Each year, approximately 1 million people are diagnosed with heart failure (HF) in the United States alone, with similar numbers reported in Europe.1 Despite therapeutic advances, roughly 50% of these individuals will die within 5 years of diagnosis.2 At the same time, most will transition to a syndrome of chronic HF, and many will be among the millions of patients hospitalized globally each year for worsening signs and symptoms. However, while these overwhelming data documenting high rates of diagnosis, morbidity, and mortality are well cited, the concept of “HF prevention” has received comparatively little attention.3 For example, the concepts of “primary prevention” and “secondary prevention” have long been embedded within routine care of atherosclerotic cardiovascular disease. The impetus for prevention of ASCVD events is juxtaposed with discrete communication of clinical risk, with guidelines including numerical absolute risk thresholds signaling initiation of primary prevention statins or escalated secondary prevention therapy.3,4 In contrast, there remains no widely communicated paradigm for primary prevention of HF. Apart from the specific indications for implantable cardioverter-defibrillators, the terms “primary and secondary prevention” for HF are infrequently used. Aside from maintaining optimal management of risk factors and comorbidities, few other guideline recommendations for primary prevention of HF exist.5 Likewise, “secondary prevention” is viewed through the lens of “HF treatment”. Yet, when one considers that routinely deferring initiation and titration of evidence-based medical therapies among patients with HF with reduced ejection fraction (HFrEF) leads to substantial numbers of preventable deaths and hospitalizations, recognizing such therapies as secondary prevention of worsening chronic HF seems appropriate.
As we consider the utility of a schema of primary versus secondary prevention of HF, the historic paucity of data differentiating de novo versus worsening chronic HF has also been a challenge. Only in recent years have robust data defined the clinical relevance of this demarcation, characterizing distinct differences in patient profile and clinical outcomes.6,7
Yet, relatively little is known regarding the differences in presentation and precipitants in patients experiencing their index heart failure event, as compared to those experiencing worsening of chronic HF. Understanding the factors provoking each type of presentation, and whether or not these factors may vary by region of the world, may be central to conceptualizing future clinical and research efforts regarding primary prevention of de novo HF, and secondary prevention of worsening chronic HF.
In this issue of the Journal, Tromp and colleagues present an analysis from the global REPORT-HF registry characterizing precipitants of HF hospitalization among 18,533 patients enrolled across 358 centers and 7 world regions.8 Patient across the spectrum of ejection fraction (EF) were included. Overall, there were 7,902 (43%) patients with new-onset HF, and 10.651 (57%) with worsening chronic HF. For both types of patients, approximately ~40% of patients were reported has having either no precipitant for their HF hospitalization, or an unknown precipitant. Among the other options for precipitants, acute coronary syndrome (ACS) comprised the most common precipitant for de novo HF hospitalizations (18%), and this was the case across most regions, with the exception of hypertension and arrhythmogenic causes most common among North American and Western European patients. Among patients with worsening chronic HF and a known precipitant, non-adherence to diet or medication was reported by investigators as the most common, with pneumonia/respiratory tract infection second most common. Similarly, the investigators found certain precipitants to track with EF phenotype (e.g., uncontrolled hypertension more common in HFpEF, ACS more common in HFmrEF, non-adherence more in HFrEF). The investigators concluded their analysis by exploring the link between type of precipitant and patient outcome. For both in-hospital and 1-year post-discharge mortality, risk of death was lowest for those precipitated by uncontrolled hypertension, intermediate for precipitants such as none, unknown, ACS, and non-adherence, and highest for worsening renal function. The prognostic value of type of precipitant was maintained after adjustment for potential confounders.
The authors are to be congratulated for a provocative analysis leveraging the strengths of a large global HF registry inclusive of both new-onset and worsening HF. Nonetheless, important points and some limitations should be acknowledged. In doing so, it is perhaps most useful to separately consider worsening chronic HF (“secondary prevention of HF events”) and de novo HF (“primary prevention of HF events”).
Prevention of Worsening Chronic Heart Failure
The current work brings to the forefront how frequently clinicians attribute “non-adherence” or “non-compliance” to diet or medications as the cause of hospitalization for worsening HF. Indeed, non-adherence was deemed the trigger for HF hospitalization in >1 in 10 patients with worsening HF, and >1 in 10 patients overall with HFrEF. However, although this terminology is pervasive across medicine, labeling a patient as “non-compliant” is often not constructive and fails to inform the “why”. Barriers to adherence (e.g., social determinants, cognitive impairment, health literacy, caregiver support, substance abuse) vary widely, as does their ability to be modified. At the same time, despite its firm place in HF dogma, the strong emphasis generally placed on low sodium diet is entirely disproportionate to the weak and inconsistent evidence supporting it, as it currently sits at a level of evidence C (i.e., expert opinion) in ACC/AHA guidelines.9 Thus, while potentially convenient for clinicians seeking explanation, routinely holding non-adherence to salt restriction (which may be impractical or unrealistic for many patients) as responsible for worsening HF is inherently problematic and not patient-centered. The SODIUM-HF trial scheduled to be reported later this year may shed much-needed further evidence on this issue.10
Although traditional patient-focused and clinical factors are well represented among the potential precipitants for HF hospitalization captured in REPORT-HF, by comparison, clinician-focused factors and overarching gaps in quality of care are not included among the possibilities (Figure). Most notably, the authors do not include data on medications prescribed prior to hospital admission, and the REPORT-HF registry did not consider “suboptimal use evidence-based medical therapy” as a potential precipitant of worsening HF. The lack of inclusion as a potential precipitant for investigators to choose is perhaps symbolic of clinician thinking on the topic in routine practice. Indeed, clinical events or “acts of commission” (e.g., new ischemia, new arrhythmia, ingestion of a salty meal) are often convenient for labeling as triggers of clinical worsening.11 Less commonly may we consider “acts of omission”, such as recognizing that the months of care preceding the index HF hospitalization were marked by absence of one or more medications proven to prevent worsening HF events, despite the patient having been eligible to receive them.11 Among US patients with HFrEF eligible for therapy, 1 in 4 do not receive a renin-angiotensin system inhibitor, 1 in 3 do not receive an evidence-based beta-blocker, and 2 in 3 do not receive a mineralocorticoid receptor antagonist.12 Early adoption of sodium glucose cotransporter-2 inhibitor (SGLT2i) therapy is also likely to be slow and varied. Based on robust evidence from multiple randomized trials, each of these 4 medications, individually, has been proven to reduce the relative risk of HF hospitalization by ~20–45%. Should it not be that the non-use of such strongly preventative medications is considered a precipitant of subsequent HF hospitalization? Likewise, among the effects of these medications are reductions in arrhythmias13, prevention of worsening kidney function14, and lowering of blood pressure15, benefits which directly address many of the worsening HF precipitants perceived by investigators in REPORT-HF. Thus, while perturbations in hemodynamics and renal function certainly require urgent attention when patients present with worsening HFrEF, it may be important to consider absence of comprehensive disease-modifying medical therapy as the frequent root cause of the clinical deterioration. Likewise, for patients presenting with worsening HFpEF, lack of SGLT2i prescription (in an otherwise eligible patients) could be considered a cause of HF hospitalization.16 Such recognition of gaps in evidence-based therapy causing HF hospitalizations may also inform how we consider scenarios where no specific precipitant is perceived, as was the case in ~40% of hospitalizations in REPORT-HF.
Figure. Potential precipitants of hospitalization for worsening chronic heart failure.
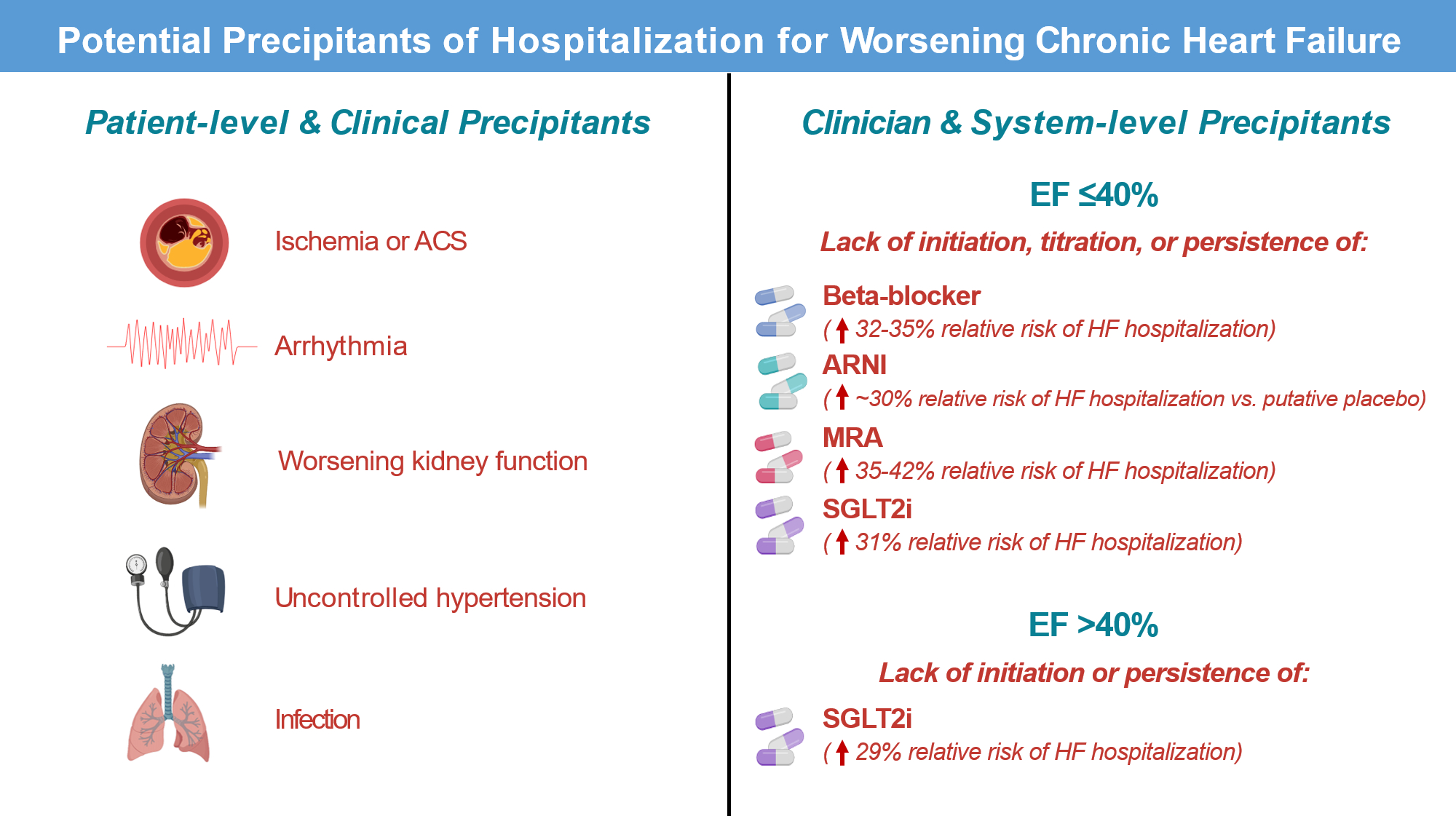
It should be recognized that eligible patients failing to receive maximally tolerated or target doses of evidence-based therapies during the outpatient phase leads to preventable hospitalizations for heart failure and deaths. Figure is not meant to be all-inclusive of all potential precipitants.
Prevention of De Novo Heart Failure
With regards to new-onset HF, while ASCVD and ACS are known common causes of acute HF, Tromp et al. highlight the relative contributions of worsening/uncontrolled hypertension, arrhythmias, and other factors. These data reaffirm the importance of many evidence-based, yet relatively unheralded, strategies for the primary prevention of HF. For example, the SPRINT investigators showed that stricter blood pressure control reduced the relative risk of incident HF by 38%.17 Likewise, among patients with type 2 diabetes or chronic kidney disease, SGLT2i reduce risk of incident HF by ~30–35%, and now carry regulatory indications and guideline recommendations for HF prevention.18 New advances in durable lipid lowering therapies hold promise in halting atherosclerotic plaque formation or promoting regression with treatment early in life.19 Advances in remote monitoring, wearables, and digital technologies used within routine life (e.g., smartwatches) may augment ability to detect atrial fibrillation and initiate treatment prior to development of clinical HF. For all primary prevention strategies, considerations of cost-effectiveness and value remain important, and for some interventions like SGLT2i therapy, validated risk scores may identify enriched patient subsets where allocation is most efficient and likely to prevent sizeable numbers of HF events.20 Yet, for other interventions where cost and access to the interventions are relative non-issues (e.g., blood pressure control, statin therapy), diffuse and intensive quality improvement initiatives remain urgently needed to adequately incentivize clinicians and patients, improve performance measures, and reduce the number of patients being newly diagnosed with HF each year.
Conclusion
Despite several available therapeutic options, HF continues to carry significant unmitigated risk in the general population. Traditional structures for HF identification and treatment should be expanded to include “primary prevention” of HF events among high-risk patients. When HF does develop, clinicians should be keenly aware that deference of early, comprehensive disease-modifying therapy among eligible patients is a cause of recurrent HF events, and that such downstream events could be prevented with timely provision of therapy. Indeed, quadruple medical therapy for HFrEF should not only be viewed as “treatment of HF”, but also as secondary prevention of worsening HF and death. Ensuring at-risk patients receive optimal treatment for primary and secondary prevention will require renewed emphasis and investments in identifying and correcting barriers to therapeutic implementation. While the challenge is large, the enormous benefits that would be realized for patients and public health globally make the prioritization of HF prevention well worth it.
Acknowledgments
DISCLOSURES:
Dr. Bhatt has received consulting fees from Sanofi Pasteur and Verve Therapeutics, served on the medical advisory board of Cohere Health, and is supported by National Heart, Lung and Blood Institute T32 post-doctoral training grant T32HL094301. Dr. Fonarow reports research support from the National Institutes of Health, consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Janssen, Medtronic, Merck, and Novartis. Dr. Greene has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, and Sanofi; has received speaker fees from Boehringer Ingelheim; and serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Merck, Sanofi, and Vifor.
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143(8):e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CJ, Ordonez-Mena JM, Roalfe AK, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ. 2019;364:l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene SJ, Butler J, Fonarow GC. Contextualizing Risk Among Patients With Heart Failure. JAMA. 2021;326(22):2261–2262. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. [DOI] [PubMed] [Google Scholar]
- 5.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. [DOI] [PubMed] [Google Scholar]
- 6.Greene SJ, Hernandez AF, Dunning A, et al. Hospitalization for Recently Diagnosed Versus Worsening Chronic Heart Failure: From the ASCEND-HF Trial. J Am Coll Cardiol. 2017;69(25):3029–3039. [DOI] [PubMed] [Google Scholar]
- 7.Greene SJ, Triana TS, Ionescu-Ittu R, et al. Patients Hospitalized for De Novo Versus Worsening Chronic Heart Failure in the United States. J Am Coll Cardiol. 2021;77(7):1023–1025. [DOI] [PubMed] [Google Scholar]
- 8.Tromp J, Beusekamp J, Ouwerkerk W, et al. Regional Differences in Precipitating Factors of Hospitalization for Acute Heart Failure: Insights from the REPORT-HF Registry. Eur J Heart Fail. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 10.Colin-Ramirez E, Ezekowitz JA, investigators S-H. Rationale and design of the Study of Dietary Intervention Under 100 MMOL in Heart Failure (SODIUM-HF). Am Heart J. 2018;205:87–96. [DOI] [PubMed] [Google Scholar]
- 11.Greene SJ, Fonarow GC. Clinical inertia and medical therapy for heart failure: the unintended harms of ‘first, do no harm’. Eur J Heart Fail. 2021;23(8):1343–1345. [DOI] [PubMed] [Google Scholar]
- 12.Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351–366. [DOI] [PubMed] [Google Scholar]
- 13.Curtain JP, Jackson AM, Shen L, et al. Effect of sacubitril/valsartan on investigator-reported ventricular arrhythmias in PARADIGM-HF. Eur J Heart Fail. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced. Circulation. 2021;143(4):310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson AM, Jhund PS, Anand IS, et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur Heart J. 2021;42(36):3741–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Packer M, Butler J, Zannad F, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021;144(16):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group SR, Wright JT Jr., Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. [DOI] [PubMed] [Google Scholar]
- 19.Musunuru K, Chadwick AC, Mizoguchi T, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–434. [DOI] [PubMed] [Google Scholar]
- 20.Pandey A, Vaduganathan M, Patel KV, et al. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail. 2021;9(3):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]


