Abstract
Multi-city epidemiologic studies examining short-term (daily) differences in fine particulate matter (PM2.5) provide evidence of substantial spatial heterogeneity in city-specific mortality risk estimates across the United States. Because PM2.5 is a mixture of particles, both directly emitted from sources or formed through atmospheric reactions, some of this heterogeneity may be due to regional variations in PM2.5 toxicity. Using inverse variance weighted linear regression, we examined change in percent change in mortality in association with 24 “exposure” determinants representing three basic groupings based on potential explanations for differences in PM toxicity – size, source, and composition. Percent changes in mortality for the PM2.5-mortality association for 313 core-based statistical areas and their metropolitan divisions over 1999–2005 were used as the outcome. Several determinants were identified as potential contributors to heterogeneity: all mass fraction determinants, vehicle miles traveled (VMT) for diesel total, VMT gas per capita, PM2.5 ammonium, PM2.5 nitrate, and PM2.5 sulfate. In multivariable models, only daily correlation of PM2.5 with PM10 and long-term average PM2.5 mass concentration were retained, explaining approximately 10% of total variability. The results of this analysis contribute to the growing body of literature specifically focusing on assessing the underlying basis of the observed spatial heterogeneity in PM2.5-mortality effect estimates, continuing to demonstrate that this heterogeneity is multifactorial and not attributable to a single aspect of PM.
Keywords: Air pollution, particulate matter, mortality, heterogeneity, components
1. Introduction
Fine particulate matter (PM2.5i) is a mixture of particles, both directly emitted from sources or formed through atmospheric reactions, with aerodynamic diameters generally of 2.5 micrometers or smaller. Multi-city population-based epidemiologic studies have provided some of the strongest evidence indicating a relationship between short-term (daily) PM2.5 exposures and mortality (Baxter et al. 2017; Dai et al. 2014; Di et al. 2017a; Di et al. 2017b; Franklin et al. 2007; Franklin et al. 2008; Krall et al. 2013; Zanobetti and Schwartz 2009), and have heavily contributed to the overarching conclusion that there is a causal relationship between short-term PM2.5 exposure and mortality (U.S. EPA. 2009, 2019). While these nationally representative epidemiologic studies provide evidence of positive associations, there is evidence of city-to-city or regional heterogeneity in the magnitude of the PM2.5 mortality effect estimates (Baxter et al. 2019; Di et al. 2017b; Dominici et al. 2006; Franklin et al. 2007). The inability to explain the city-to-city or regional heterogeneity in PM2.5 mortality effect estimates observed in multi-city studies remains a key uncertainty in the examination of the relationship between short-term PM2.5 exposures and mortality.
The observed heterogeneity in PM2.5-mortality effect estimates has been hypothesized to be due to differences in source profiles and composition of PM2.5 across the United States (U.S.). As a result, numerous studies have examined the relationship between individual PM2.5 components and mortality to assess whether the observed heterogeneity can be attributed to some individual PM2.5 components being more toxic than others. However, no consistent components have been identified as being more strongly associated with mortality than others. For example, Atkinson et al. (2015) conducted a systematic review of the epidemiologic time-series literature of the relationship between particle components and mortality, finding that sulfate (SO42-), nitrate (NO3−), elemental carbon (EC), and organic carbon (OC) were positively associated with increases in all-cause, cardiovascular, and respiratory mortality. Additional studies have demonstrated associations with various metal components and mortality, such as aluminum (Franklin et al. 2008), nickel (Franklin et al. 2008; Ito et al. 2011), vanadium (Ito et al. 2011; Lippmann et al. 2013), copper (Lippmann et al. 2013; Ostro et al. 2007), and zinc (Ito et al. 2011; Ostro et al. 2007). The variability in results across these epidemiologic studies indicates that compositional differences in PM2.5 do not fully explain the heterogeneity in PM2.5 mortality effect estimates across the U.S. This variability is further reflected in an assessment of studies examining PM2.5 components and mortality in the 2019 PM Integrated Science Assessment that contributed to the conclusion that, “the evidence does not indicate that any one source or component is consistently more strongly related to health effects than PM2.5 mass.” (U.S. EPA. 2019).
More recently, studies have expanded the examination of the observed heterogeneity in PM2.5 mortality effect estimates in an attempt to address this question more broadly instead of narrowly focusing on individual PM2.5 components. Additional exploration of this uncertainty has led to examinations of whether the heterogeneity in PM2.5 mortality effect estimates can be explained by unique differences in PM2.5 component mixtures between cities (Baxter et al. 2013), in the distribution of the population potentially at greatest risk of an air pollutant-related health effect (Levy et al. 2012), and in differences in city-specific exposures to PM2.5 (Baxter et al. 2019). While each of these studies provides information to explain some of the observed heterogeneity, together they indicate a complex and multifaceted issue.
Our analyses further examine the issue of spatial heterogeneity in PM2.5-mortality effect estimates by focusing specifically on the degree to which PM mass, sources, or composition explain this heterogeneity. Using PM2.5-mortality effect estimates from analyses of 312 Core Based Statistical Areas (CBSAs) and Metropolitan Divisions (MD) across the U.S. from 1999–2005, in combination with determinants representing aspects of PM exposure and mass, sources, and components, we explore whether these determinants contribute to the observed spatial heterogeneity in mortality effect estimates using meta-regression techniques.
2. Methods
2.1. Outcome and study population
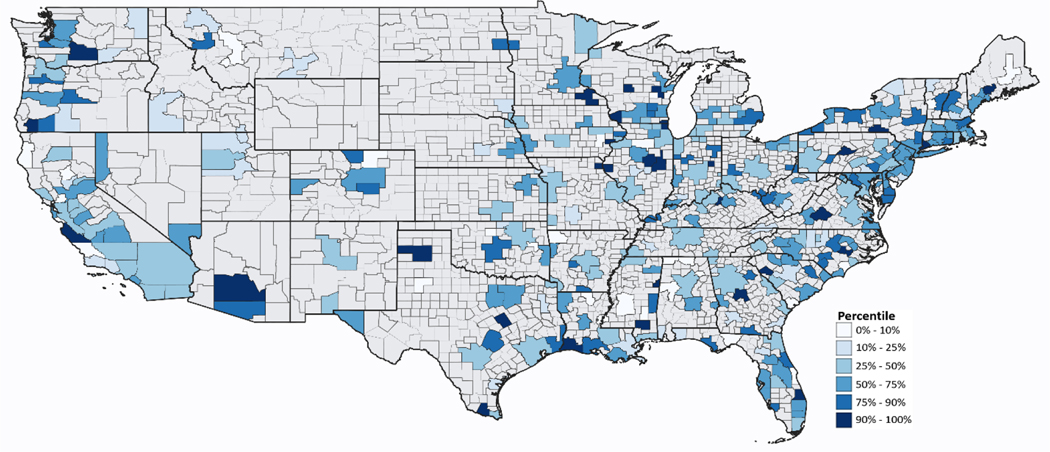
For this analysis, the study population consists of all residents of 312 core-based statistical areas (CBSA) across the U.S. for which associations (percent change) between daily counts of total non-accidental mortality and daily (24-hour average) ambient concentrations of PM2.5 were previously estimated (Figure 1) (Baxter et al. 2019). As detailed in Baxter et al. (2019), associations between daily PM2.5 from the EPA’s Air Quality System’s (AQS) Technology Transfer Network (U.S. EPA. 2020b) and individual level mortality data from the National Center for Health Statistics (http://www.cdc.gov/nchs/about.htm) were estimated at lag 1 for 1999 through 2005 using Poisson time series methods adjusting for time/season (natural spline with 7 degrees of freedom per year), day of week, and natural splines for current temperature, dew point temperature, and individual lagged temperature at lags 1–3 for each CBSA. We selected PM2.5 at lag 1 because the largest magnitude effect estimates for PM2.5 associated mortality occur within this window (Di et al. 2017a; Franklin et al. 2007; Krall et al. 2013; Ostro et al. 2007; Zanobetti and Schwartz 2009), and to be consistent with previous work (Baxter et al. 2019); in addition, we do not expect spatial determinants to differ between lags 0 and 1. Meteorological data for all U.S. cities were obtained from the U.S. Department of Commerce’s National Climatic Data Center (NCDC). The effect estimates for each CBSA are expressed as a percent change in nonaccidental mortality for a 10 μg/m3 increase in daily PM2.5 1 day before death and are used here as our outcome measure.
Figure 1:
Area-specific distribution of associations of total non-accidental mortality and fine particulate matter (PM2.5) at lag 1: 312 U.S. core-based statistical areas and their metropolitan divisions (Supplemental Table S.1)
2.2. Spatial determinants
We explored 24 determinants representing three basic groupings based on potential explanations for differences in PM toxicity – aspects of exposure or mass fraction, source, and composition; all determinants, descriptions, and sources are listed in Table 1. These groups have been previously identified as potential contributors to PM toxicity and can affect delivered dose and mode of action (Kelly and Fussell 2012; Lippmann 2012). Therefore, differences in spatial distributions of these determinants across CBSAs may help explain some of the observed heterogeneity in PM2.5-mortality associations.
Table 1:
Descriptions of determinants used to explore spatial heterogeneity in the CBSA-level effect estimates between daily PM2.5 and all-cause mortality
| Exposure determinant | Group | Description | Source for raw data | Years |
|---|---|---|---|---|
|
| ||||
| Long-term PM10 average | Mass fraction | 6-year average of daily PM10 values at the CBSA level | AQS | 1999 – 2005 |
| Long-term PM2.5 average | Mass fraction | 6-year average of daily PM5 values at the CBSA level | AQS | 1999 – 2005 |
| PM2.5 - PM10 correlation | Mass fraction | Correlation between daily PM2.5 and daily PM10 at the CBSA level | AQS | 1999 – 2005 |
| PM ratio 2.5/10 | Mass fraction | Ratio of average PM2.5 to PM10 concentrations | AQS | 1999 – 2005 |
| PM2.5 dust construction | Sources/emissions | PM2.5 emitted from construction as dust (tons) (NEI technical documentation section 3.7) | NEI | 2011 |
| PM2.5 dust paved road | Sources/emissions | PM2.5 emitted from paved roads as dust (tons) (NEI technical documentation section 3.8) | NEI | 2011 |
| PM2.5 dust unpaved road | Sources/emissions | PM2.5 emitted from unpaved roads as dust (tons) (NEI technical documentation section 3.9) | NEI | 2011 |
| PM2.5 fires wild | Sources/emissions | PM2.5 emitted from wildfires (tons) (NEI technical documentation section 5.1) | NEI | 2011 |
| PM2.5 fires prescribed | Sources/emissions | PM2.5 emitted from prescribed burning (tons) (NEI technical documentation section 5.1) | NEI | 2011 |
| PM2.5 fires agriculture field burning | Sources/emissions | PM2.5 emitted from agricultural field burning (tons) (NEI technical documentation section 5.2) | NEI | 2011 |
| PM2.5 fires all | Sources/emissions | Summed emissions from fires (tons) | NEI | 2011 |
| PM2.5 dust all | Sources/emissions | Summed emissions from dust(tons) | NEI | 2011 |
| PM2.5 dust all and ag crops/livestock | Sources/emissions | Summed emissions from fires, dust, and agricultural practices in tons | NEI | 2011 |
| PM2.5 agriculture crops livestock | Sources/emissions | PM2.5 emitted from agricultural tilling (tons) (NEI technical documentation section 3.2) | NEI | 2011 |
| VMT diesel total | Sources/emissions | Emissions related to vehicle miles traveled, vehicles designed to use diesel fuel, in tons | NEI | 2011 |
| VMT diesel per capita | Sources/emissions | Emissions related to vehicle miles traveled, vehicles designed to use diesel fuel, divided by population, in tons | NEI | 2011 |
| VMT gas total | Sources/emissions | Emissions related to vehicle miles traveled, vehicles designed to use gasoline fuel, divided by population, in tons | NEI | 2011 |
| VMT gas per capita | Sources/emissions | Emissions related to vehicle miles traveled, vehicles designed to use gasoline fuel per capita, in tons | NEI | 2011 |
| PM2.5 ammonium | Components | Estimated PM2.5 ammonium concentration averaged across 9 years | CMAQ | 1998 – 2006 |
| PM2.5 nitrate | Components | Estimated PM2.5 nitrate concentration averaged across 9 years | CMAQ | 1998 – 2006 |
| PM2.5 sulfate | Components | Estimated PM2.5 sulfate concentration averaged across 9 years | CMAQ | 1998 – 2006 |
| PM2.5 elemental carbon | Components | Estimated PM2.5 elemental carbon concentration averaged across 9 years | CMAQ | 1998 – 2006 |
| PM2.5 organic carbon | Components | Estimated PM2.5 organic carbon concentration averaged across 9 years | CMAQ | 1998 – 2006 |
AQS: EPA’s Air Quality System (https://www.epa.gov/aqs)
CBSA: Core-based Statistical Area
CMAQ: Community Multiscale Air Quality Modeling System (https://www.epa.gov/cmaq)
NEI: EPA’s National Emissions Inventory (https://www.epa.gov/air-emissions-inventories/national-emissions-inventory-nei)
VMT: vehicle miles traveled, these emission estimates are derived by first estimating the VMT and then multiplying by a fixed emission constant.
The first group includes determinants representing aspects of PM related to exposure and mass fractions: long-term average PM2.5, correlation between daily PM2.5 and daily PM10, etc. This group was chosen in an attempt to elucidate the impacts of overall mass-based exposure metrics, as when the balance of mass is shifted towards larger or smaller particles different associations with health might be observed; though there is extensive evidence indicating health effects attributed to multiple size fractions (U.S. EPA. 2019). Long-term average PM2.5 might reflect underlying particle distribution, but may also affect responses to short-term exposures through increased susceptibility (Kunzli et al. 2001; Shi et al. 2016). The ratio of PM2.5/PM10 should reflect the proportion of PM10 that is comprised of PM2.5, while the daily PM2.5-PM10 correlation may capture days with higher degrees of shared sources between PM2.5 and PM10. For these determinants, concentrations of PM were obtained from the EPA’s AQS (U.S. EPA. 2020b) within each CBSA and a county-population weighted daily average was created when there were multiple monitors.
The second group consists of determinants representing emissions and sources: PM2.5 emissions from wildland fires, diesel and gas emissions vehicle miles traveled, etc. These determinants may represent potential mixtures of chemicals particular to certain sources, some of which may produce biological responses (for example, traffic related) while others may not (for example., soil related) (Kelly and Fussell 2012). This information was sourced from the EPA’s National Emissions Inventory (NEI) (U.S. EPA 2015; U.S. EPA. 2020a), and county-level population-weighted averages were created when there were multiple counties within a CBSA.
The final group of determinants consists of specific PM2.5 components: ammonium, sulfates, nitrates, elemental carbon, and organic carbons. Component concentrations are known to vary spatially and have been identified and investigated as a source of heterogeneity previously (Kelly and Fussell 2012; U.S. EPA. 2019). For the components group, annual average PM2.5 component concentrations were estimated between 1990 and 2010 on a 36×36 km grid using the Community Multiscale Air Quality (CMAQ v 5.0.2) framework (Gan et al. 2015). Estimated concentrations were calibrated against observed concentrations from air quality networks and performed well (correlation coefficients above 0.8 for all component estimates) (Gan et al. 2015). Then, thin-plate smoothing, by means of the R software package “fields” (Nychka et al. 2018), was used to interpolate monthly average concentrations to population centroids of U.S. census tracts, and population-weighted averages were calculated across census tracts and months to obtain annual PM2.5 concentrations for each county and year, these were then averaged from 1998–2006 (Peterson et al. 2020).
All exposure determinants are mean-centered and scaled to their respective inter-quartile range (IQR).
2.3. Statistical analysis
Exposure determinants (continuous) were first examined in univariate, inverse variance weighted regression models with percent change in mortality for a 10 μg/m3 increment in PM2.5 as the outcome (i.e., a meta-regression) using the gam package in R (Hastie 2019; R Core Team 2013). Number of CBSAs in each model was allowed to vary with available exposure data. Each beta can be interpreted as the change in PM2.5 associated mean percent change in nonaccidental mortality for an IQR increase of the specific determinant, in other words a shift in the percent change in mortality associated with daily PM2.5 exposure. Models using natural cubic spline smoothing were also used to check for non-linearity (Fasiolo et al. 2020; Wood 2011), and weighted correlations were also examined. Following bivariable analysis, a final multivariable model was built using a backwards selection approach with the following inclusion criteria: 1) determinants where the univariate models were improvements over the null model, as indicated by a lower BIC; 2) determinants that were roughly linear as determined by spline models; and 3) determinants that were not highly co-linear (correlation coefficient <0.7). For co-linear determinants, the determinant with the higher F-statistic was included (Baxter et al. 2019). In these cases, the selected determinant may be representing the impact of both co-linear determinants. The multivariable model was run iteratively with the least significant determinant dropped at each iteration of the model, until all covariates were significant at the chosen critical level (p < 0.05).
3. Results
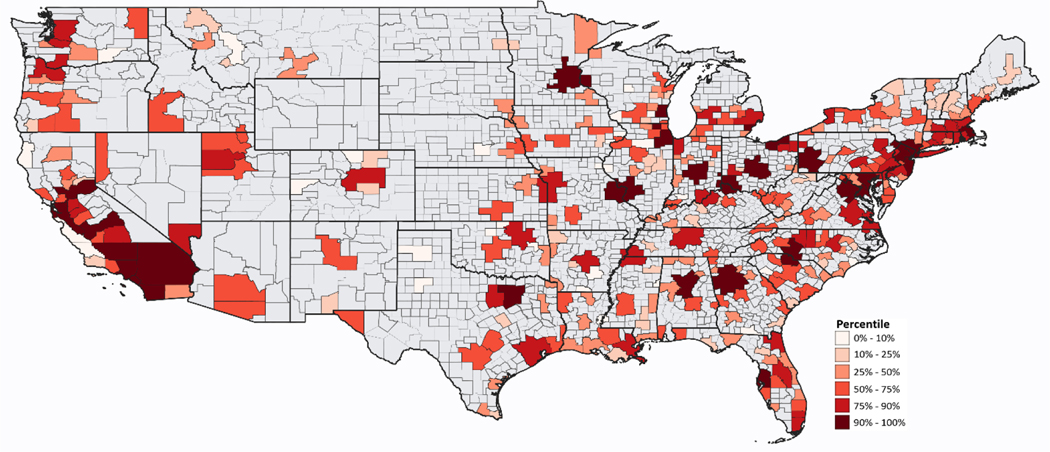
Distributions of CBSA/metropolitan division-specific health effect estimates and inverse variance weights are displayed on the maps in Figures 1 and 2 (supporting numbers in Supplemental Table S.1); the overall percent change in nonaccidental mortality for a 10 μg/m3 increase in daily PM2.5 1 day before death was 1.05%, with an IQR of 2.67. More populous areas have larger inverse-variance weights (Figure 2).
Figure 2:
Area-specific distributions of inverse variance weights for associations of total non-accidental mortality and fine particulate matter (PM2.5) at lag 1: 312 U.S. core-based statistical areas and their metropolitan divisions (Supplemental Table S.1)
Descriptive statistics for spatial determinants are presented in Table 2, with correlations between determinants presented in Supplemental Table S.2. While all included counties (n=312) had values for most PM2.5, sources/emissions determinants, and modeled component concentrations, fewer counties had data on PM10 concentration, PM2.5-PM10 daily correlation values, and PM2.5 dust construction emissions. Variation across CBSAs was generally low for mass fraction and component determinants, and higher for sources/emissions determinants. Most determinants had moderate to low correlations (|r| < 0.7); however, some were highly correlated (e.g., component determinants: ammonium, sulfate, and nitrate) and so were not included together in multi-determinant models.
Table 2:
Descriptive statistics for spatial determinants
| Exposure group | Spatial determinant | Units | N | Mean (SD) | Median (IQR) | Coefficient of variation |
|---|---|---|---|---|---|---|
|
| ||||||
| Mass fraction | PM10 long-term average | μg/m3 | 267 | 22.42 (6.23) | 23.53 (5.91) | 0.25 |
| PM2.5 long-term average | μg/m3 | 312 | 12.59 (3.61) | 12.55 (2.73) | 0.22 | |
| PM2.5-PM10 daily correlation | 238 | 0.70 (0.25) | 0.67 (0.18) | 0.26 | ||
| PM ratio 2.5/10 | 267 | 0.55 (0.13) | 0.56 (0.18) | 0.24 | ||
|
| ||||||
| Sources/emissions | PM2.5 dust construction | tons emitted/year | 241 | 137 (321) | 419 (944) | 2.25 |
| PM2.5 dust paved road | tons emitted/year | 312 | 301 (392) | 513 (597) | 1.16 | |
| PM2.5 dust unpaved road | tons emitted/year | 312 | 428 (822) | 1,065 (2,022) | 1.90 | |
| PM2.5 fires wild | tons emitted/year | 312 | 32 (248) | 712 (3,089) | 4.34 | |
| PM2.5 fires prescribed | tons emitted/year | 312 | 135 (727) | 932 (2,389) | 2.56 | |
| PM2.5 fires agriculture field burning | tons emitted/year | 312 | 9 (52) | 70 (213) | 3.06 | |
| PM2.5 fires all | tons emitted/year | 312 | 310 (1,527) | 1,713 (4,339) | 2.53 | |
| PM2.5 dust all | tons emitted/year | 312 | 1,062 (1,508) | 1,997 (3,090) | 1.55 | |
| PM2.5 dust all and ag crops/livestock | tons emitted/year | 312 | 1,570 (2,351) | 2,699 (3,555) | 1.32 | |
| PM2.5 agriculture crops livestock | tons emitted/year | 312 | 242 (782) | 702 (1,125) | 1.60 | |
| VMT diesel total | megatons emitted/year | 312 | 284 (408) | 528 (727) | 1.38 | |
| VMT diesel per capita | tons emitted/year | 312 | 882 (358) | 921 (352) | 0.38 | |
| VMT gas total | megatons emitted/year | 312 | 3,150 (5,418) | 6,680 (9,801) | 1.47 | |
| VMT gas per capita | tons emitted/year | 312 | 9,913 (2,594) | 10,191 (1,886) | 0.19 | |
|
| ||||||
| Components | PM2.5 ammonium | μg/m3 | 312 | 0.63 (0.40) | 0.61 (0.28) | 0.47 |
| PM2.5 nitrate | μg/m3 | 312 | 0.69 (0.64) | 0.70 (0.36) | 0.52 | |
| PM2.5 sulfate | μg/m3 | 312 | 2.03 (0.93) | 1.88 (0.68) | 0.36 | |
| PM2.5 elemental carbon | μg/m3 | 312 | 0.29 (0.11) | 0.31 (0.10) | 0.31 | |
| PM2.5 organic carbon | μg/m3 | 312 | 0.94 (0.30) | 0.94 (0.22) | 0.23 | |
3.1. Meta-regression results
Within the single determinant meta-regression, several determinants had some level of predictive ability for percent change in nonaccidental mortality for a 10 μg/m3 increase in daily PM2.5 1 day before death (Table 3). These included all mass fraction determinants, VMT diesel total and VMT gas per capita from sources/emissions determinants, and ammonium, nitrate, and sulfate from PM component determinants. For example, the overall percent change in mortality is 1.05% (which corresponds to a mortality rate ratio of 1.0105). Therefore, a beta of 0.34, as with the correlation between daily PM2.5 and PM10 concentrations, can be roughly interpreted as such: a correlation increase from 40% to 58% (IQR increase) would increase the average mortality association from 1.05% to 1.39%. A negative beta would be a decrease in the average mortality association, for example, a 2.72 μg/m3 increase in long-term PM2.5 average would decrease the average mortality association to 0.75% (beta of −0.30).
Table 3:
Meta-regression single determinant model results
| Exposure group | Spatial determinant | beta (95% CI)* | F-statistic | BIC lower than null model? | Adjusted r-squared (%) |
|---|---|---|---|---|---|
|
| |||||
| Mass fraction | Long-term PM10 average | −0.22 (−0.34, −0.09) | 11.38 | TRUE | 3.76 |
| Long-term PM2.5 average | −0.30 (−0.41, −0.19) | 27.55 | TRUE | 7.86 | |
| PM2.5 - PM10 correlation | 0.34 (0.20, 0.48) | 22.15 | TRUE | 8.19 | |
| PM ratio 2.5/10 | −0.16 (−0.34, 0.03) | 2.85 | TRUE | 0.69 | |
| Sources/emissions | PM2.5 dust construction | −0.01 (−0.03, 0.01) | 1.04 | FALSE | 0.01 |
| PM2.5 dust paved road | 0.01 (−0.04, 0.06) | 0.12 | FALSE | −0.28 | |
| PM2.5 dust unpaved road | −0.01 (−0.05, 0.03) | 0.19 | FALSE | −0.26 | |
| PM2.5 fires wild | 0.00 (−0.01, 0.01) | 0.15 | FALSE | −0.27 | |
| PM2.5 fires prescribed | −0.02 (−0.07, 0.03) | 0.71 | FALSE | −0.09 | |
| PM2.5 fires agriculture field burning | −0.01 (−0.05, 0.03) | 0.29 | FALSE | −0.23 | |
| PM2.5 fires all | −0.01 (−0.06, 0.05) | 0.04 | FALSE | −0.31 | |
| PM2.5 dust all | −0.01 (−0.05, 0.03) | 0.38 | FALSE | −0.20 | |
| PM2.5 dust all and ag crops/livestock | −0.01 (−0.07, 0.04) | 0.26 | FALSE | −0.24 | |
| PM2.5 agriculture crops livestock | 0.00 (−0.07, 0.07) | 0.00 | FALSE | −0.32 | |
| VMT+ diesel total | −0.04 (−0.08, −0.01) | 6.28 | TRUE | 1.67 | |
| VMT diesel per capita | −0.13 (−0.27, 0.01) | 3.43 | FALSE | 0.77 | |
| VMT gas total | −0.03 (−0.05, 0.00) | 3.89 | FALSE | 0.92 | |
| VMT gas per capita | −0.18 (−0.31, −0.04) | 6.26 | TRUE | 1.66 | |
| Components | PM2.5 ammonium | 0.24 (0.11, 0.38) | 12.75 | TRUE | 3.64 |
| PM2.5 nitrate | 0.31 (0.11, 0.52) | 9.35 | TRUE | 2.61 | |
| PM2.5 sulfate | 0.21 (0.08, 0.35) | 9.69 | TRUE | 2.72 | |
| PM2.5 elemental carbon | −0.02 (−0.11, 0.08) | 0.09 | FALSE | −0.29 | |
| PM2.5 organic carbon | 0.11 (−0.06, 0.29) | 1.54 | FALSE | 0.17 | |
beta should be interpreted as a shift from the overall daily PM2.5-mortality association (percent change in mortality) of 1.05%. For example, for every 5.91 μg/m3 increase in 6 year average PM10 the mean daily PM2.5-mortality association decreases by 0.22%.
vehicle miles traveled
The amount of variability in the PM2.5-mortality effect estimates explained by any individual determinant was generally low, with the highest adjusted r-squared value being 8.19% for the correlation between daily PM2.5 and PM10 concentrations. Following that, long-term average PM2.5 mass concentration explained 7.86% of the total variation in mortality. All other determinants with some predictive ability explained less than 5% of the total variability in PM2.5 associated percent change in mortality, with the PM2.5/10 ratio explaining the lowest at 0.69%.
Determinants considered for a multivariable meta-regression model were long-term PM10 average, long-term PM2.5 average, daily PM2.5-PM10 correlation, daily PM2.5/10 ratio, VMT diesel total, VMT gas per capita, PM2.5 ammonium, PM2.5 nitrate, and PM2.5 sulfate. Of these, PM2.5 nitrate was non-linear (see supplemental materials, spline figures). PM2.5 ammonium, PM2.5 nitrate, and PM2.5 sulfate were highly correlated; as PM2.5 ammonium had the highest F-statistic it was retained for the multivariable model. The initial multivariable model included long-term PM10 average, long-term PM2.5 average, PM2.5-PM10 correlation, PM2.5/10 ratio, VMT diesel total, VMT gas per capita, and PM2.5 ammonium. Backwards selection was performed until all included determinants were significant at the <0.05 level, leaving a final model that included only two determinants, daily correlation of PM2.5 with PM10 and long-term average PM2.5 mass concentration. Confounding between the two determinants was determined to be meaningful using a 10% change in estimate.
In the ultimate multivariable model, the adjusted percent change in nonaccidental mortality for correlation between daily PM2.5 and PM10 concentrations was 0.22 (0.06, 0.39), and for long-term average PM2.5 mass concentration was −0.17 (−0.31, −0.04). Adjusted r-squared for the final multi-determinant model was 10.38%.
4. Discussion
The goal of this meta-regression analysis was to explore if any of the available determinants related to size, source, or composition explained observed heterogeneity in the association between daily PM2.5 exposure and mortality. We identified several individual determinants that accounted for some heterogeneity, though the total variability explained for each was relatively low.
Higher long-term average PM10 and PM2.5 concentrations were associated with lower daily PM2.5-mortality effect estimates, which follows previous work showing greater health benefit of reductions (steeper relative slope) in PM2.5 in those counties achieving attainment compared to those that did not (Corrigan et al. 2018).
Higher correlation between daily PM2.5 and PM10 was associated with higher daily PM2.5-mortality effect estimates. In a study in Spain examining different size fractions, Perez et al. (2009) reported that the coarse fraction (PM10-2.5) was moderately associated with the intramodal fraction (PM2.5–1) and almost uncorrelated with PM1. While these relationships are likely to differ in different locales, it may suggest that when the daily PM2.5-PM10 correlation is higher PM2.5 may be made up of more intramodal particles than when the correlation is low. Sources and conditions that might contribute to high PM2.5-PM10 correlation include arid locales (windblown dust/dust generation), seasonality, and relative humidity conditions among others (Claiborn et al. 2000; Kegler et al. 2001; Kozákovác et al. 2018). Dosimetric studies have also shown that larger particles do not penetrate and deposit in the lower respiratory tract (U.S. EPA. 2019). The PM2.5/10 ratio was also identified as accounting for some heterogeneity, however the amount explained in this case was small (0.7% of total variability).
Emissions related to gas and diesel vehicle miles traveled were both inversely associated with daily PM2.5-mortality effect estimates; this might be explained by higher travel being an indicator for poorer exposure capture/measurement error, as the homogeneity across the MSAs may not accurately reflect intra-urban variability in PM2.5 concentrations (Dionisio et al. 2014; Dionisio et al. 2016). The PM2.5 components of ammonium, nitrate, and sulfate were all positively associated with percent change in PM2.5 related mortality. Some of these components have previously been identified as possible contributors to increase the magnitude of the PM2.5-mortality relationship (Franklin et al. 2008; Lippmann et al. 2013; U.S. EPA. 2019), and may be associated with specific source profiles for individual cities. However, estimated concentrations of these components were all highly correlated, and they can all be potentially high contributors to PM2.5 mass complicating the interpretation of these results.
Of these individual determinants, two were retained in the final multivariable model, correlation between daily PM2.5 and PM10, and long-term average PM2.5. This may be a function of the level of analysis at the CBSA, where PM2.5 mass is more spatially homogenous compared to individual components. It may also be because of measurement in general, as during the time period of this study there was limited data available for components, and emissions may not reflect direct concentrations in the same CBSA due to chemical transport.
Previous studies have investigated potential sources of heterogeneity in the PM2.5-mortality association, often focusing on PM2.5 components, sources, or related determinants; however, these studies were typically performed in fewer cities than this analysis. Several studies identified season or season-related determinants as a potential source of modification (Dai et al. 2014; Franklin et al. 2008; Lippmann et al. 2013; Zanobetti et al. 2014), suggesting that differences in sources or atmospheric chemistry across time of year are influential on the PM2.5-mortality association. Interestingly, a study performed across similar years and in a smaller number of cities did not observe seasonal differences (Krall et al. 2013). Lippmann et al. (2013) also identified sulfate and carbon monoxide (likely a traffic source indicator), among others, as having consistent associations with mortality in multi-city analyses.
Franklin et al. (2008) also examined the PM2.5 component to PM2.5 mass proportions as potential modifiers, finding sulfate as well as some metal components to be associated with higher PM2.5 related mortality. Some studies have examined regional patterns and city source profiles as a way to tease out potential sources of heterogeneity. Davis et al. (2011) found north-south differences wherein the northern U.S. cities had higher concentrations of sulfate and nitrate but noted that between city heterogeneity remained within regions. Baxter et al. (2013) examined differences in PM components and source profiles using paired cities within regions but did not identify any specific component or sources that could explain heterogeneity in mortality associations between city pairs. Across these studies, heterogeneity in effect estimates seems driven by complex interactions from a variety of determinants and no one component or source was more strongly associated with mortality than others.
This analysis adds to the existing body of literature by including more cities/CBSAs than have previously been included, and by having estimated PM component concentrations for each of these cities. However, potential limitations remain. Temporal variation for cities is likely well captured by central site monitors that are used for daily PM2.5 measurement, however these may not capture spatial variability in the sources and components within cities. Similarly, a 36 km2 model output was used for estimating component concentrations, which involved interpolating to census tracts and then aggregating to MSAs. There is likely uncertainty in estimations of component concentrations as well as in emissions sourced concentrations; low coefficients of variation may indicate a lack of power to detect effects rather than a true absence of effect due to specific components. Relatedly, we were unable to examine metal components which some have identified as potential contributors to PM toxicity, such as nickel or vanadium, as we lacked data on these components. We recognize that the data used in this analysis is older, as those were the data used for the initial mortality effect estimate estimation, and that there have been changes in source contributions to PM2.5 over time. For example, the growth in the number of wildfires over time has led to an increase in the proportion of PM2.5 emissions from wildfires (U.S. EPA. 2020a). Additionally, over the last 15 years there has been a dramatic change in the contribution of sulfate to overall PM2.5 concentrations, specifically in the eastern U.S. which can be attributed to the almost 65% reduction in SO2 concentrations that have occurred over this time period (U.S. EPA. 2019); from 2003–2005, sulfate accounted for close to 50% of overall PM2.5 mass, whereas from 2013–2015 it accounted for about a quarter to a third of mass (U.S. EPA. 2019). In CMAQ analyses, sulfate was the component with the largest total and percent decrease between 1990 and 2010 (−42%) (Peterson et al. 2020). Epidemiologic studies using more recent years of air quality data indicate that this change in the PM2.5 mixture does not impact the PM2.5-mortality association by demonstrating mortality risk estimates of similar magnitude compared to previous studies. While it is unlikely that this change will contribute to differences in PM2.5 effect estimates, it is important to recognize there have been changes in overall PM2.5 component concentrations over time. The fairly dramatic change in sulfate contributions to PM2.5 mass in the eastern U.S. and the continued relationship between short-term PM2.5 exposure and mortality provides evidence supporting that PM2.5 mass remains a good indicator of exposure, and suggests the potential that it is the mixture of PM2.5 itself that impacts health rather than an individual component. Numerous studies have shown that many PM2.5 components are associated with many health outcomes, including mortality, but no individual component has been shown to be more consistently associated with mortality than PM2.5 mass (U.S. EPA. 2019).
5. Conclusions
This study adds to the growing body of evidence indicating that the heterogeneity in PM2.5-mortality associations is multifactorial. Whereas the previous hypothesis around the observed heterogeneity was often attributed to variability in the composition of PM2.5 across locations, often with a focus on these differences being driven by an individual component, we did not observe such in this analysis. While some components did explain some of the heterogeneity, determinants related to overall PM2.5 mass were the strongest predictors. The determinants identified from this analysis can be combined with work done to explore other sources of heterogeneity, such as infiltration determinants and underlying population characteristics, to more fully explain the observed heterogeneity in PM2.5 mortality associations across the U. S.
Supplementary Material
Acknowledgements
The authors would like to gratefully acknowledge the data management and assistance of Alice Cates, and Drs. Thomas Luben and Alison Krajewski for input on early versions of the manuscript.
Funding Sources:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
AQS: Air quality system, CBSA: Core Based Statistical Area, CMAQ: Community Multiscale Air Quality modeling system, IQR: inter-quartile range, MD: Metropolitan Division, NCDC: National Climatic Data Center, NEI: Nation Emissions Inventory, PM: particulate matter, VMT Vehicle Miles Traveled
Disclaimer
The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, US EPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
References
- Atkinson RW, Mills IC, Walton HA, Anderson HR. 2015. Fine particle components and health--a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J Expo Sci Environ Epidemiol 25:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LK, Duvall RM, Sacks J. 2013. Examining the effects of air pollution composition on within region differences in pm2.5 mortality risk estimates. J Expo Sci Environ Epidemiol 23:457–465. [DOI] [PubMed] [Google Scholar]
- Baxter LK, Crooks JL, Sacks JD. 2017. Influence of exposure differences on city-to-city heterogeneity in pm2.5-mortality associations in us cities. Environ Health 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LK, Dionisio K, Pradeep P, Rappazzo K, Neas L. 2019. Human exposure factors as potential determinants of the heterogeneity in city-specific associations between pm2.5 and mortality. J Expo Sci Environ Epidemiol 29:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborn CS, Finn D, Larson TV, Koenig JQ. 2000. Windblown dust contributes to high pm25 concentrations. Journal of the Air & Waste Management Association 50:1440–1445. [DOI] [PubMed] [Google Scholar]
- Corrigan AE, Becker MM, Neas LM, Cascio WE, Rappold AG. 2018. Fine particulate matters: The impact of air quality standards on cardiovascular mortality. Environ Res 161:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Zanobetti A, Koutrakis P, Schwartz JD. 2014. Associations of fine particulate matter species with mortality in the united states: A multicity time-series analysis. Environ Health Perspect 122:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Meng Q, Sacks JD, Dutton SJ, Wilson WE, Pinto JP. 2011. Regional variations in particulate matter composition and the ability of monitoring data to represent population exposures. Sci Total Environ 409:5129–5135. [DOI] [PubMed] [Google Scholar]
- Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. 2017a. Association of short-term exposure to air pollution with mortality in older adults. JAMA 318:2446–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. 2017b. Air pollution and mortality in the medicare population. N Engl J Med 376:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Baxter LK, Chang HH. 2014. An empirical assessment of exposure measurement error and effect attenuation in bipollutant epidemiologic models. Environmental health perspectives 122:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Baxter LK, Burke J, Özkaynak H. 2016. The importance of the exposure metric in air pollution epidemiology studies: When does it matter, and why? Air Quality, Atmosphere & Health 9:495–502. [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. 2006. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasiolo M, Nedellec R, Goude Y, Wood SN. 2020. Scalable visualization methods for modern generalized additive models. Journal of computational and Graphical Statistics 29:78–86. [Google Scholar]
- Franklin M, Zeka A, Schwartz J. 2007. Association between pm2.5 and all-cause and specific-cause mortality in 27 us communities. J Expo Sci Environ Epidemiol 17:279–287. [DOI] [PubMed] [Google Scholar]
- Franklin M, Koutrakis P, Schwartz P. 2008. The role of particle composition on the association between pm2.5 and mortality. Epidemiology 19:680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan C-M, Pleim J, Mathur R, Hogrefe C, Long C, Xing J, et al. 2015. Assessment of long-term wrf--cmaq simulations for understanding direct aerosol effects on radiation” brightening” in the united states. Atmospheric Chemistry & Physics 15. [Google Scholar]
- Hastie T. 2019. Gam: Generalized additive models. R package version 1.16.1. Available: https://CRAN.R-project.org/package=gam.
- Ito K, Mathes R, Ross Z, Nadas A, Thurston G, Matte T. 2011. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in new york city. Environ Health Perspect 119:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegler SR, Wilson WE, Marcus AH. 2001. Pm 1, intermodal (pm 2.5–1) mass, and the soil component of pm 2.5 in phoenix, az, 1995–1996. Aerosol Science & Technology 35:914–920. [Google Scholar]
- Kelly FJ, Fussell JC. 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmospheric environment 60:504–526. [Google Scholar]
- Kozákovác J, Leoni C, Klán M, Hovorka J, Racek M, Koštejn M, et al. 2018. Chemical characterization of pm1–2.5 and its associations with pm1, pm2. 5–10 and meteorology in urban and suburban environments. Aerosol and Air Quality Research 18:1684–1697. [Google Scholar]
- Krall JR, Anderson GB, Dominici F, Bell ML, Peng RD. 2013. Short-term exposure to particulate matter constituents and mortality in a national study of u.S. Urban communities. Environ Health Perspect 121:1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzli N, Medina S, Kaiser R, Quenel P, Horak F Jr, Studnicka M. 2001. Assessment of deaths attributable to air pollution: Should we use risk estimates based on time series or on cohort studies? Am J Epidemiol 153:1050–1055. [DOI] [PubMed] [Google Scholar]
- Levy JI, Diez D, Dou Y, Barr CD, Dominici F. 2012. A meta-analysis and multisite time-series analysis of the differential toxicity of major fine particulate matter constituents. Am J Epidemiol 175:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M. 2012. Particulate matter (pm) air pollution and health: Regulatory and policy implications. Air Quality, Atmosphere & Health 5:237–241. [Google Scholar]
- Lippmann M, Chen LC, Gordon T, Ito K, Thurston GD. 2013. National particle component toxicity (npact) initiative: Integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res Rep Health Eff Inst:5–13. [PubMed] [Google Scholar]
- NCDC. National oceanic and atmospheric association, national climatic data center. Available: http://www.ncdc.noaa.gov/oa/ncdc.html [accessed January 2006.
- Nychka D, Hammerling D, Krock M, Wiens A. 2018. Modeling and emulation of nonstationary gaussian fields. Spatial statistics 28:21–38. [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. 2007. The effects of components of fine particulate air pollution on mortality in california: Results from calfine. Environ Health Perspect 115:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L, Medina-Ramón M, Kunzli N, Alastuey A, Pey J, Perez N, et al. 2009. Size fractionate particulate matter, vehicle traffic, and case-specific daily mortality in barcelona, spain. Environmental science & technology 43:4707–4714. [DOI] [PubMed] [Google Scholar]
- Peterson GCL, Hogrefe C, Corrigan AE, Neas LM, Mathur R, Rappold AG. 2020. Impact of reductions in emissions from major source sectors on fine particulate matter-related cardiovascular mortality. Environ Health Perspect 128:17005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria.:R Foundation for Statistical Computing. [Google Scholar]
- Shi L, Zanobetti A, Kloog I, Coull BA, Koutrakis P, Melly SJ, et al. 2016. Low-concentration pm2.5 and mortality: Estimating acute and chronic effects in a population-based study. Environ Health Perspect 124:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. 2015. 2011 national emissions inventory, version 2, technical support document.US Environmental Protection Agency Washington (DC). [Google Scholar]
- U.S. EPA. 2009. Integrated science assessment (isa) for particulate matter (final report, dec 2009). Washington, DC. [Google Scholar]
- U.S. EPA. 2019. Integrated science assessment (isa) for particulate matter (final report, dec 2019). Washington, DC. [Google Scholar]
- U.S. EPA. 2020a. Air emissions inventories: National emissions inventory (nei). Available: https://www.epa.gov/air-emissions-inventories/national-emissions-inventory-nei [accessed October 28, 2020 2020].
- U.S. EPA. 2020b. Air quality system (aqs). Available: https://www.epa.gov/aqs [accessed October 29, 2020 2020].
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 73:3–36. [Google Scholar]
- Zanobetti A, Schwartz J. 2009. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ Health Perspect 117:898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Coull BA, Gryparis A, Kloog I, Sparrow D, Vokonas PS, et al. 2014. Associations between arrhythmia episodes and temporally and spatially resolved black carbon and particulate matter in elderly patients. Occup Environ Med 71:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.