Abstract
Background:
Previous studies have demonstrated positive impacts of advance care planning (ACP) on end-of-life (EOL) care. We sought to examine trends in ACP and EOL care intensity among persons living with dementia (PLWD) who required surrogate decision-making in their final days of life.
Methods:
We analyzed the participants of the Health and Retirement Study (HRS), a nationally representative longitudinal panel study of U.S. residents, with dementia 70 years and older who required surrogate decision-making in the final days of life and died between 2000 and 2014. Based on surrogate reports after the death of a participant, our study measured the completion of three specific types of patient-engaged ACP (written EOL care instructions, assignment of a durable power of attorney for healthcare, patient engagement in EOL care discussions) and four measures of EOL care in the final days of life (death in hospital, receipt of life-prolonging treatments, limiting or withholding certain treatments, and receipt of comfort-oriented care). All analyses accounted for the complex survey design of HRS.
Results:
Among 870 adults (weighted N= 2,812,380) with dementia who died in 2000-2014 and required surrogate decision making at EOL, only 34.8% of patients participated in all three aspects of ACP, and there was not a significant increase in ACP completion between 2000 and 2014. The receipt of life-prolonging treatments in the final days of life has increased over time (adjusted change per year, 1.4 percentage points [pp]; 95% CI, 0.5pp to 2.2pp; P-for-trend=0.002), while the percentage of death in hospital, limiting or withholding certain treatments, or comfort-oriented care did not change.
Conclusions:
Our findings suggest that the rates of ACP completion have not increased over time despite its potential benefits and life-prolonging treatments are still common among PLWD who require surrogate decision-making, a population who might benefit greatly from early ACP.
Keywords: Advance care planning, end-of-life care, dementia
BACKGROUND
Dementia poses unique challenges to end-of-life (EOL) care. Most persons living with dementia (PLWD) cannot express their care preferences at the final stage of life and require surrogate decision-making. Therefore, this population may be at a higher risk of receiving EOL care that does not align with their preferences. Prior studies have raised concerns about the provision of potentially overaggressive care, such as the use of feeding tubes and intensive care units, among PLWD.1-3
Advance care planning (ACP) is a process of understanding and sharing personal values, life goals, and preferences regarding future medical care that allows individuals to have a greater influence on their EOL care.4 While the literature on the impact of ACP on healthcare utilization, health status, and goal-concordant care is mixed,5-7 randomized trials have demonstrated the ability of ACP to positively impact patients and caregivers satisfaction with care and decision-making as well as decrease caregiver burden and distress.8-11 In addition, some studies suggest that the benefits of ACP might be larger for PLWD given the progressive nature of the cognitive impairment.12,13 As a result, early initiation of ACP for PLWD has been advocated in clinical guidelines.14-16
Existing literature has shown an increase in patient-reported engagement in ACP over time among the general population21,23 and PLWD.24 However, little is known about if this is translating into the integration of ACP into decision-making at the end of life. This requires many complex steps and integration of ACP not only in the community with patients and surrogate decision-makers, but also with clinicians, policy, and healthcare systems.7 Therefore, whether ACP is becoming more prevalent among PLWD who require surrogate decision-making in their final days of life—a subgroup who may most benefit from early ACP—is an area worth investigating. Similarly, evidence is limited whether ACP completed with PLWD when they are still able to participate is associated with less aggressive EOL care among this specific population.
To address these knowledge gaps, we examined trends in surrogate-reported patient engagement in ACP and EOL treatments received among PLWD who died with dementia and required surrogate decision-making in the final days of life. We also determined the association between ACP completion and high-intensity EOL care among this population.
METHODS
Data Source
We used the public-use data from the 2002-2014 Health and Retirement Study (HRS), a nationally representative, biennial, longitudinal survey of adults aged 51 years and older, funded by the National Institute on Aging.26 The HRS participants are administered the “core” interview every two years until death, which collects information on demographics, physical and cognitive function, and medical conditions.26 After each participant’s death, a surrogate informant (such as a widow or widower) completes the “exit” interview, which collects information about the deceased participant, including the health, family, and financial situation.27 The response rates for the entire HRS population are consistently higher than 80%.28
Study Participants
We first identified those who died with dementia between 2000 and 2014. Among the participants of the 2002-2014 HRS exit interviews, we limited the sample to those with a probability of dementia of 50% or higher that is provided in the last available core interview. The probability of dementia is calculated by HRS researchers for each participant 70 years and older with self-reported race/ethnicity of non-Hispanic white, non-Hispanic black, or Hispanic based on the information from the core interviews, such as activities of daily living and multiple cognitive batteries (e.g., backward counting from 20 and delayed word recall).29,30 The cut-off of 50% has been demonstrated to classify 88% of participants correctly and used in a previous study examining health care costs of dementia.31,32 We employed this approach because very few surrogates have reported dementia as the cause of death in the HRS data.
We then identified decedents with dementia who required surrogate decision-making at end of life. We did this by using the two HRS exit interview questions: (i) “Did any decisions have to be made about the care and treatment of [First Name] during the final days of [his/her] life?” (included in the study if a surrogate responded “Yes”) and (ii) “Was [First Name] able to participate in decisions about [his/her] medical care during the final days of [his/her] life?” (included in the study if a surrogate responded “No”). Participants needed to provide answers for both questions indicating that they were appropriate for inclusion in the study. We focused on this population—PLWD who required surrogate decision-making at end of life—because early ACP is arguably the most beneficial for this population. We excluded decedents who had missing data on outcome variables or adjustment variables (n=13) as well as those who were not assigned weights by HRS (n=10). See Supplementary Figure S1 for a flow chart.
Advance Care Planning Measures
We examined whether a participant had ever completed each of the three specific types based on surrogate reports after death: i) written instructions of EOL care; ii) a legal arrangement for a specific person or persons to make decisions about medical care if the participant cannot make those decisions (i.e., a durable power of attorney for healthcare [DPOAH]); and iii) patient engagement in discussions about EOL care preferences with anyone prior to death. See the Supplementary Text S1 for the exact wording of the questions.
End-of-Life Care Measures
We used four dichotomous variables to measure EOL care based on surrogate reports: i) whether a participant died in a hospital; ii) whether a participant received life-prolonging treatments (“all care possible unconditionally in order to prolong life”); iii) whether certain treatments were limited or withheld, and iv) whether a participant received comfort-oriented care (“keeping comfortable and pain-free without taking extensive measures to prolong life”). These questions were asked separately, and surrogates could indicate that a decedent received more than one type of EOL care above. See the Supplementary Text S1 for the exact wording of the questions.
Adjustment Variables
We adjusted for the following variables in the regression models: age at death (continuous), sex, race (non-Hispanic White, non-Hispanic Black, or Hispanic), marital status (married or not married), education attainment (less than high school, graduated high school/general equivalency diploma, or at least some college), wealth categorized in quartiles (defined as the net value of total wealth including secondary residence less all debt), whether a participant was living in a nursing home at the time of death, whether a participant was covered by Medicaid at the time of death, dummy variables for each of the seven comorbidities (heart disease, hypertension, diabetes, lung disease, arthritis, stroke, and cancer), a functional limitation score (categories of 0, 1, 2, 3, 4, 5, or 6; based on the number of activities requiring assistance during the last three months of life: walking, toileting, bathing, transferring, eating, and dressing), and location of death (categorized into nine Census divisions and foreign county). All the information for adjustment variables was obtained from the exit interview, except for the information for education attainment and wealth, which was obtained from the last available core interview.
Statistical Analysis
We first estimated four separate multivariable linear regression models where the dependent variable was each of the four ACP completion variables (the completion of each of the three types of ACP as well as the completion of all three types of ACP [vs. completion of none or some types of ACP]) and the independent variable of interest was the year of death (continuous), adjusting for the characteristics of the decedents. For analyses of the change in ACP completion over time, we used a test of linear trend (i.e., the coefficient of the year of death variable) with P-value less than 0.05 considered to be statistically significant. We used linear regression models, as opposed to logistic regression models, for our binary outcomes (i.e., linear probability models) to allow better interpretation of the coefficients of the year of death variable (indicating percentage point change per year).33-35
We also estimated similar multivariable linear regression models for each of the four EOL care variables to examine the change in EOL care over time.
Secondary Analysis on Life-Prolonging Treatments
To further understand the trends in life-prolonging treatments, we compared the trends by ACP completion status for each of the four ACP variables. First, we repeated the trend analyses among those who completed ACP of interest and those who did not complete ACP of interest separately, using the same model as the main analysis with the dependent variable being the receipt of life-prolonging treatments. We then estimated P-values for the interaction terms between the year of death (continuous) and the status of ACP of interest using the total sample in order to formally test whether the trends in life-prolonging treatments differ by ACP completion status of interest.
Lastly, we examined the association between ACP status and life-prolonging treatments cross-sectionally by fitting multivariable linear regression models with the dependent variable being the receipt of life-prolonging treatments and the independent variable of interest being each of the four ACP variables, adjusting for the characteristics of the decedents. In this analysis, we used dummy variables for each year of death (as opposed to a continuous variable) to account for the secular trend.
Sensitivity Analysis
We also conducted a sensitivity analysis by excluding individuals with a functional limitation score of 0 (i.e., those who were independent in activities of daily living) because they may be more likely to prefer high-intensity EOL care compared to those with limited physical function.
All analyses accounted for the complex survey design of HRS to produce national estimates adjusted for nonresponse.36 Statistical analyses were conducted with SAS version 9.4 and Stata version 14.2. This study was deemed exempt by the Cedars-Sinai Institutional Review Board.
RESULTS
Among 2303 decedents with dementia, 1215 (52.3%) were excluded because they did not report the need for decision making during the final days of life. Among 1041 decedents with dementia who required decision-making during the final days of life, 142 (13.6%) were excluded because they made their own decisions about their medical care during the final days of life. As a result, our study included 870 decedents (see Supplementary Figure S1 for a flow chart). The mean probability of dementia among our study population was 89.7%, with 67.0% of the cohort exhibiting probabilities of dementia in excess of 90%.
Among the decedents included in our study, 279 individuals (34.8%) completed all three types of ACP across all years. Compared to decedents with no or some types of ACP, those with all three types of ACP were more likely to be older and non-Hispanic whites, have higher education and more wealth, and were less likely to be covered by Medicaid (Table 1).
Table 1.
Characteristics of the study population according to advance care planning completion status
| Characteristics | Decedents with all three types of ACP (n=279) |
Decedents with no or some types of ACP (n=591) |
P-Value |
|---|---|---|---|
| Age, median (IQR) | 88.6 (84.7-92.5) | 87.4 (83.3-91.8) | 0.02 |
| Female | 202 (73.2) | 400 (68.9) | 0.22 |
| Race/ethnicity | <0.001 | ||
| Non-Hispanic white | 255 (94.5) | 421 (79.0) | |
| Non-Hispanic black | 13 (3.0) | 110 (12.9) | |
| Hispanic | 11 (2.5) | 60 (8.2) | |
| Married | 65 (23.5) | 151 (26.7) | 0.38 |
| Education attainment | <0.001 | ||
| Less than high school | 81 (26.9) | 299 (47.4) | |
| High school/GED | 143 (54.1) | 226 (40.4) | |
| At least some college | 55 (19.0) | 66 (12.2) | |
| Wealth | 0.002 | ||
| First quartile | 79 (28.7) | 237 (38.8) | |
| Second quartile | 71 (23.9) | 152 (26.0) | |
| Third quartile | 58 (20.1) | 112 (18.6) | |
| Fourth quartile | 71 (27.4) | 90 (16.6) | |
| Medicaid coverage | 76 (26.8) | 246 (40.7) | <0.001 |
| Living in a nursing home | 191 (69.2) | 379 (65.8) | 0.42 |
| Comorbidities | |||
| Heart disease | 159 (57.5) | 316 (53.1) | 0.24 |
| Hypertension | 214 (76.5) | 445 (73.8) | 0.42 |
| Diabetes | 68 (24.8) | 159 (25.1) | 0.93 |
| Lung disease | 50 (18.3) | 115 (19.4) | 0.69 |
| Arthritis | 229 (82.4) | 468 (77.7) | 0.14 |
| Stroke | 99 (33.5) | 222 (36.6) | 0.25 |
| Cancer | 12 (4.6) | 49 (8.4) | 0.09 |
| Function limitation score | 0.60 | ||
| 0 | 105 (37.1) | 252 (41.7) | |
| 1 | 8 (2.8) | 16 (2.5) | |
| 2 | 12 (4.4) | 18 (2.9) | |
| 3 | 9 (3.2) | 22 (3.6) | |
| 4 | 9 (3.2) | 18 (3.2) | |
| 5 | 25 (9.3) | 68 (11.2) | |
| 6 | 111 (40.0) | 197 (35.0) |
Notes: The numbers are No. (%), except for age, based on the 2002-2014 Health and Retirement Study. Presented proportions and medians are weighted to be nationally representative of decedents with dementia (see the main text for the definitions of dementia). The weighted total sample size is 2,812,380. Wealth refers to the net value of total assets, including secondary residence less all debt. Functional limitation score is defined by the number of following activities requiring assistance during the last three months of life: walking, toileting, bathing, transferring, eating, and dressing (range 0-6). Abbreviations: ACP, advance care planning; GED, General Educational Development; IQR, interquartile range.
Trends in ACP Completion Measures
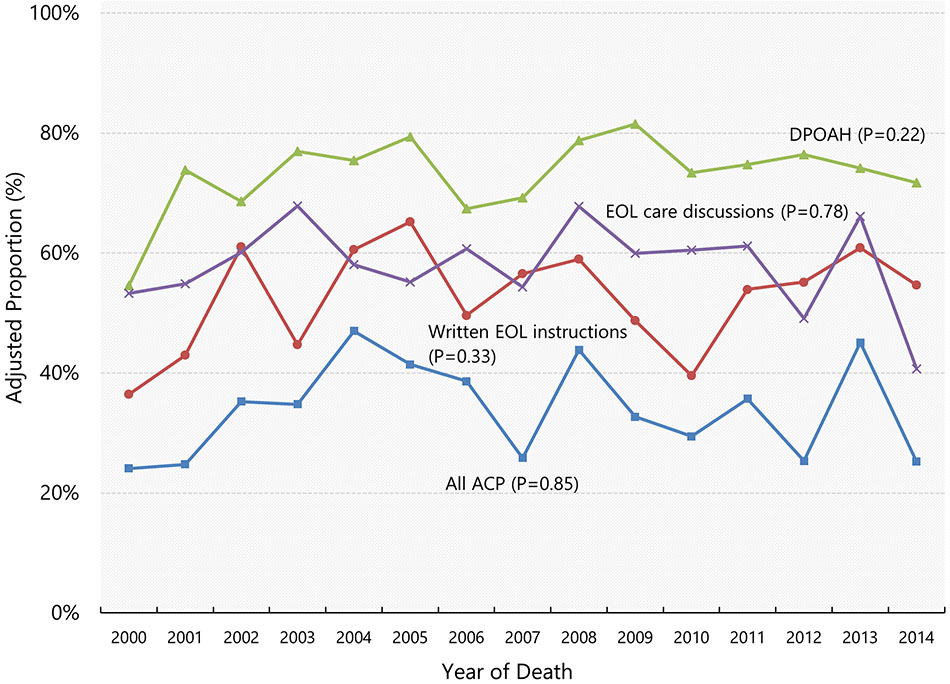
Among the decedents included in our study, the proportions of those who provided written instructions of EOL care, arranged DPOAH, and engaged in EOL discussions in 2000 were 36.4%, 54.6%, and 53.3%, respectively. Figure 1 illustrates the adjusted proportions of four ACP completion measures from 2000 through 2014. There was no evidence that the proportion changed over time for written EOL instructions (adjusted annual change, 0.4 percentage points [pp] per year; 95% CI, −0.4 to 1.2), assignment of DPOAH (0.4 pp per year; 95% CI, −0.3 to 1.1), engagement in EOL care discussion (−0.1 pp per year; 95% CI, −0.9 to 0.7), or completion of all three types of ACP (0.1 pp per year; 95% CI, −0.7 to 0.8).
Figure 1. Adjusted yearly proportions of advance care planning completion.
Data shown are adjusted proportions of end-of-life care measures among decedents with dementia who required surrogate decision-making, based on the Health and Retirement Study Exit Interview data 2002-2014. Adjusted proportions were calculated using marginal standardization. P-values are from the tests of linear trend. Abbreviations: ACP, advance care planning; DPOAH, Durable Power of Attorney for Healthcare; EOL, end-of-life.
Trends in EOL Care Measures
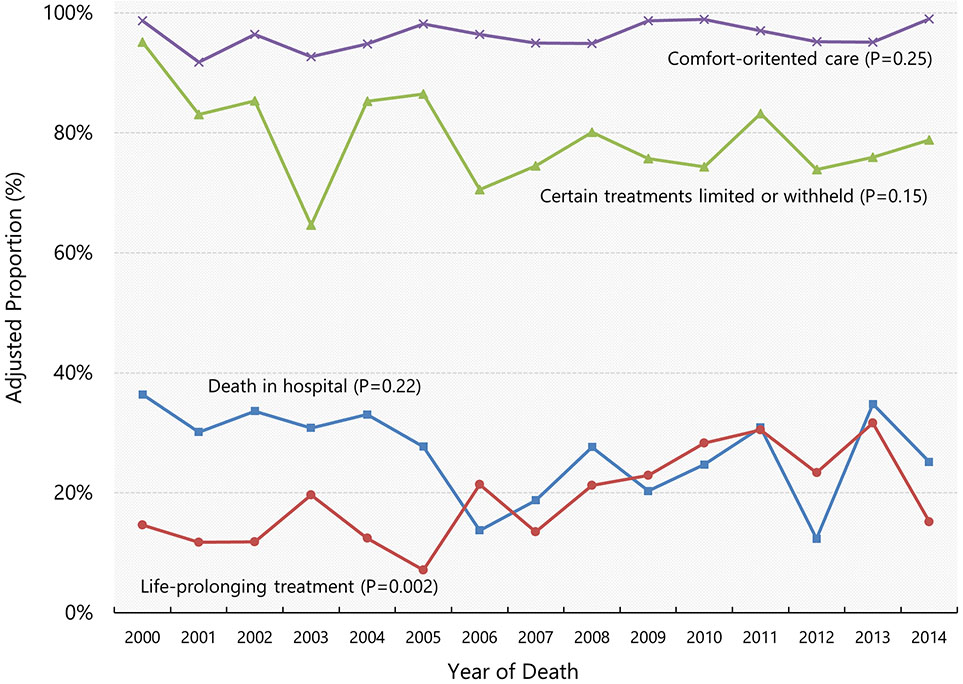
Among the decedents included in our study, the proportions of those whose surrogates reported they died in hospital, received life-prolonging treatments, had certain treatments limited or withheld, and received comfort-oriented care in 2000 were 36.4%, 14.7%, 95.1%, and 98.7%, respectively. Figure 2 illustrates adjusted yearly proportions of four EOL care measures from 2000 through 2014. The percentage of receiving life-prolonging treatments in the final days of life (14.7%) increased significantly (1.4 pp per year; 95% CI, 0.5 to 2.2) while the percentage of death in hospital (−0.5 pp per year; 95% CI, −1.2 to 0.3), limiting or withholding certain treatments (0.5 pp per year; 95% CI, −1.2 to 0.2), or comfort-oriented care (0.2 pp per year; 95% CI, −0.1 to 0.5) did not change over time. Nearly 100% of participants received comfort-oriented care throughout the observed period.
Figure 2. Adjusted yearly proportions of end-of-life care measures.
Data shown are weighted proportions of end-of-life care measures among decedents with dementia who required surrogate decision-making based on the Health and Retirement Study Exit Interview data 2002-2014. Adjusted proportions were calculated using marginal standardization. P-values are from the tests of linear trend.
Trends in Life-Prolonging Treatments by ACP Completion Status
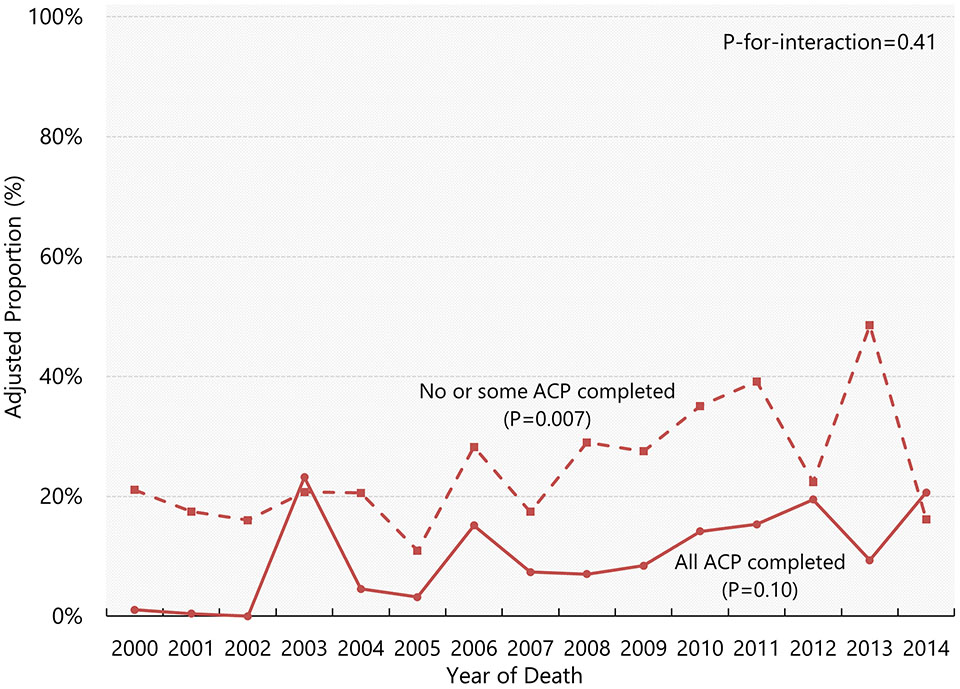
Figure 3 illustrates adjusted proportions of life-prolonging treatments from 2000 through 2014 by completion status of all three types of ACP. While the percentage of receiving life-prolonging treatments has increased over time among those who completed no or some types of advance care planning (1.5pp per year; 95% CI, 0.4pp to 2.5pp), there was no evidence that it has increased among those who completed all three types of advance care planning (0.8pp per year; 95% CI, −0.2pp to 1.8pp) (Supplementary Table S1). We found no evidence that these trends differ based on the test of interaction (P-for-interaction=0.41). The trends in life-prolonging treatments showed an increase regardless of the ACP completion status, except that there was no evidence that the rates had changed among those without DPOAH and those without EOL care discussions (Supplementary Table S1 and Supplementary Figure S2).
Figure 3. Adjusted yearly proportions of life-prolonging treatments by completion status of all three types of ACP.
Data shown are weighted proportions of life-prolonging treatments among decedents with dementia who required surrogate decision-making for (i) those who completed all three types of advance care planning (solid line) and (ii) those who completed no or some types of advance care planning (dashed line), based on the Health and Retirement Study Exit Interview data 2002-2014. Adjusted proportions were calculated using marginal standardization (estimated values beyond zero are winsorized at zero). P-values are from the tests of linear trend. P-for-interaction indicates whether the trends differ between the two groups. Abbreviations: ACP, advance care planning.
Association between ACP Completion and Life-Prolonging Treatments
Table 2 presents the association between ACP completion and life-prolonging treatments. We found that decedents with written instructions of EOL care, engagement in EOL discussions, or completion of all three types of ACP were less likely to receive life-prolonging treatments by more than 10 percentage points, compared to those who did not complete these types of ACP. There was no evidence that the percentages receiving life-prolonging treatments differed between decedents with assigned DPOAH and without.
Table 2.
Associations between ACP status and life-prolonging treatments
| Type of ACP | Adjusted Proportion (%) Receiving Life-prolonging treatments |
Adjusted Change (percentage points) [95% CI] |
P-Value | |
|---|---|---|---|---|
| Decedents with ACP |
Decedents without ACP |
|||
| Written EOL care instructions | 13.5 | 27.5 | −13.9 [−21.3, −6.6] | <0.001 |
| Assignment of DPOAH | 18.3 | 24.0 | −5.7 [−12.6, 1.2] | 0.10 |
| Engagement in EOL care discussions | 15.8 | 26.2 | −10.3 [−16.0, −4.7] | <0.001 |
| Completion of all three types of ACP | 12.8 | 23.9 | −11.1 [−17.5, −4.7] | <0.001 |
Notes: For each ACP measure, we constructed a multivariable linear regression model, adjusting for age, sex, race, marital status, education attainment, wealth categorized in quartiles, living in a nursing home, Medicaid coverage, dummy variables for each of the seven comorbidities, functional limitation score, geographic location of death, and dummy variables for the year of death. A positive adjusted change indicates that decedents with ACP of interest received more life-prolonging treatments, and a negative adjusted change indicates that those with ACP of interest received less life-prolonging treatments. Abbreviations: ACP, advance care planning; DPOAH, Durable Power of Attorney for Healthcare; EOL, end-of-life.
Sensitivity Analysis
Our sensitivity analysis by excluding individuals with a functional limitation score of 0 yielded similar findings to our main analysis (Supplementary Figures S3 and S4).
DISCUSSION
Using a nationally representative sample from HRS, we found that among PLWD who required EOL decision support by their surrogate decision-makers, only 34.8% had engaged in all three types of ACP (written EOL care instructions, assignment of DPOAH, and engagement in EOL care discussions) before the final days of life, and there was not an increase in the rate of patient-engaged ACP between 2000 and 2014 among this population. We also found an increase in the percentage who received life-prolonging treatments at EOL over time despite high rates (more than 95%) of receipt of comfort-oriented care. The upward trend in the receipt of life-prolonging treatment was less evident among those who completed all three types of ACP, and ACP completion was associated with a lower percentage of receiving life-prolonging treatments across years.
Our findings, based on surrogate reports, suggest that PLWD who required surrogate decision-making were not and still are not completing ACP at high rates before they face an end-of-life illness. Our findings differ from those of a previous study using HRS which showed an increase in rates of patient-reported ACP from 2012 to 2014 among PLWD.24 There are several potential mechanisms for differences between the two HRS studies’ findings. First, the discrepancy may be explained by the difference in the HRS sub-populations in the two studies. We studied only those who died and required surrogate decision-making at the end of life, whereas the earlier study did not focus on decedents. Second, it may be that ACP has not increased in the longer term among PLWD (we examined data over 14 years) while there are fluctuations from year to year. This explanation is supported by the high rates of DPOA since 2002 with a dip in 2006-2007 followed by stable rates. While patients and surrogates view ACP as important,7,37,38 there are well-documented barriers to ACP completion, particularly among PLWD due to lack of knowledge about the trajectory of dementia in families and lack of confidence in starting discussions among health care providers.39-41 Regardless of the mechanism, our findings suggest that ACP remains underutilized among those who may most benefit from ACP, and, as recent studies suggest,42,43 interventions leveraging implementation science that take into account the many moving parts from patient, caregiver, provider, health system, and payer perspectives may be necessary to improve communication and advance care planning in real world settings.
We observed an upward trend in life-prolonging treatments over time, likely mirroring the rising tendency to provide aggressive care in the final days of life for the general population and PLWD in recent years.44-46 However, our findings suggest the benefits of prior ACP in preventing potentially overaggressive care at end of life among PLWD. Consistent with existing literature,12,13 those who completed all three types of ACP received life-prolonging treatments at rates that were 11% lower than those without ACP.
It should be noted that HRS asks separate questions for each of the EOL care measures. Therefore, when a decedent underwent a trial of aggressive treatment at end of life then was converted to hospice care, a surrogate could affirm the receipt of both life-prolonging treatments and comfort-oriented care. Thus, this pattern of complex, step-wise decision-making in the final days of life is consistent with published literature describing high rates of intensive care unit use and burdensome transitions of care (e.g., hospitalization) at end of life, despite the frequent use of hospice services.46
It is not known whether the recent increase in life-prolonging treatments in the final days of life reflects a shift in preferences toward more aggressive care among this population. While HRS asks surrogates about the care preference documented in the written EOL care instructions, we were unable to analyze whether provided EOL care was goal-concordant due to the small sample size. In addition, consensus is lacking on optimal methods to measure goal-concordant care.47 Future studies are warranted to disentangle these complex relationships. Future studies should also evaluate the impact of surrogates making decisions for their loved ones (with and without having patient engaged ACP) on surrogate outcomes, such as decisional conflict, depression, anxiety, and post-traumatic stress disorder, to help design ACP interventions.7,48-50
Our study has limitations. First, the information on ACP completion and EOL care was solely based on surrogate-reported and thus susceptible to biases, such as recall and social desirability bias, and potentially inconsistent with the medical records.51 However, surrogate reports are routinely used and widely accepted for the research of EOL care.20,21 Second, although our study did not have sufficient power to detect small changes, we were able to detect a change of 0.9 percentage points (more than 2 standard deviations of our outcomes) per year. Third, although we adjusted for potential confounders in our analyses, it is still possible that there are uncaptured factors that can bias our estimates. Fourth, we used the probability of dementia to determine the dementia status and were not able to determine the severity, although we hypothesize that our sample includes those with more severe dementia given our sampling strategy. Lastly, because the probability of dementia is only provided for HRS participants 70 years and older with self-reported race/ethnicity of non-Hispanic white, non-Hispanic black, or Hispanic, our findings may not be generalizable to other populations such as those with early-onset dementia and in other racial and ethnic groups.
In summary, using a nationally representative sample of decedents with dementia who required surrogate decision-making, we found that there was not a significant increase in surrogate reports of patient engagement with ACP between 2000 and 2014; however, the percentage of receiving life-prolonging treatments in the final days of life increased during the same time period. We further found that, when completed, ACP is associated with less surrogate-reported life-prolonging treatments, suggesting that prior ACP that involves the patient may lead to less complicated courses at the end of life. To date, interventions based on implementation science have been successful for non-dementia population.42,43 Future research should develop and implement real-world approaches to integrating ACP early in the trajectory of illness for PLWD and study the impact of ACP on the quality of EOL care and surrogate outcomes.
Supplementary Material
Supplementary Text S1. Survey questions used to collect data about variables of interest
Supplementary Table S1. Estimations of trends in life-prolonging treatments by ACP completion status
Supplementary Figure S1. Flow chart of a study population
Supplementary Figure S2. Adjusted yearly proportions of life-prolonging treatments by advance care planning completion status
Supplementary Figure S3. Adjusted yearly proportions of advance care planning completion (excluding those with a functional limitation score of 0)
Supplementary Figure S4. Adjusted yearly proportions of end-of-life care measures (excluding those with a functional limitation score of 0)
Key Points
There was not a significant increase in advance care planning (ACP) completion between 2000 and 2014 among persons living with dementia (PLWD) who required surrogate decision-making in the final days of life.
Receipt of life-prolonging treatments in the final days of life among this population has increased over time.
When completed, ACP was associated with less surrogate-reported life-prolonging treatments.
Why Does this Paper Matter?
Our findings suggest that ACP remains underutilized among those who may most benefit from ACP despite its potential benefits.
Acknowledgments:
We thank Mourad Tighiouart, PhD for statistical advice.
Funding Information:
This study was supported by Cedars-Sinai Medical Center Clinical and Translational Science Institution (CTSI) Clinical Scholar Grant (Dr. Gotanda) and by the National Institute on Aging of the National Institutes of Health (NIH) under Award Number R01AG068633 (Dr. Tsugawa). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Sponsor’s Role:
The sponsor had no role in the design, methods, data, collections, analysis, and preparation of the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Mitchell SL, Teno JM, Kiely DK, et al. The Clinical Course of Advanced Dementia. N Engl J Med. 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell SL, Morris JN, Park PS, Fries BE. Terminal care for persons with advanced dementia in the nursing home and home care settings. J Palliat Med. 2004;7(6):808–816. [DOI] [PubMed] [Google Scholar]
- 3.Cai S, Gozalo PL, Mitchell SL, et al. Do patients with advanced cognitive impairment admitted to hospitals with higher rates of feeding tube insertion have improved survival? J Pain Symptom Manage. 2013;45(3):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudore RL, Lum HD, You JJ, et al. Defining Advance Care Planning for Adults: A Consensus Definition From a Multidisciplinary Delphi Panel. J Pain Symptom Manage. 2017;53(5):821–832.e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison RS, Meier DE, Arnold RM. What’s Wrong With Advance Care Planning? JAMA. 2021;326(16):1575–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez G, Tan WS, Virk AK, Low CK, Car J, Ho AHY. Overview of Systematic Reviews of Advance Care Planning: Summary of Evidence and Global Lessons. J Pain Symptom Manage. 2018;56(3):436–459.e425. [DOI] [PubMed] [Google Scholar]
- 7.McMahan RD, Tellez I, Sudore RL. Deconstructing the Complexities of Advance Care Planning Outcomes: What Do We Know and Where Do We Go? A Scoping Review. J Am Geriatr Soc. 2021;69(1):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson LC, Zimmerman S, Song MK, et al. Effect of the Goals of Care Intervention for Advanced Dementia: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorenbos AZ, Levy WC, Curtis JR, Dougherty CM. An Intervention to Enhance Goals-of-Care Communication Between Heart Failure Patients and Heart Failure Providers. J Pain Symptom Manage. 2016;52(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra C, Sim D, Jaufeerally FR, et al. Impact of a Formal Advance Care Planning Program on End-of-Life Care for Patients With Heart Failure: Results From a Randomized Controlled Trial. J Card Fail. 2020;26(7):594–598. [DOI] [PubMed] [Google Scholar]
- 11.Chan HY, Ng JS, Chan KS, et al. Effects of a nurse-led post-discharge advance care planning programme for community-dwelling patients nearing the end of life and their family members: A randomised controlled trial. Int J Nurs Stud. 2018;87:26–33. [DOI] [PubMed] [Google Scholar]
- 12.Nicholas LH, Bynum JP, Iwashyna TJ, Weir DR, Langa KM. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33(4):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozalo P, Teno JM, Mitchell SL, et al. End-of-Life Transitions among Nursing Home Residents with Cognitive Issues. New England Journal of Medicine. 2011;365(13):1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison Dening K, Sampson EL, De Vries K. Advance care planning in dementia: recommendations for healthcare professionals. Palliat Care. 2019;12:1178224219826579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers. 2018; https://www.nice.org.uk/guidance/ng97/chapter/Recommendations#palliative-care. Accessed June 15, 2021. [PubMed]
- 16.Alzheimer’s Association. Dementia Care Practice Recommendations. 2018; https://www.alz.org/professionals/professional-providers/dementia_care_practice_recommendations. Accessed June 15, 2021.
- 17.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon J, King D, Knapp M. Advance care planning in England: Is there an association with place of death? Secondary analysis of data from the National Survey of Bereaved People. BMJ supportive & palliative care. 2019;9(3):316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc. 2007;55(2):189–194. [DOI] [PubMed] [Google Scholar]
- 20.Silveira MJ, Kim SYH, Langa KM. Advance Directives and Outcomes of Surrogate Decision Making before Death. N Engl J Med. 2010;362(13):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710. [DOI] [PubMed] [Google Scholar]
- 22.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. Bmj. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav KN, Gabler NB, Cooney E, et al. Approximately One In Three US Adults Completes Any Type Of Advance Directive For End-Of-Life Care. Health Aff (Millwood). 2017;36(7):1244–1251. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhang Y, Prigerson H, Kaushal R, Casalino LP. Use of Advance Directives among Older U.S. Adults by Dementia Status: 2012-2016. J Palliat Med. 2019;22(12):1493–1494. [DOI] [PubMed] [Google Scholar]
- 25.Yadav KN, Gabler NB, Cooney E, et al. Approximately One In Three US Adults Completes Any Type Of Advance Directive For End-Of-Life Care. Health Affairs. 2017;36(7):1244–1251. [DOI] [PubMed] [Google Scholar]
- 26.Health and Retirement Study. The Health and Retirement Study. 2019; https://hrs.isr.umich.edu/about. Accessed June 9, 2020.
- 27.Health and Retirement Study. HEALTH AND RETIREMENT STUDY 2016 Exit: Data Description and Usage. 2018; http://hrsonline.isr.umich.edu/modules/meta/2016/exit/desc/x16dd.pdf. Accessed June 9, 2020.
- 28.Health and Retirement Study. HRS: Sample Sizes and Response Rates. 2017; https://hrs.isr.umich.edu/sites/default/files/biblio/ResponseRates_2017.pdf. Accessed September 30, 2020.
- 29.Health and Retirement Study. Gianattasio-Power Predicted Dementia Probability Scores and Dementia Classifications. 2020; https://hrsdata.isr.umich.edu/data-products/gianattasio-power-predicted-dementia-probability-scores-and-dementia-classifications. Accessed January 27, 2021.
- 30.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianattasio KZ, Ciarleglio A, Power MC. Development of Algorithmic Dementia Ascertainment for Racial/Ethnic Disparities Research in the US Health and Retirement Study. Epidemiology (Cambridge, Mass). 2020;31(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wooldridge JM. "A Binary Dependent Variable: The Linear Probability Model". Introductory Econometrics: A Modern Approach (5th ed.): Cengage Learning; 2013. [Google Scholar]
- 34.Miller S, Wherry LR. Health and Access to Care during the First 2 Years of the ACA Medicaid Expansions. N Engl J Med. 2017;376(10):947–956. [DOI] [PubMed] [Google Scholar]
- 35.Sommers BD, Maylone B, Blendon RJ, Orav EJ, Epstein AM. Three-Year Impacts Of The Affordable Care Act: Improved Medical Care And Health Among Low-Income Adults. Health Aff (Millwood). 2017;36(6):1119–1128. [DOI] [PubMed] [Google Scholar]
- 36.Health and Retirement Study. Sampling Weights: Revised for Tracker 2.0 and Beyond. 2019; https://hrs.isr.umich.edu/sites/default/files/biblio/wghtdoc_0.pdf. Accessed July 16, 2020.
- 37.O'Sullivan R, Mailo K, Angeles R, Agarwal G. Advance directives: survey of primary care patients. Can Fam Physician. 2015;61(4):353–356. [PMC free article] [PubMed] [Google Scholar]
- 38.Musa I, Seymour J, Narayanasamy MJ, Wada T, Conroy S. A survey of older peoples' attitudes towards advance care planning. Age and ageing. 2015;44(3):371–376. [DOI] [PubMed] [Google Scholar]
- 39.Lum HD, Sudore RL. Advance Care Planning and Goals of Care Communication in Older Adults with Cardiovascular Disease and Multi-Morbidity. Clin Geriatr Med. 2016;32(2):247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudore RL, Fried TR. Redefining the "planning" in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dening KH, Sampson EL, De Vries K. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2019;12:1178224219826579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fried TR, Paiva AL, Redding CA, et al. Effect of the STAMP (Sharing and Talking About My Preferences) Intervention on Completing Multiple Advance Care Planning Activities in Ambulatory Care. Ann Intern Med. 2021;174(11):1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabbard J, Pajewski NM, Callahan KE, et al. Effectiveness of a Nurse-Led Multidisciplinary Intervention vs Usual Care on Advance Care Planning for Vulnerable Older Adults in an Accountable Care Organization: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(3):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan DR, Kim H, Gozalo PL, Bunker J, Teno JM. Trends in Noninvasive and Invasive Mechanical Ventilation Among Medicare Beneficiaries at the End of Life. JAMA Intern Med. 2021;181(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teno JM, Gozalo P, Khandelwal N, et al. Association of Increasing Use of Mechanical Ventilation Among Nursing Home Residents With Advanced Dementia and Intensive Care Unit Beds. JAMA Intern Med. 2016;176(12):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teno JM, Gozalo P, Trivedi AN, et al. Site of Death, Place of Care, and Health Care Transitions Among US Medicare Beneficiaries, 2000-2015. JAMA. 2018;320(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ernecoff NC, Wessell KL, Bennett AV, Hanson LC. Measuring Goal-Concordant Care in Palliative Care Research. J Pain Symptom Manage. 2021;62(3):e305–e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song M-K, Ward SE, Hanson LC, et al. Psychological symptoms and end-of-life decision making confidence in surrogate decision-makers of dialysis patients. J Nephrol Soc Work. 2012;36:A3. [PMC free article] [PubMed] [Google Scholar]
- 49.Wendlandt B, Ceppe A, Choudhury S, et al. Risk Factors for Post-Traumatic Stress Disorder Symptoms in Surrogate Decision-Makers of Patients with Chronic Critical Illness. Ann Am Thorac Soc. 2018;15(12):1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon J, Karagiannidou M, Knapp M. The Effectiveness of Advance Care Planning in Improving End-of-Life Outcomes for People With Dementia and Their Carers: A Systematic Review and Critical Discussion. J Pain Symptom Manage. 2018;55(1):132–150.e131. [DOI] [PubMed] [Google Scholar]
- 51.Yung VY, Walling AM, Min L, Wenger NS, Ganz DA. Documentation of advance care planning for community-dwelling elders. J Palliat Med. 2010;13(7):861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text S1. Survey questions used to collect data about variables of interest
Supplementary Table S1. Estimations of trends in life-prolonging treatments by ACP completion status
Supplementary Figure S1. Flow chart of a study population
Supplementary Figure S2. Adjusted yearly proportions of life-prolonging treatments by advance care planning completion status
Supplementary Figure S3. Adjusted yearly proportions of advance care planning completion (excluding those with a functional limitation score of 0)
Supplementary Figure S4. Adjusted yearly proportions of end-of-life care measures (excluding those with a functional limitation score of 0)