Alzheimer’s disease (AD) is known to be a progressive neurodegenerative illness that attacks the brain, causing alterations in synaptic transmission, dystrophic neurites, and neuronal death brought on by the accumulation of neurofibrillary tangles in neurons and neuritic amyloid beta (Aβ) plaques in the extracellular space resulting in brain shrinkage and misshapen architecture [1]. Current therapeutic interventions in AD rely only on pharmacological treatments, which are cholinesterase inhibitors and NMDA receptor antagonist that temporarily alleviate symptoms but do not stop the progression of the disease [2].
Hence the idea of searching for a novel intervention that may be used as alternative approach to treating AD, like deep brain stimulation (DBS) is required. DBS is a novel neurosurgical procedure that has emerged over the past two decades, and is akin to a “brain pacemaker”. It entails implanting an intracranial electrode, an extension wire, and a pulse generator. DBS has proved to be one of the means for memory neuromodulation. It has been hypothesized that the timing and rhythmicity of neuromodulation is crucial for the functional activation of memory circuits, leading eventually to long-term effectiveness.
The essential nodes stimulated in the memory circuit are the nucleus basalis of Meynert (NBM), entorhinal cortex (EC) of the hippocampus, and the fornix [3]. An early network dysfunction in AD is the selective degeneration of the basal forebrain cholinergic system and the vertical limb of the diagonal band in the NBM [4]. This finding strengthens the cholinergic hypothesis, which states that the identification of neurofibrillary tangle formation and loss of cholinergic neurons due to degeneration of the NBM is a critical event leading to AD [4].
Researchers have reported that the pathological accumulation of neurofibrillary tangles occurs first in the EC. DBS triggers neurogenesis in the hippocampus, and may have a neurotrophic action, facilitating the expression of synaptic proteins, and driving the Papez circuit to improve memory [5].
The classic Papez circuit, first described by James Papez (1937), contains most of the medial temporal lobe, including the hippocampus, EC, mammillary bodies, mammillothalamic tract, anterior thalamic nucleus, and cingulum [6]. In AD, the disconnection between the different structures within the circuit of Papez network due to neural damage leads to breakdown of the network and to extensive neurodegeneration. This cascade of events can result in anterograde amnesia (an inability to create new episodic memories) in AD patients [7]. In line with this, damage to the fornix in humans is known to cause memory deficits [8]. Thus, one of our purpose is to study the effect of DBS on the neural pathways of the Papez circuit, which is one of the essential pathways of the limbic system inherent in neurovegetative function, memory, and emotional expression. This manuscript sheds new light on a new paradigm and focuses on a potentially effective compensation therapy based on non-pharmacological treatment of AD by using DBS. The inadequate treatments, the high prevalence, the high cost, and the suffering of patients and their relatives triggered us to explore new non-drug interventions, such as DBS.
The Effective Targets of DBS in AD Include the Fornix, Entorhinal Cortex, and Nucleus Basalis of Meynert in the Circuit of Papez
The Fornix
The fornix is a core white matter bundle in limbic circuits. It is an integral part of the classical circuit of Papez, a major pathway of the limbic system primarily involved in memory. Its strategic location and various connections makes it interesting for the DBS approach.
Damaged to the fornix in humans and animals can cause a severe memory deficit. Severe atrophy of the fornix is accompanied by a transition from mild/moderate cognitive impairment to AD. Thus, when fornix DBS selectively targets the CA1 and CA3 sub-regions of the hippocampus, it can successfully alleviate the cognitive deficits. It has also been shown to be effective in enhancing memory [9], aiding visuo-spatial memory, improving verbal recollection [9], reducing Aβ42-related plaques and neuroinflammation [10], increasing the extracellular level of acetylcholine (ACh), enhancing neuroplasticity, decreasing astrogliosis and microglia levels [10], and increasing metabolism [10].
The Hippocampus and Entorhinal Cortex
Holistically, the hippocampus looks like a sea-horse, described as a curved and re-curved sheet of cortex that is folded into the medial surface of the temporal lobe. The hippocampal formation and parahippocampal gyrus are the major subdivisions of the medial temporal cortex. The EC, located anteriorly in the parahippocampal gyrus, and the hippocampus form a principally unidirectional network, with input from the EC to the hippocampus, whereas the fornix is the major output structure.
The EC is divided according to cytoarchitecture and connectivity into two divisions: medial, which contains spatial neurons, and lateral, which contains neurons encoding object information, attention, and motivation [11]. There is a sturdy connection between the hippocampus and the medial and lateral EC. CA1 is the first and essential area of the hippocampus circuit that is strictly associated with the spatial memory and mainly projects to the EC and subiculum. The motivation to apply DBS directly to the EC–CA1 region is based on the fact that the earliest symptom of AD is anterograde amnesia, which begins in the EC region, while the earliest pathological changes also occur in the EC and hippocampus [12].
Nucleus Basalis of Meynert
Physiologically, the NBM is a cholinergic nucleus in the basal forebrain. It is the source of ACh in the brain: 90% of its neurons are cholinergic, it provides extensive projections to all cortical regions, and participates extensively in all cortical networks. It is a powerful nucleus that influences the neocortex and subcortical structures through the secretion of ACh, altering glial function, stabilizing cerebral blood flow, and provoking the release of nerve growth factor (NGF) [13]. Hence the importance of cortical cholinergic input from the NBM for cognitive processing and attentional processes, and hence its role as one of the targets of DBS in AD. Neuronal loss in the NBM is closely correlated with cortical cholinergic deficits and the degree of cognitive impairment in patients with AD.
The Hypothesis of the Three Axes DBS Paradigm
Here, we take a comprehensive look at the future of DBS for AD. Our hypothesis depends on the three axes DBS paradigm based on three electrodes inserted carefully into the target regions under MRI guidance. These electrodes stimulate three brain structures, beginning gradually from a low to a high frequency. Synchronization and insertion of the three electrodes is essential for complete benefit. We aim to apply cholinergic stimulation in a three-step approach. The stimulation frequencies are gradually increased from low to high, until moderate/high stimulation is reached. The first step in the protocol is to apply a low frequency (average: 50 Hz, 100 μA and 100 μs), either continuously or intermittently for a few hours per day. The second step delivers a moderate frequency (100 Hz, 100 μA and 100 μs). The results are assessed by measuring the degree of ACh release and improvement of memory function. If optimal ACh release occurs at this stage, a stimulation frequency of 100 Hz is used; if not, a higher frequency (130 Hz, 100 μA, 80 μs) is applied in the third step.
It is critical to optimize the stimulation frequency of DBS to minimize the side-effects caused either by the direct stimulation frequency or by inadvertent stimulation of limbic circuit elements and glial cells that are distant from the target.
It is important to identify which stimulation parameters are associated with a sustained memory benefit, if any, before assessing any long-term impact on disease progression. Our proposed three target structures are the NBM, the fornix, and the EC. These targets may be stimulated at different frequencies (high in the fornix, moderate in the EC, and low in the NBM). In addition, pharmacological treatment can be combined with the procedure.
Discussion
Considering the high prevalence of AD patients, and no effective treatment for AD so far, this situation requires us to explore new and effective non-drug interventions, among which DBS may be a potentially effective compensation treatment. The main strength of this insight lies in its extensive description of the Papez circuit, and on how it is adversely affected by AD, and the role of DBS as a treatment option.
Previous studies have adapted the protocol of combining pulse stimulation at a higher frequency with a low-frequency rhythm, along with the current amplitude. This has proved a promising approach both in humans and animal models, and has contributed significantly to the success of DBS in inducing memory changes. Based on successful DBS in PD, important studies have applied a continuous high-frequency stimulation protocol at 130 Hz. Despite successful experimental application of DBS in many neuropsychiatric diseases, the mechanisms behind its therapeutic effects in cognitive disorders may be quite different. This approach is still in the preliminary stages. This can be largely attributed to differences in stimulation targets, but also to differences in neuropathologies/circuitopathies.
Numerous studies conducted in animals and humans have investigated the role of the fornix. In animals, it was demonstrated that fornix DBS can improve spatial learning memory, and reduce amyloidosis and the inflammatory response in a mouse model of AD. It has been demonstrated that fornix DBS has beneficial effects on spatial [14], contextual, and recognition memory as well as cognitive function in rodents. Almost all stimulations were applied with a handheld stimulator for 1 h at parameters 2.5 V, 90 μs pulse width, and 130 Hz), similar to the report by Gondard et al., [15].
A recent study intersecting with our hypothesis was reported by Germann et al., 2021, who investigated the architecture of human memory and the neuroanatomical substrate of stimulation‐induced flashbacks in patients with mild AD undergoing bilateral fornix DBS. The authors demonstrated that a distinct diencephalic region is associated with a greater likelihood of flashbacks [16]. The authors found that involvement of the fornix and surrounding areas predicted memory events with 72% accuracy. They stressed that flashback‐inducing stimulation induces greater functional connectivity in a network of memory‐evoking and autobiographical memory‐related sites. Deeb et al., 2019, also supported this hypothesis by showing that most memory flashbacks (87%) are associated with dorsal brain contacts that probably stimulate both the fornix and the subcallosal area [17].
The memory flashbacks from brain stimulation, especially in the fornix, stress its involvement in memory during DBS and provide information regarding the neuroanatomical substrates and pathways of memory encoding and retrieval.
Concerning the EC, acute CA1-DBS can ameliorate and improve memory impairment. EC-DBS of wild-type mice can increase spatial memory and accelerate the proliferation of neural cells in the dentate gyrus. It has also been demonstrated that EC-DBS increases neurogenesis and improves contextual and spatial memory in animal models, suggesting that the pro-cognitive effects of DBS may reflect generic activation of the circuit of Papez [18]. Mann et al., 2018, also stressed the fact that chronic EC-DBS in transgenic mice remarkably decreases Aβ plaques, APP, and tau in the hippocampus [18].
In humans, DBS to EC and hippocampal neurons may be most effective for slowing AD progression, inducing personal semantic reminiscences, whereas hippocampal stimulation induces episodic memories and can influence cognitive function and hippocampal neurogenesis. Currently, no studies of chronic stimulation have targeted the EC in general, or the CA1 area specifically in humans, with the exception of Hescham et al., [19]. Chronic EC stimulation in humans is promising under certain conditions: using microstimulation (150 μA) through single, small micro-wires (100 μm) rather than large bipolar contacts in an attempt to more precisely delineate the spatial extent of stimulation.
Regarding the NBM, research has demonstrated that chronic NBM-DBS has good efficacy in AD, and if used in the early stages of AD it has beneficial effects on AD progression, enhances tonic cholinergic inputs to the cortex, and increases cognitive and metabolic activities.
Usually, low-frequency NBM–DBS in early-diagnosed AD patients aims to excite residual cholinergic neurons, causing enhanced tonic cholinergic inputs to the neocortex. In fact, AD patients who receive low-frequency NBM–DBS stimulation show no impairment or even slightly improved cognition after one year. Interestingly, AD patients who respond positively to NBM-DBS appear to have a relatively limited cognitive deficit and minor fronto-parieto-temporal cortical atrophy. This fact strengthens the hypothesis that NBM-DBS slows cognitive decline in patients with early AD by stimulating increased transmission from remaining cholinergic neurons.
NBM DBS in mice showed improvement in spatial learning. In addition, NBM DBS regulates the GABAergic, glutamatergic, and cholinergic systems; reduces the abundance of amyloid protein; and has neuroprotective effects in animals. As in humans, DBS of the NBM improves memory [20] and enhances the secretion of ACh in the neocortex in response to DBS-NBM, provoking secretion of NGF by targeting cortical neurons in wild-type rats. This is known to be essential for the survival and function of cholinergic neurons in the basal forebrain.
In rat models, the improvement in AD symptoms after regulation of other neurotransmitters besides ACh, such as glutamic acid and GABA, have been reported. This means that NBM-DBS may modulate changes in the GABA and glutamate systems and ameliorate memory in AD rats. Furthermore, it is pertinent to note that DBS-NBM increases synaptic remodeling, synaptic plasticity in the cortex, and hippocampal neurogenesis, triggers the clearance of misfolded proteins, and provokes neuroprotective responses and enhance neurotrophic support in wild-type rats [21].
Recently, a study by Nazmuddin et al. [22], examined 128 preclinical studies in animal and 12 clinical reports in humans. All studies showed that NBM-DBS, both continuously and intermittently, enhance the release of ACh in the cortex. Some of the studies showed that low-frequency stimulation (20–50 Hz) has a greater effect on cortical ACh release than high-frequency stimulation (100–200 Hz). However, other studies reported a better effect of high-frequency than low-frequency stimulation when pulses were delivered through a shorter burst of 10 s at a relatively higher stimulation amplitude (2000 μA). DBS enhances and synchronizes output and impacts neuronal and memory circuit function. Moreover, it affects neurogenesis in the hippocampus and neurotrophic activity, as well as driving the Papez memory circuit.
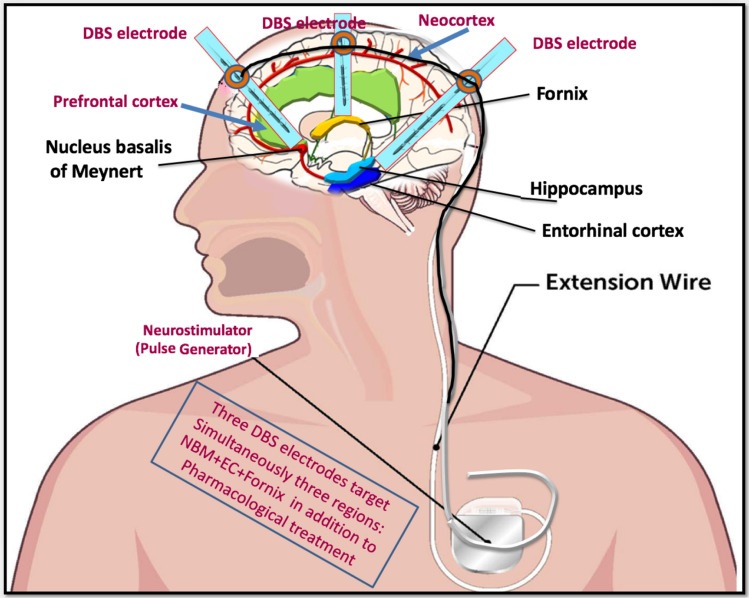
There are different opinions and controversial hypothesis about DBS. The ideal frequency of stimulation and the targets to be stimulated in AD remain the focus of controversy until we find the appropriate parameters for this devastating disease. Indeed, we rely on closed-loop DBS in these three structures – the fornix, EC, and NBM in the circuit of Papez – due to their importance as centers of memory. Notwithstanding, additional avenues such as transcranial ultrasound pulse stimulation should be examined as new add‐on therapies for AD and possibly for other neurodegenerative diseases [23] (Fig. 1).
Fig. 1.
An alternative approach that effectively targets multiple brain regions, alleviates various AD symptoms, and clarifies its pathogenic mechanism. We suggest a three-axes DBS paradigm based on three electrodes oriented to three effective brain targets, accompanied by pharmacological therapy. The first structure is the nucleus basalis of Meynert, and the other two structures are the fornix and the entorhinal cortex in the circuit of Papez, which are considered to be the major pathways of the limbic system. The three DBS electrodes are connected together and inserted into the extension wire. Due to difficulty to deliver different frequencies/amplitudes of stimulation to different targets, we suggest in the beginning (first step) to apply a low frequency (average: 50 Hz, 100 μA and 100 μs), either continuously or intermittently for a few hours per day for all three structures, if we receive no improvement, we will move on to the second step by delivering a moderate frequency (100 Hz, 100 μA and 100 μs). The results are assessed by measuring the degree of ACh release and improvement of memory function. If optimal ACh release occurs at this stage, a stimulation frequency of 100 Hz is used; if not, a higher frequency (130 Hz, 100 μA, 80 μs) is applied in the third step.
DBS is a novel means of memory neuromodulation, emotional expression, and neurovegetative function when critical nodes in the memory circuit are targeted. Continuous stimulation to these regions with high frequency stimulation (130 Hz) enhances and synchronizes output from these sites directly or even by indirect means, impacts neuronal and memory circuit function.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Talwar P, Sinha J, Grover S, Rawat C, Kushwaha S, Agarwal R, et al. Dissecting complex and multifactorial nature of Alzheimer's disease pathogenesis: A clinical, genomic, and systems biology perspective. Mol Neurobiol. 2016;53:4833–4864. doi: 10.1007/s12035-015-9390-0. [DOI] [PubMed] [Google Scholar]
- 2.Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmacotherapies for Alzheimer's disease: Beyond cholinesterase inhibitors. Pharmacol Ther. 2012;134:8–25. doi: 10.1016/j.pharmthera.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Temel Y, Boonstra J, Hescham S. The effect of fornix deep brain stimulation in brain diseases. Cell Mol Life Sci. 2020;77:3279–3291. doi: 10.1007/s00018-020-03456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu DF, Yan HH, Zhou J, Yang XD, Lu YM, Han YY. A circuit view of deep brain stimulation in Alzheimer's disease and the possible mechanisms. Mol Neurodegener. 2019;14:33. doi: 10.1186/s13024-019-0334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao S, Tang B, Wu ZY, Ure K, Sun YL, Tao HF, et al. Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature. 2015;526:430–434. doi: 10.1038/nature15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papez JW. A proposed mechanism of emotion. Arch NeurPsych. 1937;38:725. doi: 10.1001/archneurpsyc.1937.02260220069003. [DOI] [Google Scholar]
- 7.Lv Q, Du AL, Wei WS, Li YY, Liu GL, Wang XP. Deep brain stimulation: A potential treatment for dementia in Alzheimer's disease (AD) and Parkinson's disease dementia (PDD) Front Neurosci. 2018;12:360. doi: 10.3389/fnins.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vann SD, Tsivilis D, Denby CE, Quamme JR, Yonelinas AP, Aggleton JP, et al. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA. 2009;106:5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankar T, Chakravarty MM, Bescos A, Lara M, Obuchi T, Laxton AW, et al. Deep brain stimulation influences brain structure in Alzheimer's disease. Brain Stimul. 2015;8:645–654. doi: 10.1016/j.brs.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curot J, Busigny T, Valton L, Denuelle M, Vignal JP, Maillard L, et al. Memory scrutinized through electrical brain stimulation: A review of 80 years of experiential phenomena. Neurosci Biobehav Rev. 2017;78:161–177. doi: 10.1016/j.neubiorev.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumbhare D, Palys V, Toms J, Wickramasinghe CS, Amarasinghe K, Manic M, et al. Nucleus basalis of meynert stimulation for dementia: Theoretical and technical considerations. Front Neurosci. 2018;12:614. doi: 10.3389/fnins.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hescham S, Temel Y, Schipper S, Lagiere M, Schönfeld LM, Blokland A, et al. Fornix deep brain stimulation induced long-term spatial memory independent of hippocampal neurogenesis. Brain Struct Funct. 2017;222:1069–1075. doi: 10.1007/s00429-016-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondard E, Chau HN, Mann A, Tierney TS, Hamani C, Kalia SK, et al. Rapid modulation of protein expression in the rat Hippocampus following deep brain stimulation of the fornix. Brain Stimul. 2015;8:1058–1064. doi: 10.1016/j.brs.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Germann J, Elias GJB, Boutet A, Narang K, Neudorfer C, Horn A, et al. Brain structures and networks responsible for stimulation-induced memory flashbacks during forniceal deep brain stimulation for Alzheimer's disease. Alzheimers Dement. 2021;17:777–787. doi: 10.1002/alz.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeb W, Salvato B, Almeida L, Foote KD, Amaral R, Germann J, et al. Fornix-region deep brain stimulation-induced memory flashbacks in Alzheimer's disease. N Engl J Med. 2019;381:783–785. doi: 10.1056/NEJMc1905240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann A, Gondard E, Tampellini D, Milsted JAT, Marillac D, Hamani C, et al. Chronic deep brain stimulation in an Alzheimer's disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimul. 2018;11:435–444. doi: 10.1016/j.brs.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Hescham S, Jahanshahi A, Meriaux C, Lim LW, Blokland A, Temel Y. Behavioral effects of deep brain stimulation of different areas of the Papez circuit on memory- and anxiety-related functions. Behav Brain Res. 2015;292:353–360. doi: 10.1016/j.bbr.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Koulousakis P, van den Hove D, Visser-Vandewalle V, Sesia T. Cognitive improvements after intermittent deep brain stimulation of the nucleus basalis of meynert in a transgenic rat model for Alzheimer's disease: A preliminary approach. J Alzheimers Dis. 2020;73:461–466. doi: 10.3233/JAD-190919. [DOI] [PubMed] [Google Scholar]
- 21.Hotta H, Kagitani F, Kondo M, Uchida S. Basal forebrain stimulation induces NGF secretion in ipsilateral parietal cortex via nicotinic receptor activation in adult, but not aged rats. Neurosci Res. 2009;63:122–128. doi: 10.1016/j.neures.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Nazmuddin M, Philippens IHCHM, van Laar T. Electrical stimulation of the nucleus basalis of meynert: A systematic review of preclinical and clinical data. Sci Rep. 2021;11:117. doi: 10.1038/s41598-021-91391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popescu T, Pernet C, Beisteiner R. Transcranial ultrasound pulse stimulation reduces cortical atrophy in Alzheimer's patients: A follow-up study. Alzheimers Dement (N Y) 2021;7:e12121. doi: 10.1002/trc2.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]