Abstract
Objective:
Greater depressive symptoms are associated with worse cognitive functions in Parkinson’s disease (PD); however, it is unclear what underlying factors drive this association. Apathy commonly develops in PD and may be a pathway through which depressive symptoms negatively influence cognition. Prior research examining depressive symptoms, apathy, and cognition in PD is limited by being predominantly cross-sectional. This study examined the role of apathy as a within- and between-person mediator for the longitudinal relationships between depression severity and cognitive functioning in patients with early PD.
Methods:
Participants included 487 individuals newly diagnosed with PD followed annually for up to 5 years by the Parkinson’s Progression Marker Initiative. At each visit, participants completed depressive symptom measures, apathy ratings, and cognitive tests. Multi-level structural equation models examined both the within- and between-person effects of depressive symptoms on cognition through apathy, controlling for demographics and motor severity.
Results:
At the within-person level, apathy mediated the association between depressive symptoms and select cognitive functions (global cognition, attention/working memory, visuospatial functions, and immediate verbal memory; indirect effects, bootstrap p’s < 0.05). Significant between-person direct effects were found for depressive symptoms predicting apathy (boostrap p < 0.001) and lower scores on most cognitive tests (bootstrap p’s < 0.05). However, the indirect effects did not reach significance, suggesting between-person mediation did not occur.
Conclusions:
Findings suggest worsening of depressive symptoms over time in patients with PD may be a risk factor for increased apathy and subsequent decline in specific cognitive functions.
Keywords: Apathy, Cognitive Functioning, Depression, Mediation, Parkinson’s disease, Structural Equation Modeling
Objective
Parkinson’s disease (PD) is a progressive neurodegenerative disorder resulting from the loss of dopamine neurons in the substantia nigra pars compacta1. While PD is best characterized by its motor symptoms, non-motor features (including psychiatric symptoms and cognitive dysfunction) are also prominent due to disruption of shared cortico-striatal-thalamo-cortical pathways2. Changes in mood (i.e., depression) may be one of the earlier symptoms heralding progression of PD3 and later risk of developing apathy4. Apathy, defined as a disturbance in motivation leading to reduced goal-directed behavior, typically presents slightly later in the disease course relative to other mood symptoms and tends to become more prominent as the disease progresses4 (but also see Martinez-Horta et al. [2013]5 and Funkiewiez et al. [2004]6, who show apathy in early and advanced PD, respectively, not being associated with depressive symptoms or cognitive decline).
PD is typically characterized by a pattern of executive dysfunction on neuropsychological testing7, though there is heterogeneity across affected cognitive domains and in rate of progression. Depression and apathy can independently occur in PD, but their overlap with one another and cognitive dysfunction is common8–10. Several cross-sectional studies show that depressive symptoms and apathy have unique relationships with cognition in PD, such that apathy is related to worse cognitive efficiency and executive functions, while depression is negatively associated with delayed recall11–13. When both depressive symptoms and apathy are present, they predict worse cognitive performance relative to either syndrome alone14. With respect to longitudinal findings, depression severity, but not apathy, is related to cognitive decline in PD15,16. Despite studies investigating the independent and combined effects of these mood symptoms on cognition, there is limited research investigating the underlying mechanisms of these relationships in PD.
In non-PD older adults, depression is associated with poor executive functions and greater executive dysfunction is reported in the context of prominent apathy17. These results parallel findings in PD and it is hypothesized that age- and depression-related changes to dopamine receptor functioning and dopamine depletion affecting fronto-striatal networks may drive these relationships18. In an attempt to better understand potential mechanisms underlying behavioral influences to depression-cognition relationships, Funes and colleagues19 investigated whether apathy mediated the association between depression severity and cognition in older adults with depression. They found that apathy significantly mediated cognitive control performance, but not delayed recall. Although cross-sectional, this study highlights that the presence of apathy may be an important underlying factor in these relationships, particularly for frontally-mediated cognitive functions and perhaps indicative of greater fronto-subcortical structural and functional changes17.
While these cross-sectional and longitudinal studies show that depressive symptoms and apathy are linked to worse cognitive functions in PD and that apathy mediates the relationship between depressive symptoms and select cognitive functions in non-PD older adults, it is unclear whether apathy mediates the relationship between depressive symptoms and cognition over time in PD. As mood symptoms in PD may occur before cognitive dysfunction3 and apathy tends to develop with disease progression4, we examined both within- and between-person associations between depressive symptoms, apathy, and cognitive functions using a multilevel structural equation model approach in a de novo PD sample followed for up to 5 years. Specifically, we hypothesized that apathy would mediate relationships between depressive symptoms and attention/working memory, processing speed, and immediate verbal memory (and not other cognitive functions) at the within-person and between-person levels, given shared fronto-subcortical neural circuitry. Our previous work has shown that depressive symptoms predict future cognitive decline in de novo PD, but not vice versa15, supporting the directionality of our models (but also see Petkus et al., [2019]20 for alternative findings).
Methods
Participants
Data were obtained from the Parkinson’s Progression Markers Initiative (PPMI; www.ppmi-info.org/data), which is a longitudinal, multi-site observational clinical study across the United States and Europe of newly diagnosed (two years or less), untreated PD adults. The current sample included 487 de novo PD adults who were followed annually (at 1-year intervals) for up to 5 years (baseline, 1st, 2nd, 3rd, 4th, and 5th visits). Briefly, participants completed motor severity and apathy ratings, self-report measures, and cognitive tests at each visit. More comprehensive details of the study have been published elsewhere21. The Institutional Review Board at each site approved the study (in accordance with the Declaration of Helsinki) and participants provided informed consent prior to any study procedures. The parent study is registered at ClinicalTrials.gov (NCT01141023).
Measures
Depressive Symptoms
Depressive symptoms were assessed via the 15-item Geriatric Depression Scale (GDS-15)22, a self-report questionnaire whereby participants rate the presence of depressive symptoms over the previous week in a yes/no format. Total scores range from 0 to 15, with a score of 5 or more indicating clinically significant depressive symptoms. This cut-off has been validated in individuals with PD23 and is recommended for use to screen depressive symptoms in PD due to minimal overlap of individual items with motor symptoms24.
Apathy
A single-item (item 5) from the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)25 was used to assess apathy severity. The MDS-UPDRS is a physician-rated scale, with scores ranging from 0 to 4 on the apathy item (higher scores equating to greater symptoms). While other studies have used the 3-item “apathy” subscale within the GDS-15 to assess apathy severity in older adults26,27 and in advanced PD28, we previously found that such items do not adequately assess apathy in de novo PD29. More specifically, these three items did not load on to their own “apathy” factor in the PPMI sample, but rather loaded on to two separate factors: one (‘dropped activities and interests’) with other items reflecting “dysphoria” and the other two (‘prefers to stay at home’ and ‘low energy’) into their own factor. As such, the assessment of apathy in the current study was limited to the MDS-UPDRS apathy item, which we have used in previous studies15 and is considered an appropriate apathy screening measure in PD by the Movement Disorder Society30.
Cognition
Participants underwent annual neuropsychological testing. The following cognitive domains were assessed21: attention/working memory (Letter Number Sequencing [LNS]), processing speed (Symbol Digit Modalities Test [SDMT]), category fluency (Animals), visuospatial functioning (Judgment of Line Orientation [JOLO]), immediate verbal memory (Trials 1–3 on Hopkins Verbal Learning Test-Revised [HVLT-R]), and delayed verbal memory (delayed recall on HVLT-R). Raw values for each measure were used in these analyses. A global cognition composite was also calculated by averaging the normative/demographically adjusted z-scores for the above-mentioned cognitive tests for each participant at each year.
Statistical Analyses
Given the nested structure of the data (time points within individuals), multi-level structural equation models (MSEM) were conducted using AMOS31 following the guidelines provided by Preacher et al.32. MSEM allows time-varying variables to be decomposed at both the within- and between-person levels, countering conflation of the within- and between-person variance. This allows for separate evaluation of longitudinal/linear changes over time (i.e., intraindividual variability; within-person effects) and differences between individual averages (i.e., individual differences; between-person effects). See Supplemental Figure 1 for depiction. Specifically, analyses estimated the indirect effects of depressive symptoms on individual cognitive scores (i.e., attention/working memory, processing speed, category fluency, etc.) mediated by apathy, as well as the direct effects of depressive symptoms on individual cognitive scores. A separate model was conducted for each cognitive score, resulting in a total of seven models. For all models, the cognitive score was entered as the dependent variable, depressive symptoms were entered as the predictor, and apathy as the mediator. Age at baseline, education, gender, and motor severity (as measured by the MDS-UPDRS – Part III, where higher scores reflect greater motor severity) were also included in the models as covariates, with only motor severity considered as a time-varying covariate. For the global cognition model, as global cognition was calculated from demographically-corrected neuropsychological scores, demographics (i.e., age, education, and gender) were not included in the model. Given that all participants in PPMI are newly diagnosed, disease duration was not included as a covariate (as previous studies have reported average disease duration in the baseline PPMI sample to be 6.7 months33).
As recommended by Hayes34 and Preacher et al.32, indirect effects were calculated through the computation of the products of a * b (a = coefficient estimate of the relationship between the independent and mediating variables, b = the coefficient estimate of the association between the mediating and outcome variables). As some of the intercepts and slopes of the variables were not normally distributed (i.e., skewed), 2,000 iterations of bootstrapped samples35–37 was implemented to obtain more stable and valid standard errors of the indirect effects. Bootstrapping uses a bias-corrected percentile method to calculate the 95% confidence intervals (resulting in an asymmetric confidence interval from the 2.5% and 97.5% quantiles of the distribution). Effect sizes were determined via the standardized coefficients, with values > 0.6 considered as large effects38.
Results
Participant Characteristics
Demographics and clinical characteristics at baseline are presented in Table 1. Briefly, the sample had a mean age of 61.07 years (range 33 to 84 years) and some college education, with majority of the participants being male and Caucasian. On average, motor symptoms were mild and depressive and apathy symptoms were minimal at baseline.
Table 1.
Baseline characteristics (n = 487).
| Mean | SD | Range | |
|---|---|---|---|
| Age | 61.07 | 9.70 | 33 – 84 |
| Education | 15.48 | 3.10 | 5 – 26 |
| % Male | 65.10 | -- | -- |
| % Caucasian | 95.00 | -- | -- |
| UPDRS-III Motor | 20.03 | 9.20 | 2 – 51 |
| GDS-15 | 2.46 | 2.60 | 0 – 14 |
| Apathy | 0.46 | 0.60 | 0 – 3 |
| Global Cognition | 0.01 | 0.60 | −1.8 – 1.4 |
| LNS | 10.49 | 2.67 | 2 – 20 |
| SDMT | 41.19 | 10.02 | 7 – 82 |
| Animal Fluency | 20.75 | 5.46 | 8 – 41 |
| JOLO | 12.77 | 2.16 | 5 – 15 |
| HVLT-R Immediate | 24.42 | 4.90 | 9 – 36 |
| HVLT-R Delay | 8.34 | 2.55 | 0 – 12 |
Notes. Raw values are depicted, with the exception of the Global Cognition composite z-score. UPDRS = Unified Parkinson’s Disease Rating Scale; GDS-15 = 15-item Geriatric Depression Scale; LNS = Letter-Number Sequencing; SDMT = Symbol Digit Modalities Test; JOLO = Judgment of Line Orientation; HVLT-R = Hopkins Verbal Learning Test – Revised.
Multi-level Structural Equation Models
Standardized coefficients for within- and between-person mediation pathways are depicted in Figures 1–4 for statistically significant cognitive variables. Supplemental Table 1 includes values for the other (non-significant) cognitive variables.
Figure 1.
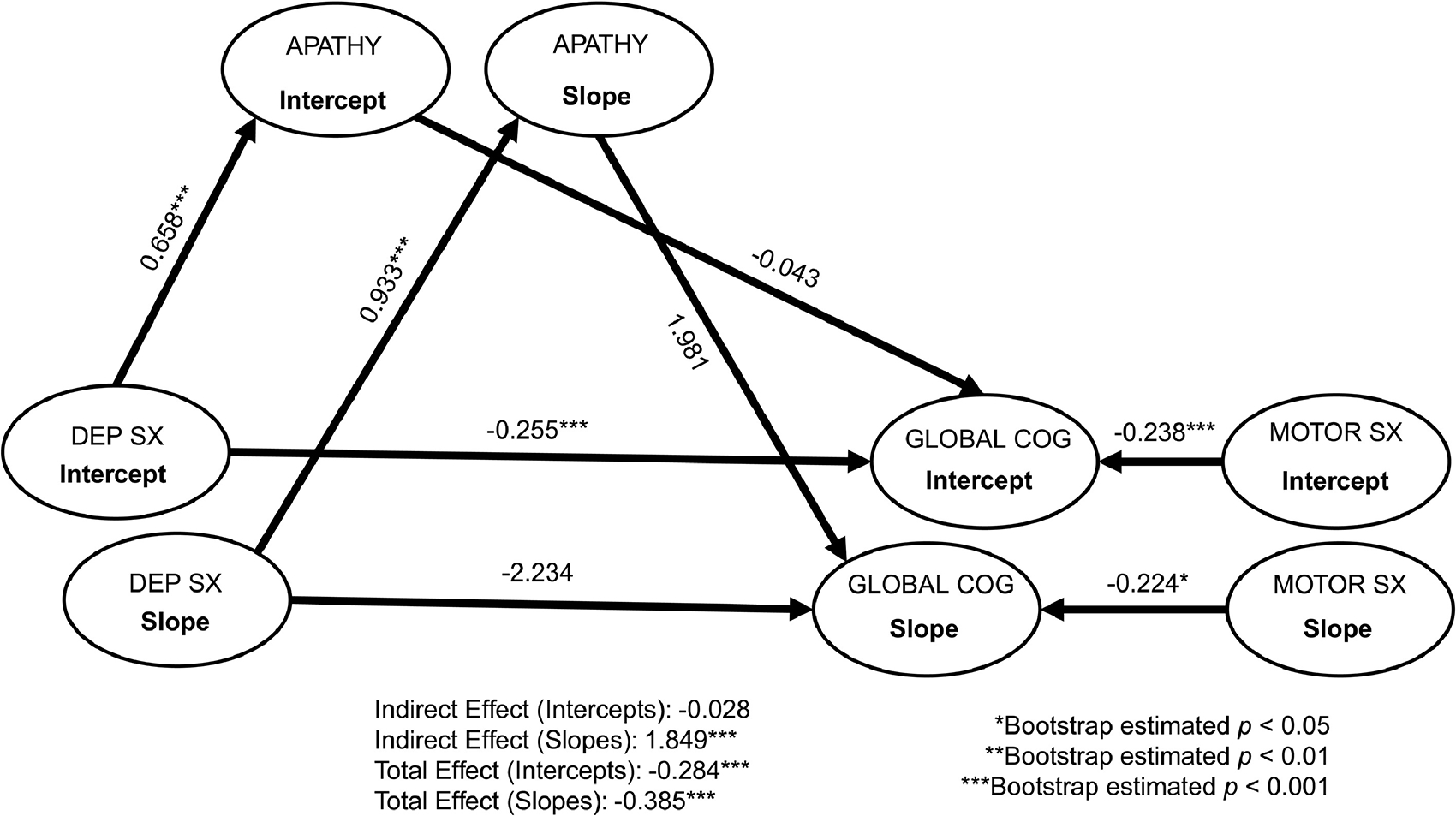
Multi-level structural equation mediation models of depressive symptoms on global cognition through apathy. Standardized coefficients are reported. Dep Sx = depressive symptoms; Global Cog = global cognition (assessed via normed composite of cognitive measures); Motor Sx = Motor symptoms. Model fit: chi-square = 739.53, df = 264, p < 0.001; Root Mean Square Error of Approximation [RMSEA] = 0.61; Comparative Fit Index [CFI] = 0.95; Normed Fit Index [NFI] = 0.92.
Figure 4.
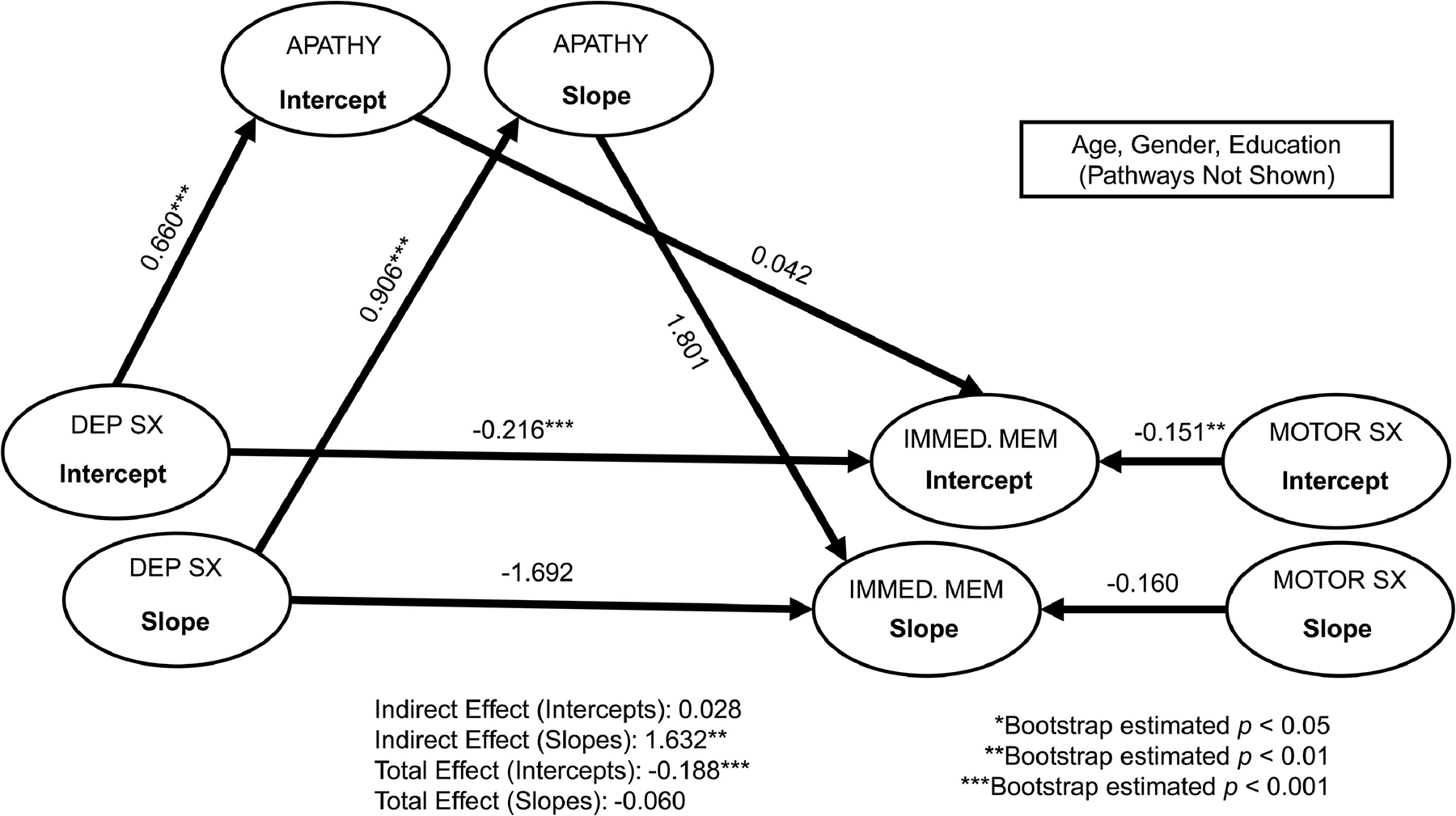
Multi-level structural equation mediation models of depressive symptoms on immediate verbal memory through apathy. Standardized coefficients are reported. For simplicity, baseline age, gender, and education covariates are not depicted. Dep Sx = depressive symptoms; Immed. Mem = immediate verbal memory (assessed via total correct on trials 1–3 of the Hopkins Verbal Learning Test – Revised); Motor Sx = Motor symptoms. Model fit: chi-square = 834.60, df = 333, p < 0.001; Root Mean Square Error of Approximation [RMSEA] = 0.56; Comparative Fit Index [CFI] = 0.93; Normed Fit Index [NFI] = 0.89.
Within-Person Effects.
As shown in Figures 1–4, after accounting for covariates, apathy significantly mediated the relationship between depressive symptoms and global cognition (standardized estimate = 1.85, bootstrap SE = 1.80, bootstrap p = 0.001), attention/working memory (standardized estimate = 1.34, bootstrap SE = 1.86, bootstrap p = 0.002), visuospatial functions (standardized estimate = 0.67, bootstrap SE = 1.28, bootstrap p < 0.05), and immediate verbal memory (standardized estimate = 1.63, bootstrap SE = 2.06, bootstrap p = 0.003) at the within-person level (indirect effects), all with large to very large effect sizes (ranging from 0.67 to 1.85). Those who experienced higher depressive symptoms also experienced more severe apathy, which was associated with lower scores in select areas of cognition. The within-person direct effect between depressive symptoms and these cognitive functions did not reach statistical significance, suggesting apathy mediated these associations. For the other cognitive functions (i.e., processing speed, category fluency, and delayed verbal memory), the within-person indirect effects for apathy as a mediator between depressive symptoms and these tasks were not statistically significant (Supplemental Table 1). Overall, the within-person direct effect between depressive symptoms and apathy was significant, as were the main effects of motor severity for certain cognitive functions. As motor severity worsened, lower scores were evident on global cognition and tasks of attention/working memory and processing speed.
Between-Person Effects.
Apathy did not mediate the relationship between depressive symptoms and any cognitive functions at the between-person level (indirect effects; Figures 1–4 and Supplemental Table 1). On average, depressive symptoms were associated with higher apathy and lower scores across most cognitive tasks (except visuospatial functions and animal fluency). Apathy was not associated with any cognitive functions. Significant effects of age, education, gender, and motor severity were found. Older age was associated with lower scores on attention/working memory, visuospatial functions, and delayed verbal memory. Higher education was associated with better cognitive functions across all measures, with the exception of animal fluency (direction of the relationship was reversed). Females had lower scores on most cognitive tasks, with the exception of visuospatial functions (significantly better than males). Higher motor severity was associated with lower cognitive scores (with the exception of delayed verbal memory).
Conclusions
The current study revealed that apathy mediated within-person, longitudinal associations between depressive symptoms and multiple aspects of cognition (global cognition, attention/working memory, visuospatial functions, and immediate verbal recall) over a 5-year period among adults with newly diagnosed PD, with large to very large effect sizes. Apathy did not mediate these associations at a between-person level, nor did apathy mediate within-person associations between depressive symptoms and processing speed, category fluency, or delayed verbal recall. This study is the first to investigate apathy as a mediating factor between depressive symptoms and cognition over time in de novo PD and has implications for mood and cognitive intervention in patients with PD.
There is high comorbidity between non-motor neuropsychiatric symptoms in PD8. It has been difficult to disentangle the unique effects of depression and apathy on cognition in PD, in part due to previous studies investigating either depression or apathy alone. There are few longitudinal studies investigating these constructs simultaneously in PD and findings generally show stronger relationships between depressive symptoms (and not apathy) with cognitive functions15,16. In lagged models, Jones et al.15 found evidence of a unidirectional relationship (i.e., changes in depressive symptoms preceding changes in cognition). Our findings add to this literature, suggesting that changes in apathy at each occasion explains the within-person relationship between depressive symptoms and select cognitive functions. Some of the largest effects in this study involved attention/working memory and immediate verbal recall, which were consistent with our hypothesis, and may reflect the shared fronto-subcortical neural circuitry between depressive symptoms, apathy, and these cognitive functions.
Interestingly, our within-person results demonstrated a significant relationship (and large effect) with visuospatial functions. While PD is primarily thought to involve fronto-subcortical processes (influencing both mood/apathy and cognition), a dual syndrome hypothesis has been proposed39, whereby the frontal lobe is related to executive functions (including working memory) and the posterior brain is related to visuospatial and memory functions. This delineation seems to map on to progression of cognitive symptoms in PD, with anterior deficits related to mild cognitive impairment (PD-MCI) and posterior deficits related to dementia (PDD) and presumed cholinergic involvement39. It may be that a subset of individuals in the current study will go on to develop dementia, given the current findings; however, this remains to be seen. It is important to highlight that the visuospatial measure used in the current study was the JOLO, which shows relationships with both prefrontal and parietal-occipital brain activation40,41. In this context, difficulties on this task may actually be representative of attention or executive dysfunction and not visuospatial functions, per se. More research on this measure and hypothesis, as well as relationships with other non-motor symptoms in PD, is needed.
At the between-person level, the indirect effect of depressive symptoms on cognition through apathy was non-existent across all of the cognitive tasks. We did, however, find significant depressive symptom and apathy relationships (on average) and greater depressive symptoms were associated with worse scores across most cognitive tasks, on average (except for visuospatial functions and category fluency). Apathy was not associated with any cognitive tasks, nor was it a significant mediator. These findings are consistent with other research showing significant depression – cognition relationships in PD13. However, the non-significant apathy – cognition findings are in contrast with literature showing significant relationships between apathy and cognitive efficiency and executive functions11,12. While a cross-sectional study in non-PD older adults did find that apathy mediated the relationship between depression and cognitive control19, the current study did not show analogous findings. This may be reflective of our sample, which includes a relatively young, newly diagnosed PD cohort with fairly low baseline levels of depressive symptoms and apathy as a whole. Moreover, O’Laughlin and colleagues42 demonstrated that cross-sectional and longitudinal mediation models may lead to different estimates, as well as interpretation, particularly for processes thought to unfold over time (such as neuropsychiatric symptoms in the context of a neurodegenerative disorder). This may be particularly relevant for the current study, given that apathy tends to become more prominent with disease progression4. These findings may also be related to the apathy measure used in the current study (i.e., single apathy item rather than use of an apathy scale) and/or limited executive function tasks in the current cognitive battery. While speculative, it may also be the case that the between-person terms (average scores) reflect longstanding apathy/depression/cognitive inefficiencies that pre-date the PD diagnosis and the within-person terms (longitudinal changes) may better reflect changes in apathy/depression/cognition secondary to the “new” PD pathology.
The present study has clinical significance at the individual level, whereby the increase in apathy explains the relationship between depressive symptoms and select cognitive functions. Given that apathy serves as a within-person mediator, early inventions aimed at reducing apathy and/or increasing behavioral activation and goal-setting may help to reduce the negative association between depressive symptoms and cognitive dysfunction. In a recent review by Goldman et al.43, exercise, cognitive and nutritional interventions, and caregiver support were all proposed as possible non-pharmacological treatments for cognitive decline in PD. These interventions may also reduce depressive symptoms and apathy, and thereby influence cognition. Other interventions aimed at targeting the cognitive control network (e.g., neuroplasticity-informed computerized cognitive remediation) shown to benefit non-PD adults with depression44 and non-invasive neuromodulation focusing on depressive and apathy symptoms45 may prove beneficial in this population.
While outside the scope of the current study, the neural mechanisms underlying mood and cognitive symptoms in PD are complex, but may overlap. Dujardin and Sgambato46 recently completed an in-depth review on the role of dopamine and non-dopamine systems in neuropsychiatric symptoms in PD. Depression in PD is likely due to multiple factors (e.g., genetic, inflammatory), including the loss of dopamine in mesocortical and mesolimbic pathways, along with noradrenaline and serotonin dysfunction, Apathy, on the other hand, seems to be the result of mesolimbic dopamine loss, though there is also evidence to suggest the role of serotonin in apathy in early PD. Cholinergic denervation may serve as a possible mechanism underlying both depression and apathy’s relationship with cognitive decline. Moreover, there appears to be separate neural signatures for depression and apathy in PD47, with depression predicting increased and apathy predicting decreased functional connectivity between frontal, subcortical, and temporal regions. There also appears to be a role for white matter architecture influencing depression – apathy – cognition relationships in PD48, which may contribute to the different motor and non-motor phenotypes.
Although the current study sheds light on a potential mediator of the relationship between depressive symptoms and cognition in PD, there are some limitations worth mentioning. The current sample is quite homogenous with respect to ethnicity and, to a lesser extent, gender. Thus, the generalizability of these findings to other ethnicities is limited. Future investigations may examine the mediational effects of apathy in more ethnically representative samples, as well as the effect of gender, as prior research in non-PD depressed older adults19 found depression-apathy-cognition relationships to be particularly salient in women. Further, formal diagnosis of depression was not made in the current study and thus results cannot be extrapolated to PD patients with psychiatric diagnosis of depression. Antidepressant use was purposefully not included in our models, as those with greater depressive symptoms are more likely to be on antidepressants, therefore removing much of the variance we were attempting to explain. Moreover, there is evidence to suggest that neither antidepressant use nor placebo are associated with improving or worsening of cognitive functions in PD over 8–24 weeks49, thus supporting our rationale for not controlling for antidepressant use, though this may be explored in future studies using the PPMI sample. Additionally, the apathy measure used in the current study came from a single item on the MDS-UPDRS and other studies have used specific apathy measures with a greater range of scores (i.e., Apathy Scale, Apathy Evaluation Scale), which may have influenced our current findings. Analyses were also limited in scope with respect to the neuropsychological measures, acknowledging that there were single to few measures to assess specific cognitive domains and the absence of certain components of executive functioning (e.g., task-switching, inhibition, problem-solving). More comprehensive cognitive assessment, including both neuropsychological measures and experimental paradigms, may improve model fits and the use of more experimental designs will increase the ability to draw conclusions about casual relationships. Finally, the current study did not investigate apathy as a transitional state between depressive symptoms and cognitive functioning, given the short interval between assessments, and future research using cross-lagged models with longer durations between assessments (e.g., baseline, year 5, and year 10) may be beneficial.
In conclusion, our findings extend previous work by examining an indirect path of depressive symptoms to cognitive functioning through apathy. Depression-cognition relationships are complex and the current study delineated one possible pathway influencing these associations at the within-person level, but not at the between-person level (i.e., cross-sectionally). Results aim to inform development of clinical interventions for improving cognition in adults with PD, in the context of their heightened risk of developing depressive symptoms and apathy along their disease trajectory.
Supplementary Material
Figure 2.
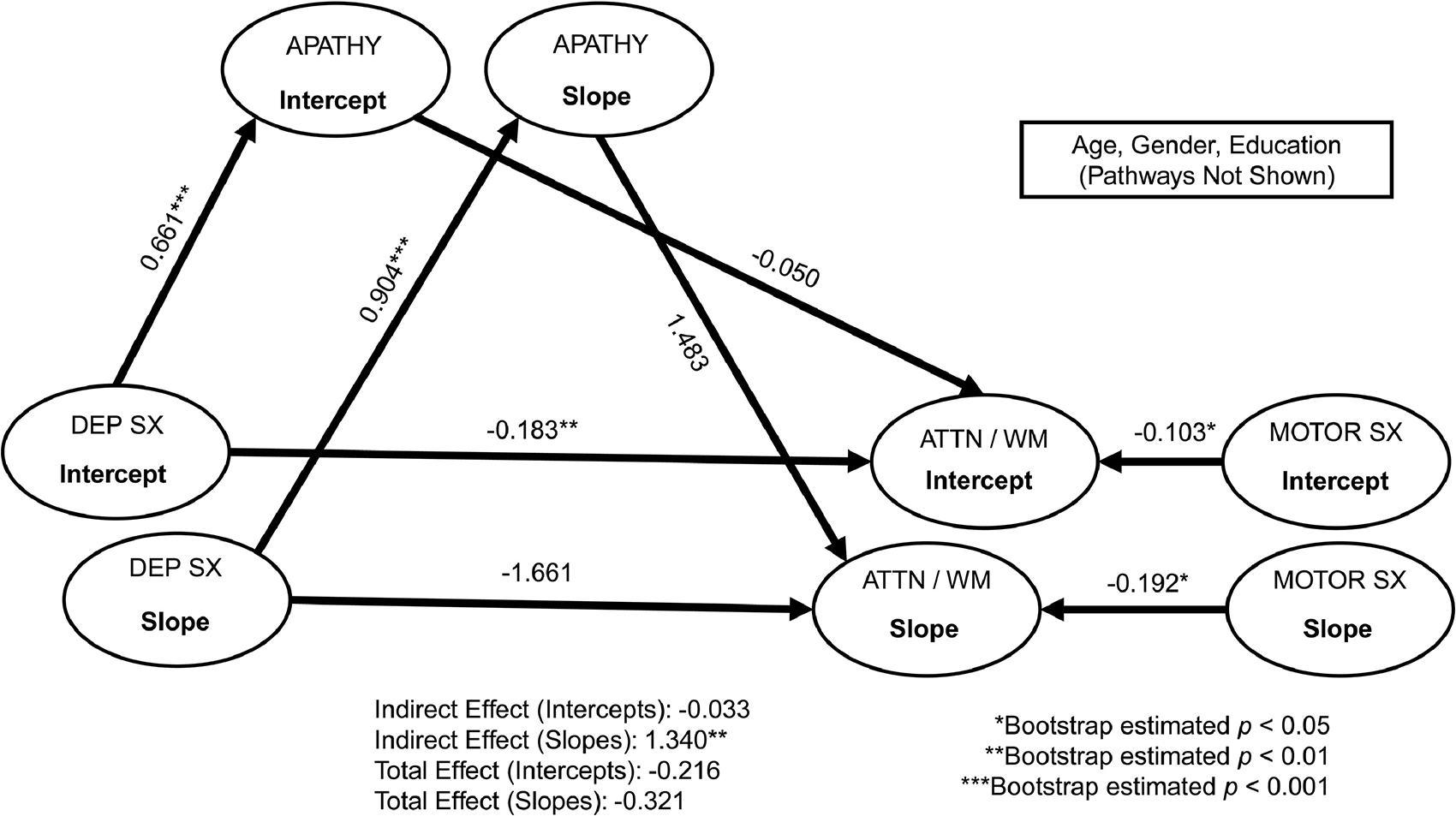
Multi-level structural equation mediation models of depressive symptoms on attention/working memory through apathy. Standardized coefficients are reported. For simplicity, baseline age, gender, and education covariates are not depicted. Dep Sx = depressive symptoms; Attn / wm = attention/working memory (assessed via Letter-Number Sequencing); Motor Sx = Motor symptoms. Model fit: chi-square = 813.94, df = 333, p < 0.001; Root Mean Square Error of Approximation [RMSEA] = 0.06; Comparative Fit Index [CFI] = 0.94; Normed Fit Index [NFI] = 0.91.
Figure 3.
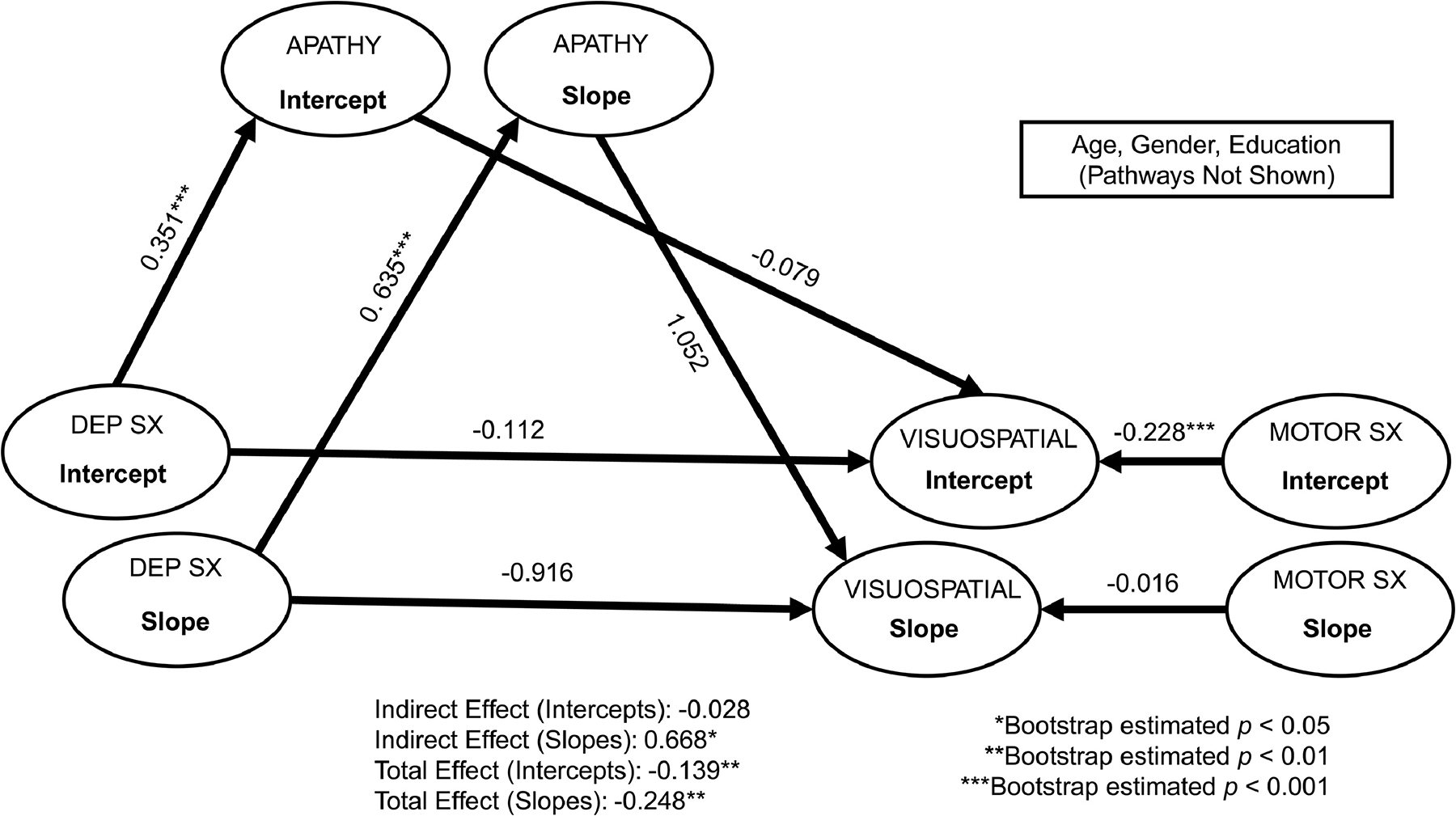
Multi-level structural equation mediation models of depressive symptoms on visuospatial functions through apathy. Standardized coefficients are reported. For simplicity, baseline age, gender, and education covariates are not depicted. Dep Sx = depressive symptoms; Visuospatial = visuospatial functions (assessed via Judgment of Line Orientation); Motor Sx = Motor symptoms. Model fit: chi-square = 800.31, df = 333, p < 0.001; Root Mean Square Error of Approximation [RMSEA] = 0.05; Comparative Fit Index [CFI] = 0.94; Normed Fit Index [NFI] = 0.90.
Highlights.
What is the primary question addressed by this study? In Parkinson’s disease (PD), both depressive symptoms and apathy can negatively affect cognition; however, these relationships are complex and research on underlying mechanisms are limited.
What is the main finding of this study? After covariate adjustment, we found an indirect, within-person path of depressive symptoms to cognition through apathy. Between-person mediation did not reach statistical significance.
What is the meaning of the finding? Disentangling within-person longitudinal depression-apathy relationships on cognition is clinically relevant, as it has implications for mood and cognitive intervention in patients with PD.
Conflicts of Interest and Source of Funding
This work was supported by National Institutes of Health (NIH) Grants SC3NS124906 (to JDJ) and U24MH100925 (to PEM). No disclosures to report.
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma PLC, AbbVie, AcureX Therapeutics, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson’s (ASAP), Avid Radiopharmaceuticals, Bial BioTech, Biogen Idec, BioLegend, Bristol-Meyers Squibb, Calico, Celgene, Dacapo Brain Science, Denali Therapeutics, Edmond J. Safra Philanthropic Foundation, Eli Lilly, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer Inc., Piramal Imaging, Prevail Therapeutics, Roche group, Sanofi-Genzyme, Servier, Takeda, TEVA, UCB, Verily, and Voyager Therapeutics.
Footnotes
Data Statement
The data has not been previously presented orally or by poster at scientific meetings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jankovic J: Parkinson’s disease: clinical features and diagnosis J Neurol Neurosurg Psychiatry 2008; 79:368–376. [DOI] [PubMed] [Google Scholar]
- 2.McGregor MM, Nelson AB: Circuit mechanisms of Parkinson’s disease Neuron 2019; 101:1942–1056. [DOI] [PubMed] [Google Scholar]
- 3.Poewe W, Seppi K, Tanner CM, et al. : Parkinson disease Nat Rev Dis Prim 2017; 3:17013. [DOI] [PubMed] [Google Scholar]
- 4.Ou R, Lin J, Liu K, et al. : Evolution of apathy in early Parkinson’s disease: A 4-years prospective cohort study Front Aging Neurosci 2021; 12:620762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Horta S, Pagonabarraga J, Fernández de Bobadilla R, et al. : Apathy in Parkinson’s disease: more than just executive dysfunction J Int Neuropsychol Soc 2013; 19:571–582. [DOI] [PubMed] [Google Scholar]
- 6.Funkiewiez A, Ardouin C, Caputo E, et al. : Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease J Neurol Neurosurg Psychiatry 2004; 75:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getz SJ, Levin B: Cognitive and neuropsychiatric features of early Parkinson’s disease Arch Clin Neuropsychol 2017; 32:769–785. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub D, Mamikonyan E: The neuropsychiatry of Parkinson disease: A perfect storm Am J Geriatr Psychiatry 2019; 27:998–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirsch-Darrow L, Marsiske M, Okun MS, et al. : Apathy and depression: Separate factors in Parkinson’s disease J Int Neuropsychol Soc 2011; 17:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oguru M, Tachibana H, Toda K, et al. : Apathy and depression in Parkinson disease J Geriatr Psychiatry Neurol 2010; 23:35–41. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield L, Cimino CR, Oelke LE, et al. : The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology 2010; 24:721–730. [DOI] [PubMed] [Google Scholar]
- 12.Varanese S, Perfetti B, Ghilardi MF, et al. : Apathy, but not depression, reflects inefficient cognitive strategies in Parkinson’s disease PLoS One 2011; 6:e17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szymkowicz SM, Dotson VM, Jones JD, et al. : Symptom dimensions of depression and apathy and their relationship with cognition in Parkinson’s disease J Int Neuropsychol Soc 2018; 24:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen ML, Aita S, Mari Z, et al. : The unique and combined effects of apathy and depression on cognition in Parkinson’s disease J Parkinsons Dis 2015; 5:351–359. [DOI] [PubMed] [Google Scholar]
- 15.Jones JD, Kurniadi NE, Kuhn TP, et al. : Depressive symptoms precede cognitive impairment in de novo Parkinson’s disease patients: Analysis of the PPMI cohort Neuropsychology 2019; 33:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirogovsky-Turk E, Moore RC, Filoteo JV, et al. : Neuropsychiatric predictors of cognitive decline in Parkinson disease: A longitudinal study Am J Geriatr Psychiatry 2017; 25:279–289. [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulos GS: Mechanisms and treatment of late-life depression Transl Psychiatry 2019; 9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor WD, Zald DH, Felger JC, et al. : Influences of dopaminergic system dysfunction on late-life depression Mol Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funes CM, Lavretsky H, Ercoli L, et al. : Apathy mediates cognitive difficulties in geriatric depression Am J Geriatr Psychiatry 2018; 26:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petkus AJ, Filoteo JV., Schiehser DM, et al. : Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson’s disease: A bidirectional analysis Neuropsychology 2019; 33:35–46. [DOI] [PubMed] [Google Scholar]
- 21.Marek K, Jennings D, Lasch S, et al. : The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol 2011; 95:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheikh JI, Yesavage JA: Geriatric Depression Scale ( Short Form ) Geriatric Depression Scale ( Short Form ) Self-Rated Version Clin Gerontol 1986; 5:165–173. [Google Scholar]
- 23.Weintraub D, Oehlberg KA, Katz IR, et al. : Test characteristics of the 15-item Geriatric Depression Scale and Hamilton Depression Rating Scale in Parkinson disease Am J Geriatr Psychiatry 2006; 14:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrag A, Barone P, Brown RG, et al. : Depression rating scales in Parkinson’s disease: Critique and recommendations Mov Disord 2007; 22:1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetz CG, Tilley BC, Shaftman SR, et al. : Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results Mov Disord 2008; 23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell J, Mathews HF, Yesavage JA: A multidimensional examination of depression among the elderly Res Aging 1993; 15:198–219. [Google Scholar]
- 27.Van Wanrooij LL, Borsboom D, Moll Van Charante EP, et al. : A network approach on the relation between apathy and depression symptoms with dementia and functional disability Int Psychogeriatrics 2019; 31:1655–1663. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub D, Xie S, Karlawish J, et al. : Differences in depression symptoms in patients with Alzheimer’s and Parkinson’s diseases: Evidence from the 15-item Geriatric Depression Scale (GDS-15) Int J Geriatr Psychiatry 2007; 22:1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szymkowicz SM, Ellis LJ, May PE: The 3-item “Apathy” subscale within the GDS-15 is not supported in de novo Parkinson’s disease patients: Analysis of the PPMI cohort J Geriatr Psychiatry Neurol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leentjens AFG, Dujardin K, Marsh L, et al. : Apathy and anhedonia rating scales in Parkinson’s disease: Critique and recommendations Mov Disord 2008; 23:2004–2014. [DOI] [PubMed] [Google Scholar]
- 31.Arbuckle JL: AMOS 2014. [Google Scholar]
- 32.Preacher KJ, Zyphur MJ, Zhang Z: A general multilevel SEM framework for assessing multilevel mediation Psychol Methods 2010; 15:209–233. [DOI] [PubMed] [Google Scholar]
- 33.Marek K, Chowdhury S, Siderowf A, et al. : The Parkinson’s progression markers initiative (PPMI) – establishing a PD biomarker cohort Ann Clin Transl Neurol 2018; 5:1460–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes AF: Beyond Baron and Kenny: Statistical mediation analysis in the new millennium Commun Monogr 2009; 76:408–420. [Google Scholar]
- 35.Gunzler D, Chen T, Wu P, et al. : Introduction to mediation analysis with structural equation modeling Shanghai Arch Psychiatry 2013; 25:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai H, Pan W: Resampling methods revisited: Advancing the understanding and applications in educational research Int J Res Method Educ 2008; 31:45–62. [Google Scholar]
- 37.Pituch KA, Stapleton LM: The performance of methods to test upper-level mediation in the presence of nonnormal data Multivariate Behav Res 2008; 43:237–267. [DOI] [PubMed] [Google Scholar]
- 38.Acock AC: A Gentle Introduction to Stata. 4th editio. Texas, Stata Press, 2014. [Google Scholar]
- 39.Kehagia AA, Barker RA, Robbins TW: Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis Neurodegener Disord 2013; 11:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar RD, Moon KLM, Neargarder S, et al. : Spatial judgment in Parkinson’s disease: Contributions of attentional and executive dysfunction Behav Neurosci 2019; 133:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesler SR, Haberecht MF, Menon V, et al. : Functional neuroanatomy of spatial orientation processing in Turner Syndrome Cereb Cortex 2004; 14:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Laughlin KD, Martin MJ, Ferrer E: Cross-sectional analysis of longitudinal mediation processes Multivariate Behav Res 2018; 53:375–402. [DOI] [PubMed] [Google Scholar]
- 43.Goldman JG, Vernaleo BA, Camicioli R, et al. : Cognitive impairment in Parkinson’s disease: A report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health npj Park Dis 2018; 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morimoto SS, Altizer RA, Gunning FM, et al. : Targeting cognitive control deficits with neuroplasticity-nased computerized cognitive remediation in patients with geriatric major depression: A randomized, double-blind, controlled trial Am J Geriatr Psychiatry 2020; 28:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oguro H, Nakagawa T, Mitaki S, et al. : Randomized trial of repetitive transcranial magnetic stimulation for apathy and depression in Parkinson’s disease J Neurol Neurophysiol 2014; 5:6. [Google Scholar]
- 46.Dujardin K, Sgambato V: Neuropsychiatric disorders in Parkinson’s disease: What do we know about the role of dopaminergic and non-dopaminergic systems? Front Neurosci 2020; 14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dan R, Růžička F, Bezdicek O, et al. : Separate neural representations of depression, anxiety and apathy in Parkinson’s disease Sci Rep 2017; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen MC, Thiery A, Tseng WYI, et al. : Apathy is associated with white matter network disruption and specific cognitive deficits in Parkinson’s disease Psychol Med 2020:1–10. [DOI] [PubMed] [Google Scholar]
- 49.Dobkin RD, Menza M, Bienfait KL, et al. : The impact of antidepressant treatment on cognitive functioning in depressed patients with Parkinson’s disease J Neuropsychiatr Clin Neurosci 2010; 22:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


