Abstract
Background and study aims Endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ) is an endoscopic procedure for treating gastric outlet obstruction (GOO). Limited data exist regarding the safety and efficacy of EUS-GJ in patients with malignant GOO with ascites. Thus, we aimed to study the outcomes and safety of EUS-GJ in GOO patients with vs. without ascites.
Patients and methods This is a retrospective cohort study of patients with malignant GOO who underwent successful EUS-GJ at a tertiary care academic center. Primary outcomes included the efficacy and safety of EUS-GJ. Secondary outcomes included 30-day readmission, reintervention, and survival utilizing Kaplan-Meier analysis.
Results A total of 55 patients (mean age of 67.0 ± 11.3 years, 40.0 % female) who underwent EUS-GJ, of whom 24 had ascites (small in 22, large in 2) were included. Clinical success was achieved in 91.7 % and 93.5 % ( P = 1.00) of patients with and without ascites, respectively. A higher rate of adverse events (AEs) was noted in patients with ascites but this was not statistically significant (37.5 % vs. 19.4 %, P = 0.13). Four patients in the ascites group (16.6 %) developed clinical evidence of peritonitis or sepsis post-EUS-GJ. Eight patients with ascites developed worsening ascites within a month of EUS-GJ. In contrast, only one patient without ascites developed evidence of new ascites. The median survival of patients was not significantly different between the two groups (patients with ascites: 129 days vs. patients without ascites: 180 days, ( P = 0.12).
Conclusions The efficacy EUS-GJ in the presence of ascites is promising; however, the safety profile remains concerning given the high rate of AEs, specifically peritonitis and sepsis.
Introduction
Malignant gastric outlet obstruction (GOO) often occurs in the setting of advanced, unresectable malignancies of the upper gastrointestinal tract 1 . This encompasses pancreaticobiliary and gastroduodenal malignancies, lymphomas, or peritoneal carcinomatosis and omental caking 2 . Malignant GOO often compromises the patient’s quality of life due to nausea, vomiting, and decreased food intake 2 . Although malignancy-associated dysmotility contributes to these symptoms, bypassing the mechanical aspect of GOO is commonly indicated for palliative purposes and improved quality of life 3 .
The most commonly employed interventions for patients with malignant GOO are surgical gastrojejunostomy (SGJ) or enteral self-expandable metallic stents (SEMS) placement 4 5 . However, SGJ is invasive and carries an adverse event (AE) rate of more than 30 % which includes delayed gastric emptying, gastroparesis, and prolonged recovery times 6 7 . Moreover, SGJ may be an inferior option to SEMS in patients with peritoneal carcinomatosis 8 . Contrarily, SEMS can be complicated by migration, or tumor ingrowth within the stent interstices, which is seen in the majority of patients who survive longer than six months 9 . Minor and major AEs occur in up to 27 %, and 9 % of patients, respectively 10 11 12 .
Endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ) has emerged as an option for the treatment of malignant GOO to overcome the limitations posed by enteral uncovered SEMS while avoiding the risks of surgical intervention in patients who may not be good surgical candidates 13 . Although data shows high clinical and technical success and relatively low AE rates, this approach remains incompletely evaluated, in the presence of ascites 13 14 15 . Although small series have reported lumen-apposing metal stents (LAMS) (AXIOS, Boston Scientific Corporation Inc., Marlborough, MA) application for EUS-GJ in patients with ascites 15 , this practice remains controversial. In a multicenter series of EUS-GJ procedures, 35 % of patients had ascites, and 33 % had peritoneal carcinomatosis. The technical success rate of the intervention was 20 % lower than patients without ascites or peritoneal carcinomatosis 16 . Acknowledging the limited data, experts suggest avoiding EUS-GJ in patients with large ascites given the risk of dehiscence and peritonitis 17 . Thus, this retrospective study aims to describe the efficacy and safety of EUS-GJ in patients with malignant GOO and ascites.
Patients and methods
We performed a retrospective review of the electronic medical records of consecutive patients with malignant GOO and ascites who underwent EUS-GJ between May 2018 and May 2021, at the Mayo Clinic, Rochester, Minnesota. The procedures were performed by a total of eight different endoscopists. All patients had permitted the use of their medical information for research review, and the study was conducted following approval by the Institutional Review Board (IRB #20–009367). The research conforms with the Declaration of Helsinki principles.
We reviewed the medical records of 58 consecutive patients with and without malignant ascites presenting for EUS-GJ for treatment of GOO, of which two patients and one patient were excluded due to technical failure of EUS-GJ and lack of post-procedural follow-up, respectively. Of the 55 included patients, 48 were inpatients and seven were outpatients. GOO symptoms included nausea, vomiting, abdominal pain, abdominal bloating, weight loss, heartburn, and early satiety. Data were abstracted retrospectively and included patient baseline characteristics, etiology and site of malignant GOO, severity of GOO, amount of ascites, presence of peritoneal carcinomatosis, prior endoscopic stent placement or dilation, Eastern Cooperative Oncology Group (ECOG) status, American Society of Anesthesiologists (ASA) status, intervention technique, prosthesis characteristics, procedure time, clinical success, length of hospital stay for inpatients, treatment outcomes, AEs, recurrence of obstructive symptoms, reinterventions, death and time to death.
Definitions and outcome assessment
The primary outcomes were efficacy defined by clinical success and safety defined by the rate of AEs. Secondary outcomes included a 30-day readmission rate, reintervention, and survival. EUS-GJ involves obtaining access to the jejunum endosonographically from the stomach and placing the LAMS across a newly formed fistulous tract thus creating a gastrojejunal bypass 18 . The degree of ascites was defined according to radiological findings on CT imaging and predefined criteria utilized by the blinded radiological reviewer assigned to each case at our institution. None of the patients had paracentesis during the 4-week period prior to the procedure. The severity of GOO was determined according to the GOO symptom score system (GOOSS; 0 = no oral intake, 1 = liquid diet only, 2 = soft diet, and 3 = low-residue or full diet) 19 . EUS-GJ technical success was defined as successful deployment of the gastroenteric LAMS determined by the reflux of methylene blue through the expanded gastric LAMS flange. Clinical success was defined as the ability to tolerate at least a liquid diet without nausea or vomiting within 5 days. AEs were defined according to the American Society of Gastrointestinal Endoscopy lexicon classification 20 . Procedure time was defined as time from endoscope insertion until endoscope retrieval. Peritonitis was defined as having a polymorphonuclear leukocytes (PMN) count of 250 cells/mm 3 or higher and a positive bacterial culture result on peritoneal fluid analysis 21 . Sepsis was defined as having positive blood cultures in conjunction with the presence or absence of peritonitis. Different EUS-GJ techniques were used at the discretion of the performing endoscopist, and all procedures were performed under general anesthesia. Technical details of the procedure have been described in previously published studies 22 23 24 . In brief, a linear echoendoscope was introduced into the stomach. The majority of EUS-GJ procedures were performed by advancing a cautery-enhanced LAMS, followed by advancing a guidewire through the LAMS catheter into the small bowel. For the indirect method, the small bowel was located and distended by infusing saline mixed with water-soluble contrast via different methods including using a nasobiliary catheter in the nasobiliary catheter-assisted method, using an ultraslim gastroscope above or through the area of obstruction in the dual scope-assisted method, and using a balloon in the balloon-assisted method. For the direct method, the 19-gauge needle was used for puncture into the jejunum under EUS guidance and contrast was injected to confirm the needle location in the jejunum. A guidewire was then passed into the jejunum followed by the LAMS deployment over the guidewire.
Statistical analysis
Data were expressed as mean and standard deviation for continuous variables or proportions, median and interquartile range (IQR) for categorical variables. Continuous data were compared using Student’s independent t-test and nonparametric Mann-Whitney U test when appropriate. Categorical data were compared using a chi-square test or Fisher’s exact test when appropriate. Survival analysis was performed using Kaplan-Meier analysis and compared using the Tarone-Ware method. Patients at the time of analysis or date of the last follow-up were censored. P < 0.05 was considered significant. The statistical analysis was performed using IBM SPSS 27.0 (IBM Corporation, Armonk, New York, United States).
Risk of bias assessment
We implement a widely adopted tool to provide a quality assessment of the risk of bias in our case series 25 . Included patients were consecutive only excluding patients not meeting the inclusion criteria, and thus representing the whole experience of our center during the study period (patients with malignant GOO, underwent successful EUS-GJ, and all qualified patients were included without omission). The exposure (EUS-GJ) was ascertained for all cases without concomitant procedures. The outcomes (efficacy, safety, and occurrence of AEs) were adequately ascertained in all cases. No alternative causes explained the outcomes. Follow-up was adequate for the assessment of the selected outcomes.
Results
Patient characteristics
A total of 55 patients were included (mean age 67.0 ± 11.3 years; 40.0 % female). Twenty-four patients (43.6 %) had ascites, of which twenty-two (91.7 %) had small ascites, and two (8.3 %) had large ascites. Thirty-one patients (54.5 %) did not have ascites. The most common etiologies of obstruction were pancreatic cancer (n = 30, 55.4 %), followed by duodenal or ampullary cancer (n = 6, 10.9 %), cholangiocarcinoma (n = 5, 9.1 %) and gastric cancer (n = 5, 9.1 %). Sites of obstruction were pylorus/antrum (n = 7, 12.7 %), duodenal bulb (n = 16, 29.1 %), second part of the duodenum (n = 25, 45.4 %), and distal duodenum (n = 7, 12.7 %). The baseline patient characteristics are shown in Table 1 .
Table 1. Baseline characteristics.
| Ascites (N = 24) | No ascites (N = 31) | P value | |
| Age (years, mean ± SD) | 68.0 ± 8.7 | 66.3 ± 13.1 | 0.60 |
| Gender (female, n (%)) | 10 (41.7) | 12 (38.7) | 0.82 |
| Symptoms of GOO (n (%)) | |||
|
20 (83.3) | 23 (74.2) | 0.42 |
|
13 (54.2) | 18 (58.1) | 0.77 |
|
3 (12.5) | 2 (6.5) | 0.64 |
|
6 (25.0) | 7 (22.6) | 0.83 |
|
1 (4.2) | 2 (6.5) | 1.00 |
|
2 (8.3) | 5 (16.1) | 0.45 |
| Severity of GOO (n (%)) | 0.36 | ||
|
19 (79.2) | 20 (64.5) | |
|
3 (12.5) | 2 (6.5) | |
|
1 (4.2) | 5 (16.1) | |
|
1 (4.2) | 4 (12.9) | |
| Cause of malignant GOO (%) | 0.97 | ||
|
2 (8.3) | 3 (9.7) | |
|
2 (8.3) | 4 (12.9) | |
|
0 (0.0) | 1 (3.2) | |
|
3 (12.5) | 2 (6.5) | |
|
14 (58.3) | 16 (51.6) | |
|
0 (0.0) | 1 (3.2) | |
|
0 (0.0) | 1 (3.2) | |
|
1 (4.2) | 0 (0.0) | |
|
1 (4.2) | 1 (3.2) | |
|
1 (4.2) | 0 (0.0) | |
|
0 (0.0) | 1 (3.2) | |
|
0 (0.0) | 1 (3.2) | |
| ECOG status, median (IQR) | 1 (0.75–2) | 1 (1–2) | 0.79 |
| ASA status, median (IQR) | 3 (3–3) | 3 (3–3) | 0.94 |
| Previous treatment attempts (%) | |||
|
5 (20.8) | 4 (12.9) | 0.48 |
|
2 (8.3) | 3 (9.7) | 1.00 |
| Peritoneal carcinomatosis (%) | 5 (20.8) | 4 (12.9) | 0.48 |
| Location of obstruction (%) | 0.12 | ||
|
1 (4.2) | 6 (19.4) | |
|
6 (25.0) | 10 (32.3) | |
|
15 (62.5) | 10 (32.3) | |
|
2 (8.3) | 5 (16.1) | |
ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; SD, standard deviation.
Clinical outcomes
The clinical success rate was 91.7 % in patients with ascites and 93.5 % in patients without ascites ( P = 1.00). In patients with ascites, clinical success was 90.9 % (n = 22) and 100 % (n = 2) in patients with small and large ascites, respectively. The EUS-GJ techniques used included nasobiliary catheter-assisted method with a guidewire (n = 30), dual-scope-assisted method with a guidewire (n = 10), balloon-assisted method with a guidewire (n = 8), and direct method with a guidewire (n = 7). Mean procedure time was 76.0 ± 42.5 minutes in the patients with ascites and 89.0 ± 55.2 minutes without ascites ( P = 0.35). After the procedure, the mean length of hospital stay was 4.8 ± 3.4 days and 3.0 ± 4.1, respectively ( P = 0.13). Duration of follow-up was longer in patients without ascites 106 days (IQR 77–200 days) vs 73.5 days (IQR 20.3–291.8 days); however, this was not statistically significant ( P = 0.11). The 30-day readmission rate was similar between the two groups: 25.0 % and 19.4 % ( P = 0.62). The most common cause for 30-day readmission was post-procedural abdominal pain that was managed conservatively. Treatment outcomes are demonstrated in Table 2 .
Table 2. Treatment outcomes.
| Ascites (N = 24) | No ascites (N = 31) | P value | |
| Procedure time (minutes, mean ± SD) | 76.0 ± 42.5 | 89.0 ± 55.2 | 0.35 |
| Technique (%) | 0.26 | ||
|
3 (12.5) | 4 (12.9) | |
|
7 (29.2) | 3 (9.7) | |
|
2 (8.3) | 6 (19.4) | |
|
12 (50.0) | 18 (58.1) | |
| Type of stent (%) | 1.00 | ||
|
24 (100.0) | 30 (96.8) | |
|
0 (0.0) | 1 (3.2) | |
| Clinical success (%) | 22 (91.7) | 29 (93.5) | 1.00 |
| 30-day readmission (%) | 6 (25.0) | 6 (19.4) | 0.62 |
| Length of hospital stay after procedure, days, mean (SD) | 4.8 ± 3.4 | 3.0 ± 4.1 | 0.13 |
| Adverse Events, no. patients (%) | 9 (37.5) | 6 (19.4) | 0.13 |
| Mild | 5 (20.8) | 3 (9.7) | |
|
1 | 2 | |
|
1 | 0 | |
|
2 | 2 | |
|
1 | 0 | |
| Moderate | 2 (8.3) | 3 (9.7) | |
|
2 | 0 | |
|
0 | 1 | |
|
0 | 1 | |
|
0 | 1 | |
|
1 | 0 | |
|
1 | 0 | |
| Severe | 2 (8.3) | 0 (0.0) | |
|
1 | 0 | |
|
1 | 0 | |
| Recurrence of obstructive symptoms (%) | 1 (4.2) | 1 (3.2) | 1.00 |
| Reintervention for recurrent GOO (%) | 1 (4.2) | 1 (3.2) | 1.00 |
| Death (%) | 14 (58.3) | 17 (54.8) | 0.80 |
| Time to death (days, median (IQR)) | 35.5 (18.5–92.3) | 110.5 (82.3–211.5) | 0.005 2 |
| Duration of follow-up (days, median (IQR)) | 73.5 (20.3–291.8) | 106.0 (77.0–200.0) | 0.11 |
SD, standard deviation; IQR, interquartile range; GOO, gastric outlet obstruction; DVT, deep vein thrombosis.
Developed additional adverse events.
Indicates statistical significance.
GOO recurrence and reintervention
In one patient (4.2 %) with ascites, recurrence of obstructive symptoms occurred secondary to partial obstruction of the gastrojejunal anastomosis with food residue and required reintervention with removal and replacement of the stent 333 days post-procedurally. In one patient (3.2 %) without ascites, recurrence of obstructive symptoms occurred, and the patient was found to have tumor progression not affecting the site of the gastrojejunostomy. However, tissue ingrowth was encountered at the site of the gastrojejunostomy necessitating removal and replacement of the stent with another stent of larger caliber for enhanced palliation 33 days post-procedurally.
Adverse events
AEs occurred in similar frequencies between the two groups (37.5 % and 19.4 % P = 0.13). In patients with ascites, AEs occurred in 36.3 % (n = 22) and 50 % (n = 2) in patients with small and large ascites, respectively. Mild AEs occurred in 20.8 % and 9.7% of patients with and without ascites, respectively. Stent mal-deployment followed by successful re-deployment in the same session was seen in one patient with ascites and in two patients without ascites. One case of late stent migration was observed in a patient with ascites. Post-procedural abdominal pain was seen in two patients with ascites and was managed conservatively. One case of deep venous thrombosis developed in a patient with ascites and was managed with anticoagulation. Moderate AEs occurred in 8.3 % and 9.7 % of patients, respectively. Stent mal-deployment followed by successful re-deployment in the same session was seen in two patients of patients with ascites. The patients subsequently developed evidence of peritonitis post-procedurally, with one of the patients having a positive blood culture and thus classified as sepsis, which then resolved after management with IV antibiotics and a full liquid diet. One case of post-procedural abdominal pain and one case of cholangitis was seen in patients without ascites, and were managed with analgesics and antibiotics, respectively. One case of tissue ingrowth that required a repeat EUS-GJ to remove and replace the stent with another stent of larger caliber was encountered in a patient without ascites. Severe AEs were seen in 8.3 % of patients with ascites and in none of the patients without ascites. One patient developed evidence of peritonitis secondary to early stent migration on post-procedure day one. The patient underwent laparotomy with repair of the gastrojejunostomy and jejunal perforation, and placement of a naso-gastric, gastrostomy and jejunostomy tubes. One patient developed concomitant peritonitis and sepsis on post-procedure day 3, and was admitted to the intensive care unit. The patient’s clinical course rapidly deteriorated and ultimately resulted in death 6 days post-procedurally.
Post-procedural ascites and paracentesis
Eight patients (33.3 %) with ascites developed evidence of worsening ascites, while sixteen patients (66.7) had stable ascites within four weeks of the procedure ( Table 3 ). One patient (1.8 %) without ascites developed evidence of ascites 3 days post-procedurally. Paracentesis was performed in five patients with worsening ascites and in three patients with stable ascites, with a median time to paracentesis of 5.5 days (IQR 3.3–20.3 days). In patients with worsening ascites, two of five had evidence of peritonitis on paracentesis. Whereas, in patients with stable ascites whose ascites was sampled, one of three of patients had evidence of peritonitis on paracentesis. All patients who underwent paracentesis were on antimicrobials. Table 4 and Table 5 describe the paracentesis results.
Table 3. Characteristics of ascites.
| Ascites (N = 24) | No ascites (N = 31) | P value | |
| Degree of ascites (%) | – | ||
|
22 (91.7) | – | |
|
2 (8.3) | – | |
|
8 (33.3) | – | – |
|
– | 1 (1.8) | – |
|
– | 3 | – |
| Paracentesis performed after procedure (%) | 8 (34.8) | 0 (0.0) | – |
| Time to paracentesis (days, median (IQR)) | 5.5 (3.3–20.3) | – | – |
| Any antimicrobials use (%) | 18 (75.0) | 24 (77.4) | 0.83 |
| Prophylactic antimicrobials use after procedure (%) | 11 (45.8) | 18 (58.1) | 0.37 |
| Duration of prophylactic antimicrobials use (days, median (IQR)) | 7.0 (3.5–10.0) | 7.0 (5.0–10.0) | 0.92 |
| Antimicrobials use for concomitant infection (%) | 8/21 (38.1) | 11/22 (50.0) | 0.43 |
| Duration of antimicrobials use (days, median (IQR)) | 14.0 (7.0–41.8) | 14.0 (10.0–19.0) | 0.95 |
IQR, interquartile range
Table 4. Status of ascites in patients with worsening ascites who underwent post-procedural paracentesis.
| Status of ascites | Worsening ascites (N = 5) | ||||
| No peritonitis (N = 3) | Peritonitis (N = 2) | ||||
| Patient A | Patient B | Patient C | Patient D | Patient E | |
| Gross appearance | Serous | Hazy | Slightly Cloudy | Cloudy and Icteric | Slightly Cloudy and Bloody |
| Total nucleated cells/mm 3 | 133 | 183 | 1,665 | 13,175 | 24,235 |
| Absolute PMN count | 9 | 20 | 1,165 | 11,462 | 21,327 |
| PMN (%) | 7 | 11 | 70 | 87 | 88 |
| Lymphocytes (%) | 7 | 59 | 15 | – | 2 |
| Monocytes/macrophages (%) | 76 | 22 | 9 | 13 | 8 |
| Bilirubin (mg/dL) | – | – | – | 9.4 | – |
| Glucose (U/L) | – | – | – | – | – |
| Total Protein (g/dL) | – | – | – | – | – |
| Albumin (g/dL) | 0.6 | – | – | – | 1.5 |
| SAAG | 2.4 | – | – | – | 2.5 |
| Culture | No growth | No growth | No growth | Klebsiella Pneumoniae complex | Escherichia coli |
PMN, polymorphonuclear leukocytes; SAAG, serum ascites albumin gradient.
Table 5. Status of ascites in patients with stable ascites who underwent post-procedural paracentesis.
| Status of ascites | Stable ascites (N = 3) | ||
| No peritonitis (N = 2) | Peritonitis (N = 1) | ||
| Patient F | Patient G | Patient H | |
| Gross appearance | Serous | Serous | Slightly cloudy and bloody |
| Total nucleated cells/mm 3 | 306 | 367 | 11,592 |
| Absolute PMN count | 55 | 121 | 10,896 |
| PMN (%) | 18 | 33 | 94 |
| Lymphocytes (%) | 36 | 26 | 1 |
| Monocytes/macrophages (%) | 43 | 34 | 5 |
| Bilirubin (mg/dL) | – | – | – |
| Glucose (U/L) | – | – | 103 |
| Total Protein (g/dL) | 1.1 | 1.3 | 0.7 |
| Albumin (g/dL) | 0.5 | 0.9 | 0.6 |
| SAAG | 2.3 | 2.5 | – |
| Culture | No growth | No growth | Leptotrichia trevisanii Campylobacter concisus/curvus |
PMN, polymorphonuclear leukocytes; SAAG, serum ascites albumin gradient.
Survival
During a median follow-up of 96 days (IQR 39–200 days), there was no statistical difference in death between the groups 58.3% (n = 14) and 54.8 % (n = 17), respectively ( P = 0.80). However, time to death was significantly shorter in patients with ascites (35.5 days [IQR 18.5–92.3 days] vs. 110.5 days [IQR 82.3–211.5 days]) ( P = 0.005). In patients with worsening ascites, death occurred in six of eight of the patients and median time to death was 26 days (IQR: 16.5–184 days). Whereas in patients with stable ascites, death occurred in eight of 16, and the median time to death was 53.5 days (IQR: 17–82.75 days), but not reaching statistical significance ( P = 0.80). Fig. 1 demonstrates the survival analysis for patients with and without ascites. The median survival was 129 days (95 % CI 32–226) in patients with ascites and 180 days (95 % CI 79–281) in patients without ascites. However, there was no statistical difference between the survival curves ( P = 0.12 by Tarone-Ware test).
Fig. 1.
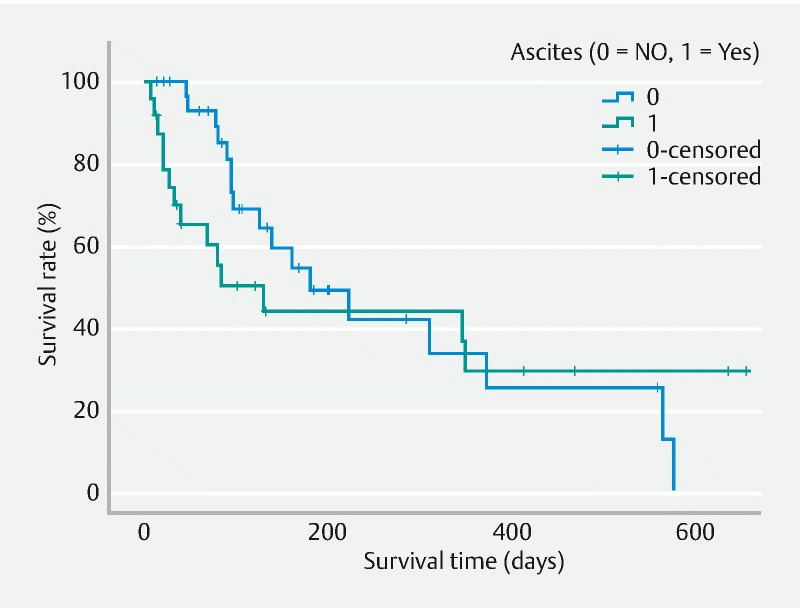
Survival analysis for patients with and without ascites.
Discussion
In this study, we examine the efficacy and safety of EUS-GJ in GOO patients with ascites. The main aim of palliative treatment in symptomatic GOO patients is to relieve obstructive symptoms and improve the quality of life. This study shows that clinical success, defined as the ability to tolerate a liquid diet, was similar in patients with and without ascites. Studies have reported clinical success rates of 83 % to 96 % in GOO patients of malignant etiologies 13 15 26 27 . These rates are comparable to our success rates of 92 % and 94 % in patients with and without ascites, respectively. There was no significant difference in the mean procedure time, mean length of post-procedural hospital stay, and 30-day readmission rate between the two groups. The most common cause for 30-day readmission was post-procedural abdominal pain that was managed conservatively. The reintervention rates were similar in both groups, at 4.2 % and 3.2 %, respectively, which are comparable to what has been reported in recent literature (3 %–11 %) 13 26 28 . Thus, our data suggest that EUS-GJ can be efficacious in the presence of ascites. Similarly, a study by Basha et al. limited to 31 patients with both malignant and benign etiologies concluded that EUS-GJ is feasible in the presence of ascites 29 .
With regards to safety, the AE rate was numerically higher in patients with ascites at 37.5 % vs. 19.4 % 13 15 26 27 30 . However, the majority of AEs in our study were mild in severity (20.8%); and the rate of severe AEs (8.3 %) was comparable to that in recent studies (10 %–11.5 %) 15 26 . The most common AE (n = 5) in our study was mal-deployment of the stent that was later salvaged with a second attempt at deployment in the same session, which occurred in three patients with ascites and in two patients without ascites. Sepsis and peritonitis were observed in four patients (16.6 %) with ascites, and no clinical evidence of infection was observed in patients without ascites. These patients were on prophylactic antibiotics pre- and post-procedurally and time to onset of infection was 1 to 3 days after EUS-GJ. The patients were subsequently managed with IV antibiotics. However, one patient who developed concomitant peritonitis and sepsis had a complicated clinical course and ultimately died 6 days after EUS-GJ. Tyberg et al. reported one procedure-related death of a patient with ascites secondary to peritonitis 15 . Other previously reported AEs include biliary obstruction, and hemoperitoneum 27 28 . Mortality was found to be accelerated in patients with ascites with a median survival of 129 days (95 % CI 32–226) in patients with ascites compared to 180 days (95 % CI 79–281) in those without ascites, but did not reach statistical significance ( P = 0.12). Nonetheless, the preliminary safety data for EUS-GJ in the presence of ascites remains contentious given the high rate of AEs, and the development of peritonitis and sepsis despite adequate utilization of peri-procedural antibiotics and therapeutic paracentesis. Thus, larger prospective studies will be required to further explore its safety. Moreover, in a retrospective study by Ge et al., EUS-GJ was shown to be a superior method of GOO palliation over enteral stenting with higher clinical success and stent durability 27 . Acknowledging the higher complications of EUS-GJ, risk-benefit discussion with the patient for shared decision making in the setting of risk mitigation with prophylactic antibiotic and paracentesis afterwards should be exercised 27 .
This study has several notable limitations worth mentioning. These limitations include the retrospective design of the study and the small overall sample size, with ensuing underpower limitation. In addition, the procedures were performed by endoscopists of varying experience and multiple techniques were implemented by these endoscopists, which affects the generalizability of the results. A selection bias was also likely present since patients were considered for endoscopic palliative intervention when they were deemed unfit for surgical interventions. The findings of this study can also be affected by the heterogeneity of etiology of GOO (i. e., type, location, and etiology) and variations in prior treatment (i. e., number of endoscopic dilation and prior stent placement).
Conclusions
In conclusion, EUS-GJ is a viable palliative option for malignant GOO in the presence and absence of ascites and demonstrates high clinical success. However, the safety profile for EUS-GJ in the presence of ascites remains controversial given the high rate of AEs, and the occurrence of peritonitis and sepsis in patients despite adequate utilization of peri-procedural antibiotics and therapeutic paracentesis. Therefore, proceeding with caution is advised in those patients with GOO and ascites, especially if they are not candidates for endoluminal interventions, such as a stent placement, or have failed this modality.
Footnotes
Competing interests Dr. Abu Dayyeh is a consultant for USGI, Medtronic, Boston Scientific, Hemostasis, Endogenex, and the recipient of research support from Apollo Endosurgery, USGI, Boston Scientific, Endogastric Solutions and Medtronic and a speaker for Olympus, Medtronic and Johnson and Johnson. Dr. Storm is a consultant for Apollo Endosurgery, GI Dynamics, Olympus, ERBE, and Enterasense, and the recipient of research support from Apollo Endosurgery, Endo-TAGSS, and Boston Scientific. Dr. Law is a consultant for ConMed and Medtronic. Dr. Chandrasekhara is a consultant for Interpace Diagnostics and Covidien LP, and a shareholder at Nevakar Corporation.
References
- 1.van Heek N T, van Geenen R C, Busch O R et al. Palliative treatment in "peri"-pancreatic carcinoma: stenting or surgical therapy? Acta Gastroenterol Belg. 2002;65:171–175. [PubMed] [Google Scholar]
- 2.Miyazaki Y, Takiguchi S, Takahashi T et al. Treatment of gastric outlet obstruction that results from unresectable gastric cancer: Current evidence. World journal of gastrointestinal endoscopy. 2016;8:165–172. doi: 10.4253/wjge.v8.i3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perone J A, Riall T S, Olino K. Palliative care for pancreatic and periampullary cancer. Surg Clin North Am. 2016;96:1415–1430. doi: 10.1016/j.suc.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surgery. 2004;28:812–817. doi: 10.1007/s00268-004-7329-0. [DOI] [PubMed] [Google Scholar]
- 5.van Hooft J E, Uitdehaag M J, Bruno M J et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest. 2009;69:1059–1066. doi: 10.1016/j.gie.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Khashab M, Alawad A S, Shin E J et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27:2068–2075. doi: 10.1007/s00464-012-2712-7. [DOI] [PubMed] [Google Scholar]
- 7.Maetani I, Tada T, Ukita T et al. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004;36:73–78. doi: 10.1055/s-2004-814123. [DOI] [PubMed] [Google Scholar]
- 8.Jaruvongvanich V, Chesta F, Baruah A et al. Palliative treatment for malignant gastrointestinal obstruction with peritoneal carcinomatosis: enteral stenting versus surgery. Endosc Int Open. 2020;8:E1487–E1494. doi: 10.1055/a-1237-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeurnink S M, Steyerberg E W, van Hooft J E et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Dormann A, Meisner S, Verin N et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543–550. doi: 10.1055/s-2004-814434. [DOI] [PubMed] [Google Scholar]
- 11.Holt A P, Patel M, Ahmed M M. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc. 2004;60:1010–1017. doi: 10.1016/s0016-5107(04)02276-x. [DOI] [PubMed] [Google Scholar]
- 12.Jeurnink S M, van Eijck C H, Steyerberg E W et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khashab M A, Bukhari M, Baron T H et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–E281. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y I, James T W, Agarwal A et al. EUS-guided gastroenterostomy in management of benign gastric outlet obstruction. Endosc Int Open. 2018;6:E363–E368. doi: 10.1055/s-0043-123468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyberg A, Perez-Miranda M, Sanchez-Ocaña R et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4:E276–E281. doi: 10.1055/s-0042-101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastelijn J B, Moons L MG, Garcia-Alonso F J et al. Patency of endoscopic ultrasound-guided gastroenterostomy in the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2020;8:E1194–e1201. doi: 10.1055/a-1214-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane M G, Khashab M A. Malignant GOO: Are duodenal stenting and surgical gastrojejunostomy obsolete? Endosc Int Open. 2020;8:E1455–E1457. doi: 10.1055/a-1231-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawod E, Nieto J M. Endoscopic ultrasound guided gastrojejunostomy. Translat Gastroenterol Hepatol. 2018;3:93. doi: 10.21037/tgh.2018.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler D G, Baron T H. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–78. doi: 10.1111/j.1572-0241.2002.05423.x. [DOI] [PubMed] [Google Scholar]
- 20.Cotton P B, Eisen G M, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Akriviadis E A, Runyon B A. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127–133. doi: 10.1016/0016-5085(90)91300-u. [DOI] [PubMed] [Google Scholar]
- 22.Itoi T, Baron T H, Khashab M A et al. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig Endosc. 2017;29:495–502. doi: 10.1111/den.12794. [DOI] [PubMed] [Google Scholar]
- 23.Itoi T, Ishii K, Ikeuchi N et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016;65:193–195. doi: 10.1136/gutjnl-2015-310348. [DOI] [PubMed] [Google Scholar]
- 24.Tyberg A, Perez-Miranda M, Sanchez-Ocana R et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4:E276–E281. doi: 10.1055/s-0042-101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murad M H, Sultan S, Haffar S et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y I, Itoi T, Baron T H et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946–2952. doi: 10.1007/s00464-016-5311-1. [DOI] [PubMed] [Google Scholar]
- 27.Ge P S, Young J Y, Dong W et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33:3404–3411. doi: 10.1007/s00464-018-06636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerdsirichairat T, Irani S, Yang J et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019;7:E144–E150. doi: 10.1055/a-0799-9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basha J, Lakhtakia S, Yarlagadda R et al. Gastric outlet obstruction with ascites: EUS-guided gastro-enterostomy is feasible. Endosc Int Open. 2021;9:E1918–E1923. doi: 10.1055/a-1642-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khashab M A, Kumbhari V, Grimm I S et al. EUS-guided gastroenterostomy: the first U.S. clinical experience (with video) Gastrointest Endosc. 2015;82:932–938. doi: 10.1016/j.gie.2015.06.017. [DOI] [PubMed] [Google Scholar]


