Abstract
Objective
Antenatal and early neonatal nutritional environment may influence later metabolic health. Infants of mothers with gestational diabetes mellitus (GDM) have higher risk for childhood obesity and metabolic syndrome (MetS). Leptin and adiponectin are known biomarkers for MetS and may guide interventions to reduce later obesity. We sought to examine the relationship between birthweight, early infancy feeding practices, and biomarkers for MetS in offspring of women with mild GDM.
Study Design
Secondary analysis of a prospective observational follow-up study on the offspring of women who participated in a multicenter randomized treatment trial on mild GDM. Children were evaluated by research coordinators and biospecimens collected at the age of 5 to 10. Plasma concentrations of leptin and adiponectin were compared between large for gestational age (LGA) and average birthweight (AGA) infants, and according to whether solid foods were introduced early (<6 months of age) or at the recommended age (≥6 months of age). Multivariable analysis adjusted for fetal sex, race/ethnicity, and maternal body mass index.
Results
Leptin and adiponectin were measured in 336 plasma samples. In bivariate analysis, compared with AGA children, LGA children had lower leptin (5.0 ng/mL [3.6–6.0] vs. 5.8 ng/mL [4.5 = 6.6], p = 0.01) and similar adiponectin (6.3 μg/mL [5.1–7.9] vs. 6.4 μg/mL [5.3–8.6], p = 0.49) concentrations. Maternal/child characteristics were similar between the early/delayed solid feeding groups. Leptin and adiponectin concentrations were similar in the early fed and delayed feeding groups (5.8 ng/mL [4.6–6.7] vs. 5.6 ng/mL [4.2–6.6], p = 0.50 and 6.4 μg/mL [5.4–8.1] vs. 6.4 μg/mL [5.1–8.8], p = 0.85, respectively). After controlling for covariates, children who were LGA and AGA at birth had similar leptin concentrations.
Conclusion
Birthweight and early infancy feeding practice are not associated with alterations in leptin and adiponectin in children of women with mild GDM.
Keywords: gestational diabetes, obesity, metabolic syndrome, breastfeeding, metabolism, infant feeding
The prevalence of childhood obesity is increasing and has reached epidemic proportion with ~21% of children in the United States now classified as obese.1 An evolving literature provides evidence linking maternal gestational diabetes mellitus (GDM) and the risk of childhood obesity and/or metabolic syndrome.2–5 It has been proposed that the risk of metabolic syndrome in the children of women affected by GDM occurs as a result of epigenetic mechanisms and dysfunctional hormonal regulation of adipocytokines during intrauterine and postnatal life.6
Adipocytokines are secreted by adipocytes and modulate the secretion of insulin in the maternal-fetal unit. Studies have identified adipocytokines, specifically leptin and adiponectin, as biomarkers of metabolic syndrome in children and adults.7,8 Adiponectin has been implicated in the development of fetal overgrowth. In GDM mothers, lower adiponectin levels are associated with macrosomic fetuses. This decrease in adiponectin stimulates fetal growth through unregulated and excessive transport of glucose, resulting in increased production of insulin growth factors.7,9
While there is evidence to support fetal programming, little research has been done to understand the potential impact of the early neonatal period on long-term programming. As supported by the American Academy of Pediatrics, breastfeeding in the neonatal period is associated with improved neonatal outcomes including decreased obesity.10 Most formulas have higher protein concentration compared with breastmilk and are associated with increased infant weight gain and childhood obesity.11 Breastmilk is known to have increased leptin concentrations compared with formula, leading to decreased hunger and food intake and increased metabolic rate resulting in leaner infants.12 The introduction of solid food, such as cereals prepared with milk or water or pureed meats and vegetables, is recommended after 6 months of age. Initiation of complementary foods before 6 months of age is associated with increased weight gain and adiposity.13 The addition of solid foods to the infant diet increases protein intake, thereby increasing circulating amnio acids that are hypothesized to stimulate insulin-like-growth factor 1.11
Children born to women with GDM are at risk of metabolic syndrome and intervention to prevent this outcome is needed. It is postulated that this risk is associated with both fetal and neonatal exposures related to maternal metabolic dysfunction (►Fig. 1). Markers of metabolic syndrome, including leptin and adiponectin, are altered in women with GDM and in children with metabolic syndrome. It is unknown if these markers differ between children that are formula fed versus breastfed and those who undergo early solid feeding versus those who experience the recommended 6-month delay in solid feeding. Therefore, our objective was to examine whether birthweight and early infancy feeding practices (timing of introduction of solid foods) are associated with biomarkers of metabolic syndrome in the child offspring of women with mild GDM.
Fig. 1.
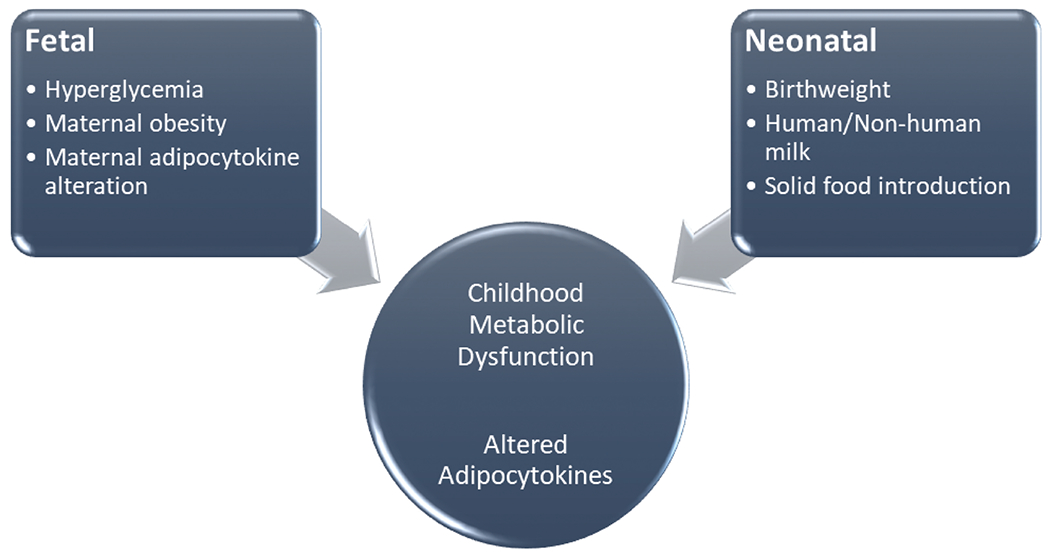
Proposed fetal and neonatal influence on childhood metabolic status in the children of women with gestational diabetes mellitus.
Materials and Methods
We performed a secondary analysis of a prospective observational follow-up study on the offspring of women who participated in a multicenter randomized treatment trial on mild GDM by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network (NICHD MFMU) from October 2002 through November 2007.14,15 Children were eligible for the follow-up study if the mother participated in the parent trial and was enrolled in a participating center that remained a part of the NICHD MFMU network from February 2012 through September 2013, 5 to 10 years following initial enrollment. Parental informed consent, and child assent when appropriate, was obtained for child participation in the follow-up study.15 The original trial and follow-up study were approved by the institutional review board of all participating centers.
At follow-up, parents were queried about breastfeeding, formula feeding, initiation of solid food and their child’s current diet, and physical activity. The children were evaluated by trained research coordinators. They had their height and weight measured, which were used to estimate child body mass index (BMI). Waist circumference was measured just above the uppermost lateral border of the right ilium of the pelvis, and an average of three measurements was used in the analysis. Blood pressure was measured and an average of two measurements was used in the analysis.
After an 8-hour fast, the children had their blood drawn. Specimens were collected per a standardized approach and analyzed at the Northwest Lipid Metabolism and Diabetes Research Laboratories for lipid panel, glucose, and insulin. Additional aliquots were collected for future research evaluation.
For this analysis, all available samples meeting inclusion criteria were analyzed. Inclusion criteria for this analysis were children who participated in the follow-up study whose mother had mild GDM during the index pregnancy (defined as abnormal glucose tolerance test with normal fasting value), had stored blood samples, and known birthweight, neonatal, and child anthropometric measurements and data on infant feeding. An exclusion criterion was birthweight less than 10th percentile. Only samples in which the maternal, neonatal, and child characteristics could be obtained were included in the analysis. Each sample was evaluated for leptin and adiponectin concentration using standardized immunoassay techniques. Leptin (R&D Systems, Minneapolis, MN) and adiponectin (BioLegend, San Diego, CA) immunoassays were performed in duplicate according to manufacturers’ instructions by investigators blinded to clinical case classification. The minimal detectable concentration for leptin and adiponectin was 15.6 and 0.78 ng/mL, respectively.
From the index study, maternal characteristics during pregnancy including age, race/ethnicity, maternal BMI, and tobacco use were evaluated. Neonatal characteristics from the index study were also evaluated including sex, birth-weight, and neonatal fat mass. Birth weight for gestational age was estimated per methods of Alexander et al16 (and personal communication) to categorize newborns into two groups: appropriate for gestational age (birthweight 10th--90th percentile) and large for gestational age (birth weight greater than 90th percentile).
Baseline maternal and child characteristics were compared by the exposures of interest, size for gestational age (LGA or AGA), and early newborn feeding (fed solid foods <6 months of age or fed solid foods ≥ 6 months of age, irrespective of formula or breastmilk as the primary nutrition source). The X2 and Fisher’s exact test were used to compare frequencies of categorical variables, and the Wilcoxon rank-sum test was used to evaluate continuous variables.
Leptin and adiponectin were evaluated as continuous outcomes. Nonparametric tests (Wilcoxon rank sum) were run for unadjusted analysis of biomarker outcomes by size for gestational age and early newborn feeding. In multivariate analysis, linear regression models were adjusted for maternal BMI, race/ethnicity, and child sex. Continuous variables were assessed to evaluate whether they were normally distributed and log-transformed when appropriate for these models. We also tested for interaction between early newborn feeding and type of infant feeding (formula fed only vs. breastfed children).
Prior to secondary analysis, estimation of the required sample size was based on a two-sided type I error of 5%. Given that the original GDM study had a 10.8% rate of LGA infants and based on published literature we assume a twofold increase in mean leptin concentration and a twofold decrease in mean adiponectin concentration, a sample size of 100 (n = 50 exposed and n = 50 unexposed) would be required for 95% power for birthweight. According to Centers for Disease Control and Prevention, ~40% of children are exposed to early feeding. Given that the follow-up GDM study enrolled 55% (500/905) of the original GDM study and an estimated 25% (n = 125) of participants were missing early feeding information, data should be available on n = 150 for early onset feeding (exposed group) and n = 225 in unexposed group. Based on change in adiponectin concentration described above, a total sample size of 80 (n = 40 exposed and n = 40 unexposed) would be required for 95% power for early newborn feeding. All available samples in which there was information regarding maternal characteristics, early infancy feeding, birthweight, and child anthropometric data were utilized for analysis.
SAS software version 9.4 was used for the analyses. All tests were two-tailed and p < 0.05 was considered statistically significant.
The study was approved by the Ohio State University Institutional Review Board.
Results
A total of 336 plasma samples were obtained from the long-term child health and mild gestational diabetes follow-up study. This represented 67% of the offspring enrolled in the initial follow-up study and 86% of the children from the initial follow-up study that provided blood samples. Nearly half, 51.5%, of the sample represented male children. Approximately 60% of the samples represented children born to women of Hispanic background. Only 36.6% of the samples were from overweight or obese children, defined by BMI at or above the 85th percentile or 95th percentile, respectively, for age and sex (►Table 1). The median age at assessment was 7 years. Compared with the initial follow-up study, our baseline characteristics were similar, except there was a higher proportion of Hispanic mothers (59.2 vs. 49.0%).
Table 1.
Maternal and child characteristics in early fed versus non-early fed offspring.
| Maternal and child characteristics | Early fed n=149 |
Not early fed n=187 |
P-Value |
|---|---|---|---|
| Neonatal Characteristics | |||
| Female | 78 (52.4) | 85 (45.5) | 0.21 |
| Birthweight (g) | 3430 (3140 - 3720) | 3400 (3064 - 3720) | 0.63 |
| Neonatal fat mass (g) | 456 (345 - 567) | 449 (326 - 573) | 0.59 |
| Neonatal fat mass ≥ 90th percentile | 15 (11.3) | 16 (9.3) | 0.57 |
| Formula fed only (never breastfed) | 32 (21.5) | 40 (21.4) | 0.98 |
| Child follow-up characteristics | |||
| Child age (years) | 7 (6 - 8) | 7 (6 - 8) | 0.67 |
| Child BMI (kg/m2) | 16.5 (15.2 - 18.6) | 16.5 (15.2 - 18.6) | 0.94 |
| Child BMI ≥ 85th percentile | 54 (36.2) | 69 (36.9) | 0.90 |
| Child waist circumference (cm) | 56.2 (52.5 - 61.2) | 56.4 (52.3 - 64.2) | 0.29 |
| Child waist circumference ≥ 90th percentile | 14 (9.4) | 24 (12.8) | 0.32 |
| Maternal characteristics at enrollment during index pregnancy | |||
| Maternal age (yr) | 29 (25 - 32) | 29 (25 - 33) | 0.92 |
| Race/ethnicity | 0.83 | ||
| Non-Hispanic white | 42 (28.2) | 57 (30.5) | |
| Hispanic | 91 (61.1) | 108 (57.8) | |
| Other/unknown | 16 (10.7) | 22 (11.8) | |
| Maternal BMI (kg/m2) | 29.7 (27.0 - 33.2) | 30.1 (27.0 - 33.3) | 0.95 |
| Maternal tobacco use | 13 (8.7) | 10 (5.4) | 0.22 |
Data are median (interquartile range) or n (%) unless otherwise specified.
A comparison of maternal, neonatal, and childhood characteristics between early fed and not early fed infants revealed no difference in child age, sex, birthweight, neonatal fat mass, type of feeding, childhood BMI, maternal race and ethnicity, maternal age, maternal BMI or maternal tobacco use (►Table 1). Furthermore, a comparison of maternal, neonatal, and childhood characteristics between AGA and LGA neonates revealed no difference in childhood sex and age or maternal race/ethnicity, age, or tobacco use. On the other hand, LGA neonates had greater neonatal fat mass (p < 0.001), percentage of neonatal fat mass ≥ 90th percentile (p < 0.001), child waist circumference (p = 0.01), child BMI (p = 0.001), child BMI ≥ 85th percentile (p < 0.001), and maternal BMI (p = 0.01) (►Table 2).
Table 2.
Maternal and child characteristics in AGA and LGA offspring.
| Maternal and child characteristics | AGA n=293 |
LGA n=43 |
P-Value* |
|---|---|---|---|
| Neonatal Characteristics | |||
| Female | 143 (48.8) | 20 (46.5) | 0.78 |
| Birthweight (g) | 3349 (3045 - 3590) | 4200 (4015 - 4320) | <.001 |
| Neonatal fat mass (g) | 432.2 (313.8 - 519.4) | 689.3 (636.9 - 756.7) | <.001 |
| Neonatal fat mass ≥ 90th percentile | 9 (3.4) | 22 (55.0) | <.001 |
| Child follow-up characteristics | |||
| Child age (years) | 7 (6 - 8) | 7 (6 - 8) | 0.76 |
| Child BMI (kg/m2) | 16.4 (15.1 - 18.4) | 17.8 (16.1 - 21.2) | 0.001 |
| Child BMI ≥ 85th percentile | 97 (33.1) | 26 (60.5) | <.001 |
| Child waist circumference (cm) | 55.9 (51.9 - 61.8) | 59.4 (54.2 - 70.7) | 0.01 |
| Child waist circumference ≥ 90th percentile | 30 (10.2) | 8 (18.6) | 0.11 |
| Maternal characteristics at enrollment during index pregnancy | |||
| Maternal age (yr) | 29 (25 - 33) | 28 (24 - 31) | 0.10 |
| Race/ethnicity | 0.08 | ||
| Non-Hispanic white | 90 (30.7) | 9 (20.9) | |
| Hispanic | 167 (57.0) | 32 (74.4) | |
| Other/unknown | 36 (12.3) | 2 (4.7) | |
| Maternal BMI (kg/m2) | 29.7 (26.4 - 33.1) | 31.7 (28.9 - 35.1) | 0.01 |
| Maternal tobacco use | 22 (7.5) | 1 (2.3) | 0.33 |
Data are median (interquartile range) or n (%) unless otherwise specified.
Based on the chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Bivariate analysis of early solid fed versus nonearly solid fed did not show any difference in leptin (5.8 ng/mL [4.5–6.7] vs. 5.6 ng/mL [4.2–6.6], p = 0.50) or adiponectin (6.4 μg/mL [5.4–8.1] vs. 6.4 μg/mL [5.1–8.8], p = 0.85) concentrations (►Table 3). There was no significant interaction between type of infant feeding (formula fed only vs. breastfed) and early solid feeding in models with either biomarker outcome. Additional bivariate analysis of LGA versus AGA showed higher leptin concentrations in AGA fetuses (5.8 ng/mL [4.5–6.6] vs. 5.0 ng/mL [3.6–6.0], p = 0.009). Adiponectin concentrations in this comparison did not show any statistically significant difference (6.4 μg/mL [5.3–8.6] vs. 6.3 μg/mL [5.1–7.9], p = 0.49) (►Table 3). Evaluation of covariables with leptin and adiponectin concentrations revealed associations with child sex (leptin only), race/ethnicity, maternal BMI (leptin only), child BMI, and child waist circumference (►Table 4). After eliminating variables that could potentially lie in the causal pathway, models were adjusted for race/ethnicity, child sex, and maternal BMI. When adjusting for race/ethnicity, child sex, and maternal BMI, we found no difference in childhood leptin concentrations between children born LGA and AGA (►Table 5).
Table 3.
Biomarker concentration evaluation in early infant feeding and birthweight (AGA vs LGA).
| Unadjusted analysis for infant feeding | |||
|---|---|---|---|
| Early fed n = 149 |
Not early fed n = 187 |
P-value* | |
| Leptin, ng/mL | 5.8 (4.6 - 6.7) | 5.6 (4.2 - 6.6) | 0.50 |
| Adiponectin, μg/mL | 6.4 (5.4 - 8.1) | 6.4 (5.1 - 8.8) | 0.85 |
| Unadjusted analysis for birthweight | |||
| AGA n = 293 |
LGA n = 43 |
||
| Leptin, ng/mL | 5.8 (4.5 - 6.6) | 5.0 (3.6 - 6.0) | 0.008 |
| Adiponectin, μg/mL | 6.4 (5.3 - 8.6) | 6.3 (5.1 - 7.9) | 0.49 |
Data are median (interquartile range).
Based on Wilcoxon rank sum test.
Table 4.
Covariable associations with leptin and adiponectin concentration
| Maternal and child characteristics | Leptin | Adiponectin | ||
|---|---|---|---|---|
| Median, IQR | p-Value | Median, IQR | p-Valuea | |
| Child sex | 0.007 | 0.28 | ||
|
| ||||
| Male | 6.1 (4.5-6.8) | 6.5 (5.3-8.8) | ||
|
| ||||
| Female | 5.5 (4.5-6.3) | 6.2 (5.2-8.3) | ||
|
| ||||
| Neonatal fat mass ≥ 90th percentile | 5.6 (4.6-6.8) | 0.88 | 6.9 (5.4-8.4) | 0.65 |
|
|
|
|||
| Neonatal fat mass < 90th percentile | 5.7 (4.5-6.6) | 6.4 (5.3-8.5) | ||
|
|
|
|||
| Type of feeding | ||||
|
| ||||
| Breastfed | 5.8 (4.3-6.6) | 0.61 | 6.3 (5.2-8.2) | 0.08 |
|
|
|
|||
| Formula fed only | 5.6 (4.7-6.6) | 6.8 (5.4-9.0) | ||
|
|
|
|||
| Child follow-up characteristics | ||||
|
| ||||
| Child BMI ≥ 85th percentile | 4.1 (3.3-5.2) | <0.001 | 7.1 (5.9-9.0) | <0.001 |
|
|
|
|||
| Child BMI < 85th percentile | 6.3 (5.6-6.9) | 5.8 (5.1-7.8) | ||
|
|
|
|||
| Child waist circumference ≥ 90th percentile | 3.6 (2.6-4.1) | <0.001 | 8.1 (5.9-10.1) | 0.003 |
|
|
|
|||
| Child waist circumference < 90th percentile | 5.9 (4.9-6.7) | 6.3 (5.2-8.2) | ||
|
|
|
|||
| Maternal characteristics at enrollment during index pregnancy | ||||
|
| ||||
| Maternal age ≥ 35 | 5.8 (4.5-6.7) | 0.30 | 6.0 (5.1-7.2) | 0.12 |
|
| ||||
| Maternal age < 35 | 5.7 (4.3-6.6) | 6.4 (5.3-8.6) | ||
|
| ||||
| Race/ethnicity | <0.001 | <0.001 | ||
|
|
|
|||
| Non-Hispanic white | 6.1 (5.6-6.8) | 5.5 (4.4-6.4) | ||
|
|
|
|||
| Hispanic | 5.2 (3.9-6.4) | 7.0 (5.7-8.8) | ||
|
|
|
|||
| Other/unknown | 6.2 (5.4-6.9) | 8.6 (6.0-10.3) | ||
|
|
|
|||
| Maternal BMI ≥ 30 (kg/m2) | 5.5 (4.0-6.6) | 0.01 | 6.4 (5.3-8.4) | 0.65 |
|
|
|
|||
| Maternal BMI < 30 (kg/m2) | 6.0 (4.9-6.7) | 6.4 (5.2-8.5) | ||
|
|
|
|||
| Maternal tobacco use | 6.1 (4.1-6.8) | 0.32 | 6.5 (5.2-8.8) | 0.71 |
|
|
|
|||
| No maternal tobacco use | 5.6 (4.5-6.6) | 6.3 (5.3-8.4) | ||
Abbreviation: BMI, body mass index; IQR, interquartile range.
Based on the Wilcoxon rank-sum test.
Table 5.
Adjusted biomarker analysis for infant feeding and birthweight
| Adjusted analysis for infant feeding | |||
|---|---|---|---|
| Early fed n = 149 |
Not early fed n = 187 |
P-value | |
| Leptin, ng/mL | 5.6 (5.3, 6.0) | 5.4 (5.1, 5.7) | 0.25 |
| Adiponectin, μg/mL | 6.6 (6.3, 7.0) | 6.7 (6.4, 7.1) | 0.72 |
| Adjusted analysis for birthweight | |||
| AGA n = 293 |
LGA n = 43 |
||
| Leptin, ng/mL | 5.5 (5.3, 5.8) | 5.0 (4.5, 5.5) | 0.05 |
| Adiponectin, μg/mL | 6.6 (6.4, 6.9) | 6.2 (5.7, 6.9) | 0.20 |
Data are adjusted mean (95% CI)
Comments
In this analysis, we observed no difference in leptin or adiponectin concentration between children of women with mild GDM born LGA versus AGA or in children who were early fed with solid food versus those not. Additionally, formula feeding only versus breastfeeding did not modify the association between early solid feeding and biomarker levels. Previous studies have shown an association between metabolic syndrome, adiposity, obesity, and insulin resistance with adipocytokines including a systemic review demonstrating an association between metabolic syndrome in adults, elevated leptin levels, and decreased adiponectin levels.17 This relationship has also been demonstrated in neonates and obese children and is further supported by Okereke et al, which showed that the offspring of women with GDM had higher umbilical cord leptin levels than the offspring of women without GDM.18 In a European cohort of children with average age of 9 years, overweight or obese children had higher leptin and leptin:adiponectin ratios.19 Children born to women with GDM have increased risk of childhood obesity and metabolic syndrome.20,21 As stated earlier, it is thought that this occurs due to fetal programming of the pancreas and hypothalamus and its subsequent effects of childhood metabolic derangements, including adipocytokine production. While other studies suggest a role of adipocytokines in fetal and neonatal metabolomics, our study does not support their relationship with birthweight or early infancy feeding characteristics, adding to a small number of studies that show a negative relationship between adipocytokines and fetal and neonatal metabolic profiles.
There is emerging evidence to support that early introduction of solid/complementary foods in infancy is associated with increased body adiposity at the age of 7 years.22 This supports an effect of nutrition on greater weight gain and higher risk for obesity in adulthood. This finding of adult obesity is hypothesized in recent studies to be due to leptin programming in the early solid feeding group of newborns. Previous studies have shown that childhood obesity is associated with maternal GDM and infant feeding patterns.23,24 These patterns were not observed in our population of early fed infants that may be due to type I and II errors. The inclusion of only mild GDM cases in the parent study, predominance of prepubertal children, and large proportion of AGA children may have resulted in the lack of association.
Mild GDM was defined as an abnormal 3-hour glucose tolerance test with a normal fasting value. This implies that most of the women represented in the study had relatively minimal hyperglycemia, as demonstrated by only 7% of the women in the treatment group requiring insulin.14 Based on research by Silverman et al, we know that maternal hyperglycemia is associated with elevated amniotic fluid insulin levels, which reflects fetal islet cell insulin production that is metabolized by the fetal kidneys.25 With minimal maternal hyperglycemia, it is possible that the effect of fetal programming via islet cell stimulation might be minimal and therefore not result in significant childhood metabolic derangements including biomarker alterations. Furthermore, there was no difference in maternal BMI between the LGA and AGA groups or early solid fed and nonearly solid fed groups in our study. Prior research has shown that maternal obesity, independent of GDM, is a risk factor for the development of cardiovascular disease, fatty liver disease, renal disease, and impaired glucose tolerance in the offspring.26 The offspring in our cohort has similar biomarker values, and although, there is a statistically significant difference in maternal BMI there is minimal clinical variation in BMI of 29.7 versus 31.7 kg/m2.
In our cohort, the mean child age represented in our samples was 7 years. There was no difference in the mean age represented in the early fed versus not early fed comparison groups (►Table 1) and AGA versus LGA comparison groups (►Table 2). Previous studies have suggested that in the offspring of women with GDM, impaired glucose tolerance or diabetes mellitus tends to manifest in the adolescent developmental period.15 Silverman et al showed that frequency of impaired glucose tolerance was significantly increased after the age of 10 years.25 Furthermore, Pettitt and Jovanovic showed the development of noninsulin-dependent diabetes and impaired glucose tolerance in the offspring of Pima Indian women with GDM peaked after the age of 15 years, well into the adolescent period.27 It is, therefore, possible that with a sample of mostly prepubertal and preadolescent children, metabolic biomarker changes associated with obesity, impaired glucose tolerance, and increased adiposity might not manifest themselves.
To detect 95% power, we estimated that we would need at least 50 LGA and 50 AGA offspring. AGA offspring represented 87% of the total sample, with only 43 LGA infants. We fell short of our goal to observe biomarker association with 95% power; however, post hoc analysis revealed this still reached 90% power. Perhaps if the cohort included more LGA offspring, we may have observed some association with birthweight and leptin and adiponectin concentrations. Of note, the data used to assign LGA status is from birthweight charts from 1999. Contemporary birthweight charts are available, and prior studies have shown that birthweight charts from 2009 and 2017 capture a larger portion of LGA infants.28 Nonetheless, previous work by Crume et al has shown that childhood obesity and adiposity may be independent of fetal growth in the offspring of women with GDM.29 Moreover, their work showed no relationship between childhood obesity and GDM exposure even when analysis adjusted for maternal prepregnancy BMI, childhood diet, and physical activity. Our work further confirms the conclusions gathered by the parent study.15 Obesity and metabolic dysfunction in this cohort is not altered by treatment of mild GDM, and we demonstrate no difference in biomarkers of metabolic dysfunction regardless of birth-weight or infant feeding practices.
Our study utilized a reliable immunoassay system to calculate cytokine concentrations, standardized anthropometric data and included a racially diverse group of offspring. Nonetheless, it is limited by potential recall bias in the reporting of infant feeding patterns, prepubertal age of offspring, and underpowered sample for detecting a difference in cytokine concentration between LGA and AGA offspring. Additional prospective work could be considered to evaluate this group of offspring once they reach adolescence.
Although no association of biomarkers with childhood obesity or infant feeding was seen in this prepubertal cohort, more long-term follow-up data may potentially indicate such metabolic alterations. Ultimately, the role of GDM as a risk factor directly implicated in postnatal programming and the causal pathway of childhood metabolic disease remains unknown.
Key Points.
Adipocytokines are markers of metabolic status.
Children of women with mild GDM may be at risk for MetS.
Biomarkers similar in LGA and AGA groups.
Biomarkers similar in early and delayed solid-fed groups.
Nonhuman milk does not modify effect of feeding practice.
Acknowledgments
The authors thank Francee Johnson, R.N., B.S.N. and Lisa Moseley, R.N. for protocol development and coordination between clinical research centers and Mark B. Landon, M.D., Elizabeth A. Thom, Ph.D. Madeline M. Rice, Ph.D., and Catherine Y. Spong, M.D. for protocol development and oversight.
Funding
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD27915, HD36801, HD34208, HD34116, HD40485, HD40500, HD27869, HD40560, HD40544, HD53097, HD40512, and HD40545) and the National Center for Advancing Translational Sciences (UL1TR001070 and UL1TR000439). Laboratory supplies funded by the Maternal Fetal Medicine Department Fund at The Ohio State University Wexner Medical Center. Comments and views of the authors do not necessarily represent views of the National Institutes of Health.
Footnotes
Presented in part at the 39th annual meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV, February 11-16, 2019.
Conflict of Interest
None declared.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015;219(219):1–8 [PubMed] [Google Scholar]
- 2.Dutton H, Borengasser SJ, Gaudet LM, Barbour LA, Keely EJ. Obesity in pregnancy: optimizing outcomes for mom and baby. Med Clin North Am 2018;102(01):87–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Phenotype of infants of mothers with gestational diabetes. Diabetes Care 2007; 30(Suppl 2):S156–S160 [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM, McIntyre HD, Cruickshank JK, et al. HAPO Study Cooperative Research Group. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35(04):780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M, Costello J. DNA methylation: an epigenetic mark of cellular memory. Exp Mol Med 2017;49(04):e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruchat SM, Hivert MF, Bouchard L. Epigenetic programming of obesity and diabetes by in utero exposure to gestational diabetes mellitus. Nutr Rev 2013;71(Suppl 1):S88–S94 [DOI] [PubMed] [Google Scholar]
- 7.Lekva T, Roland MCP, Michelsen AE, et al. Large reduction in adiponectin during pregnancy is associated with large for gestational age newborns. J Clin Endocrinol Metab 2017;102(07):2552–2559 [DOI] [PubMed] [Google Scholar]
- 8.Gnacińska M, Małgorzewicz S, Stojek M, Łysiak-Szydłowska W, Sworczak K. Role of adipokines in complications related to obesity: a review. Adv Med Sci 2009;54(02):150–157 [DOI] [PubMed] [Google Scholar]
- 9.Aye IL, Powell TL, Jansson T. Review: Adiponectin–the missing link between maternal adiposity, placental transport and fetal growth? Placenta 2013;34(Suppl):S40–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med 2012;7(05):323–324 [DOI] [PubMed] [Google Scholar]
- 11.Tang M Protein intake during the first two years of life and its association with growth and risk of overweight. Int J Environ Res Public Health 2018;15(08):1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miralles O, Sánchez J, Palou A, Picó C A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006;14(08):1371–1377 [DOI] [PubMed] [Google Scholar]
- 13.McGuire SInstitute of Medicine (IOM) Early Childhood Obesity Prevention Policies. Institute of Medicine (IOM) Early Childhood Obesity Prevention Policies. Washington, DC: The National Academies Press; 2011. Adv Nutr 2012;3(01):56–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361(14):1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landon MB, Rice MM, Varner MW, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015;38(03):445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander GR, Kogan MD, Himes JH. 1994-1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J 1999;3(04):225–231 [DOI] [PubMed] [Google Scholar]
- 17.Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management and risk stratification in a West Virginian population. Int J Med Sci 2016;13(01):25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okereke NC, Uvena-Celebrezze J, Hutson-Presley L, Amini SB, Catalano PM. The effect of gender and gestational diabetes mellitus on cord leptin concentration. Am J Obstet Gynecol 2002;187(03):798–803 [DOI] [PubMed] [Google Scholar]
- 19.Nappo A, González-Gil EM, Ahrens W, et al. Analysis of the association of leptin and adiponectin concentrations with metabolic syndrome in children: Results from the IDEFICS study. Nutr Metab Cardiovasc Dis 2017;27(06):543–551 [DOI] [PubMed] [Google Scholar]
- 20.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111(03):e221–e226 [DOI] [PubMed] [Google Scholar]
- 21.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens 2009;22(02):215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C, Howie PW. Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ 1998;316 (7124):21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baptiste-Roberts K, Nicholson WK, Wang NY, Brancati FL. Gestational diabetes and subsequent growth patterns of offspring: the National Collaborative Perinatal Project. Matern Child Health J 2012;16(01):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham MT, Brubaker K, Pruett K, Caughey AB. Risk of childhood obesity in the toddler offspring of mothers with gestational diabetes. Obstet Gynecol 2013;121(05):976–982 [DOI] [PubMed] [Google Scholar]
- 25.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995;18(05):611–617 [DOI] [PubMed] [Google Scholar]
- 26.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140 (03):387–398 [DOI] [PubMed] [Google Scholar]
- 27.Pettitt DJ, Jovanovic L. Birth weight as a predictor of type 2 diabetes mellitus: the U-shaped curve. Curr Diab Rep 2001;1 (01):78–81 [DOI] [PubMed] [Google Scholar]
- 28.Aris IM, Kleinman KP, Belfort MB, Kaimal A, Oken E. 2017 US reference for singleton birthweight percentiles using obstetric estimates of gestation. Pediatrics 2019;144(01):e20190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 2011;54(01):87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]


