Abstract
Alzheimer’s disease and related dementias (ADRD) are among the top contributors to disability and mortality in later life. As with many chronic conditions, aging is the single most influential factor in the development of ADRD. Even among older adults who remain free of dementia throughout their lives, cognitive decline and neurodegenerative changes are appreciable with advancing age, suggesting shared pathophysiological mechanisms. In this Review, we provide an overview of changes in cognition, brain morphology, and neuropathological protein accumulation across the lifespan in humans, with complementary and mechanistic evidence from animal models. Next, we highlight selected aging processes that are differentially regulated in neurodegenerative disease, including aberrant autophagy, mitochondrial dysfunction, cellular senescence, epigenetic changes, cerebrovascular dysfunction, inflammation, and lipid dysregulation. We summarize research across clinical and translational studies to link biological aging processes to underlying ADRD pathogenesis. Targeting fundamental processes underlying biological aging may represent a yet relatively unexplored avenue to attenuate both age-related cognitive decline and ADRD. Collaboration across the fields of geroscience and neuroscience, coupled with the development of new translational animal models that more closely align with human disease processes, is necessary to advance novel therapeutic discovery in this realm.
Introduction
By 2030, an estimated one in five Americans will be 65 years of age or older (1). As a consequence, the prevention and treatment of chronic age-related diseases are of growing public health significance (2). Alzheimer’s disease and related dementias (ADRD), which induce progressive cognitive and functional impairment, are among the top contributors to disability and mortality (3). As with many chronic conditions, aging is the greatest risk factor for the development of ADRD. After the age of 65, the incidence of ADRD nearly doubles every 5 years, and by the ninth decade of life, approximately one of every three adults meets criteria for dementia (4). Even among older adults who remain free of dementia throughout their lives, cognitive decline and neurodegenerative changes are appreciable with advancing age (5), suggesting shared pathophysiological mechanisms. Here we provide a concise overview of brain structure and function changes across the human lifespan, and mechanistic insights from translational studies highlighting biological aging processes as propagators of cognitive decline and neurodegenerative disease.
Cognitive changes across the lifespan
As early as the third decade of life, core cognitive abilities, including processing speed, reasoning, episodic memory, and spatial visualization, begin to decline (6). Rather than a precipitous drop in old age, multivariate growth curve models have demonstrated small yet consistent diminishment in abilities across the lifespan (7). Individual cognitive domains vary with regard to their underlying neuroanatomical substrates and may decline at different rates within individuals. In aggregate, so-called “fluid skills” such as processing speed, memory, and reasoning, which rely on integration of new information, speeded response, and problem solving, tend to decrease more saliently (5). In contrast, “crystallized skills,” such as vocabulary and fund of knowledge, which are overlearned, practiced, and enhanced by experience, typically demonstrate greater stability throughout the lifespan (6). Despite variability across domains, longitudinal studies estimate that 30% to 60% of intraindividual cognitive change is attributable to a “domain-general effect” (7), which accounts for the global declines with advancing age. Similarly, experiments conducted in rodents across the lifespan have revealed age-associated deficits in late adulthood, including decrements in spatial and avoidance learning and memory (8, 9). Mice, like humans, also experience age-related changes in sensory modalities, including hearing and vision loss, which have been linked to accelerated cognitive decline (10). A recent review summarized mechanisms driving age-associated cognitive decline with a focus on changes in synaptic plasticity and intracellular calcium homeostasis (11). Other identified mechanisms entail hallmarks of aging including epigenetic changes, cellular senescence, autophagy, mitochondrial function, and inflammation, which are discussed in greater detail in later sections.
Lifespan changes in brain morphology and function
In the absence of disease or trauma, most neurons persist throughout the lifespan, with preclinical studies suggesting that they may even outlive their host if transplanted into a longer-lived animal (12). However, in humans, cerebral gray matter volumetry gradually declines, beginning in the second decade of life, with the most appreciable changes in the frontal and parietal lobes (13, 14). Rodent models similarly indicate a reduction of gray matter volumetry in advanced age (15, 16), along with increased ventricle cerebrospinal fluid (CSF) (15) and cerebral microbleeds (16). A growing appreciation for age-associated changes in neuronal chemistry, metabolism, and morphology coincident with neuronal dysfunction and inflammation has emerged (17).
The ability to engage in new learning and memory formation, as well as other complex cognitive processes, requires coordinated action of neurons across interconnected networks. Neuronal firing patterns induce changes in synaptic plasticity that can selectively strengthen or weaken network nodes (18). In aging and neurodegenerative disease, subpopulations of neurons demonstrate reductions in intrinsic excitability, while others exude hyperexcitability, altering the signal-to-noise output (19). Aberrant hyperexcitability, in particular, has been associated with detrimental cognitive outcomes in both human and animal models (20, 21). In Caenorhabditis elegans, advancing age is associated with higher neuronal excitability, while dampening these changes enhances longevity (22). Exceptionally long-lived humans demonstrate upregulation of the RE1 silencing transcription factor (REST), as well as downregulation of genes implicated in excitatory transmission (22). More pronounced changes in neuronal hyperexcitability occur in the context of neurodegenerative disease, increasing seizure likelihood and accelerating cognitive decline (21). Neuropathological protein accumulation in Alzheimer’s disease (AD) disrupts the balance of inhibitory and excitatory synaptic transmission, propagating neuronal dysfunction and DNA damage (23, 24). Other changes that occur in aging and neurodegenerative disease, such as reduced mitochondrial efficiency and higher production of reactive oxygen species, have also been shown to alter glutaminergic signaling and induce hyperexcitability (25). In mouse models of AD, suppressing neuronal hyperexcitability with levetiracetam prevented synaptic loss and preserved cognitive functioning (26). A phase III clinical trial of AGB101, HOPE4MCI, is currently evaluating the efficacy of targeting hyperexcitability in adults with neurodegenerative disease (NCT03486938; ClinicalTrials.gov).
Changes in metabolites across the lifespan have further revealed new molecular targets that may provide insights into cognitive impairment, including those suggestive of altered myelination of the white matter tracts (27). Cerebral white matter is composed of lipid-rich myelin, which is essential for efficient neuronal transmission. In humans, age-related declines in white matter integrity are most pronounced in anterior brain regions and have been shown to contribute to poorer processing speed and executive function (28). In older rats, the myelin sheath increasingly splits and becomes untethered to the axon, which has been attributed to decline in structural proteins such as myelin basic protein and cyclic nucleotide phosphodiesterase (29, 30). Furthermore, the myelin-generating cells, oligodendrocytes, decline in normal aging (31), resulting in loss of myelination and age-related reductions in white matter integrity. White matter hyperintensities also become increasingly prevalent in older age (32). Histopathological studies attribute white matter hyperintensities to demyelination, gliosis, myelin parlor, and tissue rarefaction (33), which may be propagated by varied mechanisms including cerebral ischemia, neuroinflammation, and blood-brain barrier dysregulation (34, 35). In animal models, age-related reductions in white matter capillary density, coupled with atherosclerosis of the small perforating arteries, increase vulnerability to hypoperfusion and ischemia, further damaging the white matter (36, 37).
AD neuropathological burden in aging and disease
The pathological hallmarks of AD, the accumulation of senile plaques composed of amyloid-β (Aβ) and neurofibrillary tangles derived from the aggregation of hyperphosphorylated tau, gradually accrue over decades in the context of both normal aging and neurodegenerative disease (38). With improvements in neuroimaging techniques, Aβ and transentorhinal tau have been detected in adults beginning in middle adulthood (ages 30–49; ref. 39). Evidence from AD mouse models suggests that pathological tau may spread across the brain, converting normal tau proteins into the pathological hyperphosphorylated form (40, 41). In wild-type mice, brain extracts from humans or transgenic mice with tauopathies have been shown to induce neurofibrillary tangles that can spread from the injection site to interconnected brain regions (42, 43). Aβ has also been shown to display seeding properties (44). Furthermore, Aβ and hyperphosphorylated tau, as well as broader neuropathological proteins such as α-synuclein, may interact to accelerate the overall neuropathological burden in the brain (44, 45). In Aβ-expressing mice, the addition of human tau dampens the expression of genes involved in synaptic regulation, further inducing deleterious effects on the CNS (46).
While accumulation of Aβ and tau is linked to AD, neuropathology in old age is common even in the absence of cognitive impairment. A postmortem study of 161 cognitively unimpaired adults reported that 86% displayed at least one type of neuropathology, with approximately two-thirds displaying multiple pathologies (47). Moreover, a recent meta-analysis of 4477 adults reported that approximately one-third of individuals with intermediate to high AD neuropathology remained free of dementia throughout their lives (48). Histological evidence suggests that individuals with high neuropathological burden and normal cognition may demonstrate resistance to the synaptic degradation that typically occurs with neuropathological protein accumulation (49). Several research groups are actively exploring mechanisms mediating cognitive resiliency.
Biological aging hallmarks of cognitive decline and ADRD
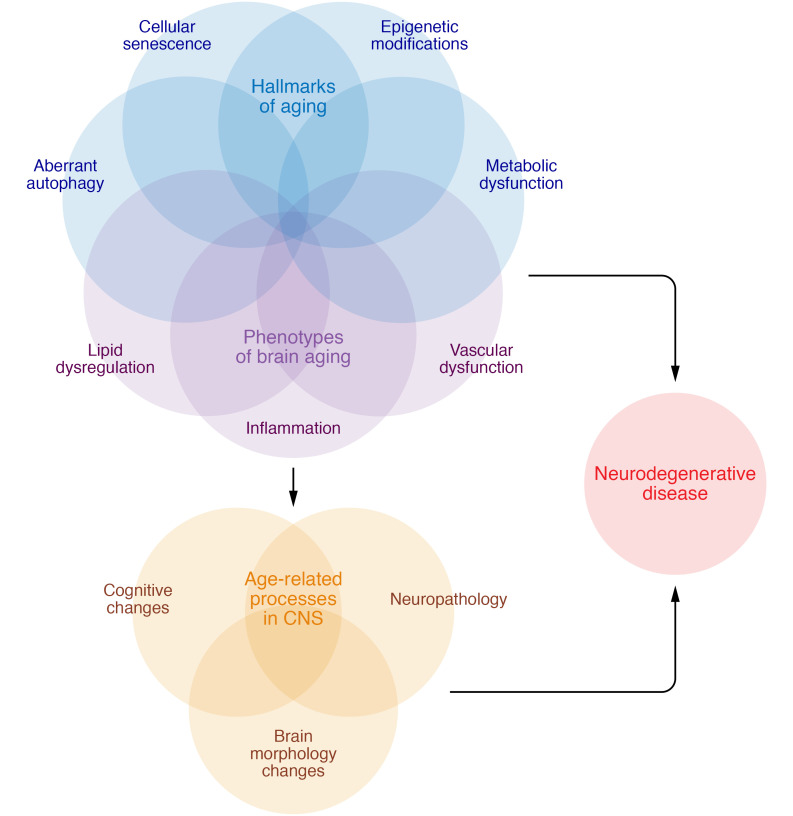
Population studies have demonstrated that aging is the single most influential risk factor for the development of sporadic ADRD (4). In addition, processes linked to neurodegenerative disease, including cognitive decline, cerebral atrophy, white matter degradation, and neuropathological protein accumulation, gradually manifest across the lifespan even among individuals who will remain free of dementia throughout their lives (5). Therefore, biological pathways underlying normal cognitive aging and ADRD are likely to overlap, existing along a continuum (50). Targeting fundamental processes underlying biological aging may represent a yet relatively unexplored avenue to attenuate both age-related cognitive decline and ADRD (51). The biology-of-aging field has made substantial gains in identifying the pathophysiological processes that contribute to biological aging and multisystem organ decline (52). In a seminal paper, Lopez-Otin et al. defined nine hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, stem cell exhaustion, altered intercellular communication, and cellular senescence (52). These aging hallmarks and others have been implicated as pathogenic factors underlying numerous chronic age-related diseases, including ADRD (Figure 1). In animal models, targeting biological aging processes has extended both lifespan and healthspan (53), suggesting the possibility that these approaches may have beneficial effects for cognitive health as well (51, 54). The following sections highlight selected aging processes that are differentially regulated in ADRD and have been mechanistically linked to pathogenesis.
Figure 1. Interactions of biological aging processes with CNS changes.
The hallmarks of aging, such as epigenetic modifications, cellular senescence, metabolic dysfunction, and aberrant autophagy, as well as other phenotypes of brain aging, including inflammation, vascular dysfunction and loss of blood brain barrier integrity, and lipid dysregulation, interact to contribute to age-related processes in the CNS, including cognitive decline, neuropathological protein accumulation, and brain morphology changes. These same factors are further dysregulated in neurodegenerative disease. Further investigations are necessary to determine the specific factors and sequences that force the transition between normative age-related changes and manifest neurodegenerative disease in some individuals while others remain cognitively resilient.
Aberrant autophagy.
The inability of postmitotic cells, such as neurons, to dilute proteotoxic burden and cellular waste through cell division increases their vulnerability to proteotoxic insults (55). Autophagy, along with the ubiquitin-proteasome system, provides relief by catabolizing proteins. Autophagy subtypes (e.g., microautophagy, chaperone-mediated autophagy, and macroautophagy) result in lysosomal degradation of substrates, including pathogenic forms of aggregate-prone proteins (i.e., Aβ, tau, and α-synuclein), lipids, dysfunctional mitochondria, and other organelles (56). Healthy neurons maintain constitutively active, highly efficient autophagy (57). Neurons in aged brains display higher levels of polyubiquitinated proteins than those in young brains; the age-associated effect becomes further elevated in the context of neurodegenerative disease (58). The requirement of autophagy activation in memory formation (59) further underscores the critical importance of its regulation for brain function. Postmortem examination of human brains with AD indicates aberrant autophagy; however, there have been conflicting reports about the directionality of dysfunction (60). Discrepancies may reflect methodological challenges associated with measuring and interpreting autophagic flux in tissue; differences in the brain regions, cell types, and species evaluated; the specific form of autophagy studied; the etiological factor(s) driving neurodegeneration; and differences in normalization controls.
Laser capture microdissection to evaluate autophagy in CA1 hippocampal neurons revealed elevated activation, but a progressive decline in lysosomal clearance across AD severity (61). Other studies indicate that Beclin-1, an autophagy-initiating protein, is reduced in AD compared with controls (62). Mechanistic studies in vitro and in vivo have demonstrated that a reduction in Beclin-1 can drive extracellular Aβ deposition (63), which protects neurons from toxic intracellular accumulation (64). Changes in Beclin-1 levels are important, as this protein negatively regulates transcription factor EB (TFEB) (65), a master transcriptional regulator of lysosome biogenesis and autophagy. Levels of nuclear (i.e., active) TFEB have been shown to progressively decrease across advancing Braak stages (66). In rodent studies, increasing TFEB reduced pathogenic tau accumulation and neurodegeneration (67); exosomal exocytosis may have contributed to the clearance of intraneuronal tau (68). Chaperone-mediated autophagy (CMA) has emerged as a critical mediator of intraneuronal tau clearance. Wild-type tau is degraded primarily through CMA; however, tau acetylation blocks CMA and redirects it toward extracellular release, increasing pathogenic spread (69–71). These studies collectively highlight the role of autophagy in eliminating intracellular neurotoxic proteins by either degrading or secreting them, as well as the essential function of extracellular clearance mechanisms for preventing the subsequent propagation of neuropathological proteins.
Mitochondrial and metabolic dysfunction.
Mitochondria utilize oxygen for cellular respiration, extracting, transferring, and producing energy from molecular substrates derived from glucose, fat, fatty acids, and amino acids. They also contribute to calcium and iron homeostasis, cell proliferation and cell death, cell signaling, and proteostasis, thereby broadly connecting mitochondrial function with cell viability and function, and other hallmarks of aging (72). The brain is a highly metabolically active organ that requires approximately 20% of the body’s basal oxygen to optimally function (73). Reactive oxygen species (ROS) are a by-product of oxidative phosphorylation that function as a critical signaling molecule; however, their accumulation (i.e., through dysfunctional mitochondria or poor antioxidant scavenging; ref. 74) can lead to oxidative stress, lipid peroxidation, and DNA damage (75, 76). Mitochondrial changes have been proposed to drive aging (i.e., the free radical theory of aging; ref. 77) and AD (i.e., the “mitochondrial cascade” hypothesis of AD; ref. 78). The critical importance of balanced mitochondrial activity is evidenced by data demonstrating lifespan extension both by the increasing of cellular metabolism and antioxidant capacity in models (79, 80) and by interventions designed to decrease mitochondrial function or enhance ROS production (81, 82). These longevity benefits may occur through a reduction in ROS production by which improving mitochondrial oxidative stress resistance increases lifespan, suggesting that a little mitochondrial stress may be beneficial (83).
Elegant studies designed to determine the role of mitochondrial dysfunction in driving aging and disease highlight its complexity. Levels of mitochondrial DNA (mtDNA) mutations increase with age; however, results from mtDNA mutator mice indicate that these mutations do not drive oxidative stress nor accelerated aging until at extreme levels far exceeding those found in aging humans (84). The level of total mtDNA decreases with age and is reduced more in AD than in cognitively normal age-matched controls (85). Single-cell analyses indicate an increase of mtDNA deletions in AD neurons (69) that is also observed in CSF (86) and blood cells (87). Through elegant cybrid experiments (which involve transferring mtDNA from donor cells to those with identical nuclear DNA but lacking mtDNA), AD mtDNA was shown to be responsible for subtle differences in mitochondrial morphology, biogenesis, and membrane potential; oxidative stress; and calcium buffering capacity (88). The observed differences in mitochondrial phenotypes that co-occur in peripheral tissues of individuals with AD compared with controls suggest that systemic changes in mitochondrial status relevant to the brain may be identified and tracked in peripheral samples. Such data provide evidence that mitochondrial dysfunction may be upstream, and not a consequence of AD neuropathology. Nevertheless, pathogenic Aβ and tau negatively impact mitochondrial function (89, 90), which may suggest that once mitochondrial dysfunction is initiated, a pathogenic feedback loop involving oxidative stress and pathogenic protein accumulation may ensue. Further studies are needed to determine whether disease conditions (like AD) represent exacerbated “normal” age-associated changes in mitochondrial function (91) or unique divergent pathogenic processes.
Cellular senescence.
Cellular senescence is a stress-induced cell state induced by macromolecular damage that culminates with cell cycle arrest and concomitant, often deleterious, secretory phenotype (92). Cells that become senescent evade cell death by upregulating antiapoptotic pathways and arresting the cell cycle. Senescent cells also secrete molecules including proinflammatory cytokines, chemokines, growth factors, extracellular remodeling proteins, and other signaling factors that alter the extracellular environment, collectively referred to as the senescence-associated secretory phenotype (SASP) (93). In the absence of senescent cell clearance, the SASP causes tissue damage, cell death, or the transition of other cells to become senescent, thus propagating the phenotype (94). With advancing age, senescent cells increase in tissues throughout the body, including the brain (95, 96).
Rodent studies have demonstrated senescent cell accumulation in the brain in response to accumulation of tau (90, 97) or Aβ protein (98); dysfunctional immune system (96); high-fat diet or obesity (99); insulin resistance (100); chronic unpredictable stress (101); environmental neurotoxins (102); and brain injury (103). Studies using postmortem human brain tissue have identified multiple senescent cell types in AD, including astrocytes (104), neurons (90, 105), microglia (106), oligodendrocyte precursor cells (98), and endothelial cells (107). Unbiased single-cell transcriptomics on dorsolateral prefrontal cortex from human AD revealed excitatory neurons as a prominent senescent cell type driven by CDKN2D (encoding p19) that overlapped with neurons bearing neurofibrillary tangles (NFTs) (105). In contrast, bioinformatics analyses of data derived from bulk tissue from healthy human tissue donors revealed that prominent senescent cell types in the brain included endothelial cells and microglia driven by CDKN1A (108). These studies, both conducted by our group, highlight potential differences in senescent cell types (a) in health versus disease; (b) possibly as a reflection of the starting material (i.e., single-cell, single-nucleus, or bulk tissue analyses); and (c) owing to differences in the predetermined criteria for senescence. Immunosenescence, described below, drives senescent cell accumulation in the brain (96). Microglia, the macrophage-like cells of the brain, clear NFT-bearing neurons that display phosphatidylserine on their surface (109). Given that microglia become senescent and dysfunctional after clearing these possibly senescent, NFT-bearing neurons (105), therapeutic strategies to help remove senescent cells from the brain may alleviate senescent cell burden, inflammation, and disease propagation (90, 98). Clinical trials are currently under way to test this approach (110, 111).
Epigenetic changes.
Epigenetic processes allow cells to integrate external stimuli into their genome to impact gene expression without altering the DNA sequence. These dynamic, reversible modifications include DNA methylation, chromatin remodeling, histone modification, and noncoding RNA regulation (microRNAs) (112). Neuronal epigenetic changes are crucial for synaptic plasticity and new memory formation (113). With age, DNA methylation in the brain trends toward global decreases, but there are sex-dependent dimorphisms (114, 115). Given that DNA methylation inhibits gene transcription, these changes may result in elevated gene expression. Genes implicated in AD, including those coding for APP, MAPT, BDNF, ABCA7, ANK1, BIB1, SORL1, and SIRT1, show differential methylation between individuals with AD and controls (116, 117). Breast cancer type 1 susceptibility protein (BRCA1), a DNA repair protein typically associated with breast cancer, is hypomethylated in AD. Elevated BRCA1 localizes to the cytosol, where it coaggregates with insoluble tau. In vitro studies suggest that this impacts neurite and dendritic spine morphology (118). Moreover, epigenetic age acceleration was found to be heritable in AD, where it was associated with neuropathological protein accumulation and cognitive decline (114, 119). Collectively these data suggest that epigenetic changes may increase AD susceptibility.
The frequency and pattern of epigenetic changes, specifically DNA methylation at CpG sites, can be used to generate an algorithm for comparing chronological age with biological age, termed an epigenetic clock. There are currently more than seven different epigenetic clocks developed for human assessments (120–126) and others for mouse models (127, 128). These differ in numbers of methylated CpGs, tissue type, and study populations. The current clocks lack correlation among them (129, 130). Nevertheless, understanding the relationships between DNA methylation, age, longevity, and age-related disease may hold promise to predict disease, including diseases relevant to the brain (131). While initial epigenetic clocks were based in blood, recent advances are moving to the brain to predict cortical age (130, 131). The recently developed Cortical clock provides evidence supporting the use of the epigenome to inform regarding brain aging and pathologies (132). The Cortical clock was trained using postmortem cortical tissue from older adults, which tracked better with AD diagnosis and Aβ deposition than clocks trained using blood. While blood-based clocks correlated with chronological age at death when applied to cortical tissue (130), only the Cortical clock significantly associated with tau and Lewy body pathology, highlighting the importance of considering tissue-specific epigenetic changes in these predictions.
Chromatin remodeling and chromatin heterogeneity (or what has been termed epigenetic noise) also increase with age. Histone acetylation tends to decrease with aging, resulting in a more condensed chromatin structure and consequent transcriptional changes (133). A recent assessment of postmortem human brain tissue revealed an upregulation of two histone acetyltransferases, H3K27ac and H3K9ac, that were linked with Aβ pathology and neurodegeneration by human proteomics data and a transgenic fly model (134, 135). Three AD mouse models and one nonhuman primate model displayed epigenetic changes that differed across models (136). This work again emphasizes the complexity of genetic and epigenetic influence on disease progression, as well the importance of matching model systems to the underlying pathogenic process in question.
Unlike the above-mentioned epigenetic alterations, microRNAs (miRNAs) influence gene expression post-transcriptionally by binding to mRNA (137). miRNAs play critical roles in AD pathology, including modulating Aβ and tau production/function, synaptic plasticity, neuronal growth, apoptosis, and inflammatory response (138). In AD, disruptions have been noted in several miRNAs, including miRNAs 9, 124, 125b, 132, 146a, and 155, which may have the potential to serve as both biomarkers and therapeutic agents (138, 139).
Vascular dysfunction and diminished blood-brain barrier integrity.
Epidemiological evidence supports an association between risk factors for cardiovascular disease, cerebrovascular dysfunction, and cognitive impairment. More than 50% of individuals with ADRD have concomitant vascular pathologies that increase with advancing age (140). Furthermore, growing evidence indicates that the molecular mechanisms associated with both vascular and ADRD pathologies act synergistically to compromise cognition (140). Vascular contributions to cognitive impairment and dementia (VCID) derive from age-related changes to the neurovascular unit (NVU), which is composed of nonfenestrated endothelial cells, pericytes, smooth muscle cells, astrocytes, microglia, oligodendroglia, and neurons (141). The NVU facilitates normal brain function by ensuring neurovascular coupling, the physiological mechanism whereby cerebral blood flow is matched to neuronal metabolic demands (142). With aging, and to a greater extent in neurodegenerative disease, there is a loss of pericytes, which has been associated with diminished cerebral blood flow delivery in both human and animal models (143). In mouse models of AD, pericyte loss has also been shown to reduce Aβ clearance, further propagating neuropathological protein accumulation (144). In addition, age-related changes in mitochondrial efficiency and the upregulation of ROS induce endothelial dysfunction, which diminishes the bioavailability of the vasodilator nitric oxide and further dampens neurovascular coupling (145).
The NVU is also important for the maintenance of the blood-brain barrier (BBB), which controls transport of substances across the endothelium into the CNS through specific transporters on both the luminal and abluminal surfaces (141, 146). BBB integrity declines in normal aging and even more dramatically in ADRD. Loss of BBB function induces capillary leakage, brain leukocyte infiltration (141), ingress of toxic substances, and upregulation of TGF-α signaling in astrocytes, resulting in disruption of the brain milieu and neuronal dysfunction (147). BBB leakage has been identified in the hippocampi of individuals with mild cognitive impairment, which correlates with CSF levels of PDGF-β, a marker of damaged pericytes (146). Loss of BBB integrity further drives neuroinflammation, which has been implicated in aging and ADRD.
Inflammaging.
It has been well established that systemic inflammation increases with age, as evidenced by higher circulating levels of proinflammatory cytokines (i.e., IL-1β, IL-6, TNF-α) and immune dysregulation (loss of vaccine efficacy, increased morbidity upon infection, rises in cancer incidence, and enhanced autoimmunity). This “inflammaging,” a term originally coined by Claudio Franceschi, is thought to contribute to systemic pathologies that develop with age, including ADRD (148, 149). Numerous studies have shown correlations between circulating proinflammatory mediators and progression of neurodegenerative diseases, suggesting that peripheral inflammation contributes to the development of chronic brain inflammation (150–154). In addition, recent studies using CSF to interrogate neuroinflammation directly in the CNS have shown mixed results. For example, in adults without measurable cognitive impairment, increased cytokine levels in the CSF were, surprisingly, associated with lower tau and Aβ levels (155). In addition, higher plasma levels of IL-12p70 and IFN-γ have been associated with protection against cognitive decline in cognitively unimpaired adults (156). Thus, it is possible that mild neuronal inflammation may provide some early protection. On the other hand, as disease etiology progresses, an association with neuroinflammatory markers, including C-reactive protein (CRP), triggering receptor expressed on myeloid cells 2 (TREM2), intercellular adhesion molecule 1 (ICAM1), IFNs, and the IL-1 family, is typically reported (157–160).
Aging elicits pleiotropic outcomes, reflecting many different factors that contribute to increased neuroinflammation; these have been extensively reviewed (161–163) and will be only briefly mentioned here. For example, brain microglia, analogous to systemic macrophages, become activated by tissue damage or pathogens and release proinflammatory mediators (reviewed in ref. 164). Inflammation can also alter Aβ clearance through effects on the NLRP3 inflammasome (165). Age-associated changes in the cells of the adaptive immune system may contribute as well. For example, the proportion of CD4+ T cells that are phenotypically suppressive, designated Tregs (expressing FOXP3), increases with age. Tregs have been shown to play both protective and pathogenic roles in neurodegenerative diseases (166, 167). Indeed, in a mouse model of AD, transient inactivation of Tregs showed improved cognition and decreased inflammation (168). T cells may also play a more direct role in neurodegenerative disease through recognition of their cognate antigen(s) through the cell surface T cell receptor (TCR) as is seen in multiple sclerosis, an autoimmune disorder in which pathogenic T cells recognizing myelin peptides damage the tissue. In pilot Aβ vaccination studies for AD, there was an induction of neuroinflammation, which in some cases led to a devastating meningoencephalitis due to proinflammatory CD4+ T cells (169). Even without immunization, autoimmune responses to neuronal peptides could develop, and in that case, one might expect to find a more restricted TCR repertoire due to selection of those antigen-specific T cells in the CNS. Indeed, this has recently been reported for CD4+ T cells in the CSF of individuals with AD (170). However, it is not clear whether the T cell clonotypes responding are “helper” T cells (CD4+FOXP3–) or “suppressive” Tregs (CD4+FOXP3+), which could be either pathogenic or protective.
Lipid dysregulation.
Genetic linkage, large-scale genome-wide association, and exome sequencing studies have also repeatedly linked lipid metabolism–related genes/loci and rare variants with AD, including apolipoprotein E (APOE), CLU, ABCA7, SORL1, TREM2, PICALM, INPP5D, and PLCG2 (reviewed in refs. 171–173). Several lipid-related gene variants, including APOE, have also been associated with human longevity (174). The first longevity-assurance gene (LAG1) discovered in yeast was found to code for a ceramide synthase (175). Ceramides comprise a class of lipids that play essential roles both as intermediates in the biosynthesis of more complex sphingolipids, and as signaling molecules that participate in a plethora of biological processes (176), including apoptosis, inflammation, insulin signaling, mitochondria function, cellular senescence, telomerase activity, and autophagy (177, 178).
Alterations in brain lipid composition occur in both normal aging and neurodegenerative disease. The brain is the richest organ in terms of lipid content and diversity, largely owing to the abundance of lipid-rich myelin (179). Lipidomics, the large-scale study of pathways and networks of cellular lipids in biological systems, has revealed specific lipid profiles associated with AD (180, 181) and aging (182). For example, early accumulation of ceramide levels in the AD brain has been consistently reported by multiple groups (183, 184). On the other hand, sulfatides, a class of sulfoglycolipids highly enriched in myelin, have been reported to be specifically and dramatically reduced at the earliest clinically recognizable stages of AD (185–187). Brain sulfatide levels in patients with AD and in animal models strongly correlate with the onset and severity of Aβ deposition (188, 189). Mechanistic studies in animal models have revealed that sulfatide deficiency in AD occurs in an isoform-specific manner (190, 191) and that sulfatide losses are sufficient to induce AD-like neuroinflammation and cognitive decline (192). Moreover, levels of the phospholipid plasmalogen have been consistently shown to decline not only in the brains of individuals with AD, but also in circulation, with ethanolamine plasmalogen deficits closely associating with disease severity (193). Notably, human brain plasmalogen levels have also been reported to decline with normal aging, decreasing dramatically by around 70 years of age (194).
Conclusions
Chronological aging is accompanied by molecular, cellular, and systems-level processes with underlying biology that may modulate susceptibility to neurodegenerative disease (50, 51, 195). Applying current insights from the biology-of-aging field to age-associated neurodegenerative diseases offers an opportunity to explore and target new cellular and molecular processes. We have focused on a few selected hallmarks of aging for which interventions are moving to clinical trials in the context of mild cognitive impairment/early AD. Though still an emerging field, geroscience-motivated approaches are appealing for the treatment of complex age-associated diseases, like AD. The synergistic interactions across biology-of-aging pathways raise optimism that effective targeting of one may exert broader beneficial influences (51, 196). As highlighted above, the transition in these cellular and molecular processes over the course of the disease is complex and may be nonlinear. Early upregulation of specific processes, such as cellular respiration and senescence, may help mitigate neurodegenerative disease changes; however, these same processes may be detrimental over time by perpetuating oxidative stress and inflammation (197). Early trials exploring geroscience-motivated approaches for the treatment of AD will provide critical information on this strategy. For example, NCT04685590, led by our team, will focus on geroscience outcomes as well as AD biomarkers and cognitive changes. Other studies are targeting mitochondrial function with NAD+ precursors (198) (NCT04078178, NCT04430517) and nutrient sensing and handling with rapamycin (NCT04200911, NCT04629495). As these early trials are under way, advances in the basic biology of aging are needed to continue shedding light on cell type specificity and interactions across biology-of-aging hallmarks, and to refine model systems through efforts including Model Organisms Development and Evaluation for Late-Onset Alzheimer’s Disease (MODEL-AD) (199). Furthermore, cross-disciplinary training and collaboration across the fields of neuroscience and geroscience will be crucial for advancing treatments that target age-related dysfunction across systems in an effort to optimize both physical and cognitive functioning throughout the lifespan (200).
Acknowledgments
This work was made possible by grants through the Alzheimer’s Drug Discovery Foundation (GC-201908-2019443); the Alzheimer’s Association Part the Cloud + Bill Gates Partnership (PTCG-20-695184); the National Institute on Aging (R21AG068731, P30AG066546); the Institute for Integration of Medicine & Science and the Center for Biomedical Neurosciences at the University of Texas Health Science Center at San Antonio; and the Coordinating Center for Claude D. Pepper Older Americans Independence Centers (U24AG059624). VRG is supported by an NIH Clinical and Translational Science Award (TL1 TR0026). MMG has received funding from the Texas Alzheimer’s Research and Care Consortium, the National Institute on Aging, the San Antonio Claude D. Pepper Older Americans Independence Center, the Alzheimer’s Association Part the Cloud + Bill Gates Partnership, and the Alzheimer’s Drug Discovery Foundation. VRG has received funding from the National Institute on Aging. JPP has received funding from the National Institute on Aging and the San Antonio Claude D. Pepper Older Americans Independence Center. MEO has received funding from the Alzheimer’s Drug Discovery Foundation, the Cure Alzheimer’s Fund, the Charleston Conference on Alzheimer’s Disease, the Older Americans Independence Center National Coordinating Center, the National Institute on Aging, the National Institute of Neurological Disorders and Stroke, the North Carolina Diabetes Research Center, and the US Department of Veterans Affairs.
Version 1. 05/16/2022
Electronic publication
Footnotes
Conflict of interest: MMG and her husband own stock in Abbvie Inc. EP and her husband are employed by and receive income from Academy Diagnostics Sleep and EEG Center.
Copyright: © 2022, Gonzales et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(10):e158453. https://doi.org/10.1172/JCI158453.
Contributor Information
Mitzi M. Gonzales, Email: GonzalesM20@uthscsa.edu.
Erin Pollet, Email: pollete@uthscsa.edu.
Juan P. Palavicini, Email: palavicinij@uthscsa.edu.
Dean L. Kellogg, Jr., Email: kelloggd@uthscsa.edu.
Ellen Kraig, Email: kraig@uthscsa.edu.
Miranda E. Orr, Email: morr@wakehealth.edu.
References
- 1. Vincent GK, ed. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. [Google Scholar]
- 2.Sierra F. Geroscience and the challenges of aging societies. Aging Med (Milton) 2019;2(3):132–134. doi: 10.1002/agm2.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tschanz JT, et al. Dementia: the leading predictor of death in a defined elderly population: the Cache County Study. Neurology. 2004;62(7):1156–1162. doi: 10.1212/01.WNL.0000118210.12660.C2. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 5.Harada CN, et al. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salthouse TA. Trajectories of normal cognitive aging. Psychol Aging. 2019;34(1):17–24. doi: 10.1037/pag0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker-Drob EM. Global and domain-specific changes in cognition throughout adulthood. Dev Psychol. 2011;47(2):331–343. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai S, Endo S. Functional aging in male C57BL/6J mice across the life-span: a systematic behavioral analysis of motor, emotional, and memory function to define an aging phenotype. Front Aging Neurosci. 2021;13:697621. doi: 10.3389/fnagi.2021.697621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnusson KR, et al. Age-related deficits in mice performing working memory tasks in a water maze. Behav Neurosci. 2003;117(3):485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- 10. Whitson HE, et al. American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc. 2018;66(11):2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radulescu CI, et al. The aging mouse brain: cognition, connectivity and calcium. Cell Calcium. 2021;94:102358. doi: 10.1016/j.ceca.2021.102358. [DOI] [PubMed] [Google Scholar]
- 12.Magrassi L, et al. Lifespan of neurons is uncoupled from organismal lifespan. Proc Natl Acad Sci U S A. 2013;110(11):4374–4379. doi: 10.1073/pnas.1217505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler G, et al. Brain structural trajectories over the adult lifespan. Hum Brain Mapp. 2012;33(10):2377–2389. doi: 10.1002/hbm.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habes M, et al. Advanced brain aging: relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl Psychiatry. 2016;6(4):e775. doi: 10.1038/tp.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maheswaran S, et al. Longitudinal regional brain volume changes quantified in normal aging and Alzheimer’s APP x PS1 mice using MRI. Brain Res. 2009;1270:19–32. doi: 10.1016/j.brainres.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Taylor EN, et al. The brains of aged mice are characterized by altered tissue diffusion properties and cerebral microbleeds. J Transl Med. 2020;18(1):277. doi: 10.1186/s12967-020-02441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Bartheld CS. Myths and truths about the cellular composition of the human brain: a review of influential concepts. J Chem Neuroanat. 2018;93:2–15. doi: 10.1016/j.jchemneu.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amelio M, Rossini PM. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: from animal models to human findings. Prog Neurobiol. 2012;99(1):42–60. doi: 10.1016/j.pneurobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Dunn AR, Kaczorowski CC. Regulation of intrinsic excitability: roles for learning and memory, aging and Alzheimer’s disease, and genetic diversity. Neurobiol Learn Mem. 2019;164:107069. doi: 10.1016/j.nlm.2019.107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberman RP, et al. Heightened cortical excitability in aged rodents with memory impairment. Neurobiol Aging. 2017;54:144–151. doi: 10.1016/j.neurobiolaging.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beagle AJ, et al. Relative incidence of seizures and myoclonus in Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal dementia. J Alzheimers Dis. 2017;60(1):211–223. doi: 10.3233/JAD-170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zullo JM, et al. Regulation of lifespan by neural excitation and REST. Nature. 2019;574(7778):359–364. doi: 10.1038/s41586-019-1647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly L, et al. Identification of intraneuronal amyloid beta oligomers in locus coeruleus neurons of Alzheimer’s patients and their potential impact on inhibitory neurotransmitter receptors and neuronal excitability. Neuropathol Appl Neurobiol. 2021;47(4):488–505. doi: 10.1111/nan.12674. [DOI] [PubMed] [Google Scholar]
- 25.Esteras N, et al. Mitochondrial ROS control neuronal excitability and cell fate in frontotemporal dementia. Alzheimers Dement. 2022;18(2):318–338. doi: 10.1002/alz.12394. [DOI] [PubMed] [Google Scholar]
- 26.Sola I, et al. Novel levetiracetam derivatives that are effective against the Alzheimer-like phenotype in mice: synthesis, in vitro, ex vivo, and in vivo efficacy studies. J Med Chem. 2015;58(15):6018–6032. doi: 10.1021/acs.jmedchem.5b00624. [DOI] [PubMed] [Google Scholar]
- 27.Ding J, et al. A metabolome atlas of the aging mouse brain. Nat Commun. 2021;12(1):6021. doi: 10.1038/s41467-021-26310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden DJ, et al. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, et al. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiyama I, et al. Ultrastructural analysis of the paranodal junction of myelinated fibers in 31-month-old-rats. J Neurosci Res. 2002;70(3):309–317. doi: 10.1002/jnr.10386. [DOI] [PubMed] [Google Scholar]
- 31.Kohama SG, et al. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age (Dordr) 2012;34(5):1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666–c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fazekas F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/WNL.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 34.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 35.Simpson JE, et al. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol. 2007;33(4):410–419. doi: 10.1111/j.1365-2990.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 36.Shao WH, et al. Stereological investigation of age-related changes of the capillaries in white matter. Anat Rec (Hoboken) 2010;293(8):1400–1407. doi: 10.1002/ar.21184. [DOI] [PubMed] [Google Scholar]
- 37.Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovasc Dis. 2002;13(s2):7–10. doi: 10.1159/000049143. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR., Jr Preclinical Alzheimer’s disease: a valid concept. Lancet Neurol. 2020;19(1):31. doi: 10.1016/S1474-4422(19)30440-5. [DOI] [PubMed] [Google Scholar]
- 39.Bischof GN, et al. Amyloid deposition in younger adults is linked to episodic memory performance. Neurology. 2016;87(24):2562–2566. doi: 10.1212/WNL.0000000000003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frost B, et al. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284(19):12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo JL, et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med. 2016;213(12):2635–2654. doi: 10.1084/jem.20160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassil F, et al. Amyloid-beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology. Neuron. 2020;105(2):260–275. doi: 10.1016/j.neuron.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinton LK, et al. Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30(21):7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickett EK, et al. Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease. Cell Rep. 2019;29(11):3592–3604. doi: 10.1016/j.celrep.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wennberg AM, et al. The influence of tau, amyloid, alpha-synuclein, TDP-43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging. 2019;77:26–36. doi: 10.1016/j.neurobiolaging.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azarpazhooh MR, et al. A third of community-dwelling elderly with intermediate and high level of Alzheimer’s neuropathologic changes are not demented: a meta-analysis. Ageing Res Rev. 2020;58:101002. doi: 10.1016/j.arr.2019.101002. [DOI] [PubMed] [Google Scholar]
- 49.Zolochevska O, Taglialatela G. Selected microRNAs increase synaptic resilience to the damaging binding of the Alzheimer’s disease amyloid beta oligomers. Mol Neurobiol. 2020;57(5):2232–2243. doi: 10.1007/s12035-020-01868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018;27(6):1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hara Y, et al. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2018;92(2):84–93. doi: 10.1212/WNL.0000000000006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Otin C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fontana L, et al. Extending healthy life span—from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaeberlein M. Translational geroscience: a new paradigm for 21st century medicine. Transl Med Aging. 2017;1:1–4. doi: 10.1016/j.tma.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 56.Scrivo A, et al. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018;17(9):802–815. doi: 10.1016/S1474-4422(18)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boland B, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28(27):6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartlett BJ, et al. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy. 2011;7(6):572–583. doi: 10.4161/auto.7.6.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glatigny M, et al. Autophagy is required for memory formation and reverses age-related memory decline. Curr Biol. 2019;29(3):435–448. doi: 10.1016/j.cub.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Loeffler DA. Influence of normal aging on brain autophagy: a complex scenario. Front Aging Neurosci. 2019;11:49. doi: 10.3389/fnagi.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bordi M, et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy. 2016;12(12):2467–2483. doi: 10.1080/15548627.2016.1239003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shibata M, et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281(20):14474–14485. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 63.Pickford F, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118(6):2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilsson P, et al. Aβ secretion and plaque formation depend on autophagy. Cell Rep. 2013;5(1):61–69. doi: 10.1016/j.celrep.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 65.Ma X, et al. Regulation of the transcription factor EB-PGC1α axis by beclin-1 controls mitochondrial quality and cardiomyocyte death under stress. Mol Cell Biol. 2015;35(6):956–976. doi: 10.1128/MCB.01091-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, et al. Transcription factor EB is selectively reduced in the nuclear fractions of Alzheimer’s and amyotrophic lateral sclerosis brains. Neurosci J. 2016;2016:4732837. doi: 10.1155/2016/4732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, et al. TFEB overexpression in the P301S model of tauopathy mitigates increased PHF1 levels and lipofuscin puncta and rescues memory deficits. eNeuro. 2016;3(2):ENEURO.0042-16.2016. doi: 10.1523/ENEURO.0042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, et al. TFEB regulates lysosomal exocytosis of tau and its loss of function exacerbates tau pathology and spreading. Mol Psychiatry. 2021;26(10):5925–5939. doi: 10.1038/s41380-020-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourdenx M, et al. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell. 2021;184(10):2696–2714. doi: 10.1016/j.cell.2021.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caballero B, et al. Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nat Commun. 2021;12(1):2238. doi: 10.1038/s41467-021-22501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alquezar C, et al. TSC1 loss increases risk for tauopathy by inducing tau acetylation and preventing tau clearance via chaperone-mediated autophagy. Sci Adv. 2021;7(45):eabg3897. doi: 10.1126/sciadv.abg3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKenna MC, et al. Energy metabolism of the brain. In: Brady ST, et al., eds. Basic Neurochemistry. 8th ed. Academic Press; 2012:200–231. [Google Scholar]
- 74.Del Río LA, et al. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot. 2002;53(372):1255–1272. [PubMed] [Google Scholar]
- 75.Egler RA, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24(54):8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 76.Venkateshappa C, et al. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem Res. 2012;37(8):1601–1614. doi: 10.1007/s11064-012-0755-8. [DOI] [PubMed] [Google Scholar]
- 77.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 78.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 80.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5(2):e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munkacsy E, Rea SL. The paradox of mitochondrial dysfunction and extended longevity. Exp Gerontol. 2014;56:221–233. doi: 10.1016/j.exger.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heidler T, et al. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11(2):183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 83.Jang JY, et al. The role of mitochondria in aging. J Clin Invest. 2018;128(9):3662–3670. doi: 10.1172/JCI120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vermulst M, et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40(4):392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 85.de la Monte SM, et al. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Lab Invest. 2000;80(8):1323–1335. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan KJ, et al. Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer’s disease. Neurobiol Aging. 2012;33(9):2210–2214. doi: 10.1016/j.neurobiolaging.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 87.Podlesniy P, et al. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol. 2013;74(5):655–668. doi: 10.1002/ana.23955. [DOI] [PubMed] [Google Scholar]
- 88.Swerdlow RH, et al. Mitochondria, cybrids, aging, and Alzheimer’s disease. Prog Mol Biol Transl Sci. 2017;146:259–302. doi: 10.1016/bs.pmbts.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilkins HM, Swerdlow RH. Mitochondrial links between brain aging and Alzheimer’s disease. Transl Neurodegener. 2021;10(1):33. doi: 10.1186/s40035-021-00261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Musi N, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Theurey P, Pizzo P. The aging mitochondria. Genes (Basel) 2018;9(1):22. doi: 10.3390/genes9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gorgoulis V, et al. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479(7374):547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 94.Yousefzadeh MJ, et al. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience. 2020;42(3):951–961. doi: 10.1007/s11357-020-00185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogrodnik M, et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20(2):e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yousefzadeh MJ, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594(7861):100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bussian TJ, et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang P, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ogrodnik M, et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29(5):1061–1077. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chow HM, et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat Neurosci. 2019;22(11):1806–1819. doi: 10.1038/s41593-019-0505-1. [DOI] [PubMed] [Google Scholar]
- 101.Lin YF, et al. Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress. Neurobiol Stress. 2021;15:100341. doi: 10.1016/j.ynstr.2021.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chinta SJ, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s Disease. Cell Rep. 2018;22(4):930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arun P, et al. Blast exposure leads to accelerated cellular senescence in the rat brain. Front Neurol. 2020;11:438. doi: 10.3389/fneur.2020.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhat R, et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dehkordi SK, et al. Profiling senescent cells in human brains reveals neurons with CDKN2D/p19 and tau neuropathology. Nat Aging. 2021;1(12):1107–1116. doi: 10.1038/s43587-021-00142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Streit WJ, Xue QS. Human CNS immune senescence and neurodegeneration. Curr Opin Immunol. 2014;29:93–96. doi: 10.1016/j.coi.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Bryant AG, et al. Cerebrovascular senescence is associated with tau pathology in Alzheimer’s disease. Front Neurol. 2020;11:575953. doi: 10.3389/fneur.2020.575953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu P, et al. The landscape of human tissue and cell type specific expression and co-regulation of senescence genes. Mol Neurodegener. 2022;17(1):5. doi: 10.1186/s13024-021-00507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brelstaff J, et al. Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 2018;24(8):1939–1948. doi: 10.1016/j.celrep.2018.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gonzales MM, et al. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): a pilot clinical trial. J Prev Alzheimers Dis. 2022;9(1):22–29. doi: 10.14283/jpad.2021.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzales MM, et al. A geroscience motivated approach to treat Alzheimer’s disease: senolytics move to clinical trials. Mech Ageing Dev. 2021;200:111589. doi: 10.1016/j.mad.2021.111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bufill E, et al. The therapeutic potential of epigenetic modifications in Alzheimer’s disease. In: Huang X, ed. Alzheimer’s Disease: Drug Discovery. Exon Publications; 2020:151–164. [PubMed] [Google Scholar]
- 113.Hwang JY, et al. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci. 2017;18(6):347–361. doi: 10.1038/nrn.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pellegrini C, et al. A meta-analysis of brain DNA methylation across sex, age, and Alzheimer’s disease points for accelerated epigenetic aging in neurodegeneration. Front Aging Neurosci. 2021;13:82. doi: 10.3389/fnagi.2021.639428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hernandez DG, et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet. 2011;20(6):1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu L, et al. Association of brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015;72(1):15–24. doi: 10.1001/jamaneurol.2014.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Jager PL, et al. Alzheimer’s disease: early alterations in brain DNA methylation at ank1, bin1, rhbdf2 and other loci. Nat Neurosci. 2014;17(9):1156–1163. doi: 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mano T, et al. Neuron-specific methylome analysis reveals epigenetic regulation and tau-related dysfunction of BRCA1 in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2017;114(45):E9645–E9654. doi: 10.1073/pnas.1707151114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levine ME, et al. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging (Albany NY) 2015;7(12):1198–1211. doi: 10.18632/aging.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hannum G, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weidner CI, et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014;15(2):R24. doi: 10.1186/gb-2014-15-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu AT, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11(2):303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levine ME, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10(4):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McEwen LM, et al. The PedBE clock accurately estimates DNA methylation age in pediatric buccal cells. Proc Natl Acad Sci U S A. 2020;117(38):23329–23335. doi: 10.1073/pnas.1820843116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dammering F, et al. The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiol Stress. 2021;15:100394. doi: 10.1016/j.ynstr.2021.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petkovich DA, et al. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25(4):954–960. doi: 10.1016/j.cmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang T, et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18(1):57. doi: 10.1186/s13059-017-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Z, et al. Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell. 2020;19(10):e13229. doi: 10.1111/acel.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grodstein F, et al. Characteristics of epigenetic clocks across blood and brain tissue in older women and men. Front Neurosci. 2020;14:555307. doi: 10.3389/fnins.2020.555307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shireby GL, et al. Recalibrating the epigenetic clock: implications for assessing biological age in the human cortex. Brain. 2020;143(12):3763–3775. doi: 10.1093/brain/awaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grodstein F, et al. The association of epigenetic clocks in brain tissue with brain pathologies and common aging phenotypes. Neurobiol Dis. 2021;157:105428. doi: 10.1016/j.nbd.2021.105428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peleg S, et al. The metabolic impact on histone acetylation and transcription in ageing. Trends Biochem Sci. 2016;41(8):700–711. doi: 10.1016/j.tibs.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 134.Nativio R, et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat Neurosci. 2018;21(4):497–505. doi: 10.1038/s41593-018-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nativio R, et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat Genet. 2020;52(10):1024–1035. doi: 10.1038/s41588-020-0696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lardenoije R, et al. Age-related epigenetic changes in hippocampal subregions of four animal models of Alzheimer’s disease. Mol Cell Neurosci. 2018;86:1–15. doi: 10.1016/j.mcn.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE. 2007;2007(367):re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 138.Wei W, et al. MicroRNAs in Alzheimer’s disease: function and potential applications as diagnostic biomarkers. Front Mol Neurosci. 2020;13:160. doi: 10.3389/fnmol.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang M, Bian Z. Alzheimer’s Disease and microRNA-132: a widespread pathological factor and potential therapeutic target. Front Neurosci. 2021;15:687973. doi: 10.3389/fnins.2021.687973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Snyder HM, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11(6):710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cai W, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. 2017;34:77–87. doi: 10.1016/j.arr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Claassen J, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487–1559. doi: 10.1152/physrev.00022.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Procter TV, et al. Interplay between brain pericytes and endothelial cells in dementia. Am J Pathol. 2021;191(11):1917–1931. doi: 10.1016/j.ajpath.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 144.Sagare AP, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Rajani RM, et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med. 2018;10(448):eaam9507. doi: 10.1126/scitranslmed.aam9507. [DOI] [PubMed] [Google Scholar]
- 146.Nation DA, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Watanabe C, et al. Aging of the vascular system and neural diseases. Front Aging Neurosci. 2020;12:557384. doi: 10.3389/fnagi.2020.557384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(s1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 149.Pilling LC, et al. Gene expression markers of age-related inflammation in two human cohorts. Exp Gerontol. 2015;70:37–45. doi: 10.1016/j.exger.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Swardfager W, et al. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 151.Kim JW, et al. Longitudinal associations between serum cytokine levels and dementia. Front Psychiatry. 2018;9:606. doi: 10.3389/fpsyt.2018.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cao W, Zheng H. Peripheral immune system in aging and Alzheimer’s disease. Mol Neurodegen. 2018;13(1):1–17. doi: 10.1186/s13024-017-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fernandes A, et al. C-reactive protein as a predictor of mild cognitive impairment conversion into Alzheimer’s disease dementia. Exp Gerontol. 2020;138:111004. doi: 10.1016/j.exger.2020.111004. [DOI] [PubMed] [Google Scholar]
- 154.Italiani P, et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J Neuroinflammation. 2018;15(1):1–12. doi: 10.1186/s12974-017-1027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Albrecht DS, et al. Early neuroinflammation is associated with lower amyloid and tau levels in cognitively normal older adults. Brain Behav Immun. 2021;94:299–307. doi: 10.1016/j.bbi.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang HS, et al. Plasma IL-12/IFN-γ axis predicts cognitive trajectories in cognitively unimpaired older adults. Alzheimers Dement. doi: 10.1002/alz.12399. [published online June 23, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nosi D, et al. Neuroinflammation: integrated nervous tissue response through intercellular interactions at the “whole system” scale. Cells. 2021;10(5):1195. doi: 10.3390/cells10051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu WT, et al. Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 2010;119(6):669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cullen NC, et al. Accelerated inflammatory aging in Alzheimer’s disease and its relation to amyloid, tau, and cognition. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rauchmann BS, et al. Soluble TREM2 and inflammatory proteins in Alzheimer’s disease cerebrospinal fluid. J Alzheimers Dis. 2020;73(4):1615–1626. doi: 10.3233/JAD-191120. [DOI] [PubMed] [Google Scholar]
- 161.Hammond TR, et al. Immune signaling in neurodegeneration. Immunity. 2019;50(4):955–974. doi: 10.1016/j.immuni.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scheiblich H, et al. Neuroimmune connections in aging and neurodegenerative diseases. Trends Immunol. 2020;41(4):300–312. doi: 10.1016/j.it.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 163.Guerrero A, et al. Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends Neurosci. 2021;44(9):714–727. doi: 10.1016/j.tins.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Casali BT, Reed-Geaghan EG. Microglial function and regulation during development, homeostasis and Alzheimer’s disease. Cells. 2021;10(4):957. doi: 10.3390/cells10040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tejera D, et al. Systemic inflammation impairs microglial aβ clearance through NLRP3 inflammasome. EMBO J. 2019;38(17):e101064. doi: 10.15252/embj.2018101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gendelman HE, Appel SH. Neuroprotective activities of regulatory t cells. Trends Mol Med. 2011;17(12):687. doi: 10.1016/j.molmed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duffy SS, et al. The role of regulatory T cells in nervous system pathologies. J Neurosci Res. 2018;96(6):951–968. doi: 10.1002/jnr.24073. [DOI] [PubMed] [Google Scholar]
- 168.Baruch K, et al. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6(1):1–12. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Agadjanyan MG, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174(3):1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 170.Joshi C, et al. CSF-derived CD4+ T-cell diversity is reduced in patients with Alzheimer clinical syndrome. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1106. doi: 10.1212/NXI.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Andrews SJ, et al. Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol. 2020;19(4):326–335. doi: 10.1016/S1474-4422(19)30435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sims R, et al. The multiplex model of the genetics of Alzheimer’s disease. Nat Neurosci. 2020;23(3):311–322. doi: 10.1038/s41593-020-0599-5. [DOI] [PubMed] [Google Scholar]
- 173.Jones L, et al. Genetic evidence for the involvement of lipid metabolism in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):754–761. doi: 10.1016/j.bbalip.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Melzer D, et al. The genetics of human ageing. Nat Rev Genet. 2020;21(2):88–101. doi: 10.1038/s41576-019-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.D’Mello NP, et al. Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J Biol Chem. 1994;269(22):15451–15459. doi: 10.1016/S0021-9258(17)40700-9. [DOI] [PubMed] [Google Scholar]
- 176.Han X. Lipidomics for studying metabolism. Nat Rev Endocrinol. 2016;12(11):668–679. doi: 10.1038/nrendo.2016.98. [DOI] [PubMed] [Google Scholar]
- 177.Wang G, Bieberich E. Sphingolipids in neurodegeneration (with focus on ceramide and S1P) Adv Biol Regul. 2018;70:51–64. doi: 10.1016/j.jbior.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Taghibiglou C, Khalaj S. Cholesterol and fat metabolism in Alzheimer’s disease. In: Adejare A, ed. Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders. Elsevier; 2017:161–193. [Google Scholar]
- 180.Han X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):774–783. doi: 10.1016/j.bbalip.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naudi A, et al. Lipidomics of human brain aging and Alzheimer’s disease pathology. Int Rev Neurobiol. 2015;122:133–189. doi: 10.1016/bs.irn.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 182. Palavicini JP, Han X. Lipids of aging. In: Musi N, Hornsby PJ, eds. Handbook of the Biology of Aging. 9th ed. Academic Press; 2021:391–404. [Google Scholar]
- 183.Han X, et al. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82(4):809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 184.Cutler RG, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(7):2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Han X, et al. Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Ann Neurol. 2003;54(1):115–119. doi: 10.1002/ana.10618. [DOI] [PubMed] [Google Scholar]
- 186.Cheng H, et al. Specific changes of sulfatide levels in individuals with pre-clinical Alzheimer’s disease: an early event in disease pathogenesis. J Neurochem. 2013;127(6):733–738. doi: 10.1111/jnc.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Irizarry MC. A turn of the sulfatide in Alzheimer’s disease. Ann Neurol. 2003;54(1):7–8. doi: 10.1002/ana.10642. [DOI] [PubMed] [Google Scholar]
- 188.Han X. The pathogenic implication of abnormal interaction between apolipoprotein E isoforms, amyloid-beta peptides, and sulfatides in Alzheimer’s disease. Mol Neurobiol. 2010;41(2–3):97–106. doi: 10.1007/s12035-009-8092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kaya I, et al. Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer’s disease mice. J Neurochem. 2020;154(1):84–98. doi: 10.1111/jnc.14999. [DOI] [PubMed] [Google Scholar]
- 190.Cheng H, et al. Apolipoprotein E mediates sulfatide depletion in animal models of Alzheimer’s disease. Neurobiol Aging. 2010;31(7):1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Han X, et al. Novel role for apolipoprotein E in the central nervous system. Modulation of sulfatide content. J Biol Chem. 2003;278(10):8043–8051. doi: 10.1074/jbc.M212340200. [DOI] [PubMed] [Google Scholar]
- 192.Qiu S, et al. Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer’s disease-like neuroinflammation and cognitive impairment. Mol Neurodegener. 2021;16(1):64. doi: 10.1186/s13024-021-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goodenowe DB, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48(11):2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 194.Rouser G, Yamamoto A. Curvilinear regression course of human brain lipid composition changes with age. Lipids. 1968;3(3):284–287. doi: 10.1007/BF02531202. [DOI] [PubMed] [Google Scholar]
- 195.Hou Y, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 196.Tchkonia T, et al. New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J Clin Endocrinol Metab. 2021;106(3):e1481–e1487. doi: 10.1210/clinem/dgaa728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nordengen K, et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J Neuroinflammation. 2019;16(1):46. doi: 10.1186/s12974-019-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Orr ME, et al. Results from a pilot study: the effects of nicotinamide riboside on mild cognitive impairment. Human/Human trials: Nutraceuticals and non-pharmacological interventions. Alzheimers Dement. 2020;16(59):e044746. doi: 10.1002/alz.044746. [DOI] [Google Scholar]
- 199.Oblak AL, et al. Model organism development and evaluation for late-onset Alzheimer’s disease: MODEL-AD. Alzheimers Dement (N Y) 2020;6(1):e12110. doi: 10.1002/trc2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hernandez AR, et al. Reuniting the body “neck up and neck down” to understand cognitive aging: the nexus of geroscience and neuroscience. J Gerontol A Biol Sci Med Sci. 2021;77(1):1–9. doi: 10.1093/gerona/glab215. [DOI] [PMC free article] [PubMed] [Google Scholar]