Abstract
Opioid addiction is a worldwide problem accentuated in the USA and European countries by the COVID-19 pandemic. The nucleus accumbens (NAc) plays an outstanding neurobiological role in opioid addiction as a part of the striatum and key component of brain reward system. The striatal GABAergic medium spiny projection neurons (MSNs) are the main neuronal type in the NAc where addiction-specific synaptic plasticity occurs. The activity of ribosomal DNA (rDNA) transcription is crucial for neural plasticity and molecular studies suggest its increase in the NAc of heroin addicts. Silver-stained argyrophilic nucleolar organizer region (AgNOR) areas visualised in neuronal nuclei in paraffin-embedded brain sections are reliable morphological estimators of rDNA transcription and thus surrogate markers for the activity of brain regions. Our study revealed increased AgNOR areas in MSNs of the left NAc in 11 heroin addicts versus 11 healthy controls from the Magdeburg Brain Bank (U-test P = 0.007). No differences were observed in another investigated part of the striatum, namely the head of caudate nucleus, which is located closely to the NAc. The results were not confounded by significant differences in the age, brain volume and time of formalin fixation existing between compared groups. Our findings suggest an increased NAc activity in heroin addicts, which is consistent with human and animal experimental data.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-022-01423-7.
Keywords: Heroin addiction, Nucleus accumbens, Medium spiny neurons, AgNOR staining
Introduction
Opioid addiction has become a nationwide epidemic within the United States of America, where the prevalence of fatal opioid overdose has steadily risen since late 1990s [1] and this rise has been notably exaggerated during the COVID-19 pandemic [2]. Besides illicitly manufactured fentanyl, whose role has been recently highlighted in the analysis of opioid fatalities, heroin remains constantly the leading substance in lethal opioid intoxications [1]. Similar phenomena are also observed in European countries [3].
The nucleus accumbens (NAc) is a key component of brain reward system and drug-induced long-term changes in neurotransmission in the NAc constitute neurobiological substrate of behavioural disturbances observed in opioid addiction (for reviews see: [4, 5]). Although reports on the NAc in human opioid addicts are scanty compared with numerous studies in animal models, both neuroimaging (for reviews see: [6, 7]) and postmortem research [8–10] suggest NAc abnormalities in heroin-dependent individuals.
GABAergic medium spiny projection neurons (MSNs) comprise a majority of all neurons in the NAc. They receive coordinated input from glutamatergic afferents (originating from prefrontal limbic areas, among others) and dopaminergic brain stem nuclei [4]. This convergent neurotransmitter release promotes heterosynaptic plasticity, which is a common feature of addiction associated with abnormal gene expression (for reviews, see: [11, 12]). The activity of ribosomal DNA (rDNA) transcription is crucial for neural plasticity [13, 14] and molecular studies suggest an increased expression of genes involved in the upregulation of this key cellular process in the NAc of heroin addicts [9, 10].
Therefore, in our current study, we hypothesized an increased rDNA transcription in MSNs of the NAc in heroin-dependent individuals who died from heroin overdose. We tested this hypothesis by the application of argyrophilic nucleolar organizer region (AgNOR) silver staining in paraffin-embedded brain sections. According to our previous studies of prefrontal cortex in suicide victims, silver-stained AgNOR areas visualised in neuronal nuclei are reliable morphological estimators of rDNA transcription in brain regions [15, 16]. Therefore, they are also surrogate markers for chronic changes in the activity of brain structures as the rRNA synthesis is fundamental for the function of their cellular components. For the evaluation whether the effect is specific for the NAc we investigated also the neighboring part (i.e. the head) of the caudate nucleus (Cd) involved in the limbic-motor interface function of the striatum [17].
Materials and methods
Characteristics of the subjects
All brains were obtained from the Magdeburg Brain Bank. Sampling and preservation of the human brain material were done in accordance with the Declaration of Helsinki, German law and the local institutional review board at the University of Magdeburg. The analysis included 11 male chronic heroin addicts who died suddenly from heroin overdose and 11 male control cases of sudden natural death from cardiopulmonary arrest (the detailed diagnostic and demographic data of investigated cases are present in the Supplementary Table). Information on clinical characteristics was extracted from the available clinical records and by structured interviews with people closely related to heroin-dependent individuals. According to the available information, the tested heroin overdose victims exclusively met the diagnostic criteria for heroin dependence, even though they occasionally used other substances (see Supplementary Table). An experienced neuropathologist ruled out qualitative neuropathological changes due to neurodegenerative disorders (such as Alzheimer disease, Parkinson disease, Pick disease), tumors, inflammatory, vascular or traumatic processes, using sections with Nissl-myelin staining and HLA-DR-, beta-amyloid-, and tau-immunostainings. None of heroin addicts was HIV-positive. A toxicological analysis of blood and urine for heroin, ethanol, and other substances of abuse was performed at each medico-legal autopsy as a part of forensic postmortem diagnostics. The cause of death in all heroin addicts was established by an experienced forensic pathologist (KT).
Tissue collection and preparation
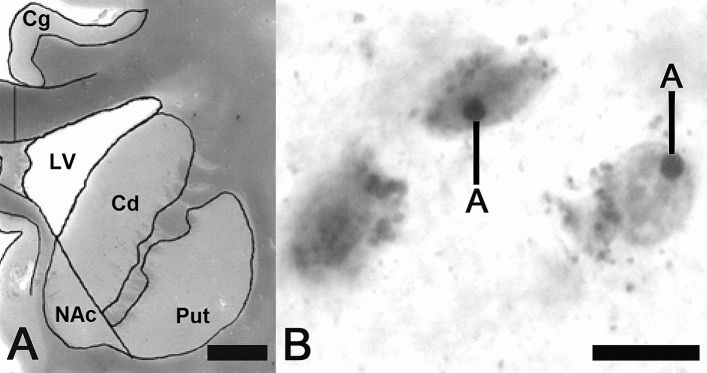
After embedding in paraffin, serial 20 µm thick transverse sections were cut along the rostrocaudal axis of the hemispheres and then mounted. Each 25th section was deparaffinised, rehydrated and stained with a combined cell and fiber staining according to Nissl (cresyl violet) and Heidenhain-Woelcke (myelin). Coronal stained sections of the brains were taken at the level where both the NAc and the head of Cd were visible (Fig. 1 A) according to established neuroanatomical criteria as presented previously in detail [8]. From Nissl-myelin stained sections, two were randomly selected and sections adjacent to them were stained for AgNOR. Consequently, two sections at the level of the NAc and the head of the Cd were used for the evaluation of AgNOR area in MSNs in these regions of interest (ROIs) bilaterally in each of the investigated cases.
Fig. 1.
A. Low magnification picture of the fragment from Nissl-myelin-stained coronal section of the left brain hemisphere of control case at the level where the investigations were carried out (NAc nucleus accumbens, Cd the head of caudate nucleus, Put putamen, Cg cingulate cortex, LV lateral ventricle, scale bar 5 mm).B Only AgNOR areas with clearly visible borders (A) were measured in the nuclei of AgNOR-stained medium spiny neurons (the neuron on the left with poorly visible AgNOR area was excluded from the evaluation); synaptic spines were present as numerous smaller black dots (scale bar 10 μm)
AgNOR staining
Silver staining was carried out as previously described [15]. Briefly, 20 µm paraffin sections were dewaxed and rehydrated through graded alcohols. The silver stain was freshly prepared by mixing 2% gelatin (dissolved in 1% aqueous formic acid) with 50% aqueous silver nitrate solution at a 1:2 ratio. The sections were incubated with this mixture in a dark moist chamber at room temperature for 45 min and subsequently washed with deionised water. Following this protocol, the AgNOR area—containing AgNORs (that are clustered, undistinguishable from each other) and representing the nucleolus—appears as clearly delineated one large black or dark brown spot in the nuclei of MSNs. Synaptic spines are also present at MSNs somata [18] and they are visible in the AgNOR staining as small dark spots surrounding medium-sized cell body (12–20 μm in diameter) (Fig. 1 B). Therefore, the most abundant neuronal population is clearly different from other neuronal types in the NAc and Cd, i.e. large and small aspiny interneurons. Compared with MSNs, they have different shapes and dimensions of cell bodies, which are devoid of somatic spines [18]. Glial cells were distinguished from neurons according to established cytomorphological criteria [19].
Quantification
In each of 4 investigated ROIs, i.e. the NAc and the Cd analysed bilaterally, AgNOR areas were determined in 100 medium spiny neurons with clearly visible AgNOR areas. The AgNOR area was proven to be the most reliable AgNOR parameter in the postmortem estimation of neuronal rDNA transcription according to our previous research [15, 16]. The neurons were selected throughout both AgNOR-stained sections available in analysed cases. The number of investigated neurons was established in accordance with diagnostic and research studies employing the AgNOR method. This method does not require an estimation of the number of cells and/or nuclei [19]. The neurons were sampled using a 400 × magnification. The AgNOR areas (composed of clustered AgNORs and representing the nucleoli) were determined using a light microscope attached to a computer image analysis system (cellSens®, Olympus, Japan). In this system, each of the neurons sampled by 400 × magnification was visualised digitally on the screen, focused, and the sharpest and longest profiles of AgNOR areas were delineated automatically by applying the circle function available in the system. As a result, the numerical values of AgNOR areas (by one large AgNOR area in each neuron) were calculated automatically. This procedure was performed separately for each of the sampled neurons. The sampled measures were averaged to derive a single set of values for each ROI in each of investigated cases (i.e. 4 mean values in each case).
Data analysis
Statistical analyses were performed with the data analysis software system STATISTICA version 10 (StatSoft®, Inc. 2011, www.statsoft.com). As normal distribution was not given for the analysed AgNOR area (i.e. significant values of Lilliefors tests were obtained), non-parametric statistical procedures were used in hierarchical mode.
First, the generalized linear/nonlinear models (GLZ) module of STATISTICA with the general custom designs (GCD) procedure was applied as an omnibus method to analyse associations between dependent variable (i.e. AgNOR area) and independent categorical variables (i.e. heroin addiction/control groups, analysed brain region, and side). The results of GCD analysis were reported automatically including the Wald statistic value, degrees of freedom, and the respective P value. Age, postmortem interval, brain volume and fixation time were considered as numerical confounding variables. Spearman correlation coefficients were calculated to determine the impact of these variables which might confound AgNOR area.
Subsequently to GCD analysis, unadjusted two-way post hoc comparisons with Mann–Whitney U test were used to detect possible differences between the study groups with respect to the variables mentioned above (i.e. AgNOR area and confounders). All statistical tests were two-tailed. Generally, P values of < 0.05 were accepted as statistically significant.
Results
Qualitative analysis of the AgNOR staining
After AgNOR staining of the NAc and Cd medium spiny neurons, borders of largest AgNOR areas (containing AgNORs that were clustered together and indistinguishable from each other) were visible (Fig. 1 B) in line with staining patterns in other neuronal populations presented in our previous research [15, 20].
Quantitative analysis of the AgNOR staining
The differences in the AgNOR area between compared groups were beyond qualitative evaluation and they could only be captured using quantitative measurements.
Cumulative analyses of results from all 4 investigated ROIs (i.e. from the NAc and Cd evaluated bilaterally, 44 mean values from heroin addicts and 44 mean control values) by GCD procedure revealed regional differences in AgNOR area (i.e. the NAc vs. Cd; Wald statistic = 9.698, df = 1, P = 0.0018) and the association of diagnosis (i.e. heroin addicts vs. controls) with brain hemisphere (Wald statistic = 4.042, df = 1, P = 0.044). In accordance with initial GCD analysis, further ROI-specific analyses by post hoc U tests revealed significant difference in the parameter related to the investigated brain region and hemisphere, i.e. an increase of the median AgNOR area in heroin addicts in the left NAc (see Table 1 and Supplementary Table).
Table 1.
Presentation of between-group comparisons regarding the evaluation of rDNA transcriptional activity in nucleus accumbens (NAc) and caudate nucleus (Cd) medium spiny neurons by the AgNOR staining
| ROI and group | AgNOR area in µm2 per nucleus Median (q1, q3, n) | U test P H/C |
|---|---|---|
| NAc right | 0.332 | |
| H | 4.015 (3.704, 4.206, 11) | |
| C | 4.230 (3.417, 4.709, 11) | |
| NAc left | 0.007 | |
| H | 4.387 (4.174, 4.788, 11) | |
| C | 3.712 (3.467, 4.211, 11) | |
| Cd right | 0.949 | |
| H | 4.585 (3.828, 5.617, 11) | |
| C | 4.645 (4.148, 5.281, 11) | |
| Cd left | 0.652 | |
| H | 4.421 (4.021, 5.092, 11) | |
| C | 4.458 (3.971, 4.769, 11) | |
ROI region of interest, H heroin addicts, C controls, q1 and q3 quartile 1 and 3, n number of cases, U-test P U-test P value (significant value is in bold)
Confounders
Age, brain volume and fixation time revealed significant differences between heroin addicts and controls (significant U test P values, see Table 2 and Supplementary Table). However, correlations analysis did not suggest that the significant increase in the AgNOR area in the left NAc of heroin addicts was driven by these confounders (see Table 2).
Table 2.
Summarised confounding variables of heroin addicts (n = 11) and control subjects (n = 11)
| A Intergroup comparisons | ||||
|---|---|---|---|---|
| BV [cm3] | Age [years] | PMI [hours] | Fixation [days] | |
| Heroin addicts: median (q, q3) | 1475 (1427, 1543) | 31 (25, 33) | 30 (16, 49) | 2922 (2185, 3815) |
| Controls: median (q1, q3) | 1354 (1263, 1398) | 46 (39, 54) | 24 (24, 44) | 240 (179, 330) |
| Statistics: | ||||
| Test | U | U | U | U |
| Characteristic value | Z = − 3.021 | Z = 2.922 | Z = 0.263 | Z = − 3.697 |
| P value | 0.0014 | 0.0019 | 0.797 | 0.00002 |
| B Correlation analysis between numerical confounding variables listed above and AgNOR area in nucleus accumbens (NAc) and caudate nucleus (Cd) medium spiny neurons | |||||
|---|---|---|---|---|---|
| ROI | Group | BV | Age | PMI | Fixation |
| NAc right | H r/P | − 0.30/0.37 | − 0.03/0.93 | 0.30/0.37 | − 0.53/0.12 |
| C r/P | − 0.36/0.28 | − 0.22/0.51 | 0.47/0.15 | 0.63/0.04 | |
| NAc left | H r/P | 0.16/0.64 | − 0.03/0.93 | − 0.02/0.96 | 0.04/0.91 |
| C r/P | 0.39/0.24 | − 0.37/0.26 | 0.39/0.24 | 0.61/0.05 | |
| Cd right | H r/P | − 0.31/0.36 | − 0.23/0.50 | − 0.39/0.23 | − 0.50/0.14 |
| C r/P | − 0.23/0.49 | − 0.21/0.55 | 0.36/0.27 | 0.24/0.48 | |
| Cd left | H r/P | − 0.35/0.29 | − 0.38/0.26 | − 0.41/0.21 | − 0.50/0.14 |
| C r/P | − 0.54/0.08 | − 0.14/0.68 | 0.67/0.03 | 0.51/0.11 | |
q1 and q3 quartile 1 and 3, BV brain volume, PMI postmortem interval, Fixation fixation time, ROI region of interest, H heroin addicts, C controls, r correlation coefficient and P P value of the Spearman’s correlation (significant values are in bold)
Discussion
Our study suggests an increased rDNA transcription in medium spiny neurons of the left NAc in heroin-dependent individuals, which seems to be not confounded by significant differences in the age, brain volume and fixation time existing between compared groups. This effect was specific for the NAc as we found no differences in another investigated part of the striatum, i.e. the head of Cd, despite the presumed involvement of the whole striatopallidal system in both emotional and behavioural functions [17] profoundly disturbed in addiction. Therefore, our results suggest a rather selective dysfunction of striatal components in heroin addiction.
The weighted gene co-expression network analysis of the NAc in heroin addicts highlights the significance of hub genes, which play a role in the regulation of rDNA transcription [10]. A transcriptome analysis of the NAc in heroin-dependent individuals revealed an increased gene expression of nuclear receptor coactivators, which are involved in many ways in the activation of this key cellular process [9]. Moreover, that study showed increased gene expression of glutamatergic receptors important for synaptic plasticity, which was a consequence of the epigenetic effect related to long-term heroin use. Other epigenetic studies provide further evidence that opioids acting via histone acetylation in the striatum of heroin addicts permit higher transcription of genes relevant for increased synaptic plasticity (for recent reviews see: [11, 12]), which is closely related to the augmented rDNA synthesis [13, 14].
However, our morphological results concerning the estimation of rDNA transcriptional activity in MNSs of striatal components in heroin-dependent individuals should be interpreted with caution. The silver staining of AgNOR areas in interphase cells is related predominantly to the multifunctional protein nucleolin (important for the rRNA synthesis) but not directly to the rRNA amount [21, 22]. The amount of nucleolin may not directly correlate with the level of rDNA transcription, because numerous factors are involved in the regulation of this crucial cellular process [23]. Nevertheless, we have recently observed in prefrontal regions of suicide victims that the decreased rDNA transcription indicated by AgNOR staining in pyramidal neurons was paralleled by the reduced rRNA synthesis evaluated by reverse transcription and quantitative polymerase chain reaction in bulk prefrontal tissue [15, 16]. The results obtained from prefrontal samples suggest that the increased AgNOR area in MSNs in the left NAc may be paralleled by the augmented rDNA transcription in this brain region. Molecular addiction research points out numerous factors which may play a role in the increased rRNA synthesis in the NAc. A key node in the intracellular regulation of rDNA transcription is the mechanistic target to rapamycin (mTOR) [24], which is upregulated in the NAc in addiction [25]. Addiction research uncovered abnormalities of different molecular components contributing to the upregulated function of mTOR [26], whose inhibition by rapamycin may add to the treatment of heroin addicts [27].
NAc activity increase in heroin-dependent individuals was also suggested by functional neuroimaging (for reviews, see: [6, 28]), where the signal mainly reflects the glutamatergic input and its local processing [29, 30]. According to the experimental data from animal models, the increased drug-evoked glutamate release from prefrontal afferents in the NAc is necessary for heroin craving [31]. Complementary to the results of both human functional neuroimaging and animal experiments, our findings suggest an increased activity of the left NAc output neurons. This effect plays hypothetically an important role in opiate-associated behavioural modifications observed in heroin addiction.
Previous postmortem study by Magdeburg group revealed a bilaterally decreased NAc volume in heroin-dependent individuals [8]. However, in structural magnetic resonance imaging study, a volume reduction was found in the left NAc [32], where also our current results suggest the abnormality specific for heroin addiction. The lateralization of NAc disorders may be considered as an addiction-specific effect proven by meta-analyses of neuroimaging data, but the exact neurobiological mechanism and significance of this phenomenon are not yet clear [7, 28]. On the other hand, however, the leftward increase in AgNOR area observed in present study may be explained by the small cohort size.
Limitations
Our study is limited by several factors: (1) Like each of postmortem analyses, our study is cross-sectional and no longitudinal data are available. (2) The cohort is small and we could only include brains of males because our brain bank does not contain postmortem brains of female heroin addicts. (3) Due to the limited clinical records, there are no reliable data on the duration of addiction nor on the consumed amount of heroin. Therefore, we cannot resolve the question whether these factors influence our results. (4) The application of paraffin-embedded tissue is a limitation of our method compared to frozen brain samples, which would allow the application of a wider set of approaches. (5) With our method, we cannot distinguish between the core and the shell regions of the NAc, which have different functional properties in accordance with their anatomical connectivity and role in reward-related responses. Moreover, we cannot distinguish between MSNs populations expressing dopamine D1- or D2 receptors, which display different physiological characteristics, exhibit different forms of drug-induced plasticity, and generally exert antagonistic effects in drug-associated behaviors [4, 5].
Conclusion
In summary, our results suggest an increased activity of rDNA transcription in MSNs in the NAc specific for the left hemisphere in male heroin addicts, which is consistent with human and animal experimental data. However, further research on larger cohorts with an involvement of molecular techniques aimed at clearly delineated regional and cellular targets within the NAc is important to substantiate these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Magdeburg Brain Collection and subsequently this investigation was supported in part by the Stanley Medical Research Foundation (Grant No. 07R-1832), the Saxony-Anhalt Ministry of Research (XN3594O/0405M, N2-OVGU), the German Ministry of Research (“BrainNet”, BMBF NBL-3/2 and 01ZZ0407) and the Alfried-Krupp-von-Bohlen-und-Halbach Foundation. We are grateful to Prof. C. Mawrin for his neuropathological expertise and to S. Funke for her excellent technical assistance.
Funding
The Magdeburg Brain Collection and subsequently this investigation was supported in part by the Stanley Medical Research Foundation (Grant No. 07R-1832), the Saxony-Anhalt Ministry of Research (XN3594O/0405 M, N2-OVGU), the German Ministry of Research („BrainNet “, BMBF NBL-3/2 and 01ZZ0407) and the Alfried-Krupp-von-Bohlen-und-Halbach Foundation.
Availability of data and materials
On behalf of all authors, the corresponding author states that the data being reported are accurate and are coming from the official source.
Code availability
Not applicable.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study has been approved by the local ethics committee of the University of Magdeburg as performed in accordance with the ethical standards laid down in the Declaration of Helsinki of 1989.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- 1.Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021;218:108350. doi: 10.1016/j.drugalcdep.2020.108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad FB, Rossen LM, Sutton P (2021) Provisional drug overdose death counts. National Center for Health Statistics. Available from: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 3.di Gaudio F, Mortali C, Tini A. Opioid epidemic spread from northern and eastern Europe to Mediterranean area. Clin Ter. 2021;172:209–210. doi: 10.7417/CT.2021.2315. [DOI] [PubMed] [Google Scholar]
- 4.Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68:816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hearing M, Graziane N, Dong Y, Thomas MJ. Opioid and psychostimulant plasticity: targeting overlap in nucleus accumbens glutamate signaling. Trends Pharmacol Sci. 2018;39:276–294. doi: 10.1016/j.tips.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fareed A, Kim J, Ketchen B, Kwak WJ, Wang D, Shongo-Hiango H, Drexler K. Effect of heroin use on changes of brain functions as measured by functional magnetic resonance imaging, a systematic review. J Addict Dis. 2017;36:105–116. doi: 10.1080/10550887.2017.1280898. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Ottino-Gonzalez J, Cupertino RB, Schwab N, Hoke C, Catherine O, Cousijn J, Dagher A, Foxe JJ, Goudriaan AE, Hester R, Hutchison K, Li CR, London ED, Lorenzetti V, Luijten M, Martin-Santos R, Momenan R, Paulus MP, Schmaal L, Sinha R, Sjoerds Z, Solowij N, Stein DJ, Stein EA, Uhlmann A, van Holst RJ, Veltman DJ, Wiers RW, Yücel M, Zhang S, Jahanshad N, Thompson PM, Conrod P, Mackey S, Garavan H. Mapping cortical and subcortical asymmetries in substance dependence: findings from the ENIGMA addiction working group. Addict Biol. 2021;26:13010. doi: 10.1111/adb.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller UJ, Truebner K, Schiltz K, Kuhn J, Mawrin C, Dobrowolny H, Bernstein HG, Bogerts B, Steiner J. Postmortem volumetric analysis of the nucleus accumbens in male heroin addicts: implications for deep brain stimulation. Eur Arch Psychiatry Clin Neurosci. 2015;265:647–653. doi: 10.1007/s00406-015-0617-x. [DOI] [PubMed] [Google Scholar]
- 9.Egervari G, Landry J, Callens J, Fullard JF, Roussos P, Keller E, Hurd YL. Striatal H3K27 acetylation linked to glutamatergic gene dysregulation in human heroin abusers holds promise as therapeutic target. Biol Psychiatry. 2017;81:585–594. doi: 10.1016/j.biopsych.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi X, Li Y, Yan P, Shi Y, Lai J. Weighted gene co-expression network analysis to explore the mechanism of heroin addiction in human nucleus accumbens. J Cell Biochem. 2020;121:1870–1879. doi: 10.1002/jcb.29422. [DOI] [PubMed] [Google Scholar]
- 11.Browne CJ, Godino A, Salery M, Nestler EJ. Epigenetic Mechanisms of opioid addiction. Biol Psychiatry. 2020;87:22–33. doi: 10.1016/j.biopsych.2019.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson BL, Oscar-Berman M, Kaplan GB. Opioid-induced structural and functional plasticity of medium-spiny neurons in the nucleus accumbens. Neurosci Biobehav Rev. 2021;120:417–430. doi: 10.1016/j.neubiorev.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes C, Smith SC, Youssef MN, Zheng JJ, Hagg T, Hetman M. RNA polymerase 1-driven transcription as a mediator of BDNF-induced neurite outgrowth. J Biol Chem. 2011;286:4357–4363. doi: 10.1074/jbc.M110.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slomnicki LP, Pietrzak M, Vashishta A, Jones J, Lynch N, Elliot S, Poulos E, Malicote D, Morris BE, Hallgren J, Hetman M. Requirement of neuronal ribosome synthesis for growth and maintenance of the dendritic tree. J Biol Chem. 2016;291:5721–5739. doi: 10.1074/jbc.M115.682161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krzyżanowska M, Steiner J, Pieśniak D, Karnecki K, Kaliszan M, Wiergowski M, Rębała K, Brisch R, Braun K, Jankowski Z, Kosmowska M, Chociej J, Gos T. Ribosomal DNA transcription in prefrontal pyramidal neurons is decreased in suicide. Eur Arch Psychiatry Clin Neurosci. 2020;270:859–867. doi: 10.1007/s00406-019-00996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzyżanowska M, Rębała K, Steiner J, Kaliszan M, Pieśniak D, Karnecki K, Wiergowski M, Brisch R, Braun K, Jankowski Z, Kosmowska M, Chociej J, Gos T. Reduced ribosomal DNA transcription in the prefrontal cortex of suicide victims: consistence of new molecular RT-qPCR findings with previous morphometric data from AgNOR-stained pyramidal neurons. Eur Arch Psychiatry Clin Neurosci. 2021;271:567–576. doi: 10.1007/s00406-021-01232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Braak E. Neuronal types in the striatum of man. Cell Tissue Res. 1982;227:319–342. doi: 10.1007/BF00210889. [DOI] [PubMed] [Google Scholar]
- 19.Polak M, Haymaker W, Johnson JE, D’Amelio F. Neuroglia and their reactions. In: Haymaker W, Adams RD, editors. Histology and Histopathology of Nervous System. Charles C: Thomas Publishing, Springfield; 1982. pp. 363–480. [Google Scholar]
- 20.Gos T, Krell D, Brisch R, Bielau H, Trübner K, Steiner J, Bernstein HG, Bogerts B. Demonstration of decreased activity of dorsal raphe nucleus neurons in depressed suicidal patients by the AgNOR staining method. J Affect Disord. 2008;111:251–260. doi: 10.1016/j.jad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Roussel P, Hernandez-Verdun D. Identification of Ag-NOR proteins, markers of proliferation related to ribosomal gene activity. Exp Cell Res. 1994;214:465–472. doi: 10.1006/excr.1994.1283. [DOI] [PubMed] [Google Scholar]
- 22.Jia W, Yao Z, Zhao J, Guan Q, Gao L. New perspectives of physiological and pathological functions of nucleolin (NCL) Life Sci. 2017;186:1–10. doi: 10.1016/j.lfs.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Neumüller RA, Gross T, Samsonova AA, Vinayagam A, Buckner M, Founk K, Hu Y, Sharifpoor S, Rosebrock AP, Andrews B, Winston F, Perrimon N. Conserved regulators of nucleolar size revealed by global phenotypic analyses. Sci Signal. 2013;6:70. doi: 10.1126/scisignal.2004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384–6391. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- 25.James MH, Quinn RK, Ong LK, Levi EM, Charnley JL, Smith DW, Dickson PW, Dayas CV. mTORC1 inhibition in the nucleus accumbens 'protects' against the expression of drug seeking and 'relapse' and is associated with reductions in GluA1 AMPAR and CAMKIIα levels. Neuropsychopharmacology. 2014;39:1694–1702. doi: 10.1038/npp.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem. 2014;130:172–184. doi: 10.1111/jnc.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR, Lu L. Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol. 2009;615:108–112. doi: 10.1016/j.ejphar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Gordon HW. Differential activation of the left and right cerebral hemispheres of individuals who use or are dependent on drugs of abuse. J Drug Abuse. 2018;4:10. doi: 10.21767/2471-853X.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 30.Sokoloff L. The physiological and biochemical bases of functional brain imaging. Cogn Neurodyn. 2008;2:1–5. doi: 10.1007/s11571-007-9033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifert CL, Magon S, Sprenger T, Lang UE, Huber CG, Denier N, Vogel M, Schmidt A, Radue EW, Borgwardt S, Walter M. Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci. 2015;265:637–645. doi: 10.1007/s00406-014-0564-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
On behalf of all authors, the corresponding author states that the data being reported are accurate and are coming from the official source.
Not applicable.