Abstract
Compared with normal cells, tumor cells mainly obtain energy through aerobic glycolysis. Hexokinase 2 (HK2) plays a key role in the regulation of tumor cell aerobic glycolysis, and targeting HK2 has become a new strategy for cancer treatment. However, little is known about the role of HK2 in colon cancer and the regulation of its targeted inhibitors. In this study, we found that the expression of HK2 in colorectal cancer tissues was significantly higher than that in adjacent tissues, and the expression level of HK2 in metastatic colorectal cancer was further increased. Meanwhile, the expression level of HK2 was closely related to clinical TNM stage and outcome of colorectal cancer patients. We provide here evidence that HK2 inhibitor 3-Bromopyruvate acid (3-BP) can significantly inhibit the survival and proliferation of colon cancer cells, and induce apoptosis through mitochondrial apoptosis signaling pathway. In addition, we found that 3-BP can also induce endoplasmic reticulum stress in colon cancer cells, the mechanism may be through the increase of intracellular calcium concentration. In vitro and in vivo experiments showed that inhibition of endoplasmic reticulum stress could further increase the proliferation inhibition and apoptosis induced by 3-BP. Collectively, our results show that HK2 is highly expressed in colorectal cancer. 3-BP, an inhibitor of HK2, can induce apoptosis and endoplasmic reticulum stress in colon cancer cells. Endoplasmic reticulum stress plays a protective role in cell death induced by 3-BP. This result suggested that targeting HK2 and endoplasmic reticulum stress may be a valuable strategy in targeted and combination therapy of colon cancer.
Keywords: Colon cancer, Hexokinase 2·apoptosis, Endoplasmic reticulum stress, 3-Bromopyruvate acid
Introduction
In both sexes, the colorectal cancer (CRC) is the third largest malignant tumor in the world, next to lung cancer and breast cancer, with a relatively high mortality rate (Siegel et al. 2022; Katona and Weiss 2020; Ma et al. 2019). Surgery plus chemotherapy is still the main treatment for colorectal cancer, but patients with advanced colorectal cancer cannot bear surgery; meanwhile, chemotherapy tolerance is widespread; the survival period of patients with colorectal cancer has not been significantly improved (Wang et al. 2021). In recent years, with the emergence of precision medicine and targeted therapy, finding effective molecular targets and combined medication strategy has become an effective way to improve the prognosis of colorectal cancer (Rawla et al. 2019).
The change of metabolic pattern is one of the important characteristics that tumor cells are different from normal cells, especially glucose metabolism (Ganapathy-Kanniappan 2018). Fast growing tumor cells gain energy mainly through glycolysis, even under aerobic conditions. This phenomenon was first discovered by scientist Warburg in 1956, called “Warburg effect” (also known as aerobic glycolysis) (WARBURG 1956; Vander Heiden et al. 2009). The abnormal expression of glycolysis related genes involved in the process of tumor cell aerobic glycolysis, including glucose transporter (GLUT), hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) (Ma and Zong 2020; Reinfeld et al. 2022). These abnormal genes not only participate in the regulation of cell proliferation, but also affect the invasion, migration, and tumor microenvironment of cancer cells (Lunt and Vander Heiden 2011; Teoh and Lunt 2018). Among these kinases, HK2 is the most widely studied. Research shows that HK2 is highly expressed in a variety of cancers, and many inhibitors targeting HK2 have been developed, among which 3-Bromopyruvate acid (3-BP) is the most widely used and researched (Garcia et al. 2019; Ko et al. 2019). In addition to targeting HK2, the mechanism of action of 3-BP and the strategy of combination therapy are not clear.
Endoplasmic reticulum (ER) is one of the important organelles in eukaryotic cells, which is involved in the process of protein synthesis, folding, and transportation (Oakes and Papa 2015). Calcium concentration in endoplasmic reticulum is very important to maintain the normal function of endoplasmic reticulum and protein folding (Kania et al. 2019). Studies have shown that endoplasmic reticulum is extremely sensitive to various injury factors, including DNA damage, hypoxia and nutrient deficiency, pH, and stimulation of various drugs (Bahar et al. 2019). Under the stimulation of various factors, a large number of misfolded proteins and unfolded proteins gather in the endoplasmic reticulum cavity, which causes the imbalance of calcium ion balance (Siwecka et al. 2019; Chern et al. 2019). This phenomenon is called endoplasmic reticulum stress (ERS). When ERS occurs in a short time or to a lesser extent, cells can maintain homeostasis in the endoplasmic reticulum through unfolded protein response (UPR) to ensure cell survival. However, when the stress persists or the stress damage exceeds the protective ability of cell survival, the endoplasmic reticulum-related apoptosis pathway is initiated, leading to cell apoptosis (Liang et al.2021). Although ERS is involved in the mechanism of action of a variety of anticancer drugs, its effect on apoptosis induced by 3-BP in colon cancer cells is not clear.
In this research, we first found the relationship between HK2 expression and survival and metastasis in clinical colorectal cancer tissue samples, and further explored the effect of HK2 inhibitor 3-BP on survival, proliferation, and apoptosis of colon cancer cells in vitro and in vivo. Meanwhile, we further clarify the effect and mechanism of 3-BP on ERS, and the role of ERS in the apoptosis of colon cancer induced by 3-BP. This study provides a theoretical basis for the clinical treatment of colorectal cancer by targeting HK2 and endoplasmic reticulum stress.
Materials and methods
Drugs and antibody
3-Bromopyruvic acid (HY-19992), Tauroursodeoxycholate Sodium (HY-19696A), and Sodium 4-phenylbutyrate (HY-15654) were purchased from MCE (MedChemExpress, USA). HK2 antibody was purchased from abcam (Cambrige, UK). Cleaved caspase-3, Bax, Bcl-2, Cytochrome C, Bip, PDI, eIF-2a, p-eIF-2a, ATF4, and Tubulin were purchased from Cell Signaling Technology (MA, USA).
Tissue specimens
Formalin-fixed paraffin-embedded colon cancer samples and normal tissues were obtained from the department of Pathology, the First Affiliated Hospital of Xinxiang Medical University. The patient did not receive any treatment before the operation. All patients were definitely diagnosed as colon cancer by H&E staining. The project was approved by the Medical Ethics Committees of Xinxiang Medical University.
Immunohistochemistry (IHC)
Paraffin-embedded tissues were selected according to H&E staining. The thickness of each tissue was 2 µm, and the sections were processed with immunohistochemistry kit (Beyotime, China). The slides were incubated overnight at 4 °C with HK2 antibody. The next day, it was incubated with the second antibody for 2 h, and then stained with DAB and counterstained with Hematoxylin. The results were judged and counted by two pathologists.
Cell culture
Human colon cancer cell lines, RKO and HCT-116, were obtained from Procell (Wuhan, China). Cells were cultured in DMEM/High glucose medium (Gibco, USA) with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin (Gibco, USA), 100 µg/ml streptomycin (Gibco, USA), and incubated in a 37 °C humidified incubator with 5% CO2.
In vitrocytotoxicity assay (CCK8)
RKO and HCT-116 cells were seeded in 96-well plates at 1.2 × 104 per well. Cells were exposed to 3-BP alone or combination with sodium phenylbutyrate and sodium taurousodeoxycholate for 12 h and then added CCK-8 to each well for incubation for 2 h and tested the absorbance value at 520 nm.
Colony-forming assay
Cells were seeded into 6-well plates with 100 or 500 cells in each well, and were cultured in DMEM/High glucose medium with 10% FBS. The cells were incubated in a 37 ℃ 5% CO2 and saturated humidity incubator for 2–3 weeks. When visible clones appeared in the culture dish, the culture was terminated. Wash carefully with PBS twice. Add 5 ml of methanol fix for 15 min. Remove the fixative solution, and add appropriate amount of crystal violet and dye for 10–30 min.
Flow cytometric analysis for apoptosis
RKO and HCT-116 cells were cultured in 6-well plates and treated with different concentratios of 3-BP or combination with sodium phenylbutyrate and sodium taurousodeoxycholate. Then the cells were harvested, and washed twice with ice-cold PBS. Then, cells were incubated with 100 µL Annexin V-FITC binding buffer (Beyotime, China), 5 µL Annexin V-FITC, and 5 µL propidium iodide (PI) for 30 min. The sample was examined by flow cytometry (CytoFLEX, USA).
Mitochondrial membrane potential detection (ΔΨm)
RKO and HCT-116 cells were plated in six-well plates and given various concentrations of 3-BP alone or combination with sodium phenylbutyrate and sodium taurousodeoxycholate. After that, cells were stained with 10 µg/mL JC-1 (JC-1 Mitochondrial Membrane Potential Detection Kit, Beyotime, China) for 20 min in the incubator (37 ℃, 5% CO2), and cells were rinsed with HBSS twice to remove the nonspecific background staining. Then cells were analyzed using a flow cytometer (CytoFLEX, USA), and cells emitting a bright red fluorescence represented the aggregate mitochondria.
Western blotting
After drug treatment, the cell pellet was resuspended in RIPA lysis buffer (Beyotime, China) and centrifuged at 13,000 rpm at 4 °C for 15 min. Determination of protein concentration was done by BCA protein quantitative kit (Beyotime, China). The protein (30–60 µg) is separated by 10% SDS-PAGE and transferred to the polyvinylidene fluoride (PVDF) membranes (Millipore, Germany) and blocked in 5% nonfat skim milk at room temperature for 2 h and incubated overnight at 4 °C with the primary antibody. It is then incubated at room temperature with the secondary antibody for 1 h. Expose the PVDF film using an imaging system (Bio-Rad, USA).
Immunofluorescence analysis
RKO and HCT116 cells were seeded on coverslips at a density of 2 × 104 per well overnight. After the cells were treated with 3-BP, the cells were washed with PBS 3 times, fixed, and perforated. Cells were incubated with primary antibody PDI (1:100) antibody at 4 °C overnight. Next day, the coverslips were incubated with rhodamine-conjugated goat antibodies against rabbit (Abcam). Finally, DAPI was used to stain the nuclei. The results were scanned and observed by confocal microscope (Nikon A1R, Japan).
Cytoplasmic calcium detection
The Ca2+ sensitive fluorescent dye Fluo-4/AM (Thermo fisher Scientific, USA) was used to measure Ca2+ concentration according to the manufacturers’ protocols. Prior to exposure to various experimental conditions, the cells were incubated with Fluo-4/AM for 30 min at 37 ℃. Cell samples were then analyzed by fluorescence microscope.
In vivo assay
HCT116 cells (1.5 × 107/mouse) were inoculated subcutaneously on the right side of BALB/c nude mice (female). When the tumor volume reached 100 mm3, the mice were randomly divided into four groups, control, 3-BP (15 mg/kg), TUDCA (3 mg/kg), and 3-BP + TUDCA. The drug was injected intraperitoneally three times a week. Tumors were measured with digital calipers, and tumor volumes were calculated by the formula: Volume = Length × (Width)2/2.
Statistics
Chi-square test was used for rate comparison (SPSS 18.0). Kaplan–Meier was used for overall survival analysis. T-test was used to compare the values between the two groups, and ANOVA (one-way analysis of variance) was used for three groups and more than three groups. Comparisons were considered to be statistically significantly different when p < 0.05.
Results
Upregulation of HK2 was associated with the poor clinical outcome and TNM classification of colon cancer
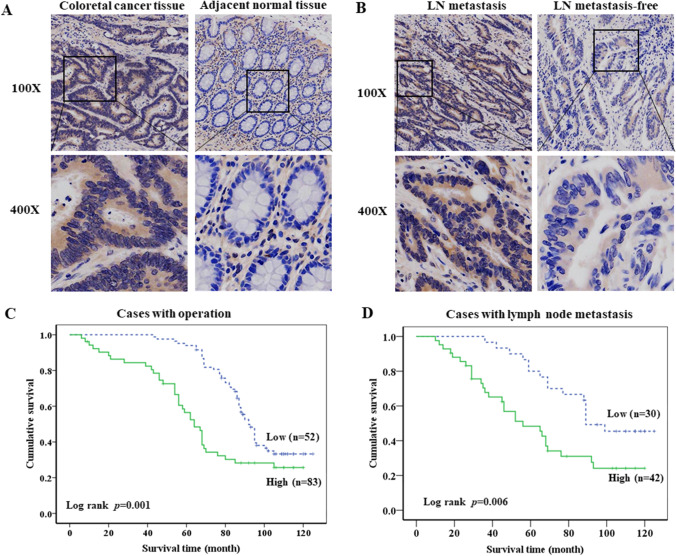
We first detected the expression of HK2 protein in 135 primary colon cancer tissues by IHC. Kaplan–Meier was used to analyze the correlation between HK2 expression and overall survival. The results of IHC showed that HK2 protein was mainly expressed in cytoplasm, and the expression of HK2 in colon cancer tissues was significantly higher than that in adjacent normal tissues (Fig. 1A). In addition, the expression of HK2 in colon cancer with lymph node (LN) metastasis was significantly higher than that in colon cancer without LN metastasis (Fig. 1B). Kaplan–Meier survival analysis showed that the patients with high HK2 expression had poorer overall survival (p = 0.001) than the patients with low HK2 expression (Fig. 1C). Meanwhile, the colon cancer patients with LN metastasis clearly had poorer overall survival (p = 0.006) than the patients without LN metastasis (Fig. 1D).
Fig. 1.
Upregulation of HK2 was associated with the poor clinical outcome. A IHC detected the HK2 protein expression in colorectal cancer and paired adjacent normal tissues. B IHC detected the HK2 protein expression in colorectal cancer with or without lymph node metastasis. C Kaplan–Meier analysis of the HK2 expression on overall survival of colorectal cancer patients. D Kaplan–Meier analysis of the HK2 expression on overall survival of colorectal cancer patients with lymph node metastasis
Associations between clinicopathological features and immunohistochemical HK2 status are shown in Table 1. The results showed that HK2 expression was related to tumor size (p = 0.001), lymph node infiltration (p = 0.021), and metastasis (p = 0.013), but not to age, gender, and differentiation. These results suggest that the high expression of HK2 is closely related to clinical outcome and TNM stage of colon cancer patients.
Table 1.
Association between the clinicopathological features and HK2 status in colon cancer
| Characteristics | N | HK2 expression | P | |
|---|---|---|---|---|
| Low | High | |||
| Gender | ||||
| Male | 60 | 22 | 38 | 0.725 |
| Female | 75 | 30 | 45 | |
| Age | ||||
| ≥ 55 | 92 | 38 | 54 | 0.35 |
| < 55 | 43 | 14 | 29 | |
| T stage | ||||
|
T1-T2 T3-T4 |
72 63 |
37 15 |
35 48 |
0.001** |
| N stage | ||||
| Nx-0 | 65 | 33 | 32 | 0.021* |
| N1-2 | 70 | 19 | 51 | |
| M stage | ||||
| M0 | 59 | 30 | 29 | 0.013* |
| M1 | 76 | 22 | 54 | |
| Differentiation | ||||
| Well | 39 | 16 | 23 | 0.864 |
| Moderately | 53 | 21 | 32 | |
| Poorly | 33 | 15 | 18 | |
HK2 inhibitors inhibited the survival, proliferation, and induced apoptosis in colon cancer cells
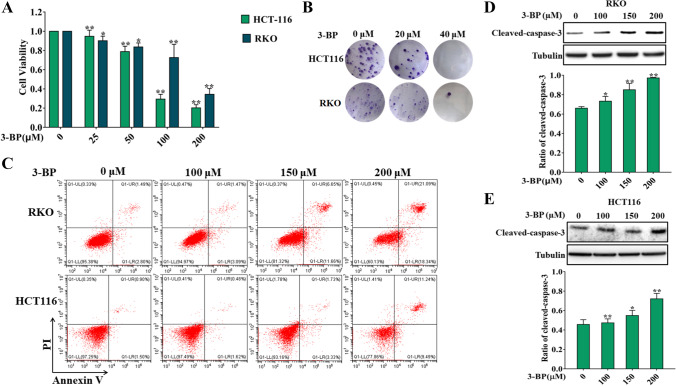
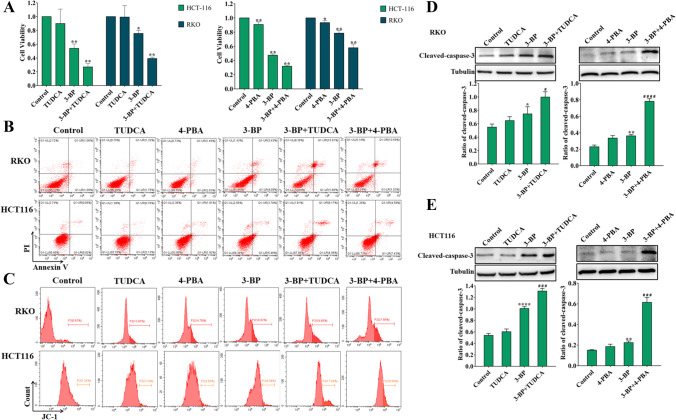
These results above indicated that HK2 is involved in the development of colon cancer. We further investigated the effect of HK2 on the biological behavior of colon cancer cells. We first examined the effect of HK2 inhibitor 3-Bromopyruvate acid (3-BP) on the survival and proliferation of colon cancer cells. The results showed that 3-BP could significantly reduce the survival rate and inhibit the proliferation rate of colon cancer cells (Fig. 2A, B). We further examined the effect of 3-BP on apoptosis of colon cancer cells. Flow cytometry showed that 3-BP increased the apoptosis rate of colon cancer cells (Fig. 2C). Meanwhile, western blotting results showed that 3-BP significantly increased the expression of cleaved caspase-3 in RKO and HCT116 cells (Fig. 2D, E). These results suggest that HK2 inhibitor can inhibit the proliferation and survival of colon cancer cells and induce apoptosis.
Fig. 2.
HK2 inhibitors inhibited the proliferation, survival, and induced apoptosis in colon cancer cells. A The cell viability of HCT116 and RKO was detected by MTT assay for 24 h. B The cell proliferation of HCT116 and RKO was detected by colony-forming assay. C Flow cytometry detected the apoptotic rate of HCT116 and RKO induced by 3-BP for 12 h. D Western blotting detected the expression of cleaved caspase-3 of HCT116 and RKO induced by 3-BP for 24 h (*p < 0.05, **p < 0.01 vs control group)
HK2 inhibitor–induced colon cancer cell death through mitochondrial apoptosis signalling pathway
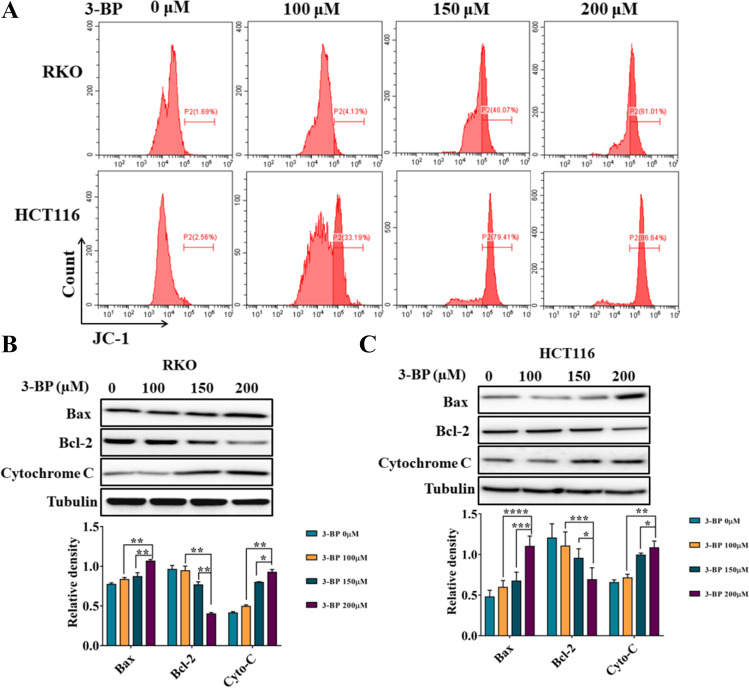
On the basis of the above detection of apoptosis, we detected the changes of mitochondrial apoptosis signal pathway. The flow cytometry was used to detect the changes of mitochondrial membrane potential. As shown in Fig. 3A, 3-BP can reduce mitochondrial membrane potential of RKO and HCT116. In addition, western blotting showed that 3-BP increased the expression of cytochrome C and Bax and decreased the expression of Bcl-2 protein (Fig. 3B, C). These results show that 3-BP can induce the activation of mitochondrial apoptosis signaling pathway.
Fig. 3.
HK2 inhibitor induced colon cancer cell death through mitochondrial apoptosis signaling pathway. A Flow cytometry detected the changes of mitochondrial membrane potential of RKO and HCT116 induced by 3-BP for 12 h. B, C Western blotting detected the expression of Bax, Bcl-2, and cytochrome C of RKO and HCT116 induced by 3-BP for 24 h (*p < 0.05, **p < 0.01, ***p < 0.001 vs control group)
3-BP–induced endoplasmic reticulum stress by affecting cytoplasmic calcium concentration.
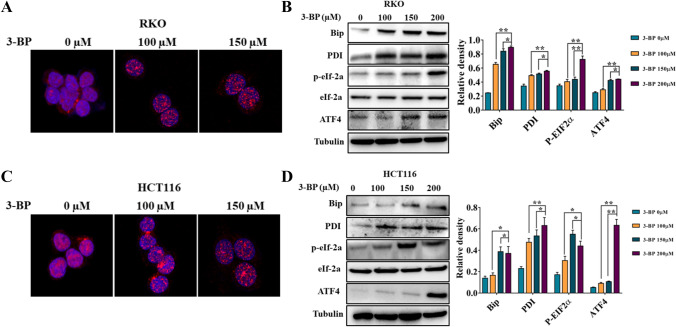
Studies have shown that endoplasmic reticulum stress plays an important role in anti-tumor drug-induced apoptosis, but the role of endoplasmic reticulum stress in HK2 inhibitor–induced apoptosis is still unclear (Lu et al. 2020; Wang et al. 2014). We first detected the distribution of PDI in colon cells treated by 3-BP. The results showed that 3-BP changed the distribution of PDI protein in RKO and HCT116 cells (Fig. 4A, C). Western Blotting further detected endoplasmic reticulum stress-related proteins; the results showed that 3-BP significantly increased the expression of Bip, PDI, p-eIf-2a, and ATF4 (Fig. 4B, D). These results suggested that 3-BP can induce endoplasmic reticulum stress in colon cancer. In order to find out the reason of endoplasmic reticulum stress induced by 3-BP, the cytosolic calcium concentration was detected by the calcium ion concentration detection kit. The results showed that 3-BP could cause the increase of cytoplasmic calcium concentration (Fig. 5). These results suggest that 3-BP can induce endoplasmic reticulum stress in colon cancer cells by increasing the concentration of cytosolic calcium.
Fig. 4.
3-BP–induced endoplasmic reticulum stress in colon cancer cells. A, C Immunofluorescence analysis examined the distribution of PDI protein treatment with 3-BP in RKO and HCT116. B, D Western blotting detected the expression of Bip, PDI, ATF4, eIf-2a, and p-eIf-2a of RKO and HCT116 induced by 3-BP (*p < 0.05, **p < 0.01 vs control group)
Fig. 5.
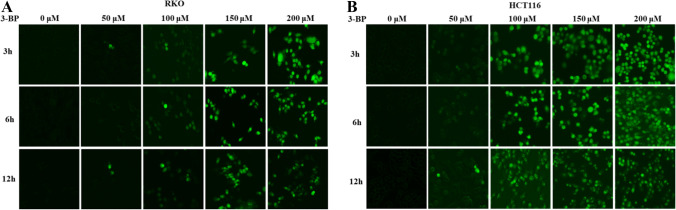
3-BP increased the cytoplasmic calcium concentration of colon cancer cells. A, B Fluorescence microscope detected the cytoplasmic calcium concentration of RKO and HCT116 induced by 3-BP
Inhibition of endoplasmic reticulum stress increased 3-BP–induced cell death in colon cancer cells
We selected two endoplasmic reticulum stress inhibitors Sodium 4-phenylbutyrate (4-PBA) and sodium taurousodeoxycholate (TUDCA) to elucidate the role of endoplasmic reticulum stress in 3-BP–induced colon cancer cell death. The results showed that the combination of ER stress inhibitors further increased 3-BP–induced RKO and HCT116 cell death compared with 3-BP alone (Fig. 6A). Apoptosis detection showed that the combination could also further increase the mitochondria-associated apoptosis induced by 3-BP. These results showed that targeted endoplasmic reticulum stress could increase the cytotoxicity of colon cancer induced by 3-BP (Fig. 6B–E).
Fig. 6.
Inhibition of endoplasmic reticulum stress increased 3-BP–induced cell death in colon cancer cells. A The cell viability was measured of RKO and HCT116 treated by 3-BP with or without endoplasmic reticulum stress inhibitor 4-PBA and TUDCA for 24 h. B, C Flow cytometry detected the apoptotic rate of RKO and HCT116 treated by 3-BP with or without 4-PBA and TUDCA for 12 h. D, E Western blotting detected the expression of cleaved caspase-3 of RKO and HCT116 treated by 3-BP with or without 4-PBA and TUDCA for 24 h (*p < 0.05 vs control group, #p < 0.05 vs 3-BP group)
3-BP and combination with TUDCA suppressed colon cancer growth in nude mice
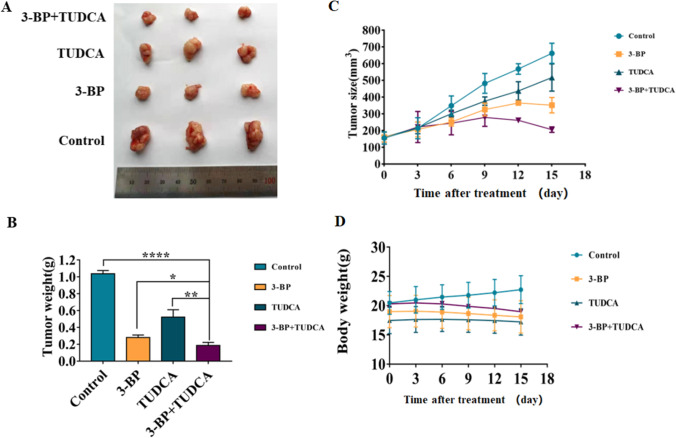
In order to determine the effect of 3-BP and 3-BP combined with endoplasmic reticulum stress inhibitor on the growth of colon cancer cells in vivo, we established a colon cancer xenograft tumor model using HCT116 cells in BALB/C nude mice. As shown in Fig. 7, 3-BP inhibited the growth of colon cancer in vivo, and 3-BP combined with TUDCA further reduced tumor tissue volume and weight without significant effect on animal weight. The results were consistent with the cell experiment in vitro, suggesting that the combined drug has a good inhibitory effect.
Fig. 7.
3-BP and combination with TUDCA suppressed colon cancer growth in nude mice. A HCT116 xenografts were divided into 4 groups, control, 3-BP (15 mg/kg), TUDCA (3 mg/kg), and 3-BP + TUDCA. On day 15 post first injection, xenograft tissues were removed and photographed. B The weight of tumor tissue in each group was measured. C, D In the course of drug action, tumor volume and animal weight were measured (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
Aerobic glycolysis is one of the most important characteristics of tumor cells that distinguish them from normal cells. Various enzymes hexokinases (HKs), phosphofructokinase (PFK), and pyruvate kinase (PK) are involved in the regulation of aerobic glycolysis in tumor cells (Kobliakov 2019). Among these enzymes, hexokinase showed abnormal expression in a variety of malignant tumors, and hexokinase targeting has become a new target for cancer therapy. Five hexokinases have been identified in human cells HK1, HK2, HK3, HK4, and HKDC1, of which HK2 has been studied extensively and deeply (Garcia et al. 2019). In this study, we first examined the expression of HK2 in normal colon tissues, colon tissues, and colon tissues with lymph node metastasis. We found that the expression of HK2 was higher in colon tissues and highest in colorectal tissues with lymph node metastasis. In addition, high HK2 expression was associated with poor prognosis, regardless of lymph node metastasis. At the same time, we found through statistical analysis that the expression of HK2 is also correlated with the clinicopathological classification of colorectal cancer. The results suggest that targeting HK2 may effectively control the occurrence and development of colon cancer. However, whether HK2 inhibitor can actually inhibit the proliferation of colon cancer cells or induce apoptosis is unclear.
3-Bromopyruvic acid (3-BP) is an ATP–depleting molecule in normal cells, mainly by inhibiting hexokinase II (Sadowska-Bartosz and Bartosz 2013). It has been reported that 3-BP as an anticancer agent can inhibit the growth of tumor cells (Dai et al. 2019; Chen et al. 2018). However, the effect of 3-BP on the biological behavior of colon cancer cells is still unclear. Our results show that different concentrations of 3-BP can significantly reduce the survival rate and proliferation rate of two colon cancer cells. The results of apoptosis experiments show that 3-BP can significantly increase the apoptotic rate of colon cancer cells and the expression of apoptosis-related proteins. We know that the mitochondrial apoptosis signaling pathway occupies the main position in the process of apoptosis. By examining the mitochondrial membrane potential and mitochondrial apoptosis-related molecular proteins, we found that 3-BP can obviously activate the mitochondrial apoptosis signaling pathway. However, it is not clear whether other signal pathways participate in or prevent this process.
The endoplasmic reticulum is a very important organelle in eukaryotic cells (Mollinedo and Gajate 2021). It plays an important role in protein transportation and folding, lipid biosynthesis, and maintaining the necessary calcium ion concentration in the cell (Bettigole and Glimcher 2015). The existence of the endoplasmic reticulum is critical to maintaining cell stability. Studies have shown that when eukaryotic cells are stimulated by a variety of external factors and internal signaling pathways, the endoplasmic reticulum function can be abnormal, which is mainly manifested as an increase in the number of unfolded and misfolded proteins (Cubillos-Ruiz et al. 2017; Lee et al. 2018). To alleviate this phenomenon, the endoplasmic reticulum induces a stress response, a process we call the unfolded protein response (Salaroglio et al. 2017). In recent years, with the widespread use of anti-tumor drugs and the deepening of research, it has been discovered that there is a very close relationship between anti-tumor agents and endoplasmic reticulum stress (Zhou et al. 2019; Cheng et al. 2018). It may be that drugs have caused intracellular balance disorders and abnormal signaling pathways. However, the effect of HK2 inhibitor 3-BP on endoplasmic reticulum stress has not been reported.
The results of immunofluorescence and western blotting showed that 3-BP can significantly change the distribution of PDI and increase the expression of endoplasmic reticulum stress-related proteins. It suggests that 3-BP can induce endoplasmic reticulum stress in colon cancer cells. Further testing found that 3-BP can increase the cytoplasmic calcium ion concentration of colon cancer cells. Interestingly, this phenomenon occurred before the increase in the expression levels of the endoplasmic reticulum stress-related pathway proteins, suggesting that 3-BP may induce the occurrence of endoplasmic reticulum stress by affecting the calcium ion concentration in the endoplasmic reticulum.
Studies have shown that the impact of endoplasmic reticulum stress on cell fate is bidirectional (Urra et al. 2016). Under the stimulation of related factors, endoplasmic reticulum stress can not only alleviate the cytotoxicity caused by stimulation, but also induce cell death through the endoplasmic reticulum stress apoptosis signaling pathway (Johnson et al. 2018; Chen et al. 2017). Based on the detection of endoplasmic reticulum stress-related pathway molecules, we detected the expression of endoplasmic reticulum apoptosis-related proteins CHOP and cleaved caspase-4 (not shown), and found that 3-BP did not increase the expression level of related proteins, suggesting that reticulum stress may play a protective role in the death of colon cancer cells caused by 3-BP. To clarify this problem, we selected two endoplasmic reticulum stress inhibitors 4-PBA and TUDCA in combination with 3-BP for colon cancer cells, and found that the combination with endoplasmic reticulum stress inhibitors can significantly increase the decrease in cell survival rate and the increase in apoptosis induced by 3-BP. In order to further determine the effect of this combination, we constructed a model of colon cancer in nude mice. In vivo experimental results also showed that the combination can further inhibit the growth of colon cancer cells.
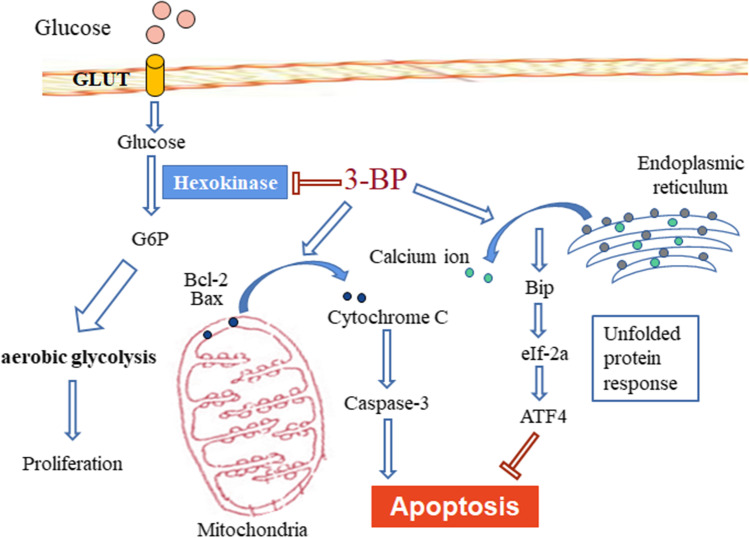
This study clarified that the HK2 inhibitor 3-BP inhibited cell proliferation by reducing the glycolysis level of colon cancer cells, and induced apoptosis through the activation of the mitochondrial apoptosis signaling pathway. At the same time, in colon cancer cells, 3-BP also induces the occurrence of endoplasmic reticulum stress by regulating the concentration of calcium ions in the endoplasmic reticulum, and this process is a reverse effect on the apoptosis induced by 3-BP (Fig. 8). This study provides a theoretical and experimental basis for clarifying the mechanism of 3-BP, and also provides a new direction for the clinical targeting of HK2 in the treatment of colon cancer and the proposal of new combination drug strategies.
Fig. 8.
Schematic diagram demonstrating the potential mechanism of 3-BP induction of ER-stress and apoptosis of mitochondrial pathway in colon cancer cells. 3-BP can inhibit the proliferation of colon cancer cells by inhibiting the activity of HK2 kinase. In addition, 3-BP can induce apoptosis and death in colon cancer cells by activating mitochondrial apoptosis signal pathway. At the same time, 3-BP can induce endoplasmic reticulum stress by affecting the concentration of endoplasmic reticulum calcium, and the occurrence of endoplasmic reticulum stress alleviates the apoptosis induced by 3-BP. Combined with endoplasmic reticulum stress inhibitor can further increase the apoptosis of colon cancer cells induced by 3-BP
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81702891 and U1804173), Zhongyuan Qianren Jihua of Henan Province (No. ZYQR201810153), Henan province young and middle-aged health science and technology innovation talent project (No. YXKC2021044), Joint construction project of Henan Medical Science and technology research plan (No. LHGJ20190452), Natural Science Foundation of Henan Province (No. 202300410326), Science and Technology Program foundation of Henan Province, China (No. 172102310651), Key Projects of 2018 Plan for Scientific Research in Colleges and Universities of Henan Province (No. 18A310022), and Teaching and Research Cultivation Project of School of Basic Medical Sciences, Xinxiang Medical College (No. JCYXYKY202021).
Author contribution
Na Li and Wei Su designed the experiments and wrote the manuscript. JTZ, SYL, XLJ, QL, LL, PX,GDW, MML, WJG, TSZ, QQW, and WS performed the experiments. Shuya Lu and Jiateng zhong contributed equally to this work. All the authors reviewed the results and approved the final version of the manuscript.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiateng Zhong and Shuya Lu contributed equally to this work
Contributor Information
Jiateng Zhong, Email: jtzhong@xxmu.edu.cn.
Wei Su, Email: hnswei@163.com.
Na Li, Email: Lina@xxmu.edu.cn.
References
- Bahar E, Kim JY, Yoon H. Chemotherapy resistance explained through endoplasmic reticulum stress-dependent signaling. Cancers (basel) 2019;11:338. doi: 10.3390/cancers11030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107–138. doi: 10.1146/annurev-immunol-032414-112116. [DOI] [PubMed] [Google Scholar]
- Cheng X, Feng HR, Wu HX, et al. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018;431:105–114. doi: 10.1016/j.canlet.2018.05.046. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wei L, Zhang X, et al. 3-Bromopyruvate sensitizes human breast cancer cells to TRAIL-induced apoptosis via the phosphorylated AMPK-mediated upregulation of DR5. Oncol Rep. 2018;40:2435–2444. doi: 10.3892/or.2018.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZK, Wu QJ, Ding Y. YD277 Suppresses triple-negative breast cancer partially through activating the endoplasmic reticulum stress pathway. Theranostics. 2017;7:2339–2349. doi: 10.7150/thno.17555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern YJ, Wong JCT, Cheng GSW, et al. The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer. Cell Death Dis. 2019;100:504. doi: 10.1038/s41419-019-1687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Radin DP, Leonardi D. PINK1 depletion sensitizes non-small cell lung cancer to glycolytic inhibitor 3-bromopyruvate: involvement of ROS and mitophagy. Pharmacol Rep. 2019;71:1184–11890. doi: 10.1016/j.pharep.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53:667–682. doi: 10.1080/10409238.2018.1556578. [DOI] [PubMed] [Google Scholar]
- Garcia SN, Guedes RC, Marques MM. Unlocking the potential of HK2 in cancer metabolism and therapeutics. Curr Med Chem. 2019;26:7285–7322. doi: 10.2174/0929867326666181213092652. [DOI] [PubMed] [Google Scholar]
- Johnson CE, Dunlop EA, Seifan S, et al. Loss of tuberous sclerosis complex 2 sensitizes tumors to nelfinavir-bortezomib therapy to intensify endoplasmic reticulum stress-induced cell death. Oncogene. 2018;37:5913–5925. doi: 10.1038/s41388-018-0381-2. [DOI] [PubMed] [Google Scholar]
- Kania E, Pająk B, Orzechowski A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed Res Int. 2019;2015:352794. doi: 10.1155/2015/352794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158:368–388. doi: 10.1053/j.gastro.2019.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobliakov VA. The mechanisms of regulation of aerobic glycolysis (Warburg Effect) by oncoproteins in carcinogenesis. Biochemistry (mosc) 2019;84:1117–2112. doi: 10.1134/S0006297919100018. [DOI] [PubMed] [Google Scholar]
- Ko YH, Niedźwiecka K, Casal M, Pedersen PL, Ułaszewski S. 3-Bromopyruvate as a potent anticancer therapy in honor and memory of the late Professor André Goffeau. Yeast. 2019;36:211–221. doi: 10.1002/yea.3367. [DOI] [PubMed] [Google Scholar]
- Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res. 2018;16:1073–1076. doi: 10.1158/1541-7786.MCR-18-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Khoonkari M, Avril T, Chevet E, Kruyt FAE. The unfolded protein response as regulator of cancer stemness and differentiation: mechanisms and implications for cancer therapy. Biochem Pharmacol. 2021;192:114737. doi: 10.1016/j.bcp.2021.114737. [DOI] [PubMed] [Google Scholar]
- Lu G, Luo H, Zhu X. Targeting the GRP78 pathway for cancer therapy. Front Med (lausanne) 2020;7:351. doi: 10.3389/fmed.2020.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Ma L, Dong L, Chang P. CD44v6 engages in colorectal cancer progression. Cell Death Dis. 2019;10:30. doi: 10.1038/s41419-018-1265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zong X. Metabolic symbiosis in chemoresistance: refocusing the role of aerobic glycolysis. Front Oncol. 2020;10:5. doi: 10.3389/fonc.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C. Direct endoplasmic reticulum targeting by the selective alkylphospholipid analog and antitumor ether lipid edelfosine as a therapeutic approach in pancreatic cancer. Cancers (basel) 2021;13:4173. doi: 10.3390/cancers13164173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P, Barsouk A, Hadjinicolaou AV, Barsouk A. Immunotherapies and targeted therapies in the treatment of metastatic colorectal cancer. Med Sci (basel) 2019;7:83. doi: 10.3390/medsci7080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfeld BI, Rathmell WK, Kim TK, Rathmell JC. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell Mol Immunol. 2022;19:46–58. doi: 10.1038/s41423-021-00727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska-Bartosz I, Bartosz G. Effect of 3-bromopyruvic acid on human erythrocyte antioxidant defense system. Cell Biol Int. 2013;37:1285–1290. doi: 10.1002/cbin.10160. [DOI] [PubMed] [Google Scholar]
- Salaroglio IC, Panada E, Moiso E, et al. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol Cancer. 2017;16:91. doi: 10.1186/s12943-017-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Siwecka N, Rozpędek W, Pytel D, et al. Dual role of endoplasmic reticulum stress-mediated unfolded protein response signaling pathway in carcinogenesis. Int J Mol Sci. 2019;20:4354. doi: 10.3390/ijms20184354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh ST, Lunt SY. Metabolism in cancer metastasis: bioenergetics, biosynthesis, and beyond. Wiley Interdiscip Rev Syst Biol Med. 2018;10:10. doi: 10.1002/wsbm.1406. [DOI] [PubMed] [Google Scholar]
- Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer. 2016;2:252–262. doi: 10.1016/j.trecan.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WA, Groenendyk J, Michalak M. Endoplasmic reticulum stress associated responses in cancer. Biochim Biophys Acta. 2014;1843:2143–2149. doi: 10.1016/j.bbamcr.2014.01.012. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Xu SW, Wang W, Piao SZ, Mao XL, Zhou XB, Wang Y, Wu WD, Ye LP, Li SW. Identification and validation of a novel DNA damage and DNA repair related genes based signature for colon cancer prognosis. Front Genet. 2021;12:635863. doi: 10.3389/fgene.2021.635863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Lu QQ, Liu JT, et al. Melatonin increases the sensitivity of hepatocellular carcinoma to sorafenib through the PERK-ATF4-Beclin1 pathway. Int J Biol Sci. 2019;15:1905–1920. doi: 10.7150/ijbs.32550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.