Abstract
Currently, there are no internationally accepted consensus guidelines for pathologic evaluation of post-therapy pancreatectomy specimens. The Neoadjuvant Therapy Working Group of Pancreatobiliary Pathology Society was formed in 2018 to review grossing protocols, literature and major issues, and to develop recommendations for pathologic evaluation of post-therapy pancreatectomy specimens. The working group generated the following recommendations: 1. Systematic and standardized grossing and sampling protocols should be adopted for pancreatectomy specimens for treated pancreatic ductal adenocarcinoma (PDAC). 2. Consecutive mapping sections along the largest gross tumor dimension are recommended to validate tumor size by histology as required by the CAP cancer protocol. 3. Tumor size of treated PDACs should be measured microscopically as the largest dimension of tumor outer limits that is bound by viable tumor cells, including intervening stroma. 4. The MD Anderson grading system for tumor response has better correlation with prognosis and better inter-observer concordance among pathologists than does the CAP system. 5. A case should not be classified as complete response unless entire pancreas, peripancreatic tissues, ampulla of Vater, common bile duct, and duodenum adjacent to pancreas are submitted for microscopic examination. 6. Future studies on tumor response of lymph node metastases, molecular and/or immunohistochemical markers, as well as application of artificial intelligence in grading tumor response of treated PDAC are needed. In summary, systematic standardized pathologic evaluation, accurate tumor size measurement, and reproducible tumor response grading to neoadjuvant therapy are needed for optimal patient care. The criteria and discussions provided here may provide guidance towards these goals.
Keywords: Pancreatic ductal adenocarcinoma, neoadjuvant therapy, gross examination, tumor size, tumor response grade, survival, lymph node metastasis
INTRODUCTION
Patients with pancreatic ductal adenocarcinoma (PDAC) have a poor prognosis with a dismal five-year survival rate of approximately 8.5% (1). The conventional adjuvant therapy approach has been shown to improve the survival of patients with PDAC compared to surgical resection alone (2–4). However, PDAC often recurs after surgical resection and the survival for patients with PDAC has improved only slightly in the last 25 years (5). As an alternative treatment approach, neoadjuvant therapy is increasingly used to treat patients with potentially resectable PDAC (6). Currently, neoadjuvant therapy is the standard of care recommended by the National Comprehensive Cancer Network (NCCN) for patients with borderline resectable PDAC and high-risk patients with resectable disease (7).
Systematic pathologic examination and reporting of post-therapy pancreatic resection specimen is important both for post-operative management and for prognostication for patients with PDAC (8–16). However, pathologic evaluation of pancreas specimens resected for PDAC after neoadjuvant therapy is a complex and challenging task for pathologists. These specimens typically have extensive therapy-induced fibrosis obscuring the demarcation between the tumor and adjacent non-neoplastic pancreatic parenchyma, which makes the gross identification of tumor, tumor borders, and tumor size measurement required for staging extremely difficult (17–19). So far, there are no reliable markers to distinguish between cancer-associated desmoplasia and therapy-induced fibrosis (20). The presence of pre-existing fibrosis related to obstructive changes, unrelated to neoadjuvant treatment, in a proportion of patients further complicates this analysis. For patients whose tumors have marked treatment response, the tumor may not be grossly identifiable, and it may require extensive sampling to identify the microscopic foci of viable carcinoma (17). Currently, there are no internationally accepted consensus guidelines for the pathologic evaluation of post-therapy pancreatectomy specimens.
Methods
The Neoadjuvant Therapy Working Group of the Pancreatobiliary Pathology Society (PBPS) was formed in August of 2018 and consists of seven expert pancreatic and gastrointestinal pathologists from Asia (YM), Europe (IE) and North America (RC, DSA, MH, VD and HW). The working group was tasked by the PBPS to analyze the present state of the literature and to provide recommendations for the pathologic evaluation of post-therapy pancreatectomy specimens. The working group particularly focused on the gross examination, tumor size measurement, and the histologic grading of tumor response to neoadjuvant therapy. The members of the working group met online quarterly. The results from these meetings and discussions were collated and presented to the members of Education Committee and Executive Committee of PBPS (CL, RPG, MDR, OB, HW, DA, RHH, AK, DSK and VA). For the gross examination of post-therapy pancreatectomy specimens, the working group worked in close collaboration with the Grossing Working Group of PBPS (DSA, DD, GEK, JS, KTJ, OB, and VA) and reviewed the grossing protocols from different institutions. The conclusions presented below are based on the work and consensus from this working group for the pathologic evaluation of post-therapy pancreas specimens.
Open comments and votes from the PBPS members
The manuscript with all figures and tables were sent by emails to all PBPS members for online voting and open comments through Survey Monkey from June 29 to July 29, 2021 (one month) with two additional reminders sent at the ends of the first two weeks and the third week of open commenting period, respectively. The members were asked to vote “Yes” or “No” on the question: “Do you conceptually agree with the consensus statements/recommendations in this white paper attached to the email sent? Your positive comments, suggestions and/or concerns are appreciated.”. The comments from members were either posted online or sent by email to Dr. Huamin Wang. Submitted comments were collated and discussed among the working group members and incorporated into the consensus recommendations.
Results
1. Gross examination, consecutive mapping sections for tumor size measurement and sampling of treated PDAC specimens
Gross examination and adequate sampling form the foundation for accurate evaluation of the pathology of pancreatectomy specimens for PDAC after neoadjuvant therapy (17, 21). Currently, there is no standardized grossing protocol and there are significant variations from center to center in intra-operative evaluation, grossing, and sampling/mapping of the tumor and margins (22). The Neoadjuvant Therapy Working Group and the Grossing Working Group of the PBPS reviewed the grossing protocols from different institutions worldwide and from the literature. For pancreaticoduodenectomy specimens, the two most commonly used grossing methods are the bivalving approach and the axial sectioning approach (23). Currently, there is not enough data to favor either bivalving or axial sectioning approach for PDAC after neoadjuvant therapy. The working group suggests the following method for grossing based on consecutive mapping sections for tumor size measurement and sampling of treated PDAC specimens.
The College of American Pathologists (CAP) cancer protocol and the Guide of the International Collaboration on Cancer Reporting (ICCR, iccr-cancer.org) for PDAC recommend that gross measurement of tumor size should be validated by microscopic examination (24, 25). After serial sectioning of the pancreas at 3–5 mm thickness, the largest dimension of the treated tumor should be identified and measured grossly. To validate gross measurement of tumor size by histology, consecutive mapping sections along the largest tumor dimension or whole mount sections of the pancreas should be submitted. The tumor size can be measured microscopically across the reassembled consecutive sections or whole mount sections (Figure 1). Although whole mount sections would be ideal for tumor mapping, this method is routinely available only in few academic institutions. In addition to the consecutive mapping sections, other sections that should be included from a post-neodjuvant pancreaticoduodenectomy specimen are the same as those recommended for the pancreaticoduodenectomy specimen for treatment naïve PDAC. For patients with borderline resectable disease, segmental or patch resection of superior mesenteric vein (SMV) or portal vein (PV) is often performed with pancreaticoduodenectomy after neoadjuvant therapy. In these cases, vein margins and the rest of the resected vein with underlying tumor should be submitted to document the vein margins, involvement of the vein, and the depth of tumor invasion into the SMV/PV (16, 17). Since vein resection is often small and could be missed during gross examination, the working group suggests that the surgeons should label the resected vein with a stich for optimal identification.
Figure 1.

Cut surface of pancreatic head with pancreatic ductal adenocarcinoma after neoadjuvant therapy show extensive fibrosis in both tumor and adjacent pancreas, which makes gross determination of tumor size very difficult. Schematic drawing of the consecutive mapping sections across the largest diameter of the tumor is shown (A). Microscopic tumor size measurement of 3.3 cm across the reassembled consecutive mapping sections after histologic review of viable tumor on hematoxylin and eosin (H & E) stained sections (B). The holes on the H & E sections represent 3.0 mm needle punches to harvest tissue for tumor bank, which has no or minimal impact on microscopic tumor size measurement.
Given the difficulties in identifying tumor in patients who had a marked response to neoadjuvant therapy, the optimal approach would involve histologic evaluation of the entire tumor or suspicious tumor area (s). Considering the cost effectiveness, however, the group suggested that smaller tumors (2.0 cm or less) should be entirely submitted for histologic examination. Larger tumors (> 2.0 cm) may be sampled generously with representative sections (at least 2 sections per cm of the tumor should be sampled). If a pancreatectomy specimen for PDAC after neoadjuvant therapy has no grossly identifiable tumor or the initially submitted sections have no viable PDAC, the working group supports the approach of entire submission or rigorous extensive sampling of the pancreas and peripancreatic soft tissue, common bile duct, ampulla of Vater, and duodenum adjacent to the pancreas. If warranted, multiple levels of the fibrotic/pathologic areas and immunohistochemical stains for pan-cytokeratin may be performed to rule out microscopic foci of residual invasive carcinoma before the case can be classified as complete pathologic response (no viable tumor identified). A similar extensive sampling approach may need to be applied to cases that are found to have only minimal viable tumor in the initial sections prior to categorizing the case as minimal residual tumor (MD Anderson or CAP grade 1 response).
The AJCC/UICC Staging Manual (8th edition) and the current CAP protocol require to examine a minimum of 12 lymph nodes from a pancreatectomy specimen. Although treatment including radiation therapy can render lymph nodes particularly difficult to identify grossly, with proper dissection, a conventional pancreaticoduodenctomy specimen after neoadjuvant therapy typically yields more than 12 lymph nodes. If this number cannot be achieved, then a comment indicating that the specimen was re-visited and the peripancreatic adipose tissue has been submitted extensively for microscopic examination is warranted. Careful dissecting and palpating of the peripancreatic and retroperitoneal soft tissue should be considered to maximize the number of lymph nodes in pancreatectomy specimens (26).
2. Tumor size measurement of treated PDAC by histology
The current AJCC/UICC staging system for PDAC (8th edition) uses only tumor size for assigning pT1 to pT3 categories: pT1 tumor ≤ 2 cm (pT1a ≤ 0.5 cm, pT1b > 0.5 cm and < 1 cm, and pT1c ≥ 1 cm and ≤ 2 cm), pT2 tumor > 2 cm and ≤ 4 cm, and pT3 tumor > 4 cm (27). Recent studies showed that the 8th AJCC/UICC ypT stage performed better than the 7th AJCC ypT stage in stratifying patients based on survival in treated PDAC patients (28, 29). However, the working group recognized that accurate tumor size measurement in post-therapy pancreatectomy specimens is extremely challenging due to the following factors: 1. Therapy-induced fibrosis is often extensive in both tumor and adjacent pancreatic tissue, which makes it difficult to identify tumor borders grossly, especially for those patients who had marked responses to neoadjuvant therapy (Figure 2A and 2B). 2. PDAC is a highly invasive carcinoma, which often invades into the adjacent pancreatic/peripancreatic tissue or other adjacent organs beyond the grossly identified tumor, and often responds heterogeneously to neoadjuvant therapy in different areas of the tumor. Microscopic foci of viable residual neoplastic cells are often scattered randomly in the treated tumor bed, intermingled with adjacent benign pancreatic parenchyma, or invading peripancreatic soft tissue, bile duct or duodenum (Figure 2B–2F) (30). Therefore, the gross measurement of tumor size of treated PDAC is often inaccurate and can either over- or underestimate the true tumor size. As described above, the working group agreed with the CAP cancer protocol and ICCR guidelines that gross measurement of tumor size of treated PDAC should be validated by microscopic examination. However, an international consensus on the method for microscopic tumor size measurement is currently missing. The working group recommended that the largest dimension of the entire area bound by viable neoplastic cells, including intervening stroma and/or non-neoplastic pancreas, should be measured as the size of a treated PDAC, as illustrated in Figure 3. Thus, if two foci of viable carcinoma are separated by 1 cm of fibrosis, the intervening area of fibrosis would be included in the measurement. This method is recommended for the standardized pathologic evaluation of post-neoadjuvant specimens of breast cancer by an international working group (31). A recent study by Chatterjee et al. showed that the tumor size/ypT stage measured using this method correlated well with patient survival and other clinicopathologic parameters in a large cohort of treated PDAC patients (28).
Figure 2.
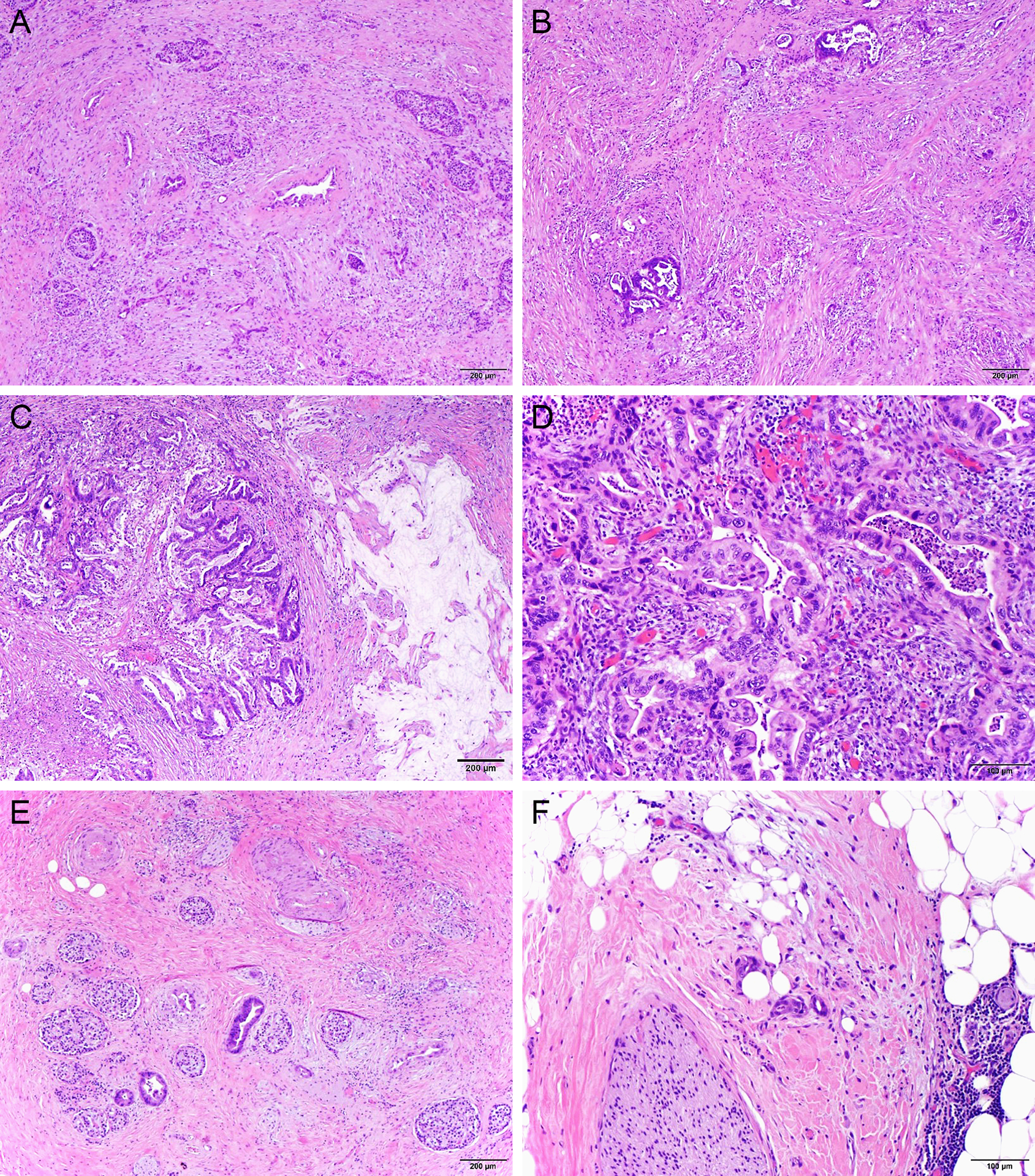
Representative micrographs show a case of treated pancreatic ductal adenocarcinoma with extensive fibrosis in both pancreas adjacent to tumor (A) and in tumor (B); heterogeneous tumor responses in different areas of the tumor (B-D), microscopic foci of residual adenocarcinoma invading into the adjacent pancreatic tissue (E) and peripancreatic soft tissue (F). The tumor shows major response with extensive fibrosis (B), necrosis and acellular mucin (C) in some areas, but no significant response in other areas of the tumor (D).
Figure 3.

Schematic drawing to illustrate microscopic measurement of tumor size in pancreatectomy for pancreatic ductal adenocarcinoma after neoadjuvant therapy.
3. Histologic Grading of Tumor Response of Pancreatic Ductal Adenocarcinoma to Neoadjuvant Therapy
Histopathologic evaluation of PDAC response to neoadjuvant treatment provides valuable information, which may help the clinicians select more effective post-operative adjuvant therapy regimens and may also serve as a prognostic marker for these patients (9, 32). Currently, three major tumor response grading systems for PDAC are commonly used, including the Evans, CAP, and MD Anderson grading systems (Table 1). While the Evans system is based on the percentage of tumor cell destruction, the CAP and MD Anderson systems are based on the amount and the percentage of residual viable tumor cells relative to the tumor bed, respectively (Figure 4). The CAP system is widely used in North America since it is part of the CAP cancer protocol for pancreatic exocrine tumors. Our working group agreed with the 2019 Amsterdam International Consensus Meeting on tumor response grading systems for resected PDAC after neoadjuvant therapy that tumor response grading should be based on the residual viable tumor component instead of tumor regression (33). Although the 2019 Amsterdam International Consensus Meeting endorsed the CAP system, this meeting also unanimously agreed that the criteria of the CAP system should be improved by using objective or quantitative criteria to evaluate the extent of viable tumor rather than the subjective terms including “single cells or rare small groups of cancer cells ” or “extensive residual tumor”, which may lead to significant variations of subjective interpretation by different pathologists (33). It should be noted that the CAP system, which is adopted from a modified Ryan scheme originally proposed for treated rectal cancer, is also used to evaluate tumor response for treated carcinomas of all gastrointestinal sites, including the esophagus, esophagogastric junction, stomach, rectum, and anus (17).
Table 1.
Three Major Tumor Response Grading Systems for Patients With Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy
| Evans Grading System, which is based on the percentage of tumor cell destruction |
| Grade I: Little (<10%) or no tumor cell destruction |
| Grade IIa: Destruction of 10%−50% of tumor cells |
| Grade IIb: Destruction of 51%−90% of tumor cells |
| Grade III: Few (<10%) viable-appearing tumor cells |
| Grade IV: No viable tumor cells |
| CAP Grading System, which is based on the amount of residual viable tumor cells |
| Grade 0: No viable cancer cells (complete response) |
| Grade 1: Single cells or rare small groups of cancer cells (near complete response) |
| Grade 2: Residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells (partial response) |
| Grade 3: Extensive residual cancer with no evident tumor regression (poor or no response) |
| MD Anderson Grading System which is based on the percentage of viable tumor cells |
| Grade 0: No viable cancer cells (complete response) |
| Grade 1: Minimal residual carcinoma (single cells or small groups of cancer cells, <5% viable residual carcinoma in treated tumor bed) |
| Grade 2: 5% or more viable residual carcinoma cells in treated tumor bed |
Figure 4.
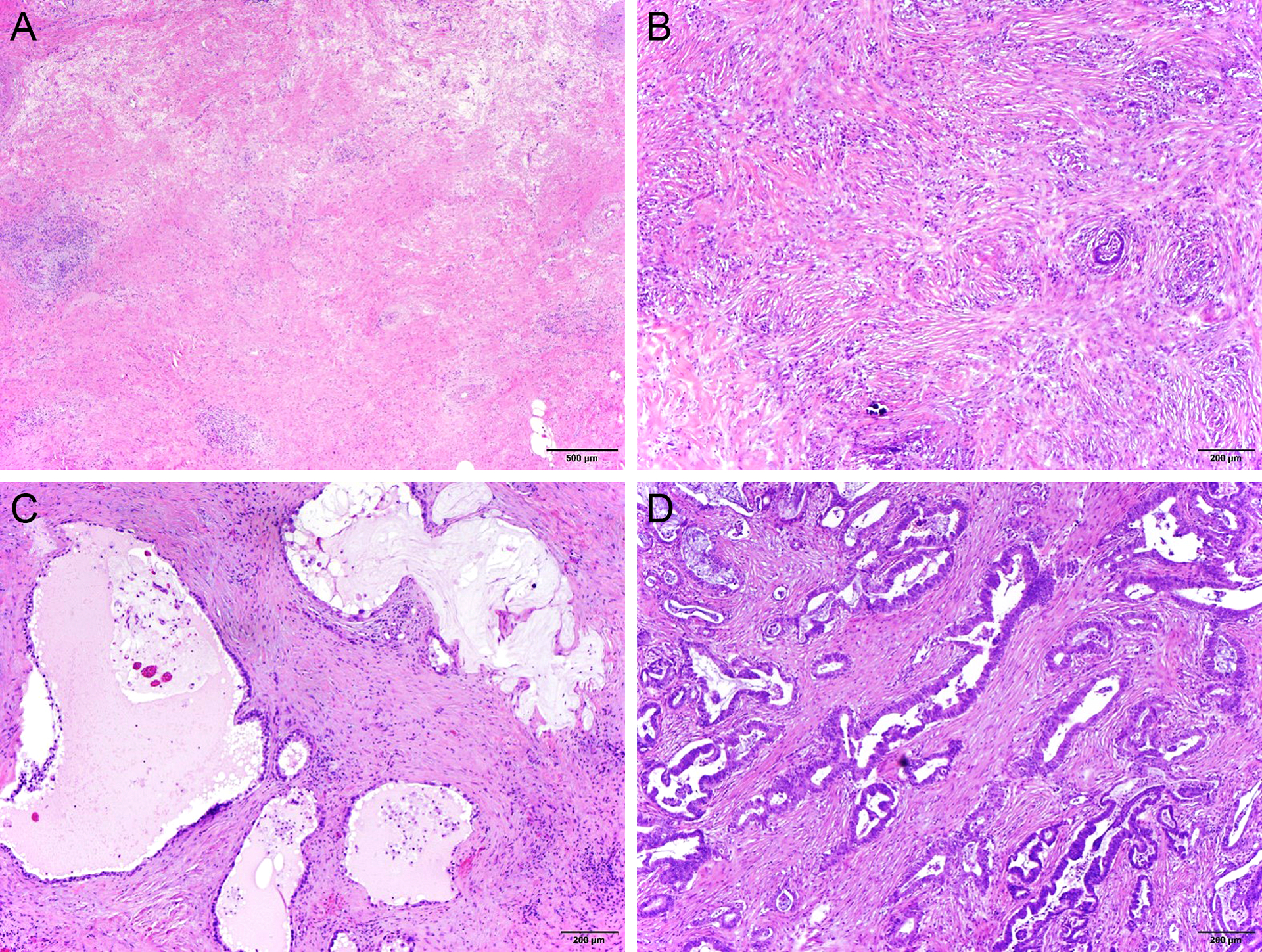
Representative micrographs illustrating College of American Pathologists (CAP) and the MD Anderson (MDA) tumor response grading. Complete pathologic response (MDA grade 0/CAP grade 0, A); MDA grade 1 (CAP grade 1, B); MDA grade 2 (CAP grade 2, C); and MDA grade 2 (CAP grade 3, D) tumor response to neoadjuvant therapy.
The MD Anderson system was proposed by Chatterjee et al. and validated by Lee et al. after comparing the clinical and prognostic significance of the CAP and Evans systems in two large cohorts of patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy at MD Anderson Cancer Center (9, 32). In both cohorts, patients with MD Anderson grade 0 response or MD Anderson grade 1 response had better disease-free and overall survival than those with MD Anderson grade 2 response (Figure 5A and 5B). In contrast, no significant differences in either disease-free survival or overall survival were observed in both cohorts between the patients with CAP grade 2 and those with grade 3 response (Figure 5C and 5D) or among the patients with Evans grades I, IIa and IIb (9, 32). The MD Anderson system also showed significant correlation with the ypT, ypN, margin status and tumor recurrence after resection (9, 32). Their findings have been validated by two recent studies: a study of 147 treated PDAC patients from Royal North Shore Hospital (Sydney, Australia) by Chou et al. and a multi-institutional study of 97 treated patients with PDAC from Japan by Matsuda et al (34, 35). Similar to the results from the MD Anderson cohorts, both studies showed no difference in either overall or disease-free survival between the patients with CAP grade 2 and those with CAP grade 3 response or among the patients with Evans grades I, IIa and IIb response (34, 35).
Figure 5.

Kaplan-Meier survival curves of disease-free survival and overall survival stratified by the MD Anderson (A and B) and CAP (C and D) tumor response grading in 398 patients from MD Anderson Cancer Center who received neoadjuvant therapy and then had a pancreaticoduodenectomy. No difference in either disease-free survival or overall survival is observed between groups with CAP grade 2 and grade 3 responses (C and D).
The optimal tumor response grading system for treated PDAC should be easy to implement and reproducible. Recent studies showed that the MD Anderson system has significantly better inter-observer agreement among pathologists than the CAP or Evans TRG systems (34–36). N Kalimuthu et al. showed concordant grading in 79% cases (11/14) using the MD Anderson system, compared to 14% (2/14) for the CAP system, and 7% (1/14) for the Evans system among four gastrointestinal pathologists (36). Matsuda et al. examined the concordant grading between two pathologists, who reviewed 97 treated PDAC cases, and showed a concordant rate of 96%, 72% and 59% using the MD Anderson, CAP and Evans systems, respectively. The inter-observer concordance (kappa value) for MDA, CAP, and Evans’ grading systems were 0.65, 0.5, and 0.34, respectively (35). Similarly, the reported kappa values between two pathologists reported in Chou’s study were 0.691, 0.431 and 0.307 for the MD Anderson, CAP and Evans systems, respectively. These data demonstrate that the MD Anderson grading system is a more reproducible tumor response grading scheme compared to the CAP or Evans systems.
Given the findings from the above-mentioned studies that patients with CAP grade 2 and CAP grade 3 show no difference in survival and that the CAP grading system performs significantly worse than the MD Anderson system in inter-observer concordant studies (9, 32, 34–36), the working group fully support that the 3-tier MD Anderson grading system could be used as an alternative grading system for CAP cancer protocol.
4. Histologic evaluation of lymph nodes
Metastatic PDAC in regional lymph node(s) may also show significant response to neoadjuvant therapy (Figure 6). Studies on the prognostic significance of micrometastasis or isolated tumor cells in lymph nodes after neoadjuvant therapy are limited. Micrometastasis (≤2.0 mm) or isolated tumor cells in axillary lymph node are associated with worse overall survival compared to those with true-negative node status in breast cancer patients after neoadjuvant therapy although patients with micrometastasis had better survival than those with macrometastasis (37). For neoadjuvant-treated PDAC, currently, any amount of viable tumor cells in a lymph node is regarded as “metastasis”. There is no “isolated tumor cell” category defined for the pancreas. Also, unlike in other organs, it is much less common to see fibroinflammatory response or mucin pool without any documentable carcinoma cells consistent with regressed tumor in the lymph node(s) from pancreatectomy specimens based on the consensus experiences of the authors. When this is encountered, in the absence of any other lymph node metastasis, the suspect lymph node may be examined with multiple section levels and cytokeratin stains to identify the carcinoma cells.
Figure 6.

Lymph node metastasis of pancreatic ductal adenocarcinoma showing marked treatment effect with fibrosis and scattered individual viable tumor cells (A) or fibrosis and mucin pools and rare small clusters of viable tumor cells (B).
5. Online voting results and open comments from PBPS members
During our open comments and voting period, we received total of 51 votes from 228 members. 47 (92.2%) voted “Yes” and conceptually agree with the consensus statements/recommendations in this white paper and 4 (7.8%) voted “No”. Total of 21 comments from members were received, mainly regarding the optimal vs practical sampling approach, the tumor response grading systems, the role of immunohistochemistry in identifying minimal residual tumor, and pathologic evaluation of lymph node metastasis with treatment effect. All comments related to the consensus recommendations have been incorporated in this manuscript.
Discussion
Systematic pathologic evaluation of the pancreatectomy specimens after neoadjuvant therapy provides critical information not only for the prognosis, but also for the subsequent clinical management of PDAC patients (8, 11–14, 28, 32, 34). However, there are no standardized grossing and sampling protocols for pancreatectomy specimens after neoadjuvant therapy (22). The expert working group of the PBPS, composed of dedicated pancreatic pathologists from three continents, identified the following relevant open issues concerning the pathological examination of pancreatic resection specimens after neoadjuvant treatment: a) gross examination, tumor mapping sections and sampling, b) measurement of the tumor size (both macro- and microscopically), c) the use of a TRG based on clinical evidence, d) the histological assessment of lymph nodes. Other clinically relevant issues, such as intra-operative frozen sections, the determination of the margin status (R-status), the assessment of tumor involvement of resected vessels and other adjacent organs, tumor banking etc. are common to pancreatectomy for PDAC without neoadjuvant therapy and have been critically assessed elsewhere (17, 38, 39). The main points on gross and microscopic examination of pancreatectomy specimens for treated PDAC are summarized in Table 2.
Table 2.
The main points on gross and microscopic examination of pancreatectomy specimens for treated pancreatic ductal adenocarcinoma
|
Currently, there is not enough data to favor either bivalving or axial sectioning approach for pancreaticoduodenectomy specimens resected for PDAC after neoadjuvant therapy. The working group agreed that both bivalving and axial sectioning approach may be used at the preference of the pathologist for pancreaticoduodenectomy specimens resected for PDAC after neoadjuvant therapy. Evolving literature indicates that both approaches provide comparable results, with their respective advantages and disadvantages (40). A multi-institutional, prospective study to compare the bivalving and axial sectioning approaches for pancreaticoduodenectomy specimens in the setting of neoadjuvant treatment is currently on-going.
Tumor size measurement is extremely difficult for PDAC after neoadjuvant therapy. The working group recognized that it is difficult to identify the borders of treated tumor by gross inspection and that microscopically, the tumor often extends beyond the grossly identified tumor borders. Submission of consecutive mapping sections or whole mount section along the largest tumor dimension in one cross-section of the pancreas may not always capture the largest tumor diameter. Alternatively approaches include submission of tumor mapping sections on two or more cross-sections of the pancreas or the entire possible tumor areas in order to identify the largest tumor diameter even though these approaches would significantly increase the number of sections. When no tumor is grossly identified, the optimal approach is to submit with mapping the entire pancreas and peripancreatic soft tissue, ampulla of Vater, common bile duct and duodenal wall adjacent to pancreas to identify microscopic residual tumor and to accurately measure the tumor size if microscopic residual tumor is identified. A case should not be classified as complete response unless the entire pancreas, peripancreatic soft tissues, the ampulla of Vater, the common bile duct, and the duodenum adjacent to pancreas are submitted for microscopic examination, and if warranted, multiple levels of the fibrotic/pathologic areas.
Although the CAP grading system is widely used in North America since the adoption of the CAP cancer protocol for pathology reporting of exocrine pancreatic tumor, the prognostic value of the CAP grading has not been validated. Multiple recent studies showed no difference in survival between the patients with CAP grade 2 and those with CAP grade 3 response (9, 32, 34, 35). In addition, the criteria for CAP grading is not well-defined and may lead to significant interobserver variations among the pathologists when applying this grading system (34–36). Currently there is no universally accepted tumor response grading system for treated PDAC. Recent studies showed that MD Anderson grading system correlates better with patient survival and has significantly better inter-observer concordance among the pathologists than the CAP grading system (9, 32, 34, 35). One argument against the MD Anderson system is that it classifies the majority (>80%) of PDAC patients as grade 2 response. This is also the major reason why the 2019 Amsterdam International Consensus Meeting and the ICCR endorsed the CAP system (19, 33). The working group recommends that future prospective or retrospective studies are needed to determine whether PDAC patients with MD Anderson grade 2 response can be further subclassified into two clinically/prognostically meaningful groups using well-defined criteria. In the recently proposed RNS system, Chou et al. subclassified patients with MD Anderson grade 2 response into RNS grade 2 (11% to 75% viable tumor cells) and RNS grade 3 (>75% viable tumor cells). They showed that patients with RNS grade 3 had shorter survival than those with RNS grade 2 response in their patient population. However, the clinical or prognostic significance of this newly proposed RNS grade 3 could not be validated using the data set of 398 patients from MD Anderson. In addition, considering the fact that untreated PDAC are typically characterized by extensive desmoplastic stroma and a relatively low percentage of tumor cells, 75% viable tumor cells as a cut off for grade 3 proposed by the RNS system seems to be too high for treated PDAC.
Other recently published grading systems include the residual tumor index (RTI), which is calculated as a product of the percentage of residual viable carcinoma and tumor size in centimeters, and a grading system from Japan based on the area of residual tumor (ART), have also been shown to correlate with patient survival (35, 41). Given the difficulties in measuring tumor size and estimating the percentage of residual viable tumor cells for treated PDAC cases, the determination of RTI is difficult and may not be suitable for daily practice. The Japanese ART system scores tumor response as 0 (no remaining viable tumor), 1, 2, 3, and 4 (viable tumor spanning ≤ 1, > 1 and ≤ 2, > 2 and ≤ 3, and > 3 4x power objective fields, respectively). Although this system is objective and easy to use, this grading system is more reflective of residual tumor size rather than the amount of residual viable tumor cells.
The working group also recognizes that there are different neoadjuvant regimens employed throughout the world. In some institution, such as MD Anderson Cancer Center, neoadjuvant therapy includes chemoradiation, while in other institutions, most patients receive chemotherapy alone. In addition, some institutions prefer FOLFIRINOX, while others prefer gemcitabine plus nab-paclitaxel as the regimen for neoadjuvant chemotherapy. It is possible that different grading systems may be required for optimal grading of tumor response based on the neoadjuvant regimen employed. In addition, the timing of surgery after completion of neoadjuvant therapy may also has significant impact on the tumor response grading since tumor may re-grow after cessation of neoadjuvant therapy. Future studies are needed to examine the effect of different neoadjuvant therapy regimens with or without radiation and the timing of surgery after completion of neoadjuvant therapy on tumor response grading.
Several other important issues remain to be studied for grading tumor response to neoadjuvant therapy. 1. Metastatic PDAC in regional lymph node(s) may also show significant response to neoadjuvant therapy. Heterogeneous tumor response may not only occur within the primary tumor, but also in the involved lymph nodes. However, none of the current tumor response grading systems for treated PDAC includes the response of metastatic PDAC in regional lymph nodes. Based on limited data, it is extremely rare to see viable metastatic PDAC in regional lymph nodes in patients with complete pathologic response (10). The response of lymph node metastasis either alone or in combination with the tumor response grading of the primary PDAC may prove to be a valuable parameter for overall response to neoadjuvant therapy. How to incorporate the response of lymph node metastasis or the size of nodal metastasis after neoadjuvant therapy into the tumor response grading system would be an important and interesting topic for future studies. 2. Although some institutions routinely submit the entire treated PDAC for histologic examination, others are doing representative sections of treated PDAC. The variations in sampling protocols among different institutions would significantly affect the accuracy of tumor response grading. Differences in sampling and inter-observer variation in interpreting the tumor response grading would also hinder the multi-institutional studies on treated PDAC. The optimal and cost-effective sampling protocol of pancreatectomy specimens for PDAC after neoadjuvant therapy remains to be determined. 3. The current tumor response grading system also does not include any molecular/immunohistochemical markers. In the era of precision/personalized medicine, molecular and/or immunohistochemical markers or artificial intelligence approaches may provide valuable information about the tumor responsiveness to neoadjuvant therapy, not only for patient prognosis, but also for selection of more effective post-operative adjuvant chemotherapy or targeted therapy.
In summary, pathologic examination, tumor size measurement and histologic tumor response grading in pancreatectomy specimens for PDAC after neoadjuvant therapy are challenging and difficult. Systematic standardized grossing and sampling protocols and meticulous microscopic examination are important not only for tumor size measurement, but also for accurate histologic grading of tumor response to neoadjuvant therapy. The working group support that the 3-tier MD Anderson grading system could be used as an alternative grading system for CAP cancer protocol since it has been shown to be not only a good prognostic marker for PDAC patients after neoadjuvant therapy, but also to have superior inter-observer agreement among pathologists.
Grant Support
HW: The National Institutes of Health grants (1R01CA196941, 1R01CA195651, U01CA196403, P01CA117969, and P50CA221707)
JS: The National Institutes of Health grant (K08CA234222)
The authors have no conflicts of interest or funding to disclose.
REFERENCES
- 1.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and −3(v1) trials. Br J Cancer. 2009;100:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–512. [DOI] [PubMed] [Google Scholar]
- 5.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 6.Youngwirth LM, Nussbaum DP, Thomas S, et al. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. J Surg Oncol. 2017;116:127–132. [DOI] [PubMed] [Google Scholar]
- 7.Tempero MA, Malafa MP, Al-Hawary M, et al. NCCN Clinical Practice Guidelines in Oncology, Pancreatic adenocarcinoma (Version 1.2021). 2020. Available at: https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. Accessed January 25, 2021.
- 8.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SM, Katz MH, Liu L, et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40:1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee D, Rashid A, Wang H, et al. Tumor invasion of muscular vessels predicts poor prognosis in patients with pancreatic ductal adenocarcinoma who have received neoadjuvant therapy and pancreaticoduodenectomy. Am J Surg Pathol. 2012;36:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–277. [DOI] [PubMed] [Google Scholar]
- 13.Fischer LK, Katz MH, Lee SM, et al. The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology. 2016;68:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Katz MH, Lee SM, et al. Superior Mesenteric Artery Margin of Posttherapy Pancreaticoduodenectomy and Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Am J Surg Pathol. 2015;39:1395–1403. [DOI] [PubMed] [Google Scholar]
- 15.Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagaria TS, Wang H, Chatterjee D, et al. Pathology of Treated Pancreatic Ductal Adenocarcinoma and Its Clinical Implications. Arch Pathol Lab Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman DJ, Krasinskas AM. Assessing treatment effect in pancreatic cancer. Arch Pathol Lab Med. 2012;136:100–109. [DOI] [PubMed] [Google Scholar]
- 19.Verbeke C, Haberle L, Lenggenhager D, et al. Pathology assessment of pancreatic cancer following neoadjuvant treatment: Time to move on. Pancreatology. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Haeberle L, Insilla AC, Kapp AC, et al. Stroma composition and proliferative activity are related to therapy response in neoadjuvant treated pancreatic ductal adenocarcinoma. Histol Histopathol. 2021:18332. [DOI] [PubMed] [Google Scholar]
- 21.Nagaria TS, Wang H. Modification of the 8(th) AJCC staging system of pancreatic ductal adenocarcinoma. Hepatobiliary Surg Nutr. 2020;9:95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeberle L, Esposito I. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soer E, Brosens L, van de Vijver M, et al. Dilemmas for the pathologist in the oncologic assessment of pancreatoduodenectomy specimens : An overview of different grossing approaches and the relevance of the histopathological characteristics in the oncologic assessment of pancreatoduodenectomy specimens. Virchows Arch. 2018;472:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbeke C, Brosens L, Campbell F, et al. Carcinoma of the Exocrine Pancreas Histopathology Reporting Guide. International Collaboration on Cancer Reporting. Sydney, Australia; 2020. [DOI] [PubMed] [Google Scholar]
- 25.Burgart LJ, Shi C, Adsay NV, et al. Protocol for the Examination of Specimens From Patients With Carcinoma of the Pancreas 2020. Available at: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates. Accessed January 26, 2021.
- 26.Adsay NV, Basturk O, Altinel D, et al. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol. 2009;22:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual. New York: Springer International Publishing; 2017. [Google Scholar]
- 28.Chatterjee D, Katz MH, Foo WC, et al. Prognostic Significance of New AJCC Tumor Stage in Patients With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant Therapy. Am J Surg Pathol. 2017;41:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlitter AM, Jesinghaus M, Jager C, et al. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121–129. [DOI] [PubMed] [Google Scholar]
- 30.Fujikura K, Hutchings D, Braxton AM, et al. Intraductal pancreatic cancer is less responsive than cancer in the stroma to neoadjuvant chemotherapy. Mod Pathol. 2020;33:2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015;28:1185–1201. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen BV, Tutucu F, van Roessel S, et al. Amsterdam International Consensus Meeting: tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod Pathol. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Chou A, Ahadi M, Arena J, et al. A Critical Assessment of Postneoadjuvant Therapy Pancreatic Cancer Regression Grading Schemes With a Proposal for a Novel Approach. Am J Surg Pathol. 2021;45:394–404. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda Y, Ohkubo S, Nakano-Narusawa Y, et al. Objective assessment of tumor regression in post-neoadjuvant therapy resections for pancreatic ductal adenocarcinoma: comparison of multiple tumor regression grading systems. Sci Rep. 2020;10:18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.N Kalimuthu S, Serra S, Dhani N, et al. Regression grading in neoadjuvant treated pancreatic cancer: an interobserver study. J Clin Pathol. 2017;70:237–243. [DOI] [PubMed] [Google Scholar]
- 37.Fisher ER, Wang J, Bryant J, et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. [DOI] [PubMed] [Google Scholar]
- 38.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol.29:127–141. [DOI] [PubMed] [Google Scholar]
- 39.Dhall D, Shi J, Allende DS, et al. Towards a More Standardized Approach to Pathologic Reporting of Pancreatoduodenectomy Specimens for Pancreatic Ductal Adenocarcinoma: Cross-continental and Cross-specialty Survey From the Pancreatobiliary Pathology Society Grossing Working Group. Am J Surg Pathol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Roessel S, Soer EC, van Dieren S, et al. Axial slicing versus bivalving in the pathological examination of pancreatoduodenectomy specimens (APOLLO): a multicentre randomized controlled trial. HPB (Oxford). 2021. [DOI] [PubMed] [Google Scholar]
- 41.Panni RZ, Gonzalez I, Hartley CP, et al. Residual Tumor Index: A Prognostically Significant Pathologic Parameter in Neoadjuvant-treated Pancreatic Ductal Adenocarcinoma. Am J Surg Pathol. 2018;42:1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]


