgA molecule in patients with IgAN [13]. By analyzing an animal model of IgAN, Yamaji et al. [14] have shown that the glomerular capillary IgA deposition may represent “overflowed” IgA excessively deposited in the mesangial area, which is determined by the balance between deposition and clearance of IgA in glomeruli. Accordingly, patients with IgAN with glomerular capillary deposition may be more sensitive to COVID-19 vaccination and have even more excessive production of Gd-IgA1 than other cases of COVID-19 vaccination-induced IgAN. Although we did not measure serum Gd-IgA1 levels in our patients, the relatively massive deposition of IgA in the mesangial area observed in our cases supports this hypothesis.
In summary, we report three cases of IgAN with gross hematuria and heavy proteinuria following SARS-CoV-2 vaccination. Histopathologically, all three cases were characterized by IgA deposition in the glomerular capillary walls. IgAN with glomerular capillary IgA deposition is a rare subtype of IgAN associated with heavy proteinuria and poor response to conventional therapies. The current case report shows SARS-CoV-2 vaccination may induce de novo or relapsing IgAN with glomerular capillary IgA deposition.
Declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Negrea L, Rovin BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487. doi: 10.1016/j.kint.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahim SEG, Lin JT, Wang JC. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021;100:238. doi: 10.1016/j.kint.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park K, Miyake S, Tai C, et al. Letter regarding: a case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int Rep. 2021;6:2246–2247. doi: 10.1016/j.ekir.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennette JC, D’Agati VD, Olson JL, et al. Heptinstall’s pathology of the kidney. 7th ed. Wolters Kluwer; 2014.
- 5.Shima Y, Nakanishi K, Mukaiyama H, et al. Clinicopathological significance of glomerular capillary IgA deposition in childhood IgA nephropathy. Pediatr Nephrol. 2021;36:899–908. doi: 10.1007/s00467-020-04772-4. [DOI] [PubMed] [Google Scholar]
- 6.Bellur SS, Troyanov S, Cook HT, et al. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant. 2011;26:2533–2536. doi: 10.1093/ndt/gfq812. [DOI] [PubMed] [Google Scholar]
- 7.Alvarado AS, Andeen NK, Brodsky S, et al. Location of glomerular immune deposits, not codeposition of immunoglobulin G, influences definitive renal outcomes in immunoglobulin A nephropathy. Nephrol Dial Transplant. 2018;33:1168–1175. doi: 10.1093/ndt/gfx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andeen NK, Jefferson JA, Akilesh S, et al. IgA-dominant glomerulonephritis with a membranoproliferative pattern of injury. Hum Pathol. 2018;81:272–280. doi: 10.1016/j.humpath.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Nasr SH, D’Agati VD. IgA-dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract. 2011;119:c18–c25. doi: 10.1159/000324180. [DOI] [PubMed] [Google Scholar]
- 10.Kusano T, Takano H, Kang D, et al. Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum Pathol. 2016;49:135–144. doi: 10.1016/j.humpath.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE. 2021;16:e0249499. doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrieri M, Francavilla B, Fiorelli D, et al. Nasal and salivary mucosal humoral immune response elicited by mRNA BNT162b2 COVID-19 vaccine compared to SARS-CoV-2 natural infection. Vaccine. 2021;9:1499. doi: 10.3390/vaccines9121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaji K, Suzuki Y, Suzuki H, et al. The kinetics of glomerular deposition of nephritogenic IgA. PLoS ONE. 2014;9:e113005. doi: 10.1371/journal.pone.0113005. [DOI] [PMC free article] [PubMed] [Google Scholar]