Abstract
Background and study aims Endoscopic submucosal dissection (ESD) is a standard method for minimally invasive resection of superficial gastrointestinal tumors. The pocket creation method (PCM) facilitates ESD regardless of location in the gastrointestinal tract. The aim of this systematic review and meta-analysis is to evaluate the effectiveness and safety of ESD for superficial neoplasms in the upper and lower gastrointestinal tract comparing the PCM to the non-PCM.
Methods Randomized controlled, prospective, and retrospective studies comparing the PCM with the non-PCM were included. Outcomes included en bloc resection, R0 resection, dissection speed, delayed bleeding and perforation. Pooled odds ratios (ORs) with 95 % confidence intervals (CIs) using the Mantel-Haenszel random effect model were documented.
Results Eight studies including gastric, duodenal, and colorectal ESD were included. The en bloc resection rate was significantly higher in the PCM group than the non-PCM group (OR 3.87, 95 %CI 1.24–12.10 P = 0.020). The R0 resection rate was significantly higher in the PCM group than the non-PCM group (OR 2.46, 95 %CI 1.14–5.30, P = 0.020). The dissection speed was significantly faster in the PCM group than the non-PCM group (mean difference 3.13, 95 % CI 1.35–4.91, P < 0.001). The rate of delayed bleeding was similar in the two groups (OR 1.13, 95 %CI 0.60–2.15, P = 0.700). The rate of perforation was significantly lower in the PCM group than the non-PCM group (OR 0.34, 95 %CI 0.15–0.76, P = 0.009).
Conclusions The PCM facilitates high-quality, fast and safe colorectal ESD. Further studies are needed regarding the utility of PCM in ESD of the upper gastrointestinal tract.
Introduction
Endoscopic submucosal dissection (ESD) became the gold standard for the minimally invasive resection of superficial gastrointestinal tumors. The difficulty associated with this technique is largely influenced by the location of the tumor. An R0 resection, meaning an en bloc resection with negative margins, is mandatory for high-quality ESD regardless of the tissue resected. Positive vertical margins can severely limit the clinical significance of ESD. To achieve an R0 resection, stabilized endoscopic maneuvering is important even in difficult locations. Endoscopic mucosal resection (EMR) is not useful for the resection of large superficial gastrointestinal tumors that sometimes have severe submucosal fibrosis, and EMR of such tumors is associated with an increased risk of perforation and positive vertical margins. ESD is a more sophisticated technique than EMR 1 . In difficult locations, vertical and/or distant approaches are sometimes inevitable, and ESD without clear visualization of the submucosa may result in damage to the muscularis or tumor.
We first reported the pocket creation method (PCM) to facilitate ESD 2 . The PCM is useful to achieve an en bloc resection regardless of location throughout most of the gastrointestinal tract. In summary, the PCM begins with a minimal mucosal incision at least 1 cm from the edge of a superficial lesion. Subsequently, several shallow dissecting passes enable the tip of the endoscope to enter the submucosa. Then, submucosal dissection is performed with clear visualization of the submucosa and muscularis without a circumferential mucosal incision. After complete dissection under the lesion, the pocket is opened from the gravity side and an en bloc resection is accomplished ( Fig. 1 ). There are five reasons that the PCM facilitates ESD: 1) prevention of dispersion of the injected solution due to a minimal mucosal incision without a circumferential incision; 2) traction and counter traction is provided in the pocket using a conical transparent hood ( Fig. 2a ;) 3) a vertical approach can be changed to a tangential approach regardless of location by entering the pocket ( Fig. 2b , Fig. 2c ); 4) a specimen with a less-cauterized thick submucosa by selecting the dissection line just above the muscularis due to clear visualization of the submucosa stretched by the conical hood in the pocket ( Fig. 2a ); and 5) the effect of cardiopulmonary movement is minimized by stabilizing the tip of the endoscope in the pocket ( Fig. 2c ) 3 . In the non-PCM, submucosal dissection is performed after a partial or fully circumferential mucosal incision. The aim of this systematic review and meta-analysis is to evaluate effectiveness and safety of ESD for superficial neoplasms in the entire gastrointestinal tract using the PCM compared to the non-PCM.
Fig. 1.
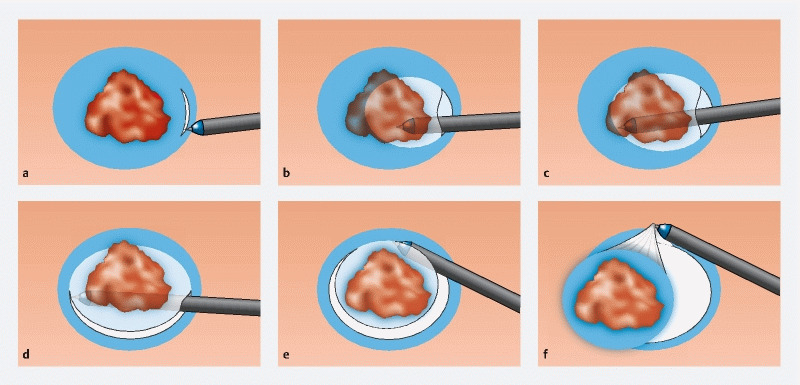
Procedure for the pocket creation method (PCM). a Minimal mucosal incision followed by submucosal dissection allowing the tip of a conical hood on the tip of the endoscope to enter the submucosa. b Extending the pocket by dissecting the submucosa. c Dissecting under the lesion without a circumferential incision. d Opening the pocket from the gravity side. e Opening the pocket on the non-gravity side. f Completion of en bloc resection.
Fig. 2.
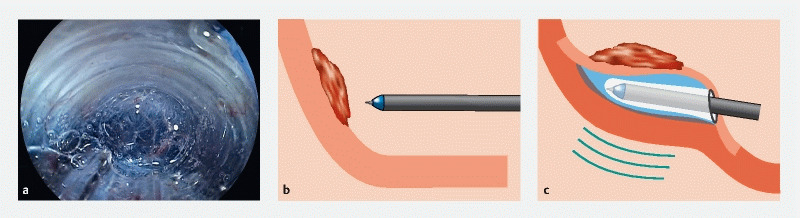
Advantages of the pocket creation method (PCM). a Endoscopic view showing the well-visualized submucosa in the pocket. The conical transparent hood naturally provides both traction and counter traction to stretch the submucosal tissue. b Vertical approach against the lesion. c The PCM changes a vertical to a tangential approach by creating the pocket. The fixed tip of the endoscope is less influenced by cardiopulmonary movement.
Methods
This systematic review and meta-analysis were registered in the International Prospective Register of Systematic Review (PROSPERO, ID: CRD42020208735). We included randomized controlled trials (RCT), prospective and retrospective studies comparing the PCM with the non-PCM for ESD of superficial gastrointestinal tumors. Evaluated outcomes are the R0 resection rate, en bloc resection rate, dissection speed, and the occurrence of delayed bleeding and perforation.
Search strategy
Medline (PubMed), ISI the Web of Science, EMBASE and Cochrane Library were searched with following keywords: (“pocket creation method” or “conventional method”) and “endoscopic submucosal dissection” on June 18, 2021. Language was limited to English. The search period was from 2014 to 2021.
Study selection
Abstracts and titles of screened articles were independently reviewed by the first and second authors (S.S. and Y.H.). Duplicate studies were excluded. Full text articles were also independently assessed by the two authors. In case of controversy, the first and second authors discussed the issue with another coauthor to reach a consensus.
Data extraction and quality assessment
We extracted the following data: first author, year of publication, study period, country, study design, treated organ, number of patients, age, gender, number of lesions, size of lesion, en bloc resection, R0 resection, dissection speed, delayed bleeding and perforation. After the first author extracted these data, the second author verified the data. In case of a lack of critical data, we requested further data from the corresponding authors by direct contact.
Risk of bias
The Cochrane criteria were used to estimate the risk of bias for RCTs 4 . The Risk of Bias Assessment tool for Non-randomized Studies was used to estimate risk of bias for non-RCTs 5 .
Statistical analysis
To compare the PCM with the non-PCM, we used Review Manager (RevMan) Version 5.4, The Cochrane Collaboration, 2020. Pooled odds ratios (ORs) with 95 % confidence intervals (CIs) were calculated with the Mantel-Haenszel random effect model, because great diversity among clinical studies was expected. Interstudy heterogeneity was evaluated by the chi-squared test with I 2 statistic 6 . The I 2 values were divided into low (0 %–40%), moderate (41 %–75 %) and high (76 %–100 %) heterogeneity. For dissection speed, mean difference was calculated. P < 0.05 was considered statistically significant.
Results
Study selection
The study flowchart for this review is shown in Fig. 3 . The first search identified 636 studies, and finally eight studies were selected. Two studies from our own group 7 8 were excluded due to many patients overlapping with subjects in another study 9 . The eight studies included five retrospective studies 3 9 10 11 12 , two RCTs 13 14 and one prospective study 15 . Overall, eight studies including 1,585 ESDs were analyzed.
Fig. 3.
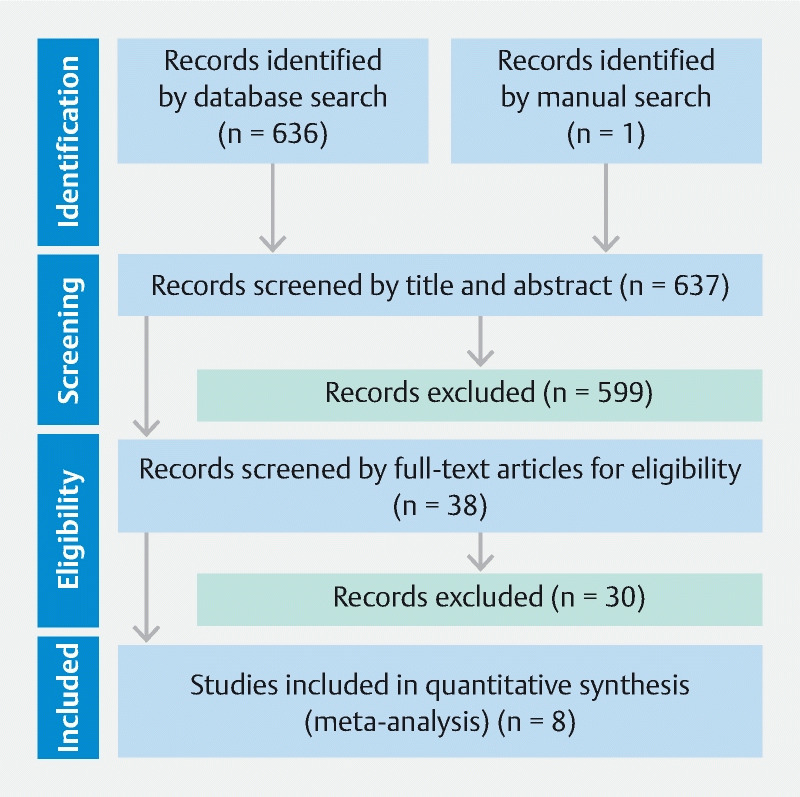
Study flowchart
Characteristics of studies included
All studies were reported from Japan. The years of publication are from 2016 to 2021 ( Table 1 ). Treated organs include the stomach (n = 2), duodenum (n = 1) and colorectum (n = 5). One study regarding gastric ESD was limited to lesions involving the pyloric ring 12 , and one study of lower gastrointestinal ESD excluded rectal lesions 9 . Two studies used the water immersion technique during the PCM 14 15 . We extracted data for non-severe fibrosis in one study 10 . One study only included laterally spreading tumors of the colorectum 14 . Three patients in one study 9 were also included in a subsequent study 13 , and these three patients were excluded from the initial study 9 .
Table 1. Characteristics of the eight studies evaluated.
| First author | Year | Study period | Design | Organ | Method | Patients, n | Age, mean | Male, n | Lesion, n | Size of lesion, mm, mean |
| Kitamura 12 | 2021 | 2006–2019 | Retrospective | Stomach | PCM | 20 | 72.1 ± 11.5 | 13 | 20 | 24.6 ± 11.6 |
| Non-PCM | 46 | 72.7 ± 8.2 | 29 | 46 | 24.4 ± 13.2 | |||||
| Harada 15 | 2018 | 2017 | Prospective | Stomach | PCM | 48 | 75.6 ± 6.8 | 42 | 48 | 16.0 ± 6.6 |
| Non-PCM | 48 | 75.2 ± 7.1 | 44 | 48 | 17.6 ± 11.3 | |||||
| Miura 3 | 2016 | 2006–2015 | Retrospective | Duodenum | PCM | 28 | 59.6 ± 10.9 | 16 | 28 | 30.5 ± 21.5 |
| Non-PCM | 17 | 62.4 ± 12.7 | 12 | 17 | 21.0 ± 8.1 | |||||
| Kanamori 11 | 2017 | 2014–2016 | Retrospective | Colorectum | PCM | 47 | 67.5 ± 11.1 | 32 | 47 | 29.1 ± 11.0 |
| Non-PCM | 49 | 69.7 ± 9.2 | 33 | 49 | 31.1 ± 9.4 | |||||
| Yoshida 10 | 2018 | 2006–2017 | Retrospective | Colorectum | PCM | 37 | 65.2 ± 13.5 | 18 | 37 | 31.1 ± 19.3 |
| Non-PCM | 500 | 67.6 ± 10.6 | 282 | 500 | 37.3 ± 15.3 | |||||
| Takezawa 9 | 2019 | 2010–2017 | Retrospective | Colon | PCM | 266 | 67.0 ± 9.9 | 153 | 278 | 35.3 ± 13.7 |
| Non-PCM | 248 | 67.0 ± 10.0 | 143 | 262 | 35.7 ± 16.2 | |||||
| Harada 14 | 2019 | 2017–2018 | RCT | Colorectum | PCM | 46 | 69.9 ± 10.4 | 29 | 46 | 26.4 ± 6.2 |
| Non-PCM | 45 | 68.9 ± 14.1 | 26 | 45 | 26.8 ± 7.1 | |||||
| Yamashina 13 | 2020 | 2016–2018 | RCT | Colorectum | PCM | 59 | 69.3 ± 9.9 | 34 | 59 | 33.4 ± 11.7 |
| Non-PCM | 55 | 67.8 ± 9.4 | 33 | 55 | 31.7 ± 11.5 |
PCM, pocket-creation method; CM, conventional method; RCT, randomized-controlled trial.
Risk of bias
Risk of bias assessments are shown in Table 2 and Table 3 . In retrospective studies, one study adopted propensity score matching to diminish the effects of confounding variables 15 .
Table 2. Risk of bias assessment for randomized-controlled studies.
| First author | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessments | Incomplete outcome data | Selective outcome reporting | Other bias |
| 2019, Harada | Low | Low | High | Unclear | Low | Low | Unclear |
| 2020, Yamashina | Low | Low | High | Unclear | Low | Low | Unclear |
Table 3. Risk of Bias Assessment for Non-randomized Studies (RoBANS).
| First author | Selection of participants | Confounding variables | Measurement of exposure | Blinding of outcome assessments | Incomplete outcome data | Selective outcome reporting |
| 2021, Kitamura | High | High | Low | Unclear | Low | Low |
| 2018, Harada | Low | Low | Low | Unclear | Low | Low |
| 2016, Miura | High | High | Low | Unclear | Low | Low |
| 2017, Kanamori | High | High | Low | Unclear | Low | Low |
| 2018, Yoshida | High | High | Low | Unclear | Low | Low |
| 2019, Takezawa | High | High | Low | Unclear | Low | Low |
En bloc and R0 resection rates
The en bloc resection rate was significantly higher in the PCM group than the non-PCM group (OR 3.87, 95 %CI 1.24–12.10 P = 0.020) with low heterogeneity ( I 2 = 13 %), although most studies did not show a significant difference independently except one ( Fig. 4a ) 9 . The R0 resection rate was significantly higher in the PCM group than the non-PCM group (OR 2.46, 95%CI 1.14–5.30, P = 0.020) with low heterogeneity ( I 2 = 40 %) ( Fig .4b ). Therefore, the PCM provides higher local curability compared to the non-PCM.
Fig. 4.
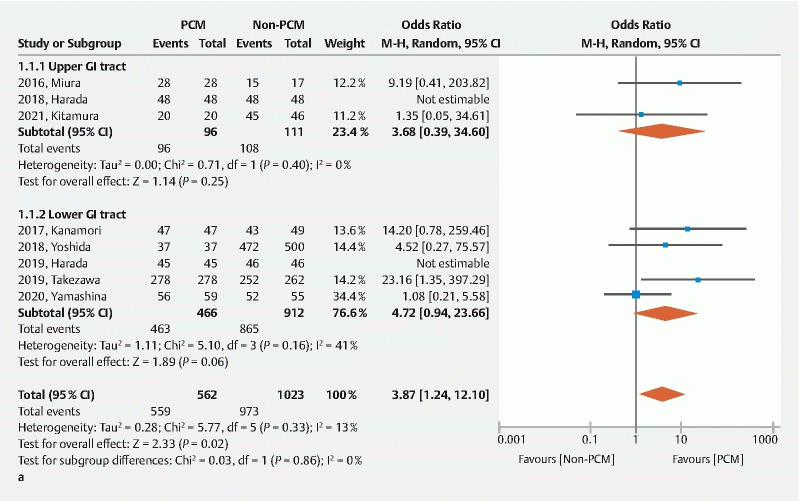
A Forest plot comparing the pocket creation method (PCM) to the non-PCM. a En bloc resection rate.
Fig. 4.
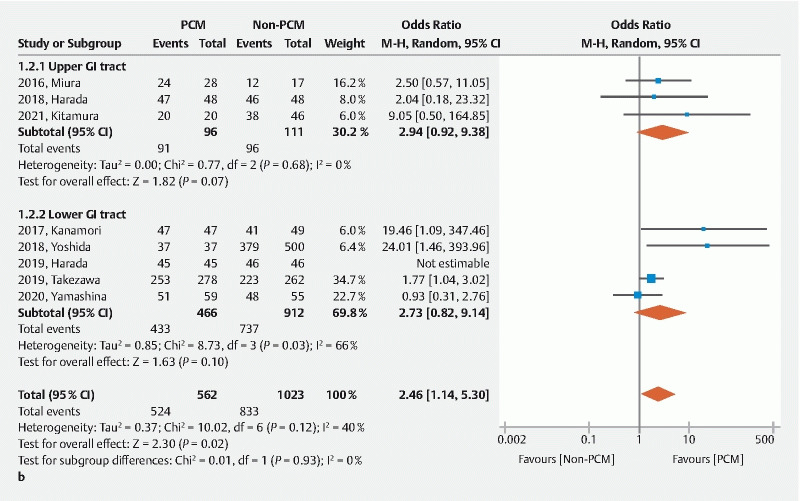
A Forest plot comparing the pocket creation method (PCM) to the non-PCM. b R0 resection rate.
Dissection speed
Seven of the eight studies reported the dissection speed. The dissection speed was significantly faster in the PCM group than the non-PCM group (mean difference 3.13, 95 %CI 1.35–4.91, P < 0.001) with moderate heterogeneity ( I 2 = 47 %) ( Fig. 4c ). The PCM decreases the time needed for lengthy ESD procedures.
Fig. 4.
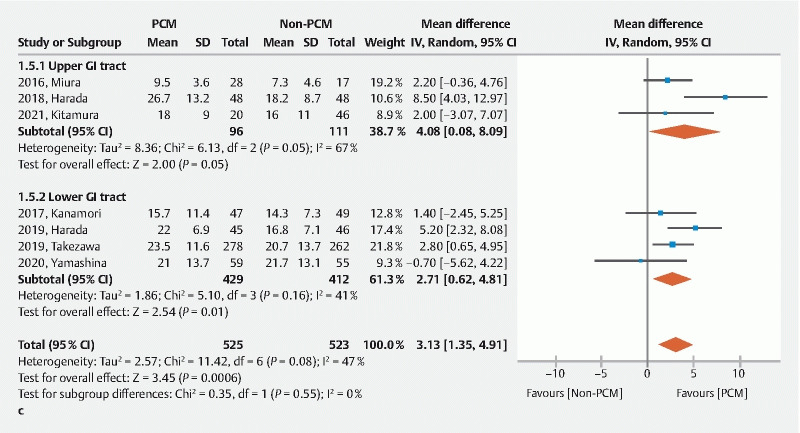
A Forest plot comparing the pocket creation method (PCM) to the non-PCM. c Dissection speed.
Safety
The rate of delayed bleeding was similar between the two groups (OR 1.13, 95 %CI 0.60–2.15, P = 0.700) ( Fig. 4d ). The rate of perforation was significantly lower in the PCM group than the non-PCM group (OR 0.34, 95 %CI 0.15–0.76, P = 0.009) without heterogeneity ( I 2 = 0 %) ( Fig. 4e ). Use of the PCM results in safer ESD compared to the non-PCM. The PCM facilitates safe ESD.
Fig. 4.
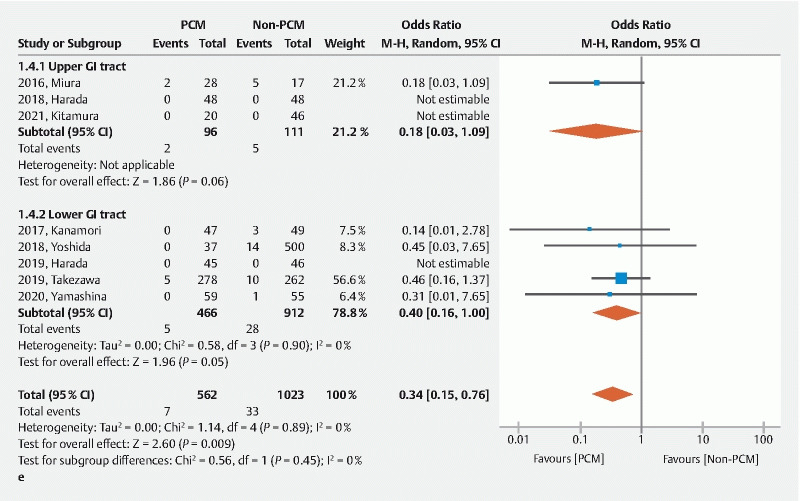
A Forest plot comparing the pocket creation method (PCM) to the non-PCM. e Rate of perforation. CI, confidence interval; IV, inverse variance; M-H, Mantel-Haenszel; SD, standard deviation.
Fig. 4.
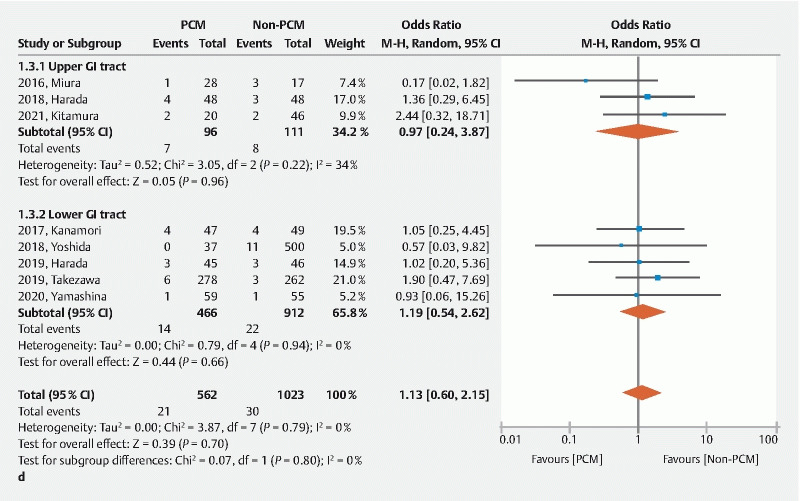
A Forest plot comparing the pocket creation method (PCM) to the non-PCM. d Rate of delayed bleeding.
Discussion
This quantitative review reveals significant superiority of the PCM over the non-PCM in ESD of lesions of the gastrointestinal tract regarding en bloc resection, R0 resection, dissection speed and perforation. The PCM improves curability and safety regardless of the organ where the lesion is located. En bloc resection is especially important because piecemeal resections make it impossible to confirm a negative margin and increases the rate of local recurrence (10–23.5 %) 16 . Since ESD is an advanced endoscopic technique compared with EMR, extensive training is necessary to attain competence to perform safe ESD. The location influences the difficulty of ESD due to factors such as a vertical approach, strong bending, presence of haustra and the pyloric ring. The PCM was developed and disseminated to conquer these difficult circumstances and provide a shortcut to learning ESD for beginner endoscopists. Past systematic reviews and meta-analyses regarding the PCM were limited to colorectal ESD 17 18 . This is the first quantitative review evaluating the effectiveness and safety of the PCM in both the upper and lower gastrointestinal tract.
This study demonstrates that the PCM facilitates R0 resection. Confirmation of a negative pathological margin and the presence/absence of lymphovascular invasion from resected specimens obtained by en bloc resection is important to determine future treatment strategies. Indications for additional surgical resection are discussed based on the depth of submucosal invasion, presence/absence of lympho-vascular invasion and budding grade 19 . Because these important factors are determined from examination of the submucosa, the resected specimen must have a thick less-cauterized submucosa to provide the information needed to decide on optimal treatment. The PCM provides clear submucosal visualization and stretching of submucosal tissue aided by the traction and countertraction provided by use of a small-caliber-tip transparent (ST) hood, which enables the operator to select the dissection level in the submucosa and divide submucosal tissue with minimum thermal damage. The ST hood facilitates entering the pocket and provides clear vision through its transparent wall enveloped in the pocket. The dissection level just above the muscularis (deep submucosa) is essential to keep a thick submucosa with the resected specimen which enables safe dissection by avoiding fat and branched vasculature generally located in the superficial submucosa. To assess local curability, a negative vertical margin is more important than a negative horizontal margin. Local recurrence at a positive horizontal margin is generally managed by additional endoscopic resection. A high R0 resection rate with a thick less-cauterized submucosa obtained by using the PCM provides important information to determine future treatment.
Greater dissection speed during ESD decreases the physical burden for the patient as well as the endoscopist. When using the non-PCM, the initial circumferential incision enhances dispersion of injected solution which decreases the traction in the submucosa. Dissection without a pocket in the stomach or duodenum is sometimes difficult due to cardiopulmonary movement. Dissection in a shallow submucosa increases bleeding which prolongs the procedure to achieve hemostasis. The PCM diminishes these time-consuming events and results in overall faster dissection.
This quantitative review shows a low rate of perforation in the PCM group. There are two reasons for this. First, the PCM can change a vertical approach to a tangential approach. As shown in Fig. 2 , the PCM can avoid a vertical approach by entering the pocket and changing the direction. A tangential approach is essential to achieve safe ESD and provides stable maneuvering. Second, traction and countertraction when using the PCM provide clear visualization of the submucosa and muscularis. The PCM can complete ESD without the need for dedicated traction devices. The PCM surely makes difficult ESD easier, and it also makes standard ESD safer and faster. Therefore, the PCM is useful without the need for special devices and can be used as far as the endoscope reaches 20 .
This study has acknowledged limitations. First, all studies originated from Japan. Data from western countries are necessary to generalize these results. Second, experience and skill levels of endoscopists vary. Third, seven of nine studies were non-RCTs. Since these retrospective studies used historical controls, this time-frame shift may influence the learning curve of endoscopists and the evolution of ESD devices. These factors may work to the advantage of the PCM group. Fourth, the resection method used was not blinded to the endoscopists. Fifth, heterogeneity exists among studies in the definition of R0 resection, delayed bleeding and procedure time. Sixth, only three studies regarding upper gastrointestinal ESD were included without esophageal ESD.
Conclusions
The PCM facilitates high-quality and safe colorectal ESD. Further studies are needed regarding the utility of PCM in ESD of the upper gastrointestinal tract.
Acknowledgments
The authors are grateful for detailed unpublished data provided by Dr Hideaki Harada (New Tokyo Hospital, Chiba, Japan), Dr Akira Kanamori (Dokkyo Medical University, Tochigi, Japan) and Dr Takeshi Yamashina (Osaka Red Cross Hospital, Osaka, Japan).
Footnotes
Competing interests Drs. Hayashi, Miura, and Yano have received honoraria from the Fujifilm Corporation. Dr. Yamamoto has a consultant relationship with the Fujifilm Corporation and has received honoraria, grants, and royalties from the company.
Supplementary material :
References
- 1.Shinozaki S, Hayashi Y, Lefor A K et al. What is the best therapeutic strategy for colonoscopy of colorectal neoplasia? Future perspectives from the East. Dig Endosc. 2016;28:289–295. doi: 10.1111/den.12566. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi Y, Sunada K, Takahashi H et al. Pocket-creation method of endoscopic submucosal dissection to achieve en bloc resection of giant colorectal subpedunculated neoplastic lesions. Endoscopy. 2014;46:E421–E422. doi: 10.1055/s-0034-1377438. [DOI] [PubMed] [Google Scholar]
- 3.Miura Y, Shinozaki S, Hayashi Y et al. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8–14. doi: 10.1055/s-0042-116315. [DOI] [PubMed] [Google Scholar]
- 4.Higgins J P, Altman D G, Gøtzsche P C et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S Y, Park J E, Lee Y J et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Higgins J P, Thompson S G, Deeks J J et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashina T, Hayashi Y, Fukuda H et al. The pocket-creation method may facilitate endoscopic submucosal dissection of large colorectal sessile tumors. Endosc Int Open. 2020;8:E1021–e1030. doi: 10.1055/a-1190-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto H, Hayashi Y, Miura Y et al. Pocket-creation method facilitates endoscopic submucosal dissection of colorectal laterally spreading tumors, non-granular type. Endosc Int Open. 2017;5:E123–E129. doi: 10.1055/s-0042-122778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takezawa T, Hayashi Y, Shinozaki S et al. The pocket-creation method facilitates colonic endoscopic submucosal dissection (with video) Gastrointest Endosc. 2019;89:1045–1053. doi: 10.1016/j.gie.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida N, Naito Y, Yasuda R et al. The efficacy of the pocket-creation method for cases with severe fibrosis in colorectal endoscopic submucosal dissection. Endosc Int Open. 2018;6:e975–e983. doi: 10.1055/a-0593-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori A, Nakano M, Kondo M et al. Clinical effectiveness of the pocket-creation method for colorectal endoscopic submucosal dissection. Endosc Int Open. 2017;5:e1299–e1305. doi: 10.1055/s-0043-118744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitamura M, Miura Y, Shinozaki S et al. The pocket-creation method facilitates endoscopic submucosal dissection of gastric neoplasms involving the pyloric ring. Endosc Int Open. 2021;9:e1062–e1069. doi: 10.1055/a-1403-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashina T, Nemoto D, Hayashi Y et al. Prospective randomized trial comparing the pocket-creation method and conventional method of colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:368–379. doi: 10.1016/j.gie.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Harada H, Nakahara R, Murakami D et al. Saline-pocket endoscopic submucosal dissection for superficial colorectal neoplasms: a randomized controlled trial (with video) Gastrointest Endosc. 2019;90:278–287. doi: 10.1016/j.gie.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Harada H, Murakami D, Suehiro S et al. Water-pocket endoscopic submucosal dissection for superficial gastric neoplasms (with video) Gastrointest Endosc. 2018;88:253–260. doi: 10.1016/j.gie.2018.04.2331. [DOI] [PubMed] [Google Scholar]
- 16.Hotta K, Saito Y, Matsuda T et al. Local recurrence and surveillance after endoscopic resection of large colorectal tumors. Dig Endosc. 2010;22:S63–S68. doi: 10.1111/j.1443-1661.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 17.Pei Q, Qiao H, Zhang M et al. Pocket-creation method versus conventional method of endoscopic submucosal dissection for superficial colorectal neoplasms: a meta-analysis. Gastrointest Endosc. 2021;93:1038–1.046E7. doi: 10.1016/j.gie.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Chen T, Tan Y et al. Pocket-creation method improves efficacy of colorectal endoscopic submucosal dissection: a system review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;33:1241–1246. doi: 10.1097/MEG.0000000000001864. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Itabashi M, Shimada Y et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe S, Wu S YS, Ego M et al. Efficacy of Current traction techniques for endoscopic submucosal dissection. Gut Liver. 2020;14:673–684. doi: 10.5009/gnl19266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


