Introduction
Granuloma annulare (GA) is a benign, self-limited skin condition of unknown etiology. However, it is postulated to reflect a hypersensitivity reaction to triggers such as trauma, medications, malignancy, viral infections, and vaccinations, with some forms associated with diabetes mellitus and dyslipidemia.1, 2, 3 In early 2021, authors described the first case of generalized GA associated with pneumococcal vaccination developing <2 weeks after a patient received the pneumococcal conjugate vaccine.2 Although the patient was asymptomatic and the disease self-limited, the exact pathogenesis of this disease process remains unknown.2
To date, only 13 cases of GA developing after vaccination have been documented, 8 of which were in response to the bacillus Calmette-Guérin vaccine and none of which have been in response to the COVID-19 vaccine.2 Of interest, 76.92% of all cases described in response to vaccination have represented the generalized subtype of GA and, on average, presented within the first 8 weeks following vaccine administration.2 Most cases were reported in younger patients, which might reflect the higher frequency of vaccinations in this segment.2 Herein, to our knowledge, we report the first documented case of generalized GA occurring after COVID-19 vaccination. We moreover present a review of the literature regarding GA eruptions associated with COVID-19.
Case report
A 58-year-old woman with a history of hyperlipidemia and bipolar disorder presented to our dermatology clinic because of a 3-month rash, which began 2 weeks after her second dose of the Pfizer COVID-19 vaccine. The rash began on her trunk and spread to her upper and lower extremities. She endorsed mild pruritus. Examination revealed multiple papules coalescing into plaques with central clearing on the back, flank, inguinal folds, and extremities (Fig 1). Based on morphology, a diagnosis of generalized GA was favored, and a punch biopsy provided confirmation (Fig 2). Laboratory tests revealed a normal complete blood cell count, comprehensive metabolic panel, thyroid function, and hemoglobin A1c; HIV and hepatitis panels were negative. A real-time polymerase chain reaction test result for SARS-CoV-2 was negative. The patient had elevated levels of total cholesterol (299 mg/dL; reference range, <200 mg/dL) and low-density lipoprotein-cholesterol (196 mg/dL; reference range, <100 mg/dL). Treatment was initiated with an intramuscular injection of triamcinolone 40 mg and clobetasol 0.05% topical cream with improvement 1 month later, at which point she was started on hydroxychloroquine 200 mg twice a day following clearance from an ophthalmologist. At followup 2 months later, there was a reduction and flattening of the skin lesions (Fig 3). She was continued on hydroxychloroquine 200 mg twice a day and topical clobetasol. The patient was advised to receive the COVID-19 booster dose through a shared decision-making process, as the benefits of vaccination were deemed to be greater than the possible risk of a GA flare. The patient followed up 2 weeks after receiving the Pfizer COVID-19 booster dose, complaining of new lesions, increased itching, and redness of her rash, which started less than a week after the third dose (Fig 4). The patient was instructed to continue her current treatment, and hydroxyzine 25 mg every night at bedtime was prescribed.
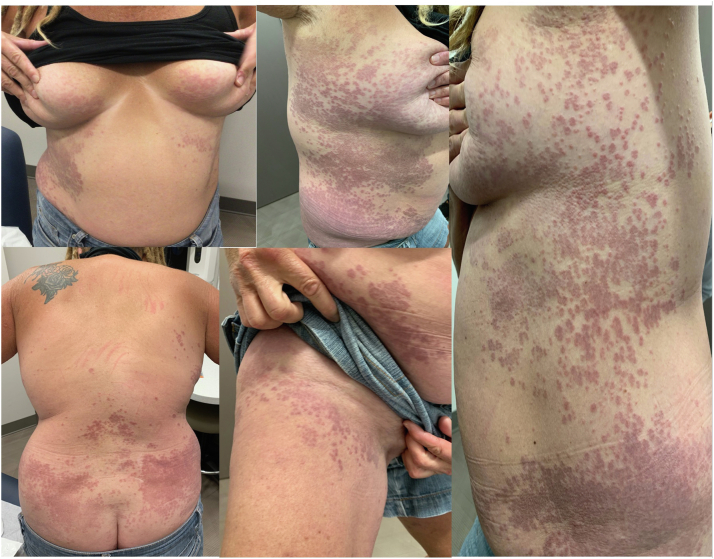
Fig 1.
Skin findings on initial visit with erythematous papules with central clearing coalescing into annular plaques on the breasts, lower portion of the back, flanks, and inguinal folds.
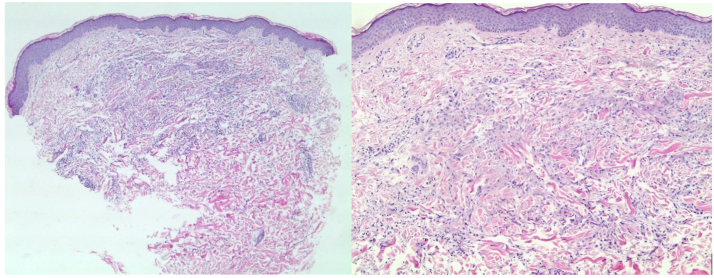
Fig 2.
Hematoxylin-eosin–stained slides (left, original magnification: ×50; right, original magnification: ×100) of a punch biopsy, showing an interstitial histiocytic infiltrate in the papillary dermis in a palisading array with central necrobiosis consistent with granuloma annulare.
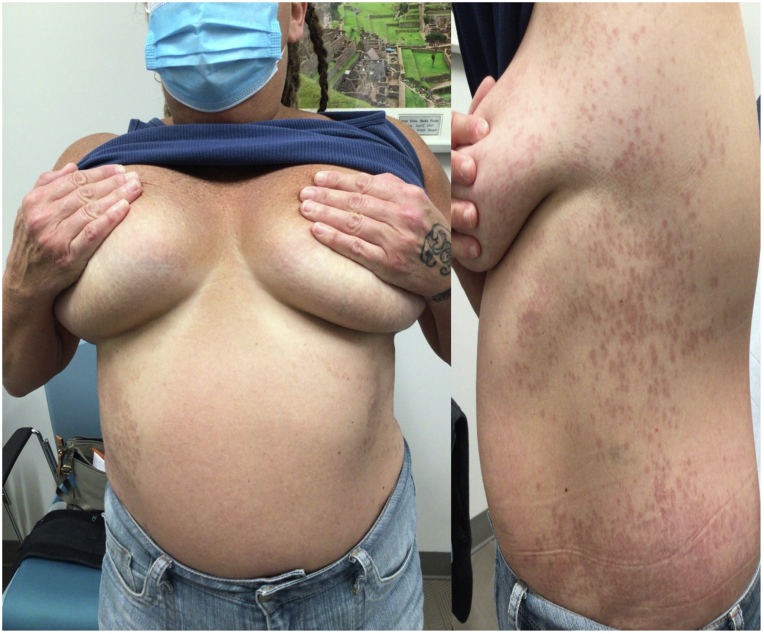
Fig 3.
Significant clinical improvement was observed after 2 months of therapy.
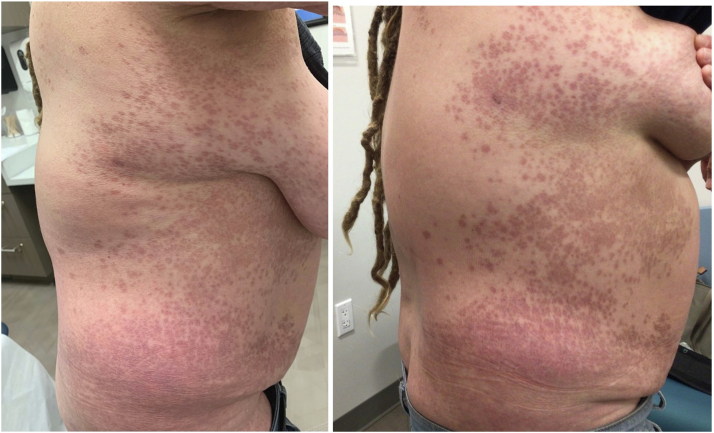
Fig 4.
The patient’s disease flares 2 weeks after she received the booster dose (left, before the booster dose; right, after the booster dose).
Discussion
The etiology of GA is unknown but is postulated to be a delayed-type hypersensitivity to a stimulus.1,4,5 There have been several associations with generalized GA, including diabetes mellitus, malignancy, thyroid disease, dyslipidemia, HIV, and hepatitis B and C. Generalized GA has a prolonged course that is poorly responsive to treatment and comprises 8% to 15% of all cases.5 Of the 13 cases of vaccine-induced GA in the literature, 8 occurred after the bacillus Calmette-Guérin vaccine, and none were reported to develop after administration of the COVID-19 vaccine.2 Seventy-six percent of all cases described to occur after vaccination have represented the generalized form of GA and, on average, presented within 8 weeks following vaccination.2
Amidst the COVID-19 pandemic, there have been several cases of GA occurring shorty after infection with the SARS-CoV-2. In 2021, Monte-Serrano et al6 described a patient who developed localized GA after SARS-CoV-2 infection. The patient was a 53-year-old woman with biopsy-proven GA on her left hand coinciding with the onset of headache, anosmia, and dysgeusia. A real-time polymerase chain reaction test result for SARS-CoV-2 was positive. Testing of her skin biopsy specimen revealed a similar pattern of immunohistochemistry-based cytoplasmic staining in the GA histiocytes with the viral spike protein of SARS-CoV-2.6 However, similar results were also seen in negative control specimens of patients diagnosed with GA in 2019—before the pandemic. This finding could be explained by lack of specificity against the spike proteins of SARS-CoV-2.6 Moreover, the real-time polymerase chain reaction test result for SARS-CoV-2 of the patient’s skin specimen was negative, further supporting the notion that GA represents a reaction pattern to a stimulus, in this case SARS-CoV-2 infection, as opposed to a direct result of the virus causing the skin lesions.6
As discussed by Akinosoglou et al,7 there has been a well-established correlation with hyperactivation of the immune system, resulting in an inordinate cytokine release that led to multiorgan failure and death in patients with COVID-19. It has been postulated that molecular mimicry plays a role in this acute autoimmune response, with antibodies to SARS-CoV-2-spike glycoproteins cross-reacting with host peptides that contain a similar protein arrangement.7,8 Talotta et al9 reasoned that those predisposed to autoimmune and autoinflammatory conditions, receiving a nucleic acid vaccine may be at risk for an adverse immunologic effect.9 In patients with autoimmune disease who have received a COVID-19 vaccination, severe adverse effects have not surpassed those experienced from receiving other age appropriate vaccines.
Although GA is considered a benign disease, it can cause significant distress, especially in generalized cases. Our patient has a history of hyperlipidemia, which, along with diabetes, has been cited as a likely risk factor for GA; however, more studies are needed to better substantiate this relationship.3,4 To our knowledge, no studies have evaluated the time sequence between dyslipidemia and the onset of generalized GA. Dyslipidemia was considered as a possible etiology; however, given her 10-year history of hyperlipidemia and treatment with a statin, we concluded that the presentation of generalized GA 2 weeks after COVID-19 vaccination was more likely attributed to the vaccination rather than the hyperlipidemia. It is important to acknowledge that since the majority of cases of GA are idiopathic, the onset of generalized disease in our patient following administration of the COVID-19 vaccine could simply be a temporal coincidence. That being said, the patient’s flare developing after the booster dose does provide more evidence to support the vaccine as a contributor.
The nebulous nature of the etiology of GA is likely the culprit reason for our patient not experiencing generalized GA after her first dose of the vaccine. It is also possible that threshold titers of a stimulatory antigen were not present until after the second vaccine dose or that immunologic memory was naïve until the second dose; initial testing of the Pfizer and Moderna vaccines showed that a relatively weak immune response was elicited after the first dose; a more robust response followed, when the second dose was given.10 This may be the reason why the patient experienced a flare only after her second dose and is the reason why current guidelines recommend a 2-dosage schedule for the aforementioned COVID-19 vaccines. Additionally, the patient experienced the flare less than 1 week after receiving the COVID-19 booster dose, which fact further supported the correlation between the vaccine and her disease. Upon exposure to the booster dose, the faster onset of disease flares is consistent with delayed-type hypersensitivity reactions that result from subsequent exposure to the stimulus. To our knowledge, our case is the first reported in the literature of generalized GA occurring after COVID-19 vaccination. Clinicians should be aware that this may occur, as vaccination rates increase.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Balin S.J., Barnhill R.L. In: Dermatology. 4th ed. Bolognia J.L., Schaffer J.V., Cerroni L., editors. Elsevier; 2017. Benign melanocytic neoplasms; pp. 1981–1982. [Google Scholar]
- 2.García-Gil M.F., Álvarez-Salafranca M., Martínez García A., Ara-Martín M. Generalized granuloma annulare after pneumococcal vaccination. An Bras Dermatol. 2021;96(1):59–63. doi: 10.1016/j.abd.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbieri J.S., Rosenbach M., Rodriguez O., Margolis D.J. Association of granuloma annulare with type 2 diabetes, hyperlipidemia, autoimmune disorders, and hematologic malignant neoplasms. JAMA Dermatol. 2021;157(7):817–823. doi: 10.1001/jamadermatol.2021.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piette E.W., Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75(3):457–465. doi: 10.1016/j.jaad.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Nambiar K.G., Jagadeesan S., Balasubramanian P., Thomas J. Successful treatment of generalized granuloma annulare with pentoxifylline. Indian Dermatol Online J. 2017;8(3):218–220. doi: 10.4103/2229-5178.206119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monte-Serrano J., García-Gil M.F., García-García M., Casas-Flecha I., Matovelle-Ochoa C., Ara-Martín M. Granuloma annulare triggered by SARS-CoV-2 infection: immunohistochemical staining. Dermatol Ther. 2021;34(3) doi: 10.1111/dth.14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akinosoglou K., Tzivaki I., Marangos M. Covid-19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. 2021;226:108721. doi: 10.1016/j.clim.2021.108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol. 2021;224:108665. doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston E.H. Necessity of 2 doses of the Pfizer and Moderna COVID-19 vaccines. JAMA. 2021;325(9):898. doi: 10.1001/jama.2021.1375. [DOI] [PubMed] [Google Scholar]