Abstract
Objective:
To establish if cessation of testosterone (T) therapy reverses T-induced acyclicity in a transgender mouse model that allows for well-defined T cessation timing.
Design:
Experimental laboratory study using a mouse model.
Setting:
University-based basic science research laboratory.
Animals:
A total of 10 C57BL/6NHsd female mice were used for this study.
Intervention(s):
Postpubertal C57BL/6NHsd female mice received subcutaneously implanted T enanthate (n = 5 mice) or control (n = 5 mice) pellets. Pellets were surgically removed after 6 weeks to ensure T cessation, after which mice were followed 4 cycles after resumption of cyclicity.
Main Outcome Measure(s):
Primary outcomes included daily vaginal cytology and weekly T levels before, during, and after T enanthate or placebo pellet implantation and removal. Additional secondary outcomes included ovarian follicle distribution and corpora lutea numbers, body metrics, and terminal diestrus hormone levels.
Result(s):
T-treated mice (100%) resumed cycling within one week of T pellet removal after 6 weeks of T therapy. T levels were significantly elevated during T therapy (p<0.0001) and fell to control levels with surgical pellet removal. There were no detectable differences 4 cycles later in follicle counts, corpora lutea formation, diestrus hormone levels, or body metrics with the exception of persistent increased clitoral area (p<0.0001) between T-treated mice and controls. One T-treated mouse was sacrificed early due to vaginal prolapse and not included in subsequent analyses.
Conclusion(s):
Our results demonstrate a close temporal relationship between estrous cycle return and T levels dropping to control levels following T pellet removal. The return of regular cyclic ovulatory function is also supported by the formation of corpora lutea and the lack of detectable differences in key reproductive parameters as compared to controls four cycles after T cessation. These results may be relevant to understanding the reversibility of T-induced amenorrhea and possible anovulation in transgender men interested in pausing T to pursue pregnancy or oocyte donation. Results may be limited by duration of T treatment, lack of functional testing, and physiological differences between mice and humans.
Keywords: testosterone, transgender, mouse model, ovary, reversibility
INTRODUCTION
Individuals whose gender identity does not align with their sex assigned at birth may identify as transgender. An estimated 1.4 million adults in the United States self-identify as transgender, comprising about 0.6% of the population (1); however, the true number may be higher due to surrounding stigma and limited data collection. Transgender individuals may seek medical treatment in order to affirm their gender identity through gender-affirming hormone treatment and/or surgery (2). For transgender men, testosterone (T) is typically used for masculinizing hormone therapy through intramuscular, subcutaneous, or transdermal routes of administration. Effects of T administration include the development of male physical contours, deepening of the voice, increased facial and body hair, and clitoral growth along with changes in body composition such as the redistribution of fat and changes in muscle mass (3).
Physicians have limited data to draw from when counseling patients about fertility after starting T therapy. Studies to date regarding gender-affirming T therapy and reproduction may leave us with “more questions than answers” (4). As such, counseling about fertility preservation is currently recommended prior to starting gender-affirming T therapy (2,5,6). T therapy has been shown to typically lead to the cessation of menses and the induction of ovarian characteristics of polycystic ovary morphology including collagenization of the tunica albuginea, stromal hyperplasia, and luteinization of stroma cells (4). Studies suggest that many transgender men do not preserve their gametes before beginning T therapy, potentially due to the expensive, invasive, and time-consuming nature of ovarian fertility preservation methods (7,8); however, some of these individuals may later be interested in using their gametes for reproduction.
For individuals interested in fertility after beginning T therapy, there has been minimal research regarding the reversibility of menstrual suppression and possible anovulation if T therapy is paused for reproductive purposes. There are anecdotal reports of clinics pausing T therapy for 1 to 6 months prior to ovarian stimulation for oocyte harvesting, although others have not paused T therapy at all (4). Given the health risks to the fetus posed by in utero T exposure, pausing T therapy is essential before attempting to carry a pregnancy (9). Light et al., (2014) surveyed 41 transgender men with successful birth outcomes, of which 25 had previously been on T. Of these 25 with previous T exposure, 80% resumed menses within 6 months of stopping T and 20% conceived while amenorrhoeic (10). Others have also reported anecdotal resumption of menses in some transgender men following T cessation for reproductive purposes, although timing and menstrual cycle architecture has not been well-characterized. In qualitative interviews of 15 transgender men pursuing fertility preservation, Armuand et al., noted that for 7 of the men who had already begun T, T therapy was discontinued until menstruation resumed, which took approximately 3–6 months (11). A case series on transgender and gender-nonconforming patients undergoing IVF included one transgender man who had previously started T and resumed menses one month after discontinuation of T therapy (12). In a comparison of ovarian stimulation for fertility preservation including 7 transgender men with prior T therapy, the time off T prior to simulation was 1–13 months and all were described as not experiencing amenorrhea at cycle start (13). A comparison of assisted reproductive technology (ART) outcomes in a cohort of transgender men, of which 16 had been on prior T therapy, noted that 13 (81.2%) of these men experienced menses resumption after stopping T for 1–12 months before their ART cycle (14).
We have developed a mouse model to allow for study of the impact of gender-affirming T therapy on reproductive function (15). For postpubertal female mice treated for six weeks with male range T therapy, we noted that T induced the cessation of cyclicity with persistent diestrus, a decrease in terminal LH levels, and an increase in atretic cyst-like late antral follicles with a lack of corpora lutea (15). The continued persistence or reversibility of these T-induced reproductive alterations is yet to be fully established. The objective of this study was to investigate the reversibility of T-induced changes on estrous cyclicity in a mouse model mimicking the T therapy used by transgender men that also allows for well-defined timing of T cessation.
MATERIALS AND METHODS
Ethical approval
Animal studies were completed in compliance with the protocol approved at the University of Michigan (PRO00007618) by the Institutional Care & Use Committee (IACUC).
Experimental design
This study used ten C57BL/6NHsd female mice (Envigo, Indianapolis, IN, USA). Mice were housed in groups of five in ventilated cages within a non-barrier facility with 12-hour light/dark cycles and free access to food and water at the University of Michigan, Ann Arbor. Mice were administered T therapy via a subcutaneously implanted T enanthate pellet (5 mg / pellet, 60-day release, n = 5 mice) or a placebo pellet (n = 5 mice) (Innovative Research of America, Sarasota, FL, USA). At the time of pellet administration, mice were 9–10 weeks old with a body mass of 18.6 ± 0.7 g (mean ± SD). After 6 weeks of T therapy, pellets were surgically removed under isoflurane to ensure well-defined timing of T cessation. Daily vaginal cytology and weekly blood were collected. After resumption of cyclicity and 4 estrous cycles, mice were sacrificed during diestrus and their ovaries were harvested for histological analysis. We estimated we would need at least n = 3 mice per group to detect expected differences in T levels and percent cyclicity between T-treated and control mice at 90% power.
Vaginal cytology
Vaginal cytology was performed daily throughout the study, for at least 2 cycles prior to T therapy, throughout the 6-week T therapy period, and subsequently following T cessation to track 4 estrous cycles. Stages of the estrous cycle were based on the distribution of cornified epithelial cells, nucleated epithelial cells, and leukocytes (16).
Weekly blood collection and serum hormone analysis
Blood was collected weekly from the lateral tail vein. Collections did not exceed 0.5% of body weight. Cardiac puncture under isoflurane anesthesia was used to collect terminal blood. Blood samples were stored overnight at 4°C. The next day, blood samples were centrifuged for 10 min (8100 g) and serum was collected and stored at −20°C. Peptide hormone analysis for luteinizing hormone (LH) and follicle stimulating hormone (FSH) and steroid hormone analysis (testosterone, estradiol, progesterone) were performed at the Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction. The reportable ranges that can be detected with a CV of <20% for the different hormonal measures were: 0.10–16 ng/mL (or 0.20–32 ng/mL with a 2x dilution) for the Testosterone Mouse & Rat IBL ELISA, 0.04–75.0 ng/mL for the LH Mouse & Rat in house protocol RIA, 2.1–45 ng/mL for the FSH Mouse & Rat in house protocol RIA, 0.15–40 ng/mL for the Progesterone Mouse & Rat IBL ELISA, and 3–300 pg/mL for the Estradiol Mouse & Rat CALBIOTECH ELISA.
Body weights and measures
Mice were weighed weekly prior to blood collection. On the day of sacrifice, the uterus and liver were weighed prior to fixation. Weights of the collected ovaries were not taken due to a desire to preserve extra ovarian structures (e.g. rete ovarii, oviducts). Images of external clitoral structures were taken while mice were under anesthesia and supine. ImageJ was used to measure clitoral length and width.
Histological analysis
Bouin’s fixative was used to fix ovaries along with extraovarian structures. All samples were sent to the Histology Core in the School of Dentistry at the University of Michigan for processing. Samples embedded in paraffin were sectioned serially with a thickness of 5 μm. Hematoxylin and eosin were used to stain every other slide.
Ovarian morphometry
Follicle counts were performed for every 10th section throughout one entire ovary per mouse at 20–40x magnification to identify primordial, primary, and secondary follicles using a light microscope (DM1000, Leica, Germany, 22 ± 3 (mean ± SD) slides examined per ovary). Experimental groups were blinded prior to analysis. Follicle distribution was recorded by follicle type in an ovary. An oocyte surrounded by a single layer of squamous granulosa cells was identified as a primordial follicle. An oocyte surrounded by a single layer of cuboidal granulosa cells were identified as a primary follicle, while secondary follicles had multiple layers (two or more) of cuboidal granulosa cells. Primordial and primary follicles were counted when a nucleus was present, and secondary follicles when the nucleolus was present, in order to prevent overcounting. Every 10th section throughout one entire ovary per mouse was imaged using a light microscope (DM1000, Lecia, Germany) at 5x magnification for counting corpora lutea and antral follicles and images were examined alongside each other to prevent overcounting. Antral follicles were identified based on the presence of an antral cavity. Late cyst-like antral follicles that did not have an oocyte connected to granulosa cells and had an attenuated granulosa cell layer (adapted from (17)) were labelled as atretic. Corpora lutea were identified by their increased eosinophilic cytoplasmic staining in discrete rounded structures.
Statistical analysis
GraphPad Prism 8 was used to analyze data with a single mouse being the unit of analysis. Results of a Shapiro-Wilk normality test were used to determine parametric or non-parametric testing. Parametric testing included ordinary one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test and Welch’s t-test. Non-parametric testing utilized Mann-Whitney tests. P<0.05 was considered the threshold for statistical significance. For analysis purposes, hormone levels below the level of detection were treated as value set for the lower limit of quantification.
RESULTS
T-induced persistent diestrus promptly reverses after T levels drop
All T-treated mice stopped cycling and entered persistent diestrus within one week of T pellet implantation (Fig. 1B, representative examples Fig. 1C). Notably, within one week of T cessation as defined by pellet removal, 100% of T-treated mice resumed cycling (Fig. 1B, representative examples Fig. 1C). All control mice implanted with a placebo pellet cycled throughout the pre-treatment, post-implantation, and post-removal periods (Fig. 1B, representative examples Fig. 1C). Longitudinal serum T levels (ng/mL, mean ± SD) were comparable at week 0 in control (0.37 ± 0.08) and T-treated (0.32 ± 0.09) mice prior to pellet implantation. Mice implanted with T pellets had elevated T levels over six weeks of pellet release (Week 1: 20 ± 3, Week 2: 12 ± 2, Week 3: 9 ± 2, Week 4: 7 ± 1, Week 5: 6.4 ± 0.6, Week 6: 5 ± 1), which were significantly higher (p<0.0001) than control levels (Week 1: 0.35 ± 0.10, Week 2: 0.34 ± 0.12, Week 3: 0.29 ± 0.10, Week 4: 0.36 ± 0.07, Week 5: 0.38 ± 0.12, Week 6: 0.26 ± 0.06). Following pellet removal at six weeks, T levels of T-treated mice (Week 7: 0.51 ± 0.03, Week 8: 0.53 ± 0.06, Week 9: 0.45 ± 0.17) were comparable to control levels (Week 7: 0.47 ± 0.11, Week 8: 0.50 ± 0.16, Week 9: 0.61 ± 0.18) (Fig. 1A). Subcutaneously implanted T pellets were relatively intact upon surgical removal (Fig. 1A).
Figure 1.
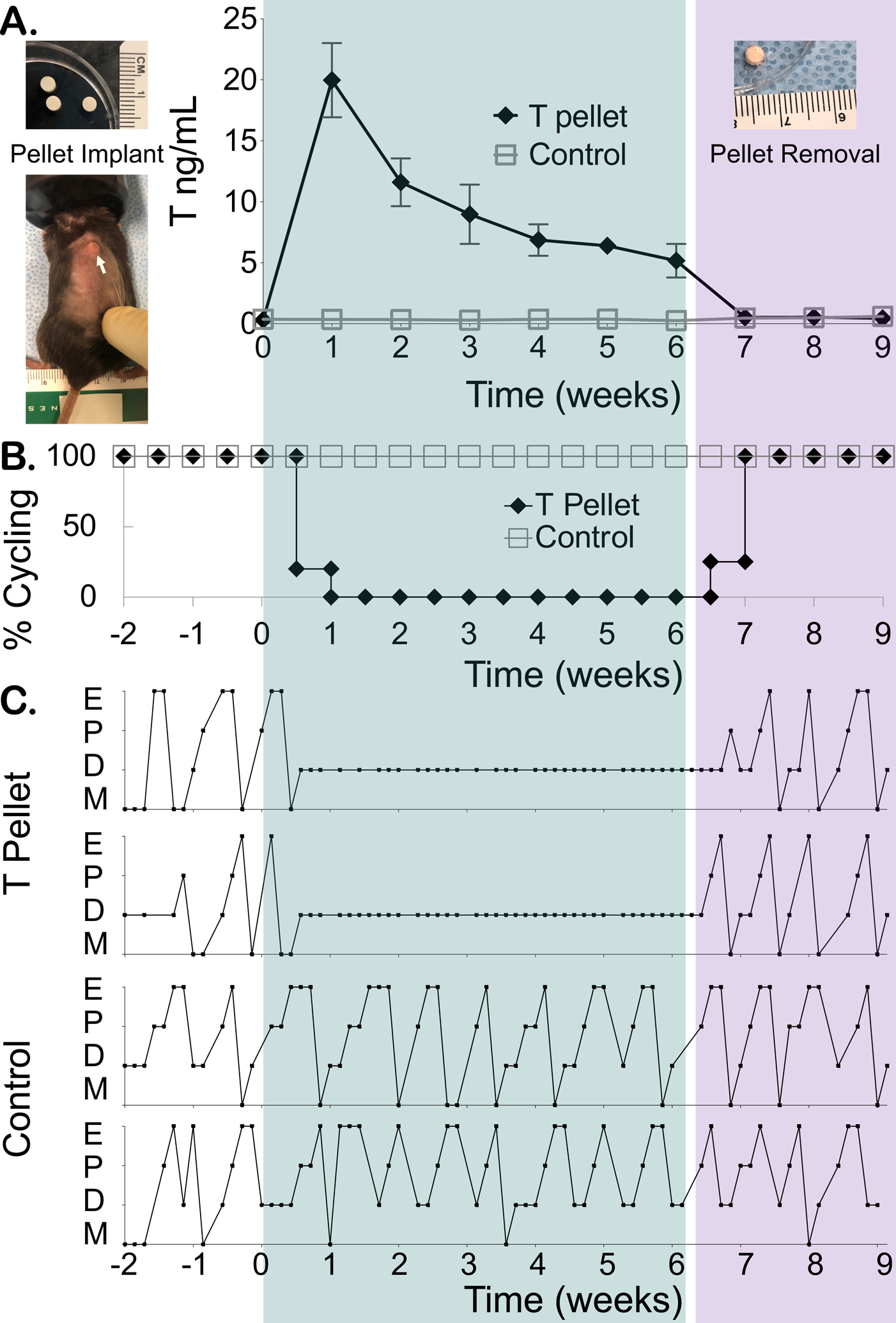
Reversibility of T-induced estrous cycle changes after T cessation. (A) Mice were subcutaneously implanted with a placebo (control) or T enanthate pellet at week 0, which was then removed after 6 weeks. Longitudinal weekly T levels were measured before pellet implantation (week 0), during T therapy (weeks 1–6) and following pellet removal (weeks 7–9) (mean ± SD, error bars shorter than symbol not shown, arrow points to subcutaneously implanted pellet). (B) All mice treated with T pellets at week 0 stopped cycling and demonstrated persistent diestrus until pellet removal at week 6, at which point estrous cyclicity resumed. (C) Two representative mice implanted with T pellets showed persistent diestrus during T therapy and prompt resumption of cyclicity following pellet removal, while two representative control mice continued cycling throughout the study (E = estrus, M = metestrus, D = diestrus, P = proestrus). Green shaded rectangle highlights 6 weeks of T therapy and purple shaded rectangle highlights 3 weeks following T cessation.
Corpora lutea noted with comparable follicle distribution following T cessation
Representative hematoxylin and eosin-stained control and post T ovaries, antral follicles, and corpora lutea are displayed in Figure 2. After resumption of cyclicity, previously T-treated mice demonstrated corpora lutea formation and did not significantly differ from controls in regard to follicular distribution counts. No difference was detected in primordial follicles post T (171 ± 15) as compared to controls (160 ± 44) (Fig. 3A), in primary follicles post T (44 ± 21) as compared to controls (41 ± 5) (Fig. 3B), or in secondary follicles post T (26 ± 7) as compared to controls (25 ± 8) (Fig. 3C). No difference was detected in the total number of antral follicles post T (33 ± 6) or in atretic late antral follicles post T (0.5 ± 1) as compared to the respective controls (30 ± 10), (0.8 ± 0.4) (Fig. 4D, 4E). Corpora lutea counts (5 ± 2) in post T mice also did not differ significantly as compared to control mice (8 ± 4) (Fig. 4F).
Figure 2.
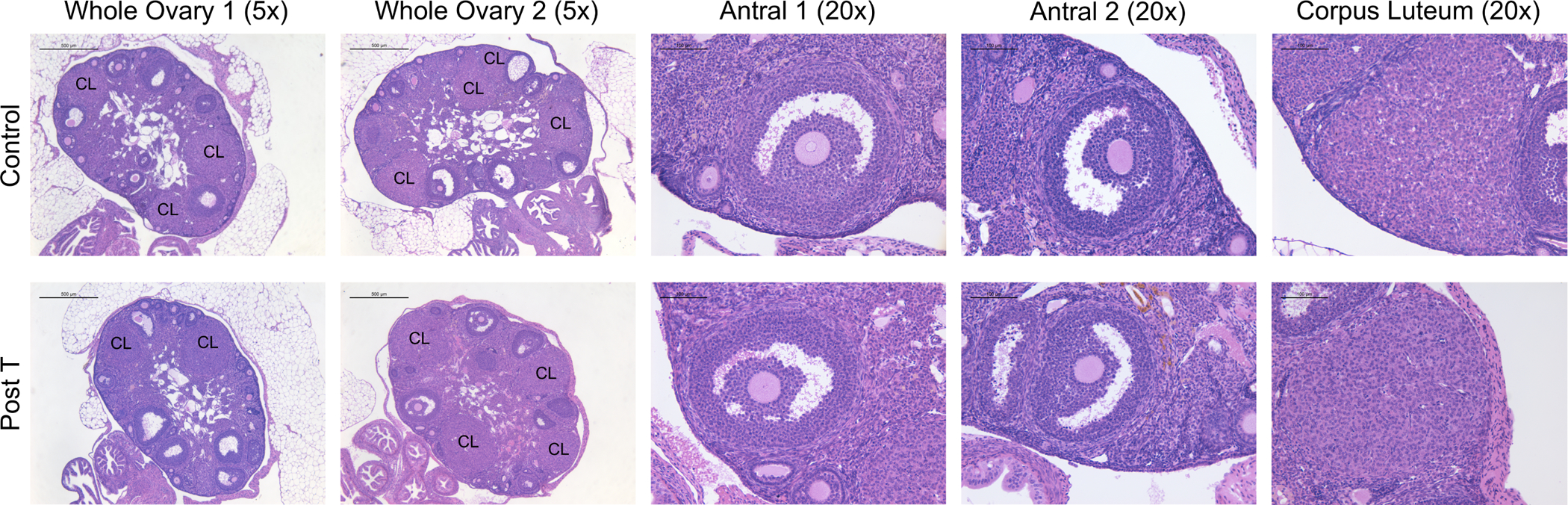
Comparable Ovarian Histology. Corpora lutea were noted in both control ovaries (row 1) and post T ovaries (row 2) (columns 1 and 2, 5x, hematoxylin and eosin stain, scale 500 μm). Post T mice were sacrificed after T cessation and 4 estrous cycles. Examples of higher magnification antral follicles (Columns 3 and 4, 20x, scale 100 μm) and corpora lutea (Column 5, 20x, scale 100 μm) for control ovaries (row 1) and post T ovaries (row 2).
Figure 3.
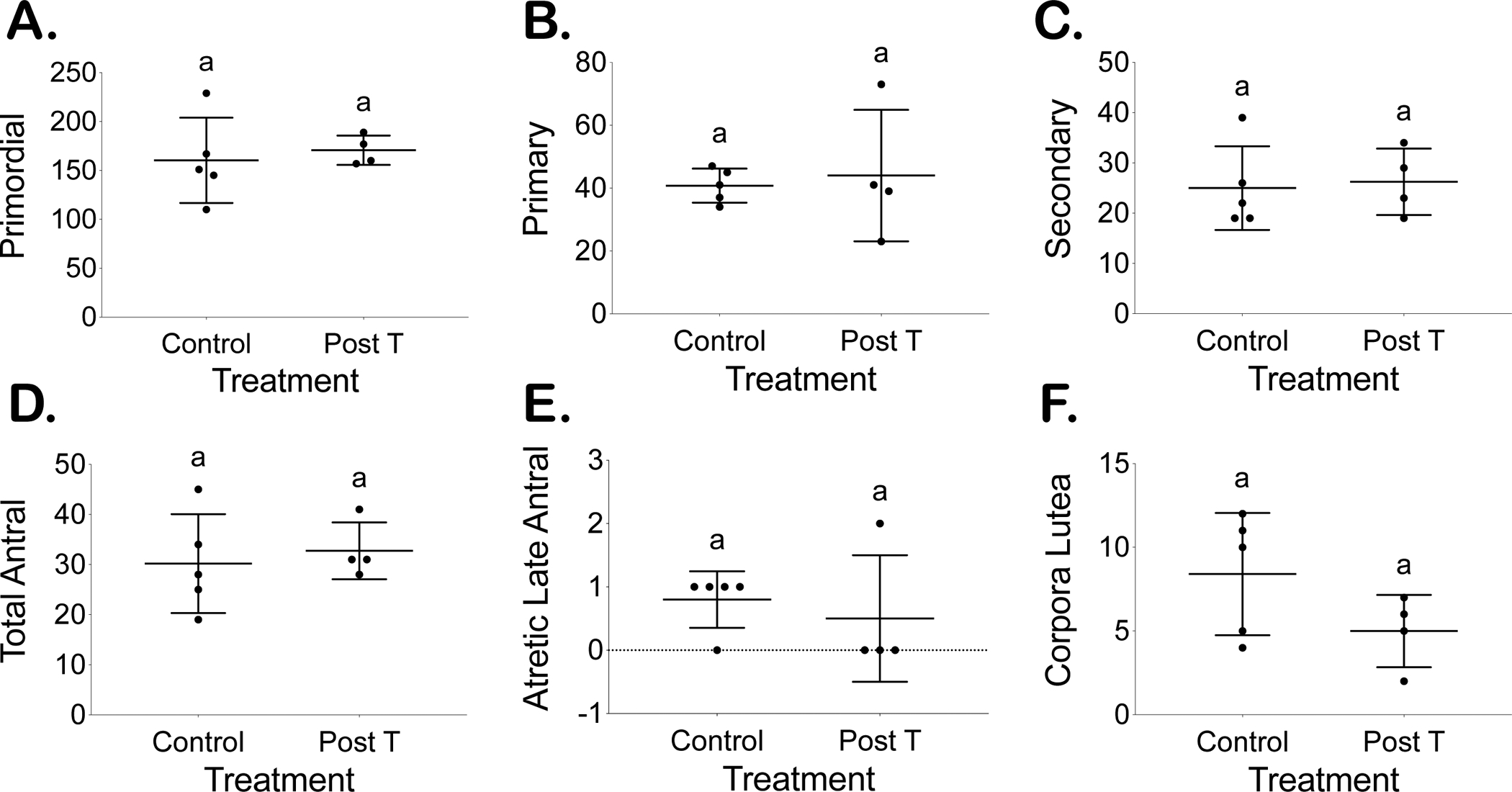
Comparable follicle counts from every 10th section between placebo (control) and T-treated mice sacrificed 4 cycles after T cessation (post T) for primordial (A), primary (B), secondary (C), total antral (D), and atretic late antral (E) follicles in addition to corpora lutea (F).
Figure 4.
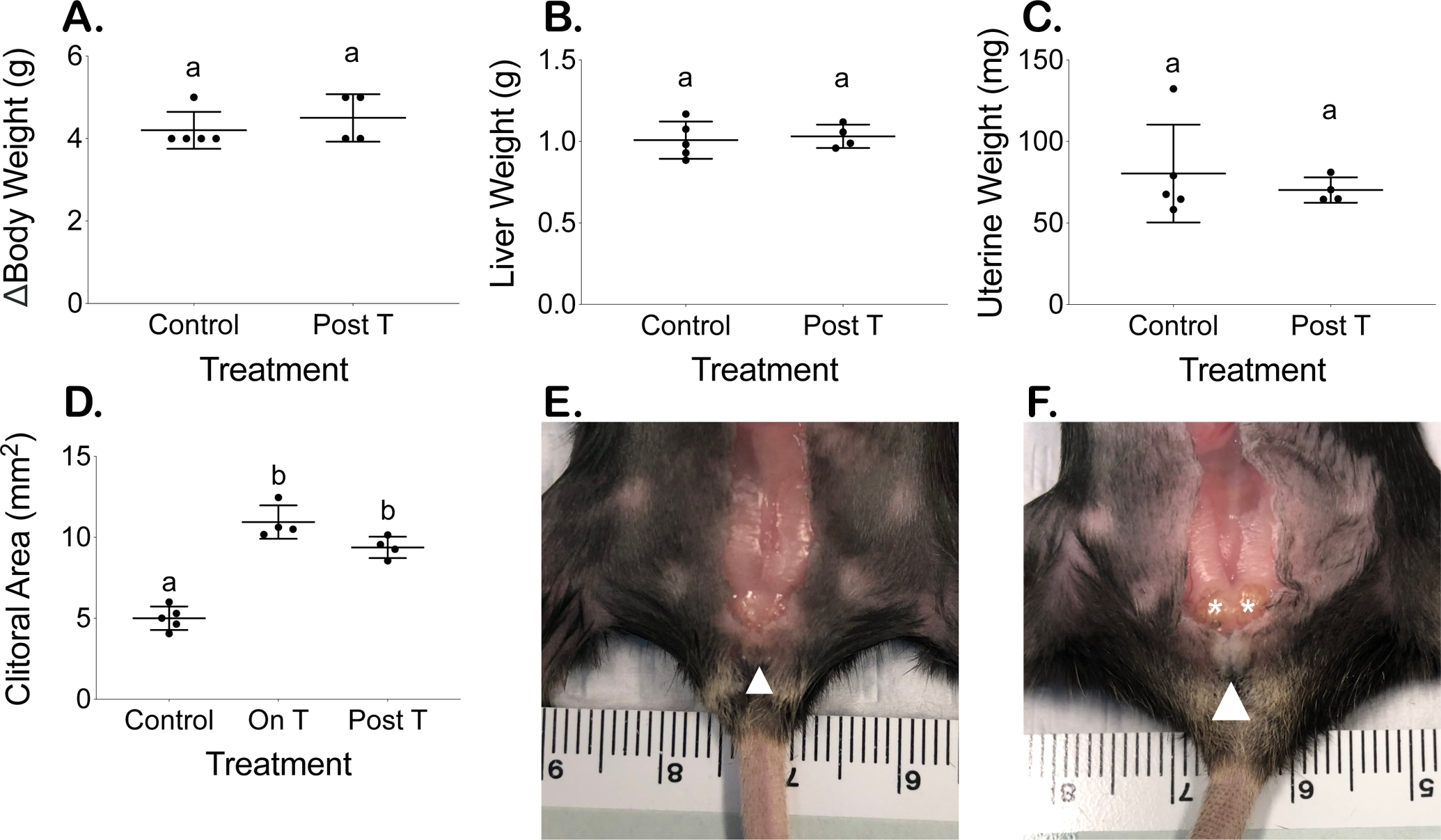
Terminal body measurements comparable except for persistent clitoromegaly. (A) Body weight increase over approximately 9 weeks, (B) terminal liver weight (normalized to terminal average of 22.9 g mouse), and (C) terminal uterine weight (normalized to terminal average of 22.9 g mouse) (mean ± SD). Increased clitoral area measured at 6 weeks on T remains persistently elevated 4 estrous cycles following T cessation (D) (mean ± SD). Clitoral size remains enlarged in post T mice (F) compared to control mice (E) with white arrowheads identifying clitoral structure and asterisks indicating glands enlarged by T therapy that are not readily visible in the control.
Body metrics comparable except for persistent T-induced clitoromegaly
The overall change in body weight (g) over approximately 9 weeks did not significantly differ between control (4.2 ± 0.4) and T-treated mice (4.5 ± 0.6) (Fig. 4A). Normalized terminal liver weights and uterine weights were also comparable between control mice (liver: 1.01 ± 0.11 g, uterus: 80 ± 30 mg) and mice post T therapy (liver: 1.03 ± 0.07 g, uterus: 70 ± 8 mg) (Fig. 4B and C). T-Treated mice demonstrated significantly increased clitoral areas after 6 weeks on T, which persisted 4 cycles after T cessation (On T 10.9 ± 1.0 mm2; Post T 9.4 ± 0.7 mm2) as compared to the clitoral areas of control mice (5.0 ± 0.7 mm2) (Fig. 4D, p<0.0001). The T-induced external clitoral size differences (white arrowheads) and internal gland enlargement (asterisks) are shown for an example mouse post T (Fig. 4F) as compared to a control mouse (Fig. 4E).
Terminal hormone levels comparable following T cessation
Collection of terminal hormones occurred during diestrus 4 estrous cycles after T pellet removal or from parallel diestrus controls. Post cessation of T therapy, there were no detectable differences in levels (mean ± SD) of LH, FSH, progesterone, or estradiol in post T mice (LH 0.2 ± 0.1 ng/mL; FSH 4 ± 3 ng/mL; progesterone 23 ± 2 ng/mL; estradiol 3.9 ± 0.8 pg/mL) as compared to control mice (LH 0.5 ± 0.3 ng/mL; FSH 7 ± 7 ng/mL; progesterone 18 ± 5 ng/mL; estradiol 4.8 ± 1.0 pg/mL) (Fig. 5).
Figure 5.
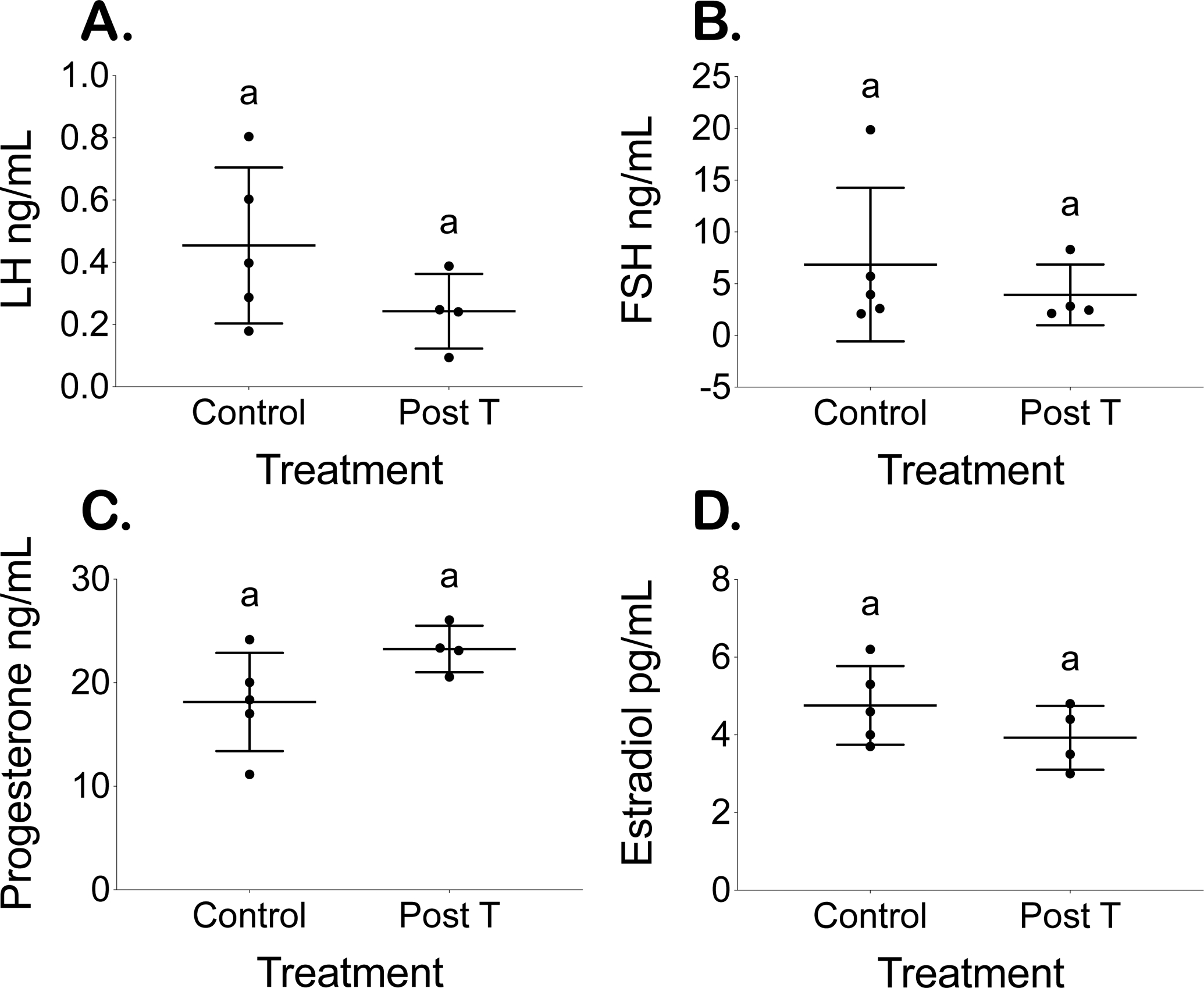
Comparable terminal hormone levels. Terminal (A) LH, (B) FSH, (C) Progesterone, and (D) Estradiol levels for placebo (control) and T-treated mice 4 cycles after T cessation (post T) (mean ± SD).
DISCUSSION
In this study, following pellet removal, T-treated mice in persistent diestrus all resumed cycling within a week. Pellet removal allows for a more precise assessment of estrous cycle reversibility timing than has previously been examined.
Our previous study investigating the effects of injectable T enanthate on postpubertal female mice demonstrated T-induced persistent diestrus with a lack of corpora lutea (15). Others have also noted T-induced persistent diestrus with a lack of corpora lutea in peripubertal mice treated with silastic T implants for 12 weeks (18). One recent study found that female mice injected weekly with T cypionate experienced persistent diestrus with a significant reduction in corpora lutea (19). This group went on to follow some of their mice after T cessation and note a resumption of cyclicity, but given that they only measured T levels once in the washout period (6 weeks after their last T injection), they were not able to tightly couple estrous cycle return timing to current hormonal milieu. A limitation to models using injectable T, including our previous study, is that subcutaneously injected hormones suspended in oil may take multiple weeks to fully washout (20). This makes precise determination of T cessation timing difficult to ascertain.
Given the lack of corpora lutea we previously noted during T therapy in T-treated female mice (15), it is promising to see corpora lutea formation 4 cycles after resumption of cyclicity following T cessation. Coupled with prompt estrous cycle return and no detectable differences in terminal diestrus hormone levels, these results suggest possible resumption of regular ovulatory cyclic function. We further assessed the ovarian follicular distribution and did not detect any significant differences between mice after T cessation as compared to their parallel controls. Terminal body metrics, including overall body weight increase, diestrus uterine weight, and liver weights were comparable between mice after T cessation as compared to controls, with the exception of a slight, but not significant reduction in T-induced clitoromegaly. In contrast to our study, Bartels et al. reported that T-induced clitoromegaly was no longer visually apparent 6–7 weeks after their last T injection, although they did not report measurements or note if they saw any T-induced internal clitoral gland changes (19).
Limitations to our study include the 6-week duration of T administration, which may not be reflective of more long-term gender-affirming T in humans. In addition, one T-treated mouse had to be sacrificed early due to vaginal prolapse that began during T therapy. Further investigation of T-induced structural changes to the vaginal epithelium and pelvic floor as well as longer-term assessments of T-induced reproductive changes are needed to more completely understand the reproductive consequences of T for transgender men. While mouse models of gender-affirming T therapy may not fully reflect human physiology, many similarities exist between murine and human reproductive function regulation and ovarian follicle development (21), such that findings from mouse models can be utilized to inform future clinical research directions.
Conclusions
In summary, we have shown prompt reversibility of acyclicity in female mice treated with T enanthate pellets for 6 weeks following well-defined pellet removal. After 4 cycles following T pellet removal, we did not detect differences in key reproductive parameters between T-treated mice and controls. Although further work is needed to understand the reversibility of T-induced amenorrhea and possible anovulation in transgender men interested in pausing T to pursue pregnancy or egg donation, our results suggest that cycle return may be tightly coupled to T levels dropping.
ACKNOWLEDGEMENTS
The authors thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for performing hormone analyses.
FUNDING
National Institutes of Health (R01-HD098233 to M.B.M., F30-HD100163 and T32-HD079342 to H.M.K.), American Society for Reproductive Medicine / Society for Reproductive Endocrinology and Infertility Grant to M.B.M., University of Michigan Office of Research funding (U058227) to A.S. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant (P50-HD28934).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
No authors report competing interests.
REFERENCES
- 1.Flores AR, Herman JL, Gates GJ, Brown TNT. How Many Adults Identify As Transgender in the United States? [Internet]. 2016. Available from: https://williamsinstitute.law.ucla.edu/wp-content/uploads/How-Many-Adults-Identify-as-Transgender-in-the-United-States.pdf
- 2.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab 2017;102:1–35. [DOI] [PubMed] [Google Scholar]
- 3.T’Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V. Endocrinology of Transgender Medicine. Endocr Rev 2019;40:97–117. [DOI] [PubMed] [Google Scholar]
- 4.Moravek MB, Kinnear HM, George J, Batchelor J, Shikanov A, Padmanabhan V, et al. Impact of Exogenous Testosterone on Reproduction in Transgender Men. Endocrinology 2020;161:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgenderism 2011;13:165–232. [Google Scholar]
- 6.Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril 2015;104:1111–5. [DOI] [PubMed] [Google Scholar]
- 7.Baram S, Myers SA, Yee S, Librach CL. Fertility preservation for transgender adolescents and young adults: a systematic review. Hum Reprod Update 2019;25:694–716. [DOI] [PubMed] [Google Scholar]
- 8.Auer MK, Fuss J, Nieder TO, Briken P, Biedermann SV., Stalla GK, et al. Desire to Have Children Among Transgender People in Germany: A Cross-Sectional Multi-Center Study. J Sex Med 2018;15:757–67. [DOI] [PubMed] [Google Scholar]
- 9.Wolf CJ. Effects of Prenatal Testosterone Propionate on the Sexual Development of Male and Female Rats: A Dose-Response Study. Toxicol Sci 2002;65:71–86. [DOI] [PubMed] [Google Scholar]
- 10.Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender Men Who Experienced Pregnancy After Female-to-Male Gender Transitioning. Obstet Gynecol 2014;124:1120–7. [DOI] [PubMed] [Google Scholar]
- 11.Armuand G, Dhejne C, Olofsson JI, Rodriguez-Wallberg KA. Transgender men’s experiences of fertility preservation: a qualitative study. Hum Reprod 2017;32:383–90. [DOI] [PubMed] [Google Scholar]
- 12.Broughton D, Omurtag K. Care of the transgender or gender-nonconforming patient undergoing in vitro fertilization. Int J Transgenderism 2017;18:372–5. [Google Scholar]
- 13.Adeleye AJ, Cedars MI, Smith J, Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet 2019;36:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung A, Sakkas D, Pang S, Thornton K, Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril 2019;112:858–65. [DOI] [PubMed] [Google Scholar]
- 15.Kinnear HM, Constance ES, David A, Marsh EE, Padmanabhan V, Shikanov A, et al. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum Reprod 2019;34:2009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cora MC, Kooistra L, Travlos G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol Pathol 2015;43:776–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell ASL, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014;155:3146–59. [DOI] [PubMed] [Google Scholar]
- 18.Aflatounian A, Edwards MC, Rodriguez Paris V, Bertoldo MJ, Desai R, Gilchrist RB, et al. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. J Endocrinol 2020;245:381–95. [DOI] [PubMed] [Google Scholar]
- 19.Bartels CB, Uliasz TF, Lestz L, Mehlmann LM. Short-term testosterone use in female mice does not impair fertilizability of eggs: implications for the fertility care of transgender males. Hum Reprod 2020;1–10. [DOI] [PubMed] [Google Scholar]
- 20.Deanesly R, Parkes AS. Note on the subcutaneous absorption of oils by rats and mice, with special reference to the assay of œstrin. J Physiol 1933;78:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters KA, Allan CM, Handelsman DJ. Rodent Models for Human Polycystic Ovary Syndrome. Biol Reprod 2012;86:1–12. [DOI] [PubMed] [Google Scholar]


