Abstract
Background and study aims Esophagogastroduodenoscopy (EGD), the most common method used for diagnosing upper gastrointestinal diseases, is often limited by the presence of foam and mucous. Thus, this study was designed to detect whether the combination of simethicone with N-acetyl cysteine (NAC) as premedication before EGD improves mucosal visualization.
Patients and methods A total of 768 consenting patients were enrolled in this prospective, double-blind, randomized placebo-controlled trial in four groups (A: simethicone + N-acetyl cysteine; B: simethicone alone; C: NAC alone; and D: placebo). After 20 minutes of consuming the corresponding solution, EGD was done and multiple images were obtained from the esophagus, stomach, and duodenum. Based on the various images obtained, the study parameters were calculated. Statistical Analysis Software (SAS) was used to analyze the results using Kruskal-Wallis with the Bonferroni correction method.
Results The study population consisted of 57 % men and the mean age was 44.18 years. Each group was randomized with 192 participants. Group A (combination of simethicone + NAC) premedication had the lowest total mucosal visibility score of 8.31, a significantly lower score for mucous/bubbles obscuring the vision, and less time to complete the procedure. Also, 81 % of the participants in group A did not require flushing to clear the mucous/bubbles. There were no side effects due to this premedication in any of the groups.
Conclusions Using simethicone and NAC combined for premedication may improve the quality of EGD.
Introduction
Esophagogastroduodenoscopy (EGD) is one of the most common methods for diagnosing and treating upper gastrointestinal diseases 1 . However, foam and bubbles usually accumulate in the esophagus, stomach, and duodenum, obscuring endoscopic visibility of gastrointestinal mucosa. This increases the opportunity for missing subtle smaller lesions 2 3 , resulting in decreased diagnostic accuracy and also affecting patient comfort due to prolonged procedure time 4 5 .
Simethicone (polydimethylsiloxane and silicon dioxide) has proven to be a good defoaming agent as an endoscopic premedication to remove bubbles 6 7 . Simethicone works by reducing the surface tension of air bubbles, causing small bubbles to coalesce and collapse to release trapped air 8 . N-acetylcysteine (NAC), a mucolytic agent, has also been used as premedication to improve endoscopic visibility by removing the mucous overlying the gastrointestinal mucosa 9 10 .
The usage of premedication regimens differs among various countries and different centers. East Asian countries like Japan 11 , Korea 12 , and a few others use premedication in routine practice before EGD. However Western 13 and West Asian countries neither use it as their routine practice nor has it been published in their guidelines. Many articles were published regarding this practice from East Asian countries only. The recommended premedication combination before EGD is not a current standard of practice in this part of the world. There is a lack of literature regarding this aspect from the Indian subcontinent. Therefore, the current study was undertaken to test the hypothesis that a combination of simethicone and NAC as premedication and its effects on endoscopic mucosal visibility. The primary outcome of the evaluation was to determine the total mucosal visibility score (TMVS) and the secondary outcome was to study the total duration of the procedure, the total number of lesions detected, overall mucus, and patient overall comfort during the procedure.
Patients and methods
Study design
This was a prospective, double-blind, randomized placebo-controlled trial in a 2 × 2 factorial design conducted in our gastroenterology department. Participants were randomly assigned to one of four groups in a 1:1:1:1 ratio to receive simethicone + NAC, simethicone alone, NAC alone, or placebo.
Eligibility criteria for participants
Eligible participants were all adults > 18 years of age undergoing diagnostic EGD. Exclusion criteria were patients with partial or complete gastric outlet obstruction, minimum fasting < 6 hours, history of active/recent gastrointestinal bleed including melena, pregnancy, history of post-gastric surgery, phenylketonuria, or suspected hypersensitivity to the study drugs.
Study location
The study took place in the Gastroenterology Department of Pondicherry Institute of Medical Sciences, Puducherry from September 2017 to March 2018.
Intervention
The participants in the study were asked to drink a bottle containing 90 mL of solution, 20 minutes before the procedure. Each bottle comprised 15 mL of emulsion + 75 mL of water. The composition of emulsion for group A was simethicone 150 mg + NAC- 600 mg group B was simethicone 150 mg alone, group C was NAC 600 mg alone, and in group D was just plain water as placebo.
Outcome measures
EGD was done and multiple images were taken from the esophagus, stomach, and duodenum. The TMVS of each segment was measured as the primary outcome. The total duration of the procedure, amount of water used to flush the mucous or bubbles, need for suction, and lesions detected during the procedure were also assessed by the endoscopists as a secondary outcome measure.
The mucosal visibility was scored from 1 to 4 with little modification from the previously described studies 14 . Score 1 was no adherent mucus on the gastric mucosa; 2 was a small amount of mucus on the gastric mucosa, but not obscuring vision; 3 was a moderate amount of mucus on the gastric mucosa, obscuring less than one-third visibility of the mucosa; and 4 was a large amount of mucus on the gastric mucosa, obscuring more than one-third of the mucosa. The total mucosal visibility score was calculated from the sum of scores obtained from seven different sites such as the esophagus, fundus, upper corpus, lower corpus, antrum, duodenal bulb, and the second part of duodenum.
The total duration of the procedure was recorded in minutes. The amount of water used to flush was measured in milliliters and recorded. Also, the degree of visualization was studied by verifying mucous/bubbles in the lumen. It was graded with scores of 1 to 4, according to Bertoni et al 15 , Keeratichananont et al 7 , and McNally et al 16 . The scores were: 1, no bubbles; 2, minimal air bubbles, 3, moderate; and 4, abundant air bubbles. The patient’s tolerability/comfort to the procedure was assessed later by using a questionnaire (excellent/good/tolerable discomfort/intolerable discomfort/totally intolerable) at the end of the procedure.
Sample size estimation
Based on the literature evidence, 80 % power was considered with an alpha error of 5 %, beta error of 20 %, and error of margin 0.1, and the difference in mean ± SD of the TMVS between placebo and others as 2 ± 0.5. The sample size was estimated as 768 for all groups and the individual group calculated sample size was 192 participants.
Study approval
The study was approved by the Institution Research Committee Board and Institutional Ethics Committee. The study was also registered with the Clinical Trials Registry of India (CTRI/2017 /09 /009913). Informed written consent was received from all patients before enrollment in the trial.
Patient recruitment and randomization
Patients were randomly assigned to one of four groups following simple randomization procedures. Informed consent was obtained. Allocation was done by an attendant nurse using sealed envelopes. Both the patients and the endoscopist were kept blinded to the allocation. The intervention solutions in all groups were matched by their color, volume, and method of administration.
Statistical methods
Statistical analysis was performed using pairwise comparison utilizing Kruskal-Wallis test with Bonferroni correction to evaluate the P values. P < 0.05 was considered to be significant. Analysis was done using a Statistical Analysis Software (SAS) program.
Results
A total of 842 patients were enrolled prospectively into the study, following which 74 patients were excluded due to various reasons summarized in the flowchart ( Fig. 1 ). The rest of the 768 participants were randomized into four groups (simethicone + NAC, simethicone alone, NAC alone, and placebo), and none of them were lost to follow-up during the study period. The study population consisted of 57 % men and the mean age was 44.18 years. Dyspepsia was the most common indication for gastroscopy followed by abdominal pain. There were no significant differences in the baseline characteristics among the four groups ( Table 1 ).
Fig. 1.
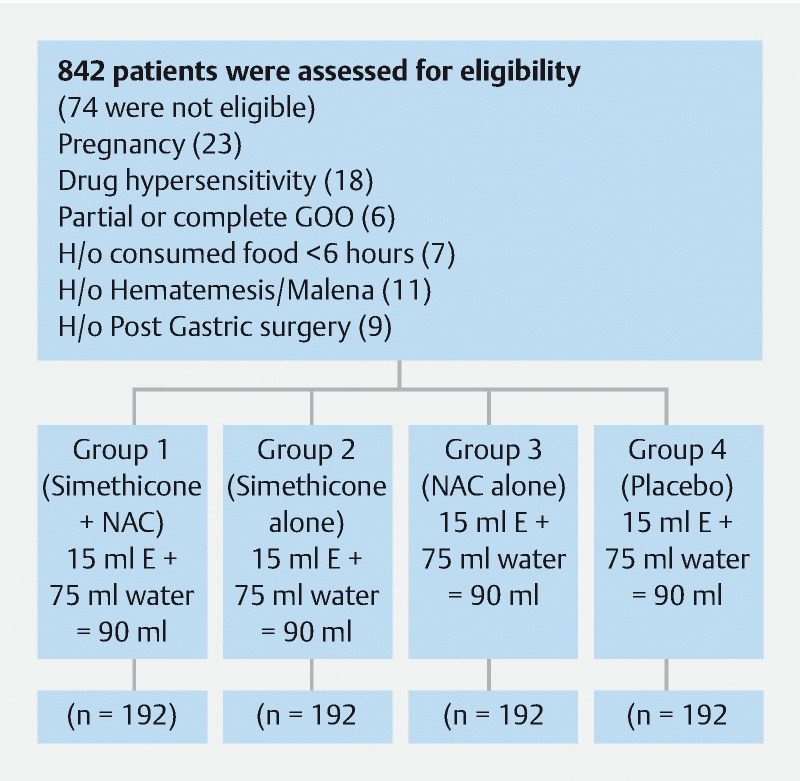
Flowchart depicting patient enrollment procedure.
Table 1. Baseline characteristics of all four groups.
| Variables | Group A (n = 192) | Group B (n = 192) | Group C (n = 192) | Group D (n = 192) |
| Age (mean ± SD) | 45.46 ± 14.56 | 44.45 ± 14.59 | 43.42 ± 13.55 | 43.40 ± 13.09 |
| Sex (M/F) | 106 (55.2 %) | 106 (55.2 %) | 125 (65.1 %) | 101 (52.6 %) |
| Clinical diagnosis | ||||
|
60 | 67 | 60 | 58 |
|
51 | 47 | 46 | 54 |
|
36 | 32 | 30 | 32 |
|
32 | 31 | 39 | 34 |
|
13 | 15 | 17 | 14 |
| Sedation given (midazolam 3 mg IV) | 190 | 189 | 192 | 191 |
SD, standard deviation; CLD, chronic liver disease; PHT, portal hypertension; GERD, gastroesophageal reflux disease; IV, intravenous.
Mucosal visibility score
Mucosal visibility scores (MVS) in various regions were studied for all groups and is summarized in Table 2 . Groups A, B, and C had better MVS for all regions than the placebo group. The MVS for group A was less than that for the other groups in all the regions of the gastrointestinal tract except for the lower corpus and esophagus and the difference also was statistically significant. When the total MVS was compared between the study groups, group A had a lower TMVS score (8.31 ± 1.73) than all other groups and the difference also was statistically significant ( P < 0.05).
Table 2. Mucosal visibility scores for various regions studied and total mucosal visibility score.
| MVS in various regions & TMVS | Mucosal visibility Score (Mean ± SD) | P value 1 | ||||||
| Group – A | Group – B | Group – C | Group – D | ABCD | AB | AC | AD | |
| Esophagus | 1.04 ± 0.23 | 1.13 ± 0.34 | 1.03 ± 0.18 | 1.17 ± 0.48 | < 0.05 | < 0.05 | > 0.05 | < 0.05 |
| Fundus | 1.24 ± 0.44 | 1.66 ± 0.53 | 1.86 ± 0.54 | 1.95 ± 0.53 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Upper corpus | 1.24 ± 0.95 | 1.68 ± 0.54 | 1.78 ± 0.63 | 1.70 ± 0.62 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Lower corpus | 1.28 ± 0.57 | 1.14 ± 0.41 | 1.62 ± 0.69 | 1.63 ± 0.67 | < 0.05 | > 0.05 | < 0.05 | < 0.05 |
| Antrum | 1.28 ± 0.50 | 1.75 ± 0.59 | 2.19 ± 0.83 | 2.72 ± 0.92 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Duodenal bulb | 1.17 ± 0.40 | 1.53 ± 0.53 | 1.89 ± 0.65 | 1.80 ± 0.67 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| D2 | 1.06 ± 0.35 | 1.04 ± 0.18 | 1.09 ± 0.30 | 1.09 ± 0.28 | 0.23 | |||
| TMVS | 8.31 ± 1.73 | 9.93 ± 2.19 | 11.46 ± 2.4 | 12.06 ± 2.3 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Difference in mean compare to Group A | – | 1.62 | 3.15 | 3.75 | ||||
MVS, mucosal visibility score; TMVS, total mucosal visibility score; SD, standard deviation.
One-way analysis of variance and Tukey’s multiple comparison was used to calculate P value between groups.
The difference in the mean MVS in group A versus groups B, C and D was 1.62, 3.15, and 3.75 respectively.
In addition, statistical analysis was performed using the Kruskal-Wallis test with Bonferroni ( Table 3 ) correction to evaluate the P value for all other groups (B, C, and D) for all areas of the gastrointestinal tract in comparison with group A.
Table 3. Pairwise comparison using Kruskal-Wallis test with Bonferroni correction.
| Location | Contrast | Bon _p | Bonp |
| 1 | 1 V 2 | 0.03985 | < 0.05 |
| 1 | 1 V 3 | 1.00000 | 1 |
| 1 | 1 V 4 | 0.00126 | < 0.05 |
| 2 | 1 V 2 | 0.00000 | < 0.05 |
| 2 | 1 V 3 | 0.00000 | < 0.05 |
| 2 | 1 V 4 | 0.00000 | < 0.05 |
| 3 | 1 V 2 | 0.00000 | < 0.05 |
| 3 | 1 V 3 | 0.00000 | < 0.05 |
| 3 | 1 V 4 | 0.00000 | < 0.05 |
| 4 | 1 V 2 | 0.06622 | 0.066 |
| 4 | 1 V 3 | 0.00000 | < 0.05 |
| 4 | 1 V 4 | 0.00000 | < 0.05 |
| 5 | 1 V 2 | 0.00000 | < 0.05 |
| 5 | 1 V 3 | 0.00000 | < 0.05 |
| 5 | 1 V 4 | 0.00000 | < 0.05 |
| 6 | 1 V 2 | 0.00000 | < 0.05 |
| 6 | 1 V 3 | 0.00000 | < 0.05 |
| 6 | 1 V 4 | 0.00000 | < 0.05 |
| 7 | 1 V 2 | 0.00000 | < 0.05 |
| 7 | 1 V 3 | 0.00000 | < 0.05 |
| 7 | 1 V 4 | 0.00000 | < 0.05 |
| 8 | 1 V 2 | 0.27276 | 0.273 |
| 8 | 1 V 3 | 0.00000 | < 0.05 |
| 8 | 1 V 4 | 0.00000 | < 0.05 |
Location 1: esophagus; 2: stomach (fundus); 3: stomach (upper corpus); 4: stomach (lower corpus); 5: antrum, 6: duodenal bulb; 7: II part of duodenum, 8: number of lesions identified.
Results indicated that the P value was significant ( < 0.05) for all the groups and all the regions except for the groups B and C for the gastrointestinal regions (stomach [lower corpus]) and esophagus respectively, where P > 0.05. Also, the P value was > 0.05 for the total number of lesions identified in the stomach (fundus).
Mucous/bubbles obscuring the vision and length of time to perform the procedure
Among Group A, 78.6 % of patients had no air bubbles (score 1) obscuring the vision, whereas only 67.7 %, 50.52 %, and 33.85 % of patients had score 1 in groups B, C, and D, respectively ( Fig. 2 ).
Fig. 2.
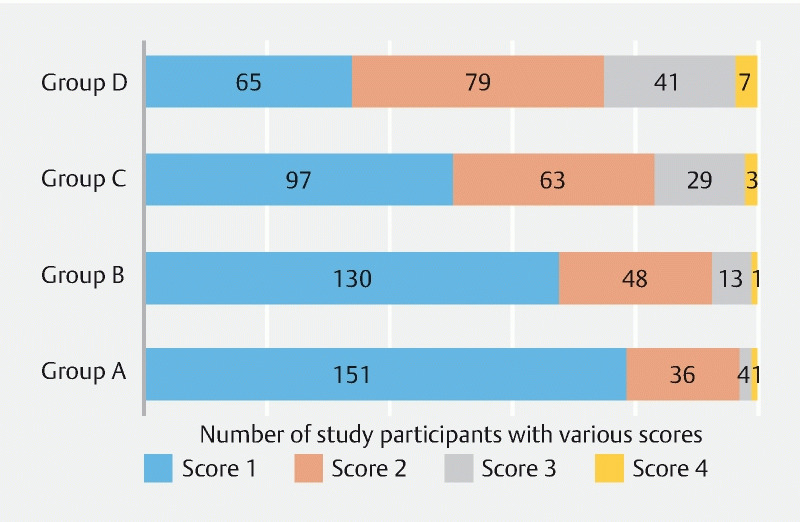
Scoring of mucous/bubbles obscuring vision in the various groups.
Group A had the shortest mean time (5.27 ± 1.28) required to complete the upper gastrointestinal endoscopy procedure and the difference was statistically significant when compared with other groups. However, the time difference among groups A and B was not statistically significant ( Fig. 3 ).
Fig. 3.
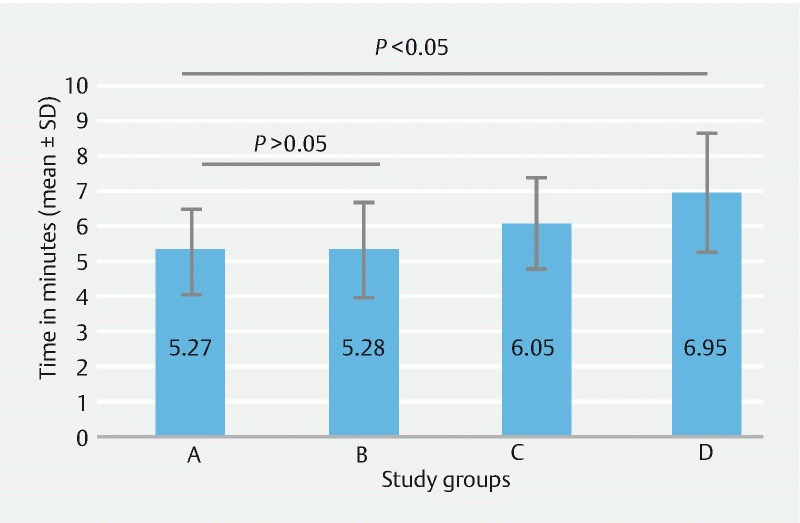
Amount of time required to complete the procedure in the various groups.
Results of additional parameters like overall bubbles, overall mucous, number of lesions identified, and flushing evaluated for total study subjects are shown in Table 4 . Overall bubbles and mucous were checked for all study subjects in terms of percentages and the number of incidences and the number of bubbles/mucous grades were recorded and reported. Similarly, the number of lesions detected and suction required for all the study subjects were noted and reported in terms of frequency and percentage.
Table 4. Results of additional parameters evaluated for total study subjects.
| In % | Frequency | Percent | |
| Overall bubbles | < 25 % | 519 | 67.7 |
| 25–50 % | 200 | 26.1 | |
| 50–75 % | 38 | 5.0 | |
| > 75 % | 10 | 1.3 | |
| Total | 767 | 100.0 | |
| Overall mucous | < 25 % | 545 | 71.1 |
| 25–50 % | 185 | 24.1 | |
| 50–75 % | 28 | 3.7 | |
| > 75 % | 9 | 1.2 | |
| Total | 767 | 100.0 | |
| No. lesions identified | 0 | 288 | 37.5 |
| 1 | 283 | 36.9 | |
| 2 | 120 | 15.6 | |
| 3 | 53 | 6.9 | |
| 4 | 23 | 3.0 | |
| Total | 767 | 100.0 | |
| Flushing | Suction | 64 | 8.3 |
| < 50 mL | 111 | 14.5 | |
| > 50 mL | 160 | 20.9 | |
| No Flushing | 430 | 56.1 | |
| Total | 765 | 99.7 |
Need for flushing and number of lesions detected
The amount of water needed for flushing with or without suction to provide adequate visualization of mucosa was studied and compared between the groups. Nearly 81 % of the study population in group A required no suction or flushing, whereas flushing + /- the suction was not required in 68.22 %, 50 %, and 27.08 % of participants in groups B, C, and D respectively. Also, lesions were detected in more patients (72 of 192; 37.5 %) in group A compared to the other groups with a P < 0.05; however, the P value was not significant between groups A and B ( Table 5 ).
Table 5. Number of patients in whom lesions were detecte3 d.
| In % | Frequency | Percent | |
| Overall bubbles | < 25 % | 519 | 67.7 |
| 25–50 % | 200 | 26.1 | |
| 50–75 % | 38 | 5.0 | |
| > 75 % | 10 | 1.3 | |
| Total | 767 | 100.0 | |
| Overall mucous | < 25 % | 545 | 71.1 |
| 25–50 % | 185 | 24.1 | |
| 50–75 % | 28 | 3.7 | |
| > 75 % | 9 | 1.2 | |
| Total | 767 | 100.0 | |
| No. lesions identified | 0 | 288 | 37.5 |
| 1 | 283 | 36.9 | |
| 2 | 120 | 15.6 | |
| 3 | 53 | 6.9 | |
| 4 | 23 | 3.0 | |
| Total | 767 | 100.0 | |
| Flushing | Suction | 64 | 8.3 |
| < 50 mL | 111 | 14.5 | |
| > 50 mL | 160 | 20.9 | |
| No Flushing | 430 | 56.1 | |
| Total | 765 | 99.7 |
Tolerability/comfort during the procedure and adverse reactions to study drugs
The tolerability/comfort during the procedure was analyzed. In groups A, B and C, an experience of good to tolerable discomfort was reported by 98 %, 98 %, and 96 % of participants, respectively, compared to 86 % in group D. Table 6 lists data on patient comfort evaluation for all the groups. Feedback for each of the patient comfort parameters was reported in terms of the percentage of the total study population.
Table 6. Data on patient comfort evaluation for all groups.
| Parameters | Score/grade | Frequency | Percent |
| Overall comfort | Excellent | 732 | 95.4 |
| Good | 18 | 2.3 | |
| Tolerable discomfort | 12 | 1.6 | |
| Intolerable discomfort | 5 | .7 | |
| Total | 767 | 100.0 | |
| Feedback on taste | Delicious | 1 | .1 |
| Good | 757 | 98.7 | |
| Acceptable | 9 | 1.2 | |
| Total | 767 | 100.0 | |
| Feedback on comfort of drinking | Excellent | 36 | 4.7 |
| Good | 708 | 92.3 | |
| Fair | 21 | 2.7 | |
| Poor | 2 | .3 | |
| Total | 767 | 100.0 |
Adverse effects due to study drug
No adverse effects were noted on using the study drug for study participants.
Discussion
Optimal mucosal visualization without the hindrance of mucous, foam, or bubbles is very important while doing EGD, particularly in the era of image-enhanced endoscopy 11 . EGD is said to be satisfactory when several factors, such as adequate mucosal visibility, adequate duration of the procedure (approximately 7 to 8 minutes/procedure), better patient comfort, and detection of more lesions are achieved 17 . Improving the quality of endoscopy is very important, as it plays a critical role in detecting early neoplastic lesions in the upper gastrointestinal tract 5 13 17 . To accomplish these parameters, the endoscopists in some eastern countries are using certain premedication agents like antifoaming agents (simethicone/dimethicone/dimethyl polysiloxane) and mucolytics (Pronase/NAC). Water alone, if used as a premedication, can only move the foam/bubbles or mucous from one area to another in the stomach, hence, it may not reveal entirely clear visible mucosa an improve the quality of EGD. In this study, we analyzed the effectiveness of using premedication agents alone and in combination (simethicone + NAC) in comparison with placebo.
TMVS has been used in various studies in different ways 7 10 14 17 18 . In our study, we a score similar to the one described by Bertoni et al 15 and Keeratichananont et al 7 . A randomized study from Taiwan showed that use of Pronase with simethicone and NAC with simethicone resulted in a better TMVS than simethicone with water and plain simethicone alone 9 .
A British randomized study showed that patients who took simethicone and Pronase combined for premedication in a drink had better mucosal visibility when compared to patients who drank simethicone alone and simethicone with Pronase flush during EGD 17 . Another large study by Chang et al compared simethicone (100 mg) in 5-mL and 100-mL doses with and without NAC (200 mg). The authors found that the group with a combination of simethicone and NAC had better mucosal visibility than the other groups. Also, the study stated that the amount of time taken to complete the procedure and the number of patients in whom lesions were detected were not significantly different between the groups 14 .
Another multicenter randomized study compared the effectiveness of premedication before EGD in five different groups, simethicone (200 mg) alone, simethicone with NAC (500 mg), simethicone with NAC (1000 mg), and two control groups (placebo and 100 mL of water). In that study, the authors concluded that premedication with simethicone + NAC (500 mg) and simethicone + NAC (1000 mg) resulted in better gastric visibility than simethicone alone, 100 mL of water or placebo 19 .
In contrast to previous studies, an interesting study from Iran by Asl et al showed that simethicone alone as premedication 20 minutes before EGD resulted in better mucosal visibility than the combination of dimethicone and NAC 10 .
In the present study, TMVS was better in the group with a combination of simethicone and NAC compared to placebo. However, there was no significant difference between the combination group (simethicone + NAC) and simethicone alone in the total length of the procedure or the number of patients in whom lesions were detected.
Bertoni et al., Keeratichananont et al., and McNally et al evaluated the usefulness of simethicone with placebo and found that patients who took simethicone had less mucous/foam or bubbles 7 15 16 . Similarly, in the present study, the same kind of scale was used and the group who were given simethicone + NAC had fewer air bubbles/mucous compared to simethicone alone, NAC alone, and placebo.
The total length of time to complete the procedure was also assessed in various studies 14 19 . It was expected that in patients with worse TMVS, more time might be needed to complete the procedure, as more endoscopic flushing is required. However, in these studies, it was concluded that there was no significant difference in the total duration of the procedure among the combination premedication group and the group who were given mucolytics or anti-foaming agents alone or placebo. But in the present study, we found that combination premedication (simethicone + NAC) was associated with shorter and more comfortable procedures when compared to other groups.
The suction or endoscopic flushing required to get better gastric mucosal visibility was also studied by Bhandari et al, who found that a premedication drink is better than endoscopic flushes with simethicone 17 . In the present study, we found that nearly 81 % of patients in the combination premedication group did not require suction or endoscopic flushes versus 68.22 %, 50 %, and 27.08 % in groups B, C, and D, respectively.
The number of patients in whom lesions were detected was evaluated in a study by Chang et al 14 and they inferred that there were not significantly more with combination premedication compared to other groups. Monrroy et al stated that the number of lesions detected between the intervention and control groups was not significantly higher 19 . Even in the present study, the number of patients in whom lesions were detected was significantly higher in the simethicone + NAC premedication group compared to other groups. However, In the present study, the total number of lesions detected in each group was not studied, as it was not included in the scope of the research. Also, whether usage of premedications such as antifoaming agents and mucolytics may help better detect neoplastic lesions in early stages has to be answered in future studies.
The dose of simethicone appears to be a minor factor determining affecting mucosal visibility. Instead, premedication volume and timing are the major factors contributing to optimal visualization. A large volume of liquid premedication covers more surface area, leading to a larger reaction surface. A comparison of simethicone dosing in volumes of 30, 50, and 90 mL of water showed that 90 mL of liquid premedication reduced the foam and bubbles more effectively than a small, 30-mL volume of water 9 . However, this was not included in the scope of the present study.
Our study had several strengths. It was a large, randomized placebo-controlled trial. It compared the two agents alone and in combination with placebo. Because the intervention was implemented in patients with different age groups, both sexes, different diagnoses, these results can be generalized to other circumstances. The study also has several limitations. The TMVS is still a subjective scoring and it may vary with individual endoscopist. Many variations of TMVS still exist. Thick bile, food debris, and blood may still obscure vision. Whether improved visualization leads to superior detection of early mucosal lesions is still a question to be answered in future large randomized trials.
Conclusions
The results indicated that the primary evaluation of TMVS was minimal for patients premedicated with simethicone + NAC (group A) during endoscopy compared to other groups premedicated with simethicone alone (B), NAC alone (C), and placebo (water). The secondary evaluation parameters showed reduced mucous/bubbles, shorter procedure time, and increased comfort during the endoscopy procedure in patients who received premedication. Also, the number of patients in whom lesions were detected was smaller in the placebo group than in the premedication group.
Hence, the use of an antifoaming agent and mucolytic as premedication may improve the quality of EGD by increasing gastric mucosal visibility.
Acknowledgements
The authors acknowledge the Dean of Research and Research Committee members at Pondicherry Institute of Medical Sciences for their outstanding support during this research. We also acknowledge Delvin Formulations, India, for their efforts in making the study drug combination of simethicone + N-acetyl cysteine (Endoview) available for our study.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
References
- 1.Park W G, Shaheen N J, Cohen J et al. Quality indicators for EGD. Gastrointest Endosc. 2015;81:17–30. doi: 10.1016/j.gie.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 2.Veitch A M, Uedo N, Yao K et al. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Amp Hepatol. 2015;12:660. doi: 10.1038/nrgastro.2015.128. [DOI] [PubMed] [Google Scholar]
- 3.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2013;26:11–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen B T. Quality assurance for endoscopists. Best Pract Res Clin Gastroenterol. 2011;25:349–360. doi: 10.1016/j.bpg.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kwan V, Devière J. Endoscopy essentials: preparation, sedation, and surveillance. Endoscopy. 2008;40:65–70. doi: 10.1055/s-2007-967046. [DOI] [PubMed] [Google Scholar]
- 6.Ahsan M, Babaei L, Gholamrezaei A et al. Simethicone for the preparation before esophagogastroduodenoscopy. Diagn Ther Endosc. 2011;2011:484532. doi: 10.1155/2011/484532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keeratichananont S, Sobhonslidsuk A, Kitiyakara T et al. The role of liquid simethicone in enhancing endoscopic visibility prior to esophagogastroduodenoscopy (EGD): A prospective, randomized, double-blinded, placebo-controlled trial. J Med Assoc Thail. 2010;93:892–897. [PubMed] [Google Scholar]
- 8.Brecevic L, Bosan-Kilibarda I, Strajnar F. Mechanism of antifoaming action of simethicone. J Appl Toxicol JAT. 1994;14:207–211. doi: 10.1002/jat.2550140311. [DOI] [PubMed] [Google Scholar]
- 9.Chang C-C, Chen S-H, Lin C-P et al. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: an endoscopist-blinded, prospective, randomized study. World J Gastroenterol. 2007;13:444–447. doi: 10.3748/wjg.v13.i3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asl S MKH, Sivandzadeh G R. Efficacy of premedication with activated Dimethicone or N-acetylcysteine in improving visibility during upper endoscopy. World J Gastroenterol WJG. 2011;17:4213–4217. doi: 10.3748/wjg.v17.i37.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uedo N, Yao K. Endoluminal diagnosis of early gastric cancer and its precursors: bridging the gap between endoscopy and pathology. Adv Exp Med Biol. 2016;908:293–316. doi: 10.1007/978-3-319-41388-4_14. [DOI] [PubMed] [Google Scholar]
- 12.Cho Y K. How to improve the quality of screening endoscopy in korea: national endoscopy quality improvement program. Clin Endosc. 2016;49:312–317. doi: 10.5946/ce.2016.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisschops R, Areia M, Coron E et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016;48:843–864. doi: 10.1055/s-0042-113128. [DOI] [PubMed] [Google Scholar]
- 14.Chang W-K, Yeh M-K, Hsu H-C et al. Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol Hepatol. 2014;29:769–774. doi: 10.1111/jgh.12487. [DOI] [PubMed] [Google Scholar]
- 15.Bertoni G, Gumina C, Conigliaro R et al. Randomized placebo-controlled trial of oral liquid simethicone prior to upper gastrointestinal endoscopy. Endoscopy. 1992;24:268–270. doi: 10.1055/s-2007-1010479. [DOI] [PubMed] [Google Scholar]
- 16.McNally M PR, Maydonovitch C L, Wong C RKH. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988;34:255–258. doi: 10.1016/s0016-5107(88)71324-3. [DOI] [PubMed] [Google Scholar]
- 17.Veitch A M, Uedo N, Yao K et al. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol. 2015;12:660–667. doi: 10.1038/nrgastro.2015.128. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari P, Green S, Hamanaka H et al. Use of Gascon and Pronase either as a pre-endoscopic drink or as targeted endoscopic flushes to improve visibility during gastroscopy: a prospective, randomized, controlled, blinded trial. Scand J Gastroenterol. 2010;45:357–361. doi: 10.3109/00365520903483643. [DOI] [PubMed] [Google Scholar]
- 19.Woo J G, Kim T O, Kim H J et al. Determination of the optimal time for premedication with pronase, dimethylpolysiloxane, and sodium bicarbonate for upper gastrointestinal endoscopy. J Clin Gastroenterol. 2013;47:389–392. doi: 10.1097/MCG.0b013e3182758944. [DOI] [PubMed] [Google Scholar]
- 20.Monrroy H, Vargas J I, Glasinovic E et al. Use of N-acetylcysteine plus simethicone to improve mucosal visibility during upper GI endoscopy: a double-blind, randomized controlled trial. Gastrointest Endosc. 2018;87:986–993. doi: 10.1016/j.gie.2017.10.005. [DOI] [PubMed] [Google Scholar]


