Abstract
Obsessive-compulsive disorder (OCD) is a disabling condition that often begins in childhood. Genetic studies in OCD have pointed to SLC1A1, which encodes the neuronal glutamate transporter EAAT3, with evidence suggesting that increased expression contributes to risk. In mice, midbrain Slc1a1 expression supports repetitive behavior in response to dopaminergic agonists, aligning with neuroimaging and pharmacologic challenge studies that have implicated the dopaminergic system in OCD. These findings suggest that Slc1a1 may contribute to compulsive behavior through altered dopaminergic transmission; however, this theory has not been mechanistically tested. To examine the developmental impact of Slc1a1 overexpression on compulsive-like behaviors, we therefore generated a novel mouse model to perform targeted, reversible overexpression of Slc1a1 in dopaminergic neurons. Mice with life-long overexpression of Slc1a1 showed a significant increase in amphetamine (AMPH)-induced stereotypy and hyperlocomotion. Single-unit recordings demonstrated that Slc1a1 overexpression was associated with increased firing of dopaminergic neurons. Furthermore, dLight1.1 fiber photometry showed that these behavioral abnormalities were associated with increased dorsal striatum dopamine release. In contrast, no impact of overexpression was observed on anxiety-like behaviors or SKF-38393-induced grooming. Importantly, overexpression solely in adulthood failed to recapitulate these behavioral phenotypes, suggesting that overexpression during development is necessary to generate AMPH-induced phenotypes. However, doxycycline-induced reversal of Slc1a1/EAAT3 overexpression in adulthood normalized both the increased dopaminergic firing and AMPH-induced responses. These data indicate that the pathologic effects of Slc1a1/EAAT3 overexpression on dopaminergic neurotransmission and AMPH-induced stereotyped behavior are developmentally mediated, and support normalization of EAAT3 activity as a potential treatment target for basal ganglia-mediated repetitive behaviors.
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating neuropsychiatric condition characterized by persistent intrusive thoughts (obsessions) and/or repetitive behaviors (compulsions) (1). OCD affects 2–3% of the general population (2) and is a leading cause of disability (3). Available first-line treatments include exposure therapy with response prevention (E/RP) and serotonin-reuptake inhibitors (SRIs) (4–6); however, full remission is achieved in only 10–20% of patients (7). Several other neuropsychiatric disorders, including Tourette Syndrome (TS) and Autism Spectrum Disorders (ASD), also include repetitive behavior and show elevated co-occurrence rates with OCD (8–10). Approximately half of individuals with OCD have onset in childhood (11), suggesting that it has developmental origins. A greater understanding of the neurobiological underpinnings of OCD, and related disorders, is needed to develop novel treatments.
Converging evidence indicates that dysfunction of basal ganglia circuits contributes to abnormal repetitive behaviors characteristic of OCD and related disorders, such as TS (12–14). Structural neuroimaging finds abnormal caudate volume in OCD and TS (15–19), and functional imaging demonstrates abnormal activity in basal ganglia circuits in these disorders (20–25). Neuronal recordings also demonstrate abnormal activity in the caudate, globus pallidus, thalamus and subthalamus (26–30), supporting the hypothesis that dysregulated basal ganglia signaling is linked to obsessive-compulsive and tic symptoms.
Evidence also indicates involvement of the dopaminergic system in OCD and TS. For example, in humans, dopaminergic agents such as amphetamine (AMPH) and L-DOPA can trigger repetitive behaviors and can exacerbate or worsen tics in patients with TS (31–36). Molecular imaging studies in OCD and TS also find altered dopamine transporter or D2 receptor binding in the striatum (37–39). Notably, increased neuromelanin signal, a marker of dopaminergic neurons, has been reported in pediatric OCD (40). Increased striatal intrasynaptic dopamine release is also implicated in TS from PET imaging findings (41, 42). Consistent with these findings, augmentation with D2 antagonists is a common pharmacological strategy for SRI-resistant OCD and TS (43–46). Finally, data from a wide range of animal models of repetitive behavior suggests that alterations in dopaminergic transmission contribute to abnormal repetitive behavior (47, 48).
Family and twin studies demonstrate substantial heritability of OC symptoms, with greater support for heritability in early-onset OCD (49). Genetic linkage (50, 51) and association (52–54) studies point to the SLC1A1 gene located on chromosome 9p24, which encodes the neuronal glutamate transporter Excitatory Amino Acid Transporter 3 (EAAT3/EAAC1), with strongest association in males and in early-onset OCD. Notably, the OCD families contributing to linkage at the chromosome 9p24 region and association to SLC1A1 also included individuals with Tic Disorder or TS (50, 52, 53), highlighting the co-occurrence and shared genetic risk of these repetitive behavior disorders. Most of the findings have clustered at the 3’ gene region with the greatest evidence for association with the rs301430C allele, which is linked to increased expression in brain tissue and in cell models (54, 55). Of note, however, a meta-analysis found only a modest association of SLC1A1 variants with OCD (56), and modestly sized genome-wide association studies have reported no genes reaching genome-wide significance in OCD (57, 58). SLC1A1/EAAT3 is expressed broadly throughout the brain, including in spiny projection neurons in the striatum (59, 60) and midbrain dopaminergic neurons (61, 62). Within neurons, EAAT3 is localized to peri-synaptic regions where it modulates glutamate signaling (63, 64). This is consistent with evidence of altered glutamate signaling in OCD and TS from cerebrospinal fluid, magnetic resonance spectroscopy, and gene expression studies (65–71).
Recently, Delgado-Acevedo and colleagues (72) used CaMKIIα-Cre to activate transgenic overexpression of EAAT3 in glutamatergic neurons in the cortex, striatum, and hippocampus, finding increased anxiety-like and grooming behavior. In a follow-up study (73), the authors found decreased baseline dopamine levels and increased AMPH-induced dopamine release in the dorsal striatum, suggesting that CaMKIIα-Cre-based EAAT3 overexpression impacts dopaminergic signaling in the striatum; although AMPH-induced locomotor activity was not affected. Here, we investigated the functional impact of EAAT3 manipulation on circuits implicated in OCD. We focused on EAAT3 activity in dopaminergic neurons because of the well-established importance of dopamine in repetitive behaviors (37, 43, 47, 48, 74), and our previous work in EAAT3 knockout mice (75) that suggested involvement of midbrain EAAT3 expression in pharmacologically induced-repetitive behaviors. Here, we therefore directly tested the hypothesis that increased EAAT3 expression in dopaminergic neurons leads to increased compulsive-like behavior. We used a flexible Slc1a1 knock-in cassette (76) to generate mice with selective and reversible overexpression of EAAT3 in tyrosine hydroxylase (TH)-expressing neurons in both development and adulthood to determine the impact on dopamine signaling and basal ganglia-mediated repetitive behavior.
Materials and methods
A summary is provided here. Additional details are available in Supplementary Information.
Mice
All experimental studies were approved by the New York State Psychiatric Institute or the University of Pittsburgh Institutional Animal Care and Use Committees. Slc1a1-STOP-tetO mice (75) were crossed to Pgk1-flpo mice (77) to obtain Slc1a1-tetO mice that were then crossed to the Th-tTA line (78) to obtain Slc1a1-Th-OE and control mice. EAAT3 expression was regulated by doxycycline-supplemented chow (Envigo, Madison, Wisconsin, USA) and was quantified using real-time qRT-PCR and immunoblots. Adult male and female mice and littermate controls were used for all experiments.
Behavioral assays
Behavioral procedures were adapted from (75). Two separate cohorts of naïve, Slc1a1-Th-OE mice and littermate controls were used to test baseline anxiety-like and repetitive (elevated plus maze, light-dark emergence, and marble-burying), and drug-induced (AMPH-induced locomotion, AMPH-induced stereotypy, and SKF-38393-induced grooming) behaviors. Developmental effects were tested in separate, naïve cohorts that were administered doxycycline/regular chow. Rescue experiments were tested separately, in naïve cohorts, using a longitudinal design and in drug-naïve mice. Timelines for each experiment, including the ages of mice, are provided in the figures.
In vivo single-unit recordings
Naïve, adult chloral hydrate anesthetized mice were implanted with a glass electrode filled with 2M NaCl into the VTA (AP −3.2, ML +/−0.6, DV −3.5mm). Neurons were sampled from 6 equally spaced tracks. Traces were processed using NeuroScope software. Dopamine neurons were identified by the broad action potential width (>3 msec) and tri-phasic waveform (79–81). Bursts were defined as per the classical criteria of Grace and Bunney (79, 80). ISI distributions, burst properties, and firing rates for each dopamine neuron were quantified. Recording locations were histologically confirmed at the end of the experiment.
In vivo fiber photometry
Naïve, adult isoflurane anesthetized mice were injected with 0.3 ul of AAV5-CAG-dLight1.1 (82) into the dorsal striatum (AP +1.0, ML +/−1.5, DV −3.2 mm) followed by implantation of a fiber optic cannula (200 um core, 0.48 NA, Doric, Quebec, Canada) above the site of virus injection. 3 weeks after virus expression dopamine imaging was conducted using a fiber photometry system (Neurophotometrics) with capabilities for 470 nm and 410 nm excitation wavelengths. Raw 470 nm and 410 nm signals were extracted using MATLAB (Mathworks, R2015a); extracted signals were baseline-corrected to remove the slope (Origin(Pro), Version 2021) and then standardized using the median and standard deviation of the 20 min baseline period (83). The standardized 410 nm signal was then regressed onto the standardized 470 nm signal. The regressed 410 nm signal was then subtracted from the standardized 470 nm signal to obtain dF/F values.
Statistical analysis
Data analysis was performed with GraphPad Prism (version 9.0.0, GraphPad Software, San Diego, CA, USA). Two-tailed, unpaired t test, 2-way RM ANOVA, or 3-way RM ANOVA was used to analyze the primary data. Locomotion data was analyzed using non-linear curve fit and area under curve (AUC) analysis. Table S1 in Supplementary Information shows a summary of all statistics. Secondary analysis using sex as an independent variable is presented in Sex as a biological variable in Supplementary Information. All data are reported as the mean +/− SEM (standard error of mean).
Results
EAAT3 is selectively and reversibly overexpressed in Slc1a1-Th-OE mice
We took advantage of the Flexible Accelerated STOP TetO-knockin (FAST) system (76) to selectively overexpress EAAT3 in TH-positive neurons via the tetracycline controlled transcriptional activator (tTA) (Figure 1, Supplementary Figures S1–2). We first created Slc1a1-tetO mice by crossing to a Pgk1-flpo line (77) to delete the STOP cassette from Slc1a1-STOP-tetO mice (75). To obtain TH-positive neuron-restricted overexpression, Slc1a1-tetO mice were crossed to mice expressing tTA under control of the Th promotor (Th-tTA) (78), yielding mice that were homozygous for the Slc1a1-tetO allele and possessed one copy of the Th-tTA transgene (Slc1a1-tetOhomo/Th-tTA+, termed Slc1a1-Th-OE or Th-OE mice) (see Supplementary Information for detailed breeding scheme). In the absence of doxycycline (tet-OFF), tTA binds and activates the tetO promotor, leading to increased expression of the tetO-associated gene (Figure 1A). As expected, western blot analysis of midbrain whole cell-lysis extracts (Figure 1B) showed increased EAAT3 protein expression in life-time overexpressing Slc1a1-Th-OE mice in comparison with littermate controls (Figure 1C, unpaired t (22) = 6.435, P < 0.0001). Administration of doxycycline-supplemented chow in adulthood resulted in restoration of expression in Slc1a1-Th-OE mice to littermate control group levels (Figure 1D, unpaired t (10) = 0.01105, P = 0.9142). The increased EAAT3 protein expression in Slc1a1-Th-OE mice was restricted to the midbrain; no genotype differences in EAAT3 expression were observed in regions outside the midbrain, including cortex, hippocampus, and striatum (Supplementary Figure S2). Together, these results indicate efficient and selective induction and reversal of midbrain EAAT3 overexpression in Slc1a1-Th-OE mice (76, 84, 85).
Figure 1. EAAT3 is selectively and reversibly overexpressed in Slc1a1-Th-OE mice.
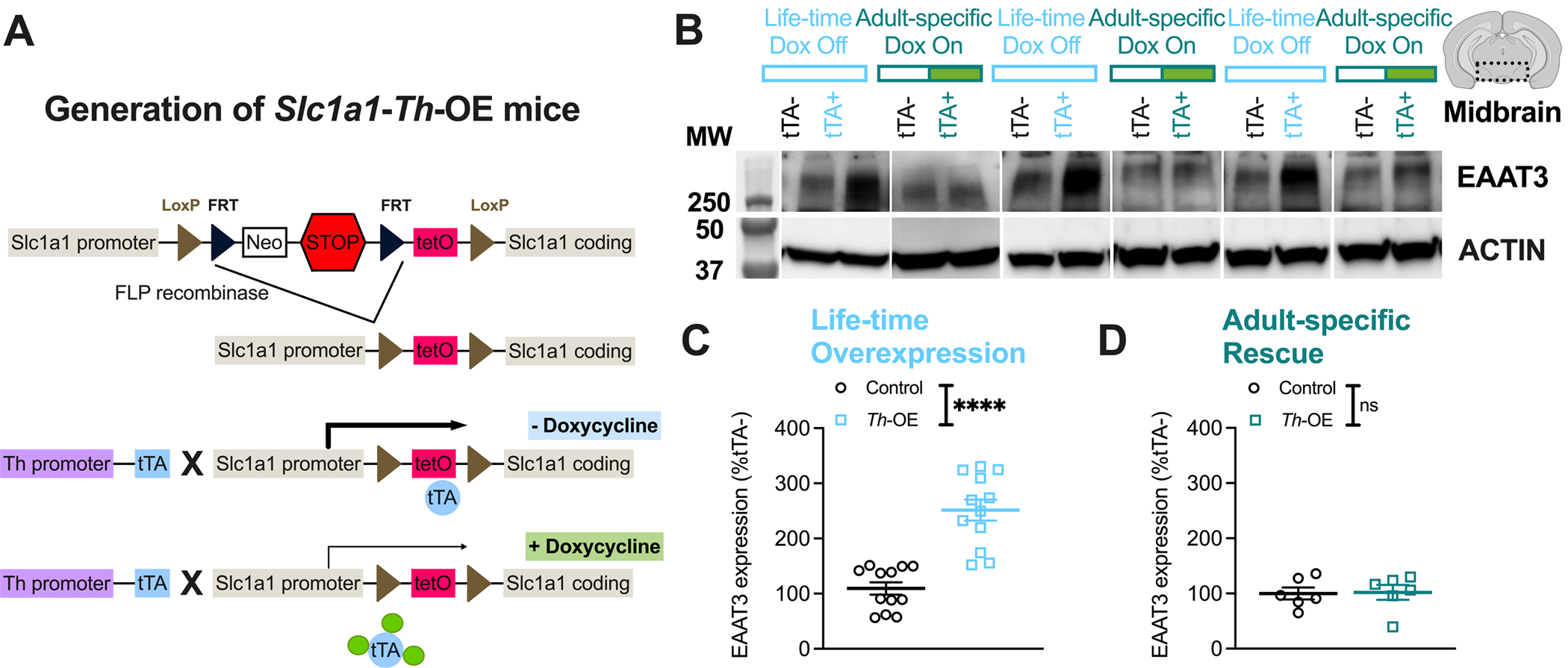
(A) Generation of Slc1a1-Th-OE mice. Slc1a1-STOP-tetO mice were crossed with Pgk1-flpo deleter line to generate Slc1a1-tetO mice that were then crossed with Th-tTA line to generate tTA-mediated overexpression of EAAT3 selectively in TH-expressing neurons. (Neo, PGK-EM7-NEO minigene; STOP, Stop signal; tetO, tetracycline operon; tTA, tetracycline transactivator). (B) Representative western blot images showing increased midbrain EAAT3 expression in Slc1a1-Th-OE (Th-OE) mice following administration of regular chow and restored expression following administration of doxycycline-supplemented chow. Molecular weight (MW) in kDA is indicated on the left. (C) EAAT3 overexpression (****P < 0.0001, n = 12 per genotype), and (D) rescue of expression following treatment with doxycycline-supplemented chow (nsP, not significant, n = 6 per genotype). Also see Figure 4H for qRT-PCR, Supplementary Figure S1 for full blot image and Supplementary Figure S2 for regional protein expression.
Slc1a1-Th-OE mice show no baseline changes in anxiety-like or repetitive behavior
We next evaluated life-time overexpressing Slc1a1-Th-OE mice and littermate controls for baseline anxiety-like and repetitive behavioral phenotypes (Supplementary Figures S3, S4). In the open field (a novel environment), Slc1a1-Th-OE mice showed a slight but significant increase in novelty-induced locomotion in comparison with littermate controls over 30 minutes of exploration (Supplementary Figure 3A, third order polynomial least squares fit, F (4, 106) = 2.483, P = 0.0481). Despite this small increase in locomotion in Slc1a1-Th-OE mice during the first open field exposure, no significant time by genotype interaction was found across subsequent days of exposure (Supplementary Figure 3A), indicating preserved habituation in Slc1a1-Th-OE mice. We found no differences in indices of anxiety-like behavior in the open field (Supplementary Figure S4). No significant genotype differences were found in time spent in the open sections of the elevated zero maze (considered a more anxiety-provoking environment) (Supplementary Figure S3B) or in the time spent or distance traveled in the lit chamber in the light-dark test (Supplementary Figure S3C). Finally, we found no significant genotype differences in the number of marbles buried in the marble burying test (Supplementary Figure S3D), or in baseline grooming behavior in a novel cage (Figure 2E).
Figure 2. Amphetamine reveals increased basal ganglia-dependent repetitive behavior in Slc1a1-Th-OE mice.
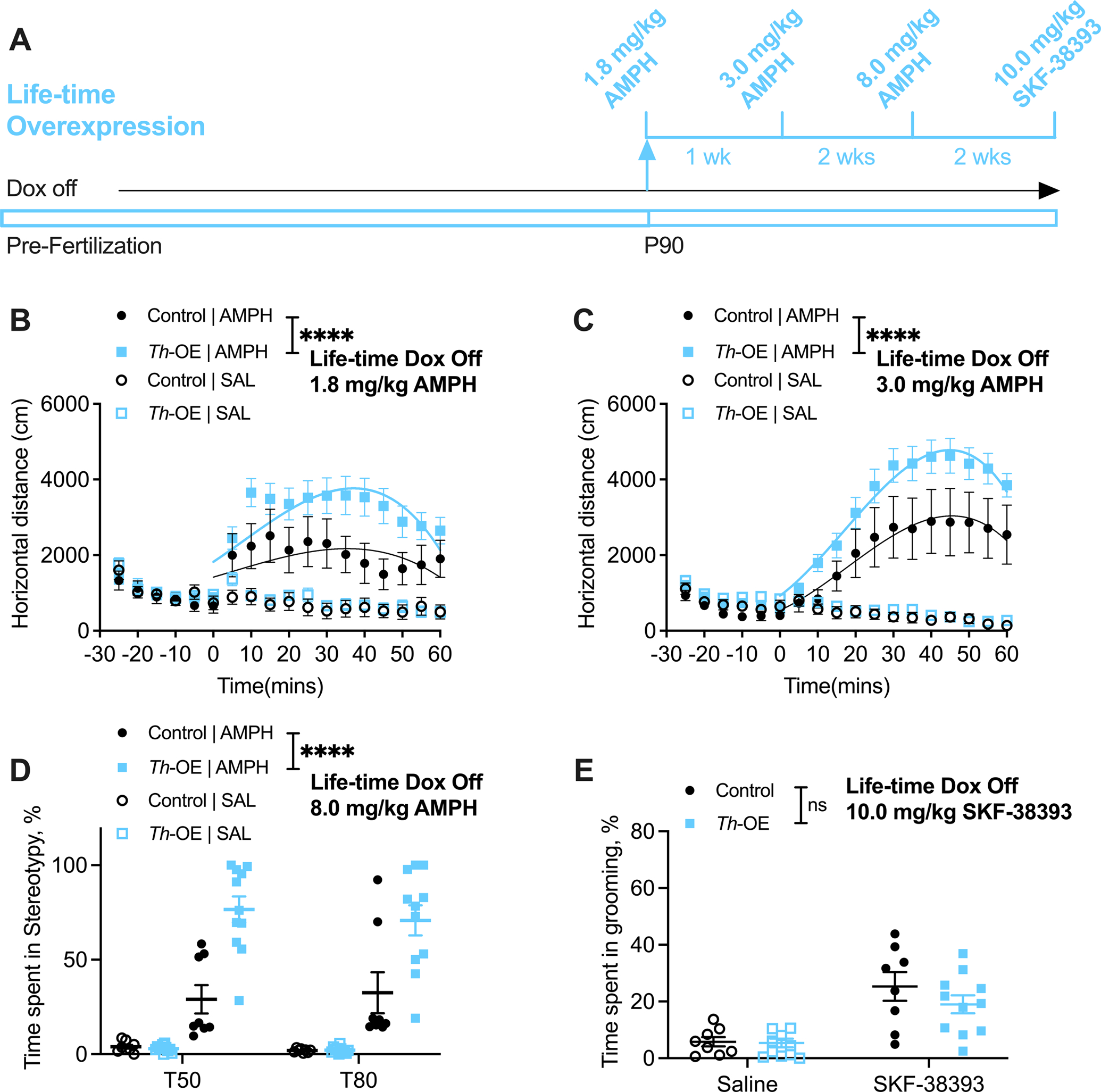
(A) Experimental timeline. For all panels, n = 8 control, 11 Th-OE mice. No genotype differences were found in basal locomotion in saline-injected Th-OE and control mice in a familiar environment (B, third order polynomial least squares fit, t = 0–60; F (4, 334) = 1.349, P = 0.2515). Th-OE mice show significantly increased locomotor response to (B) low and (C) moderate doses of AMPH. (D) Th-OE mice display increased stereotypic behavior following 8.0 mg/kg AMPH dose. (E) SKF-38393-induced grooming response is not altered in Th-OE mice. ****P < 0.0001; nsP, not significant. Also see Supplementary Figures S3, S4 for baseline anxiety-like and repetitive behaviors and Supplementary Figures S5, S6 for tet-operator and tTA transgene control experiments.
Amphetamine reveals increased basal ganglia-dependent repetitive behavior in Slc1a1-Th-OE mice
To examine whether life-time overexpression of Slc1a1 would lead to an increase in basal ganglia-mediated repetitive behavior mirroring the decrease in AMPH-induced repetitive behaviors in Slc1a1-STOP-tetO mice (75), Slc1a1-Th-OE mice and littermate controls were evaluated for AMPH-induced locomotion and stereotypic behavior (Figure 2). Injection of low dose AMPH (1.8 mg/kg) resulted in significantly increased locomotor activity in Slc1a1-Th-OE mice relative to littermate controls (Figure 2B, third order polynomial least squares fit, t = 0–60; F (4, 334) = 13.47, P < 0.0001). At a moderate dose (3.0 mg/kg), this difference became even more pronounced (Figure 2C, third order polynomial least squares fit, t = 0–60; F (4, 334) = 16.12, P < 0.0001). At the highest dose tested (8.0 mg/kg), video-scoring of stereotypic behavior at 50- and 80-mins post-injection revealed a significant drug by genotype interaction and a significant main effect of genotype (Figure 2D, 3-way RM ANOVA; drug × genotype interaction, F (1, 34) = 26.73, P < 0.0001; drug, F (1, 34) = 139.8, P < 0.0001, genotype, F (1, 34) = 25.84, P < 0.0001), indicating increased stereotypic activity in Slc1a1-Th-OE mice. To examine stereotypic behavior that is independent of pre-synaptic dopamine release, we acutely challenged Slc1a1-Th-OE mice and littermate controls with the D1 agonist SKF-38393. While there was a significant main effect of drug, there was no significant drug by genotype interaction or main effect of genotype (Figure 2E, 2-way RM ANOVA; drug × genotype interaction, F (1, 17) = 1.219, P = 0.2849; drug, F (1, 17) = 38.53, P < 0.0001, genotype, F (1, 17) = 0.9749, P = 0.3373). These results indicate that EAAT3 overexpression impacts dopamine-induced repetitive behavior via a pre-, not post-synaptic, mechanism.
Because gene knock-ins and transgene insertions can cause unintended changes in native gene expression and function (86–91), we evaluated the impact of 1) the Slc1a1-tetO operator, and 2) the Th-tTA transgene independent of the Slc1a1-tetO operator in mice that were administered regular chow. While mice that were homozygous for the Slc1a1-tetO allele (Slc1a1-tetOhomo/Th-tTA-) displayed ~50% reduction in EAAT3 expression compared to WT mice (Supplementary Figure S5B), they did not show significant differences in AMPH-induced responses or basal dopaminergic transmission (Supplementary Figure S5C–M). We similarly observed no significant effect of the Th-tTA transgene alone on AMPH-induced responses (Supplementary Figure S6).
Anesthetized Slc1a1-Th-OE mice show increased dopamine neuron burst firing
To determine whether life-time overexpression of EAAT3 in dopaminergic neurons alters neuron activity patterns in vivo, we recorded spontaneous action potential firing in anesthetized Slc1a1-Th-OE mice and littermate controls (Figure 3A–I). Putative dopamine neurons were identified by the established long duration (>3 msec), triphasic waveform and by their tonic irregular firing and intermittent bursts (79–81, 92) (Figure 3B), defined per the standard 80/160 ms criteria (79). We found an increased proportion of short duration inter-spike intervals (ISIs) in life-time overexpressing Slc1a1-Th-OE mice relative to littermate controls (Figure 3C, 2-way RM ANOVA: ISI × genotype, F (50, 500) = 5.254, P < 0.0001; ISI, F (50, 500) = 103.5, P < 0.0001, genotype, F (1, 10) = 6.269, P = 0.0312; Sidak’s multiple comparisons: P < 0.0001 for ISIs 30–90 msec). Evaluation of burst parameters revealed that Slc1a1-Th-OE mice displayed an increased percentage of total spikes fired in bursts (Figure 3D, unpaired t (10) = 4.684, P = 0.0009), spikes per burst (Figure 3E, unpaired t (10) = 4.905, P = 0.0006), and increased burst set rate (Figure 3F, unpaired t (10) = 2.825, P = 0.0180). We did not find differences in average burst ISI (Figure 3G, unpaired t (10) = 1.191, P = 0.2612); however, average before-burst ISI trended toward a decrease in Slc1a1-Th-OE mice (Figure 3H, unpaired t (10) = 2.030, P = 0.0699). Overall, we found increased firing rate in Slc1a1-Th-OE mice (Figure 3I, unpaired t (10) = 3.808, P = 0.0034).
Figure 3. Slc1a1-Th-OE mice show increased dopamine transmission.
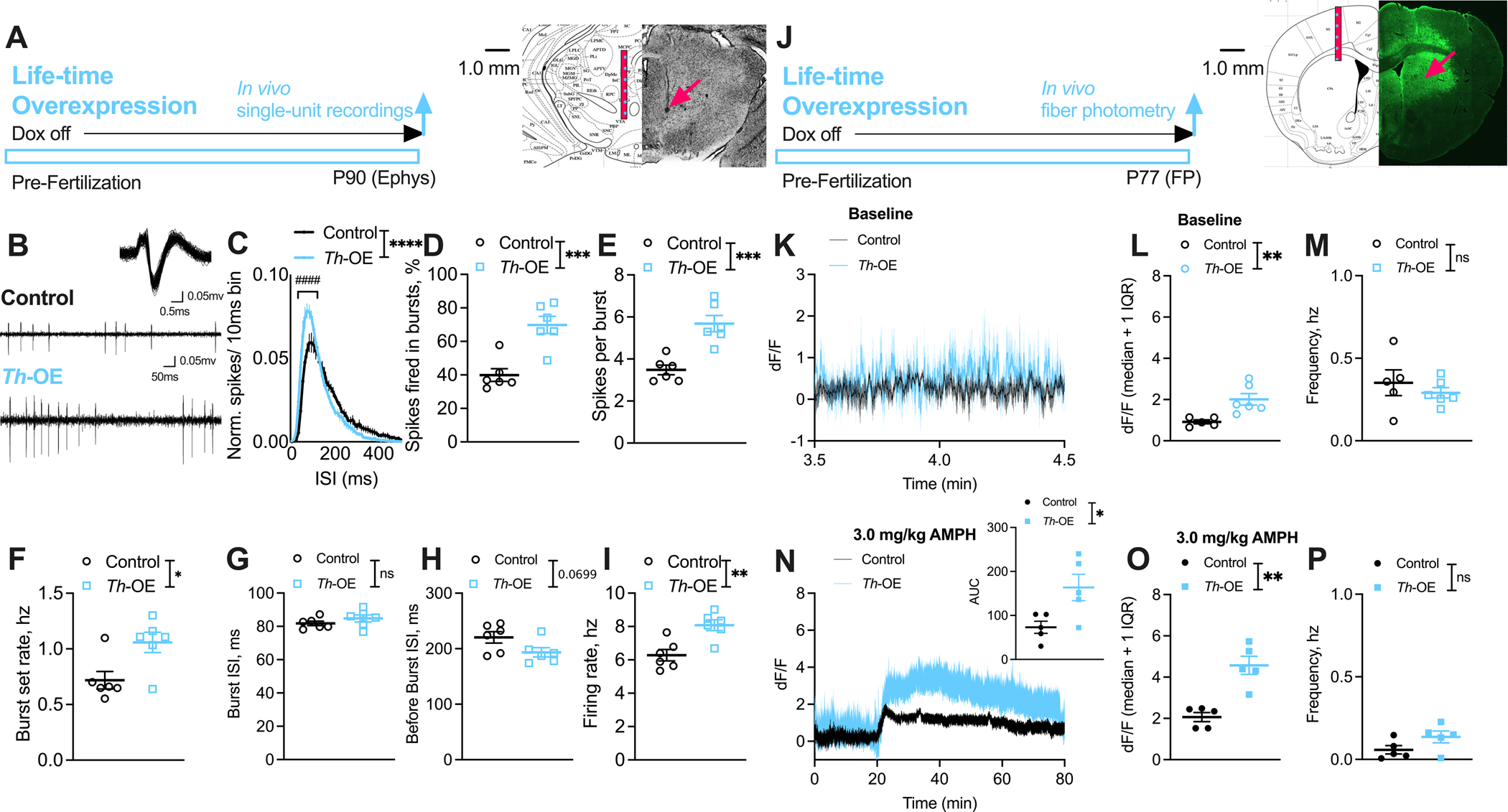
(A-I) Single-unit recordings. (A) Representative coronal section showing electrode track (arrow) through the VTA recording region. (B) Representative extracellularly recorded dopaminergic neuron waveform (overlay of ~100 spikes) (Upper) and spiking patterns (Lower) from control and Th-OE mice. For all ephys panels, n = 62 cells/ 6 control mice, 59 cells/ 6 Th-OE mice. (C) Normalized ISI histograms from putative dopaminergic neurons showing increased proportion of short duration ISIs in Th-OE mice. Th-OE mice display increased (D) % spikes fired in bursts, (E) spikes per burst and (F) burst set rate. (G) Average burst ISI is unaltered and (H) average before-burst ISI show trend level decreases in Th-OE mice. (I) Overall firing rate is increased in Th-OE mice. (J-P) dLight1.1 fiber photometry. (J) Representative coronal section showing optic fiber placement and dLight1.1 expression in the dorsal striatum. (K) Dopaminergic transients during baseline evaluation period. For all baseline panels, n = 5 control, 6 Th-OE mice. (L) Th-OE mice show increased fluorescence (median) compared to littermate control mice. (M) No genotype differences were observed in transient frequency. (N) Th-OE mice display increased fluorescence after AMPH challenge in comparison with littermate controls. For all AMPH panels, n = 5 per genotype. (O) Increased dopaminergic transient fluorescence (median), and (P) unaltered transient frequency in Th-OE mice compared to littermate controls. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; ####P < 0.0001; nsP, not significant.
Awake, freely moving Slc1a1-Th-OE mice show increased baseline and AMPH-induced dopamine release in dorsal striatum
To determine whether life-time overexpression of EAAT3 in TH-positive neurons alters dopamine release, we measured dopamine transients in freely moving mice with fiber photometry (Figure 3J–P). To visualize dopamine release, we infused an AAV vector encoding dLight1.1 (AAV5-CAG-dLight1.1), a highly-specific, intensity-based dopamine sensor (82), into the dorsal striatum of Slc1a1-Th-OE and littermate control mice. Optic fibers were simultaneously implanted (Figure 3J). After 3 weeks, we reliably detected dopamine transients during a 20-minute baseline evaluation period in an empty cage (Figure 3K). We used a stringent threshold of one interquartile range (IQR) above median and a minimum of 100 msec duration (to account for dLight1.1 decay time (82)) to detect dopamine transients in each group. Analysis of these dopamine transients revealed significantly increased median fluorescence (Figure 3L, unpaired t (9) = 3.379, P = 0.0081) but unaltered transient frequency (Figure 3M, unpaired t (9) = 0.7751, P = 0.4582) in Slc1a1-Th-OE mice in comparison with littermate controls. We next challenged mice with a 3.0 mg/kg dose of AMPH and monitored fluorescence for 60 mins. As expected, AMPH induced a significant elevation of dLight1.1 signal compared to baseline in both groups. AUC analysis of the 60-minute post-AMPH injection period revealed significantly increased fluorescence in Slc1a1-Th-OE mice compared with littermate controls (Figure 3N, unpaired t (8) = 2.740, P = 0.0254). Like pre-AMPH, we found increased median fluorescence of dopamine transients (Figure 3O, unpaired t (8) = 5.065, P = 0.0010) but unaltered transient frequency (Figure 3P, unpaired t (8) = 1.788, P = 0.1115) following AMPH in Slc1a1-Th-OE mice compared to littermate controls.
Overexpression of EAAT3 during development is necessary for increased AMPH-induced locomotion and stereotypic behavior in Slc1a1-Th-OE mice
To determine whether the effects of EAAT3 overexpression are developmental in origin, we administered doxycycline-supplemented chow to Slc1a1-Th-OE mice and littermate controls for 2 months, and then switched them to regular chow for 1 month to induce adult-specific tTA activation (termed ‘adult-specific overexpressing mice’, Figure 4M–R). Slc1a1-Th-OE mice and littermate controls that were administered doxycycline-supplemented chow for whole-life (termed ‘life-time rescue mice’, Figure 4A–F) or regular chow for whole-life (termed ‘life-time overexpressing mice’, Figure 4G–L) served as control groups and were run in parallel to the adult-specific overexpressing mice.
Figure 4. Overexpression of EAAT3 during development is necessary for increased AMPH-induced locomotion and stereotypic behavior in Slc1a1-Th-OE mice.
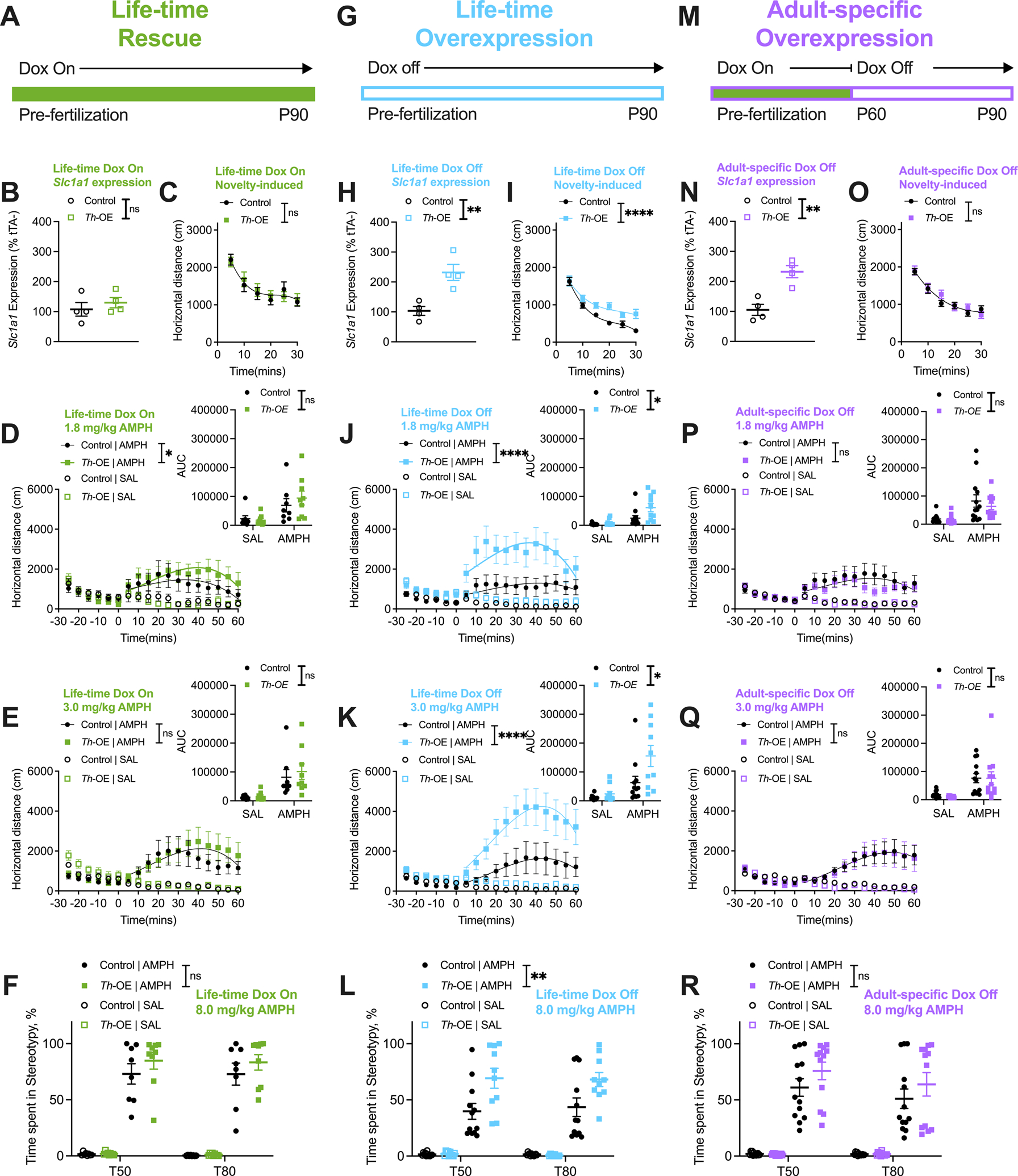
(A-F) Life-time rescue experiments. (B) qRT-PCR showing complete rescue of Slc1a1 mRNA expression in Th-OE mice (n = 4 per genotype). For all life-time rescue behavior panels, n = 8 controls, 9 Th-OEs. (C) Life-time rescue Th-OE mice display novelty-induced locomotion that is indistinguishable from littermate controls. (D) Life-time rescue Th-OE mice show a slightly increased locomotor response to a low dose of AMPH via curve-fit analysis; however, no genotype differences are observed in AUC analysis (D, inset). (E) Locomotor response of life-time rescue Th-OE mice to a moderate AMPH dose (AUC, 2-way RM ANOVA; drug × genotype interaction, F (1, 15) = 0.2081, P = 0.6548; drug, F (1, 15) = 18.74, P = 0.0006, genotype, F (1, 15) = 0.3003, P = 0.5917) is indistinguishable from littermate controls. (F) Stereotypic activity at the high AMPH dose in life-time rescue Th-OE mice does not differ from controls. (G-L) Life-time overexpression experiments. (H) qRT-PCR showing increased Slc1a1 mRNA expression in Th-OE mice (n = 4 per genotype). For all life-time overexpression behavior panels, n = 12 controls, 10 Th-OEs. (I) Life-time overexpressing Th-OE mice show increased novelty-induced locomotion. (J-K) Life-time overexpressing Th-OE mice show increased locomotor activation at (J) low (AUC, 2-way RM ANOVA; drug × genotype interaction, F (1, 20) = 4.446, P = 0.0478; drug, F (1, 20) = 23.90, P < 0.0001, genotype, F (1, 20) = 5.347, P = 0.0315), and (K) moderate (AUC, 2-way RM ANOVA; drug × genotype interaction, F (1, 20) = 4.915, P = 0.0384; drug, F (1, 20) = 24.34, P < 0.0001, genotype, F (1, 20) = 5.997, P = 0.0237) doses of AMPH. (L) Stereotypic behavior at 8.0 mg/kg AMPH dose is elevated in Th-OE mice. (M-R) Adult-specific overexpression experiments. (N) qRT-PCR showing increased Slc1a1 expression in Th-OE mice (n = 4 per genotype). For all adult-specific overexpression behavior panels, n = 13 controls, 12 Th-OEs. (O) Adult-specific overexpressing Th-OE mice show novelty-induced locomotor behavior that is indistinguishable from littermate control mice. (P-Q) Locomotor responses in Th-OE mice to (P) low (AUC, 2-way RM ANOVA; drug × genotype interaction, F (1, 23) = 0.3956, P = 0.5356; drug, F (1, 23) = 18.19, P = 0.0003, genotype, F (1, 23) = 0.6658, P = 0.4229), and (Q) moderate (AUC, 2-way RM ANOVA; drug × genotype interaction, F (1, 23) = 0.06689, P = 0.7982; drug, F (1, 23) = 19.62, P = 0.0002, genotype, F (1, 23) = 0.07915, P = 0.7810) doses of AMPH are comparable to control group responses. (R) No genotype differences are observed in AMPH-induced stereotypic behavior in adult-specific overexpressing mice. ****P < 0.0001; **P < 0.01; *P < 0.05; nsP, not significant. Also see Supplementary Figure S7 for anxiety-like measures.
As expected, whole-life tTA suppression by doxycycline resulted in rescue of Slc1a1 expression via qRT-PCR of the midbrain (Figure 4B, unpaired t (6) = 0.7816, P = 0.4642). We found no genotype differences in novelty-induced locomotion (Figure 4C, third order polynomial least squares fit, t = 0–30; F (4, 94) = 0.3311, P = 0.8564) or anxiety-like behavior measures (Supplementary Figure S7A–C) in life-time rescue mice. We found a slight increase in AMPH-induced locomotor activity in Slc1a1-Th-OE mice at 1.8 mg/kg via curve fit analysis (Figure 4D, third order polynomial least squares fit, t = 0–60; F (4, 298) = 2.774, P = 0.0273); however, no significant drug by genotype interaction or a main effect of genotype was found in AUC analysis at this dose (Figure 4D, inset, 2-way RM ANOVA; drug × genotype interaction, F (1, 15) = 1.341, P = 0.2650; drug, F (1, 15) = 21.85, P = 0.0003, genotype, F (1, 15) = 0.2310, P = 0.6377). Locomotor activity at 3.0 mg/kg (Figure 4E, third order polynomial least squares fit, t = 0–60; F (4, 298) = 2.144, P = 0.0754) and stereotypic behavior at 8.0 mg/kg (Figure 4F, 3-way RM ANOVA; drug × genotype interaction, F (1, 30) = 1.581, P = 0.2183; drug, F (1, 30) = 341.4, P < 0.0001, genotype, F (1, 30) = 2.017, P = 0.1659) in life-time rescue Slc1a1-Th-OE mice was comparable to controls.
As above, administration of regular chow to life-time overexpressing mice resulted in increased Slc1a1 expression in Slc1a1-Th-OE mice (Figure 4H, unpaired t (6) = 4.168, P = 0.0059), and life-time overexpressing Slc1a1-Th-OE mice displayed robust increases in novelty-induced locomotion (Figure 4I, third order polynomial least squares fit, t = 0–30; F (4, 124) = 9.196, P < 0.0001). No genotype differences were observed in open-field anxiety-like measures (Supplementary Figure S7D–F). AMPH-induced locomotor activation in response to 1.8 mg/kg (Figure 4J, third order polynomial least squares fit, t = 0–60; F (4, 388) = 20.86, P < 0.0001) and 3.0 mg/kg (Figure 4K, third order polynomial least squares fit, t = 0–60; F (4, 388) = 22.41, P < 0.0001) was significantly increased in life-time overexpressing Slc1a1-Th-OE mice; AMPH-induced stereotypic behavior at 8.0 mg/kg (Figure 4L, 3-way RM ANOVA; drug × genotype interaction, F (1, 40) = 12.34, P = 0.0011; drug, F (1, 40) = 193.7, P < 0.0001, genotype, F (1, 40) = 11.88, P = 0.0013) was also significantly elevated.
Remarkably, adult-specific overexpression of Slc1a1 (Figure 4N, unpaired t (6) = 4.662, P = 0.0035) failed to increase novelty-induced locomotion in Slc1a1-Th-OE mice (Figure 4O, third order polynomial least squares fit, t = 0–30; F (4, 142) = 0.6595, P = 0.6212). Furthermore, no genotype differences were found in AMPH-induced locomotor response at 1.8 mg/kg (Figure 4P, third order polynomial least squares fit, t = 0–60; F (4, 442) = 2.341, P = 0.0544) or 3.0 mg/kg (Figure 4Q, third order polynomial least squares fit, t = 0–60; F (4, 442) = 0.04487, P = 0.9962) in adult-specific overexpressing mice. Stereotypic behavior at the 8.0 mg/kg AMPH dose (Figure 4R, 3-way RM ANOVA; drug × genotype interaction, F (1, 46) = 2.605, P = 0.1134; drug, F (1, 46) = 199.5, P < 0.0001, genotype, F (1, 46) = 2.407, P = 0.1277) in Slc1a1-Th-OE mice was also comparable to controls. These data suggest that increased EAAT3 activity during early development may increase susceptibility to pharmacologically-induced compulsive-like behavior.
Reversal of EAAT3 overexpression in adulthood rescues AMPH-induced locomotor and stereotypic behavior and dopamine neuron activity
To evaluate whether the heightened AMPH-induced repetitive behavior in life-time Slc1a1-Th-OE mice could be rescued by normalizing EAAT3 overexpression in adulthood, we performed a series of longitudinal experiments. We first assessed AMPH-induced behavior prior to 4 weeks of tTA suppression by doxycycline-supplemented chow (see Fig 1D for rescue of EAAT3 expression). As above, we found robust increases in AMPH-induced locomotion at the low (Figure 5B, third order polynomial least squares fit, t = 0–60; F (4, 370) = 9.326, P < 0.0001) and moderate doses (Figure 5D, third order polynomial least squares fit, t = 0–60; F (4, 370) = 16.33, P < 0.0001), and increased AMPH-induced stereotypic behavior at 8.0 mg/kg (Figure 5F, 3-way RM ANOVA; drug × genotype interaction, F (1, 38) = 13.89, P = 0.0006; drug, F (1, 38) = 126.9, P < 0.0001, genotype, F (1, 38) = 9.839, P = 0.0033) in Slc1a1-Th-OE mice. After 4 weeks of doxycycline-supplemented chow, we retested AMPH-induced behavior (Figure 5A) and found significantly reduced AMPH responses in Slc1a1-Th-OE mice compared to controls–after doxycycline treatment, Slc1a1-Th-OE mice actually displayed less locomotor activity at the low dose than controls (Figure 5C, third order polynomial least squares fit, t = 0–60; F (4, 370) = 5.153, P = 0.0005). Locomotor activation at the moderate AMPH dose (Figure 5E, third order polynomial least squares fit, t = 0–60; F (4, 370) = 2.105, P = 0.0796), and stereotypic behavior at the high AMPH dose (Figure 5G, 3-way RM ANOVA; drug × genotype interaction, F (1, 38) = 0.05213, P = 0.8206; drug, F (1, 38) = 96.66, P < 0.0001, genotype, F (1, 38) = 0.040704, P = 0.8411) was comparable to controls.
Figure 5. Reversal of EAAT3 overexpression in adulthood rescues AMPH-induced locomotion and stereotypic behavior and dopaminergic neuron activity.
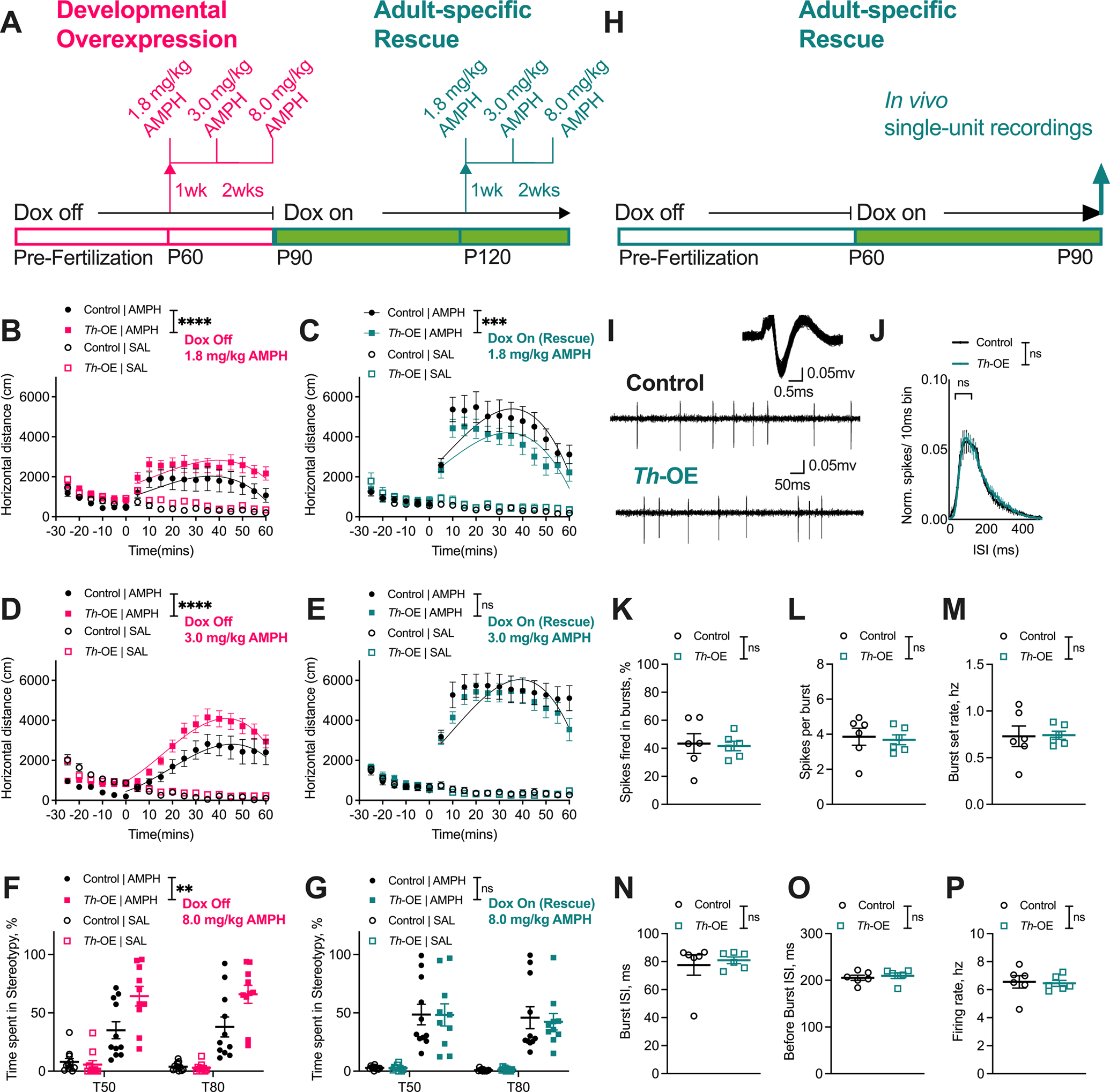
(A) Behavior timeline. For all behavioral panels, n = 11 controls, 10 Th-OEs. Developmentally overexpressing Th-OE mice showed increased locomotor activation at low (B) and moderate (D) doses of AMPH and increased stereotypic behavior at 8.0 mg/kg AMPH dose (F). Following doxycycline administration to these same mice, Th-OE mice showed (C) reduced locomotor activation in response to low AMPH dose in comparison with control mice. (E) Locomotor activation of rescue Th-OE mice was indistinguishable from control mice at the moderate AMPH dose. (G) Further, adult-specific rescue Th-OE mice showed AMPH-induced stereotypic behavior that was comparable to the control group. (H) Single-unit recordings timeline. (I) Representative dopaminergic neuron waveform and spiking patterns from naïve adult-specific rescue Th-OE and control animals. For all ephys panels, n = 55 cells/ 6 control mice, 46 cells/ 6 Th-OE mice. (J) Normalized ISI histograms showing no changes in proportion of short duration ISIs in adult-specific rescue Th-OE mice. (K) % spikes fired in bursts, (L) spikes per burst, (M) burst set rate, (N) average burst ISI, (O) average before-burst ISI, and (P) firing rate are not altered in adult-specific rescue Th-OE mice in comparison to control mice. ****P < 0.0001; ***P < 0.001; **P < 0.01; nsP, not significant. Also see Supplementary Figures S8, S9 for naïve adult-specific rescue behavioral experiments.
To eliminate the potential confound of repeated exposure to high-dose AMPH, a separate cohort of drug naïve mice were switched to doxycycline-supplemented chow at 2 months of age and then evaluated for pharmacological-induced behaviors starting at 4 months (Supplementary Figure S8). We found a slightly increased locomotor response at the low dose in Slc1a1-Th-OE mice compared to littermate controls in this cohort (Supplementary Figure S8C, third order polynomial least squares fit, t = 0–60; F (4, 298) = 6.411, P < 0.0001) and no differences at the moderate (Supplementary Figure S8D) or high AMPH doses (Supplementary Figure S8E), or in SKF-38393-induced grooming behavior (Supplementary Figure S8F).
To determine whether the increased dopaminergic neuron spike firing in life-time overexpressing Slc1a1-Th-OE mice could be rescued by normalizing EAAT3 expression, both adult naïve Slc1a1-Th-OE and littermate control mice were placed on doxycycline-supplemented chow (Figure 5H). One month after initiating doxycycline, no significant genotype differences were observed in the proportion of short duration ISIs in adult-specific rescue Slc1a1-Th-OE mice compared to controls (Figure 5J, 2-way RM ANOVA: ISI × genotype, F (50, 500) = 0.1851, P > 0.9999; ISI, F (50, 500) = 60.06, P < 0.0001, genotype, F (1, 10) = 3.171, P = 0.1053). Furthermore, no significant genotype differences were observed in percentage of total spikes fired in bursts (Figure 5K, unpaired t (10) = 0.2226, P = 0.8283), spikes per burst (Figure 5L, unpaired t (10) = 0.3098, P = 0.7631), burst set rate (Figure 5M, unpaired t (10) = 0.1082, P = 0.9160), average burst ISI (Figure 5N, unpaired t (10) = 0.4365, P = 0.6717), and average before-burst ISI (Figure 5O, unpaired t (10) = 0.5456, P = 0.5973). Finally, no genotype differences were observed in firing rate (Figure 5P, unpaired t (10) = 0.2049, P = 0.8417) after adult-specific administration of doxycycline. Together, these results indicate that normalizing EAAT3 expression in adulthood leads to both normalization of AMPH-induced behaviors and of dopamine neuron firing.
Discussion
Here, using a novel transgenic strategy, we found that overexpression of Slc1a1/EAAT3 in TH-positive neurons can increase AMPH-induced hyperlocomotion and repetitive behavior. This was accompanied by increased baseline burst firing of dopaminergic neurons in anesthetized mice and increased dopamine release in striatum in awake, freely moving animals. Surprisingly, we also observed a developmental effect of Slc1a1/EAAT3 expression on AMPH-induced repetitive behavior. Specifically, we found that both developmental and adult overexpression of Slc1a1 are necessary to induce increases in AMPH-induced hyperlocomotion and stereotypy, whereas neither by itself is sufficient. Our findings identify dopaminergic Slc1a1/EAAT3 activity over the life course as a mechanism that shapes susceptibility to AMPH-induced compulsive-like behavior, and provide a causal link between elevated dopaminergic EAAT3 activity that begins in early development and increased stereotypic activity induced by AMPH. Lastly, we found that rescue of Slc1a1 expression in adulthood is sufficient to restore AMPH-induced responses, suggesting normalization of EAAT3 as a potential treatment strategy in basal ganglia-mediated repetitive behaviors.
Our data indicate that altered EAAT3 expression in dopaminergic neurons impacts baseline dopamine release, as well as AMPH-induced release and downstream repetitive behavior, as is seen in some people with OCD or TS (31–34). We did not, however, observe increased grooming, a behavior that is also dependent upon the basal ganglia and has face validity for OCD (48, 93, 94). We also did not find increased anxiety-like behavior, despite the prominence of anxiety in OCD. The increased grooming and anxiety-like behavior previously reported with a CamKII-Cre-mediated transgenic approach (72, 73) may indicate that EAAT3 overexpression in other brain regions or circuits is responsible for other OCD-relevant phenotypes. Future work will be necessary to fully disentangle the cell type-specific and developmental impact of EAAT3 on OCD-relevant circuits and behavior.
We observed decreased expression of EAAT3 in Slc1a1-tetO mice, our controls for the Slc1a1-Th-OE mice, in comparison to wildtype mice. Importantly, there was no difference in baseline dopaminergic neuron activity or AMPH-induced behaviors in these animals, supporting their use as the appropriate controls. Previous work in EAAT3 heterozygous mice similarly found intact baseline anxiety-like and compulsive-like behaviors, AMPH-induced response, and glutamate and dopamine levels (95), suggesting that a larger change in EAAT3 expression is necessary to induce a functional change.
We previously reported that constitutive ablation of Slc1a1/EAAT3 caused decreased AMPH-induced locomotion and stereotypy, as well as decreased SKF-38393-induced grooming behavior (75). Rescue of midbrain EAAT3 expression in this previous study using a constitutive viral Cre promotor partially rescued the AMPH response in these animals but was not specific to a neuronal subtype (75). Based on previous studies, midbrain GABAergic and dopaminergic neurons were candidates for this rescue effect (62, 96, 97). Unfortunately, transgenic lines that drive Cre expression in GABAergic neurons yield broad expression that extends beyond the midbrain (98), and it has been difficult to specifically drive Cre expression in GABAergic neurons using viral vectors (99, 100). Furthermore, transgenic animals that drive dopaminergic Cre expression (101, 102) could not be used to further localize this effect because we found that they have baseline changes in AMPH response (87). Our data in Slc1a1-Th-OE mice support and refine previous imaging, pharmacologic, and transgenic work that implicates altered dopaminergic transmission in compulsive behavior and provide Slc1a1-driven increases in dopaminergic transmission during development as a pathophysiological mechanism that contributes to susceptibility to pharmacologically-induced compulsive-like behavior.
The impact of EAAT3 overexpression on dopaminergic transmission and AMPH-induced repetitive behavior could result from multiple potential mechanisms. Notably, AMPH triggers endocytosis of EAAT3 upon entry into dopamine neurons and leads to potentiation of glutamatergic transmission via activation of extra-synaptic NMDA receptors (59, 61, 62). Although we did not directly evaluate glutamate transmission in dopamine neurons, it is conceivable that long-term decreases in exposure to glutamate due to overexpression of EAAT3 leads to compensatory increases in NMDA receptor expression and/or sensitivity. Indirect evidence for altered NMDA receptor function comes from our in vivo recording data where, compared with control animals, an increase in burst firing is observed in EAAT3 overexpressing mice. Notably, EAAT3 also plays roles in supplying glutamate for GABA synthesis (96, 97) and cysteine for glutathione synthesis (103, 104), so alternative mechanisms could also account for the changes in dopaminergic neuron burst firing, striatal dopamine release, and AMPH response in overexpressing mice. Future studies examining glutamate release (105), receptor subunit composition of glutamate and GABA receptors, and the impact on other glutamate transporters’ function, will be important for revealing molecular mechanisms underlying increased dopaminergic transmission in Slc1a1-Th-OE mice.
We observed a slight increase in response to 1.8 mg/kg AMPH in Slc1a1-Th-OE mice (Fig 4D, S8C) despite life-time suppression of overexpression by doxycycline. This increase was significant in the curve fit analysis but not the AUC analysis, and no change in response was seen to higher dose AMPH, suggesting a small effect size. It seems possible that this change reflects a small amount of residual EAAT3 overexpression, which could be assessed in future experiments using higher doses of doxycycline. Remarkably, inducing Slc1a1/EAAT3 overexpression in adulthood alone did not lead to increases in AMPH-induced locomotion or stereotypy. Failure to induce increases in AMPH response in adult mice is especially intriguing as the effects of both EAAT3 overexpression and knockdown (Figure 5 and (75)) can still be rescued in adulthood. Our data showing the necessity of both developmental and adult EAAT3 overexpression in the induction of increases in AMPH-induced repetitive behavior also parallel genetic data that show a stronger association between SLC1A1 and early-onset OCD (50–53, 55). These temporal-specific aspects of EAAT3 function may reflect the protracted course of dopaminergic system development that continues into adolescence (106, 107). Our data highlight the need to study the role of genetic influences during early developmental epochs when cognitive circuits and processes are maturing. Future work will be needed to evaluate potential effects of altered dopaminergic signaling on OCD-relevant cognitive tasks, such as reversal learning or set shifting (108–111).
Finally, we found that the effects of EAAT3 overexpression in TH-positive neurons are reversible. Specifically, restoration of normal levels of expression in adult animals reverses the increases in dopaminergic neuron spiking activity and the novelty- and AMPH-induced increases in locomotion and AMPH-induced stereotypic behavior, indicating that maintenance of these phenotypes requires continuing EAAT3 overexpression in TH-positive neurons. This could suggest therapeutic potential of EAAT3 inhibition for abnormal repetitive behaviors, including those that have an early onset. The apparent over-rescue of the 1.8 mg/kg AMPH response in Slc1a1-Th-OE mice following doxycycline-mediated suppression in the longitudinal study (Fig 5C) is intriguing and could suggest opposite impacts of developmental and adult expression of EAAT3. However, the lack of any behavioral differences in response to 3.0 mg/kg and 8.0 mg/kg of AMPH suggest that any such effect is small.
In contrast to the robust and reversible effects on AMPH-induced repetitive behaviors, we observed minimal or no change in several other behaviors. Increased dopaminergic burst firing in Slc1a1-Th-OE mice did align with a slight increase in locomotion in a novel environment, which is known to induce a transition from tonic to burst firing of dopaminergic neurons (112–116). However, we did not observe changes in baseline anxiety-like or repetitive behavior. This is not altogether surprising, as the directionality of the relationship between anxiety and dopaminergic activity may vary by brain region based on the literature. For example, although genetic suppression of dopaminergic neuron firing promotes anxiety-like behavior (117), infusions of dopaminergic agonists in limbic forebrain regions can also increase behavioral measures of anxiety (118). Indeed, evidence from pharmacological (119) and imaging studies (120) lend support for both hypodopaminergic and hyperdopaminergic states leading to anxiety in humans, highlighting the need to study the cellular targets of dopaminergic neurons that mediate anxiety phenotypes. In contrast with our specific overexpression in TH-positive neurons, a recent study evaluated the impact of forebrain-specific overexpression of EAAT3 and found increased anxiety-like and grooming behavior (72), suggesting a possible separate role of forebrain Slc1a1/EAAT3 expression in these phenotypes. Intriguingly, in a prior study, mice with CaMKIIα-Cre-dependent Slc1a1 overexpression exhibited reduced basal dopamine levels in the striatum (73). Decreased basal dopaminergic tone may reflect changes in dopamine D2-mediated inhibition, which was found to be increased in this line (73). Because CaMKIIα is broadly expressed in the forebrain (121, 122), and is expressed in heterogenous cell types, the increased anxiety-like and compulsive behaviors at baseline could potentially result from changes in multiple brain regions.
As noted above, there is substantial evidence suggesting that OCD and TS are etiologically linked. Tic disorders were present in the pediatric OCD populations in which linkage and association with SLC1A1 was initially studied (50, 52, 53). Multiple carriers of a loss of function variant in HDC, which segregated perfectly with TS (123), had co-occurring OCD (33). Of relevance, AMPH can trigger compulsive-like behavior in humans and exacerbate tics in individuals with TS (31, 34), as well as increase stereotypies in Hdc-deficient mice (33). Interestingly, an early case-control study of SLC1A1 in schizophrenia identified a single-nucleotide polymorphism (rs7022369) that was associated with increased SLC1A1 expression in postmortem tissue (124); however, a recent well-powered genome-wide association study of schizophrenia did not find genome wide significance for SLC1A1, making its role in schizophrenia unclear (125). Our findings of increased AMPH sensitivity in Slc1a1-Th-OE mice are reminiscent of the increased AMPH sensitivity that is observed in rodent models of schizophrenia and in human patients (126), potentially warranting examination of schizophrenia-relevant behavior in these animals.
One caveat of these studies is that we cannot exclude the contribution of perturbed norepinephrine signaling in the observed phenotypes. AMPH can induce endocytosis of EAAT3 in cultured locus coeruleus (LC) norepinephrine neurons, indicating that AMPH also potentiates glutamatergic signaling in this cell population (127). Notably, norepinephrine-dopamine co-release from the LC is extensive and has been shown to be involved in novelty-associated memory formation (112, 128, 129). Dopamine release from norepinephrine neurons could thus be an additional mechanism by which EAAT3 modulates dopamine-dependent behaviors. However, we do not expect this is a significant contributor to these results because our previous experiments with midbrain viral injections would not have rescued EAAT3 expression in the LC (75).
In summary, we used a circuit- and cell-type specific approach to begin to dissect the impact of overexpression of the OCD candidate gene Slc1a1/EAAT3. We found that dopamine neuron overexpression of EAAT3 that begins in early life leads both to increased AMPH-induced stereotypic behavior and increased dopaminergic transmission in the striatum. Importantly, overexpression of Slc1a1/EAAT3 in dopaminergic neurons did not affect anxiety-like or self-grooming behavior, suggesting that direct effects of EAAT3 manipulation in dopaminergic neurons affect some but not all OCD-relevant behaviors. Surprisingly, adult-specific overexpression of Slc1a1/EAAT3 failed to recapitulate the phenotypes that emerged after induction of overexpression in early development, suggesting that the effects of EAAT3 overexpression are developmentally mediated. Our electrophysiological data suggest that alterations in dopaminergic transmission during developmentally sensitive periods may be a mechanism underlying the effects of Slc1a1/EAAT3 overexpression, warranting additional work to understand the dynamics of glutamatergic and dopaminergic signaling in basal ganglia circuits during early developmental periods. Finally, our electrophysiological and behavioral data following reversal of EAAT3 overexpression suggest that EAAT3 inhibitors should be evaluated as a potential treatment for basal ganglia-mediated repetitive behavior.
Supplementary Material
Acknowledgements
We would like to thank Ziwen Wang and Hanxiao Liu for assistance with general laboratory support. This work was funded by NIH Grant MH114296 (to J.V. and S.E.A.).
Conflict of Interest
Dr. Veenstra-VanderWeele has received research support from the National Institutes of Health, the Simons Foundation, Roche, and Janssen. He has served on advisory boards for Roche, Autism Speaks, the Brain and Behavior Research Foundation, and the Autism Biomarkers Consortium for Clinical Trials. He has served as associate editor of Autism Research, as a special issue editor for Pediatrics, and on the editorial boards of JAMA Psychiatry, the Journal of Autism and Developmental Disorders, Autism, and the Journal of Neurodevelopmental Disorders. Dr. Ahmari has received research support from the National Institutes of Health, the International OCD Foundation, the One Mind Foundation, the Brain Research Foundation, the Klingenstein-Simons Foundation, the McKnight Foundation, and the Foundation for OCD Research. She serves on advisory boards for the International OCD Foundation, ORCHARD, and the Brain and Behavior Research Foundation. She serves as an associate editor of Neuropsychopharmacology. The other authors disclose no biomedical financial interests or potential conflicts of interest.
References
- 1.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- 2.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Depression and other common mental disorders: global health estimates. World Health Organization; 2017. [Google Scholar]
- 4.Ost LG, Havnen A, Hansen B, Kvale G. Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993–2014. Clin Psychol Rev. 2015;40:156–69. [DOI] [PubMed] [Google Scholar]
- 5.Soomro GM, Altman D, Rajagopal S, Oakley-Browne M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008(1):CD001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. Br J Psychiatry. 1999;174:297–303. [DOI] [PubMed] [Google Scholar]
- 8.Meier SM, Petersen L, Schendel DE, Mattheisen M, Mortensen PB, Mors O. Obsessive-Compulsive Disorder and Autism Spectrum Disorders: Longitudinal and Offspring Risk. PLoS One. 2015;10(11):e0141703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012;21(8):451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivarsson T, Melin K, Wallin L. Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD). Eur Child Adolesc Psychiatry. 2008;17(1):20–31. [DOI] [PubMed] [Google Scholar]
- 11.Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 1999;8(3):445–60. [PubMed] [Google Scholar]
- 12.Haber SN, Heilbronner SR. Translational research in OCD: circuitry and mechanisms. Neuropsychopharmacology. 2013;38(1):252–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmari SE, Dougherty DD. Dissecting Ocd Circuits: From Animal Models to Targeted Treatments. Depress Anxiety. 2015;32(8):550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vicente AM, Martins GJ, Costa RM. Cortico-basal ganglia circuits underlying dysfunctional control of motor behaviors in neuropsychiatric disorders. Curr Opin Genet Dev. 2020;65:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis. Am J Psychiatry. 2017;174(1):60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg DR, Keshavan MS, O’Hearn KM, Dick EL, Bagwell WW, Seymour AB, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54(9):824–30. [DOI] [PubMed] [Google Scholar]
- 17.Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52(5):393–8. [DOI] [PubMed] [Google Scholar]
- 18.Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J Psychosom Res. 2009;67(6):559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan X, Zhang S, Wang W, Su X, Li J, Yang X, et al. Gray matter abnormalities in Tourette Syndrome: a meta-analysis of voxel-based morphometry studies. Transl Psychiatry. 2021;11(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxter LR Jr., Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44(3):211–8. [DOI] [PubMed] [Google Scholar]
- 21.Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989;46(6):518–23. [DOI] [PubMed] [Google Scholar]
- 22.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55(4):326–33. [DOI] [PubMed] [Google Scholar]
- 23.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53(7):595–606. [DOI] [PubMed] [Google Scholar]
- 24.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62–70. [DOI] [PubMed] [Google Scholar]
- 25.Worbe Y, Malherbe C, Hartmann A, Pelegrini-Issac M, Messe A, Vidailhet M, et al. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135(Pt 6):1937–46. [DOI] [PubMed] [Google Scholar]
- 26.Guehl D, Benazzouz A, Aouizerate B, Cuny E, Rotge JY, Rougier A, et al. Neuronal correlates of obsessions in the caudate nucleus. Biol Psychiatry. 2008;63(6):557–62. [DOI] [PubMed] [Google Scholar]
- 27.Welter ML, Burbaud P, Fernandez-Vidal S, Bardinet E, Coste J, Piallat B, et al. Basal ganglia dysfunction in OCD: subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transl Psychiatry. 2011;1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priori A, Giannicola G, Rosa M, Marceglia S, Servello D, Sassi M, et al. Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neurosci Biobehav Rev. 2013;37(6):1063–8. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang P, Hallett M, Zhang X, Li J, Zhang Y, Li Y. Neuronal activity in the globus pallidus internus in patients with tics. J Neurol Neurosurg Psychiatry. 2009;80(10):1075–81. [DOI] [PubMed] [Google Scholar]
- 30.Marceglia S, Servello D, Foffani G, Porta M, Sassi M, Mrakic-Sposta S, et al. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov Disord. 2010;25(3):300–8. [DOI] [PubMed] [Google Scholar]
- 31.Shakeri J, Farnia V, Karimi AR, Tatari F, Juibari TA, Alikhani M, et al. The prevalence and clinical features of amphetamine-induced obsessive compulsive disorder. Drug Alcohol Depend. 2016;160:157–62. [DOI] [PubMed] [Google Scholar]
- 32.Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8(12):1140–9. [DOI] [PubMed] [Google Scholar]
- 33.Baldan LC, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81(1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madruga-Garrido M, Mir P. Tics and other stereotyped movements as side effects of pharmacological treatment. Int Rev Neurobiol. 2013;112:481–94. [DOI] [PubMed] [Google Scholar]
- 35.Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shale H, Fahn S, Mayeux R. Tics in a patient with Parkinson’s disease. Mov Disord. 1986;1(1):79–83. [DOI] [PubMed] [Google Scholar]
- 37.Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol Psychiatry. 2004;55(10):1041–5. [DOI] [PubMed] [Google Scholar]
- 38.Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, et al. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42(1):306–14. [DOI] [PubMed] [Google Scholar]
- 39.Hienert M, Gryglewski G, Stamenkovic M, Kasper S, Lanzenberger R. Striatal dopaminergic alterations in Tourette’s syndrome: a meta-analysis based on 16 PET and SPECT neuroimaging studies. Transl Psychiatry. 2018;8(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsh R, Pagliaccio D, Fitzgerald K. Neuromelanin-Sensitive MRI as a Marker of Dopamine Hyperactivity and CBT Response in Children With Obsessive-Compulsive Disorder. Biological Psychiatry. 2021;89(9, Supplement):S79–S80. [Google Scholar]
- 41.Singer HS, Szymanski S, Giuliano J, Yokoi F, Dogan AS, Brasic JR, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159(8):1329–36. [DOI] [PubMed] [Google Scholar]
- 42.Ernst M, Zametkin AJ, Jons PH, Matochik JA, Pascualvaca D, Cohen RM. High presynaptic dopaminergic activity in children with Tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(1):86–94. [DOI] [PubMed] [Google Scholar]
- 43.Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11(7):622–32. [DOI] [PubMed] [Google Scholar]
- 44.Albert U, Carmassi C, Cosci F, De Cori D, Di Nicola M, Ferrari S, et al. Role and clinical implications of atypical antipsychotics in anxiety disorders, obsessive-compulsive disorder, trauma-related, and somatic symptom disorders: a systematized review. Int Clin Psychopharmacol. 2016;31(5):249–58. [DOI] [PubMed] [Google Scholar]
- 45.Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers. 2017;3:16097. [DOI] [PubMed] [Google Scholar]
- 46.Du JC, Chiu TF, Lee KM, Wu HL, Yang YC, Hsu SY, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255–64. [DOI] [PubMed] [Google Scholar]
- 47.Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauls DL. The genetics of obsessive compulsive disorder: a review of the evidence. Am J Med Genet C Semin Med Genet. 2008;148C(2):133–9. [DOI] [PubMed] [Google Scholar]
- 50.Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, et al. Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet. 2002;114(5):541–52. [DOI] [PubMed] [Google Scholar]
- 51.Willour VL, Yao Shugart Y, Samuels J, Grados M, Cullen B, Bienvenu OJ 3rd, et al. Replication study supports evidence for linkage to 9p24 in obsessive-compulsive disorder. Am J Hum Genet. 2004;75(3):508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veenstra-VanderWeele J, Kim SJ, Gonen D, Hanna GL, Leventhal BL, Cook EH Jr. Genomic organization of the SLC1A1/EAAC1 gene and mutation screening in early-onset obsessive-compulsive disorder. Mol Psychiatry. 2001;6(2):160–7. [DOI] [PubMed] [Google Scholar]
- 53.Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778–85. [DOI] [PubMed] [Google Scholar]
- 54.Wendland JR, Moya PR, Timpano KR, Anavitarte AP, Kruse MR, Wheaton MG, et al. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66(4):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):769–76. [DOI] [PubMed] [Google Scholar]
- 56.Stewart SE, Mayerfeld C, Arnold PD, Crane JR, O’Dushlaine C, Fagerness JA, et al. Meta-analysis of association between obsessive-compulsive disorder and the 3’ region of neuronal glutamate transporter gene SLC1A1. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(4):367–79. [DOI] [PubMed] [Google Scholar]
- 57.Stewart SE, Yu D, Scharf JM, Neale BM, Fagerness JA, Mathews CA, et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry. 2013;18(7):788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2015;20(3):337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underhill SM, Ingram SL, Ahmari SE, Veenstra-VanderWeele J, Amara SG. Neuronal excitatory amino acid transporter EAAT3: Emerging functions in health and disease. Neurochem Int. 2019;123:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98(4):1007–18. [DOI] [PubMed] [Google Scholar]
- 61.Li MH, Underhill SM, Reed C, Phillips TJ, Amara SG, Ingram SL. Amphetamine and Methamphetamine Increase NMDAR-GluN2B Synaptic Currents in Midbrain Dopamine Neurons. Neuropsychopharmacology. 2017;42(7):1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron. 2014;83(2):404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate tranporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8(2):108–16. [DOI] [PubMed] [Google Scholar]
- 64.Otis TS, Brasnjo G, Dzubay JA, Pratap M. Interactions between glutamate transporters and metabotropic glutamate receptors at excitatory synapses in the cerebellar cortex. Neurochem Int. 2004;45(4):537–44. [DOI] [PubMed] [Google Scholar]
- 65.Brennan BP, Rauch SL, Jensen JE, Pope HG Jr. A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biol Psychiatry. 2013;73(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology. 2009;34(12):2489–96. [DOI] [PubMed] [Google Scholar]
- 67.Piantadosi SC, Chamberlain BL, Glausier JR, Lewis DA, Ahmari SE. Lower excitatory synaptic gene expression in orbitofrontal cortex and striatum in an initial study of subjects with obsessive compulsive disorder. Mol Psychiatry. 2019. [DOI] [PubMed] [Google Scholar]
- 68.Mahone EM, Puts NA, Edden RAE, Ryan M, Singer HS. GABA and glutamate in children with Tourette syndrome: A (1)H MR spectroscopy study at 7T. Psychiatry Res Neuroimaging. 2018;273:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson GM, Pollak ES, Chatterjee D, Leckman JF, Riddle MA, Cohen DJ. Brain monoamines and amino acids in Gilles de la Tourette’s syndrome: a preliminary study of subcortical regions. Arch Gen Psychiatry. 1992;49(7):584–6. [DOI] [PubMed] [Google Scholar]
- 70.Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310(5746):317–20. [DOI] [PubMed] [Google Scholar]
- 71.Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, et al. Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatr Genet. 2011;21(2):90–7. [DOI] [PubMed] [Google Scholar]
- 72.Delgado-Acevedo C, Estay SF, Radke AK, Sengupta A, Escobar AP, Henriquez-Belmar F, et al. Behavioral and synaptic alterations relevant to obsessive-compulsive disorder in mice with increased EAAT3 expression. Neuropsychopharmacology. 2019;44(6):1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Escobar AP, Martinez-Pinto J, Silva-Olivares F, Sotomayor-Zarate R, Moya PR. Altered Grooming Syntax and Amphetamine-Induced Dopamine Release in EAAT3 Overexpressing Mice. Front Cell Neurosci. 2021;15:661478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denys D, de Vries F, Cath D, Figee M, Vulink N, Veltman DJ, et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013;23(11):1423–31. [DOI] [PubMed] [Google Scholar]
- 75.Zike ID, Chohan MO, Kopelman JM, Krasnow EN, Flicker D, Nautiyal KM, et al. OCD candidate gene SLC1A1/EAAT3 impacts basal ganglia-mediated activity and stereotypic behavior. Proc Natl Acad Sci U S A. 2017;114(22):5719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka KF, Ahmari SE, Leonardo ED, Richardson-Jones JW, Budreck EC, Scheiffele P, et al. Flexible Accelerated STOP Tetracycline Operator-knockin (FAST): a versatile and efficient new gene modulating system. Biol Psychiatry. 2010;67(8):770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y, Wang C, Sun H, LeRoith D, Yakar S. High-efficient FLPo deleter mice in C57BL/6J background. PLoS One. 2009;4(11):e8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tillack K, Aboutalebi H, Kramer ER. An Efficient and Versatile System for Visualization and Genetic Modification of Dopaminergic Neurons in Transgenic Mice. PLoS One. 2015;10(8):e0136203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4(11):2877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2. [DOI] [PubMed] [Google Scholar]
- 81.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 2018;360(6396). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martianova E, Aronson S, Proulx CD. Multi-Fiber Photometry to Record Neural Activity in Freely-Moving Animals. J Vis Exp. 2019(152). [DOI] [PubMed] [Google Scholar]
- 84.Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338(6103):128–32. [DOI] [PubMed] [Google Scholar]
- 85.Shinozaki Y, Shibata K, Yoshida K, Shigetomi E, Gachet C, Ikenaka K, et al. Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation. Cell Rep. 2017;19(6):1151–64. [DOI] [PubMed] [Google Scholar]
- 86.Qiu L, Rivera-Perez JA, Xu Z. A non-specific effect associated with conditional transgene expression based on Cre-loxP strategy in mice. PLoS One. 2011;6(5):e18778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chohan MO, Esses S, Haft J, Ahmari S, Veenstra-VanderWeele J. Altered baseline and amphetamine-mediated behavioral profiles in dopamine transporter Cre (DAT-Ires-Cre) mice compared to tyrosine hydroxylase Cre (TH-Cre) mice. Psychopharmacology (Berl). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kramer DJ, Aisenberg EE, Kosillo P, Friedmann D, Stafford DA, Lee AY, et al. Generation of a DAT-P2A-Flpo mouse line for intersectional genetic targeting of dopamine neuron subpopulations. Cell Rep. 2021;35(6):109123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143(1):27–32. [DOI] [PubMed] [Google Scholar]
- 90.Steinmetz NA, Buetfering C, Lecoq J, Lee CR, Peters AJ, Jacobs EAK, et al. Aberrant Cortical Activity in Multiple GCaMP6-Expressing Transgenic Mouse Lines. eNeuro. 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costa KM, Schenkel D, Roeper J. Sex-dependent alterations in behavior, drug responses and dopamine transporter expression in heterozygous DAT-Cre mice. Sci Rep. 2021;11(1):3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci U S A. 2014;111(20):7450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(5):710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez LF, Henriquez-Belmar F, Delgado-Acevedo C, Cisternas-Olmedo M, Arriagada G, Sotomayor-Zarate R, et al. Neurochemical and behavioral characterization of neuronal glutamate transporter EAAT3 heterozygous mice. Biol Res. 2017;50(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003;23(6):2040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22(15):6372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nathanson JL, Jappelli R, Scheeff ED, Manning G, Obata K, Brenner S, et al. Short Promoters in Viral Vectors Drive Selective Expression in Mammalian Inhibitory Neurons, but do not Restrict Activity to Specific Inhibitory Cell-Types. Front Neural Circuits. 2009;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lahti L, Achim K, Partanen J. Molecular regulation of GABAergic neuron differentiation and diversity in the developing midbrain. Acta Physiol (Oxf). 2013;207(4):616–27. [DOI] [PubMed] [Google Scholar]
- 101.Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci. 2005;25(29):6721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, et al. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis. 2006;44(8):383–90. [DOI] [PubMed] [Google Scholar]
- 103.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9(1):119–26. [DOI] [PubMed] [Google Scholar]
- 104.Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, et al. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Annals of neurology. 2011;69(3):509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Muller JA, et al. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat Methods. 2018;15(11):936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rothmond DA, Weickert CS, Webster MJ. Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. BMC Neurosci. 2012;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 2012;32(46):16223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powell SB, Khan A, Young JW, Scott CN, Buell MR, Caldwell S, et al. Early Adolescent Emergence of Reversal Learning Impairments in Isolation-Reared Rats. Dev Neurosci. 2015;37(3):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Radke AK, Kocharian A, Covey DP, Lovinger DM, Cheer JF, Mateo Y, et al. Contributions of nucleus accumbens dopamine to cognitive flexibility. Eur J Neurosci. 2019;50(3):2023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116(1):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mar AC, Horner AE, Nilsson SR, Alsio J, Kent BA, Kim CH, et al. The touchscreen operant platform for assessing executive function in rats and mice. Nat Protoc. 2013;8(10):1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537(7620):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McNamara CG, Tejero-Cantero A, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17(12):1658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. 2012;15(9):1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–9. [DOI] [PubMed] [Google Scholar]
- 116.Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci. 2001;13(4):819–28. [DOI] [PubMed] [Google Scholar]
- 117.Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14(5):620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bananej M, Karimi-Sori A, Zarrindast MR, Ahmadi S. D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J Psychopharmacol. 2012;26(4):564–74. [DOI] [PubMed] [Google Scholar]
- 119.Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14(2):123–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11(12):1381–2. [DOI] [PubMed] [Google Scholar]
- 121.Wang X, Zhang C, Szabo G, Sun QQ. Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain Res. 2013;1518:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cook SG, Bourke AM, O’Leary H, Zaegel V, Lasda E, Mize-Berge J, et al. Analysis of the CaMKIIalpha and beta splice-variant distribution among brain regions reveals isoform-specific differences in holoenzyme formation. Sci Rep. 2018;8(1):5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette’s syndrome. N Engl J Med. 2010;362(20):1901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Horiuchi Y, Iida S, Koga M, Ishiguro H, Iijima Y, Inada T, et al. Association of SNPs linked to increased expression of SLC1A1 with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(1):30–7. [DOI] [PubMed] [Google Scholar]
- 125.Pardinas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peleg-Raibstein D, Yee BK, Feldon J, Hauser J. The amphetamine sensitization model of schizophrenia: relevance beyond psychotic symptoms? Psychopharmacology (Berl). 2009;206(4):603–21. [DOI] [PubMed] [Google Scholar]
- 127.Underhill SM, Colt MS, Amara SG. Amphetamine Stimulates Endocytosis of the Norepinephrine and Neuronal Glutamate Transporters in Cultured Locus Coeruleus Neurons. Neurochem Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6(6):657–64. [DOI] [PubMed] [Google Scholar]
- 129.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A. 2016;113(51):14835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


