Abstract
Background
Hypertension treatment in older adults can decrease mortality, cardiovascular events, including heart failure, cognitive impairment, and stroke risk, but may also lead to harms such as syncope and falls. Guidelines recommend targeting preventive interventions with immediate harms and delayed benefits to patients whose life expectancy exceeds the intervention's time to benefit (TTB). Our objective was to estimate a meta‐analyzed TTB for stroke prevention after initiation of more intensive hypertension treatment in adults aged ≥65 years.
Methods
Studies were identified from two Cochrane systematic reviews and a search of MEDLINE and Google Scholar for subsequent publications until August 31, 2021. We abstracted data from randomized controlled trials comparing standard (untreated, placebo, or less intensive treatment) to more intensive treatment groups in older adults (mean age ≥ 65 years). We fit Weibull survival curves and used a random‐effects model to estimate the pooled annual absolute risk reduction (ARR) between control and intervention groups. We applied Markov chain Monte Carlo methods to determine the time to ARR thresholds (0.002, 0.005, and 0.01) for a first stroke.
Results
Nine trials (n = 38,779) were identified. The mean age ranged from 66 to 84 years and study follow‐up times ranged from 2.0 to 5.8 years. We determined that 1.7 (95%CI: 1.0–2.9) years were required to prevent 1 stroke for 200 persons (ARR = 0.005) receiving more intensive hypertensive treatment. Heterogeneity was found across studies, with those focusing on tighter systolic blood pressure control (SBP < 150 mmHg) showing longer TTB. For example, in the SPRINT study (baseline SBP = 140 mmHg, achieved SBP = 121 mmHg), the TTB to avoid 1 stroke for 200 patients treated was 5.9 years (95%CI: 2.2–13.0).
Conclusions
More intensive hypertension treatment in 200 older adults prevents 1 stroke after 1.7 years. Given the heterogeneity across studies, the TTB estimates from individual studies may be more relevant for clinical decision‐making than our summary estimate.
Keywords: hypertension, stroke, time to benefit
Short abstract
See related Editorial by Mark A. Supiano in this issue.
Key points
More intensive hypertension treatment in 200 older adults prevents 1 stroke after 1.7 years.
For older adults with baseline systolic blood pressures (SBP) below 150 mmHg, the time to benefit (TTB) of more intensive hypertension treatment appears to be substantially longer than 1.7 years; for older adults with baseline SBP above 190 mmHg, the TTB appears to be shorter than 1.7 years.
Why does this paper matter?
Since the overwhelming majority of older adults have a life expectancy >1.7 years, our results suggest that almost all older adults with hypertension would benefit from treatment.
INTRODUCTION
Hypertension is a strong and common modifiable risk factor for stroke. In the United States, hypertension affects 47% adults (245 million) and 76% of older adults ≥65 years of age (48.0 million). 1 , 2 The prevalence of stroke is strongly age‐dependent, and hypertension dramatically increases the risk of stroke in older adults. 3 , 4 Data from Framingham Study suggests that hypertension doubles the risk of stroke in older adults age 65–94, with a relative risk (RR) of 1.9 in men and 2.3 in women. 5 While hypertension treatment has been shown to reduce stroke risk, 6 it is less clear when stroke reduction occurs. In contrast, the harms of hypertension treatment, which include orthostatic hypotension, syncope, falls, and electrolyte abnormalities, appear to occur soon after treatment initiation. 7 , 8 , 9 For example, the risk of falls and fractures was found to be increased in the first 7–45 days after the initiation of antihypertensive medications. 10 , 11 , 12 , 13 , 14 Thus, while hypertension treatment decreases stroke risk over time, it can also lead to an increased risk for adverse effects.
For interventions with short‐term potential harms and long‐term potential benefits, we previously proposed a framework for individualizing prevention decisions that focuses on a patient's life expectancy and an intervention's time to benefit (TTB). 15 Older adults with a limited life expectancy should avoid preventive interventions with an extended TTB, since these older adults would be exposed to the up‐front harms of the intervention with little chance they would survive to experience the benefits. Although many indexes to predict life expectancy for older adults have been validated and are available through websites such as ePrognosis (ePrognosis.ucsf.edu), the TTB for hypertension treatment to prevent strokes is unclear.
To help clinicians identify, which patients are most likely to benefit from hypertension treatment (and which patients are more likely to be harmed), our objective was to determine the TTB of hypertension treatment for the primary prevention of stroke. We conducted a survival meta‐analysis of major randomized clinical trials to determine the TTB for various stroke absolute risk reduction (ARR) thresholds. Specifically, we sought to quantify how many years of hypertension treatment was necessary before 1 stroke was prevented for 500 older adults treated (TBB to reach an ARR of 0.002). Similarly, we sought to quantify the TTB for ARR of 0.005 (1 stroke prevented for 200 treated) and TBB for ARR of 0.01 (1 stroke prevented for 100 treated).
METHODS
Literature search
The Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) statement guidelines were followed (Table S1). One reviewer (VSH) identified randomized control trials from two systematic reviews (2017 Cochrane review entitled, “Blood pressure targets for hypertension in older adults” 16 and 2019 Cochrane review entitled, “Pharmacotherapy for hypertension in adults 60 years or older” 17 ). We also searched MEDLINE–PubMed and Google Scholar for subsequently published relevant studies up until August 31, 2021 using the search terms blood pressure, hypertension, antihypertensive, older, elderly, and stroke. The trial names, authors, and references of included trials and published systematic reviews were screened for other potential trials. The search strategy is detailed in Figure 1. Since this project relied on previously published data, the Committee on Human Research at the University of California, San Francisco determined that this did not meet the definition of human subjects research and thus did not require review.
FIGURE 1.
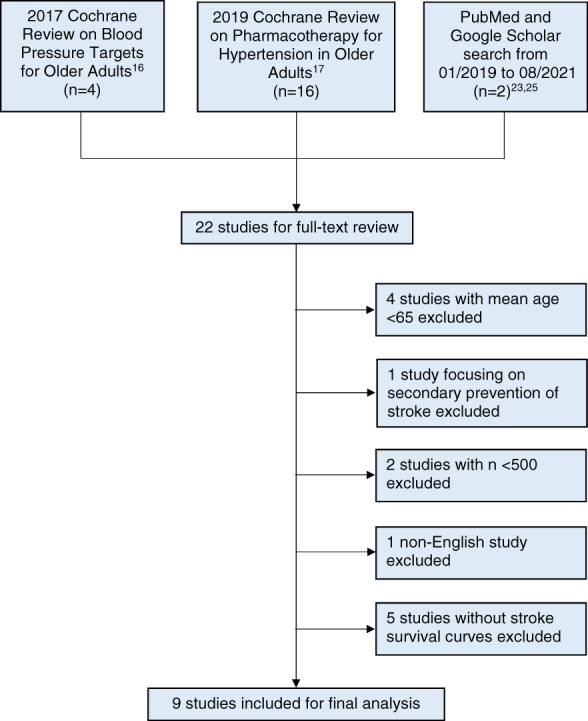
Study identification and selection
Eligibility criteria
This study focused on the primary prevention of stroke in older adults. Thus, we excluded trials with a mean age <65 years, trials focusing on secondary stroke prevention, smaller studies with n < 500 and non‐English studies. Since our outcome was the time to stroke prevention, we required studies to present time to stroke data and excluded studies without stroke survival curves.
Data extraction
We utilized previously validated methods to quantify data from published survival curves. Specifically, we scanned the published survival curves and used the program DigitizeIt to reconstruct the data underlying the survival curves. 18 As a sensitivity analysis, we manually quantified the scanned survival curves from two studies and compared the manual quantification with the DigitizeIt program results.
Outcomes of interest
Our primary outcome was time to stroke reduction after initiation of more intensive blood pressure treatment.
Statistical methodology
Our statistic of interest was the “time to benefit,” which is not routinely reported by individual studies. In order to calculate the TTB for each study, we fit random‐effects Weibull survival curves using the annual event data for the control and intervention groups, allowing both the scale and shape Weibull parameters to vary for each arm of the study. We then used 100,000 Markov Chain Monte Carlo (MCMC) simulations to acquire point estimates, standard errors, and confidence intervals for mortality rates in control and intervention arms of each study. From this model, we obtained estimates of time to specific ARR thresholds (ARR = 0.002, 0.005 and 0.01) for each study. Similar ARR thresholds have been used in previously published time to benefit analyses and risk prediction tools for shared decision‐making. 19 , 20
Next, we pooled the estimates from each study using a random‐effects meta‐analysis model. We employed the χ2 test of homogeneity and I 2 statistics (with p < 0.05 and I 2 > 50%, respectively, indicating significant heterogeneity) to determine variability in the intervention effects across studies due to statistical heterogeneity. MCMC computations were conducted in SAS 9.4, estimates for individual studies were obtained using R 3.4.0 and a random‐effects meta‐analysis was conducted in STATA 14.2. We utilized similar methods to estimate TTB for cancer screening in previously published studies. 21 , 22
RESULTS
We examined the meta‐analyses conducted by the Cochrane Collaboration in 2017 16 and 2019 17 and identified 22 studies (Figure 1). A search of Google scholar and Medline for subsequently published studies revealed two studies, which met our inclusion criteria: 1) a post hoc analysis 23 of the SPRINT study 24 and 2) the STEP trial. 25 Of the 22 studies that underwent full‐text review, 13 studies 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 were excluded. Four studies were excluded because the mean age of participants was <65 years. One study focusing on secondary prevention of stroke was excluded. Two studies with n < 500 were excluded and one non‐English study was excluded. Finally, five studies did not present survival curves or time to stroke data and were also excluded. Two excluded studies met more than one exclusion criterion (Table 1).
TABLE 1.
Characteristics of excluded studies
| Cochrane review | Study a | Reason(s) for exclusion | ||||
|---|---|---|---|---|---|---|
| Mean age <65 years | Secondary prevention of stroke | Sample size (n < 500) | Non‐English study | No stroke survival curve | ||
| Garrison 16 2017 | JATOS 26 2008 | x | ||||
| VALISH 27 2010 | x | |||||
| Steurer 28 2016 | x | |||||
| Musini 17 2019 |
Carter 29 1970 |
x | ||||
| VA‐II 30 1970 | x | X | x | |||
| HSCSG 31 1974 | x | x | ||||
| ATTMH 32 1981 | x | |||||
| Kuramoto 33 1981 | x | |||||
| Sprackling 34 1981 | X | |||||
| EWPHBPE 35 1989 | x | |||||
| SHEP‐P 36 1989 | x | |||||
| MRC‐TMH 37 1992 | x | |||||
| HYVET P 38 2003 | x | |||||
Abbreviations: ATTMH, Australian therapeutic trial in mild hypertension; EWPHBPE, European working party on high blood pressure in the elderly; HSCSG, hypertension‐stroke cooperative study group; HYVET P, hypertension in the very elderly trial pilot; JATOS, Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients; MRC‐TMH, medical research council trial of treatment of mild hypertension; n, number of participants; SHEP‐P, systolic hypertension in the elderly program pilot; VA‐II, Veterans administration cooperative study group on antihypertensive agents; VALISH, Valsartan in elderly isolated systolic hypertension.
Studies are listed chronologically.
We identified nine randomized controlled trials 24 , 25 , 39 , 40 , 41 , 42 , 43 , 44 , 45 that met our inclusion criteria (total n = 38,779). The trials ranged in size from 724 to 9361 participants. The mean age in the included trials ranged from 66 to 84 years. Five of the studies were placebo‐controlled studies, one study compared observation only (no treatment) to active hypertension treatment, and three studies focused on standard hypertension treatment compared to more intensive hypertension treatment. Older trials focused on patients with a higher baseline systolic blood pressure (SBP), resulting in a higher stroke risk. For example, our earliest included study (Coope 1986 39 ) had a baseline SBP of 196 mmHg with a 9.5% stroke risk in the standard treatment group. On the other hand, our most recent included study (STEP 2021 25 ) had a baseline SBP of 146 mmHg with a 1.7% stroke risk in the standard treatment group. Across the studies, the mean follow‐up ranged from 2.0 to 5.8 years. The RR of stroke reported by each individual study ranged from 0.55 to 0.89. The stroke risk in the standard treatment group varied widely across studies, ranging from 1.5% to 10.0%. Characteristics of included studies are summarized in Table 2.
TABLE 2.
Characteristics of Included Studies
| Study a | n | Mean Age (Range), y | Intervention Groups | Mean SBP b (Std Tx/Int Tx), mmHg | Mean FU, y | ΔSBP achieved, mmHg | Stroke Risk in Std Tx, % | Stroke RR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Std Tx | Int Tx | Baseline | Achieved | |||||||
| Coope 39 1986 | 884 |
69 (60–79) |
Obs only | A | 196.4/196.7 | 180.4/162.3 | 4.4 | −18.1 | 9.5 |
0.58(0.36–0.94) |
| SHEP 40 1991 | 4736 | 72 (>60) | P | A | 170.1/170.5 | 155.1/144.0 | 4.5 | −11.1 | 6.3 |
0.65(0.50–0.83) |
|
STOP 41 1991 |
1627 |
76 (70–84) |
P | A | 195.0/195.0 | 186.0/167.0 | 2.1 | −19.0 | 6.5 |
0.55(0.35–0.85) |
| MRC‐O 42 1992 | 4396 |
70 (65–74) |
P | A | 184.7/184.7 | 165.0/151.6 | 5.8 | −13.5 | 6.1 |
0.76(0.58–0.97) |
| Syst‐Eur 43 1997 | 4695 |
70 (>60) |
P | A | 173.9/173.8 | 160.9/150.8 | 2.0 c | −10.1 | 3.4 |
0.58(0.41–0.84) |
| HYVET 44 2008 | 3845 |
84 (80–105) |
P | A | 173.0/173.0 | 158.3/143.5 | 2.1 | −14.8 | 3.5 |
0.74(0.52–1.05) |
|
Wei 45 2013 |
724 |
77 (>70) |
Std Tx | Int Tx | 160.3/158.8 | 149.7/135.7 | 4.0 | −14.0 | 10.0 |
0.58(0.35–0.97) d |
|
SPRINT 24 2015 |
9361 |
68 (>50) |
Std Tx | Int Tx | 139.7/139.7 | 134.6/121.4 | 3.3 c | −13.1 | 1.5 |
0.89(0.63–1.25) |
|
STEP 25 2021 |
8511 |
66 (60–80) |
Std Tx | Int Tx | 146.0/146.1 | 135.9/126.7 | 3.3 c | −9.3 | 1.7 |
0.67(0.47–0.97) |
Abbreviations: ΔSBP, difference in SBP Achieved = (Intensive SBP achieved – Standard SBP achieved); A, active treatment; CI, confidence interval; FU, follow‐up; HYVET, hypertension in the very elderly trial; Int Tx, intensive treatment; MRC‐O, medical research council trial of treatment of hypertension in older adults; n, number of participants; Obs, observation; P, placebo; RR, relative risk; SBP, systolic blood pressure; SHEP, systolic hypertension in the elderly program; SPRINT, systolic blood pressure intervention trial; Std Tx, standard treatment; STEP, strategy of blood pressure intervention in elderly hypertensive patients; STOP, Swedish trial in old patients with hypertension; Syst‐Eur, systolic hypertension in Europe; y, years.
Studies are listed chronologically.
Sitting or supine SBP.
Reported as median (mean follow‐up years was not provided by the study).
Calculated by the authors of this article (CI was not provided by the study).
Our survival meta‐analysis suggested that the benefit of more intensive hypertension treatment increased steadily with longer follow‐up. (Figure 2). For example, at 1 year, 0.3 strokes were prevented for 100 persons treated with more intensive hypertension treatment. At 3 and 5 years after treatment initiation, 1.0 and 1.8 strokes were prevented for 100 persons treated with more intensive hypertension treatment, respectively.
FIGURE 2.
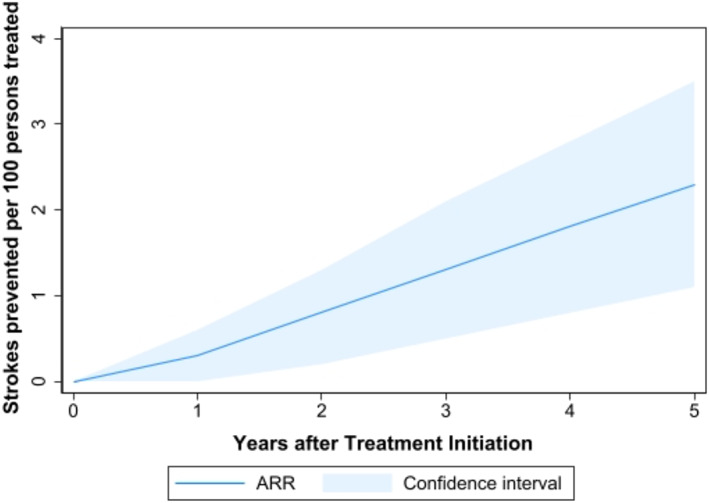
Meta‐analyzed absolute risk reduction over time with more intensive blood pressure treatment. The dark blue line signifies the difference in number of stroke events between standard and more intensive blood pressure treatment groups. The light blue areas above and below the line indicate the upper and lower 95% confidence limits of the ARR, respectively
We determined that it took 3.0 years (95%CI: 1.8–4.9) for more intensive hypertension treatment to prevent 1 stroke in 100 older patients treated (ARR = 0.01; Figure 3C and Table S2). Similarly, 200 older adults would need to receive more intensive blood pressure treatment (ARR = 0.005; Figure 3B and Table S2) for 1.7 years (95%CI: 1.0–2.9) to prevent 1 stroke and 500 older adults would need more intensive blood pressure treatment (ARR = 0.002; Figure 3A and Table S2) for 0.9 years (95%CI: 0.5–1.7) to avoid 1 stroke.
FIGURE 3.

Forest plots for time to benefit to achieve ARR thresholds. (A) Forest plot for ARR = 0.002. (B) Forest plot for ARR = 0.005. (C) Forest plot for ARR = 0.01
We found evidence of substantial heterogeneity in TTB across studies (Figure 3 and Table S2). For example, while the pooled time to prevent 1 stroke per 200 persons treated (ARR = 0.005) was 1.7 years (95%CI: 1.0–2.9), the equivalent TTB for individual trials ranged from 0.7 years (95%CI: 0.2–1.8) in the STOP study to 5.9 years (95%CI: 2.2–13.0) in the SPRINT study. For all ARR thresholds, statistical tests for heterogeneity indicated that the variability between individual studies was larger than would be expected by chance (p = 0.02 at ARR = 0.002; p = 0.01 at ARR = 0.005; p < 0.001 at ARR = 0.01). Furthermore, I 2 values ranged from 52% at ARR = 0.002 to 72.2% at ARR = 0.01, suggesting substantial statistical heterogeneity. 46
Earlier trials with higher baseline blood pressures and higher stroke risk appeared to have shorter times to benefit than more recent trials with lower baseline blood pressures and lower stroke risk. For example, the time to prevent 1 stroke per 200 persons treated was 0.7 years (95%CI: 0.2–1.8) in the STOP study (published in 1991, baseline SBP of 195 mmHg and stroke risk of 6.5%). In contrast, the time to prevent 1 stroke per 200 persons treated was 5.9 years (95%CI: 2.2–13.0) in the SPRINT study (published in 2015, baseline SBP of 140 mmHg and stroke risk of 1.5%).
DISCUSSION
In this survival meta‐analysis of 9 randomized trials of hypertension treatment in older adults, the TTB to prevent 1 stroke per 200 older adults treated with more intensive hypertension therapy was 1.7 years. Since the overwhelming majority of older adults have a life expectancy >1.7 years, our results suggest that almost all older adults with hypertension would benefit from treatment.
We found evidence of heterogeneity in TTB across studies, with most earlier trials focusing on older adults with higher baseline blood pressures and higher stroke risk showing a TTB <1.7 years (for ARR threshold of 0.005) and more recent studies focusing on older adults with lower baseline blood pressures and lower stroke risk showing TTB >1.7 years (for ARR threshold of 0.005). Given the clinical and statistical heterogeneity across studies, individual patients may be best served by focusing on TTB results from an individual study rather than focusing on our pooled TTB results. For example, for patients whose hypertension is already relatively well‐controlled with SBP near 140 mmHg and are being considered for treatment intensification to a target SBP of 120 mmHg, the SPRINT TTB results are likely to be most relevant. For these patients, the TTB to avoid a stroke is more likely to range from 4.0 years (ARR of 0.002) to 7.8 years (ARR of 0.01) rather than our pooled TTB estimates (0.9 years for ARR = 0.002 to 3.0 years for ARR = 0.01). On the other hand, for patients whose hypertension is poorly controlled with an SBP over 190 mmHg, the STOP TTB results are likely to be more relevant, with TTB to avoid stroke ranging from 0.4 years (ARR of 0.002) to 1 year (ARR of 0.01). Thus, although our summary TTB results provide a global estimate for the primary prevention of stroke with antihypertensive treatment, individual patients will likely be better served by focusing on TTB results from individual studies with participants that most closely resemble the patient.
Potential harms of antihypertensive medications
The preponderance of evidence from observational studies, meta‐analyses, and randomized trials suggests that more intensive hypertension treatment increases the risk for adverse events such as hypotension, syncope, and falls. 47 Leipzig and colleagues showed in a systematic review and meta‐analysis published in 1999 that while diuretics were associated with an increased risk of falls, the association between falls and the use of other classes of antihypertensive medications did not reach statistical significance. 48 In a meta‐analysis published in 2009, Woolcott and colleagues updated the Leipzig study and found that antihypertensive medications increased the risk of falls. 49 A recent meta‐analysis of randomized trial data showed that while the more intensive blood pressure treatment did not increase the risk of falls, but did increase the risk of hypotension and syncope. 50 Taken together, these results suggest that more intensive blood pressure treatment can lead to hypotension, syncope, and falls.
Recent studies have suggested that the risk of falls and fractures is transiently increased shortly after initiation and intensification of blood pressure treatment. Butt and colleagues demonstrated that within 45 days of new initiation of antihypertensive medications, the risk of injurious falls requiring hospital care increased 69% and the risk of hip fracture increased 43%. 11 , 12 Shimbo and colleagues found that antihypertensive medication initiation and intensification was associated with an increased risk of serious fall injury for 15 days, with attenuated nonsignificant associations beyond 15 days. 13 Berry and colleagues showed that the risk of hip fracture increased two‐fold in the first 14 days after initiation of diuretic antihypertensives. 14 These and other studies were summarized in a recent meta‐analysis, which concluded that the risk of falls is highest immediately after antihypertensive medication intensification, with chronic use of antihypertensive medications not being associated with falls. 10 Thus, emerging evidence suggests that antihypertensive medication initiation and intensification may result in a short‐term but transiently elevated risk of falls and fractures.
The findings from these observational studies are supported by the SPRINT study, 24 which found a borderline increase in the number of injurious falls (defined as life threatening or requiring an emergency room visit) in the intensive versus standard treatment groups (HR = 2.22, p = 0.05). The study also showed that intensive treatment also led to increased hypotension (HR = 2.24, p < 0.001) and syncope (HR = 2.13, p = 0.005) compared to standard treatment. While SPRINT focused on older hypertensive patients without common diseases such as diabetes, stroke, and congestive heart failure, the rates of adverse effects are expected to be much higher in the relatively less healthy patient population that was excluded from the study. Consequently, these results highlight the importance of postural blood pressure measurements and the close monitoring of orthostatic symptoms during initiation or intensification of antihypertensive medications.
While hip fracture and injurious falls are clinically important harms, falls without injury can also lead to long‐term harms. Falls and dizziness are potent risk factors for fear of falling, 51 , 52 which has been shown to be associated with restriction or avoidance of daily activities, loss of independence, reduction in social activity, depression, and a reduction in quality of life. 53 , 54 Thus, while noninjurious falls are clearly less immediately harmful than injurious falls or fractures, they are not benign and can lead to long‐term adverse effects for older adults.
Individualizing hypertension treatment decisions
Our results can help inform and individualize hypertension treatment discussions. There is overwhelming evidence that improved blood pressure control can significantly reduce cardiovascular disease morbidity and mortality. For most older adults, the benefit of avoiding stroke and subsequent permanent disability is of paramount importance. Since these older adults place great value in avoiding stroke, they may focus on a relatively small ARR (i.e., ARR = 0.002) as clinically significant and choose to initiate or continue hypertension treatment as long as their life expectancy is >0.9 years. In contrast, other older adults with a preexisting fear of falling may feel that the risks of hypotension and falls are substantial. They may focus on larger ARRs for stroke (i.e., ARR = 0.01) instead, and choose to initiate or continue hypertension treatment as long as their life expectancy is >3.0 years. By presenting a spectrum of times to benefit across a range of ARRs, our study can inform treatment discussions for older adults prioritizing avoiding stroke as well as for older adults prioritizing avoiding hypotension and falls.
Hypertension treatment decisions need to be informed by benefits and harms beyond stroke prevention and falls. Hypertension treatment benefits beyond stroke include decreased all‐cause mortality and decreased major adverse cardiovascular outcomes, including heart failure and myocardial infarction, and decreased incidence of cognitive impairment. 16 , 17 , 49 , 55 Hypertension treatment harms beyond hypotension, syncope, and falls include hyperkalemia and acute kidney injury, 49 which are usually reversible. 17 For older adults with substantial frailty or comorbidities, contextual factors such as polypharmacy may also be important considerations. Thus, individualized hypertension treatment decisions should account for the benefits beyond stroke prevention as well as for the risks beyond falls and fractures.
Heterogeneity
Heterogeneity was observed to increase with larger ARR thresholds and longer times to benefit. Specifically, at ARR of 0.002, I 2 = 52.0% (p = 0.034). The I 2 value increases to I 2 = 60.1% (p = 0.01) and I 2 = 72.2% (p < 0.001) at ARRs of 0.005 and 0.01, respectively. The I 2 statistic estimates the proportion of the variation in individual study results that are due to heterogeneity rather than chance 46 and an I 2 value of 50% to 90% is generally considered to suggest “moderate” to “substantial” heterogeneity. 56
The observed statistical heterogeneity in our study results is likely due to important clinical differences between included studies (Table 2). For example, the SPRINT study 24 showed the longest times to benefit (Figure 3 and Table S2). These findings are likely due to its relatively modest efficacy (RR = 0.89) and low baseline risk of stroke (1.5%). SPRINT's modest RR and low baseline risk of stroke, and the subsequently long TTB, can be attributed to its focus on well‐controlled hypertension (mean baseline SBP of 139.7 mmHg; target SBP <140 mmHg in standard treatment group versus <120 mmHg in intensive treatment group) and the active treatment of both intervention groups. The STEP study 25 also showed long times to benefit, most likely due to lower baseline SBP (146 mmHg) and lower baseline risk of stroke (1.7%). In general, more recent trials (such as the SPRINT and STEP trials) focused on older adults with lower baseline blood pressures and lower stroke risk, resulting in longer estimates for TTB thresholds.
In contrast to SPRINT and STEP, earlier trials focused on older adults with higher blood pressures and higher stroke risk, resulting in shorter estimates of TTB. For example, the STOP study 41 showed the shortest TTB. This result is most likely due to a combination of relatively high efficacy (RR = 0.55) and higher risk of stroke (6.5%) in participants with poorly controlled hypertension (mean baseline SBP of 195 mmHg). In general, earlier studies (such as STOP) focused on older adults with higher blood pressures and higher stroke risk, resulting in shorter estimates of TTB.
Strength and limitations
A major strength of our study is that, to our knowledge, this is the first study to use quantitative methods to determine the TTB for the primary prevention of stroke with more intensive hypertension treatment in adults with a mean age ≥ 65 years. Thus, our study fills a critical gap, informing discussions between clinicians and patients about when the benefits of more intensive antihypertensive therapy are most likely.
One limitation is that, because SBP treatment targets have evolved over time, there is a substantial range in blood pressures in both the standard and intensive treatment arms across the included studies. Thus, there is clinical and methodologic heterogeneity that likely contributed to the observed statistical heterogeneity. 56 We utilized random‐effects meta‐analysis methods, which incorporate an assumption of underlying heterogeneity to more flexibly account for variable treatment effects across studies. 57 When we discovered evidence of heterogeneity, we followed recommended best practices to explore the potential causes of heterogeneity and found that baseline blood pressure and baseline stroke risk to be likely contributing factors to the observed heterogeneity. While heterogeneity is moderate to substantial, we believe the fundamental similarities in our included studies (comparison of stroke rates among patients randomized to standard versus intensive blood pressure treatment) allow for a meaningful pooled summary measure. However, as noted previously, evidence of heterogeneity suggests that for a given patient, TTB estimates from an individual study may be more relevant than our pooled TTB estimates.
A second potential limitation of our study is that only the SPRINT study provided age‐stratified results, precluding the presentation of age‐stratified meta‐analyses. Thus, our study included participants as young as 50 years in SPRINT and as young as 60 years in 4 other included studies (Table 2). Since stroke risk increases almost exponentially with age, it is possible that our TTB to stroke reduction is an underestimate for the oldest patients. Since mean ages in our included studies ranged from 64 to 84 years, our TTB estimates are likely most relevant for older adults in this age range.
Finally, because all of our included studies focused on primary stroke prevention after the initiation or intensification of antihypertensive medications, it is unclear whether our results can be extrapolated to inform de‐prescribing decisions.
CONCLUSIONS
In this meta‐analysis, we found that 200 adults aged ≥65 years would need to be treated for 1.7 years to avoid 1 stroke. Since the overwhelming majority of older adults have a life expectancy >1.7 years, these results suggest that almost all older adults with hypertension would benefit from treatment. We found substantial heterogeneity across studies suggesting that for older adults with poorly controlled hypertension (i.e., SBP >190 mmHg), the TTB to prevent 1 stroke for 200 persons treated is likely substantially shorter than 1.7 years. Conversely, for older adults with relatively well‐controlled hypertension (i.e., SBP <150 mmHg), the TTB to prevent 1 stroke for 200 persons treated is likely substantially longer than 1.7 years.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors have read and approved the submission of this manuscript. Vanessa S. Ho and Sei J. Lee conceived the study and designed the protocol. Vanessa S. Ho performed the literature search, selected the studies, and extracted the survival data, which was reviewed by Sei J. Lee. Vanessa S. Ho, Irena S. Cenzer, Brian T. Nguyen, and Sei J. Lee synthesized the data. Vanessa S. Ho wrote the first draft of the article. All authors critically revised successive drafts of the article and approved the final version. Sei J. Lee obtained funding. Vanessa S. Ho and Sei J. Lee are the study co‐guarantors. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
SPONSOR'S ROLE
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Table S1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 Checklist and PRISMA 2020 for Abstracts Checklist.
Table S2. Time to Benefit for the Primary Prevention of Stroke for Older Adults Treated with More Intensive Hypertension Treatment.
Ho VS, Cenzer IS, Nguyen BT, Lee SJ. Time to benefit for stroke reduction after blood pressure treatment in older adults: A meta‐analysis . J Am Geriatr Soc. 2022;70(5):1558‐1568. doi: 10.1111/jgs.17684
See related Editorial by Mark A. Supiano in this issue.
An oral presentation based in part on the study findings was given at Plenary Paper Session at the American Geriatrics Society Annual Scientific meeting on June 24, 2020.
Funding informationThis work is supported by the facilities and resources of the Veterans Affairs Medical Center, San Francisco, CA. Sei J. Lee was also supported by grants from the National Institute on Aging (K24AG066998 and T35AG026736)
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) . Estimated Hypertension Prevalence, Treatment, and Control Among U.S. Adults Tables. Million Hearts website. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence-tables.html. Updated March 22, 2021. Accessed June 2, 2021.
- 2. National Center for Health Statistics, Centers for Disease Control and Prevention . National Health and nutrition examination survey (NHANES), 2015–2018.
- 3. Takahashi I, Geyer SM, Nishi N, et al. Lifetime risk of stroke and impact of hypertension: estimates from the adult health study in Hiroshima and Nagasaki. Hypertens Res. 2011;34(5):649‐654. doi: 10.1038/hr.2011.7 [DOI] [PubMed] [Google Scholar]
- 4. Fang XH, Zhang XH, Yang QD, et al. Subtype hypertension and risk of stroke in middle‐aged and older Chinese: a 10‐year follow‐up study. Stroke. 2005;37(1):38‐43. doi: 10.1161/01.str.0000195005.65998.38 [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB. Risk stratification in hypertension: new insights from the Framingham study. Am J Hypertens. 2000;13(1 pt 2):3S‐10S. doi: 10.1016/s0895-7061(99)00252-6 [DOI] [PubMed] [Google Scholar]
- 6. Perry HM Jr, Davis BR, Price TR, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the systolic hypertension in the elderly program (SHEP). JAMA. 2000;284(4):465‐471. doi: 10.1001/jama.284.4.465 [DOI] [PubMed] [Google Scholar]
- 7. Aronow WS. Managing the elderly patient with hypertension: current strategies, challenges, and considerations. Expert Rev Cardiovasc Ther. 2020;18(2):117‐125. doi: 10.1080/14779072.2020.1732206 [DOI] [PubMed] [Google Scholar]
- 8. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588‐595. doi: 10.1001/jamainternmed.2013.14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Correa A, Rochlani Y, Khan MH, Aronow WS. Pharmacological management of hypertension in the elderly and frail populations. Expert Rev Clin Pharmacol. 2018;11(8):805‐817. doi: 10.1080/17512433.2018.1500896 [DOI] [PubMed] [Google Scholar]
- 10. Kahlaee HR, Latt MD, Schneider CR. Association between chronic or acute use of antihypertensive class of medications and falls in older adults. A systematic review and meta‐analysis. Am J Hypertens. 2017;31(4):467‐479. doi: 10.1093/ajh/hpx189 [DOI] [PubMed] [Google Scholar]
- 11. Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of falls on initiation of antihypertensive drugs in the elderly. Osteoporos Int. 2013;24(10):2649‐2657. doi: 10.1007/s00198-013-2369-7 [DOI] [PubMed] [Google Scholar]
- 12. Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med. 2012;172(22):1739‐1744. doi: 10.1001/2013.jamainternmed.469 [DOI] [PubMed] [Google Scholar]
- 13. Shimbo D, Barrett Bowling C, Levitan EB, et al. Short‐term risk of serious fall injuries in older adults initiating and intensifying treatment with antihypertensive medication. Circ Cardiovasc Qual Outcomes. 2016;9(3):222‐229. doi: 10.1161/CIRCOUTCOMES.115.002524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry SD, Zhu Y, Choi H, Kiel DP, Zhang Y. Diuretic initiation and the acute risk of hip fracture. Osteoporos Int. 2013;24(2):689‐695. doi: 10.1007/s00198-012-2053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. 2013;310(24):2609‐2610. doi: 10.1001/jama.2013.282612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garrison SR, Kolber MR, Korownyk CS, McCracken RK, Heran BS, Allan GM. Blood pressure targets for hypertension in older adults. Cochrane Database Syst Rev. 2017;8(8):CD011575. doi: 10.1002/14651858.CD011575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musini VM, Tejani AM, Bassett K, Puil L, Wright JM. Pharmacotherapy for hypertension in adults 60 years or older. Cochrane Database Syst Rev. 2019;6(6):CD000028. doi: 10.1002/14651858.CD000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Leeuw J, Visseren FL, Woodward M, et al. Predicting the effects of blood pressure‐lowering treatment on major cardiovascular events for individual patients with type 2 diabetes mellitus: results from action in diabetes and vascular disease: Preterax and Diamicron MR controlled evaluation. Hypertension. 2015;65(1):115‐121. doi: 10.1161/HYPERTENSIONAHA.114.04421 [DOI] [PubMed] [Google Scholar]
- 20. Llewellyn‐Thomas HA, Paterson JM, Carter JA, et al. Primary prevention drug therapy: can it meet patients' requirements for reduced risk? Med Decis Mak. 2002;22(4):326‐339. doi: 10.1177/0272989X0202200411 [DOI] [PubMed] [Google Scholar]
- 21. Lee SJ, Boscardin WJ, Stijacic‐Cenzer I, Conell‐Price J, O'Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta‐analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. doi: 10.1136/bmj.e8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang V, Boscardin WJ, Stijacic‐Cenzer I, Lee SJ. Time to benefit for colorectal cancer screening: survival meta‐analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h2228. doi: 10.1136/bmj.h1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leitão L, Soares‐Dos‐Reis R, Neves JS, Baptista RB, Bigotte Vieira M, Mc Causland FR. Intensive blood pressure treatment reduced stroke risk in patients with albuminuria in the SPRINT trial. Stroke. 2019;50(12):3639‐3642. doi: 10.1161/STROKEAHA.119.026316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The SPRINT Research Group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373(22):2103‐2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood‐pressure control in older patients with hypertension. N Engl J Med. 2021;385:(14):1268‐1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 26. JATOS Study Group . Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31(12):2115‐2127. doi: 10.1291/hypres.31.2115 [DOI] [PubMed] [Google Scholar]
- 27. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56(2):196‐202. doi: 10.1161/HYPERTENSIONAHA.109.146035 [DOI] [PubMed] [Google Scholar]
- 28. Steurer J. Intensive Blutdruckbehandlung vs. weniger intensive Behandlung [intensive blood pressure treatment vs. less intensive treatment]. Praxis (Bern 1994). 2016;105(4):225‐226. doi: 10.1024/1661-8157/a002281 [DOI] [PubMed] [Google Scholar]
- 29. Carter AB. Hypotensive therapy in stroke survivors. Lancet. 1970;1(7645):485‐489. doi: 10.1016/s0140-6736(70)91577-1 [DOI] [PubMed] [Google Scholar]
- 30. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm hg. JAMA. 1970;213(7):1143‐1152. doi: 10.1001/jama.1970.03170330025003 [DOI] [PubMed] [Google Scholar]
- 31. Hypertension‐Stroke Cooperative Study Group . Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229(4):409‐418. doi: 10.1001/jama.1974.03230420021019 [DOI] [PubMed] [Google Scholar]
- 32. The National Heart Foundation of Australia . Treatment of mild hypertension in the elderly. A study initiated and administered by the National Heart Foundation of Australia. Med J Aust. 1981;2(8):398‐402. [PubMed] [Google Scholar]
- 33. Kuramoto K, Matsushita S, Kuwajima I, Murakami M. Prospective study on the treatment of mild hypertension in the aged. Jpn Heart J. 1981;22(1):75‐85. doi: 10.1536/ihj.22.75 [DOI] [PubMed] [Google Scholar]
- 34. Sprackling ME, Mitchell JR, Short AH, Watt G. Blood pressure reduction in elderly: a randomised controlled trial of methyldopa. BMJ. 1981;283(6300):1151‐1153. doi: 10.1136/bmj.283.6300.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Staessen J, Bulpitt C, Clement D, et al. Relation between mortality and treated blood pressure in elderly patients with hypertension: report of the European working party on high blood pressure in the elderly. BMJ. 1989;298(6687):1552‐1556. doi: 10.1136/bmj.298.6687.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perry HM Jr, Smith WM, McDonald RH, et al. Morbidity and mortality in the systolic hypertension in the elderly program (SHEP) pilot study. Stroke. 1989;20(1):4‐13. doi: 10.1161/01.str.20.1.4 [DOI] [PubMed] [Google Scholar]
- 37. Medical Research Council Working Party . MRC trial of treatment of mild hypertension: principal results. Medical Research Council working party. BMJ. 1985;291(6488):97‐104. doi: 10.1136/bmj.291.6488.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the hypertension in the very elderly trial. J Hypertens. 2003;21(12):2409‐2417. doi: 10.1097/00004872-200312000-00030 [DOI] [PubMed] [Google Scholar]
- 39. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ. 1986;293(6555):1145‐1151. doi: 10.1136/bmj.293.6555.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265(24):3255‐3264. [PubMed] [Google Scholar]
- 41. Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP‐hypertension). Lancet. 1991;338(8778):1281‐1285. doi: 10.1016/0140-6736(91)92589-t [DOI] [PubMed] [Google Scholar]
- 42. MRC Working Party . Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ. 1992;304(6824):405‐412. doi: 10.1136/bmj.304.6824.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350(9080):757‐764. doi: 10.1016/s0140-6736(97)05381-6 [DOI] [PubMed] [Google Scholar]
- 44. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887‐1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 45. Wei Y, Jin Z, Shen G, et al. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J Clin Hypertens (Greenwich). 2013;15(6):420‐427. doi: 10.1111/jch.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 47. Sink KM, Evans GW, Shorr RI, et al. Syncope, hypotension, and falls in the treatment of hypertension: results from the randomized clinical systolic blood pressure intervention trial. J Am Geriatr Soc. 2018;66(4):679‐686. doi: 10.1111/jgs.15236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta‐analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47(1):40‐50. doi: 10.1111/j.1532-5415.1999.tb01899.x [DOI] [PubMed] [Google Scholar]
- 49. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons [published correction appears in Arch Intern Med. 2010 Mar 8;170(5):477]. Arch Intern Med. 2009;169(21):1952‐1960. doi: 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 50. Albasri A, Hattle M, Koshiaris C, et al. Association between antihypertensive treatment and adverse events: systematic review and meta‐analysis. BMJ. 2021;372:n189. doi: 10.1136/bmj.n189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37(1):19‐24. doi: 10.1093/ageing/afm169 [DOI] [PubMed] [Google Scholar]
- 52. Howland J, Peterson EW, Levin WC, Fried L, Pordon D, Bak S. Fear of falling among the community‐dwelling elderly. J Aging Health. 1993;5(2):229‐243. doi: 10.1177/089826439300500205 [DOI] [PubMed] [Google Scholar]
- 53. Delbaere K, Close JC, Brodaty H, Sachdev P, Lord SR. Determinants of disparities between perceived and physiological risk of falling among elderly people: cohort study. BMJ. 2010;341:c4165. doi: 10.1136/bmj [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Legters K. Fear of falling. Phys Ther. 2002;82(3):264‐272. doi: 10.1093/ptj/82.3.264 [DOI] [PubMed] [Google Scholar]
- 55. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553‐561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deeks JJ, JPT H, Altman DG. (editors). Chapter 10: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook [Google Scholar]
- 57. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 Checklist and PRISMA 2020 for Abstracts Checklist.
Table S2. Time to Benefit for the Primary Prevention of Stroke for Older Adults Treated with More Intensive Hypertension Treatment.


