Abstract
Hospitalized patients with coronavirus disease 2019 (COVID-19), particularly those admitted to the intensive care unit (ICU) are at high risk of morbidity and mortality. Several observational studies have described hemostatic derangements and thrombotic complications in patients with COVID-19. The aim of this review article is to summarize the current evidence on pathologic findings, pathophysiology, coagulation and hemostatic abnormalities, D-dimer’s role in prognostication epidemiology and risk factors of thrombotic complications, and the role of prophylactic and therapeutic anticoagulation in patients with COVID-19. While existing evidence is limited in quality, COVID-19 appears to increase micro-and macro-vascular thrombosis rates in hospitalized and critically ill patients, which may contribute to the burden of disease. D-dimer can be used for risk stratification of hospitalized patients, but its role to guide anticoagulation therapy remains unclear. Evidence of higher quality is needed to address the role of therapeutic anticoagulation or high-intensity venous thromboembolism prophylaxis in COVID-19 patients.
Take-home points
-
•
The prevalence of venous thromboembolism (VTE) in hospitalized COVID-19 patients is high, therefore, clinicians should have a high index of suspicion.
-
•
The pathophysiology of thrombosis is likely related to a combination of SARS-CoV-2 direct endothelial injury and dysregulated inflammation causing coagulation activation.
-
•
The current evidence on the value of D-dimer guided therapy is limited.
-
•
The rate of VTE post-hospital discharge is very low, supporting the safety of current discharge practice without VTE prophylaxis in most patients.
-
•
The role of higher-intensity VTE prophylaxis or therapeutic anticoagulation in critically ill COVID-19 patients without documented or suspected VTE remains uncertain.
-
•
Therapeutic anticoagulation in hospitalized non-critically ill patients with COVID-19 may improve outcomes but more research is warranted.
Abbreviations: ACE-2, Angiotensin-Converting Enzyme-2; ARDS, acute respiratory distress syndrome; aPTT, activated partial thromboplastin time; BMI, body mass index; COVID-19, coronavirus disease 2019; CI, confidence interval; CrI, credible interval; DVT, deep venous thrombosis; DAD, diffuse alveolar damage; DIC, disseminated intravascular coagulation; ICU, intensive care unit; IQR, interquartile range; ELSO, Extracorporeal Life Support Organization; FDP, fibrin degradation products; HR, hazard ratio; PT, prolonged prothrombin time; PE, pulmonary embolism; NETs, neutrophil extracellular traps; OR, odds ratio; MD, mean difference; MI, myocardial infarction; UFH, unfractionated heparin; ROC, receiver operating curve; SIC, sepsis-induced coagulopathy
Keywords: Anticoagulation, COVID-19, Thrombosis, Venous thromboembolism prophylaxis, Critically ill, D-dimer level
1. Introduction
Hospitalized patients with coronavirus disease 2019 (COVID-19), particularly those admitted to the intensive care unit (ICU) with acute respiratory distress syndrome (ARDS) are at high risk of morbidity and mortality [1], [2]. Studies described hemostatic derangements in patients with COVID-19, including elevated D-dimer and fibrinogen levels, thrombocytopenia, and prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT). These abnormalities are more frequently observed in patients with severe disease [2], [3]. In addition, post-mortem studies described a high prevalence of micro- and macro-vascular thrombosis, microangiopathy, pulmonary vasculitis [4], [5], pulmonary embolism (PE), and deep venous thrombosis (DVT) [5], [6].
Rapid recognition of the relationship between COVID-19 and thrombosis, and the paucity of high-quality evidence resulted in considerable variation in clinical practice [7]. Both intensified thromboprophylaxis and full-dose therapeutic anticoagulation have been used in patients with COVID-19, despite unclear data on the impact upon patient outcomes and safety [8], [9], [10].
In this review, we summarize the current evidence on the pathologic findings, pathophysiology, derangements in coagulation, the role of D-dimer in prognostication, epidemiology and risk factors of thrombosis, and the role of prophylactic and therapeutic anticoagulation in patients with COVID-19.
2. Data sources
We searched MEDLINE for studies of thrombosis in patients with COVID-19, published between December 1st, 2019 and October 30, 2021, with no language or study design restrictions. In addition, we reviewed reference lists of identified reports and searched medRxiv.org for pre-print articles. The results of the review was summarized qualitatively.
3. Pathologic findings
Post-mortem findings in COVID-19 describe pathologic micro- and macro-vascular thromboses, possibly due to an inflammatory thrombotic state.
3.1. Microvascular thrombosis
Multiple post-mortem studies compared the histopathological features in deceased COVID-19 patients to deceased non-COVID-19 viral pneumonia patients and uninfected controls. While most viral-associated ARDS manifest diffuse alveolar damage (DAD), COVID-19 has distinctive angiocentric features, including severe endothelial injury associated with intracellular virus and cell membrane disruption, and widespread vascular thrombosis with microangiopathy and alveolar capillary occlusion [4], [11], [12].
Other findings include small pulmonary vessel thromboses and alveolar capillary fibrin microthrombi, up to nine times more common among patients with COVID-19 than those with influenza, including both damaged and preserved lung parenchyma [4], [5], [6], [13]. Interestingly, microvascular thrombosis may be present in less severe disease. A case series of five patients with COVID-19 lacking DAD features also reported significant deposits of terminal complement components in the pulmonary microvasculature, further supporting a prominent vascular process [14]. Fox et al. described post-mortem findings in four patients, the small pulmonary vessels showed platelets and fibrin causing aggregation of inflammatory cells with entrapment of neutrophils and small thrombi formation [15].
Thrombotic phenomena were not limited to the pulmonary vasculature; thromboembolic events were also found in the kidneys, with 30% of the examined samples showing thrombi in the glomerular capillaries [5]. A recent systematic review summarized the above histopathological findings classified according to the system involved [12].
3.2. Macrovascular thrombosis
Autopsy studies also describe the presence of thrombi in large vascular beds, most frequently pulmonary vasculature. In one post-mortem series (n = 12 patients), previously undiagnosed DVT was found in 58% of patients in whom venous thromboembolism (VTE) was not suspected, with PE identified as the direct cause of death in four patients [6]. Similarly, a case-series of 80 post-mortem COVID-19 cases identified DVT in about 40% of them.
Limb ischemia, ischemic stroke, myocardial infarction, and prostatic venous plexus thrombosis have also been reported [5], [6], [12].
3.3. Mechanisms of thrombosis
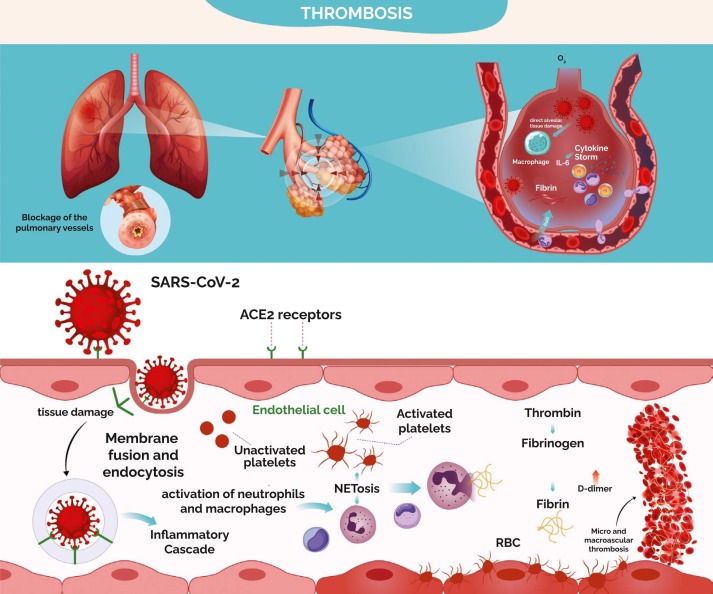
Researchers proposed several mechanisms for COVID-19 associated thrombosis related to SARS-CoV-2′s affinity for the Angiotensin-Converting Enzyme-2 (ACE-2) receptors, which are extensively expressed in the vascular endothelium leading to direct endothelial damage and exocytosis ( Fig. 1) [12], [13], [16], [17], [15]. Endothelial tissue damage induced by SARS-CoV-2 results in hyper-inflammatory response, apoptosis, tissue factor exposure and release (von Willebrand factor, interlukin-6, and P-selectin), coagulation cascade activation along with leukocytes and platelets recruitment followed by aggregation, which in turn causes microvascular thrombosis [15], [18]. In addition, the virus will also trigger innate immunity resulting in mononuclear phagocyte activation, which up-regulates tissue factors, pro-inflammatory cytokine release, and complement activation contributing to cytokine storms [19]. More importantly, neutrophils were found to play a major role in micro-thrombi formation through enhanced extracellular traps (NETs) release resulting in further tissue damage, release of platelets-derived factor, thrombin generation and fibrin deposition, inhibition of fibrinolysis, and platelets-neutrophils aggregation, resulting in immune-thrombosis [19], [20], [21], [22]. Initially this process is limited to the injured lungs, but the hemostatic dysregulation can spread to other vascular beds leading to micro and macro-vascular thrombosis [16].
Fig. 1.
Pathophysiology of COVID-19 associated thrombosis.
4. Coagulation parameters
4.1. D-dimer and thrombotic complications
About 43–60% of patients with COVID-19 present with elevated D-dimer levels ( Table 1) [1], [23], [24]. Higher D-dimer levels are noted in patients with VTE compared to those without [25]. Multivariable regression analysis showed an association between D-dimer level> 1570 ng/mL and increased risk of asymptomatic DVT among non-critically ill patients (adjusted odds ratio [OR] 9.1, 95% confidence interval [CI] 1.1–70.1) [26]. A retrospective study of 429 hospitalized COVID-19 patients found a strong association between D-dimer> 2500 ng/mL on presentation to the hospital and thrombotic complications during hospitalization (adjusted OR 6.79, 95% CI 2.39–19.30) [27]. Another retrospective study (n = 3334) found an incremental dose-response between D-dimer level and VTE, where a D-dimer level> 10,000 ng/mL was associated with an adjusted hazard ratio (HR) of 32.63 (95% 17.2–61.89) for VTE [28]. It is also important to highlight that elevated D-dimer levels can be associated with higher odds of bleeding, particularly when thrombocytopenia is present [27]. High-quality diagnostic accuracy studies are lacking; therefore, D-dimer level should be interpreted carefully, considering the clinical context.
Table 1.
Studies on D-dimer levels in COVID-19 patients.
| Author/Country | Study Type | Patients (n/total) | Population | Outcomes |
|---|---|---|---|---|
| Zhang[29] China | Retrospective | 140 | Hospitalized COVID-19 patients | Admission D-dimer was greater in severe vs non-severe cases (400 vs 200 ng/mL, p < 0.001) |
| Tang[30] Wuhan, China | Retrospective | 183 | COVID-19 pneumonia | Non-survivors revealed significantly higher D-dimer [2120 ng/mL (770–5270) vs 610 ng/mL (350–1290), P = <0.001], longer PT [15. 5 vs 13.6, P < 0.001], and APTT [44.8 vs 41.2, P = 0.096] compared to survivors on admission. |
| Zhou[2] Wuhan, China | Retrospective | 54/191 Non-survivors |
Survivors vs non-survivors | Levels of D-dimer were elevated in non-survivors (>1000 ng/mL) compared with survivors and increased with illness deterioration (In-hospital death OR 18.42, 95% CI 2.64–128.55), P = 0.0033. PT was also longer in non-survivors 12.1 vs 11.4, P = 0.0004. |
| Wu[31] China | Retrospective | 201 | COVID-19 pneumonia patients who developed ARDS or died |
D-dimer and INR were higher in patients with ARDS vs those without (D-dimer 1160 vs 520 ng/mL, p < 0.001, unadjusted HR 1.03; INR 11.70 vs 10.60 s, p < 0.001, unadjusted HR 1.56). Among ARDS patients, D-dimer was greater in non-survivors (3950 vs 490 ng/mL, p 0.001, unadjusted HR 1.02) while aPTT was lower (24.10 vs 29.60 s, p 0.04). |
| Wang[32] Wuhan, China | Retrospective | 138 | ICU vs non-ICU COVID-19 pneumonia | On admission, D-dimer was elevated in ICU vs non-ICU patients (414,000 vs 166,000 ng/mL, p < 0.001) and in non-survivors versus survivors. No difference in PT, aPTT, or platelets. |
| Guan[1] China | Retrospective | 1099 | Hospitalized COVID-19 patients | Admission D-dimer > 500 ng/L was seen in 69.4% of patients with the primary endpoint (ICU admission, mechanical ventilation or death) vs 44.2% without these endpoints. |
| Huang[33] Wuhan, China | Prospective | 41 | ICU vs non-ICU COVID-19 patients | On admission, D-dimer and PT were elevated in ICU vs non-ICU patients (2400 vs 500 ng/mL, P = 0.0042), (12.2 vs 10.7 s, P = 0.012); respectively. No statistically significant difference in aPTT or platelets. |
| Han[34] China | Prospective | 94 + 40 healthy controls | Hospitalized COVID-19 patients compared to healthy controls | COVID-19 cases had lower anti-thrombin (85.46 vs 98.82%, p < 0.001), higher D-dimer (10,360 vs 260 ng/mL, p < 0.001), higher fibrin/FDP (33.83 vs 1.55 mg/L, p < 0.001), higher fibrinogen (5.02 vs 2.90 g/L, p < 0.001), and lower PT (80.59 vs 96.86%, p 0.001).D -dimer and FDP differed between severe and mild cases of COVID-19. |
| Liu[35] China | Prospective | 12 vs 10 control | SARS-CoV-2 infected patients given dipyridamole with prophylactic anticoagulation | There was an increase in lymphocyte and platelet count, decreasing D-dimer level in patients receiving DIP treatment compared to the control group. Seven out of 12 were discharged home. |
| Lippi[23] Italy | Pooled analysis of 4 RCTs | 553 | 22% severe COVID-19 | D-dimer was higher in COVID-19 patients with severe disease than those without (WMD: 2970 mg/L, 95% CI 2470–3460 mg/L, I2 94%, P < 0.001). |
| Chen[36] China | Retrospective | 1859 | Hospitalized patients from 7 Chinese centers | Cox regression showed an association between in-hospital mortality and activated partial thromboplastin (aPTT) per second increase (HR =1.04 [1.02, 1.05]; P < 0.001) and Log10 D-dimer per mg/l increase (HR =3.00 [2.17, 4.16]; P < 0.001) |
| Koleilat[37] USA | Retrospective case-control | 135 | Patients with COVID-19 who had duplex scanning | DVT occurred in 18 (13.3%) patients compared to 72/711 (10.1) patients who were COVID-19 negative or untested. Patients with DVT had higher D-dimer levels – both first and average in-hospital. |
4.2. D-dimer and prognosis
Elevated D-dimer is associated with severe disease and death among patients with COVID-19 [38], [34], [36], [30], [39], [40]. A meta-analysis of 5 observational studies (n = 553 patients) demonstrates that D-dimer levels are higher in critically ill patients compared to non-severe cases (mean difference (MD) 2970 ng/mL, 95% CI 2470–3460) [23]. A retrospective study of 343 COVID-19 patients showed a strong association between D-dimer level ≥ 2000 ng/mL and death (unadjusted HR 51.5, 95% CI 12–206) [40].
Although D-dimer appears to be prognostically relevant in COVID-19 outcomes, no study prospectively validated the use of D-dimer to guide therapy. In addition, variations in test assays and cutoff values make it challenging to pool and generalize these data in the clinical setting [41].
4.3. Prothrombin time (PT) and activated partial thrombin time (aPTT)
PT and aPTT, but not thrombin time, are usually prolonged in patients with COVID-19 compared to healthy controls [42]. However, the proportion of patients with abnormal results varies [36], [30], [43]. The association between coagulation parameters and clinical outcomes is unclear. A case series of 35 patients with COVID-19 showed that over 90% of patients with an increased aPTT demonstrated the presence of lupus anticoagulant and factor XII deficiency, but the VTE rates were low (1/35 patients, 2.85% had confirmed PE) [44]. On the other hand, several studies have linked coagulation times to VTE. Prolonged aPTT was associated with a higher incidence of VTE, while PT was higher in ICU than non-ICU cases [45], [33]. Another study showed an association between thromboembolic events and prolonged PT and aPTT (aHR 4.1, 95% CI 1.9–9.1) [46]. Tang et al. compared COVID-19 survivors and non-survivors, and reported a longer PT in the latter group, but not aPTT [30]. Conversely, a retrospective study of 1859 patients with COVID-19 reported a 4% relative increase in death with each 1-second increase in aPTT (HR 1.04, 95% CI 1.02–1.05, per 1-second increase) [36]. The ability of PT on admission to discriminate between survivors and non-survivors is poor, with an area under the receiver operating curve (ROC) curve of 0.64 [24].
4.4. Other coagulation parameters
Compared to healthy controls, patients with COVID-19 have higher fibrinogen, fibrin degradation products (FDP) and antithrombin levels [47]. A prospective study of 150 ICU patients with COVID-19 showed markedly increased fibrinogen levels, factor VIII and von Willebrand factor antigen, while factor V and antithrombin levels were normal [43]. FDP levels appear to be associated with increased disease severity and mortality [34], [30]. Several studies used global coagulation tests to assess the pathophysiology of COVID-19 associated coagulopathy. In a case series of 24 critically ill patients, thromboelastography showed increased fibrin generation, greater clot strength and reduced fibrinolysis, suggesting that COVID-19 associated coagulopathy is inconsistent with disseminated intravascular coagulopathy but rather hypercoagulability with a severe inflammatory state [48]. Another study of 22 patients with critical COVID-19 in whom rotational thromboelastography showed shorter clot formation time and increased maximum clot firmness, suggests a state of hypercoagulability [49].
5. Thrombotic events
5.1. Thrombotic events in hospitalized patients
While VTE incidence varies between studies, it is likely highest among patients hospitalized in ICU ( Table 2, Supplementary Table 1) [8], [13], [14], [22], [24], [26], [34], [39], [40], [41], [42], [43], [44], [45], [33], [46], [47], [48], [49], [50], [51].
Table 2.
Systematic reviews summarizing the prevalence of venous thromboembolism in inpatients population.
| Author | Number of studies/number of patients (n) | Population | VTE | DVT | PE |
|---|---|---|---|---|---|
| Chi[50] | 11 (n = 1981) | Hospitalized COVID-19 patients (ward/ ICU) |
23.9% (95% CI 16.2–33.7%) despite anticoagulation ICU (30.4%, 95% CI 19.6–43.9%) Ward (13.0%, 95% CI 5.9–26.3%) |
11.9% (95% CI 6.3–21.3%) ICU (10.6%, 4.5–23.2%) Ward (13.6%, 5.2–31.1%) |
11.6% (95% CI 7.5–17.5%) ICU (15.7%, 9.4–25.0%) Ward (5.6%, 2.4–12.4%) |
| Kollias[52] | 47 (n = 6459) | COVID-19 patients who were screened or assessed for PE or DVT. (ward/ ICU) |
NR | 32 studies 27% (95% CI 21–34%) |
17 studies 32% (95% CI 25–40%) |
| Nopp[53] | 86 (n = 33,970) | Hospitalized COVID-19 patients (ward/ ICU) |
14.1% (95% CI 11.6–16.9) ICU (22.7%, 95% CI 18.1–27.6) Ward (7.9%, 95% CI 5.1–11.2) With US screening (40.3%, 95% CI 27.0–54.3) Without US screening (9.5%, 95% CI 7.5–11.7) |
ICU (18.7%, 95% CI 12.6–25.6) Ward (4.1%, 95% CI 2.3–6.4) |
ICU (13.7%, 95% CI 10.0–17.9) Ward (3.5%, 95% CI 2.2–5.1) |
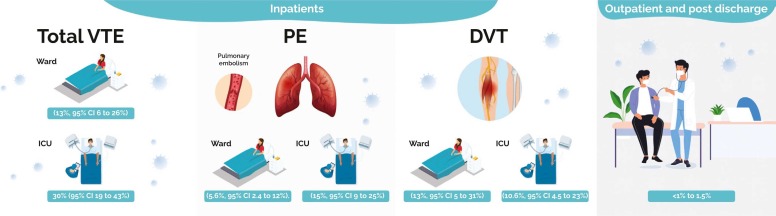
A systematic review of 11 observational studies showed a pooled VTE event rate in ICU patients of 30% (95% CI 19–43%) compared to a pooled event rate in ward patients of 13% (95% CI 6–26%,) [50]. In nine studies, clinical suspicion was the trigger to investigate for PE; five studies used regular screening for asymptomatic DVT. PE was more common in the ICU (15%, 95% CI 9–25%) than in ward patients (5.6%, 95% CI 2.4–12%) whereas DVT event rates were similar in ICU patients (10%, 95% CI 4.5–23%) and non-ICU patients (13%, 95% CI 5–31%), ( Fig. 2) [50].
Fig. 2.
The pooled incidence of venous thromboembolism in inpatients and outpatient population.
Arterial thrombosis is less common than VTE irrespective of disease severity, though reported rates vary widely. Some studies demonstrated a low incidence of acute coronary syndrome (0.0–2.1% in ICU) and 1.0% in general ward, limb ischemia (0.7% in ICU) and mesenteric artery ischemia (0.7% in ICU) [43], [51], [54]. However, a large retrospective study reported a much higher incidence of myocardial infarction (MI) and stroke, especially in ICU patients when compared to non-ICU patients (13.9% vs. 7.3% and 3.7% vs. 0.9%, respectively) [28]. This may be related to variations in event definitions and detection: myocardial injury appears to be common (i.e. increased cardiac troponin level) ranging between 7.2% and 36%, though actual rates of coronary artery thrombosis are unclear [55], [32].
After MI, ischemic stroke is the second most frequently reported arterial thrombotic event (incidence 0.9–3.7% for non-ICU and ICU patients, respectively) [28]. A case series of patients< 50 years old from the Extracorporeal Life Support Organization (ELSO) registry reported a higher rates of mechanical complications in COVID-19 patients on ECMO support in comparison to historic 2019 rates [56].
5.2. Thrombotic events after hospital discharge
Six observational studies (n = 9024 patients) reported on thrombosis in COVID-19 patients after hospital discharge, ( Table 3) [57], [58], [59], [60], [61], [62]. All studies reported a low incidence of VTE with a VTE rate ranging from< 1–1.5%, (Fig. 2) [57], [59], [60], [61], [62]. A prospective study (n = 102) reported on the outcomes of patients six weeks post-hospital discharge with ultrasound screening with or without computed tomography, with an incidence of VTE less than 1% [57]. Of note, around 8% of patients received VTE prophylaxis post-hospital discharge. A retrospective study (n = 1877) evaluated COVID-19 patients at six weeks post-hospital discharge, VTE occurred in 0.5% of patients at a median of 8 days (range 3–33 days) [58]. Another prospective study (n = 1529), reported a cumulative rate of symptomatic VTE post-hospital discharge of 0.2% (95%CI 0.1%−0.6%) [60].
Table 3.
Incidence and prevalence of venous thromboembolism in the outpatient population.
| Author | Design | Population | Thromboprophylaxis during Hospitalization | Post discharge Thromboprophylaxis | Indication for VTE Detection | N | D-Dimer, ng/mL | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Engelen[57] | Prospective | COVID-19 patients post discharge | prophylactic or intermediate dose of LMWH (no description of dosages) | 8% received prophylactic LMWH post discharge | Screening US for all patients CTPA or VQ screening for ICU patients or those with D-dimer ≥ 2 μg/mL |
102 | 590 | 6 weeks post-hospital discharge |
| Patell[59] | Retrospective | Discharged patients with confirmed COVID-19 | NR | 13 patients received prophylactic dose anticoagulation (10 LMWH, 2 DOACs, 1 UFH); they were excluded from the analysis | VTE diagnosis based on clinical suspicion | 163 | 30 days | |
| Roberts[58] | Retrospective | COVID-19 patients post-discharge | Thromboprophylaxis during admission to all Weight based dosing (<50 kg: enoxaparin 20 mg OD or 5000 units BID, 50–100 kg: 40 mg OD or 5000 units BID, 101–150 kg: 40 mg BID or 80 mg OD or UFH 5000 units TID, >150 kg: 60 mg BID or 120 mg OD or 5000 units TID) |
None | Testing for VTE was only done in symptomatic patients | 1877 | NR | 6 weeks post-hospital discharge |
| Rashidi[60] | Prospective | Discharged patients with suspected or confirmed COVID-19 | 1490 (97%) received VTE prophylaxis (enoxaparin 40–60 mg/daily, UFH 5000 IU/QID) | None | VTE diagnosis based on clinical suspicion | 1529 | NR | 45–55 days |
| Giannis[61] | Prospective | Discharged patients with confirmed COVID-19 | 82% of patients received VTE prophylaxis | 12.7% received extended VTE prophylaxis with DOAC or LMWH (enoxaparin 40 mg SQ daily) for 30 days in patients with an IMPROVE VTE score of ≥ 4 or D-dimer ≥ 2X ULN. | VTE diagnosis based on clinical suspicion | 4906 | 340 ± 710 | Up to 90 days post-discharge |
| Eswaran[62] | Retrospective | Discharged patients with confirmed COVID-19 | NR | 190 (42.5%) patients were discharged on VTE prophylaxis. | VTE diagnosis based on clinical suspicion | 447 | 30 days post-discharge |
The largest prospective data comes from the CORE registry (n = 4906) [61], the rates of VTE, arterial thrombosis, and major bleeding post-hospital discharge were 1.5%, 1.7%, 1.7%, respectively.
6. Risk factors for thrombosis in COVID-19 patients
Few studies described thrombosis risk factors in COVID-19 patients. A prospective observational study of 3334 COVID-19 patients showed an increased risk of thrombosis with a D-dimer level> 10,000 ng/mL (HR 7.09, 95% CI 4.69–10.71), age ≥ 75 years (adjusted HR 1.62, 95% CI 1.13–2.33), coronary artery disease (adjusted HR 1.52, 95% CI 1.22–1.90), male srx (HR 1.51, 95% CI 1.25–1.83), and history of MI (adjusted HR 1.43, 95% CI 1.01–2.03) [28]. Moreover, venous or arterial thrombotic event was an independent risk for death (adjusted HR 1.82, 95%CI 1.54–2.15). Similarly, other studies showed an association between D-dimer level above 1500 ng/mL and VTE in hospitalized COVID-19 patients [1], [26], [45].
In the CORE registry (n = 4906), several factors were associated with VTE and arterial thrombosis post-hospital discharge including age over 75 years (OR 4.33, 95% CI 3.47–5.40), coronary artery disease (OR 2.49, 95% CI 1.78–3.39), heart failure (OR 1.99, 95% CI 1.31–3), end-stage renal disease (OR 2.86, 95% CI 2.1–3.91), and D-dimer> 1380 (OR 1.81, 95% CI 1.43–2.31) [61]. However, It is unclear whether D-dimer in COVID-19 patients is merely a marker of current thrombosis, a predictor of a future thrombotic event, or both.
7. Role of anticoagulants
7.1. Pharmacologic VTE prophylaxis
We identified six retrospective observational studies of VTE prophylaxis in COVID-19 [10], [63], [64], [65], [66], [67] and one RCT [68], ( Table 4).
Table 4.
Effect of anticoagulation in COVID-19 patients.
| Author | Study Design/ Type of VTE therapy | Patients (n/total) | Population | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|
| VTE Prophylaxis versus no VTE prophylaxis | ||||||
| Tang[63] | Retrospective | 99/449 Received heparin | Severe COVID-19 | LMWH (40–60 mg/day) or UFH (10,000–15,000 U/day) ≥ 7 days (prophylactic) | No VTE prophylaxis | 28-day mortality were lower in heparin users in patients with SIC score ≥ 4 (OR 0.372, 95% CI 0.154–0.901, P = 0.029), or D-dimer > 6 ULN (OR 0.442, 95% CI 0.226–0.867, P = 0.017). |
| Rentsch[67] | Retrospective | 3627/4297 received VTE prophylaxis | Confirmed cases of COVID-19 | Subcutaneous LMWH or UFH | No VTE prophylaxis | 30-day mortality was higher among patients who did not receive VTE prophylaxis (18.7%) compared to patients receiving prophylactic anticoagulation (14.3%), (HR 0.73, 95% CI 0.66–0.81). The bleeding risk was similar (HR 0.87, 95% CI 0.71–1.05). |
| High-intensity VTE prophylaxis versus conventional VTE prophylaxis | ||||||
| Daughety[65] | Retrospective | 192 | Confirmed severe COVID-19 patients requiring ICU admission or D-dimer > 2.5 mg/L | Enoxaparin 0.5 mg/kg BID or heparin infusion titrated to anti-factor Xa levels 0.3–0.5 U/mL in patients with renal failure (CrCl < 30 mL/min) | Standard-dose thrombo-prophylaxis enoxaparin 40 mg OD if weight < 100 kg and 60 mg OD if weight > 100 kg or 5000 U of UFH TID in patients with renal failure. | The mortality risk was higher among patients who developed thrombotic events (uOR 1.8, 95% CI 0.72–4.5) with lower rates of VTE in patients using the escalated-dose prophylaxis. |
| Sadeghipour[68] | RCT | 600 | COVID-19 ICU patients | Enoxaparin 1 mg/kg daily | Enoxaparin 40 mg daily | There was no difference in the composite outcome of venous and arterial thrombosis, treatment with ECMO, and 30-day mortality in the higher-intensity group (45.7%) compared to the standard group (44.1%). |
| Taccone[10] | Retrospective Therapeutic and prophylactic |
40 | Mechanically ventilated COVID-19 patients | high-dose prophylaxis (6 patients received continuous therapeutic infusion; dose range 1,500_2200 IU/hr, and 12 patients received subcutaneous enoxaparin 4000 IU BID |
enoxaparin subcutaneous 4000 IU once daily) | The use of high-regimen VTE prophylaxis was associated with a lower occurrence of PE (2/18; 11%) than standard regimen (11/22, 50%), (OR 0.13, 95% CI 0.02–0.69, p = 0.02) after adjustment for confounders. 6(46%) patients with PE and 14 (52%) patients without PE died at ICU discharge (OR 0.79, 0.24–3.26, p = 0.99). |
| Therapeutic anticoagulation versus high-intensity or conventional VTE prophylaxis | ||||||
| Nadkarni[66] | Retrospective | 4389 | Confirmed SARS-CoV-2 infection | Therapeutic LMWH or UFH, and DOACs | Prophylactic LMWH or UFH, and DOACs | The use of therapeutic anticoagulation compared to prophylactic anticoagulation is associated with lower in-hospital mortality and intubation. |
| Trinh[9] | Retrospective Therapeutic |
245 | COVID-19 positive patients admitted to ICU requiring MV | 161 Patients therapeutic anticoagulation for a minimum of 5 days (heparin 15 units/kg/hr with and without a bolus; or LMWH 1 mg/kg BID) | 83 patients VTE prophylaxis (heparin 5000 units subcut BID to TID, or enoxaparin 40 mg BID if the GFR >30 mL/min or 40 mg OD if GFR ≤30 mL/min |
Therapeutic anticoagulation for at least five days reduced the rate of death by 79.1% (HR 0.21, 95% CI 0.10–0.46, p < 0.001). The absolute rate of death in the therapeutic was lower than the prophylactic, 34.2% vs. 53.0%, (p < 0.005). |
| Motta[69] | Retrospective | 501 | COVID-19 patients admitted to the hospital | Enoxaparin 1 mg/kg BID or 1.5 mg/kg daily or IV heparin | Enoxaparin 30 or 40 mg daily or heparin 5000 units TID | The risk of in-hospital mortality was higher in patients receiving therapeutic anticoagulation (OR 2.3. 95% CI 1–4.9, P = 0.04) |
| Paranjpe[8] | Retrospective | 2773 | hospitalized COVID-19 patients 395 Intubated patients |
786 (28%) received systemic anticoagulation with a median duration was 3 days (IQR: 2–7 days). | Prophylactic dose of anticoagulation or no prophylaxis | In-hospital mortality (29.1%) for intubated patients treated with anticoagulation vs. (62.7%) in patients who did not receive anticoagulation. Longer duration of anticoagulation was associated with lower risk of mortality (aHR 0.86, 95% CI 0.82–0.89). Bleeding were more common among intubated (30/395; 7.5%) vs. non-intubated patients (32/2378; 1.35%). |
| Lemos[7] | RCT | 20 | COVID-19 patients on mechanical ventilation and D-dimer > 1000 ng/mL | Therapeutic enoxaparin 1 mg/kg either BID or OD, adjusted per age and CrCl | UFH 5000 IU TID (weight < 120 kg) and 7500 IU TID (weight > 120 kg) or enoxaparin 40 mg OD (weight < 120 kg) and 40 mg BID (weight > 120 Kg) | Therapeutic anticoagulation showed improvement in gas exchange using the PaO2/FiO2 ratio (163, 95% CI 133–193 at baseline, and 261, 95% CI 230–293 after 14 days, p = 0.0004) and successful liberation from mechanical ventilation (HR 4.0, 95% CI 1.035–15.053, p = 0.03) and more ventilator free days (15, IQR 6–16 versus 0, IQR 0–11, p = 0.03). |
| Lopes[70] | RCT | 615 | Hospitalized COVID-19 patients with elevated D-dimer | Therapeutic anticoagulation (Rivaroxaban 20 mg or 15 mg daily in stable patients, or LMWH/ UFH in unstable patients) followed by rivaroxaban 20 mg daily for 30 days | Standard prophylaxis with either LMWH or UFH | Therapeutic anticoagulation was not different from prophylactic with regards to clinical outcomes (death, duration of hospitalization, duration of supplemental oxygen up to 30 days), 34.8% vs 41.3%, respectively. The risk of bleeding was higher in therapeutic anticoagulation group (8% vs 2%). |
| REMAP-CAP ATTACC ACTIV-4a[71], [72] | open-label, adaptive, multiplatform RCT | 1074 | Critically ill COVID-19 patients | Therapeutic anticoagulation with heparin | conventional thromboprophylaxis | Therapeutic anticoagulation compared to conventional thromboprophylaxis group showed similar hospital survival (aOR 0.88, 95% CrI 0.67–1.16) and days free of organ support (aOR 0.87, 95% CrI 0.70–1.08) with a similar risk of bleeding (3.1% vs 2.4%). |
| HEP-COVID[73] | Blinded multicenter RCT | 257 | Critically ill and non-critically ill COVID-19 patients with D-dimer > 4 times ULN or SIC score of ≥ 4 | Therapeutic-dose LMWH 1 mg/kg BID | Standard prophylactic or intermediate-dose LMWH or UFH | Therapeutic anticoagulation was associated with reduced major thromboembolism and death compared with standard VTE thromboprophylaxis among inpatients with COVID-19 with very elevated D-dimer levels (RR 0.46; 95% CI 0.27–0.81, P = 0.004) but not in ICU patients (RR 0.92, 95% CI 0.62–1.39, P = 0.71). |
| RAPID[74] | Open-label adaptive RCT | 465 | Moderately ill COVID-19 patients with high D-dimer levels ULN | Therapeutic dose of heparin | Prophylactic dose of heparin | In hospitalized non-critically ill COVID-19 patients with elevated levels of D-dimer, therapeutic anticoagulation was not associated with decrease in the composite primary outcome of death, invasive or non-invasive mechanical ventilation or ICU admission at day 28. However, the odds of death with therapeutic anticoagulation was lower (OR 0.22, 95% CI 0.07–0.65, P = 0.006). |
| Others | ||||||
| Yin[64] | Retrospective | 99/449 | Severe COVID-19 versus non-COVID-19 patients | LMWH (40–60 mg/day) or UFH (10,000–15,000 U/day) ≥ 7 days | LMWH (40–60 mg/day) or UFH (10,000–15,000 U/day) ≥ 7 days | D-dimer > 3.0 μg/mL (6 ULN), significantly lower mortality in heparin users than nonusers was found in COVID group (32.8% vs. 52.4%, P = 0.017). |
*aHR: adjusted hazard ratio, APTT: activated thromboplastin time; BID: twice a day; CI: confidence interval; Crl: credible interval; CrCl: creatinine clearance; DVT: deep venous thrombosis; DOAC: direct oral anticoagulant; FDP: fibrinogen degradation products, ISTH: International Society on Thrombosis and Haemostasis; Kg: kilogram; LMWH: low molecular weight heparin, OR: odds ratio, PE: pulmonary embolism; PT: prothrombin time, PaO2/FiO2 ratio: the ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2), VTE: venous thromboembolism; RCT: randomized controlled trial; sHR: sub-distribution hazard ratio, SIC: sepsis-induced coagulopathy, UFH: unfractionated heparin, ULN: upper limit of normal, WMD: weighted mean difference.
7.1.1. Observational studies
A retrospective study evaluated 449 hospitalized patients with severe COVID-19, of which 99 patients (22%) received VTE prophylaxis for at least seven days. There was no significant difference in 28-day mortality between those receiving VTE prophylaxis versus those not (30.3% vs. 29.7%, P = 0.910) [63]. Nevertheless, when using a sepsis-induced coagulopathy (SIC) score for diagnosing early disseminated intravascular coagulation (DIC) [75], VTE prophylaxis was associated with lower 28-day mortality in patients with SIC score ≥ 4 (40.0% vs. 64.2%, P = 0.029) or D-dimer concentration more than six times the upper limit of normal (32.8% vs. 52.4%, P = 0.017). In these studies, a surprisingly high proportion of patients did not receive VTE prophylaxis. In another observational study (n = 4297), 3627 (84.4%) patients received prophylactic anticoagulation and initiated within 24 h [67]. The incidence of 30-day mortality was higher among patients who did not receive VTE prophylaxis (18.7%) compared to patients who received prophylactic anticoagulation (14.3%), (HR 0.73, 95% CI 0.66–0.81). Interestingly, therapeutic anticoagulation use was associated with lower mortality (HR 0.81, 95 CI% 0.73–0.90) without increased risk of bleeding (HR 0.87, 95% CI 0.71–1.05) [67].
Another retrospective observational study (n = 4389) assessed association between the use of prophylactic or therapeutic anticoagulation and mortality, intubation and bleeding [66]. Authors found an association between therapeutic or prophylactic anticoagulation use and lower in-hospital mortality and intubation [66]. This observation came at the expense of a higher risk of major bleeding in patients using therapeutic anticoagulation (3%) when compared to prophylactic (1.7%) or no anticoagulation (1.9%).
Several studies attempted to evaluate the effects of anticoagulants according to risk stratification tools. When stratified by D-dimer level> 3000 ng/mL, COVID-19 patients receiving VTE prophylaxis had lower 28-day mortality than those without (32% vs. 52%, P = 0.017) [64]. Viscoelastic coagulation tests have also been used to risk-stratify [76]. In one case series, patients were given an intensified prophylactic dose (nadroparin 6000 IU twice a day or 8000 IU twice a day in patients with BMI>35 kg/m2), along with clopidogrel based on viscoelastic testing. None of the patients developed VTE; however, 7 patients died due to refractory hypoxemia and multiorgan failure. Given the competing risks of bleeding and thrombosis, clinical trials are needed to investigate the potential benefit of such intensified thromboprophylaxis in risk-stratified groups.
These observational studies come with inherit risk of bias due to confounding, survival bias. Therefore, their results should be interpreted with caution especially if the results contradict the results of high quality RCTs that were not available at the time of their publication.
7.1.2. Randomized controlled trials
A multicenter superiority RCT (n = 600) of ICU COVID-19 patients showed no difference in the primary composite outcome of venous or arterial thrombosis, ECMO institution, and 30-day mortality with the use of higher-intensity prophylaxis (enoxaparin 1 mg/kg daily) compared to standard prophylaxis (enoxaparin 40 mg daily) [68]. The pre-specified secondary non-inferiority analysis with respect to major bleeding also failed to meet the non-inferiority criteria (non-inferiority margin OR 1.8). It showed a higher bleeding risk in the higher-intensity prophylaxis (OR 1.83, 1-sided 97.5% CI 0.00–5.93.).
7.2. Therapeutic anticoagulation
Six retrospective observational studies and six RCTs assessed the use of therapeutic anticoagulation in hospitalized COVID-19 patients with mixed results, (Table 4) [7], [8], [9], [10], [66], [77], [69], [70], [78], [73], [74], [71], [72].
7.2.1. Observational studies
Several observational studies reported conflicting results with few studies demonstrating either reduced or similar in-hospital mortality rates in patients receiving therapeutic anticoagulation compared to those receiving VTE prophylaxis or no prophylaxis, along with a similar risk of bleeding [8], [9], [10], [66], [77]. In contrast, a retrospective study (n = 501) showed higher in-hospital mortality in patients receiving preemptive therapeutic anticoagulation compared to standard VTE prophylaxis (OR 2.3, 95% CI 1–4.9) [69].
7.2.2. Randomized controlled trials in critically ill patients
A small single-center study randomized 20 patients with critical COVID-19 to either VTE prophylaxis or therapeutic anticoagulation [7]. Therapeutic anticoagulation showed an improvement in gas exchange using the PaO2/FiO2 ratio (from 163, 95% CI 133–193 at baseline to 261, 95% CI 230–293 after 14 days, p < 0.001), successful liberation from mechanical ventilation (HR 4.0, 95% CI 1.04–15.05, p = 0.03), and more ventilator-free days (15 days, IQR 6–16 versus 0 days IQR 0–11, p = 0.03). However, this study was underpowered due to low event rates.
The REMAP-CAP, ATTACC, and ACTIV-4a trials randomized ICU patients with COVID-19 to receive either therapeutic anticoagulation or VTE prophylaxis. After the second interim analysis, the recruitment in these trials was terminated due to futility as pre-specified in the protocol [71]. These trials enrolled a total of 1074 patients and were analyzed as one trial using Bayesian statistics. Authors defined severe COVID-19 cases as those requiring ICU admission for respiratory or hemodynamic support. The median D-dimer level was 800 ng/mL, and only 47% of the patients had a D-dimer> twice the upper limit of normal. There was no difference in organ support free days (aOR 0.87, 95% credible interval [CrI] 0.70–1.08), in-hospital survival (aOR 0.88, 95% CrI 0.67–1.16), or major bleeding [71]. Another open-label RCT (n = 615) compared therapeutic versus prophylactic anticoagulation in hospitalized COVID-19 patients with elevated D-dimer. In the therapeutic anticoagulation arm, patients received either 20 mg of rivaroxaban or LMWH/UFH depending on the acuity of illness, followed by rivaroxaban up to 30 days [70]. The use of therapeutic anticoagulation did not reduce the risk of 30-day composite outcome of time to death, hospital length of stay, or the duration of supplemental oxygen use in hospitalized patients but increased the risk of major bleeding compared to VTE prophylaxis (8.4% vs 2.3%, respectively) [70].
These trials showed lower rates of thrombotic events with the use of therapeutic anticoagulation compared to usual care which included both low-dose and higher-intensity prophylaxis (6.4% vs 10.4%), however, this did not translate into reduction in mortality or other important outcomes [71]. However, it is challenging to impact mortality in critically ill patients due to various reasons including the heterogeneous population, variable causes of death even if the intervention seems to be effective in other secondary outcomes [79].
7.2.3. Randomized controlled trials in hospitalized non-critically ill patients
The same trials REMAP-CAP, ATTACC addressing the role of therapeutic anticoagulation (LMWH or UFH) compared to the standard of care thromboprophylaxis in hospitalized non-critically ill patients (n = 2219) were stopped after meeting the superiority criteria [72]. The use of therapeutic anticoagulation was associated with a higher rate of organ support-free days at day 21 (aOR 1.27, 95% Crl 1.03–1.58) and higher absolute difference in survival rates to hospital discharge rate without the need for organ support (80.2% vs 76.4%), but it was statistically not significant (95% Crl 0.5–7.2) [72]. In support of this, the HEP-COVID, a blinded multicenter RCT of 257 COVID-19 hospitalized critically ill and non-critically ill patients with D-dimer levels> 4 times ULN or SIC ≥ 4 [73]. Compared with standard or higher-intensity VTE thromboprophylaxis, therapeutic anticoagulation was associated with lower rates of mortality and major thromboembolism among inpatients with COVID-19 with high D-dimer levels (RR 0.46; 95% CI 0.27–0.81, P = 0.004) but not in critically ill patients (RR 0.92, 95% CI 0.62–1.39, P = 0.71) [73]. The RAPID open-label RCT(465 patients), showed a similar results of lower mortality in COVID-19 patients with elevated D-dimer levels receiving therapeutic anticoagulation compared to prophylactic anticoagulation (1.8% vs 7.6%) but no difference in the composite primary outcome of death, invasive and non-invasive mechanical ventilation and ICU admission (OR 0.69, 95% CI 0.43–1.10) [74].
In summary, little evidence supports a benefit of full-dose anticoagulation in critically ill patients with COVID-19 compared to standard VTE prophylaxis. However, there are about 70 ongoing RCTs assessing the role of anticoagulation in different populations (outpatient, hospitalized and critically ill inpatients) with various types of pharmacological agents [80].
8. Heparin resistance in COVID-19 patients
In a retrospective study, 14 of 69 COVID-19 ICU patients received therapeutic anticoagulation with either unfractionated heparin (UFH) (n = 10) or LMWH (n = 5) [81]. Around 80% of patients on UFH developed heparin resistance, and all patients on LMWH had an unexplained suboptimal anti-factor Xa level. Although intriguing, we need high-quality studies to validate the results before making clinical inferences. Heparin resistance is rarely reported in critically ill patients; however, data from patients undergoing cardiac surgery, which are usually high risk for heparin resistance, reported this phenomenon in around 20% of patients showing evidence of heparin resistance [82].
9. Clinical Implications
Despite some limitations, the current body of evidence has several clinical implications. First, the high prevalence of VTE in COVID-19 patients should prompt clinicians to use pharmacologic VTE prophylaxis in hospitalized patients without contraindications [83]. Second, although it appears unlikely that intensified VTE prophylaxis or therapeutic anticoagulation improves clinical outcomes in COVID-19 patients without documented VTE, clinicians may have a lower threshold to investigate for VTE, especially in patients with elevated D-dimer (e.g., six times of normal or>10,000 ng/mL), coronary artery disease, and in those> 65 years of age [26], [28], [45]. Third, measuring D-dimer in critically ill COVID-19 patients may help clinicians risk-stratify patients for further VTE testing, especially when the degree of hypoxemia is disproportionate to the findings in chest radiographs. Lastly, the low rates of VTE post-hospital discharge suggest the safety of standard discharge practice without VTE prophylaxis.
10. Knowledge and research gaps
We identified several key research gaps for further study, (Supplementary Table 2). Many of these questions are already being addressed prospectively, and given the rapid development of research into COVID-19, it is anticipated that definitive data which can inform clinical practice is forthcoming.
Supplementary Table 2. Clinical research gaps in thrombosis and COVID-19.
11. Conclusions
While existing evidence is limited in quantity and quality, COVID-19 appears to increase micro-and macro-vascular thrombosis rates in critically ill patients, which may contribute to the burden of disease. The clinical impact upon patients without critical illness is less clear. More research is needed to inform clinical practice.
Financial support
No funding or sponsorship was received for this review to be conducted or for the publication of this article.
Declarations
-
•
Ethics approval and consent to participate: not applicable.
-
•
Consent for publication: not applicable.
-
•
Availability of data and materials: not applicable.
-
•
Competing interests: the authors declare that they have no competing interests.
-
•
Funding: no funding or sponsorship was received for this review to be conducted or for the publication of this article.
-
•
Authors’ contributions: Zainab Al Duhailib was responsible about critical review of the data, and preparing the first draft of the manuscript, graphs, tables, and editing the final draft of the manuscript. Simon Oczkowski was responsible about conception of the project. All authors were responsible about manuscript editing of the first draft and subsequent drafts, methodology review, critical review of the data.
Acknowledgements
No available funding source.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.05.003.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. PubMed PMID: 32109013; PubMed Central PMCID: PMCPMC7092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. PubMed PMID: 32171076; PubMed Central PMCID: PMCPMC7270627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. PubMed PMID: 32437596; PubMed Central PMCID: PMCPMC7412750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. PubMed PMID: 32364264; PubMed Central PMCID: PMCPMC7496150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. PubMed PMID: 32374815; PubMed Central PMCID: PMCPMC7240772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemos A.C.B., do Espirito Santo D.A., Salvetti M.C., Gilio R.N., Agra L.B., Pazin-Filho A., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. PubMed PMID: 32977137; PubMed Central PMCID: PMCPMC7503069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. PubMed PMID: 32387623; PubMed Central PMCID: PMCPMC7202841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinh MA, Chang DR, Govindarajulu US, Kane E., Fuster V., Kohli-Seth R., et al. Therapeutic Anticoagulation Is Associated with Decreased Mortality in Mechanically Ventilated COVID-19 Patients. medRxiv, 2020:2020.05.30.20117929. 〈doi:10.1101/2020.05.30.20117929〉.
- 10.Taccone F.S., Gevenois P.A., Peluso L., Pletchette Z., Lheureux O., Brasseur A., et al. Higher Intensity Thromboprophylaxis Regimens and Pulmonary Embolism in Critically Ill Coronavirus Disease 2019 Patients. Crit Care Med. 2020;48(11):e1087–e1090. doi: 10.1097/CCM.0000000000004548. PubMed PMID: 32769623; PubMed Central PMCID: PMCPMC7437413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142(12):1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. PubMed PMID: 32755393; PubMed Central PMCID: PMCPMC7497892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshmukh V., Motwani R., Kumar A., Kumari C., Raza K. Histopathological observations in COVID-19: a systematic review. J Clin Pathol. 2021;74(2):76–83. doi: 10.1136/jclinpath-2020-206995. PubMed PMID: 32817204. [DOI] [PubMed] [Google Scholar]
- 13.Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. PubMed PMID: 32415579; PubMed Central PMCID: PMCPMC7225095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. PubMed PMID: 32299776; PubMed Central PMCID: PMCPMC7158248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenstein C.J., Solomon S.D. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. PubMed PMID: 32877231; PubMed Central PMCID: PMCPMC7580651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colling M.E., Kanthi Y. COVID-19-associated coagulopathy: An exploration of mechanisms. Vasc Med. 2020;25(5):471–478. doi: 10.1177/1358863×20932640. PubMed PMID: 32558620; PubMed Central PMCID: PMCPMC7306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. PubMed PMID: 15141377; PubMed Central PMCID: PMCPMC7167720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. PubMed PMID: 32558075; PubMed Central PMCID: PMCPMC7323352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. PubMed PMID: 32788101; PubMed Central PMCID: PMCPMC7305763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. PubMed PMID: 32473124; PubMed Central PMCID: PMCPMC7255143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217(12) doi: 10.1084/jem.20201129. PubMed PMID: 32926098; PubMed Central PMCID: PMCPMC7488868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. PubMed PMID: 32597954; PubMed Central PMCID: PMCPMC7472714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G., Favaloro E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. PubMed PMID: 32246450; PubMed Central PMCID: PMCPMC7295300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long H., Nie L., Xiang X., Li H., Zhang X., Fu X., et al. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. Biomed Res Int. 2020;2020:6159720. doi: 10.1155/2020/6159720. PubMed PMID: 32596339; PubMed Central PMCID: PMCPMC7301188 ICMJE Form for Disclosure of Potential Conflicts of Interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K., et al. Acute pulmonary embolism and COVID-19. Radiology. 2020;297(3) doi: 10.1148/radiol.2020201955. PubMed PMID: 32407256; PubMed Central PMCID: PMCPMC7706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macias M., Toledo-Samaniego N., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. PubMed PMID: 32405101; PubMed Central PMCID: PMCPMC7219400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. PubMed PMID: 32492712; PubMed Central PMCID: PMCPMC7378457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. PubMed PMID: 32702090; PubMed Central PMCID: PMCPMC7372509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. PubMed PMID: 32077115. [DOI] [PubMed] [Google Scholar]
- 30.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. PubMed PMID: 32073213; PubMed Central PMCID: PMCPMC7166509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. PubMed PMID: 32167524; PubMed Central PMCID: PMCPMC7070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. PubMed PMID: 32031570; PubMed Central PMCID: PMCPMC7042881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. PubMed PMID: 31986264; PubMed Central PMCID: PMCPMC7159299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. PubMed PMID: 32172226. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Li Z., Liu S., Sun J., Chen Z., Jiang M., et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020;10(7):1205–1215. doi: 10.1016/j.apsb.2020.04.008. PubMed PMID: 32318327; PubMed Central PMCID: PMCPMC7169892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L., Yu J., He W., Chen L., Yuan G., Dong F., et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34(8):2173–2183. doi: 10.1038/s41375-020-0911-0. PubMed PMID: 32546725; PubMed Central PMCID: PMCPMC7296516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koleilat I., Galen B., Choinski K., Hatch A.N., Jones D.B., Billett H., et al. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2021;9(1):36–46. doi: 10.1016/j.jvsv.2020.06.012. PubMed PMID: 32593770; PubMed Central PMCID: PMCPMC7315975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. PubMed PMID: 32353746; PubMed Central PMCID: PMCPMC7177070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. PubMed PMID: 32077115. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. PubMed PMID: 32306492; PubMed Central PMCID: PMCPMC7264730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thachil J., Longstaff C., Favaloro E.J., Lippi G., Urano T., Kim P.Y., et al. The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on fibrinolysis. J Thromb Haemost. 2020;18(9):2408–2411. doi: 10.1111/jth.14956. PubMed PMID: 32881272; PubMed Central PMCID: PMCPMC7307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., He L., Chen H., Lu S., Xiong Y., Liu J., et al. Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID-19 patients: Retrospective analysis. Int J Lab Hematol. 2020 doi: 10.1111/ijlh.13273. PubMed PMID: 32592539; PubMed Central PMCID: PMCPMC7361562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. PubMed PMID: 32367170; PubMed Central PMCID: PMCPMC7197634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. PubMed PMID: 32369280; PubMed Central PMCID: PMCPMC7217555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. PubMed PMID: 32271988; PubMed Central PMCID: PMCPMC7262324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. PubMed PMID: 32291094; PubMed Central PMCID: PMCPMC7146714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., He L., Chen H., Lu S., Xiong Y., Liu J., et al. Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID-19 patients: Retrospective analysis. Int J Lab Hematol. 2020;42(6):766–772. doi: 10.1111/ijlh.13273. PubMed PMID: 32592539; PubMed Central PMCID: PMCPMC7361562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. PubMed PMID: 32302438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., et al. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. PubMed PMID: 32316063; PubMed Central PMCID: PMCPMC7295272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S., et al. Venous Thromboembolism among Hospitalized Patients with COVID-19 Undergoing Thromboprophylaxis: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9(8) doi: 10.3390/jcm9082489. PubMed PMID: 32756383; PubMed Central PMCID: PMCPMC7463975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. PubMed PMID: 32353746; PubMed Central PMCID: PMCPMC7177070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollias A., Kyriakoulis K.G., Lagou S., Kontopantelis E., Stergiou G.S., Syrigos K. Venous thromboembolism in COVID-19: A systematic review and meta-analysis. Vasc Med. 2021;26(4):415–425. doi: 10.1177/1358863×21995566. PubMed PMID: 33818197; PubMed Central PMCID: PMCPMC8024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pr Thromb Haemost. 2020 doi: 10.1002/rth2.12439. PubMed PMID: 33043231; PubMed Central PMCID: PMCPMC7537137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. PubMed PMID: 32381264; PubMed Central PMCID: PMCPMC7192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. PubMed PMID: 32517963; PubMed Central PMCID: PMCPMC7279721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. PubMed PMID: 32987008; PubMed Central PMCID: PMCPMC7518880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Engelen MMVT, Balthazar T., Claeys E., Gunst J., Jacquemin M., Janssens S., Lorent N., Liesenborghs L., Peerlinck K., Pieters G., Rex S., Sinonquel P., Van der Linden L., Van Laer C., Vos R., Wauters J., Wilmer A., Verhamme P., Vandenbriele C.. Incidence of Venous Thromboembolism in Patients Discharged after COVID-19 Hospitalisation [abstract]. Res Pract Thromb Haemost.4 (Suppl 1).
- 58.Roberts L.N., Whyte M.B., Georgiou L., Giron G., Czuprynska J., Rea C., et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. doi: 10.1182/blood.2020008086. PubMed PMID: 32746455; PubMed Central PMCID: PMCPMC7483432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. PubMed PMID: 32766883; PubMed Central PMCID: PMCPMC7483433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rashidi F., Barco S., Kamangar F., Heresi G.A., Emadi A., Kaymaz C., et al. Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: Prospective results from a multi-center study. Thromb Res. 2021;198:135–138. doi: 10.1016/j.thromres.2020.12.001. PubMed PMID: 33338976; PubMed Central PMCID: PMCPMC7836837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giannis D., Allen S.L., Tsang J., Flint S., Pinhasov T., Williams S., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838–2847. doi: 10.1182/blood.2020010529. PubMed PMID: 33824972; PubMed Central PMCID: PMCPMC8032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eswaran H., Jarmul J.A., Shaheen A.W., Meaux D., Long T., Saccoccio D., et al. Vascular thromboembolic events following COVID-19 hospital discharge: Incidence and risk factors. Res Pract. Res Pract Thromb Haemost. 2021;5(2):292–295. doi: 10.1002/rth2.12485. PubMed PMID: 33733027; PubMed Central PMCID: PMCPMC7938613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. PubMed PMID: 32220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2021;51(4):1107–1110. doi: 10.1007/s11239-020-02105-8. PubMed PMID: 32246317; PubMed Central PMCID: PMCPMC7124128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daughety M.M., Morgan A., Frost E., Kao C., Hwang J., Tobin R., et al. COVID-19 associated coagulopathy: Thrombosis, hemorrhage and mortality rates with an escalated-dose thromboprophylaxis strategy. Thromb Res. 2020;196:483–485. doi: 10.1016/j.thromres.2020.10.004. PubMed PMID: 33091700; PubMed Central PMCID: PMCPMC7557260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P.R., Pujadas E., et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. PubMed PMID: 32860872; PubMed Central PMCID: PMCPMC7449655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi: 10.1136/bmj.n311. PubMed PMID: 33574135; PubMed Central PMCID: PMCPMC7876672 at www.icmje.org/coi_disclosure.pdf and declare: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (U01-AA026224, U24-AA020794, U01-AA020790, U10-AA013566) and by the Department of Veterans Affairs Health Services Research and Development (C19 20-405) and Office of Research and Development (MVP000). Research was also sponsored by the Laboratory Directed Research and Development Program (LOIS:10074) of Oak Ridge National Laboratory, managed by UT-Battelle, LLC for the US Department of Energy under contract DE-AC05-00OR22725. JAB reports consulting with Amgen, Bayer, JanOne, and Janssen. He serves on the data safety monitoring committee for Novartis. PMH is supported by grants from National Heart, Lung, and Blood Institute, VA Health Services Research & Development, and University of Colorado School of Medicine. He has a research agreement with Bristol-Myers Squibb through the University of Colorado. He serves as the deputy editor for Circulation: Cardiovascular Quality and Outcomes. IJD reports grants from UK National Health Service National Institute for Health Research and has received unrestricted research grants and holds shares in GlaxoSmithKline, outside of the submitted work. All other authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Investigators I., Sadeghipour P., Talasaz A.H., Rashidi F., Sharif-Kashani B., Beigmohammadi M.T., et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. PubMed PMID: 33734299; PubMed Central PMCID: PMCPMC7974835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motta J.K., Ogunnaike R.O., Shah R., Stroever S., Cedeno H.V., Thapa S.K., et al. Clinical Outcomes With the Use of Prophylactic Versus Therapeutic Anticoagulation in Coronavirus Disease 2019. Crit Care Explor. 2020;2(12) doi: 10.1097/CCE.0000000000000309. PubMed PMID: 33354679; PubMed Central PMCID: PMCPMC7746209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopes R.D., de Barros E.S.P.G.M., Furtado R.H.M., Macedo A.V.S., Bronhara B., Damiani L.P., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–2263. doi: 10.1016/S0140-6736(21)01203-4. PubMed PMID: 34097856; PubMed Central PMCID: PMCPMC8177770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Investigators R.-C., Investigators A.C.-a, Investigators A., Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. PubMed PMID: 34351722; PubMed Central PMCID: PMCPMC8362592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Investigators A., Investigators A.C.-a, Investigators R.-C., Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. PubMed PMID: 34351721; PubMed Central PMCID: PMCPMC8362594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spyropoulos A.C., Goldin M., Giannis D., Diab W., Wang J., Khanijo S., et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern Med. 2021;181(12):1612–1620. doi: 10.1001/jamainternmed.2021.6203. PubMed PMID: 34617959; PubMed Central PMCID: PMCPMC8498934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sholzberg M., Tang G.H., Rahhal H., AlHamzah M., Kreuziger L.B., Ainle F.N., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. PubMed PMID: 34649864; PubMed Central PMCID: PMCPMC8515466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M., et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578. PubMed PMID: 31410983. [DOI] [PubMed] [Google Scholar]
- 76.Ranucci M., Ballotta A., Di Dedda U., Baryshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. PubMed PMID: 32302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tremblay D., van Gerwen M., Alsen M., Thibaud S., Kessler A., Venugopal S., et al. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood. 2020;136(1):144–147. doi: 10.1182/blood.2020006941. PubMed PMID: 32462179; PubMed Central PMCID: PMCPMC7332896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarychanski R. Therapeutic Anticoagulation in Critically Ill Patients with Covid-19 – Preliminary Report. medRxiv. 2021:2021.03.10.21252749. 〈doi:10.1101/2021.03.10.21252749〉.
- 79.Vincent J.L., Marini J.J., Pesenti A. Do trials that report a neutral or negative treatment effect improve the care of critically ill patients? No. Intensive Care Med. 2018;44(11):1989–1991. doi: 10.1007/s00134-018-5220-y. PubMed PMID: 29947879. [DOI] [PubMed] [Google Scholar]
- 80.Talasaz A.H., Sadeghipour P., Kakavand H., Aghakouchakzadeh M., Kordzadeh-Kermani E., Van Tassell B.W., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. PubMed PMID: 33741176; PubMed Central PMCID: PMCPMC7963001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White D., MacDonald S., Bull T., Hayman M., de Monteverde-Robb R., Sapsford D., et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287–291. doi: 10.1007/s11239-020-02145-0. PubMed PMID: 32445064; PubMed Central PMCID: PMCPMC7242778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ranucci M., Isgro G., Cazzaniga A., Ditta A., Boncilli A., Cotza M., et al. Different patterns of heparin resistance: therapeutic implications. Perfusion. 2002;17(3):199–204. doi: 10.1191/0267659102pf562oa. PubMed PMID: 12017388. [DOI] [PubMed] [Google Scholar]
- 83.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020 doi: 10.1016/j.chest.2020.05.559. PubMed PMID: 32502594; PubMed Central PMCID: PMCPMC7265858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.