Abstract
The loss of skeletal muscle mass and size, or muscle atrophy, is a common human experience, linked to disability, for which there are no widely accepted pharmacological therapies. Piezo1 is a mechanosensitive cation channel that opens upon alteration of the plasma membrane lipid bilayer, such as through increased membrane tension. In this issue of the JCI, Hirata et al. identified Piezo1 and its downstream effectors, Krüppel-like factor 15 (KLF15) and interleukin-6 (IL-6), as an important signaling pathway in a murine model of disuse atrophy. Through genetic and pharmacological modulation of the pathway, the authors demonstrated that immobilization resulted in downregulation of Piezo1 and basal intracellular calcium concentration ([Ca2+]i), increasing expression of Klf15 and its downstream target Il6 and thereby inducing muscle atrophy. Piezo1 has been considered a therapeutic target for diverse disorders, including atherosclerosis and kidney fibrosis, and with this publication should now also be considered a viable target for disuse atrophy.
Muscle atrophy
Skeletal muscle atrophy triggers frailty, disability, and death across the lifespan and across the globe. It is associated with numerous etiologies, including chronic systemic disease, disuse, aging, denervation, and intrinsic disorders of muscle, thus affecting a large proportion of humanity. Despite the widespread prevalence and considerable consequences of muscle atrophy in terms of quality and quantity of life, there are very few therapeutic options beyond rehabilitative and nutritional therapies (1). Muscle atrophy, while not homogeneous in its etiology or pathophysiology, is recognized to reflect predominantly a shift in balance between protein synthesis and degradation, principally driven by the interaction of the anabolic insulin-like growth factor-1 (IGF-1)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway and the catabolic transcription factor forkhead box O (FoxO) and atrogenes (such as MuRF1 and MAFbx; refs. 2, 3). Other important modulators of muscle mass, many of which interact with the IGF-1/Akt/mTOR pathway, include myostatin, androgens, AMPK/PGC1α, IKKβ/NF-κB, and inflammatory cytokines (4–9).
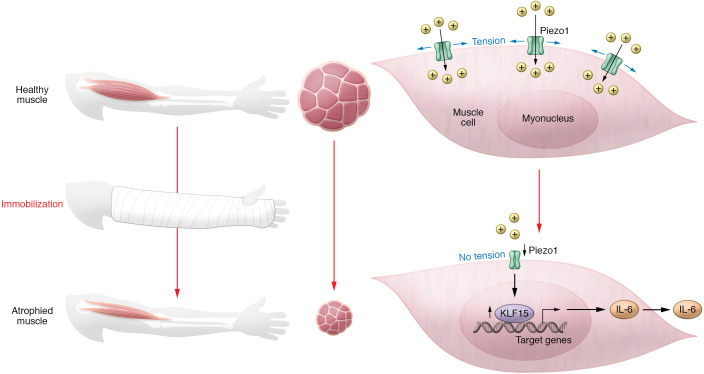
In this issue of the JCI, Hirata et al. profile an emerging, and potentially druggable, signaling pathway regulating muscle atrophy. The authors performed a series of comprehensive genetic and pharmacological experiments identifying the Piezo1/KLF15/IL-6 pathway mediating muscle atrophy following immobilization, such as occurs with limb casting (10). While the transcription factor Krüppel-like factor 15 (KLF15) and the cytokine interleukin-6 (IL-6) have been independently implicated in some forms of muscle atrophy, the association of Piezo1 with muscle atrophy represents an upstream event (11, 12). Piezo1 is a mechanosensitive cation channel that opens upon alteration of the plasma membrane lipid bilayer, such as through increased membrane tension (13). Hirata et al. proposed a process by which a reduction in mechanical stimulation during immobilization leads to downregulation of Piezo1. Reduced Piezo1 channel activity and gene expression would lower basal intracellular calcium concentrations ([Ca2+]i), increase KLF15 expression and, through KLF15 binding to the promoter region of IL6, increase IL-6–induced muscle atrophy (Figure 1). This narrative was supported by a dramatic reduction in Piezo1 and an increase in Klf15 and Il6 mRNA in skeletal muscle after limb immobilization in mice. The authors demonstrated that Piezo1 in myotubes was activated by mechanical stimuli and involved in the maintenance of basal [Ca2+]i. GsMTx-4, a pharmacological inhibitor of Piezo1, phenocopied atrophy induced by increased expression of Klf15 and Il6 while conversely, Yoda-1, an allosteric positive modulator of Piezo1, blunted the upregulation of the same genes after immobilization in mice. Downstream, tissue-specific knockout of Klf15 abrogated Il6 upregulation and muscle atrophy. Neutralizing antibodies against IL-6 prevented immobilization-induced upregulation of atrogenes and muscle atrophy. To address the translatability of these preclinical findings, human muscle biopsy samples from patients casted for fracture were compared with those from patients several months out from fracture and casting and the authors demonstrated that PIEZO1 mRNA was reduced, KLF15 showed a trend toward increased expression, and IL6 and various atrogenes were increased (10).
Figure 1. A reduction in mechanical stimulation during immobilization leads to downregulation of Piezo1 and muscle atrophy.
During muscle immobilization, such as through limb casting, the muscle atrophies primarily through a reduction in myofiber size. Hirata et al. (10) provide evidence that muscle atrophy occurs through decreased expression and activation of the cation channel Piezo1, which is sensitive to mechanical tension. Absent Piezo1 activation, an increase in the transcription factor KLF15 modulates the expression of multiple target genes, including IL6.
During immobilization, downregulation of Piezo1 and upregulation of Klf15 were observed in the non–satellite cell fraction, which contained multinucleated myofibers, fibroblasts, and endothelial cells. Conversely, Piezo1 and KLF15 changes were not observed in the satellite cell fraction, which corresponded with Pax7pos muscle stem cells (MuSCs), suggesting that the Piezo1/KLF15/IL-6 pathway of muscle atrophy occurs predominantly if not exclusively in myofibers (10). However, recently, two intriguing papers have also demonstrated key roles of Piezo1 in MuSC function, implicating Piezo1 in MuSC fusion and muscle regeneration (14, 15). MuSCs play a major role in muscle growth and regeneration but there is little evidence to support their role in acute muscle atrophy. Therefore, currently, from the flurry of recent papers on Piezo1 in skeletal muscle, it appears that the cation channel is critical in both myofiber and MuSC physiology where it is similarly sensitive to stretch and responsible for calcium influx but where it potentially activates different downstream signaling, gene expression, and cell functions.
A druggable target for muscle wasting
Piezo1 represents a potential druggable target in the quest to halt muscle wasting. There are still mechanistic gaps in the understanding of how Piezo channels are modulated by muscle activity. However, since Piezo1 is linked to multiple diseases, there is already good knowledge on the druggability of the channel with a wealth of structural and functional data, including an understanding of potential allosteric sites that can support rational design of putative isoform-selective Piezo modulators (16). Until then, the safety profile of Piezo1 modulators remains to be determined, since it is rather promiscuously expressed and since there are data suggesting that modulation of both Piezo1 and KLF15 may need careful titration to avoid adverse effects on muscle growth and regeneration (15, 17). Notwithstanding the fact that there are certainly many downstream effectors of Piezo1 activity, IL-6 may be a good alternative target in this pathway and there are already several approved IL-6 inhibitors, including anti–IL-6 receptor and anti–IL-6 monoclonal antibodies (18).
Hirata et al. identify Piezo1 as a relevant upstream target in muscle atrophy, warranting future exploration (10). Like any good study, it raises many additional questions: (a) How is Piezo1 modulated in mature myofibers and satellite cells in atrophy, degeneration, and regeneration? (b) Is there crosstalk between this pathway and other major known pathways directing muscle atrophy? (c) How does Ca2+ influx from the Piezo1 channel modulate KLF15 expression and what is the interplay with other Ca2+ sources, such as through voltage-gated calcium channels and the ryanodine receptor? (d) Is this pathway important in both acute and chronic processes involved in muscle atrophy? (e) And perhaps most importantly, are these findings generalizable to other etiologies of muscle atrophy? Hopefully, many of these questions will be answered while moving molecules on the path to clinical development.
Version 1. 05/16/2022
Electronic publication
Footnotes
Conflict of interest: RJ and KRW are employees of and own stock in F. Hoffmann-La Roche.
Copyright: © 2022, Jagasia et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(10):e159668. https://doi.org/10.1172/JCI159668.
See the related article at A Piezo1/KLF15/IL-6 axis mediates immobilization-induced muscle atrophy.
Contributor Information
Ravi Jagasia, Email: ravi.jagasi@roche.com.
Kathryn R. Wagner, Email: kathryn_r.wagner@roche.com.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiaffino S, et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 3.Wilburn D, et al. Shared and distinct mechanisms of skeletal muscle atrophy: A narrative review. Ageing Res Rev. 2021;71:101463. doi: 10.1016/j.arr.2021.101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest. 2021;131(9):e148372. doi: 10.1172/JCI148372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder PJ, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369–386. doi: 10.1210/er.2017-00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai D, et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Pascoe AL, et al. Controversies in TWEAK-Fn14 signaling in skeletal muscle atrophy and regeneration. Cell Mol Life Sci. 2020;77(17):3369–3381. doi: 10.1007/s00018-020-03495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuttle CSL, et al. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res Rev. 2020;64:101185. doi: 10.1016/j.arr.2020.101185. [DOI] [PubMed] [Google Scholar]
- 10.Hirata Y, et al. A Piezo1/KLF15/IL-6 axis mediates immobilization-induced muscle atrophy. J Clin Invest. 2022;132(10):e154611. doi: 10.1172/JCI154611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu N, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13(2):170–182. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz-Cánoves P, et al. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma N, et al. Piezo1 regulates the regenerative capacity of skeletal muscles via orchestration of stem cell morphological states. Sci Adv. 2022;8(11):eabn0485. doi: 10.1126/sciadv.abn0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quiroga HPO, et al. Fine-tuning of Piezo1 expression and activity ensures efficient myoblast fusion during skeletal myogenesis. Cells. 2022;11(3):393. doi: 10.3390/cells11030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alper SL. Genetic diseases of PIEOZO1 and PIEZO2 dysfunction. Curr Top Membr. 2017;79:97–134. doi: 10.1016/bs.ctm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Morrison-Nozik A. Glucocorticoids enhance muscle endurance and ameliorate Duchenne muscular dystrophy through a defined metabolic program. Proc Natl Acad Sci U S A. 2015;112(49):E6780–E6789. doi: 10.1073/pnas.1512968112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choy EH, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]