Abstract
Worldwide reports have produced conflicting data on perinatal outcomes during the COVID-19 pandemic. This systematic review and meta-analysis addressed the effect of mitigation measures against COVID-19 on preterm birth, stillbirth, low birth weight, and NICU admission during the first nine months of the pandemic.
A search was performed using MEDLINE, Embase and SCOPUS for manuscripts published up until 24th May 2021. Studies that reported perinatal outcomes (preterm birth, stillbirth, low birth weight, NICU admission) during the COVID-19 pandemic with a pre-pandemic control period were included. Risk of bias assessment was performed using ROBINS-I tool. RevMan5 was used to perform meta-analysis with random-effects models. A score of the stringency of mitigation measures was calculated from the Oxford COVID-19 Government Response Tracker.
Thirty-eight studies of moderate to serious risk of bias were included, with varied methodology, analysis and regional mitigation measures, using stringency index scores. There was no overall effect on preterm birth at less than 37 weeks (OR 0.96, 95% CI 0.92–1.00). However, there was a reduction in preterm birth at less than 37 weeks (OR 0.89, 95% CI 0.81–0.98) and 34 weeks (OR 0.56, 95% CI 0.37–0.83) for iatrogenic births and in singleton pregnancies. There was also a significant reduction in preterm births at less than 34 weeks in studies with above median stringency index scores (OR 0.71, 95% CI 0.58–0.88). There was no effect on risk of stillbirth (OR 1.04, 95% CI 0.90–1.19) or birth weight. NICU admission rates were significantly reduced in studies with above median stringency index scores (OR 0.87, 95% CI 0.78–0.97). The reduction in preterm births in regions with high mitigation measures against SARS-CoV-2 infection is likely driven by a reduction in iatrogenic births. Variability in study design and cohort characteristics need to be considered for future studies to allow further investigation of population level health measures of perinatal outcomes.
Keywords: COVID-19, SARS-CoV-2, Mitigation measures, Perinatal outcomes, Preterm birth, Stillbirth
Introduction
The COVID-19 pandemic has led to myriad changes in how pregnant women live their lives, while also affecting logistics of worldwide maternity services. The impact of these changes on pregnancy outcomes, including preterm birth, remains unclear.
Early in the pandemic, a substantial reduction in very low birthweight neonates and a rise in stillbirth rates were reported from Ireland [1] and London [2] respectively. Subsequently, studies from USA [3] and Botswana [4] reported a substantial reduction in preterm birth rates below 28 and 32 weeks gestation, while others, from China [5], [6] and London [2], reported no difference in overall preterm birth rates before 37 completed weeks gestation.
Approaches to comparing obstetric outcomes between the pandemic and pre-pandemic months have led to diverse analyses, which are challenging to compare due to the different variables addressed and the extent of analyses. Establishment of the COVID-19 pandemic cohorts almost exclusively coincides with the implementation of SARS-CoV-2 mitigation measures, such as hand hygiene, face masks and community lockdown with social and travel restrictions. However, the stringency of these measures differed significantly between countries and studies, so the relationship with perinatal outcomes has not been addressed.
The initial systematic review of maternal and fetal outcomes by Chmielewska et al. [7] found that during the first year of the pandemic there was an increase in stillbirths, with high income countries also reporting a reduction in preterm birth before 37 weeks, particularly spontaneous births. The publication of additional studies potentially modified the results of the subsequent meta-analysis by Yang et al. [8] who identified a reduction in preterm births before 37 weeks, with a decrease in spontaneous and iatrogenic births, with no effect on the stillbirth rate.
This current study is a systematic review and meta-analysis of published evidence on the relationship between SARS-CoV-2 mitigation measures and perinatal outcomes, with extensive subgroup analysis. Subgroup analysis included assessment of risk of preterm birth, stillbirth, low birth weight and neonatal intensive care (NICU) admission by stringency of lockdown, by singleton and multiple order births, single centre studies, multicentre studies, and countries’ economic income. Focusing on the first nine months of the pandemic allowed assessment of fewer strains of SARS-CoV-2 without potential confounding effects from vaccination. These results are crucial to understanding whether any true changes in population obstetric outcomes have occurred, and if so, how they inform future maternity care and public health measures.
Methods
This meta-analysis was registered with PROSPERO (CRD42021254880), and PRISMA and MOOSE guidelines were followed. We aimed to address how neonatal outcomes changed during the early Covid-19 pandemic compared to pre-pandemic, stratified by level of mitigation measures.
Search strategy
An electronic search was performed using MEDLINE, Embase and Scopus databases for manuscripts published until 24th May 2021. Reference lists were searched for additional studies. Abstracts and full-text articles were screened by two independent reviewers (SH (MEDLINE, Embase and Scopus), VW (MEDLINE and Embase) and NC (Scopus)) with studies excluded as shown in the PRISMA flowchart (Fig. 1 ).
Fig. 1.
PRISMA flowchart [13].
We searched for studies that reported on the impact of mitigation measures during the COVID-19 pandemic on perinatal outcomes, including preterm births, stillbirths, low birthweight infants or neonatal intensive care unit (NICU) admission. Keywords (‘COVID-19′ OR ‘coronavirus’ OR ‘SARS-CoV-2′) AND (‘preterm birth’ OR ‘preterm delivery’ OR ‘stillbirth’ OR ‘intrauterine death’ OR ‘birthweight’ OR ‘neonatal intensive care admission’) were used. Studies were included that compared birth outcomes during a pre-pandemic period with those during the COVID-19 pandemic where mitigation measures against SARS-CoV-2 were implemented.
The following exclusion criteria were applied: case reports, systematic reviews, studies without control cohorts pre-mitigation measures, non-English language and studies restricted only to women positive for SARS-CoV-2 infection. The full results of the Scopus database search are in Supplementary Table S.4.
Data extraction and outcome measures
The following data were extracted by two independent reviewers (SH and AW): country of study, regional or national cohort, mitigation measures implemented, primary outcomes, definition of preterm birth, inclusion criteria, exclusion criteria, methods used for analysis, data source, cohort size, number of cases of SARS-Cov-2 infection in the cohort. Study methodology, cohort characteristics and the timing of the cohort to the degree of mitigation measures were assessed.
The extent of mitigation measures against the transmission of SARS-CoV-2, including hand hygiene advice, physical distancing, closure of public services, travel restrictions, were assessed using the Oxford COVID-19 Government Response Tracker [9]. The published daily stringency index score (out of 100) for the duration of the exposure cohort was used to calculate a mean stringency index score. Regional or state score was used in preference to national scores, where appropriate. Countries were characterised as low, lower-middle, upper-middle or high income using the World Bank income classifier [10].
The primary outcome assessed the effect of the pandemic on preterm birth rates. Quantitative synthesis was performed for preterm birth at less than 37 weeks, less than 34 weeks, less than 32 weeks and less than 28 weeks gestation, Table 2 (Forest plots shown in Fig. 2, Fig. 3 and supplementary data). Where available, spontaneous and iatrogenic preterm birth rates were reviewed. Secondary outcomes included stillbirth rates, very low birth weight (less than 1500 g), extremely low birth weight (less than 1000 g) and NICU admission.
Table 2.
Pooled analysis for odds of preterm birth during COVID-19 pandemic versus pre-pandemic period.
| Subgroup analysis | Less than 37 weeks |
Less than 34 weeks |
Less than 32 weeks |
Less than 28 weeks |
Stillbirth |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Total sample size | Odds ratio (95% Confidence interval) | Number of studies | Total sample size | Odds ratio (95% Confidence interval) | Number of studies | Total sample size | Odds ratio (95% Confidence interval) | Number of studies | Total sample size | Odds ratio (95% Confidence interval) | Number of studies | Total sample size | Odds ratio (95% Confidence interval) | |
| All (unadjusted) | 31 | 2696892 | 0.96 (95% CI 0.92 to 1.00) | 10 | 195143 | 0.85 (95% CI 0.72 to 1.01) | 14 | 2563242 | 0.96 (95% CI 0.80 to 1.15) | 11 | 2390547 | 0.94 (95% CI 0.83 to 1.06) | 16 | 1013235 | 1.02 (95% CI 0.89–1.17) |
| High stringency index score (>median) | 14 | 275116 | 0.95 (95% CI 0.88 to 1.04) | 4 | 138522 | 0.71 (95% CI 0.58 to 0.88) | 8 | 218355 | 0.88 (95% CI 0.73 to 1.06) | 2 | 14526 | 0.85 (95% CI 0.25 to 2.92) | 11 | 459871 | 1.07 (95% CI 0.88 to 1.29) |
| Low stringency index score (<median) | 16 | 1708209 | 0.95 (95% CI 0.89 to 1.01) | 6 | 56621 | 0.98 (95% CI 0.79 to 1.22) | 5 | 1631320 | 0.94 (95% CI 0.88 to 1.00) | 8 | 1662454 | 0.92 (95% CI 0.78 to 1.08) | 5 | 553364 | 0.97 (95% CI 0.75 to 1.17) |
| Singletons only | 14 | 1435386 | 0.91 (95% CI 0.87 to 0.95) | 6 | 172173 | 0.83 (95% CI 0.69 to 0.99) | 8 | 1364467 | 0.92 (95% CI 0.79 to 1.08) | 6 | 1243966 | 0.95 (95% CI 0.75 to 1.20) | 7 | 275217 | 1.04 (95% CI 0.77–1.42) |
| Singletons and multiples | 17 | 1261506 | 1.00 (95% CI 0.94 to 1.06) | 4 | 22970 | 0.89 (95% CI 0.61 to 1.30) | 6 | 1198775 | 1.04 (95% CI 0.76 to 1.42) | 5 | 1146581 | 0.93 (95% CI 0.78 to 1.10) | 9 | 738018 | 1.05 (95% CI 0.91–1.22) |
| Single centre studies | 17 | 228494 | 0.96 (95% CI 0.86 to 1.07) | 7 | 169356 | 0.83 (95% CI 0.65 to 1.06) | 4 | 139356 | 0.75 (95% CI 0.41 to 1.38) | 3 | 31311 | 0.76 (95% CI 0.43 to 1.32) | 8 | 171014 | 1.21 (95% CI 0.84–1.74) |
| Multicentre studies | 13 | 2460372 | 0.95 (95% CI 0.91 to 1.00) | 3 | 25787 | 0.89 (95% CI 0.70 to 1.13) | 10 | 2423886 | 1.01 (95% CI 0.83 to 1.23) | 8 | 2359236 | 0.96 (95% CI 0.85 to 1.09) | 8 | 842221 | 0.99 (95% CI 0.88–1.12) |
| High income settings | 25 | 2645593 | 0.96 (95% CI 0.92 to 1.00) | 9 | 183620 | 0.85 (95% CI 0.69 to 1.04) | 13 | 2551719 | 0.97 (95% CI 0.80 to 1.18) | 11 | 2390547 | 0.94 (95% CI 0.83–1.06) | 13 | 982003 | 0.95 (95% CI 0.81–1.12) |
| Middle income settings | 6 | 51599 | 1.00 (95% CI 0.81 to 1.23) | 1 | – | – | 1 | – | – | 0 | – | – | 3 | 31232 | 1.19 (95% CI 0.98–1.45) |
| Spontaneous birth | 6 | 58105 | 0.95 (95% CI 0.80 to 1.14) | 2 | 7016 | 0.75 (95% CI 0.51 to 1.10) | 0 | – | – | 0 | – | – | 0 | – | – |
| Iatrogenic birth | 5 | 48749 | 0.89 (95% CI 0.81 to 0.98) | 2 | 7016 | 0.56 (95% CI 0.37 to 0.83) | 0 | – | – | 0 | – | – | 0 | – | – |
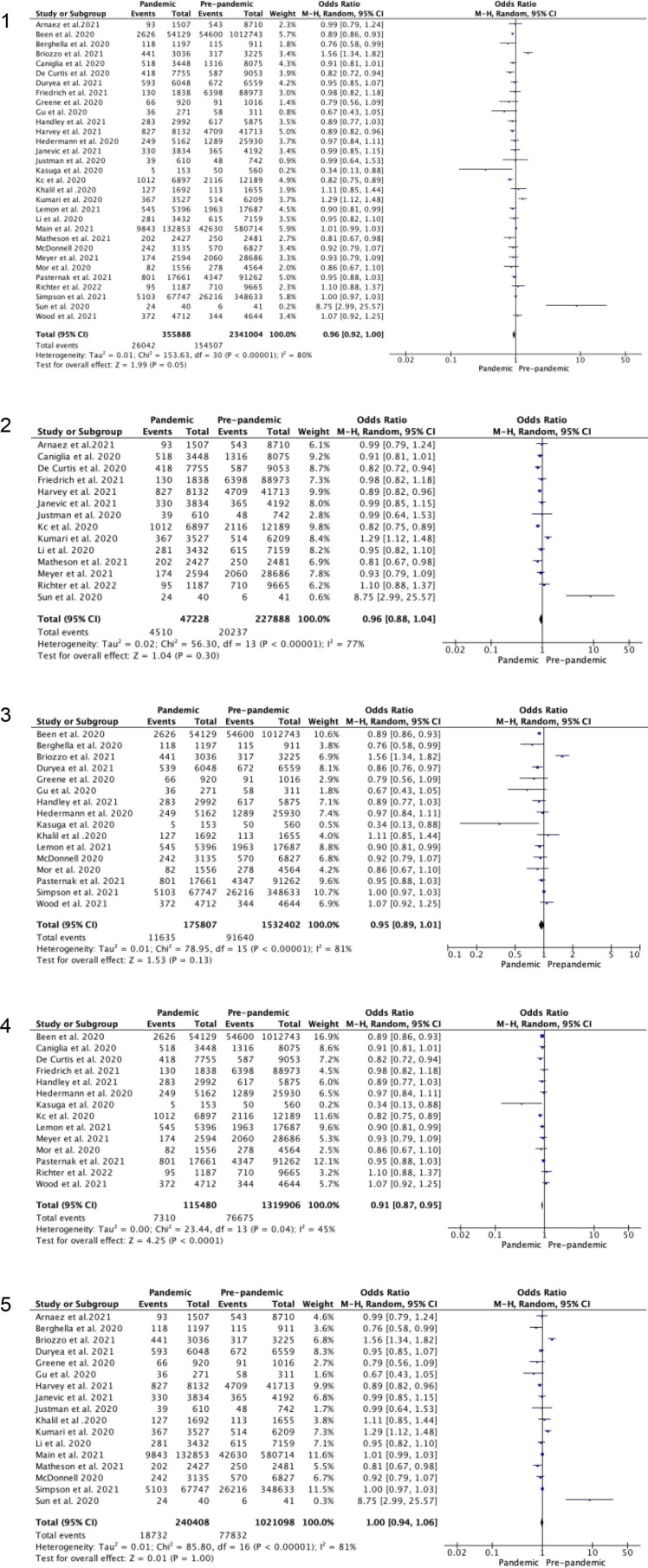
Fig. 2.
Random-effects meta-analysis for odds of preterm birth at less than 37 weeks gestation during the COVID-19 pandemic versus pre-pandemic period. 2.1 Unadjusted odds ratio. 2.2 Studies in high (>median) stringency index regions. 2.3 Studies in low (<median) stringency index. 2.4 Studies with only singleton births. 2.5 Studies including singleton and multiple order births.
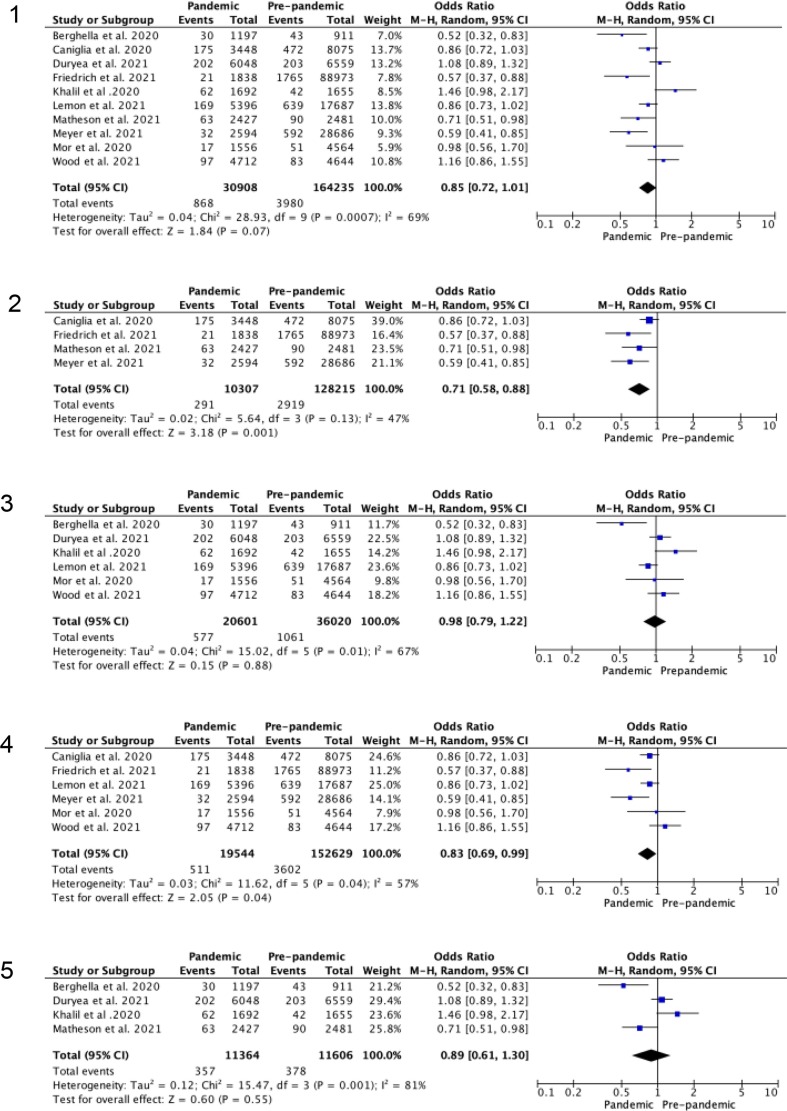
Fig. 3.
Random-effects meta-analysis for odds of preterm birth at less than 34 weeks gestation during the COVID-19 pandemic versus pre-pandemic period. 3.1 Unadjusted odds ratio. 3.2 Studies in high (>median) stringency index regions. 3.3 Studies in low (<median) stringency index. 3.4 Studies with only singleton births. 3.5 Studies including singleton and multiple order births.
Pre-planned subgroup analysis was performed for studies with high (greater than median) and low (less than median) stringency index scores, studies including only singleton pregnancies, studies with singletons and multiple order births, studies from single centre units, studies from multicentre units (including national studies), studies from high income settings, and those from middle-income settings.
Studies were assessed for risk of bias using the Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool [11] by two independent authors (SH and NC). Risk of bias gradings were assigned overall following each of the seven individual domains; bias due to confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, outcome measurement, section of the reported result.
Results were pooled using Review Manager 5.4.1 (RevMan5). Between-study heterogeneity was assessed using the I2 statistic. Meta-analysis was performed using the Mantel-Haenszel method and random-effects models, with estimation of the between-study variance calculated with the DerSimonian and Laird method. There was no public involvement in the study and no funding was sought.
Results
General characteristics
As shown in the PRISMA flowchart (Fig. 1), 566 papers were identified, with duplicates removed (n = 42). Titles were screened for eligibility, with 6 records published before 2020 removed. Abstracts were assessed for 518 studies with 480 excluded. Full papers were assessed for 48 studies of which 38 were included in the systematic review (Table 1 ).
Table 1.
Study characteristics.
| Author | Region, Country | Area covered by study | World Bank classification of national income* | Control cohort timeframe | Exposure cohort timeframe | Mean Oxford Stringency index during exposure cohort (range) | Were mitigation measures described |
|---|---|---|---|---|---|---|---|
| Arnaez et al.[37] | Castilla-y-León, Spain | Multicentre | High | 15th March to 3rd May 2015 to 2019 | 15th March to 21st June 2020 | 75.61 (41.20 to 85.19) | Yes |
| Been et al.[41] | Netherlands | National | High | 1st Oct 2010 to 31st March 2020 | 9th March to 16th July 2020 | 65.44 (11.11 to 78.7) | Yes |
| Berghella et al.[3] | Philadelphia, USA | Single centre | High | 1st March to 31st July 2019 | 1st March to 31st July 2020 | 65.03** (16.67 to 95.48) | No |
| Briozzo et al.[38] | Montevideo, Uruguay | Single centre | High | 15th March to 30th September 2019 | 15th March to 30th September 2020 | 50.99 (32.41 to 72.22) | No |
| Caniglia et al.[4] | Botswana | Multicentre | Upper-middle | 1st January to 31st July 2017 to 2019 | 1st January to 31st July 2020 | 86.11 (61.11 to 86.11) | No |
| De Curtis et al.[39] | Lazio, Italy | Single centre | High | 1st March to 31st May 2019 | 1st March to 31st May 2020 | 81.80 (63.89 to 93.52) | No |
| Dell’Utri et al.[40] | Mia, Italy | Single centre | High | 23rd February to 24th June 2019 | 23rd February to 23rd June 2020 | 76.24 (52.78 to 93.52) | Yes |
| Duryea et al.[36] | Texas, USA | Single centre | High | 1st May to 31st October 2019 | 1st May to 31st October 2020 | 57.39 (51.39 to 72.69) | No |
| Friedrich et al.[14] | Israel | Single centre | High | 19th April to 27th June 2019, and 21st March 2011 to 18th April 2020 | 19th March to 27th June 2020 | 78.06 (69.44 to 92.59) | No |
| Greene et al.[15] | California, USA | Single centre | High | 1st January to 29th February 2020 | 1st March to 30th April 2020 | 67.76** (16.67 to 90.91) | Yes |
| Gu et al.[5] | Jiangsu Province, China | Single centre | Upper-middle | 1st January to 28th February 2019 | 1st January to 29th February 2020 | 53.87** (0 to 81.02) | No |
| Handley et al.[16] | Philadelphia, USA | Multicentre | High | 1st March to 30th June 2018 & 2019 | 1st March to 30th June 2020 | 65.64** (16.67 to 85.19) | No |
| Harvey et al.[17] | Tennessee, USA | Multicentre | High | 22nd March to 30th April 2015 to 2019 | 22nd March to 30th April 2020 | 73.94** (66.67 to 75.93) | No |
| Hedermann et al.[18] | Denmark | National | High | 12th March to 14th April 2015 to 2019 | 12th March to 14th April 2020 | 70.18 (37.96 to 72.22) | Yes |
| Janevic et al.[19] | New York City, USA | Multicentre | High | 28th March to 31st July 2019 | 28th March to 31st July 2020 | 79.65** (77.22 to 82.41) | No |
| Justman et al.[20] | Israel | Single centre | High | 1st March to 30th April 2019 | 1st March to 30th April 2020 | 73.69 (37.96 to 72.22) | No |
| Kasuga et al.[21] | Japan | Single centre | High | 1st April to 30th June 2017 to 2019 | 1st April to 30th June 2020 | 38.51 (25.93 to 45.37) | No |
| Kc et al.[12] | Nepal | Multicentre | Lower-middle | 1st January to 20th March 2020 | 21st March to 30th May 2020 | 94.10 (58.33 to 96.30) | Yes |
| Khalil et al.[2] | England, UK | Single centre | High | 1st October 2019 to 31st January 2020 | 1st February to 14th June 2020 | 52.82 (8.33 to 79.63) | No |
| Kirchengast et al.[22] | Venna, Austria | Single centre | High | 1st January to 29th February 2020 and 1st January 2005 to 31st December 2019 | 1st March to 31st July 2020 | 56.18 (11.11 to 81.48) | Yes |
| Kugelman et al.[23] | Haifa, Israel | Single centre | High | 15th March to 12th April 2019 | 15th March to 12th April 2020 | 83.52 (62.96 to 94.44) | Yes |
| Kumar et al.[45] | New Delhi, India | Single centre | Lower-middle | 1st March to 30th September 2020 | 1st March to 30th September 2020 | 72.67 (10.19 to 100) | No |
| Kumari et al.[25] | Jodhpur, India | Multicentre | Lower-middle | 15th January to 24th March 2020 | 25th March to 2nd June 2020 | 90.78 (75.46 to 100) | No |
| Lemon et al.[35] | Pittsburgh, USA | Single centre | High | 1st January 2018 to 31st January 2020 | 1st April 2020 to 27th October 2020 | 65.73 (53.24 to 85.19) | No |
| Li et al.[6] | Wuhan, China | Single centre | Upper-middle | 1st January 2019 to 22nd January 2020 | 23rd January to 24th March 2020 | 83.75** (62.04 to 86.11) | Yes |
| Maeda et al.[42] | Japan | National | High | 8th January to 29th April 2019 | 8th January to 28th April 2020 | 28.97 (2.78 to 47.22) | Yes |
| Main et al.[26] | California, USA | Multicentre | High | 1st April to 31st July 2016 to 2019 | 1st April to 31st July 2020 | 72.08** (67.59 to 82.41) | No |
| Matheson et al.[43] | Melbourne, Australia | Multicentre | High | 1st July to 30th September 2019 | 1st July to 30th September 2020 | 73.20 (68.06 to 75.46) | Yes |
| McDonnell et al.[27] | Dublin, Ireland | Single centre | High | 1st January to 31st July 2018 and 2019 | 1st January to 31st July 2020 | 47.17 (0 to 90.74) | Yes |
| Meyer et al.[28] | Tel Aviv, Israel | Single centre | High | 20th March to 27th June 2019 and 20th March to 27th June 2011 to 2019 | 20th March to 27th June 2020 | 80.80 (75.00 to 94.44) | No |
| Mor et al.[29] | Israel | Single centre | High | 21st February to 30th April 2017 to 2019 | 21st February to 30th April 2020 | 66.72 (19.44 to 94.44) | No |
| Pasternak et al.[30] | Sweden | National | High | 1st April to 31st May 2015 to 2019 | 1st April to 31st May 2020 | 64.54 (59.26 to 64.81) | Yes |
| Philip et al.[1] | Ireland | Single centre | High | 1st January to 30th April 2001 to 2019 | 1st January to 30th April 2020 | 33.78 (0 to 90.74) | Yes |
| Richter et al.[44] | New York City, USA | Single centre | High | 16th March to 15th May 2019 | 16th March to 15th May 2020 | 78.88 (63.89 to 82.41) | Yes |
| Simpson et al.[31] | Ontario, Canada | Multicentre | High | 15th March to 30th September 2015 to 2019 | 15th March to 30th September 2020 | 63.87** (24.07 to 70.83) | No |
| Stowe et al.[32] | England, UK | National | High | 1st April to 30th June 2019 | 1st April to 30th June 2020 | 74.90 (67.59 to 79.63) | No |
| Sun et al.[33] | Sao Paulo, Brazil | Single centre | Upper-middle | 11th March to 11th June 2019 | 11th March to 11th June 2020 | 73.72 (11.11 to 81.02) | No |
| Wood et al.[34] | Boston, USA | Multicentre | High | 1st April to 31st July 2019 | 1st April to 31st July 2020 | 70.36** (62.04 to 75.93) | No |
* Based on GNI per capita 2021.
** Regional/state stringency index score.
All studies were assessed as moderate risk of bias, with the exception of Kc et al. [12] which was assessed as serious risk of bias, Supplementary Table S.1.
Study characteristics and methodology are shown in Table 1 and Supplementary Table S.2 respectively. All studies adopted a retrospective approach to data collection, conducting a comparison of obstetric outcomes during the pandemic with the months immediately preceding lockdown, corresponding months in 2019 and longer cohorts over the preceding two to nine years. Thirty-three studies [1], [2], [3], [4], [5], [6], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40] used comparative cohorts for analysis, with five studies [12], [41], [42], [43], [44] employing interrupted time series analysis. Sixteen studies [3], [4], [12], [19], [23], [26], [28], [30], [31], [36], [37], [41], [43], [15], [16], [17] provided adjusted and unadjusted results. Publication bias was assessed for outcomes with 10 or more studies and the funnel plots are shown in Supplementary Figure S.9.
Seven studies were performed in middle income countries (three lower-middle [12], [25], [45], four upper-middle [33], [4], [5], [6]), with the remaining 31 studies occurring in high income countries. Eleven studies were conducted in Europe [1], [2], [18], [22], [27], [30], [32], [37], [39], [40], [41], 12 in Asia [5], [6], [12], [14], [20], [21], [23], [25], [28], [29], [42], [45], 11 in North America [3], [19], [26], [31], [44], [15], [16], [17], [34], [35], [36], two in South America [33], [38], one in Africa [4], and one in Australasia [43], A description of the COVID-19 mitigation measures in place was provided in 15 studies [1], [6], [12], [15], [18], [22], [23], [27], [30], [37], [40], [41], [42], [43], [44]. There was considerable variability in the mean stringency index score between exposed cohorts, from 28.97 [42] to 94.08 [12].
Sixteen studies [4], [12], [25], [26], [34], [37], [16], [17], [18], [19], [30], [31], [32], [41], [42], [43] reported multicentre data and 22 studies [5], [6], [14], [33], [35], [36], [38], [39], [44], [45], [1], [2], [3], [20], [21], [22], [23], [27], [28], [29] reported data from single centres. Mothers testing positive for SARS-CoV-2 were noted in 13 [2], [3], [6], [19], [28], [30], [31], [34], [35], [45], [14], [15], [16] out of the 38 studies, with five studies [4], [5], [20], [22], [38] having no identified infections, three studies [21], [22], [44] excluding patients who tested positive and a further 16 studies [1], [12], [17], [18], [23], [29], [32], [33], [36], [37], [25], [26], [27], [39], [40], [41], [42], [43] not reporting rates of SARS-CoV-2 infection. Women with multiple pregnancies were excluded in 16 studies [1], [4], [12], [14], [16], [18], [21], [22], [34], [35], [39], [41], [44], [28], [29], [30].
Primary outcomes studied included preterm birth (n = 24) [12], [21], [22], [30], [31], [34], [35], [2], [3], [4], [16], [17], [18], [19], [26], [27], [28], [37], [38], [39], [41], [42], [43], [44], stillbirth (n = 12) [2], [4], [12], [16], [20], [27], [37], [39], [45], [30], [31], [32], hospitalisation rates (n = 2), ‘pregnancy complications’ or ‘adverse outcomes’ (n = 4) [4], [21], [27], [38], ‘perinatal results’ (n = 2) [21], [38], effect of service change (n = 4) [15], [20], [36], [40], admission to hospital (n = 4) [23], [25], [33], [40], ‘haematological impact’ (n = 1) [14], NICU admission rates (n = 2) [42], [44], placental abruption, stillbirth, term NICU admission and low umbilical cord pH (n = 1) [36] and the prevention of COVID-19 spread in hospital (n = 1) [5].
Preterm birth rates
Preterm birth rates were reported in 34 out of 38 (89.5%) studies [12], [22], [2], [3], [4], [5], [6], [14], [15], [16], [18], [19], [20], [25], [26], [27], [28], [29], [30], [31], [33], [34], [35], [36], [37], [38], [39], [41], [42], [43], [44], [45], [46]. Three studies were excluded from the meta-analysis at less than 37 weeks; Kumar et al. [45] as their data was for stillbirths exclusively, Kirchengast et al. [22] as they recognised preterm as less than 36 weeks, and Maeda et al. [42] as they were unable to provide data on total number of births. There was a wide range of gestational breakdown of preterm birth rates. Birth at various gestational ages up to 37 weeks was reported by 20 studies [14], [26], [39], [41], [43], [2], [3], [4], [17], [18], [19], [20], [28], [29], [30], [31], [34], [35], [36], [37], with 11 studies [5], [6], [12], [15], [16], [21], [25], [27], [33], [38], [44] reporting only gestational age of less than 37 weeks as a group.
For the 31 studies included in the random-effects meta-analysis, the unadjusted odds of preterm birth at less than 37 weeks gestation during the pandemic period compared (355888 births) to pre-pandemic period (2341004 births) was 0.96 (95% CI 0.92–1.00), Figure 2.1. The corresponding funnel plot, Figure S9.1, suggests a low risk of publication bias with clustering around the average. The lower portion of the funnel plot is empty suggesting there may be some small-scale studies not showing an association that are missing.
When only singleton pregnancies (14 studies) [4], [12], [14], [18], [21], [34], [35], [39], [41], [44], [28], [29], [30] are considered, the reduction in odds of preterm birth before 37 weeks becomes significant OR 0.91 (95% CI 0.87–0.95), Figure 2.4, but this is not true when multiple pregnancies are added. Six studies [3], [16], [25], [34], [35], [43] reported spontaneous preterm birth and five studies [3], [16], [25], [35], [43] iatrogenic preterm birth at less than 37 weeks gestation, with a significant reduction in iatrogenic birth (OR 0.89, 95% CI 0.81–0.98) but no effect seen in spontaneous preterm birth (OR 0.95, 95% CI 0.80–1.14). Subgroup analysis for single centre and multicentre studies, and high and middle income countries found no difference in odds of preterm birth at less than 37 weeks, although there was an overall trend to a reduction (Table 2).
Preterm birth at less than 34 weeks was reported by 10 studies [14], [28], [29], [43], [2], [3], [4], [34], [35], [36], with no overall reduction on meta-analysis, OR 0.85 (0.72–1.01), Figure 3.1. This reduction became significant when only singleton deliveries were studied, OR 0.83 (95% CI 0.69–0.99). When studies were divided by the stringency of COVID-19 mitigation measures, there was a significant reduction in preterm birth before 34 weeks when the stringency index for the study period was greater than the median, OR 0.71 (95% CI 0.58–0.88), Figure 3.2. This was not seen for studies with stringency index scores less than the median, OR 0.98 (95% CI 0.79–1.22), Figure 3.3. When iatrogenic preterm birth at less than 34 weeks was isolated, this also showed a significant reduction, OR 0.56 (95% CI 0.37–0.83), with no effect seen on spontaneous births, OR 0.75 (95% CI 0.51 to 1.10). The odds of birth at less than 32 weeks and less than 28 weeks did not show a reduction on overall meta-analysis or subgroup analysis, although a non-significant trend to reduced odds of preterm birth was apparent.
Stillbirth rate
Nineteen studies [2], [4], [12], [16], [20], [25], [31], [32], [36], [37], [39], [40], [43], [45], [27], [28], [29] reported stillbirth rates during the pandemic period with three studies excluded from the meta-analysis: McDonnall et al. [27] due to a lack of control cohort data, Khalil et al. [2] as there was cohort cross-over with Stowe et al., [32] and Kc et al. [12] as it reported in-hospital stillbirths only. Pooled analysis of 255,968 births during the pandemic and 757,267 during the control cohorts, identified no significant effect on the rates of stillbirth (OR 1.02, 95% CI 0.89–1.17). Subgroup analysis for stringency index score, economic setting, single or multicentre studies and the inclusion of multiple pregnancies did not show statistically significant effects on stillbirth rates during the pandemic period, although there was a non-significant trend towards increased rates, Table 2.
Low birth weight
The incidence of low birth weight was described in seven studies, but definition varied: neonates weighing less than the 10th and 3rd centile [43], less than 2500 g [12], [27], [33], [38], less than 1500 g [1], [17], [22], [37] and less than 1000 g [1], [22], [37]. Pooled analysis of the four studies reporting very low birth weight (less than 1500 g) and three reporting extremely low birth weight (less than 1000 g) showed no effect comparing rates during the pandemic with pre-pandemic when considering the full data or subgroup analysis, Table S.3.
Neonatal intensive care admission
Admissions to neonatal intensive care (NICU) were reported by 12 studies [2], [5], [14], [15], [20], [23], [28], [29], [31], [36], [43], [44], with one study [36] excluded from the meta-analysis which only reported full-term admissions to NICU. There was a non-significant change in admission during the pandemic period, OR 0.96 (95% CI 0.86–1.08). However, subgroup analysis showed a significant reduction in studies with above median stringency index scores OR 0.87 (95% CI 0.78–0.97), Table S.3.
Discussion
Main findings
This systematic review identified a reduction in preterm birth rates at less than 34 weeks gestation in countries with above median COVID-19 mitigation measures, measured by the Oxford COVID-19 Government Response Tracker [9]. This may be driven by a reduction in iatrogenic births.
Consideration of stillbirth rates in combination with preterm birth rates is vital; the consequence of delayed diagnosis of pregnancy complications, for example fetal growth restriction, with expedited preterm delivery, may increase stillbirth rates. However, this was not demonstrated, with stillbirth rates varying widely. Stillbirth rates appeared unchanged in the larger studies, and were not significantly different in the meta-analysis, supporting the impression that the smaller studies’ results were prone to error linked to publication bias. However, as the study populations were broad and inclusive, a potential change in risk for women with pre-existing risk factors for stillbirth cannot be excluded.
Studies reporting on low and very low birth weight reported contradictory findings, but they were small and potentially underpowered.
A significant reduction in risk of NICU admission during the pandemic period was observed in studies conducted in areas with above median stringency index scores. This may be linked to the reduction in iatrogenic preterm births.
The results presented here are not consistent with previous meta-analyses [7], [8]. The increase in stillbirth rates reported by Chmielewska et al. [7] was not replicated, probably due to the publication and inclusion of additional studies in our review. The effect of economic setting on preterm birth rates could not be validated in this meta-analysis. The recent review by Yang et al. [8] found a reduction in preterm birth at less than 37 weeks in single centre studies, but this was not replicated. This may be due to the inclusion of different data presented within single studies, e.g. for different control cohorts or different durations of follow-up in the pandemic group [14], [37], [41].
Strengths and limitations
A strength of this review is the large number of studies included. Where necessary, authors were contacted to clarify original data [41], [42], [44]. Extensive pre-planned subgroup analysis was performed to explore differences in reported outcomes, and uniquely on severity of mitigation measures. Inclusion of an unselected population, low and high-risk pregnancies, allowed the assessment of effects of population-level changes on perinatal outcomes.
The time frame of this review was limited to the first 9 months of the pandemic, when fewer variants of SARS-CoV-2 were present, there was no vaccination programme and more defined mitigation measures were in place.
Potential limitations include the retrospective nature of the included studies, the lack of inclusion of grey literature, and single centre studies potentially missing changes from attendance at different hospitals with movement restrictions. The definition of perinatal outcomes varied, limiting the inclusion of some studies in the meta-analysis.
Interpretation of Findings
The mixed methodology of cohort and interrupted time series analysis made direct comparison of the studies more challenging. The concurrent reporting of preterm birth and stillbirth rates was mixed; 15 studies [2], [4], [12], [14], [16], [20], [25], [36], [37], [39], [43], [28], [29], [30], [31] reporting both and 18 studies [3], [5], [6], [15], [21], [22], [26], [27], [33], [34], [38], [41], [42], [44], [17], [18], [19] reporting preterm birth rates without stillbirth rates. Stillbirths were excluded in five studies [6], [22], [35], [41], [43]. This lack of combined preterm birth and stillbirth data makes interpreting the linked effects of the outcomes more difficult. The 11 studies [5], [6], [12], [15], [16], [21], [25], [27], [33], [42], [44] that reported only preterm births at less than 37 weeks may have missed any effect at earlier gestations. Adjusted risk analysis was not always provided, and the variables accounted for were wide ranging and inconsistent. The inclusion, or exclusion, of women positive for SARS-CoV-2 infection may alter the rates of preterm birth, particularly iatrogenic, but inclusion of these women was poorly reported. Sixteen of the 38 studies failed to identify positive cases in their cohort and only one study [16] considered this independently, finding no significant effect on spontaneous or iatrogenic preterm birth rates.
The mitigation measures varied between and within countries and were only described in half of the studies. With widespread national and local lockdowns, risk of spontaneous preterm birth may have reduced with improved hygiene and reduced exposure to other pathogens [47], [48]. However, meta-analysis demonstrated no effect on spontaneous preterm births. Social circumstances may also have influenced psychological and physical stress for pregnant women and changes in provision of maternity services, as well as women’s attitudes towards attending maternity services, in turn affecting care journeys and outcomes [49]. All these factors are more likely to impact on a subgroup of women already at risk of adverse perinatal outcomes, e.g. multiple pregnancies. As most women have no adverse perinatal outcomes, it is possible that an effect would be lost in a sample which included women who were never at increased risk in the first place. Analysis of these high risk groups was not possible in this review but may reveal effects not seen at the population level, as found in a new study from Melbourne, Australia [50].
There is significant variation in study design and analysis, and the ideal methodology has yet to be realised. Early studies suggested significant changes in preterm birth and stillbirth rates but the larger studies that followed did not demonstrate this. These allowed for a greater degree of selection bias and background trends and showed no significant differences in these outcomes. This review suggests that the varying cohort timings in relation to when the COVID-19 mitigation measures were deployed, may have affected the perinatal outcomes. Most studies specified their ‘exposure’ cohort for the duration of the mitigation measures. However, this may not capture the effect of mitigation measures on perinatal outcomes, particularly for those women who delivered within the first few weeks and had spent very little time pregnant with restrictions in place. For those women, any effect on perinatal outcomes may well be the result of altered maternity care provision and access. Three studies [1], [4], [27] included January 2020 (pre-pandemic in most countries) in the exposed cohort, potentially diluting any effect of mitigation measures. Studies which followed up the cohort for greater duration, or provided multiple time-periods for control and exposed cohorts showed that the risk of adverse perinatal outcomes varied with the different time points [1], [4], [14], [18], [22], [27], [28], [37], [41].
As the COVID-19 pandemic continues, further analysis is needed on the effect of mitigation measures on perinatal outcomes, particularly for those women who were in the first and second trimesters during the strictest lockdown periods. The data to permit an analysis of high-risk subgroups is not yet available. Future studies should carefully consider the cohort design and analysis. A proposed study design to address this could prospectively follow women from conception, comparing mothers who conceived in the same period, rather than a cohort based on delivery date.
Conclusions
Pooled analysis of population data during the COVID-19 pandemic revealed a significant reduction in preterm births at less than 34 weeks gestation in areas with high SARS-CoV-2 mitigation measures, such as community lockdown. There was also a reduction in preterm births at less than 37 and 34 weeks in singleton pregnancies, and in iatrogenic preterm births at less than 37 and 34 weeks. No corresponding significant effects were seen on stillbirth rates.
A prospective study design could permit the assessment of exposure to mitigation measures during conception, first and second trimesters and allow in depth analysis of the effect of these measures on perinatal outcomes.
Contribution to authorship
SH, BM and MB designed the study. SH, NC and VW (all investigators) conducted the literature review and SH and AW performed the data extraction and analysis. SH and NC performed the risk of bias assessment. SH and MB wrote the first draft of the manuscript. SH, DR, BM and MB contributed to data interpretation and commented on all versions of the manuscript. All authors approved this version of the manuscript prior to submission.
Details of ethics approval
No approval was required.
Funding
No funding was received.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thank you to the authors who provided additional primary data for use in this meta-analysis.
Biographies

Dr Sarah Hawco, MBChB, PhD. Specialty Registrar in Obstetrics and Gynaecology in North of Scotland Deanery, UK. MBChB at the University of Aberdeen and Doctor of Philosophy (PhD) at the University of Dundee in Cancer Biology. Interest in women’s health research, in particular gynaecological oncology and research methodology.

Dr Daniel L. Rolnik FRANZCOG, MSc, MD, PhD, Diploma in Fetal Medicine (Fetal Medicine Foundation, UK). Specialty training in Obstetrics and Gynaecology, Master of Sciences (MSc) by the University of São Paulo, Brazil. Subspecialist in Fetal Medicine by the Fetal Medicine Foundation / King’s College Hospital, London, UK. Doctor of Philosophy (PhD), Manchester Metropolitan University, Manchester, UK. Master of Public Health (MPH) candidate at Harvard University, USA. Consultant in Obstetrics and Gynaecology, Maternal-Fetal Medicine at Monash Medical Centre and a Senior Lecturer at Monash University, Melbourne, Australia.

Dr Andrea Woolner, PhD, MBChB, BSc, PgCert, MRCOG, DFSRH. Senior Clinical Lecturer in Obstetrics and Gynaecology at the Aberdeen Centre for Women's Health Research and Honorary Consultant Obstetrician NHS Grampian. Her research interests include - early pregnancy including recurrent miscarriage; preterm birth; preconception health; reproductive health; and improving pregnancy outcomes.

Dr Natalie J. Cameron, MBChB, BScMedSci(Hons). Academic Foundation Programme Trainee in North of Scotland Deanery, UK. MBChB and BScMedSci at the University of Aberdeen. Research interests include modelling outcomes in reproductive medicine and improving outcomes of preterm birth.

Dr Victoria Wyness, BSc (Hons) Medicine, MBChB, MRCOG. Specialty Registrar in Obstetrics and Gynaecology in North of Scotland Deanery. BSc (Hons) Medicine from University of St. Andrews and MBChB at the University of Aberdeen.

Professor Ben W. Mol, PhD, MD, BSc, FRANCOG. Professor of Obstetrics and Gynaecology at Monash University where he has held continuous NHMRC funding, including a prestigious investigator grant, since his arrival in 2018. He is focused on the organisation of multi-centric evaluative research in Obstetrics, Gynaecology and Fertility.

Dr Mairead Black, PhD, MBChB, MRCOG, MSc. Dr Mairead Black is a senior clinical lecturer in obstetrics at the University of Aberdeen and honorary consultant obstetrician at Aberdeen Maternity Hospital. She obtained her PhD in 2016. Her research focuses on improving maternity care experiences for women and optimising outcomes of pregnancy for women and their offspring. She uses qualitative and quantitative research methods. She has a particular interest in improving maternity care for women with multiple health conditions and those with social disadvantage.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejogrb.2022.05.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Philip R.K., Purtill H., Reidy E., Daly M., Imcha M., McGrath D., et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Heal. 2020;5(9):e003075. doi: 10.1136/bmjgh-2020-003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil A., Von Dadelszen P., Draycott T., Ugwumadu A., O’Brien P., Magee L. Change in the Incidence of Stillbirth and Preterm Delivery during the COVID-19 Pandemic. J Am Med Assoc. 2020;324(7):705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghella V., Boelig R., Roman A., Burd J., Anderson K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020;2(4):100258. doi: 10.1016/j.ajogmf.2020.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caniglia E.C., Magosi L.E., Zash R., Diseko M., Mayondi G., Mabuta J., et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol. 2021;224(6):615.e1–615.e12. doi: 10.1016/j.ajog.2020.12.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu X.X., Chen K., Yu H., Liang G.Y., Chen H., Shen Y. How to prevent in-hospital COVID-19 infection and reassure women about the safety of pregnancy: Experience from an obstetric center in China. J Int Med Res. 2020;48(7) doi: 10.1177/0300060520939337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Yin H, Jin Z, Zhang H, Leng B, Luo Y, et al. Impact of Wuhan lockdown on the indications of cesarean delivery and newborn weights during the epidemic period of COVID-19. PLoS One. 2020 Aug 1;15(8 August). [DOI] [PMC free article] [PubMed]
- 7.Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Heal. 2021;9(6):e759–72. [DOI] [PMC free article] [PubMed]
- 8.Yang J., D’Souza R., Kharrat A., Fell D.B., Snelgrove J.W., Murphy K.E., et al. COVID-19 pandemic and population-level pregnancy and neonatal outcomes: a living systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2021;100(10):1756–1770. doi: 10.1111/aogs.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale T., Angrist N., Goldszmidt R., Kira B., Petherick A., Phillips T., et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021;5(4):529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 10.Bank W. World Bank Country and Lending Groups – World Bank Data Help Desk [Internet]. The World Bank. 2020 [cited 2021 May 24]. p. 1–8. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519#High_income%0Ahttps://datahelpdesk.worldbank.org/knowledgebase/articles/906519.
- 11.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KC A, Gurung R, Kinney M V., Sunny AK, Moinuddin M, Basnet O, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Heal. 2020 Oct 1;8(10):e1273–81. [DOI] [PMC free article] [PubMed]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. Vol. 372. BMJ Publishing Group; The BMJ: 2021. (The PRISMA 2020 statement: An updated guideline for reporting systematic reviews). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich L., Levin G., Maixner N., Bart Y., Tsur A., Yinon Y., et al. Hematologic adaptation to mask-wearing among pregnant women and obstetrical outcome during the coronavirus disease 2019 pandemic. Int J Gynecol Obstet. 2021;154(2):297–303. doi: 10.1002/ijgo.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene NH, Kilpatrick SJ, Wong MS, Ozimek JA, Naqvi M. Impact of labor and delivery unit policy modifications on maternal and neonatal outcomes during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020 Nov 1;2(4):100234. [DOI] [PMC free article] [PubMed]
- 16.Handley S.C., Mullin A.M., Elovitz M.A., Gerson K.D., Montoya-Williams D., Lorch S.A., et al. Changes in Preterm Birth Phenotypes and Stillbirth at 2 Philadelphia Hospitals during the SARS-CoV-2 Pandemic, March-June 2020. J Am Med Assoc. 2021;325(1):87. doi: 10.1001/jama.2020.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey E.M., McNeer E., McDonald M.F., Shapiro-Mendoza C.K., Dupont W.D., Barfield W., et al. Association of Preterm Birth Rate with COVID-19 Statewide Stay-at-Home Orders in Tennessee. JAMA Pediatr. 2021;175(6):635. doi: 10.1001/jamapediatrics.2020.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedermann G., Hedley P.L., Bækvad-Hansen M., Hjalgrim H., Rostgaard K., Poorisrisak P., et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):93–95. doi: 10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janevic T., Glazer K.B., Vieira L., Weber E., Stone J., Stern T., et al. Racial/Ethnic Disparities in Very Preterm Birth and Preterm Birth before and during the COVID-19 Pandemic. JAMA Netw Open. 2021;4(3):e211816. doi: 10.1001/jamanetworkopen.2021.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Justman N., Shahak G., Gutzeit O., Ben Zvi D., Ginsberg Y., Solt I., et al. Lockdown with a Price: The impact of the COVID-19 Pandemic on Prenatal Care and Perinatal Outcomes in a Tertiary Care Center. Isr Med Assoc J. 2020;22(9):533–537. [PubMed] [Google Scholar]
- 21.Kasuga Y., Tanaka M., Ochiai D. Preterm delivery and hypertensive disorder of pregnancy were reduced during the COVID-19 pandemic: A single hospital-based study. J Obstet Gynaecol Res. 2020 Dec 7;46(12):2703–2704. doi: 10.1111/jog.14518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchengast S, Hartmann B. Pregnancy outcome during the first covid 19 lockdown in Vienna, Austria. Int J Environ Res Public Health. 2021;18(7). [DOI] [PMC free article] [PubMed]
- 23.Kugelman N, Lavie O, Assaf W, Cohen N, Sagi-Dain L, Bardicef M, et al. Changes in the obstetrical emergency department profile during the COVID-19 pandemic. J Matern Neonatal Med. 2020;0(0):1–7. [DOI] [PubMed]
- 24.Kumar J. Effect of COVID-19 on maternal and neonatal services. Vol. 9, The Lancet Global Health. Elsevier Ltd; 2021. p. e113. [DOI] [PMC free article] [PubMed]
- 25.Kumari V, Mehta K, Choudhary R. COVID-19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob Heal. 2020;8(9):e1116–7. [DOI] [PMC free article] [PubMed]
- 26.Main E.K., Chang S.-C., Carpenter A.M., Wise P.H., Stevenson D.K., Shaw G.M., et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224(2):239–241. doi: 10.1016/j.ajog.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonnell S., McNamee E., Lindow S.W., O’Connell M.P. The impact of the Covid-19 pandemic on maternity services: A review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020 Dec;1(255):172–176. doi: 10.1016/j.ejogrb.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer R., Bart Y., Tsur A., Yinon Y., Friedrich L., Maixner N., et al. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2021;224(2):234–237. doi: 10.1016/j.ajog.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mor M., Kugler N., Jauniaux E., Betser M., Wiener Y., Cuckle H., et al. Impact of the COVID-19 Pandemic on Excess Perinatal Mortality and Morbidity in Israel. Am J Perinatol. 2021;38(04):398–403. doi: 10.1055/s-0040-1721515. [DOI] [PubMed] [Google Scholar]
- 30.Pasternak B., Neovius M., Söderling J., Ahlberg M., Norman M., Ludvigsson J.F., et al. Preterm Birth and Stillbirth During the COVID-19 Pandemic in Sweden: A Nationwide Cohort Study. Ann Intern Med. 2021;174(6):873–875. doi: 10.7326/M20-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson AN, Snelgrove JW, Sutradhar R, Everett K, Liu N, Baxter NN. Perinatal Outcomes During the COVID-19 Pandemic in Ontario, Canada. JAMA Netw open. 2021;4(5):e2110104. [DOI] [PMC free article] [PubMed]
- 32.Stowe J., Smith H., Thurland K., Ramsay M.E., Andrews N., Ladhani S.N. Stillbirths During the COVID-19 Pandemic in England, April-June 2020. JAMA. 2021 Jan 5;325(1):86. doi: 10.1001/jama.2020.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S.Y., Guazzelli C.A.F., Morais L.R., Dittmer F.P., Augusto M.N., Soares A.C., et al. Effect of delayed obstetric labor care during the COVID-19 pandemic on perinatal outcomes. Int J Gynecol Obstet. 2020;151(2):287–289. doi: 10.1002/ijgo.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood R., Sinnott C., Goldfarb I., Clapp M., McElrath T., Little S. Preterm Birth During the Coronavirus Disease 2019 (COVID-19) Pandemic in a Large Hospital System in the United States. Obstet Gynecol. 2021 Mar;137(3):403–404. doi: 10.1097/AOG.0000000000004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemon L, Edwards RP, Simhan HN. What is driving the decreased incidence of preterm birth during the coronavirus disease 2019 pandemic? Am J Obstet Gynecol MFM. 2021;3(3):100330. [DOI] [PMC free article] [PubMed]
- 36.Duryea E.L., Adhikari E.H., Ambia A., Spong C., McIntire D., Nelson D.B. Comparison between In-Person and Audio-Only Virtual Prenatal Visits and Perinatal Outcomes. JAMA Netw Open. 2021;4(4):1–9. doi: 10.1001/jamanetworkopen.2021.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnaez J., Ochoa-Sangrador C., Caserío S., Gutiérrez E.P., Jiménez M.D.P., Castañón L., et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur J Pediatr. 2021;180(6):1997–2002. doi: 10.1007/s00431-021-03984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briozzo L., Tomasso G., Viroga S., Nozar F., Bianchi A. Impact of mitigation measures against the COVID 19 pandemic on the perinatal results of the reference maternity hospital in Uruguay. J Matern Neonatal Med. 2021 doi: 10.1080/14767058.2021.1874911. [DOI] [PubMed] [Google Scholar]
- 39.De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(4):F456. [DOI] [PMC free article] [PubMed]
- 40.Dell’Utri C., Manzoni E., Cipriani S., Spizzico C., Dell’Acqua A., Barbara G., et al. Effects of SARS Cov-2 epidemic on the obstetrical and gynecological emergency service accesses. What happened and what shall we expect now? Eur J Obstet Gynecol Reprod Biol. 2020;254:64–68. doi: 10.1016/j.ejogrb.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Been J.V., Burgos Ochoa L., Bertens L.C.M., Schoenmakers S., Steegers E.A.P., Reiss I.K.M. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Heal. 2020;5(11):e604–e611. doi: 10.1016/S2468-2667(20)30223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda Y., Nakamura M., Ninomiya H., Ogawa K., Sago H., Miyawaki A. Trends in intensive neonatal care during the COVID-19 outbreak in Japan. Arch Dis Child Fetal Neonatal Ed. 2021;106(3):F327–F329. doi: 10.1136/archdischild-2020-320521. [DOI] [PubMed] [Google Scholar]
- 43.Matheson A., McGannon C.J., Malhotra A., Palmer K.R., Stewart A.E., Wallace E.M., et al. Prematurity Rates During the Coronavirus Disease 2019 (COVID-19) Pandemic Lockdown in Melbourne. Australia Obstet Gynecol. 2021;137(3):405–407. doi: 10.1097/AOG.0000000000004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richter F., Strasser A.S., Suarez-Farinas M., Zhao S., Nadkarni G.N., Jabs E.W., et al. Neonatal outcomes during the COVID-19 pandemic in New York City. Pediatr Res. 2022;91(3):477–479. doi: 10.1038/s41390-021-01513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar M., Puri M., Yadav R., Biswas R., Singh M., Chaudhary V., et al. Stillbirths and the COVID-19 pandemic: Looking beyond SARS-CoV-2 infection. Int J Gynecol Obstet. 2021;153(1):76–82. doi: 10.1002/ijgo.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasuga Y., Miyakoshi K., Nishio H., Akiba Y., Otani T., Fukutake M., et al. Mid-trimester residual cervical length and the risk of preterm birth in pregnancies after abdominal radical trachelectomy: a retrospective analysis. BJOG An Int J Obstet Gynaecol. 2017;124(11):1729–1735. doi: 10.1111/1471-0528.14688. [DOI] [PubMed] [Google Scholar]
- 47.Olsen S.J., Azziz-Baumgartner E., Budd A.P., Brammer L., Sullivan S., Pineda R.F., et al. Decreased Influenza Activity During the COVID-19 Pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan S.G., Carlson S., Cheng A.C., Chilver M.BN., Dwyer D.E., Irwin M., et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Eurosurveillance. 2020;25(47) doi: 10.2807/1560-7917.ES.2020.25.47.2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jardine J., Relph S., Magee L.A., Dadelszen P., Morris E., Ross‐Davie M., et al. Maternity services in the UK during the coronavirus disease 2019 pandemic: a national survey of modifications to standard care. BJOG An Int J Obstet Gynaecol. 2021;128(5):880–889. doi: 10.1111/1471-0528.16547. [DOI] [PubMed] [Google Scholar]
- 50.Rolnik DL, Matheson A, Liu Y, Chu S, McGannon C, Mulcahy B, et al. The impact of COVID-19 pandemic restrictions on pregnancy duration and outcomes in Melbourne, Australia. Ultrasound Obstet Gynecol. 2021 Jul 26. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.