Abstract
Objectives
Determine whether there are different longitudinal patterns of treatment burden in people living with multiple chronic conditions (MCC) and, if so, explore predictors that might reveal potential routes of intervention.
Methods
We analyzed data from a prospective mailed survey study of 396 adults living with MCC in southeastern Minnesota, USA. Participants completed a measure of treatment burden, the Patient Experience with Treatment and Self-management (PETS), and valid measures of health-related and psycho-social concepts at baseline, 6, 12, and 24 months. Latent class growth mixture modeling (LCGM) determined trajectories of treatment burden in two summary index scores of the PETS: Workload and Impact. Multivariable logistic regressions were used to identify independent predictors of the trajectories.
Results
LCGM supported a 2-class model for PETS Workload, including a group of consistently high workload (N = 69) and a group of consistently low workload (N = 311) over time. A 3-class model was supported for PETS Impact, including groups of consistently high impact (N = 62), consistently low impact (N = 278), and increasing impact (N = 51) over time. Logistic regression analyses showed that the following factors were associated with patterns of consistently high or increasing treatment burden over time: lower health literacy, lower self-efficacy, more interpersonal challenges with others, and worse subjective reports of physical and mental health (all p < .05).
Conclusions
Different longitudinal patterns of treatment burden exist among people with MCC. Raising health literacy, enhancing self-efficacy, and lessening the effects of negative social interactions might help reduce treatment burden.
Keywords: Burden of treatment, multimorbidity, latent class growth model, social determinants
Introduction
Many people with multiple chronic conditions (MCC) must seamlessly integrate complex treatment and self-care into their lives. The “workload” involved with self-managing chronic conditions (e.g., understanding medical information, taking medications, maintaining medical appointments, monitoring health) and its “impact” on quality of life (e.g., participating in social roles and activities) are key elements of treatment burden.1,2 Treatment burden is the work of self-management, the impact of this work on functioning and well-being, and stressors that exacerbate burden like medical financial concerns and challenges interacting with the healthcare system.3,4 Today, new patient-reported measures are making it easier to study treatment burden in people with multimorbidity.1,5–8 Still, little is known about if and how treatment burden fluctuates over time as well as whether there are different patterns of fluctuation across people with MCC. Knowing this could have implications for intervention, including whether it is critical to intervene upon treatment burden at all and if so, who might benefit from intervention.
Numerous correlative studies have shown treatment burden to be associated with a host of adverse consequences, including worse adherence to medical regimens,7,9–11 problems navigating healthcare systems,12,13 and poorer health-related quality of life.1,5,6,14 The importance of attending to treatment burden may hinge on the degree to which it persists over time and the extent to which it can be modified. If fleeting, then treatment burden would not be expected to have significant long-term impacts on clinical or person-centered outcomes and would therefore be less important to directly intervene upon to address known long-term outcomes. However, if enduring, treatment burden could act to catalyze a negative spiral of consequences such as lower adherence to medical regimens and exacerbations of health conditions, ultimately resulting in poorer clinical and person-centered outcomes.3,15
We attempt to address these questions by analyzing data from a prospective observational survey study to explore whether different longitudinal patterns of treatment burden exist in people with MCC. If found to exist, we will then identify what (if any) factors might predict these patterns to clarify potential indicators of problematic burden and possible targets for future intervention. Recent evidence from small-scale studies of certain individual chronic conditions like diabetes and cystic fibrosis suggests that treatment burden may be modifiable. 16
Methods
Design
A prospective, observational survey study was conducted with adults living with MCC in the midwestern United States. Surveys were administered at a baseline (first) assessment and at 6, 12, and 24 months post-baseline. The aim of the survey study was to examine the prospective validity of a recently developed patient-reported measure of treatment burden. We report here on a secondary analysis of these data. Findings of the original validation study are available elsewhere.1,2
Setting
The resources of the Rochester Epidemiology Project (REP) research infrastructure 17 helped to identify a community sample of English-speaking adults (of at least 20 years of age) with MCC living in Olmsted County in southeastern Minnesota, USA between 1 July 2015 and 30 June 2016. The REP links the medical records of local health care providers for virtually the entire Olmsted County population; hence, it can serve as a sampling frame for residents of the region. 18
Participants
Surveys were mailed to a random sample of 1496 persons with MCC stratified by age (20–49, 50–64, 65+), race (white, non-white), number of medical-record confirmed diagnosed conditions (2–3, 4+), and presence/absence of at least one incident condition diagnosed within the past year. Those eligible for the study had received an International Classification of Diseases (ICD) diagnostic code from a health care provider for two or more of 27 chronic conditions including conditions identified by the Department of Health and Human Services as public health priorities of the nation.19,20 Persons with ICD codes for anxiety, hearing problems, vision problems, irritable bowel/Crohn’s disease, atopic dermatitis/psoriasis, back problems, or headaches were also included as these conditions were identified by clinical co-investigators as having high treatment burden. The rationale for the participant selection criteria was to obtain a sample diverse in number and types of chronic conditions, age, and race for the purpose of prospective validation of a measure of treatment burden. Change in treatment burden was expected for some participants in line with hypotheses of the original validation study (see Eton et al., 2020). 1 Those with severe cognitive impairments (e.g., dementia, Alzheimer’s, stroke) or severe mental illness (e.g., psychotic disorder) were excluded.
Survey
A survey booklet, cover letter, and a privacy authorization form Health Insurance Portability and Accountability Act (HIPAA) were mailed at baseline to the eligible sample. All baseline responders confirmed alive were subsequently mailed a follow-up survey at 6, 12, and 24 months post-baseline. At each assessment, two repeat mailings were sent to those not responding within 3 weeks of the initial mailing, with a phone call reminder preceding the final mailing. Participants were compensated US$10 for each completed survey.
The primary outcomes of the study were two aggregate scores of treatment burden derived from the Patient Experience with Treatment and Self-management (PETS), version 2.0. The PETS is a multi-domain measure of patient perceived treatment burden that has been shown to be valid, reliable, and responsive to change in people with MCC.1,2,7 It is based on a conceptual framework derived from input from people with MCC and triangulated against the content of other chronic disease measures.3,4,21 This framework also served as conceptual foundation for another self-report measure of treatment burden. 6 In this study, the Workload and Impact aggregate summary scores from the PETS were used. The Workload score is an aggregated summary of the scores of four PETS domain scales: medical information, taking medications, medical appointments, and monitoring health. These four scales measure the difficulty performing the work associated with treatment and self-management. The Impact score is an aggregated summary of the scores of the PETS role/social activity limitations and physical/mental exhaustion scales. These two scales measure the personal impact of treatment and self-management on well-being. The Workload and Impact indices have been psychometrically supported as second-order (i.e., higher-level) dimensions of treatment burden in recent confirmatory factor analyses. 2 Scores of these indices demonstrate evidence of known-groups validity and responsiveness to change. 1 We chose to focus on these two summary scores instead of the six individual domain scales to reduce the number and complexity of the analyses. Workload and impact represent two fundamental components of treatment burden.3,4,22 PETS scales assessing other external challenges (e.g., medical expenses, difficulty with healthcare services) were not considered for this analysis.
The items constituting the aggregate scores appear in the Appendix (Supplemental file 1). The recall period for the items of these scales is the past 4 weeks. Standard PETS scoring was used with raw scores converted to a 0 to 100 scale with a higher score indicating more treatment burden.1,7 Workload and Impact summary scores represent the mean score of the contributing scales and can be calculated if >50% of those scales are non-missing. Baseline, 6, 12, and 24 month PETS administrations were used to derive trajectories of Workload and Impact scores over time with completion of at least two assessment times required to generate a trajectory (see analyses below).
Several other established measures were embedded within the survey booklet to identify associations of treatment burden with various personal, health-related, and psycho-social constructs. The following measures were included in the surveys mailed at all four time points (baseline, 6, 12, and 24 months): (1) two items from the Centers for Disease Control and Prevention’s Healthy Days measure to assess the number of poor physical and poor mental health days in the last 30 days23,24; (2) a single-item screener of subjective health literacy: “How often do you have problems learning about your medical condition because of difficulty understanding written information? (all of the time, most of the time, some of the time, little of the time, none of the time)” 25 ; (3) an investigator-derived item to determine if the respondent had a set routine for all of their self-management (yes/no)26,27; (4) the Perceived Medical Condition Self-Management scale (PMCSM) to assess self-efficacy or the belief in one’s ability to successfully self-manage chronic conditions 28 ; (5) the ENRICHD Social Support Instrument (ESSI) to assess perceived available emotional, informational, and instrumental support from others 29 ; (6) the interpersonal challenges scale of the PETS to assess bother associated with negative relations with social network members about one’s healthcare (e.g., feeling dependent on others, tension with others, lack of understanding from others)2,7; and (7) the Life Engagement Test (LET) to assess the extent to which a person engages in activities that are personally valued and meaningful (i.e., life purpose). 30 A list and brief descriptions of the measures included in the survey appear in Table 1.
Table 1.
Measures included in the surveys at baseline, 6, 12, and 24 months.
| Treatment Burden |
| Patient Experience with Treatment and Self-management [PETS], version 2.0¶ |
| Personal, Health-related, and Psycho-social constructs |
| Centers for Disease Control and Prevention Healthy Days measure (physical and mental health)‡ |
| Subjective health literacy (single-item screen)§ |
| Set routine for self-management (investigator-derived item)† |
| Perceived Medical Condition Self-Management Scale (self-efficacy)¶¶ |
| ENRICHD Social Support Instrument (perceived available social support)‡‡ |
| Interpersonal challenges scale from PETS (negative relations with social network members)§§ |
| Life Engagement Test (life purpose)* |
PETS = Patient experience with treatment and self-management. ¶Higher PETS scores = greater treatment burden; ‡Indicates the number of unhealthy days in the past 30. Higher score = more unhealthy days; §“How often do you have problems learning about your medical condition because of difficulty understanding written information? (all of the time (1), most of the time (2), some of the time (3), little of the time (4), none of the time (5))”; †“I have a set routine for all of my self-management” (no = 0, 1 = yes).
¶¶Higher score=greater self-efficacy; ‡‡Higher score = more perceived social support from others; §§Higher score = more perceived interpersonal challenges with other social network members about healthcare; *Higher score = higher perceived purpose in life.
Demographic data on age, race/ethnicity, marital, education and employment status, and household income were collected on the survey. Gender as well as the number and types of chronic conditions were extracted from the electronic medical record. Given that this is a secondary analysis of data collected for the purpose of validating a measure, the sample size for this particular analysis was not determined a priori.
Data analysis
Analyses were conducted in two steps. First, trajectories of Workload and Impact were identified with latent class growth mixture (LCGM) modeling using the Mplus software program 8.3, assuming linear change over time. Linear change was assumed because prior research has shown that PETS scores are responsive to patient changes over two time points. 1 However, there is no evidence currently available that would suggest that treatment burden can also change in a non-linear (i.e., quadratic) manner across multiple time points. These analyses provide insight into patterns of change in the outcomes. LCGM models are regression-based models that assume that individuals in the sample do not necessarily come from one underlying population but from multiple latent sub-populations. In this analysis, the latent growth factors (intercepts and slopes) were estimated by repeated measures across four time points. LCGM modeling aims to find the optimal number of sub-populations that share similar patterns of scores. A one-class model was first determined, assuming one underlying population, and subsequently an additional class was added one at a time with model fit indices inspected at each step. Optimal number of classes was determined according to the following standard fit criteria31,32: (1) a lower Bayesian Information Criterion (BIC), (2) a higher entropy value (range from 0 to 1), a measure of the accuracy of classification into latent classes with a higher value indicating better classification, (3) average posterior probabilities of ≥0.80 where the posterior probabilities refer to the probability that an individual is classified into a given class, and (4) a significant Lo, Mendell, and Rubin likelihood ratio test (LMR-LRT) that compares the fit of the tested model with the fit of a model with exactly one fewer class than the tested model. 33 The choice for the optimal number of classes was made considering interpretability, parsimony, and class size. Individuals were classified into classes based on their posterior probabilities. While guidance varies on the appropriate sample size required for growth mixture modeling, a Monte Carlo simulation study has indicated that a sample of at least 300 is required with high class separation of up to six classes and four time points, while the sample size needed is lower if fewer classes are extracted. 34 A maximum likelihood estimation procedure was used to account for missing data of up to two time points.
Second, bivariate analyses conducted in SAS Studio 3.81 were used to explore differences in baseline personal, health-related, and psycho-social factors among the trajectory classes. Factors showing statistically significant differences among classes were subsequently entered as predictors of trajectory membership into multivariable logistic regressions. Those measured as continuous variables (i.e., self-efficacy, health literacy, social support, interpersonal challenges, life engagement, physical and mental health) were standardized. Results of the multivariable logistic regressions are reported as odds ratios (OR) with corresponding 95% confidence intervals (CI). For any latent trajectory(ies) that reflected change in treatment burden over time, follow-up descriptive analyses were conducted on the longitudinal scores of the significant predictors to explore whether there were any trends in these scores over time. To facilitate comparison, predictor scores were converted to standardized z-scores and their means plotted across the four time points.
Ethics approval
The study was approved as minimal risk with the use of oral consent by Institutional Review Boards at the Mayo Clinic (#14–008629) and Olmsted Medical Center (#022-OMC-16), the institutions that co-administer the REP. A signed HIPAA form was required before use of any protected health information extracted from the medical record.
Patient and public involvement
Patients were not involved in the development of the research questions, study design, data analysis and interpretation, or the drafting of the article for this report. However, the content of the PETS treatment burden measure used in this study, including the wording of its items, was informed by extensive input from people living with MCC.3,4,7
STROBE guideline
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the reporting of an observational cohort study 35 are included with this report in Supplemental file 2.
Results
Characteristics of the sample
Of the original sample of 1496 persons identified, 443 returned a completed baseline survey (30% response). As detailed in Lee et al. 2020, 2 when compared with those who were mailed a survey but never responded, survey responders were older, more likely to be of white race, and had slightly more diagnosed conditions. A total of 396 of the 443 baseline responders (89%) also completed one or more of the follow-up surveys and could therefore be included in the trajectory analysis. Reasons for non-responsiveness to any of the surveys were not compiled. Descriptive statistics of the 396 people eligible for inclusion in the analyses appear in Table 2. Median age of the sample was 63 years (range: 20–98 years), and there were more women than men (63% vs 37%). Most (78%) were White/Caucasian, college educated (75%), married/partnered (61%), and not currently employed (57%). Most (55%) had a household income below that of the median for U.S. households in 2017 (US$61,372). Median number of diagnosed conditions was 5, with the most common diagnoses being hyperlipidemia (55%), hypertension (54%), low back disorder (51%), arthritis (49%), diabetes (48%), and depression (42%). Only 6% of respondents had a diagnosis of an incident condition (i.e., a condition diagnosed within a year of the baseline survey).
Table 2.
Descriptive characteristics of the sample (N = 396).
| Age | |
| Median (range) | 63 years (20–98 years) |
| Gender | |
| Female | 249 (63%) |
| Male | 147 (37%) |
| Race | |
| White/Caucasian | 307 (78%) |
| Black/African American | 29 (7%) |
| Asian | 25 (6%) |
| Mixed | 13 (3%) |
| Native American | 1 (<1%) |
| Unknown | 21 (5%) |
| Ethnicity | |
| Hispanic, Spanish, or Latino origin | 15 (4%) |
| Education status | |
| College educated | 295 (75%) |
| No more than high school | 90 (23%) |
| Missing | 11 (3%) |
| Marital status | |
| Married/partnered | 240 (61%) |
| Not married | 161 (36%) |
| Missing | 12 (3%) |
| Employment status | |
| Not employed | 224 (57%) |
| Employed (full or part) | 149 (38%) |
| Missing | 23 (6%) |
| Household income (2017 U.S. median: US$61,372) | |
| Below median | 216 (55%) |
| At or above median | 152 (38%) |
| Missing | 28 (7%) |
| Total number of diagnosed conditions | |
| Median | 5.0 conditions |
| Range | 2 to 13 conditions |
| 2–3 conditions | 88 (22%) |
| 4–5 conditions | 150 (38%) |
| 6+ conditions | 158 (40%) |
| Incident diagnosis within past year§ | |
| Yes | 24 (6%) |
| No | 372 (94%) |
| Types of diagnosed conditions | |
| Hyperlipidemia | 218 (55%) |
| Hypertension | 214 (54%) |
| Low back disorder¶ | 203 (51%) |
| Arthritis | 194 (49%) |
| Diabetes (type 1 or 2) | 192 (48%) |
| Depression | 167 (42%) |
| Vision problems | 150 (38%) |
| Anxiety | 113 (29%) |
| Cancer | 108 (27%) |
| Cardiac arrhythmia | 101 (26%) |
| Coronary artery disease | 75 (19%) |
| Hearing problems | 63 (16%) |
| Chronic kidney disease | 48 (12%) |
| Chronic obstructive pulmonary disease | 42 (11%) |
| Substance abuse | 38 (10%) |
| Headache | 33 (8%) |
| Osteoporosis | 30 (8%) |
| Congestive heart failure | 30 (8%) |
| Psoriasis | 19 (5%) |
| Crohn’s disease | 17 (4%) |
| Hepatitis | 8 (2%) |
| Human immunodeficiency virus | 2 (<1%) |
| PETS workload score baseline | |
| Mean (standard deviation) | 25.3 (18.2) |
| PETS impact score baseline | |
| Mean (standard deviation) | 26.2 (24.4) |
PETS = Patient experience with treatment and self-management.
§Presence of at least one condition diagnosed within a year of the baseline survey.
¶Includes osteopathic conditions such as disc displacement/degeneration, spondylosis, spinal stenosis, sciatica, and post-laminectomy syndromes.
Workload and impact trajectories
The results of the fit indices for the LCGM models with one to six trajectories for PETS Workload are presented at the top of Table 3. The LMR likelihood ratio test indicated that a 2-class model is the most parsimonious and best fitting as the test was no longer significant after two classes. BIC values also increased after the 2-class model indicating worsening fit. The average posterior probability for the 2-class model was high and above threshold at 0.88, and the model had an entropy value of 0.70, indicating accurate classification of persons into classes. As shown in Figure 1, the two-trajectory model indicated a group showing consistently high workload (N = 69, 18% of total) and another group showing consistently low workload (N = 311, 82% of total). Note, a workload classification could not be determined for 16 participants due to missing responses on the PETS.
Table 3.
Fit indices for the latent class growth mixture models of patient experience with treatment and self-management workload and impact.
| Workload | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of classes | BIC¶ | Entropy† | Average posterior Probability (min–max)‡ | LMR likelihood ratio test§ | Number of people in each trajectory class | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 1 | 10444.1 | NA | 1 | NA | 380 | |||||
| 2 | 10432.3 | 0.699 | 0.88 (0.83–.93) | Significant | 311 | 69 | ||||
| 3 | 10433.0 | 0.713 | 0.87 (0.84–.89) | Not significant | 168 | 48 | 164 | |||
| 4 | 10435.2 | 0.720 | 0.82 (0.74–.88) | Not significant | 157 | 51 | 18 | 154 | ||
| 5 | 10442.2 | 0.786 | 0.90 (0.84–.99) | Not significant | 132 | 1 | 157 | 27 | 63 | |
| 6 | 10449.5 | 0.809 | 0.71 (0–.91) | Not significant | 131 | 0 | 15 | 139 | 70 | 25 |
| Impact | ||||||||||
| Number of classes | BIC | Entropy | Average posterior Probability (min–max) | LMR likelihood ratio test | Number of people in each trajectory class | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 1 | 11930.1 | NA | 1 | NA | 391 | |||||
| 2 | 11871.6 | 0.816 | 0.93 (0.90–.96) | Significant | 305 | 86 | ||||
| 3 | 11843.3 | 0.799 | 0.85 (0.75–.95) | Significant | 278 | 51 | 62 | |||
| 4 | 11830.3 | 0.817 | 0.85 (0.79–.95) | Not significant | 65 | 20 | 257 | 49 | ||
| 5 | 11830.7 | 0.822 | 0.85 (0.74–.94) | Not significant | 259 | 18 | 12 | 64 | 38 | |
| 6 | 11833.5 | 0.817 | 0.84 (0.71–.91) | Not significant | 13 | 27 | 245 | 15 | 19 | 72 |
BIC = Bayesian Information Criterion; LMR = Lo, Mendell, and Rubin.
¶Bayesian Information Criterion (BIC), a lower BIC value indicates better fit.
†Entropy value ranges from 0 to 1 with a higher value indicating more accurate classification into latent classes.
‡Average of the probabilities that individuals are classified into a latent class. A good fit is indicated by an average posterior probability of ≥0.80.
§Lo, Mendell, and Rubin likelihood ratio test. A significant result indicates a better fit for the tested model (K) compared to the model with one fewer classes (K-1). NA = not applicable.
Figure 1.
Two-trajectory model of patient experience with treatment and self-management workload (N = 380).
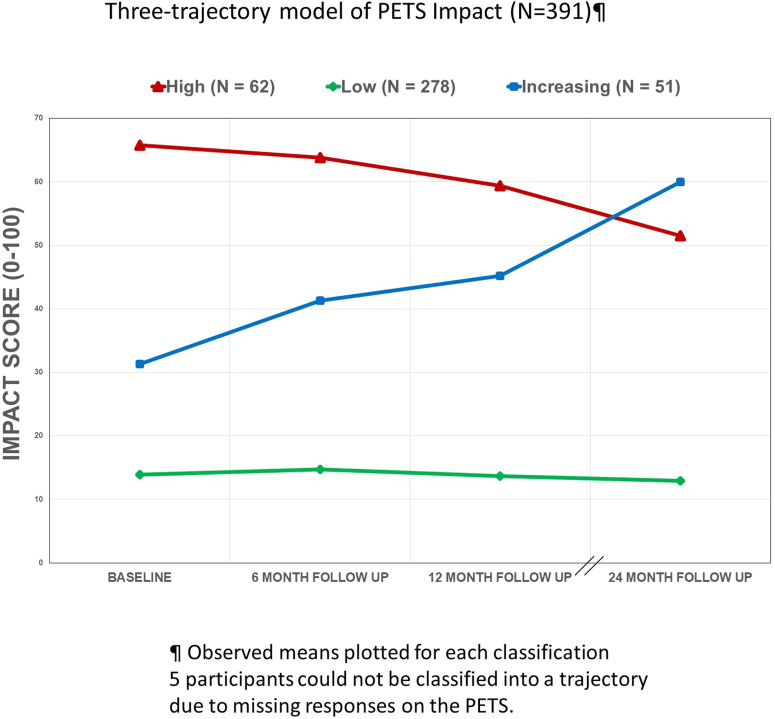
The results of the fit indices for the LCGM models with one to six trajectories for PETS Impact are indicated at the bottom of Table 3. The LMR likelihood ratio test indicated that a 3-class model is the most parsimonious and best fitting for impact as the test was no longer significant after three classes. Further, decreases in BIC values were smaller after the 3-class model and BIC values began to increase after the 4-class model indicating worsening fit. The entropy values were similar across models, with all being around 0.80. The average posterior probability was highest for the 2-class model (0.93). We chose the 3-class model based on interpretability and the acceptable fit statistics. As shown in Figure 2, the three-trajectory model indicated a group showing consistently high impact (N = 62, 16% of total), another group showing consistently low impact (N = 278, 71% of total), and a third group showing increasing impact (N = 51, 13% of total). Note, an impact classification could not be determined for five participants due to missing responses on the PETS. Supplementary Table 1 in Supplemental file 3 shows observed means of the Workload and Impact scores for each of the classes at the four time points.
Figure 2.
Three-trajectory model of patient experience with treatment and self-management impact (N = 391).
Predictors of workload and impact trajectories
Descriptive statistics of the baseline personal, health-related, and psycho-social variables for the burden trajectories are presented in Supplementary Table 2 (Workload) and Supplementary Table 3 (Impact), both located in Supplemental file 3. Workload classes significantly differed across age, education level, household income, mental health diagnoses, self-efficacy, health literacy, self-reported physical and mental health, social support, interpersonal challenges, life engagement, and whether one has a set routine for self-management. Impact classes significantly differed across age, household income, mental health diagnoses, self-efficacy, health literacy, self-reported physical and mental health, social support, interpersonal challenges, life engagement, and whether one has a set routine for self-management.
Multivariable logistic regressions identified which factors were most strongly and independently predictive of treatment burden class among the variables found to be associated with these classes in the bivariate analyses. A binomial logistic regression was used for the analysis of workload (2 classes) while a multinomial logistic regression was used for the analysis of impact (3 classes). As shown in Table 4, compared with participants classified as consistently low in workload, those classified as consistently high in workload reported lower self-efficacy (OR 0.5, 95% CI 0.3–0.9), lower health literacy (OR 0.6, 95% CI 0.4–0.9), and higher interpersonal challenges with others (OR 2.4, 95% CI 1.5–3.7). There was also some evidence that lower age (OR 0.6, 95% CI 0.3–1.0) and reporting more mentally unhealthy days (OR 1.6, 95% CI 1.0–2.6) were associated with high workload class (p < .10), although these associations did not attain a conventional level of significance.
Table 4.
Binomial logistic regression analysis to distinguish between the workload trajectories.
| Low (ref)¶ versus high | ||
|---|---|---|
| OR (95% CI)* | p | |
| Person-related factors | ||
| Age in years | 0.6 (0.3, 1.0) | .06 |
| Education (0 = high school or below; 1 = college) | 0.9 (0.3, 2.5) | .77 |
| Median household income (U.S. in
2017) (0 = below; 1 = above) |
1.0 (0.4, 2.7) | .94 |
| Health-related factors | ||
| Mental health diagnoses (0 = no; 1 = yes) | 0.9 (0.3, 2.6 | .90 |
| Unhealthy physical health days (past 30)† | 1.2 (0.7, 1.8) | .57 |
| Unhealthy mental health days (past 30)† | 1.6 (1.0, 2.6) | .07 |
| Self-management routine (0 = no; 1 = yes) | 0.9 (0.4, 2.4) | .89 |
| Psycho-social factors | ||
| Self-efficacy‡ | 0.5 (0.3, 0.9) | .02 |
| Health literacy§ | 0.6 (0.4, 0.9) | .009 |
| Social support¶¶ | 0.8 (0.5, 1.2) | .25 |
| Interpersonal challenges§§ | 2.4 (1.5, 3.7) | <.001 |
| Life purpose†† | 1.0 (0.6, 1.8) | .89 |
Note. Only variables statistically significant in the bivariate analyses are entered into the regression (see Supplementary Table 2 located in Supplementary file 3).
¶ref indicates the “reference” category and is identified as the category of comparison in each pairwise comparison of trajectory classes (ref = 0).
*OR: odds ratio; CI: confidence interval. Bold type indicates p < .05; Italic type indicates p < .10.
†From the Centers for Disease Control and Prevention’s Healthy Days measure: higher = more unhealthy days reported in the past 30 days.
‡From the Perceived Medical Condition Self-management Scale: Higher score = greater self-efficacy.
§From the single-item screener: “How often do you have problems learning about your medical condition because of difficulty understanding written information? (all of the time (1), most of the time (2), some of the time (3), little of the time (4), none of the time (5)).”
¶¶From the ENRICHD Social Support Instrument: Higher score = more perceived social support from others.
§§From the Patient Experience with Treatment and Self-management Interpersonal Challenges scale: Higher score = more perceived interpersonal challenges with others.
††From the Life Engagement Test: Higher score = higher perceived purpose in life.
Table 5 shows the multinomial logistic regression results for impact. Compared with participants classified as consistently low in impact, those classified as increasing in impact reported more physically unhealthy days (OR 2.1, 95% CI 1.4–3.1) and higher interpersonal challenges with others (OR 1.8, 95% CI 1.1–2.9). Furthermore, compared with those classified as consistently low in impact, those classified as consistently high in impact reported more mentally unhealthy days (OR 1.9, 95% CI 1.02–3.4), lower health literacy (OR 0.6, 95% CI 0.4–0.9), and higher interpersonal challenges with others (OR 5.5, 95% CI 3.1–9.8). Finally, compared to those classified as increasing in impact, those classified as consistently high in impact reported higher interpersonal challenges with others (OR 3.2, 95% CI 1.8–5.5). There was also some evidence that lower self-efficacy (OR 0.5, 95% CI 0.2–1.0) was associated with classification into high impact (p < .10), although this association did not attain a conventional level of significance.
Table 5.
Multinomial logistic regression analysis to distinguish between the impact trajectories.
| Low (ref)¶ versus increasing | Low (ref) vs. high | Increasing (ref) versus high | ||||
|---|---|---|---|---|---|---|
| OR (95% CI)* | p | OR (95% CI) | p | OR (95% CI) | p | |
| Person-related factors | ||||||
| Age in years | 0.8 (0.4, 1.5) | .24 | 0.8 (0.4, 1.5) | .47 | 1.0 (0.6, 2.0) | .91 |
| Median household income (U.S. in 2017) (0 = below; 1 = above) | 0.9 (0.4, 1.9) | .71 | 0.6 (0.2, 1.9) | .43 | 0.8 (0.2, 2.3) | .62 |
| Health-related factors | ||||||
| Mental health diagnoses (0 = no; 1 = yes) | 1.8 (0.8, 4.1) | .17 | 1.0 (0.3, 3.4) | .99 | 0.6 (0.3, 1.3) | .38 |
| Unhealthy physical health days (past 30)† | 2.1 (1.4, 3.1) | < .001 | 1.5 (0.8, 2.5) | .18 | 0.7 (0.4, 1.2) | .20 |
| Unhealthy mental health days (past 30)† | 1.0 (0.6, 1.7) | .99 | 1.9 (1.02, 3.4) | .04 | 1.9 (1.0, 3.6) | .06 |
| Self-management routine (0 = no; 1 = yes) | 0.8 (0.3, 1.8) | .58 | 0.5 (0.2, 1.4) | .17 | 0.6 (0.2, 1.8) | .37 |
| Psycho-social factors | ||||||
| Self-efficacy‡ | 0.8 (0.5, 1.3) | .41 | 0.5 (0.2, 1.0) | .06 | 0.6 (0.3, 1.3) | .18 |
| Health literacy§ | 0.9 (0.6, 1.4) | .74 | 0.6 (0.4, 0.9) | .03 | 0.6 (0.4, 1.0) | .07 |
| Social support¶¶ | 0.7 (0.5, 1.1) | .13 | 1.0 (0.6, 1.7) | .99 | 1.4 (0.8, 2.3) | .27 |
| Interpersonal challenges§§ | 1.8 (1.1, 2.9) | .03 | 5.5 (3.1, 9.8) | < .001 | 3.2 (1.8, 5.5) | < .001 |
| Life purpose†† | 1.0 (0.6, 1.6) | .99 | 1.1 (0.6, 2.0) | .79 | 1.1 (0.6, 2.1) | .79 |
Note. Only variables statistically significant in the bivariate analyses are entered into the regressions (see Supplementary Table 3 located in Supplementary file 3).
¶ref indicates the “reference” category and is identified as the category of comparison in each pairwise comparison of trajectory classes (ref = 0).
*OR: odds ratio; CI: confidence interval. Bold type indicates p < .05; Italic type indicates p < .10.
†From the Centers for Disease Control and Prevention’s Healthy Days measure: higher = more unhealthy days reported in the past 30 days.
‡From the Perceived Medical Condition Self-management Scale: Higher score = greater self-efficacy.
§From the single-item screener: “How often do you have problems learning about your medical condition because of difficulty understanding written information? (all of the time (1), most of the time (2), some of the time (3), little of the time (4), none of the time (5)).”
¶¶From the ENRICHD Social Support Instrument: Higher score = more perceived social support from others.
§§From the Patient Experience with Treatment and Self-management Interpersonal Challenges scale: Higher score = more perceived interpersonal challenges with others.
††From the Life Engagement Test: Higher score = higher perceived purpose in life.
Follow-up descriptive analyses of the one trajectory reflecting change in treatment burden, the increasing Impact class, were conducted by plotting the longitudinal standardized z-scores of significant predictors identified in the multivariable logistic regression analyses. This was done to explore trends in the longitudinal scores of these predictors that might coincide with the increases in impact. The small sample size precluded the use of more formal statistical testing of the longitudinal trend of predictor scores; hence, mean z-scores were plotted. As shown in Supplementary Figure 1 located in Supplemental file 4, individuals with increasing Impact from baseline to follow-up reported concurrent increases in the number of physically unhealthy days, the number of mentally unhealthy days, and interpersonal challenges as well as small declines in self-efficacy across the same time span. Health literacy appeared somewhat stable over time in this group.
Discussion
The primary aim of this study was to determine whether distinct patterns of treatment burden exist in people with multimorbidity over an extended period (2 years). Latent class trajectory analyses of two summary index scores from the PETS, a comprehensive measure of treatment burden, supported discrete longitudinal trajectories of each outcome. Two trajectories of PETS Workload (i.e., difficulty in self-managing chronic conditions) were revealed, including a pattern of consistently high workload and one of consistently low workload over time. Furthermore, three trajectories of PETS Impact (i.e., impact of treatment burden on well-being) were supported, including patterns of consistently high impact, consistently low impact, and increasing impact over time. Demographic, health-related, and psycho-social factors assessed at baseline were found to discriminate these patterns in bivariate analyses. Multivariable regression analyses were used to determine which of these factors most strongly and independently predict the various patterns, controlling for other variables in the model. Logistic regression analyses showed that participants reporting lower subjective health literacy, lower self-efficacy, and greater bother from negative interactions with social network members about healthcare issues were more likely to be classified into a pattern reflecting higher treatment burden. Additionally, poorer self-reported physical and mental health were associated with patterns of worse impact (i.e., consistently high or increasing impact). Follow-up exploratory analyses showed that changes in the impact of treatment and self-management on well-being appear to coincide with changes in physical and mental health, self-efficacy, and interpersonal challenges with others.
The study has noteworthy strengths and weaknesses. The use of a valid, multi-dimensional measure of treatment burden allowed for the study of unique facets of the construct in people coping with MCC. The assessment schedule built in multiple measurements of treatment burden over an extended period (2 years) allowing for modeling of burden trajectories. Furthermore, use of an extensive survey battery and diagnostic data extracted from the electronic medical record meant that a multitude of predictive factors were available for analysis, with multivariable regression analyses enabling tests of independent predictors of treatment burden. However, weaknesses of the study may limit the generalizability of its findings. First, analyses were conducted on survey responses of a self-selected sample of persons from one region of the United States. Second, recall bias is always a potential limitation when self-report surveys are used. Third, most respondents were white (78%) and highly educated (75% college educated). Fourth, the low response rate to the baseline survey (30%) may have introduced some bias in the estimates of treatment burden, especially if non-responders felt too burdened by their treatment to respond to a survey. If this is true, then the actual proportions of people in the high and worsening burden classes may have been underestimated. Finally, while findings would seem to indicate that different underlying patterns of treatment burden exist in people with MCC, we cannot be certain that these are the only burden trajectory patterns that exist. Future analyses with larger and more diverse samples are needed to investigate this.
To our knowledge, this is the first study to profile treatment burden scores of people with MCC prospectively over multiple time points. Many recent studies employing novel measures of treatment burden have focused on cross-sectional relationships,7,8,10,14,36–38 providing limited understanding of treatment burden at a single moment in time. The few prospective studies available focus on testing responsiveness to change of select measures across limited follow-up, precluding in-depth exploration of longitudinal profiles of treatment burden.5,6 While survey length may have discouraged some from participating, the use of an extensive battery allowed for examination of a variety of demographic, health-related, and psycho-social factors and their relationship with different facets of treatment burden. Some of the factors found to distinguish the burden trajectories in this study have been found to be associated with treatment burden in other studies. For example, younger age,6,37 lower levels of education, 26 more financial difficulties,7,38 poorer self-rated health,6,27 and presence of depression or other mental health problems, 6 have all been associated with higher treatment burden. Furthermore, robust associations of subjective health literacy and self-efficacy with treatment burden have been observed, both in studies using the PETS1,7 and in studies using other treatment burden measures.5,14,36,38 However, the PETS is the only existing measure that features multi-item scales of different treatment burden domains, including summary indices for workload and impact. While other measures achieve brevity and simplicity in scoring,5,6,8,38 they sacrifice comprehensive assessment of this complex, multi-faceted construct.
Notably, this is the first quantitative study to show a relationship between negative interactions with social network members and higher treatment burden. Recent qualitative studies point to the importance of social relationships, but they tend to highlight the role of supportive actions from network members in mitigating treatment burden.39,40 As this analysis shows, and consistent with the chronic illness self-management literature, social relationships can be a “double-edged” sword having both positive and negative aspects. 41 Negative exchanges with close network members, while occurring less often than positive interactions, are frequently related to worse health outcomes 42 with some studies showing that the health-damaging effects of negative exchanges outweigh the health-enhancing effects of supportive exchanges. 43 Findings from the analyses reported here were consistent with this as interpersonal challenges with others were more consistently and robustly predictive of treatment burden than was social support.
It is also important to highlight a few factors that were not associated with the burden trajectories in either the bivariate analyses, the multivariable regression analyses, or both, especially since they have been found to be associated with treatment burden in other studies. Burden trajectory classification did not differ by the number of diagnosed conditions. As we have described elsewhere,1,27 the association between treatment burden scores and number of chronic conditions has varied across studies with some studies showing a moderate positive relationship and others showing low or no relationship. This may be due to other moderating factors such as the length of time living with the conditions (most in this study had been living with them for over a year), access to resources that may lessen burden, and/or the types and severity of diagnosed conditions. Study differences in how conditions are captured (self-report vs record extraction) may also play a role. Furthermore, while associated with burden classification in the bivariate analyses, in the multivariable regression analyses, age, income level, mental health diagnosis, and having a self-management routine were not significantly predictive of burden classification. It is possible that the influence of these factors is moderated somewhat by those factors that were found to be significant predictors (e.g., health literacy, self-efficacy, and interpersonal challenges). Alternatively, these may be “upstream” factors that influence treatment burden through mediating processes involving one or more of the health-related and psycho-social predictors. Future tests of such pathways using a causal modeling approach could shed light on this.
Clinical implications
For some, treatment burden may persist over time, while for others, it may fluctuate in tandem with personal circumstances. Comprehending how it manifests clinically in individual patients would involve periodically screening for treatment burden. Notwithstanding the limitations of our study sample, our findings seem to support that meaningful numbers of patients with MCC may be experiencing elevated and unremitting or increasing levels of treatment burden over time, and therefore might benefit from some form of supportive intervention. While identifying the presence and severity of treatment burden is important, pinpointing what contributes to it is also critical as it would allow clinicians to match type of self-management support to an identified need. The findings of this study suggest potential mechanisms through which self-management support could operate to mitigate treatment burden and potentially improve long-term outcomes, namely through shoring up deficits in health literacy and enhancing self-efficacy for self-management. Findings also highlight that healthcare providers may need to pay more attention to how patients are interacting with members of their social network. Referrals to social work or other community resources might help patients and their caregivers better understand the role that interpersonal dynamics plays in influencing treatment burden. 44 Behavioral health professionals could promote strategies that encourage appraising of self-management as a task to be shared by patients and their caregivers. Emerging evidence in diabetes is showing that communal coping, that is, collaborative interactions between patients and their partners focused on achieving self-management goals, is associated with better self-care and disease outcomes. 45 Finally, declining physical and mental health may foreshadow an increase in treatment burden, especially increased impact of self-management on well-being. However, it may be more challenging to modify these in those experiencing an exacerbation of a health condition.
Future research directions
Pragmatically speaking an important next step in work with the PETS is to precisely identify score thresholds indicative of severe treatment burden in the individual, that is, burden that could lead to poorer outcomes. While not a focus of this study, findings suggest that a workload or impact score of 50 (out of 100) might indicate clinically-severe treatment burden. However, more targeted analyses of these and other PETS domain scales are needed to determine the individual-level score benchmarks that would identify an at-risk patient who might benefit from additional clinical attention. 46 This is clinically important as our findings indicate that meaningful numbers of people may be experiencing high and unremitting treatment burden or treatment burden that substantially increases over time. Furthermore, there is a pressing need for more theoretical research of treatment burden. Theoretical foundations can inform the rationale and design of future interventions to relieve treatment burden in those with MCC. Several theories are candidates for consideration including those from the social and behavioral sciences such as stress and coping and social cognitive theories. Empirically tested frameworks with the flexibility to accommodate multiple determinants and pathways are particularly useful. Our current work is guided by a conceptual heuristic model that attempts to integrate social, behavioral, and clinical factors that we and others have found to be associated with treatment burden within the context of two established social-behavioral theories of health, Lazarus and Folkman’s transactional theory of stress and coping 47 and Bandura’s self-efficacy theory.48,49 The model is available in Supplementary Figure 2 located in Supplemental file 5. We intend to use it as a roadmap for future empirical tests of treatment burden and welcome its application by other interested investigators.
Supplemental Material
Supplemental Material, sj-pdf-1-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-2-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-3-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-4-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-5-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Acknowledgements
We thank Ms Ann Harris and Ms Wendy Daniels of the Mayo Clinic Survey Research Center for formatting, distribution, and receipt of the survey. We also thank Ms Kandace Lackore, Ms Sarah Jenkins, and Mr Richard Pendegraft for database support and Ms Bayly Bucknell for study coordination. We thank three anonymous reviewers for their important comments on an earlier version of this manuscript.
Author contributions: Study concept and design: DTE, MKL; Acquisition of data: DTE, JLS; Analysis and interpretation of data: DTE, MKL, JLS, RTA, ML, EAR; Statistical analysis: MKL; Funding acquisition: DTE; Study supervision: DTE. Each author contributed important intellectual content during manuscript drafting and revision and accepts accountability for the overall work produced. All authors approve of the final version of this manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was funded by the National Institute of Nursing Research of the National Institutes of Health (USA) under award number R01NR015441.
Data availability: The datasets generated and/or analyzed during the current study are not publicly available as they are governed by a resource sharing plan of the funded project. De-identified datasets can be made available to interested investigators upon reasonable request and approval of the corresponding author, provided that all conditions of data sharing as stipulated in the resource sharing plan are met. All requests are subject to review by the project principal and co-investigators. The PETS measure along with its scoring are available from the corresponding author.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
David T Eton https://orcid.org/0000-0001-8715-1511
References
- 1.Eton DT, Lee MK, St Sauver JL, et al. Known-groups validity and responsiveness to change of the Patient Experience with Treatment and Self-management (PETS vs. 2.0): a patient-reported measure of treatment burden. Qual Life Res 2020; 29: 3143–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MK, St Sauver JL, Anderson RT, et al. Confirmatory factor analyses and differential item functioning of the Patient Experience with Treatment and Self-Management (PETS vs. 2.0): a measure of treatment burden. Patient Relat Outcome Meas 2020; 11: 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eton DT, Ramalho de Oliveira D, Egginton JS, et al. Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Relat Outcome Meas 2012; 3: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eton DT, Ridgeway JL, Egginton JS, et al. Finalizing a measurement framework for the burden of treatment in complex patients with chronic conditions. Patient Relat Outcome Meas 2015; 6: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd CM, Wolff JL, Giovannetti E, et al. Healthcare task difficulty among older adults with multimorbidity. Med Care 2014; 52(Suppl 3): S118–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan P, Murphy M, Man MS, et al. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open 2018; 8: e019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eton DT, Yost KJ, Lai JS, et al. Development and validation of the Patient Experience with Treatment and Self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res 2017; 26: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran VT, Montori VM, Eton DT, et al. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Med 2012; 10: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. Jama 2002; 288: 2880–2883. [DOI] [PubMed] [Google Scholar]
- 10.Schreiner N, DiGennaro S, Harwell C, et al. Treatment burden as a predictor of self-management adherence within the primary care population. Appl Nurs Res 2020; 54: 151301. [DOI] [PubMed] [Google Scholar]
- 11.Vijan S, Hayward RA, Ronis DL, et al. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med 2005; 20: 479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortenblad L, Meillier L, Jonsson AR. Multi-morbidity: a patient perspective on navigating the health care system and everyday life. Chronic Illn 2018; 14: 271–282. [DOI] [PubMed] [Google Scholar]
- 13.Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011; 61: e12–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran VT, Harrington M, Montori VM, et al. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med 2014; 12: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ (Clinical Research Ed) 2009; 339: b2803. [DOI] [PubMed] [Google Scholar]
- 16.Lesage A, Leclere B, Moret L, et al. Decreasing patient-reported burden of treatment: a systematic review of quantitative interventional studies. PLoS One 2021; 16: e0245112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012; 87: 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012; 41: 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman RA, Posner SF, Huang ES, et al. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis 2013; 10: E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services . Multiple chronic conditions -- A strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. Washington, DC: U.S. Department of Health and Human Services, 2010. [Google Scholar]
- 21.Eton DT, Elraiyah TA, Yost KJ, et al. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas 2013; 4: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson RT, Eton DT, Camacho FT, et al. Impact of comorbidities and treatment burden on general well-being among women’s cancer survivors. J Patient Rep Outcomes 2021; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention . Measuring healthy days. Atlanta, GA: CDC, 2000. [Google Scholar]
- 24.Hagerty MR, Cummins RA, Ferriss AL, et al. Quality of life indexes for national policy: review and agenda for research. Soc Indic Res 2001; 55: 1–96. [Google Scholar]
- 25.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008; 23: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eton DT, Anderson RT, Cohn WF, et al. Risk factors for poor health-related quality of life in cancer survivors with multiple chronic conditions: exploring the role of treatment burden as a mediator. Patient Relat Outcome Meas 2019; 10: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eton DT, Linzer M, Boehm DH, et al. Deriving and validating a brief measure of treatment burden to assess person-centered healthcare quality in primary care: a multi-method study. BMC Fam Pract 2020; 21: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallston KA, Osborn CY, Wagner LJ, et al. The Perceived medical condition self-management scale applied to persons with HIV/AIDS. J Health Psychol 2011; 16: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD social support inventory. J Cardiopulm Rehabil 2003; 23: 398–403. [DOI] [PubMed] [Google Scholar]
- 30.Scheier MF, Wrosch C, Baum A, et al. The life engagement test: assessing purpose in life. J Behav Med 2006; 29: 291–298. [DOI] [PubMed] [Google Scholar]
- 31.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass 2008; 2: 302–317. [Google Scholar]
- 32.van de Schoot R, Sijbrandij M, Winter SD, et al. The GRoLTS-checklist: guidelines for reporting on latent trajectory studies. Struct Equ Model 2017; 24: 451–467. [Google Scholar]
- 33.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika 2001; 88: 767–778. [Google Scholar]
- 34.Kim S-Y. Sample size requirements in single- and multiphase growth mixture models: A monte carlo simulation study. Struct Equ Model 2012; 19: 457–476. [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 36.Friis K, Lasgaard M, Pedersen MH, et al. Health literacy, multimorbidity, and patient-perceived treatment burden in individuals with cardiovascular disease. A Danish population-based study. Patient Educ Couns 2019; 102: 1932–1938. [DOI] [PubMed] [Google Scholar]
- 37.Mohammed MA, Moles RJ, Hilmer SN, et al. Development and validation of an instrument for measuring the burden of medicine on functioning and well-being: the Medication-Related Burden Quality of Life (MRB-QoL) tool. BMJ Open 2018; 8: e018880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris JE, Roderick PJ, Harris S, et al. Treatment burden for patients with multimorbidity: cross-sectional study with exploration of a single-item measure. Br J Gen Pract 2021; 71: e381–e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boehmer KR, Gionfriddo MR, Rodriguez-Gutierrez R, et al. Patient capacity and constraints in the experience of chronic disease: a qualitative systematic review and thematic synthesis. BMC Fam Pract 2016; 17: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridgeway JL, Egginton JS, Tiedje K, et al. Factors that lessen the burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Prefer Adherence 2014; 8: 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav 2003; 30: 170–195. [DOI] [PubMed] [Google Scholar]
- 42.Rook KS, Charles ST. Close social ties and health in later life: strengths and vulnerabilities. Am Psychol 2017; 72: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rook KS. Social networks in later life: weighing positive and negative effects on health and well-being. Curr Dir Psychol Sci 2015; 24: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linzer M, Rogers EA, Eton DT. Reducing the burden of treatment: Addressing how our patients feel about what we ask of them. Mayo Clin Proc, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helgeson VS, Horner F, Naqvi JB. Partner involvement in Type 2 diabetes self-management: a mixed methods investigation. Diabetes Spectr. In press. DOI: 10.2337/ds21-0034. [DOI] [PMC free article] [PubMed]
- 46.King MT, Dueck AC, Revicki DA. Can methods developed for interpreting group-level patient-reported outcome data be applied to individual patient management? Med Care 2019; 57(Suppl 5 1): S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazarus R, Folkman S. Stress, coping, and appraisal. New York, NY: Springer, 1984. [Google Scholar]
- 48.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977; 84: 191–215. [DOI] [PubMed] [Google Scholar]
- 49.Bandura A. Social foundations of thought and action: a social cognitive theory. Hoboken, NJ: Prentice-Hall, 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-2-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-3-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-4-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity
Supplemental Material, sj-pdf-5-cob-10.1177_26335565221081291 for Longitudinal trajectories of treatment burden: A prospective survey study of adults living with multiple chronic conditions in the midwestern United States by David T Eton, Roger T Anderson, Jennifer L St Sauver, Elizabeth A Rogers, Mark Linze, and Minji K Lee in Journal of Comorbidity