Abstract
Exploiting cancer vulnerabilities is critical for the discovery of anticancer drugs. However, tumor suppressors cannot be directly targeted because of their loss of function. To uncover specific vulnerabilities for cells with deficiency in any given tumor suppressor(s), we performed genome-scale CRISPR loss-of-function screens using a panel of isogenic knockout cells we generated for 12 common tumor suppressors. Here, we provide a comprehensive and comparative dataset for genetic interactions between the whole-genome protein-coding genes and a panel of tumor suppressor genes, which allows us to uncover known and new high-confidence synthetic lethal interactions. Mining this dataset, we uncover essential paralog gene pairs, which could be a common mechanism for interpreting synthetic lethality. Moreover, we propose that some tumor suppressors could be targeted to suppress proliferation of cells with deficiency in other tumor suppressors. This dataset provides valuable information that can be further exploited for targeted cancer therapy.
Whole-genome CRISPR screens uncover synthetic lethal interactions for tumor suppressors.
INTRODUCTION
Cancer is at least partially a genetic disease, since multiple genetic alterations accumulate during tumorigenesis. Among them, oncogenes and tumor suppressor genes (TSGs) are the most prevalent and critical ones (1), as they play essential roles in tumorigenesis. For many known driver oncogenes, such as gain-of-function mutated receptor tyrosine kinases, highly potent and specific inhibitors have been developed. For example, inhibitors are available to target mutated EGFR (2, 3), ALK (4, 5), and BRAF (6, 7). This strategy has been proven to be clinically successful, although cancers may eventually acquire resistance to these drugs. However, unlike targetable oncogenes, tumor suppressors are not directly druggable because of their functional loss, which may arise from deletion, loss-of-function mutations, epigenetic silence, and posttranslational regulations. Thus, how to take advantage of the frequent genetic alteration of TSGs in cancer, and specifically kill these tumor cells but spare normal cells, remains a challenging issue for the development of new anticancer therapies.
The concept of synthetic lethality (SL) provides an ideal strategy to target cancers with deficiency in certain tumor suppressors (8), which is exemplified by the prominent clinical success of BRCA1/2 mutations with poly(ADP-ribose) polymerase inhibitors (9, 10). Recent studies revealed some additional SL gene interactions, such as ARID1A/ARID1B (11), ARID1A/EZH2 (12, 13), PTEN/CHD1 (14), and PTEN/SMARCA4 (15). However, these SL interactions have not been studied extensively. Moreover, CRISPR-Cas9—a powerful tool widely used to discover new anticancer targets—was recently used to conduct large-scale loss-of-function screens in hundreds of cancer cell lines (16, 17) and identified very few robust genetic vulnerabilities associated with specific TSG deficiency. This may be due to the genetic heterogeneity across cancer cell lines, which usually harbor hundreds of additional genetic mutations. It is very likely that the SL interaction between one gene and a given TSG may be blurred by alternations in many other genes. To address this issue, we established a panel of TSG knockout (KO) isogenic cell lines. We chose to conduct this study using the 293A cell line, which is a subclone of the human embryonic kidney (HEK) 293 cell line established from primary embryonal human kidney transformed with sheared human adenovirus type 5 DNA. This cell line has been widely used for many biological studies. It has relatively normal genome, and thus, the influence of other genetic alternations in these cells may be less than in other cancer cell lines. In addition, this cell line is amenable to genetic screens based on our previous studies (18, 19). The purpose of our study was to provide a comprehensive and comparative dataset for the genetic interactions across multiple TSGs, which will serve as a valuable resource for further uncovering therapeutic targets within a specific genetic context. Therefore, we generated a panel of 293A-derivative isogenic KO cells for each of the 12 common TSGs and performed genome-wide CRISPR KO screening to systematically investigate the genetic interactions between TSGs and other protein-coding genes.
RESULTS
Generation of isogeneic KO cells for a panel of TSGs
We selected 12 well-known TSGs, including ARID1A, BAP1, CDH1, KEAP1, NF1, NF2, PBRM1, PTEN, RB1, STK11, TP53, and VHL. We also included TP53BP1, a key player in DNA damage response (20), as a control. Although loss of TP53BP1 also leads to increased tumorigenesis in mice (21), its function is more restricted and is believed to be mainly involved in DNA repair.
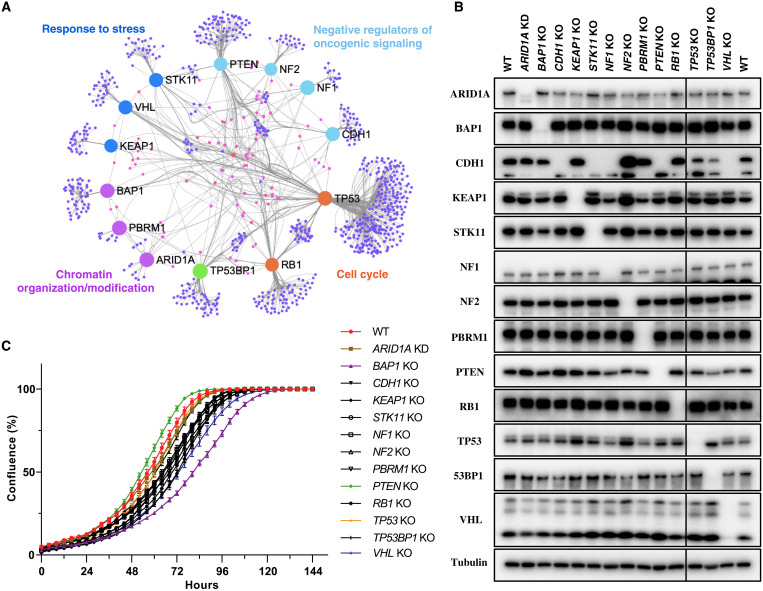
On the basis of their well-known functions, these tumor suppressors could be classified into four categories: response to stress (KEAP1, STK11, and VHL), negative regulation of oncogenic signaling (CDH1, NF1, NF2, and PTEN), chromatin organization/modification (ARID1A, BAP1, and PBRM1), and cell cycle regulation (RB1 and TP53) (Fig. 1A). In addition to their own unique interacting proteins, these tumor suppressors shared numerous nodes connecting them directly or indirectly (Fig. 1A), indicating that they may have some common genetic vulnerabilities. In general, these TSGs are altered in a broad spectrum of cancer types (fig. S1A). However, some of them have prevalent tissue specificities, such as VHL and PBRM1, which are mainly involved in kidney renal clear cell carcinoma (fig. S1B), and KEAP1 and STK11 in non–small cell lung cancer (fig. S1C). These TSGs not only engage in 6 of the top 10 frequently altered canonical signaling pathways in cancer (22) but also participate in many other important cancer-related biological processes, such as cell-cell adhesion (CDH1), hypoxia response (VHL), SWI/SNF complex–mediated chromatin remodeling (ARID1A and PBRM1), and H2AK119ub1 deubiquitinating (BAP1).
Fig. 1. Generation of a panel of isogenic cells with TSG KO.
(A) Protein-protein interaction networks among all the studied TSGs. The graph was generated on the NetworkAnalyst 3.0 platform (69) (www.networkanalyst.ca) based on the STRING interactome database (70) with a cutoff of confidence score >700 and experimental evidence required. (B) All TSG KO cells used in this study were verified by immunoblotting. WT or TSG KO cells (8 × 105) were seeded into a six-well plate, and 24 hours later, cells were harvested for immunoblotting with the indicated antibodies. (C) Cell growth curve for WT and all TSG KO cells. WT or TSG KO cells (1 × 103) were seeded into a 96-well plate, and the cell confluence was monitored with Incucyte S3 Live-Cell Analysis System. Data are presented as means ± SD of six biological replicates.
As mentioned above, the TSG KO cells were generated using the CRISPR-Cas9 technique. All the KO cells were confirmed by immunoblotting (Fig. 1B) and Sanger sequencing (fig. S1D). Among them, we found that NF1 KO cells still have one allele with in-frame deletion, but the protein level of NF1 was nearly undetectable (Fig. 1B). In addition, we noticed that CDH1 protein (E-cadherin) disappeared not only in CDH1 KO cells but also in STK11 KO, NF1 KO, PTEN KO, and VHL KO cells. This robust decrease was largely due to the change in CDH1 mRNA level, as shown by our RNA sequencing (RNA-seq) data (fig. S1E), indicating that loss of E-cadherin is frequently accompanied by loss of some TSGs in cancer progression and metastasis. Furthermore, increased AKT phosphorylation, stabilized HIF1α protein level, and stabilized NRF2 protein level were observed, respectively, in PTEN KO, VHL KO, and KEAP1 KO cells (fig. S1, F and G), which are consistent with the literature (23–27). Please note that ARID1A KO was amended to ARID1A knockdown (KD) based on several considerations that will be described later, and thus, we refer to these cells as ARID1A KD cells rather than ARID1A KO cells. We also measured growth rates of wild-type (WT) and all KO cells. Compared to WT cells, PTEN KO cells grew a little bit faster, while other TSG KO cells grew similarly or relatively slower, especially BAP1 KO and VHL KO cells (Fig. 1C). These data suggested that loss of TSGs may have some fitness advantages, but these advantages may not reflect on their growth in in vitro culture under normal growth conditions.
Genome-wide CRISPR screening using isogeneic TSG KO cells
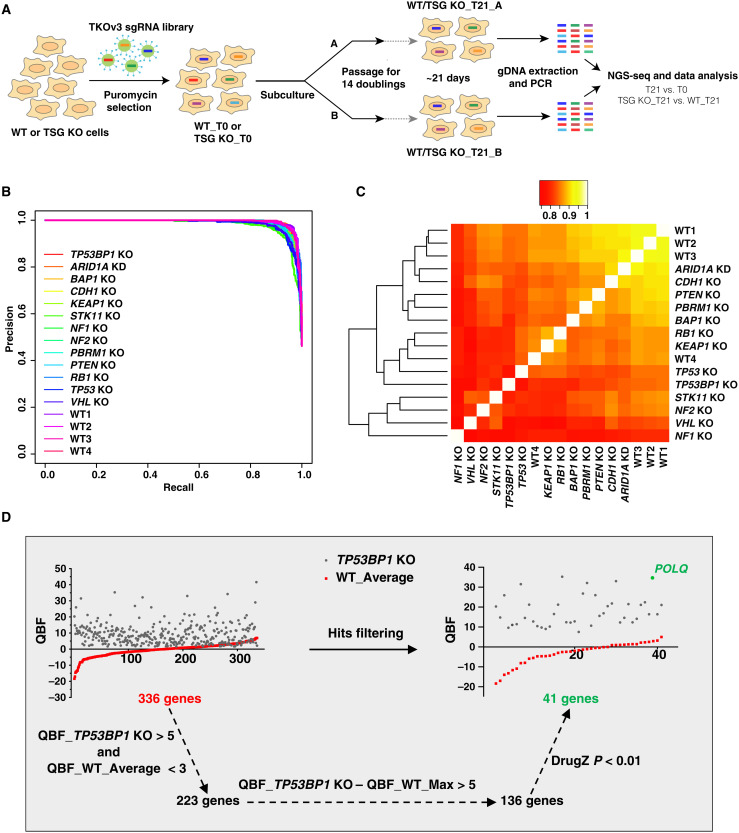
Genome-wide CRISPR KO screens were performed as previously described (18, 19). Briefly, WT and TSG KO cells were infected with lentiviruses at a low multiplicity of infection (MOI; ~0.25), which were prepared from Toronto Knock Out Library v3 (TKOv3), an all-in-one library with excellent performance containing 70,948 guide RNAs (gRNAs) targeting 18,053 protein-coding genes (28). Cells were then selected with puromycin to eliminate uninfected cells within 3 days, and one aliquot was collected as the initial population and labeled as T0; the remaining cells were divided into two replicates and further passaged for about 14 doubling cycles (~21 days) and then collected as the final population and labeled as T21. Genomic DNA was extracted from T0 and T21, amplified with barcode-tagged primers, and then gRNA abundance was determined by next-generation sequencing (Fig. 2A and table S1). Relative gRNA abundance of T0 and T21 was analyzed with the BAGEL algorithm to assign an essentiality score to each gene, and then binary essentiality calls were made (see Materials and Methods) to classify the essentiality of genes in a given background across batches. In brief, binary essentiality calls generate a file containing binary calls where 1 indicates being essential and 0 means being nonessential (table S2); BAGEL provides a log Bayes factor (BF) for each gene, where more positive scores indicate increased likelihood of essentiality in a given background (table S3). In addition to these methods, DrugZ analysis was used to compare the gRNA abundance of T21 samples of WT and TSG KO cells from the same batch, which provided gene-level depletion-normalized Z scores (normZ) and statistical significance (table S4).
Fig. 2. The pipeline of whole-genome CRISPR KO screening.
(A) Schematic diagram showing the whole workflow of our CRISPR screenings. (B) Precision-recall (PR) curves were plotted for all screens. Screen performance was evaluated by calculating PR curves with reference essential and nonessential gene lists. (C) Heatmap of Pearson correlation coefficients calculated from QBF among all screens. (D) The pipeline for SL hit filtering. Raw SL hits (genes found to be essential in TSG KO cells but not in WT cells) generated from binary essentiality calls, and the essentiality of these hits was further confirmed by QBF with cutoffs (QBF_X_TSG KO > 5 and QBF_X_WT_Average < 3). Additional cutoffs were applied to make sure that the difference between TSG KO and WT was robust (QBF_X_TSG KO − QBF_X_WT_Max > 5) and significant (DrugZ P < 0.01). X could be any gene. WT_Average and WT_Max respectively indicate the mean and maximal QBF number of four repeated WT screens. NGS-seq, next-generation sequencing; gDNA, genomic DNA.
To evaluate the quality of our screens, precision-recall curves (29) were calculated, and the result showed very high performance for all these screens (Fig. 2B). We also measured the Pearson correlation coefficient of the quantile-normalized BF (QBF) distributions. All screens showed very high correlation (>0.75), especially for screens in WT cells (>0.85) from different batches (Fig. 2C). On the basis of binary essentiality calls, we defined essential genes in WT cells as those genes that were shown to be essential for three or four times in a WT background, since we repeated screening in WT cells for all four batches. In total, we identified 1911 candidate essential genes in WT cells, which covered 91.4% (625 genes) of reference Core Essential Genes 2.0 [including 684 genes identified from 17 genome-scale KO screens in human cell lines (28)]. On the basis of the binary essentiality call approach, a preliminary hit list was generated, which shows being essential in a given TSG KO background but not in a WT background, and further evaluated by QBF (QBF_X_TSG KO > 5, QBF_X_WT_Average < 3, and QBF_X_TSG KO − QBF_X_WT_Max > 5, where X is any gene from TKOv3 library) and DrugZ (P < 0.01). QBF > 5 was chosen as a critical threshold of being essential in a given background because QBF was greater than 5 for 613 of the 625 core essential genes in 293A WT cells.
Here, we use the data from TP53BP1 KO cells to exemplify our hit filtering pipeline. The preliminary list contained 336 genes that showed SL with TP53BP1 loss. After filtering with the criteria above, the list was confined to a short list including 41 genes that we believe are high-confidence SL hits (Fig. 2D). Polymerase theta (POLQ) was identified as the top SL hit in our high-confidence list, which was recently reported to be synthetic lethal with TP53BP1 loss (30, 31).
These filters narrowed down the preliminary gene list from hundreds of genes to dozens. After filtering the data for all KO cells, we identified a total of 347 genes whose loss led to potential SL with at least one TSG or TP53BP1, with an average of 26.7 genes per screen, ranging from 71 genes (VHL KO) to 17 genes (TP53 KO) (table S5).
Global view of TSG SL screen results
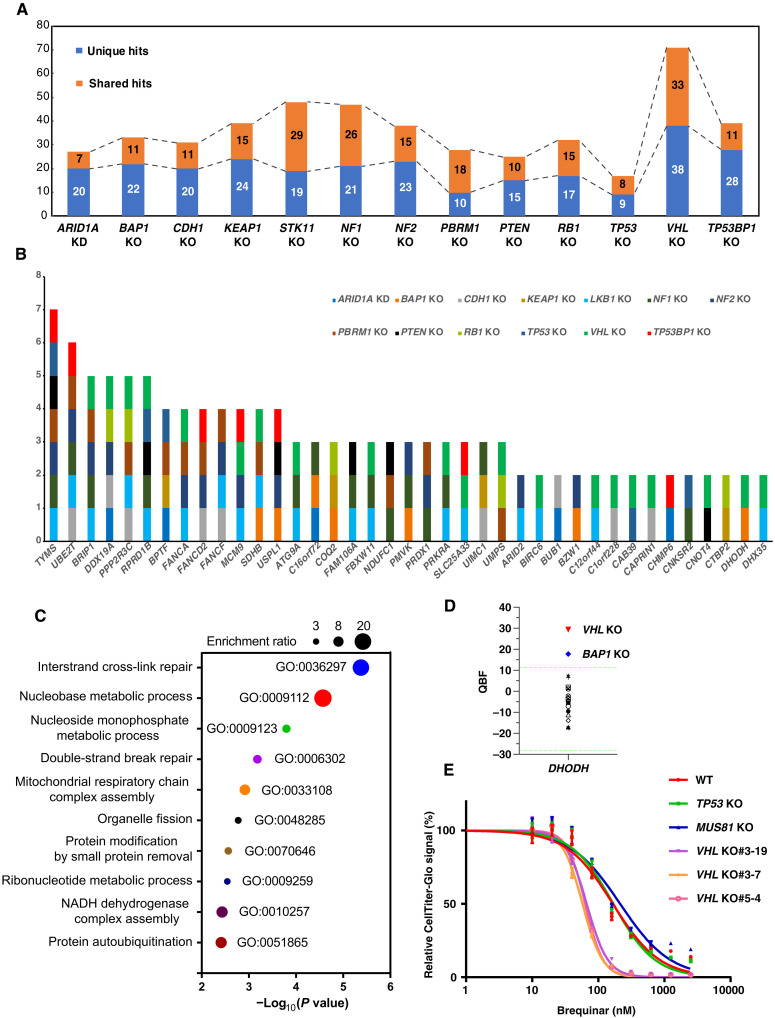
When comparing these high-confidence SL hits, we found that some of these SL genes were shared across different KO screens, ranging from 33 genes (VHL KO) to 7 genes (ARID1A KD) (Fig. 3A), which may be partially due to direct or indirect functional interactions between these TSGs (Fig. 1A). In total, we identified 81 genes that appeared in at least two KO screens (table S6); some of these appeared in multiple screens, such as TYMS and UBE2T in seven and six KO screens, respectively (Fig. 3B). Excluding these shared hits, we identified a total of 266 unique hits, which averaged to about 20.5 genes per KO screen and ranged from 38 genes (VHL KO) to 9 genes (TP53 KO) (Fig. 3A and table S7).
Fig. 3. Overview of SL hits across each TSG KO screen.
(A) A histogram showing unique and shared SL hits in each TSG KO screen. (B) A histogram showing the top shared hits that exist in multiple TSG KO screens. The full list can be found in table S6. (C) Gene ontology (GO) analysis for these 81 shared SL hits. Gene set enrichment analysis was performed on WebGestalt (71) (WEB-based gene set analysis toolkit) using the overrepresentation analysis method, and the graph was generated on GraphPad. (D) The QBF of DHODH in all screens. Light green dashed line indicates the mean QBF of all four WT screens ± 3 × SD. (E) 293A WT and several independent VHL KO clones were seeded into a 96-well plate, and 24 hours later, brequinar sodium at different concentrations was added to the cells and maintained for 3 days before CellTiter-Glo assay according to the manufacturer’s instructions. TP53 KO and MUS81 KO cells were also included as negative controls.
We first focused on the shared SL genes. Functional enrichment analysis with these 81 genes showed that gene sets linked to DNA repair and nucleotide metabolism were highly enriched (Fig. 3C). In other words, cells with loss of certain TSGs were prone to be sicker than WT cells when the cells gained mutations, resulting in DNA repair deficiency or dysregulated nucleotide metabolism. Nucleotide metabolism is critical for DNA/RNA synthesis, DNA replication, and efficient DNA repair (32), which supports uncontrolled growth of tumor cells; thus, agents targeting nucleotide metabolism are widely used for cancer chemotherapeutics. Here, we found that depletion of dihydroorotate dehydrogenase (DHODH), a rate-limiting enzyme in the de novo pyrimidine biosynthesis pathway, is synthetic lethal with VHL KO and BAP1 KO, especially for VHL KO (Fig. 3, B and D). Inhibitors of DHODH, such as leflunomide and its active metabolite teriflunomide, have been approved by the U.S. Food and Drug Administration (FDA) for rheumatoid arthritis and multiple sclerosis treatment (33, 34), and a new DHODH inhibitor, ASLAN003, was recently granted orphan drug designation by the FDA and is in a phase 2 clinical trial for the treatment of acute myeloid leukemia.
To validate our findings, 293A WT cells and several independent VHL KO cells were treated with brequinar, a potent and selective inhibitor of DHODH (35). TP53 KO and MUS81 KO cells were included as controls. VHL KO cells displayed much lower IC50 (median inhibitory concentration) than other KO cells in response to DHODH inhibition (Fig. 3E). This finding was consistently observed in several independent VHL KO clones (Fig. 3E). These data supported our screening result that VHL KO 293A cells were more sensitive to DHODH inhibition. However, when we tested other cell lines, such as MDA-MB-231 and ACHN, we found no substantial difference between WT and VHL KD cells in response to DHODH inhibition (fig. S2, A to D), and notably, ACHN cells were very insensitive to DHODH inhibition (fig. S2D).
We also examined the sensitivity of 293A BAP1 KO cells to DHODH inhibition with brequinar. The results showed that, compared with 293A WT cells, BAP1 KO cells were slightly sensitive to DHODH inhibition, especially at high concentrations of the drug (fig. S2E), which was similar to the effect of CRISPR-mediated DHODH KO. This sensitivity was not as robust as that of 293A VHL KO cells (Fig. 3E), which was consistent with our screen results (Fig. 3D). We also observed no notable difference between WT and BAP1 KD MDA-MB-231 or ACHN cells (fig. S2, B and D). In addition, we reconstituted BAP1 in UMRC6 cells (fig. S2H), a BAP1-deficient renal cancer cell line, and found that UMRC6 cells with BAP1 reexpression did not display substantial resistance to DHODH inhibition (fig. S2I).
Accumulating studies have demonstrated that the functions of certain TSGs are associated with genomic stability, and loss of them may induce DNA damage or deficiency in DNA repair (36–45), which, in turn, renders cells more sensitive to the ablation of genes involved in DNA damage response/repair or nucleotide metabolism. These findings are in agreement with our screening data. However, we should also note that frequent mutations in cancer cells could blur TSG-dependent genetic vulnerabilities in some cases, which makes it difficult to apply findings from one cell line to another, as we did here. For example, VHL loss itself could induce replication stress and DNA damage accumulation, but concomitant loss of PBRM1 could rescue this defect and maintain cell fitness (45).
Essential paralog gene pairs could be a common mechanism for interpreting SL
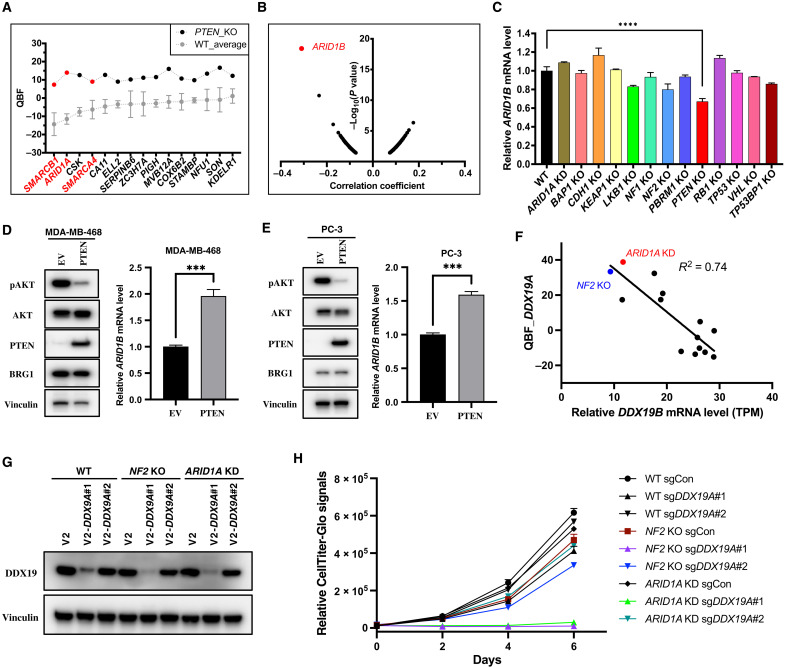
We further went through the unique hits identified in our screens and found that in PTEN KO cells, ARID1A, SMARCB1 (or BAF47), and SMARCA4 (or BRG1), which are three subunits of the SWI/SNF chromatin remodeling complex (46), were identified as high-confidence SL hits (Fig. 4A). Notably, SMARCA4 has been shown to be synthetic lethal with PTEN in prostate cancer (15), which is consistent with our screening data. However, in 293A cells, ARID1A was a stronger SL hit with PTEN than were SMARCB1 and SMARCA4.
Fig. 4. Essential paralogs underlying SL.
(A) QBF of high-confidence SL hits for PTEN KO cells. WT_Average means the mean QBF of all four repeated WT screens and is presented as means ± SD. (B) Pearson correlation analysis of gene dependency for ARID1A-mutant features in all available cell lines in the DepMap portal. (C) Relative mRNA levels of ARID1B in 293A WT and all TSG KO cells. Data are presented as means ± SD of two biological replicates. Statistical analysis was performed with one-way ANOVA. ****P < 0.0001. (D) EV- or PTEN-overexpressed MDA-MB-468 cells were seeded at 4 × 105 cells per well in a six-well plate and maintained for 48 hours, and then cells were harvested for immunoblotting with the indicated antibodies or real-time quantitative PCR to measure the mRNA levels of ARID1B. Data are presented as means + SD of three biological replicates with statistical analysis by unpaired t test. ***P < 0.001. (E) Same conditions described in (D), except that the cell line was PC-3. (F) Negative correlation between DDX19B expression and DDX19A essentiality. TPM, transcripts per kilobase million. (G) 293A WT, NF2 KO, and ARID1A KD cells were infected with lentiviruses containing LentiCRISPRv2 sgControl or two independent sgRNAs targeting DDX19A; 24 hours later, cells were selected with puromycin (2 μg/ml) for 3 days and harvested for immunoblotting with the indicated antibodies. (H) Same conditions described in (G), except that the cells after puromycin selection were seeded at 800 cells per well in a 96-well plate with four replicates, and luminescence detection was performed every 2 days according to the standard protocol.
It is well established that ARID1B shows specific vulnerability in ARID1A-mutant cancers (11), and this SL interaction is very likely due to conserved functions between ARID1B and ARID1A (47). In other words, cells with loss of either ARID1A or ARID1B are viable, but the loss of both is lethal; i.e., ARID1A and ARID1B are essential paralogs (48, 49). Consistent with these previous findings, we confirmed that ARID1B was the top SL hit for ARID1A-mutant cancer cell lines through Pearson correlation analysis via the DepMap portal (Fig. 4B).
The data mentioned above indicate that ARID1A essentiality in PTEN KO cells may arise from a change in ARID1B expression. To test this hypothesis, we first checked the mRNA level of ARID1B in 293A WT and all the KO cells we used for our screening. As shown in Fig. 4C, a significant decrease in ARID1B mRNA level was only observed in PTEN KO cells, and consistently, PTEN reconstitution in 293A PTEN KO cells up-regulated ARID1B expression (fig. S3, A and B). ARID1A KD markedly diminished the growth of PTEN KO cells (fig. S3, C and D).
To further confirm the role of PTEN in ARID1B regulation, we reconstituted PTEN expression in PTEN-deficient breast cancer cell line MDA-MB-468, and as anticipated, overexpression of PTEN significantly repressed Akt hyperphosphorylation and up-regulated ARID1B expression (Fig. 4D). A similar result was observed in a PTEN-deficient prostate cancer cell line, PC-3 (Fig. 4E). These data supported our hypothesis that PTEN loss leads to down-regulation of ARID1B expression, which may give rise to the essentiality of ARID1A or other components of the SWI/SNF complex in these cells. To further confirm this possibility, we knocked down ARID1A, SMARCA4, or SMARCB1 in empty vector (EV)– or PTEN-reconstituted MDA-MB-468 and PC-3 cells (fig. S3, E and F). In MDA-MB-468 cells, we observed that ARID1A KD caused a cell growth defect in WT (EV) cells but not in PTEN-reconstituted cells (fig. S3G). Similarly, we measured the cell growth when SMARCA4 or SMARCB1 was depleted in a WT (EV) or PTEN-reconstituted background (fig. S3H). To have a better evaluation of gene dependency, we took the ratio of sgSMARCA4 or sgSMARCB1 to sgAAVS1 in each background from days 4 and 5 as the essentiality score; a lower score means that a given gene is more essential in a given background. SMARCB1, but not SMARCA4, turned out to be more essential in the PTEN-deficient background compared with the PTEN-reconstituted background (fig. S3, I and J), indicating potential SL interaction between PTEN and SMARCB1 in MDA-MB-468 cells. However, in PC-3 cells, we only observed a trend that showed potential SL interaction between PTEN and ARID1A (fig. S3, K and L), and for SMARCA4 or SMARCB1, we observed no notable SL interactions (fig. S3M). We noticed that reconstituted PTEN expression level was much higher in MDA-MB-468 cells than in PC-3 cells (fig. S3E), and PTEN reconstitution suppressed the cell growth in MDA-MB-468 but not PC-3 cells (fig. S3, G, H, K, and M), although PTEN reconstitution markedly suppressed Akt phosphorylation in both cell lines (Fig. 4, D and E). Thus, we suspect that reconstituted PTEN expression and subsequent functional effects, such as slower growth rate, may affect whether the SL interactions could be rescued.
This type of SL due to essential paralog pairs also exists in other settings. For example, in our screens, we also found that adenosine triphosphate–dependent RNA helicase DDX19A, which has been experimentally confirmed as the essential paralog for DDX19B (49), was identified as an SL hit in several KO cells, especially in ARID1A KD and NF2 KO cells (Fig. 4F and table S5). We thus checked the mRNA levels of DDX19B in our RNA-seq data, and as predicted, all KO cells showing DDX19A dependency had a reduction in DDX19B expression (Fig. 4F). Moreover, there was a clear negative correlation between DDX19A essentiality and DDX19B expression when we included WT and all other KO cells (Fig. 4F). We confirmed that DDX19A/DDX19B exists as essential paralogs in 293A cells. Only knocking down both DDX19A and DDX19B led to a drastic decrease in cell viability (fig. S3, N and O). To further validate this SL interaction between DDX19A and ARID1A or NF2, we knocked down DDX19A with Lenti CRISPRv2 virus packaged from two independent single-guide RNAs (sgRNAs) in WT, ARID1A KD, and NF2 KO cells (Fig. 4G) and performed viability assay with CellTiter-Glo. The results showed that DDX19A ablation induced a robust decrease in cell viability in ARID1A KD or NF2 KO cells but not in WT cells (Fig. 4H), which is consistent with KD efficiency (Fig. 4G). We speculated that the viability in WT cells relied on residual relative higher DDX19B expression, since DDX19B expression was already down-regulated in ARID1A KD or NF2 KO cells (Fig. 4F), and consistently, expression of DDX19B could remarkably rescue the lethality induced by DDX19A loss in ARID1A KD or NF2 KO cells (fig. S3, P and Q). Collectively, our data suggested a principle underlying SL, in which TSG loss may result in down-regulation/inactivation of one paralog in essential paralog gene pairs and make the other paralog essential. We anticipate that these findings may not be limited to TSGs and should be further exploited for the discovery of potential new therapeutic targets.
Targeting tumor suppressors in other tumor suppressor–deficient cancers
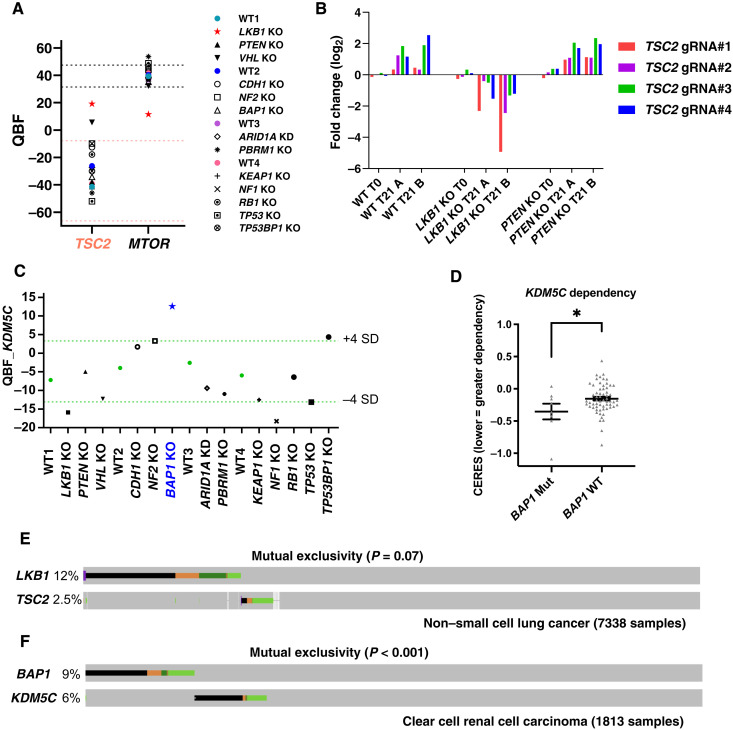
As we mentioned in Introduction, a specific TSG cannot be targeted in cancers that harbor loss-of-function mutations in that particular TSG. However, it may be possible to target TSGs in tumors that harbor loss-of-function mutations in other TSGs. For example, we showed an SL interaction between PTEN and ARID1A/SMARCA4, and this interaction could be further exploited. In addition, we also noticed that TSG TSC2 (tuberous sclerosis complex 2), a critical negative regulator of mTORC1 (50), was identified as an SL hit with STK11 (LKB1). As shown in Fig. 5A, QBF for TSC2 in LKB1 KO cells was nearly 20, which is usually considered an essential gene in any given background in 293A cells. Consistently, mTOR, which is essential for cell metabolism, growth, proliferation, and survival (51), turned out to be not that essential in LKB1 KO cells, when we compared them with WT and all other KO cells (Fig. 5A). Deep-sequencing results showed that levels of sgRNAs targeting TSC2 were dramatically decreased in LKB1 KO cells but increased in WT and PTEN KO cells (Fig. 5B). LKB1 is a master upstream kinase that is required for the activation of 5′ adenosine monophosphate-activated protein kinase (AMPK) (52) and all other AMPK-related kinase family members (53). LKB1 negatively regulates mTOR signaling via AMPK-mediated TSC2 (54, 55) and raptor (56) phosphorylation. Thus, cells with deficiency in both LKB1 and TSC2 may have higher mTOR activity than cells with TSC2 deficiency alone. Constitutive activation of mTOR in TSC2-deficient cells makes these cells sensitive to various cell death stimuli (57), such as glucose starvation (58). This susceptibility could be substantially enhanced by loss of LKB1 (52, 55), since LKB1 is a major regulator of metabolic flexibility in response to energy stress (59). The interplay between these processes may explain the SL interaction between LKB1 and TSC2, considering that energy stress may occur during screening, especially when cells reach confluence after 3 days of culture.
Fig. 5. Tumor suppressors could be potential anticancer targets in certain cancers.
(A) Relative essentiality of TSC2 and mTOR in 293A WT and TSG KO cells as shown by QBF. Light red dashed line indicates the range of the mean QBF_TSC2 from all four WT screens ± 3 × SD. Black dashed line indicates the range of the mean QBF_mTOR from all four WT screens ± 3 × SD. (B) gRNA fold changes of TSC2 in 293A WT, LKB1 KO, and PTEN KO cells. (C) KDM5C gene dependency in 293A WT and TSG KO cells as shown by QBF. Light green dashed line indicates the range of the mean QBF_KDM5C from all four WT screens ± 4 × SD. (D) KDM5C KO effects inferred by the CERES dependency scores were compared in 67 breast and kidney cancer cell lines grouped by BAP1 mutation status using data from the DepMap portal. Data are presented as means ± SEM and analyzed with an unpaired t test. *P < 0.05. (E) Oncoprint displaying mutational landscapes of LKB1 and TSC2 in patients with non–small cell lung cancer on cBioPortal. Copy number alterations were not included in this analysis. Alteration colors are the same as those shown in cBioPortal. Data were retrieved on 1 August 2021. (F) Oncoprint displaying mutational landscapes of BAP1 and KDM5C in patients with ccRCC on cBioPortal. Other conditions are the same as in (E).
Another example is KDM5C (lysine demethylase 5C, also known as JARID1C), a specific H3K4me3 and H3K4me2 demethylase that is frequently mutated and inactivated in clear cell renal cell carcinoma (ccRCC) (60, 61). KDM5C was identified as an SL hit with BAP1 (Fig. 5C). Consistently, a cancer genetic dependency study from the DepMap project also confirmed that the BAP1-mutant (but not BAP1 WT), kidney, and breast cancer cell lines were hyperdependent on KDM5C for survival (Fig. 5D). To further substantiate this potential SL interaction, we knocked down KDM5C using two independent sgRNAs in 293A WT and BAP1 KO cells (fig. S4A) and monitored cell growth with CellTiter-Glo assay daily. As shown in fig. S4B, KDM5C KD alone significantly diminished cell growth; similarly, BAP1 KO cells also grew much slower than WT cells, which was consistent with our previous result (Fig. 1C). Apparently, cells with these two gene deficiencies barely grow (fig. S4B), indicating the SL interaction between BAP1 and KDM5C in 293A cells. Similar results were observed in MCF7 cells (fig. S4, C and D). In addition, we knocked down KDM5C in EV- or BAP1-reconstituted UMRC6 cells (fig. S4E), a ccRCC cell line, and found that KDM5C KD impaired cell growth in UMRC6 EV cells but not in UMRC6 BAP1 cells (fig. S4F). KDM5C turned out to be more essential in a BAP1-deficient background compared with a BAP1-reconstituted background (fig. S4G), indicating an SL interaction between BAP1 and KDM5C in UMRC6 cells.
To determine whether the abovementioned SL interactions exist in patients with cancer, we checked The Cancer Genome Atlas (TCGA) data for somatic mutations targeting TSC2/LKB1 in non–small cell lung cancer and BAP1/KDM5C in ccRCC. As shown in Fig. 5 (E and F), LKB1 mutations tended to occur in a mutually exclusive pattern with TSC2 mutations, and the mutual exclusivity between BAP1 mutations and KDM5C mutations showed strong statistical significance. Together, our data suggest that targeting certain TSGs could be detrimental to tumors deficient in other TSGs.
Epistasis effects between tumor suppressors
We previously showed that algorithms designed to identify essential genes in pooled library dropout CRISPR screens are not suited for discovery of genes whose KO confers a fitness advantage (62). In that study, we developed an approach that estimates the fold change distribution of a genome-scale screen with a two-component Gaussian mixture model and then calculates a gene-level Z score based on the null phenotype component (Fig. 6A). This approach effectively discriminates proliferation suppressor genes, whose KO results in faster cell growth (strongly positive Z scores), from genes whose KO induces no appreciable deviation from the growth rate of the parental strain (Z scores near zero). We previously showed that virtually all proliferation suppressor genes in vitro were also tumor suppressors in vivo but that TSG KO showed variable phenotypic response across cell lines. For details, please see our previous paper (62).
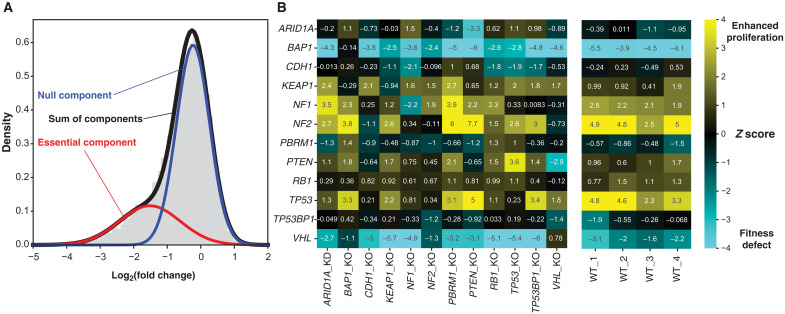
Fig. 6. Epistatic effects between TSG KOs.
(A) Calculating the Z score. The observed fold change distribution (gray histogram) is modeled as the weighted sum (black) of two Gaussian distributions. The mean and SD of the “null component” (blue), which models the fold change distribution of genes with no KO phenotype, are used to calculate a Z score for every gene. Negative Z scores indicate fitness defects (“essential genes”), and positive scores indicate faster proliferation upon gene KO. (B) Z scores of indicated TSG KOs (y axis) in TSG KO cell lines (x axis, left) or in replicate WT 293A cells (right).
We applied this mixture model Z-score approach to the parental and isogenic KO screens described here. Notably, two TSGs studied here (NF2 and TP53) showed strong proliferation suppressor phenotypes in WT 293A cells, and two others (BAP1 and VHL) showed degraded fitness (Fig. 6B). These data are consistent with the data available in DepMap, in which the mean CERES dependency scores were −0.93 for VHL and −0.45 for BAP1 but 0.14 for NF2 and 0.29 for TP53. In every case, targeting the TSG in the isogenic KO line showed no phenotype, consistent with robust loss of function in the KO cells (e.g., TP53 mean Z score in WT lines = 3.75 versus Z score = 0.23 in TP53 KO cells).
The TSG KOs show signs of epistatic effects. TP53, for example, retains its proliferation suppressor phenotype in PTEN KO cells, but loss of TP53 in CDH1, NF1, or NF2 KO cells confers no further fitness advantage. In contrast, NF2 Z score increases from a mean of 4.3 in WT cells to 7.7 in PTEN KO cells and 8.0 in PBRM1 KO cells, suggesting synergistic effects between NF2 and these two tumor suppressors (Fig. 6B). Consistent with this hypothesis, co-occurrence of mutations or copy loss in both PTEN and NF2 (P = 0.002, cBioPortal) and PBRM1 and NF2 (P < 0.001) is enriched in TCGA Pan Cancer Atlas studies.
DISCUSSION
Here, we performed whole-genome CRISPR screenings for a series of KO cells with specific TSG deficiencies and provided a useful and comparative resource that investigators can use with relatively strict or loose criteria for their own analysis, since gene essentiality may differ among different cell lines with the same TSG deficiency. The criteria we used revealed the common and unique vulnerabilities for each TSG KO in 293A cells. Mining the identified SL hits, we proposed one principle, essential paralogs could be one of the common mechanisms underlying SL, which merits further investigation and validation. Moreover, we uncovered that a TSG could be a potential anticancer target for cancers bearing another TSG deficiency, such as the SL relationship of TSC2 with STK11, KDM5C with BAP1, and SMARCA4 or ARID1A with PTEN. There are likely other meaningful implications waiting to be found on the basis of our dataset, which can help us better understand not only the cellular functions of TSGs but also how to target these deficiencies for cancer treatment.
There are a few limitations in our current study. First, screening with a monogenic CRISPR KO technique could not target all paralogs—which normally have a very similar function—at the same time, which means that one paralog can compensate for the functional consequence aroused from another paralog’s loss. As a result, these paralogs will barely show up as SL hits in any screens. For example, constitutive activation of Akt contributes to tumorigenesis in PTEN-deficient tumors (63), which should make Akt essential in PTEN KO screens. However, there are three Akt paralogs, Akt1, Akt2, and Akt3, which share a high degree of homology at the protein level but are encoded by three different genes. We observed the hyperactivation of Akt in PTEN KO cells (fig. S1F). However, none of these three Akt paralogs was identified as SL hit in PTEN KO cells, because the remaining two are likely to be sufficient in maintaining cell proliferation or survival when one paralog is knocked out in these cells. On the other hand, the relative contribution of each paralog varies with its expression among different cell lines. One may observe an SL relationship in certain cell lines when one of the paralogs is dominant and/or plays central functions.
A second limitation is that the status of most TSGs in tumors is not simply lost at the protein level as in the KO cells we generated with the CRISPR-Cas9 technique, which, in theory, can only mimic deletion and/or premature termination of translation from nonsense mutations or frameshift deletions. For most common missense mutations, there are still mutated proteins expressed in cells that may have residual activities or sometimes even new activities. Potential residual TSG functions may exist even in our CRISPR-mediated KO study. For example, the ARID1A KD cells that we generated harbor a 19–base pair deletion on early exons (fig. S1D), which should cause frameshift in its open reading frame. Consistently, we could not detect full-length ARID1A in these cells. However, new bands with smaller molecular weight were detected by an ARID1A antibody, which recognizes residues surrounding Gly1293 of human ARID1A protein (Fig. 1B). This observation raises the possibility that editing and/or frameshift mutations on early exons may result in the expression of other isoforms from downstream alternative translation initiation sites, which could partially restore ARID1A’s expression and function. This result may explain why ARID1B was not identified as an SL hit in our screen using ARID1A KD cells; thus, to avoid further confusion, we referred to these cells as ARID1A KD rather than ARID1A KO.
A third possible limitation is the inherent genetic variations in different cell lines, which make it difficult to identify strong SL partners conferred by one specific TSG deficiency. Large pan-cancer CRISPR screen datasets provide the opportunity to test possible associations between any given molecular feature and genetic dependency, which helped the identification of WRN helicase showing SL in microsatellite instability cancers (64). However, issues such as tissue-specific genetic dependency, limited numbers of cell lines with shared molecular features from the same tissue of origin, and unique genetic background in each cell line may significantly reduce SL hits because these genetic interactions may not have a high correlation coefficient or enough statistical power to draw reliable conclusions. This may partially explain the limited overlap between the hits we identified in 293A cells and those associated hits through Pearson correlation analysis via the DepMap portal.
Besides the limitations mentioned above, we also realized that the potential fitness from TSG loss may not manifest in vitro on cell proliferation or survival, especially under normal culture conditions, i.e., stress-free with sufficient nutrition/oxygen. Together, our results suggest that future endeavors on the identification of genetic vulnerabilities for cancers with a specific TSG deficiency should focus on cancer cells from the same tissue of origin that has the prevalent TSG alteration and, if possible, under relevant physiological or pathological in vivo settings.
MATERIALS AND METHODS
Cell culture
The HEK 293A cell line was purchased from Thermo Fisher Scientific and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum. PC-3 was purchased from the American Type Culture Collection (ATCC) and cultured in RPMI 1640 medium with 10% fetal bovine serum. MDA-MB-468 was obtained from ATCC and cultured in DMEM medium with 10% fetal bovine serum. All the cell lines were tested to verify that they were free of mycoplasma contamination.
Antibodies
Antibodies for ARID1A (#12354S), BAP1 (#13271S), CDH1 (#3195T), NF1 (#14623S), NF2 (#12888S), PBRM1 (#91894S), PTEN (#9188T), RB1 (#9309S), STK11 (#3050S), VHL (#68547S), TP53BP1 (#4937S), AKT (pan) (#4691S), phospho-AKT (Ser473) (#4060S), BRG1 (#49360), and HIF-1α (#14179) were purchased from Cell Signaling Technology. β-Actin (A5441), vinculin (V9264), and α-tubulin (T6199) antibodies were obtained from Sigma-Aldrich. Antibodies for KEAP1 (sc-365626) and TP53 (sc-126) were obtained from Santa Cruz Biotechnology. Anti-Nrf2 antibody (ab62352) was purchased from Abcam. DDX19 antibody (A300-547A), JARID1C/KDM5C antibody (A301-035A), and SMARCB1 antibody (A301-087A) were obtained from Bethyl Laboratories.
Immunoblotting
Cells were washed once with phosphate-buffered saline (PBS) and then directly lysed in 1× SDS sample buffer [50 mM tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 4% β-mercaptoethanol, and 0.025% bromophenol blue] and denatured by heating to 99°C for 10 min as previously described (65). Immunoblotting was performed in SDS–polyacrylamide gel electrophoresis according to the standard protocol.
Plasmids and lentivirus infection
pENTR221-PTEN plasmid was purchased from Horizon and transferred to pLenti cytomegalovirus (CMV) Neo DEST (705-1) vector through Gateway LR reaction. To stably express PTEN in MDA-MB-468 and PC-3 cells, lentiviruses carrying PTEN gene in pLenti CMV Neo DEST (705-1) vector were produced in HEK293T cells using pMD2.g and psPAX2 as packaging plasmids. The virus supernatant was filtered through a 0.45-μm filter and used to infect targeting cells in the presence of polybrene (8 μg/ml) as previously described (65). Stable cell pools were selected with G418 (600 μg/ml; Gemini Bio-Products) for 5 days before any further experiments.
Gene KO with CRISPR-Cas9
gRNAs against target genes were designed by Synthego and annealed into digested LentiCRISPRv2 plasmid according to the standard protocol. Cells were transfected and selected with puromycin (2 μg/ml) for 2 days after transfection, and single clones were sorted (if needed) and picked up for further verification by immunoblotting and Sanger DNA sequencing (fig. S1D). The gRNA sequences used for generating KO clones in this study were as follows: ARID1A-sgRNA: GAAAGCGAGGGCCCCGCCGT; BAP1-sgRNA: TCAAATGGATCGAAGAGCGC; CDH1-sgRNA: CGCCGAGAGCTACACGTTCA; KEAP1-sgRNA: GTTACGGGGCACGCTCATGG; NF1-sgRNA: CTCGTCGAAGCGGCTGACCA; NF2-sgRNA: GAACTCCATCTCGGCGTCCA; STK11-sgRNA: CCTCGGTGGAGTCGATGCGG; PTEN-sgRNA: ACAGATTGTATATCTTGTAA; PBRM1-sgRNA: TCAACCAGACTATTATGAAG; RB1-sgRNA: ATCATGTCAGAGAGAGAGCT; TP53-sgRNA: ACTTCCTGAAAACAACGTTC; TP53BP1-sgRNA: GGAAAGACTGTCCTGAACAA; VHL-sgRNA#1: CATACGGGCAGCACGACGCG. The gRNA sequences used for validation experiments in this study were as follows: DDX19A-sgRNA#1: GGAGGGTCGATTGAAGCCCA; DDX19A-sgRNA#2: TGTAATTACAGCATACACTG; DDX19B-sgRNA#1: GAAGTACCCACCTCACAGA; DDX19B-sgRNA#2: TGCTCAGCCGCGATGGCTG; SMARCB1-sgRNA: GAAGCCCGTGAAGTTCCAGC; SMARCA4-sgRNA: GCATGCTCAGAGCCACCCAG; ARID1A-sgRNA: TTTGTAGCCATCCAGTCCAA; KDM5C-sgRNA#1: AGATTCCCAATGTAGAACGG; KDM5C-sgRNA#2: CTGGCAGCCACCCTTTGCTG; AAVS1-sgRNA#1: TGTTAGGCAGATTCCTTATC; AAVS1-sgRNA#2: CCCCGTCGTTCCTGGCCCT.
Genome-wide CRISPR KO screens and data analysis
Eighty million 293A WT or TSG KO cells were infected with the lentiviral TKOv3 library at an MOI of ~0.25 to keep every gRNA representation in at least ~250 cells; 24 hours after infection, cells were selected with puromycin (2 μg/ml) for 72 hours. Next, 20 million cells were collected from the pool as T0 samples, and the rest were split into two replicates, passaged every 3 days for 21 days with at least 200-fold coverage maintained during the whole procedure. At day 21, 20 million cells were collected as T21 samples. Genomic DNA was extracted from T0 and T21 samples using the QIAamp Blood Maxi Kit (Qiagen) and polymerase chain reaction (PCR)–amplified with primers harboring Illumina TruSeq adapters with i5 and i7 barcodes. The resulting libraries were sequenced using the Illumina NextSeq 500 High Output platform to determine gRNA representations.
Raw reads from next-generation sequencing were processed as previously reported (29). The gRNA read counts were further analyzed with the BAGEL algorithm (66) to calculate gene-level fitness scores (log BF), where positive scores indicate higher confidence of gene essentiality and negative scores indicate no fitness effect from knocking out that gene. Beyond relying on a continuous range of essentiality scores, we also used an alternative approach that depends on the probabilistic framework behind the BAGEL algorithm. In this method, log BFs are converted to posterior log odds of essentiality by multiplying by a ratio of informative priors. Briefly, instead of using a flat prior ratio for all BF calculations in BAGEL, we used the bin-wise false discovery rates described previously (67), which were calculated from modeling of hundreds of cell lines from the cancer dependency map to update the prior ratio as observations were made from the screens. For each gene, we first ranked the screens from the highest BF to lowest. Then, starting with the top cell line, for each screen, we used the log prior ratios from the bin-wise false discovery rates and evaluated the posterior probability of essentiality for that gene because the prior was now based on the gene having been observed in the previous screen as essential. Last, we assigned a binary essentiality call of 1, indicating an essential gene, if the gene had a posterior log odds ratio ≥ 7, which corresponds to a posterior probability of essentiality of ~99%, or 0 otherwise. In addition, the DrugZ algorithm (68) was used to compare the difference between WT_T21 samples and TSG KO_T21 samples from the same batch, which provides gene-level normZ scores with statistical significance. A more negative normZ score indicates more gRNA depletion in the treated group (TSG KO_T21) compared with the control group (WT_T21).
Z-score analysis of proliferation suppressor genes
The fold changes of all guides targeting a gene across all replicates of a cell line screen were averaged into a gene-level fold change. The distribution of all gene-level fold changes in a sample (n = 18,053 human protein-coding genes in TKOv3) was estimated using a two-component Gaussian mixture model using sklearn.mixture.GaussianMixture. For two one-dimensional Gaussians, this fit returns the means, variances, and weights of the two components. The component with the largest weight is assumed to be the component that models genes with no KO fitness phenotype (Fig. 6A, blue curve). We then took the mean μ and variance σ2 of this component and calculated a gene-level Z score according to the standard formula
In this approach, positive Z scores indicate genes whose KO leads to increased proliferation, consistent with TSGs.
Cell growth measurement
For this experiment, 1 × 103 293A WT cells or TSG KO cells were seeded in 96-well plates with six replicates, and the confluence was monitored every 4 hours with the Incucyte S3 Live-Cell Analysis System and maintained for 6 days. For the CellTiter-Glo Luminescent Cell Viability Assay, cells with indicated treatments and numbers were seeded in a 96-well plate with four to six replicates as indicated, and luminescence detection was performed at indicated time points according to the standard protocol using a BioTek Synergy 2 Multi-Mode microplate reader.
RNA extraction and reverse transcriptase quantitative PCR
As previously described (65), WT or PTEN-reconstituted cells were washed with cold PBS, and total RNA was isolated using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. Next, 1 μg of RNA was reverse-transcribed to complementary DNA (cDNA) using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, 4368814) according to the manufacturer’s instructions. cDNA was then diluted and subjected to real-time PCR with gene-specific primers using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) and the 7500 real-time PCR system (Applied Biosystems). The primer pairs used in this study were as follows: β-actin, GCCGACAGGATGCAGAAGGAGATCA/AAGCATTTGCGGTGGACGATGGA; ARID1B#1, CGATAGGCTCAGCTAGTCGT/GCTGTGTGCTTTCATGGTGA; ARID1B#2, TGCGTCCCCTCATCTCTCCA/AGGCATCTGACTACCTGGGA.
Statistical analysis
All data were plotted as mean with SEM or with SD as indicated, and statistical analysis was performed with unpaired t test or one-way analysis of variance (ANOVA) as indicated.
Acknowledgments
We thank all members of the Chen laboratory for help and constructive discussion. We thank MD Anderson’s Science Park Next-Generation Sequencing Facility for help with CRISPR library NGS sequencing (supported by MD Anderson’s NIH Cancer Center Support Grant, P30CA016672). Editorial support was provided by B. Tutt, Scientific Editor, Research Medical Library at MD Anderson.
Funding: This work was supported in part by institutional funds and the Pamela and Wayne Garrison Distinguished Chair in Cancer Research. J.C. also received support from Cancer Prevention and Research Institute of Texas (CPRIT; RP160667 and RP180813) and NIH/NCI (CA193124, CA210929, CA216911, and CA216437). T.H. received support from NIH (R35GM130119). T.H. is also an Andrew Sabin Family Fellow and a CPRIT Scholar in Cancer Research. Z.Z. received support from CPRIT (RP180734).
Author contributions: X.F. and J.C. conceived the project and wrote the manuscript. X.F. and M.T. performed most of the experiments with assistance from G.P., D.J., C.W., Z.C., M.L., L.N., Y.X., S.L., J.-M.P., H.Z., M.H., and K.S. G.P., Z.Z., and D.J. analyzed RNA-seq data. M.D. and D.S. analyzed all CRISPR screening data with supervision from T.H.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The data and code used in this study can be found at figshare (https://doi.org/10.6084/m9.figshare.19398332.v1). All the KO cell lines generated in this study can be provided by J.C. pending scientific review and a completed material transfer agreement. Requests for the cell lines should be submitted to JChen8@mdanderson.org.
Supplementary Materials
This PDF file includes:
Figs. S1 to S4
Other Supplementary Material for this manuscript includes the following:
Tables S1 to S7
REFERENCES AND NOTES
- 1.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Mok T. S., Wu Y. L., Thongprasert S., Yang C. H., Chu D. T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., Nishiwaki Y., Ohe Y., Yang J. J., Chewaskulyong B., Jiang H., Duffield E. L., Watkins C. L., Armour A. A., Fukuoka M., Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., Fujita Y., Okinaga S., Hirano H., Yoshimori K., Harada T., Ogura T., Ando M., Miyazawa H., Tanaka T., Saijo Y., Hagiwara K., Morita S., Nukiwa T., Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Shaw A. T., Kim D. W., Nakagawa K., Seto T., Crinó L., Ahn M. J., de Pas T., Besse B., Solomon B. J., Blackhall F., Wu Y. L., Thomas M., O’Byrne K. J., Moro-Sibilot D., Camidge D. R., Mok T., Hirsh V., Riely G. J., Iyer S., Tassell V., Polli A., Wilner K. D., Jänne P. A., Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Solomon B. J., Mok T., Kim D. W., Wu Y. L., Nakagawa K., Mekhail T., Felip E., Cappuzzo F., Paolini J., Usari T., Iyer S., Reisman A., Wilner K. D., Tursi J., Blackhall F., First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Bollag G., Hirth P., Tsai J., Zhang J., Ibrahim P. N., Cho H., Spevak W., Zhang C., Zhang Y., Habets G., Burton E. A., Wong B., Tsang G., West B. L., Powell B., Shellooe R., Marimuthu A., Nguyen H., Zhang K. Y. J., Artis D. R., Schlessinger J., Su F., Higgins B., Iyer R., D’Andrea K., Koehler A., Stumm M., Lin P. S., Lee R. J., Grippo J., Puzanov I., Kim K. B., Ribas A., McArthur G. A., Sosman J. A., Chapman P. B., Flaherty K. T., Xu X., Nathanson K. L., Nolop K., Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman P. B., Hauschild A., Robert C., Haanen J. B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., Hogg D., Lorigan P., Lebbe C., Jouary T., Schadendorf D., Ribas A., O’Day S. J., Sosman J. A., Kirkwood J. M., Eggermont A. M., Dreno B., Nolop K., Li J., Nelson B., Hou J., Lee R. J., Flaherty K. T., McArthur G.; BRIM-3 Study Group , Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neil N. J., Bailey M. L., Hieter P., Synthetic lethality and cancer. Nat. Rev. Genet. 18, 613–623 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Fong P. C., Boss D. S., Yap T. A., Tutt A., Wu P., Mergui-Roelvink M., Mortimer P., Swaisland H., Lau A., O’Connor M. J., Ashworth A., Carmichael J., Kaye S. B., Schellens J. H. M., de Bono J. S., Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Farmer H., McCabe N., Lord C. J., Tutt A. N. J., Johnson D. A., Richardson T. B., Santarosa M., Dillon K. J., Hickson I., Knights C., Martin N. M. B., Jackson S. P., Smith G. C. M., Ashworth A., Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Helming K. C., Wang X., Wilson B. G., Vazquez F., Haswell J. R., Manchester H. E., Kim Y., Kryukov G. V., Ghandi M., Aguirre A. J., Jagani Z., Wang Z., Garraway L. A., Hahn W. C., Roberts C. W. M., ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat. Med. 20, 251–254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitler B. G., Aird K. M., Garipov A., Li H., Amatangelo M., Kossenkov A. V., Schultz D. C., Liu Q., Shih I. M., Conejo-Garcia J. R., Speicher D. W., Zhang R., Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat. Med. 21, 231–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K. H., Kim W., Howard T. P., Vazquez F., Tsherniak A., Wu J. N., Wang W., Haswell J. R., Walensky L. D., Hahn W. C., Orkin S. H., Roberts C. W. M., SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat. Med. 21, 1491–1496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao D., Lu X., Wang G., Lan Z., Liao W., Li J., Liang X., Chen J. R., Shah S., Shang X., Tang M., Deng P., Dey P., Chakravarti D., Chen P., Spring D. J., Navone N. M., Troncoso P., Zhang J., Wang Y. A., DePinho R. A., Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer. Nature 542, 484–488 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y., Li N., Dong B., Guo W., Wei H., Chen Q., Yuan H., Han Y., Chang H., Kan S., Wang X., Pan Q., Wu P., Peng C., Qiu T., Li Q., Gao D., Xue W., Qin J., Chromatin remodeling ATPase BRG1 and PTEN are synthetic lethal in prostate cancer. J. Clin. Invest. 129, 759–773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyers R. M., Bryan J. G., McFarland J. M., Weir B. A., Sizemore A. E., Xu H., Dharia N. V., Montgomery P. G., Cowley G. S., Pantel S., Goodale A., Lee Y., Ali L. D., Jiang G., Lubonja R., Harrington W. F., Strickland M., Wu T., Hawes D. C., Zhivich V. A., Wyatt M. R., Kalani Z., Chang J. J., Okamoto M., Stegmaier K., Golub T. R., Boehm J. S., Vazquez F., Root D. E., Hahn W. C., Tsherniak A., Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet. 49, 1779–1784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behan F. M., Iorio F., Picco G., Gonçalves E., Beaver C. M., Migliardi G., Santos R., Rao Y., Sassi F., Pinnelli M., Ansari R., Harper S., Jackson D. A., McRae R., Pooley R., Wilkinson P., van der Meer D., Dow D., Buser-Doepner C., Bertotti A., Trusolino L., Stronach E. A., Saez-Rodriguez J., Yusa K., Garnett M. J., Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 568, 511–516 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Wang C., Wang G., Feng X., Shepherd P., Zhang J., Tang M., Chen Z., Srivastava M., McLaughlin M. E., Navone N. M., Hart G. T., Chen J., Genome-wide CRISPR screens reveal synthetic lethality of RNASEH2 deficiency and ATR inhibition. Oncogene 38, 2451–2463 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M., Feng X., Su D., Wang G., Wang C., Tang M., Paulucci-Holthauzen A., Hart T., Chen J., Genome-wide CRISPR screen uncovers a synergistic effect of combining Haspin and Aurora kinase B inhibition. Oncogene 39, 4312–4322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirza-Aghazadeh-Attari M., Mohammadzadeh A., Yousefi B., Mihanfar A., Karimian A., Majidinia M., 53BP1: A key player of DNA damage response with critical functions in cancer. DNA Repair 73, 110–119 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Ward I. M., Minn K., van Deursen J., Chen J., p53 binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol. Cell. Biol. 23, 2556–2563 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Vega F., Mina M., Armenia J., Chatila W. K., Luna A., La K. C., Dimitriadoy S., Liu D. L., Kantheti H. S., Saghafinia S., Chakravarty D., Daian F., Gao Q., Bailey M. H., Liang W.-W., Foltz S. M., Shmulevich I., Ding L., Heins Z., Ochoa A., Gross B., Gao J., Zhang H., Kundra R., Kandoth C., Bahceci I., Dervishi L., Dogrusoz U., Zhou W., Shen H., Laird P. W., Way G. P., Greene C. S., Liang H., Xiao Y., Wang C., Iavarone A., Berger A. H., Bivona T. G., Lazar A. J., Hammer G. D., Giordano T., Kwong L. N., Arthur G. M., Huang C., Tward A. D., Frederick M. J., Cormick F. M., Meyerson M.; Cancer Genome Atlas Research Network, Van Allen E. M., Cherniack A. D., Ciriello G., Sander C., Schultz N., Oncogenic signaling pathways in the cancer genome atlas. Cell 173, 321–337.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stambolic V., Suzuki A., de la Pompa J. L., Brothers G. M., Mirtsos C., Sasaki T., Ruland J., Penninger J. M., Siderovski D. P., Mak T. W., Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J., Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin Jr. W. G., HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292, 464–468 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J., The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi A., Kang M. I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M., Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24, 7130–7139 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart T., Tong A. H. Y., Chan K., Van Leeuwen J., Seetharaman A., Aregger M., Chandrashekhar M., Hustedt N., Seth S., Noonan A., Habsid A., Sizova O., Nedyalkova L., Climie R., Tworzyanski L., Lawson K., Sartori M. A., Alibeh S., Tieu D., Masud S., Mero P., Weiss A., Brown K. R., Usaj M., Billmann M., Rahman M., Constanzo M., Myers C. L., Andrews B. J., Boone C., Durocher D., Moffat J., Evaluation and design of genome-wide CRISPR/SpCas9 knockout screens. G3 7, 2719–2727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart T., Chandrashekhar M., Aregger M., Steinhart Z., Brown K. R., MacLeod G., Mis M., Zimmermann M., Fradet-Turcotte A., Sun S., Mero P., Dirks P., Sidhu S., Roth F. P., Rissland O. S., Durocher D., Angers S., Moffat J., High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell 163, 1515–1526 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Wyatt D. W., Feng W., Conlin M. P., Yousefzadeh M. J., Roberts S. A., Mieczkowski P., Wood R. D., Gupta G. P., Ramsden D. A., Essential roles for polymerase θ-mediated end joining in the repair of chromosome breaks. Mol. Cell 63, 662–673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng W., Simpson D. A., Carvajal-Garcia J., Price B. A., Kumar R. J., Mose L. E., Wood R. D., Rashid N., Purvis J. E., Parker J. S., Ramsden D. A., Gupta G. P., Genetic determinants of cellular addiction to DNA polymerase theta. Nat. Commun. 10, 4286 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews C. K., Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat. Rev. Cancer 15, 528–539 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Kasarello K., Cudnoch-Jedrzejewska A., Czlonkowski A., Mirowska-Guzel D., Mechanism of action of three newly registered drugs for multiple sclerosis treatment. Pharmacol. Rep. 69, 702–708 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Haghikia A., Gold R., Multiple sclerosis: TOWER confirms the efficacy of oral teriflunomide in MS. Nat. Rev. Neurol. 10, 183–184 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Sykes D. B., Kfoury Y. S., Mercier F. E., Wawer M. J., Law J. M., Haynes M. K., Lewis T. A., Schajnovitz A., Jain E., Lee D., Meyer H., Pierce K. A., Tolliday N. J., Waller A., Ferrara S. J., Eheim A. L., Stoeckigt D., Maxcy K. L., Cobert J. M., Bachand J., Szekely B. A., Mukherjee S., Sklar L. A., Kotz J. D., Clish C. B., Sadreyev R. I., Clemons P. A., Janzer A., Schreiber S. L., Scadden D. T., Inhibition of dihydroorotate dehydrogenase overcomes differentiation blockade in acute myeloid leukemia. Cell 167, 171–186.e15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H., Pak H., Hammond-Martel I., Ghram M., Rodrigue A., Daou S., Barbour H., Corbeil L., Hébert J., Drobetsky E., Masson J. Y., di Noia J. M., Affar E. B., Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl. Acad. Sci. U.S.A. 111, 285–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Harn T., Foijer F., van Vugt M., Banerjee R., Yang F., Oostra A., Joenje H., te Riele H., Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 24, 1377–1388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thoma C. R., Toso A., Gutbrodt K. L., Reggi S. P., Frew I. J., Schraml P., Hergovich A., Moch H., Meraldi P., Krek W., VHL loss causes spindle misorientation and chromosome instability. Nat. Cell Biol. 11, 994–1001 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Shen J., Peng Y., Wei L., Zhang W., Yang L., Lan L., Kapoor P., Ju Z., Mo Q., Shih I. M., Uray I. P., Wu X., Brown P. H., Shen X., Mills G. B., Peng G., ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 5, 752–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiang L., Shah P., Barcellos-Hoff M. H., He Y. Y., TGF-β signaling links E-cadherin loss to suppression of nucleotide excision repair. Oncogene 35, 3293–3302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puc J., Keniry M., Li H. S., Pandita T. K., Choudhury A. D., Memeo L., Mansukhani M., Murty V. V. V. S., Gaciong Z., Meek S. E. M., Piwnica-Worms H., Hibshoosh H., Parsons R., Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 7, 193–204 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Negrini S., Gorgoulis V. G., Halazonetis T. D., Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Metcalf J. L., Bradshaw P. S., Komosa M., Greer S. N., Stephen Meyn M., Ohh M., K63-ubiquitylation of VHL by SOCS1 mediates DNA double-strand break repair. Oncogene 33, 1055–1065 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B., Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc. Natl. Acad. Sci. U.S.A. 89, 7491–7495 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espana-Agusti J., Warren A., Chew S. K., Adams D. J., Matakidou A., Loss of PBRM1 rescues VHL dependent replication stress to promote renal carcinogenesis. Nat. Commun. 8, 2026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittal P., Roberts C. W. M., The SWI/SNF complex in cancer—Biology, biomarkers and therapy. Nat. Rev. Clin. Oncol. 17, 435–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelso T. W. R., Porter D. K., Amaral M. L., Shokhirev M. N., Benner C., Hargreaves D. C., Chromatin accessibility underlies synthetic lethality of SWI/SNF subunits in ARID1A-mutant cancers. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson N. A., Ranzani M., van der Weyden L., Iyer V., Offord V., Droop A., Behan F., Gonçalves E., Speak A., Iorio F., Hewinson J., Harle V., Robertson H., Anderson E., Fu B., Yang F., Zagnoli-Vieira G., Chapman P., del Castillo Velasco-Herrera M., Garnett M. J., Jackson S. P., Adams D. J., Combinatorial CRISPR screen identifies fitness effects of gene paralogues. Nat. Commun. 12, 1302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dede M., McLaughlin M., Kim E., Hart T., Multiplex enCas12a screens detect functional buffering among paralogs otherwise masked in monogenic Cas9 knockout screens. Genome Biol. 21, 262 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoki K., Li Y., Zhu T., Wu J., Guan K. L., TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Laplante M., Sabatini D. M., mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C., The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R., LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw R. J., Bardeesy N., Manning B. D., Lopez L., Kosmatka M., DePinho R. A., Cantley L. C., The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91–99 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Corradetti M. N., Inoki K., Bardeesy N., DePinho R. A., Guan K. L., Regulation of the TSC pathway by LKB1: Evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 18, 1533–1538 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J., AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng S., Wu Y. T., Chen B., Zhou J., Shen H. M., Impaired autophagy due to constitutive mTOR activation sensitizes TSC2-null cells to cell death under stress. Autophagy 7, 1173–1186 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Lee C. H., Inoki K., Karbowniczek M., Petroulakis E., Sonenberg N., Henske E. P., Guan K. L., Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 26, 4812–4823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parker S. J., Svensson R. U., Divakaruni A. S., Lefebvre A. E., Murphy A. N., Shaw R. J., Metallo C. M., LKB1 promotes metabolic flexibility in response to energy stress. Metab. Eng. 43, 208–217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gossage L., Murtaza M., Slatter A. F., Lichtenstein C. P., Warren A., Haynes B., Marass F., Roberts I., Shanahan S. J., Claas A., Dunham A., May A. P., Rosenfeld N., Forshew T., Eisen T., Clinical and pathological impact of VHL, PBRM1, BAP1, SETD2, KDM6A, and JARID1c in clear cell renal cell carcinoma. Genes Chromosomes Cancer 53, 38–51 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Dalgliesh G. L., Furge K., Greenman C., Chen L., Bignell G., Butler A., Davies H., Edkins S., Hardy C., Latimer C., Teague J., Andrews J., Barthorpe S., Beare D., Buck G., Campbell P. J., Forbes S., Jia M., Jones D., Knott H., Kok C. Y., Lau K. W., Leroy C., Lin M. L., McBride D. J., Maddison M., Maguire S., McLay K., Menzies A., Mironenko T., Mulderrig L., Mudie L., O’Meara S., Pleasance E., Rajasingham A., Shepherd R., Smith R., Stebbings L., Stephens P., Tang G., Tarpey P. S., Turrell K., Dykema K. J., Khoo S. K., Petillo D., Wondergem B., Anema J., Kahnoski R. J., Teh B. T., Stratton M. R., Futreal P. A., Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463, 360–363 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lenoir W. F., Morgado M., DeWeirdt P. C., McLaughlin M., Griffith A. L., Sangree A. K., Feeley M. N., Esmaeili Anvar N., Kim E., Bertolet L. L., Colic M., Dede M., Doench J. G., Hart T., Discovery of putative tumor suppressors from CRISPR screens reveals rewired lipid metabolism in acute myeloid leukemia cells. Nat. Commun. 12, 6506 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carnero A., Blanco-Aparicio C., Renner O., Link W., Leal J. F., The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 8, 187–198 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Chan E. M., Shibue T., McFarland J. M., Gaeta B., Ghandi M., Dumont N., Gonzalez A., McPartlan J. S., Li T., Zhang Y., Bin Liu J., Lazaro J. B., Gu P., Piett C. G., Apffel A., Ali S. O., Deasy R., Keskula P., Ng R. W. S., Roberts E. A., Reznichenko E., Leung L., Alimova M., Schenone M., Islam M., Maruvka Y. E., Liu Y., Roper J., Raghavan S., Giannakis M., Tseng Y. Y., Nagel Z. D., D’Andrea A., Root D. E., Boehm J. S., Getz G., Chang S., Golub T. R., Tsherniak A., Vazquez F., Bass A. J., WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature 568, 551–556 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng X., Tubbs A., Zhang C., Tang M., Sridharan S., Wang C., Jiang D., Su D., Zhang H., Chen Z., Nie L., Xiong Y., Huang M., Nussenzweig A., Chen J., ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J. 39, e104036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim E., Hart T., Improved analysis of CRISPR fitness screens and reduced off-target effects with the BAGEL2 gene essentiality classifier. Genome Med. 13, 2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dede M., Kim E., Hart T., Biases and blind-spots in genome-wide CRISPR knockout screens. bioRxiv , 2020.01.16.909606 (2020). [Google Scholar]
- 68.Colic M., Wang G., Zimmermann M., Mascall K., McLaughlin M., Bertolet L., Lenoir W. F., Moffat J., Angers S., Durocher D., Hart T., Identifying chemogenetic interactions from CRISPR screens with drugZ. Genome Med. 11, 52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou G., Soufan O., Ewald J., Hancock R. E. W., Basu N., Xia J., NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 47, W234–W241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., Tsafou K. P., Kuhn M., Bork P., Jensen L. J., von Mering C., STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao Y., Wang J., Jaehnig E. J., Shi Z., Zhang B., WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S4
Tables S1 to S7