Abstract
Lenses that filter short-wavelength (“blue”) light are commercially marketed to improve sleep and circadian health. Despite their widespread use, minimal data are available regarding their comparative efficacy in curtailing blue light exposure while maintaining visibility. Fifty commercial lenses were evaluated using five light sources: a blue LED array, a computer tablet display, an incandescent lamp, a fluorescent overhead luminaire, and sunlight. Absolute irradiance was measured at baseline and for each lens across the visual spectrum (380–780 nm), which allowed calculation of percent transmission. Transmission specificity was also calculated to determine whether light transmission was predominantly circadian-proficient (455–560 nm) or non-proficient (380–454nm and 561–780 nm). Lenses were grouped by tint and metrics were compared between groups. Red-tinted lenses exhibited the lowest transmission of circadian-proficient light, while reflective blue lenses had the highest transmission. Orange-tinted lenses transmitted similar circadian-proficient light as red-tinted lenses but transmitted more non-circadian-proficient light, resulting in higher transmission specificity. Orange-tinted lenses had the highest transmission specificity while limiting biologically active light exposure in ordinary lighting conditions. Glasses incorporating these lenses currently have the greatest potential to support circadian sleep-wake rhythms.
Keywords: circadian rhythms, blue light, sleep, delayed sleep phase, blue blockers
Introduction
Circadian rhythms organize human physiology and behavior along a 24-hour schedule that approximates night and day. Entrainment, the process of synchronizing internal circadian oscillations with environmental conditions, is regulated by the suprachiasmatic nucleus of the hypothalamus (SCN) in response to multiple environmental cues, the most salient of which is light. Light exposure contributes to circadian entrainment by phase-aligning SCN activity with the solar cycle as well as suppressing the production of melatonin, a hormone imprinting a biological representation of night. Thus, waning light intensity at sunset triggers melatonin secretion (Gooley et al. 2011; Phillips et al. 2019), which prepares the brain and body for sleep and sleep-related restorative processes.
Circadian timekeeping and melatonin secretion are regulated by light in a wavelength-dependent fashion such that short wavelength light exerts greater influence over physiological/behavioral rhythms and melatonin secretion than long wavelength light (Zaidi et al. 2007; Chang et al. 2015; Stefani et al. 2021). This is because intrinsically photosensitive retinal ganglion cells (ipRGCs) are best stimulated by light around 480 nm (Brainard et al. 2001; Lockley et al. 2003; Zaidi et al. 2007; Chellappa et al. 2013), and these cells are the primary mechanism for reporting light exposure to the SCN (Panda et al. 2002; Ruby et al. 2002; Altimus et al. 2010; Lucas et al. 2012; Mouland et al. 2019; Diepen et al. 2021). Light in the 380–454nm range coincides with the peak sensitivity for short-wavelength (S) cones, which may suppress circadian responses and modify processing of ipRGC inputs (Mouland et al. 2019; Spitschan, Lazar, Yetik, et al. 2019). To estimate the physiologic response of ipRGCs from spectrometric measurements, melanopic lux can be calculated and relative intensities can infer excitement (Enezi et al. 2011; Lucas et al. 2014). The SCN is also influenced by the timing (Appleman et al. 2013), duration (Chang et al. 2012; Rahman et al. 2017), and pattern of light exposure (Najjar and Zeitzer 2016; Negelspach et al. 2018; Kaladchibachi et al. 2019; Rahman et al. 2021).
Although these technical details may seem trivial, their consequences are not; variations in light exposure affect arousal (Jung et al. 2010; Pilorz et al. 2016), cardiovascular output (Chellappa et al. 2017), metabolism (Plano et al. 2017), and physical and cognitive performance (Grant et al. 2021; Barger et al. 2021). Thus, managing light exposure from artificial sources may be important for various facets of physical and mental health. When stimulated with bright light at night, subjects tend to have higher self-rated alertness (Badia et al. 1991; Cajochen et al. 2000) and memory that persists through the following night (Foret et al. 1998). The consequence of this nocturnal shift is a decline in alertness the following day (Deacon and Arendt 1994). Increased alertness at night may be beneficial for shift workers (Figueiro et al. 2016), but a phase delay caused by nighttime light exposure can lead to an increase in daytime sleepiness and impaired functioning for those with a daytime work schedule. This shift in alertness occurs when subjects are exposed to bright or short wavelength light, as well as following exposure to long wavelength red light (Plitnick et al. 2010), indicating that the total visible spectrum has the potential to influence the circadian rhythmicity of alertness. In addition, the sensitivity of the non-image forming system exhibits wide interindividual variability, with some subjects exhibiting melatonin suppression responses at 10 lux and some insensitive up to 400 lux (Phillips et al. 2019).
This circadian sensitivity to the frequency, intensity, and pattern of visible light has tangible implications for modern electronic use. Nighttime exposure to light-emitting devices such as e-readers (Chang et al. 2015) and computers (Cajochen et al. 2011) can suppress melatonin onset and delay the circadian rhythms of sleep and wake (Stevens et al. 2013; Rångtell et al. 2016). Moreover, many individuals use these devices in bed before sleep (Bhat et al. 2018) when their circadian effects are most pronounced. The consequences of electronic use on circadian rhythmicity are particularly relevant for adolescents (Bruni et al. 2015) who are naturally predisposed to delayed sleep timing (van der Meijden et al. 2016). Indeed, data from adolescents and adults indicate that excessive mobile device use can reduce sleep health (Bruni et al. 2015). Of course, electronic devices are not the only problematic light source, as incandescent and fluorescent light bulbs can also elicit non-visual biological responses (Gooley et al. 2011).
Blue light blocking lenses (“blue blockers”) offer a novel approach for managing biologically active light exposure by filtering out short wavelength light. Preliminary data suggest that blue blockers can increase nighttime melatonin secretion in a laboratory setting (Sasseville et al. 2006), improve sleep quality (Shechter et al. 2018), decrease cortisol (Heo et al. 2017), and reduce nighttime arousal (van der Lely et al. 2015). Such lenses may also help shift workers to maintain consistent circadian rhythms by providing control over circadian-proficient light exposure (Sasseville and Hébert 2010; Aarts et al. 2020). Working nights or rotating shifts undermines typical sleep/wake schedules, such that shift workers often report trouble sleeping and are at greater risk of developing a circadian rhythm disorder (Wickwire et al. 2017). Again, these effects are not trivial, as shift work is associated with cancer (Yuan et al. 2019), cardiometabolic disease (Sookoian et al. 2007; Puttonen et al. 2011; Proper et al. 2016), and cognitive impairment (Weinmann et al. 2018). By comparison, blue locking lenses are a simple and affordable intervention that may prevent some of these effects.
Currently, there are no empirical studies that compare the efficacy of commercially-available blue blockers, although Spitschan and colleagues recently summarized the publicly available performance data on a number of commercial lenses (Spitschan, Lazar, and Cajochen 2019). Moreover, the utility of each blue blocker may depend on the context in which it is used; blue blockers that filter out high amounts of both circadian-proficient and non-proficient light may be better at managing morning sunlight but inappropriate for use at night with low-intensity tablets or lamps. Therefore, the present study examined the transmission performance of a range of commercial blue blockers to quantify their efficacy in filtering light transmission and specificity in blocking circadian-proficient light.
Materials and Methods
Glasses
Fifty blue blockers were selected based on one or more of the following: (1) their previous use in experiments seeking to alter light’s non-visual effects (spectra may or may not have been provided in these published reports), (2) existing advertising claims regarding blocking circadian-proficient or “blue” light, and/or (3) whether the lenses were tinted and/or filtered in some other way (and thus would be expected to curtail light exposure). Lenses were excluded if they were prescription-only, unavailable in the U.S. market, used mirrored lenses, or involved magnification or distortion.
Light Exposure
Five light sources were used in the analysis: a blue LED array (Philips GoLite Blu Model: HF3422/60, Philips Respironics, Monroeville, PA, USA) specifically designed for emitting bright, circadian-proficient light; a 10-inch computer tablet (Apple iPad Display, Apple, Cupertino, CA, USA) with 500 nits brightness set to a fully white screen at maximal screen brightness; an incandescent light bulb (Feit Electric Model: CEOM60/927/6 (N1)) set in a table lamp to represent a typical home lighting scene for reading or other dim-light activities; a fluorescent ceiling light (GE Model: 25613 F32T8/SPX41/ECO – T8s) with translucent cover to represent a typical office/work environment; and sunlight measured in Tucson, Arizona on April 15th, 2021 at approximately 1:00pm facing east.
Light Measurement
Figure 1 depicts the measurement strategy. Light measurements were obtained using a FLAME spectrometer (Ocean Insight Model: FLAME-S-UV-VIS) attached to an optic fiber (Ocean Insight Model: 727-733-2447) with the cosine corrector (Ocean Insight Model: CC-3-UV-S) aimed at the light source. The spectrometer was calibrated for absolute irradiance (μW/cm2/nm) using a DH3 plus halogen light source (Ocean Insight Model: UV-VIS-NIR). Before any measurements with glasses were taken, the irradiance measured from each light source was captured and used as a baseline.
Figure 1.
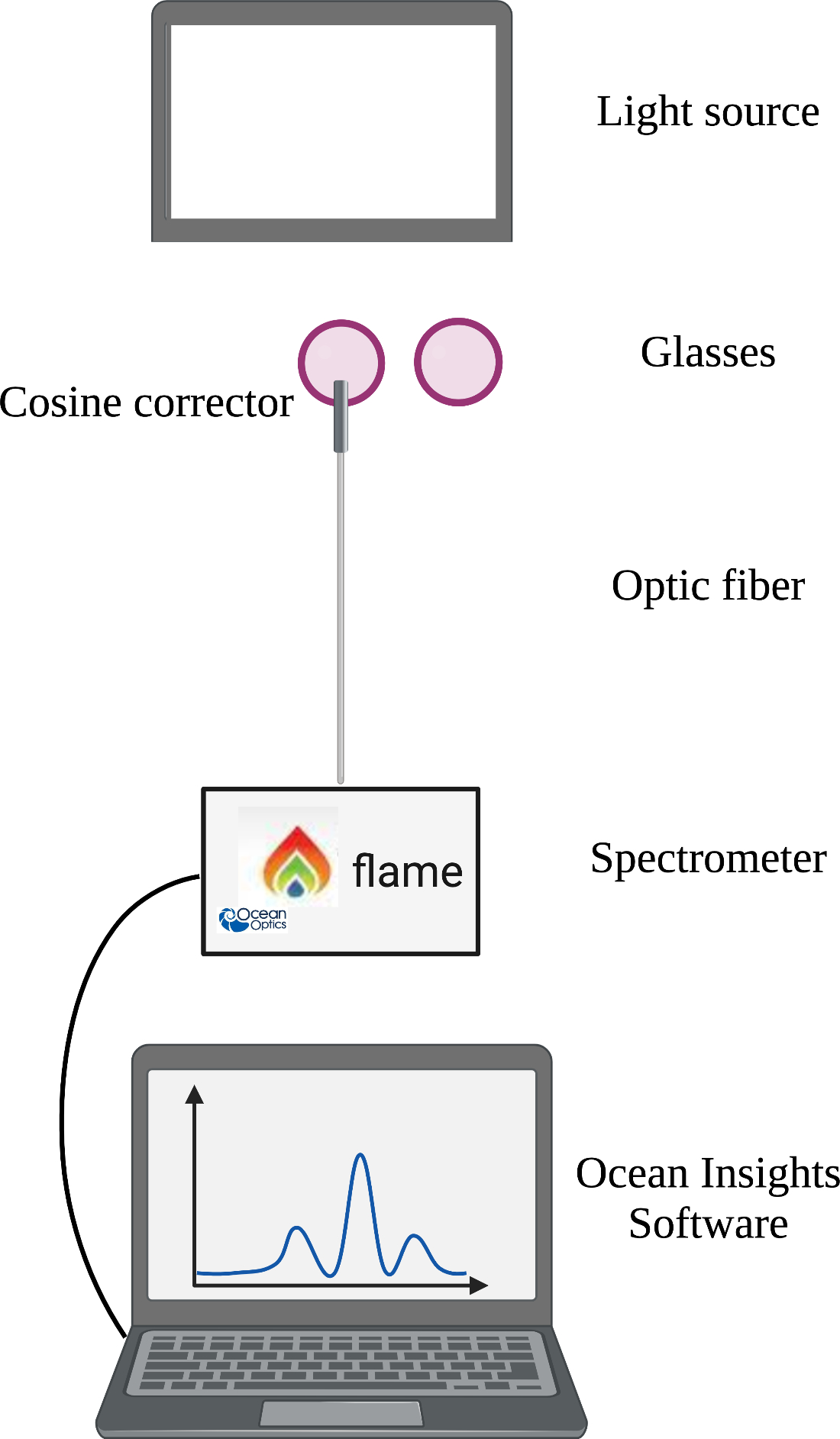
Experimental setup depicting the arrangement of the light source, lenses, cosine corrector, spectrometer, and computer output. Image created with BioRender.com
For the blue light array, computer tablet, and incandescent lamp, the probe was positioned 3 feet from the light source. For the fluorescent ceiling light, measurements were taken at eye-level when seated approximately 3 feet below the luminaire. Sunlight measurements were taken with the probe parallel to the ground and aimed at the horizon. In each setting, the lens was placed 1 inch in front of the probe to represent the typical distance between the lens (when worn within an eyeglass frame) and the eye, to gather the expected corneal irradiance. For indoor settings, all measurements were taken in the same windowless room with all other light sources disabled except for a recording computer, which was maximally dimmed and faced away from the light source and probe. The reference irradiance was measured for each light source free of any intervening lens. All measurements were taken using Ocean Optics software (OceanView 2.0 Software) and repeated three times.
Statistical Analysis
Visible light was divided into two ranges: circadian-proficient (455–560nm) and non-proficient (380–454nm and 561–780nm) based on prior studies showing that light in the former range impacted circadian rhythms and the pupillary light response through ipRGCs and photoreceptor cells (Thapan et al. 2001; Wright and Lack 2001). Non-circadian-proficient light was designated to include 380–454 and 561–780nm to exclude light that does not directly activate the ipRGCs (Wright and Lack 2001; Zaidi et al. 2007; Gooley et al. 2010; Mouland et al. 2019; Spitschan, Lazar, Yetik, et al. 2019). Absolute irradiance values for each nanometer wavelength were then summed across each spectral range (circadian-proficient versus non-circadian-proficient) by blue blocker and light source.
Beyond absolute irradiance, two additional metrics were calculated to compare blue blocker performance. Percent transmission was calculated as the irradiance from each blue blocker divided by the baseline irradiance of each light source and multiplied by 100, thus reflecting how much light was transmitted in each spectrum (circadian-proficient and total visible spectrum).
Transmission specificity was also calculated to determine whether a blue blocker preferentially transmitted non-circadian-proficient over circadian-proficient light (thus, whether the blue blocker was indeed blocking blue light). Transmission specificity was defined as the difference between percent transmission of non-circadian-proficient light and circadian-proficient light:
For example, a lens that transmitted 0% of circadian-proficient light and 100% non-proficient light would have a specificity of 100%. Conversely, lenses that transmitted 100% of the circadian-proficient light and 0% of the non-proficient light would have a specificity of −100%.
Melanopic lux was calculated following the toolbox found as supplementary online material from Lucas et al. (2014). In accordance with the author’s recommendations, cyanopic lux, rhodopic lux, chloropic lux, and erythropic lux are also reported (Lucas et al. 2014). These additions allow for a more complete picture into the alteration of light transmission across the lens groups.
Results
The irradiance spectra for each lens-tint, as well as the baseline irradiance, are plotted for each light source in Figure 2. In general, red-tinted and both orange-tinted lenses had the lowest transmission values and the highest specificity, while reflective blue lenses had the highest transmission values and the lowest specificity.
Figure 2.
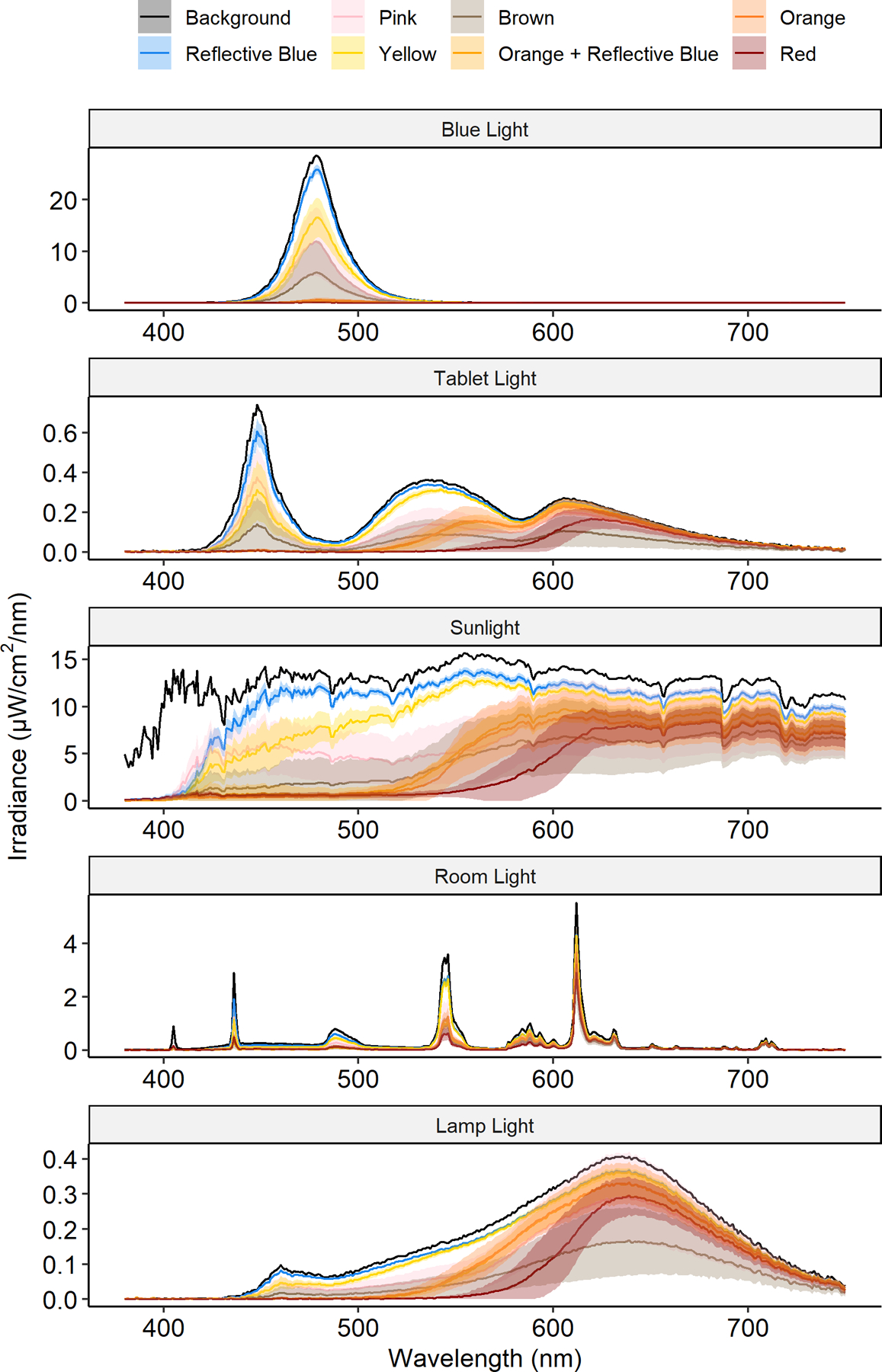
The spectral irradiance of light penetrating each lens tint with 95% confidence bands (shaded areas) across the visible light spectrum for each light condition. Reflective blue lenses had the highest light transmission across the visual spectrum under all lighting conditions, while red lenses had the lowest transmission within the circadian-proficient range.
These visual differences are reiterated in Table 1, which presents the mean and 95% confidence intervals for irradiance and percent transmission for each lens tint across light sources (values for individual blue blockers are available in Table S1). Circadian-proficient transmission ranged from 76.5% to 92.2% for reflective blue lenses, 0.62% to 17.22% for red-tinted lenses and 2.9% to 28.2% for orange-tinted lenses. Similar patterns were seen for total spectrum transmission.
Table 1.
Circadian-range transmission, total transmission, and percentages are reported across each lens group and light source.
| Light Source: | Blue Light | Tablet Light | Sunlight | Room Light | Lamp Light | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Circadian (450–560nm) | Irradiance 1 | 95% CI | Transmission 2 | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission |
| Baseline (No Lens) | 2357.12 | 67.78 | 4075.65 | 141.15 | 33.59 | ||||||||||
| RBL | 2125.64 | [2056.08, 2195.2] | 90.18% | 62.46 | [61.34, 63.58] | 92.15% | 3579.06 | [3458.75, 3699.37] | 87.82% | 108 | [103.48, 112.52] | 76.51% | 29.68 | [29.17, 30.19] | 88.36% |
| YTL | 1402.06 | [1099.55, 1704.57] | 59.48% | 51.07 | [46.01, 56.13] | 75.35% | 2753.1 | [2446.39, 3059.81] | 67.55% | 94.6 | [84.68, 104.52] | 67.02% | 24.87 | [22.56, 27.18] | 74.04% |
| PTL | 990.38 | [446.34, 1534.42] | 42.02% | 26.96 | [12.01, 41.91] | 39.78% | 1475.25 | [596.56, 2353.94] | 36.20% | 48.76 | [21.53, 75.99] | 34.54% | 13.09 | [5.66, 20.52] | 38.97% |
| BTL | 491.3 | [−20.9, 1003.5] | 20.84% | 15.54 | [0.62, 30.46] | 22.93% | 781.02 | [56.65, 1505.39] | 19.16% | 31.07 | [18.74, 43.4] | 22.01% | 7.37 | [0.73, 14.01] | 21.94% |
| OTL | 68.24 | [14.28, 122.2] | 2.90% | 12.74 | [4.9, 20.58] | 18.80% | 467.25 | [196.23, 738.27] | 11.46% | 39.82 | [30.4, 49.24] | 28.21% | 5.98 | [2.59, 9.37] | 17.80% |
| OBL | 54.79 | [19.39, 90.19] | 2.32% | 11.97 | [8.05, 15.89] | 17.66% | 580.92 | [404.57, 757.27] | 14.25% | 31.81 | [28.62, 35] | 22.54% | 5.33 | [3.8, 6.86] | 15.87% |
| RTL | 14.64 | [4.82, 24.46] | 0.62% | 1.18 | [0.22, 2.14] | 1.74% | 216.94 | [112.5, 321.38] | 5.32% | 24.3 | [14.36, 34.24] | 17.22% | 0.78 | [0.14, 1.42] | 2.32% |
| Total Range (380–780nm) | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission | Irradiance | 95% CI | Transmission |
| Light Source | 2478.19 | 178.76 | [28.17, 59.69] | 14490.74 | 345.7 | 182.54 | |||||||||
| RBL | 2211.81 | [2138.93, 2284.69] | 89.25% | 160.63 | [157.13, 164.13] | 89.86% | 11772.06 | [11408.92, 12135.2] | 81.24% | 255.82 | [245.26, 266.38] | 74.00% | 162.17 | [159.61, 164.73] | 88.84% |
| YTL | 1457.51 | [1139.6, 1775.42] | 58.81% | 135.47 | [123.32, 147.62] | 75.78% | 10133.05 | [9585.87, 10680.23] | 69.93% | 233.85 | [214.47, 253.23] | 67.65% | 155.62 | [149.47, 161.77] | 85.25% |
| PTL | 1048.25 | [480.89, 1615.61] | 42.30% | 97.85 | [62.77, 132.93] | 54.74% | 7219.33 | [4126.78, 10311.88] | 49.82% | 160.66 | [96.79, 224.53] | 46.47% | 114.55 | [66.75, 162.35] | 62.75% |
| BTL | 523.01 | [−4.25, 1050.27] | 21.10% | 52.59 | [11.47, 93.71] | 29.42% | 5229.08 | [2870.14, 7588.02] | 36.09% | 115.62 | [87.38, 143.86] | 33.45% | 69.15 | [29.23, 109.07] | 37.88% |
| OTL | 86.48 | [31.55, 141.41] | 3.49% | 76.63 | [59.89, 93.37] | 42.87% | 5697.66 | [4084.36, 7310.96] | 39.32% | 153.18 | [124.35, 182.01] | 44.31% | 122.22 | [99.91, 144.53] | 66.96% |
| OBL | 69.89 | [35.96, 103.82] | 2.82% | 78.63 | [69.8, 87.46] | 43.99% | 6272.39 | [5620.62, 6924.16] | 43.29% | 142.51 | [131.01, 154.01] | 41.22% | 120.47 | [107, 133.94] | 66.00% |
| RTL | 28.63 | [14.12, 43.14] | 1.16% | 43.93 | [28.17, 59.69] | 24.57% | 4644.41 | [3591.3, 5697.52] | 32.05% | 113.58 | [100.97, 126.19] | 32.86% | 88.46 | [65.57, 111.35] | 48.46% |
Irradiance: area under the curve for each blue blocker averaged within groups
Transmission = (Irradiance_Lens/Irradiance_(Light Source))*100
Figure 3 demonstrates the transmission of circadian-proficient light as a function of total light transmission for each blue blocker, grouped by lens-tint and light source. Located at the bottom right of each plot are blue blockers that transmit much of the total light available in the emission but very little circadian-proficient light. Reflective blue lenses consistently transmitted the most light, regardless of spectra, while red, orange with reflective blue, and orange tinted lenses had the highest transmission of total light for the least transmission of circadian-proficient light. The close correlation between circadian-proficient and total light filtering under the blue light condition (far left panel) was due to the blue LED array only emitting circadian-proficient light (thus providing a useful proof-of-concept). For the four other lighting conditions, the orange and red-tinted lenses were mainly found in the lower right quadrant because they transmit very little circadian-proficient light while enabling passage of much of the remaining emission, in some cases as much as 75%. This was not the case for the reflective blue and yellow-tinted groups, which transmitted a high degree of both circadian-proficient and total light.
Figure 3.
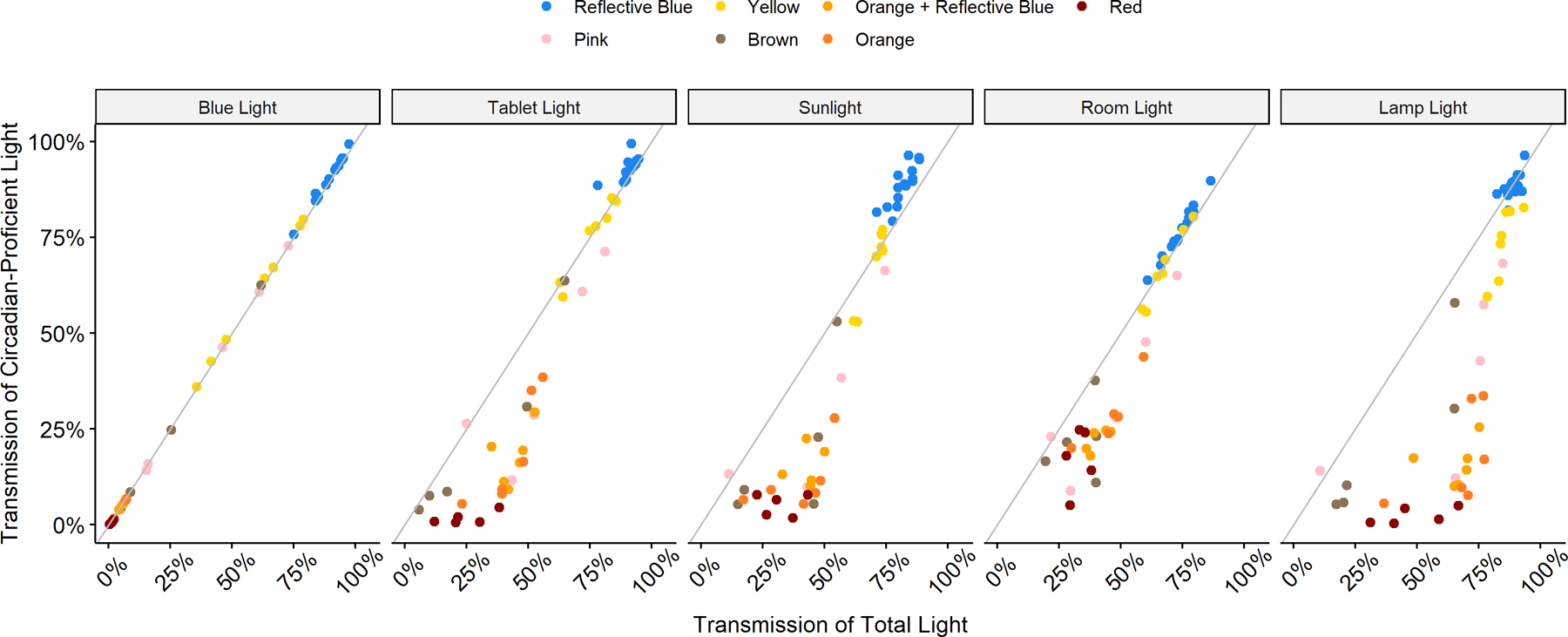
A scatterplot showing the percent transmission of circadian-proficient light (y-axis) as a function of total light transmitted (x-axis). Lenses in the bottom righthand quadrant had greater total light transmission without increasing circadian-proficient light transmission.
Table 2 and Figure 4 provide the transmission specificity values for each lens-tint across each light source to demonstrate how effective each lens-tint was in filtering out circadian-proficient light while retaining non-circadian-proficient light. Again, reflective blue lenses had the worst performance (in many cases filtering more non-circadian-proficient light than circadian-proficient light, indicated as a negative specificity) while orange and red tinted lenses had higher transmission specificity. Additionally, pink and brown tinted lenses had the greatest intra-group variability in transmission specificity. A visual representation of the data regarding transmission specificity are presented as boxplots in Figure 4. It should be noted that the generally higher transmission specificity exhibited by all the blue-blocker groups under “lamp light” is a byproduct of the lighting source -- an incandescent bulb with longer wavelength emissions (see Figure 2).
Table 2:
Transmission specificity across each lens group and light source.
| Light Source: | Blue Light | Tablet Light | Sunlight | Room Light | Lamp Light | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specificity1 | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| RBL | −19.00 | [−24.40, −13.60] | −3.68 | [−5.94, −1.42] | −9.15 | [−11.20, −7.10] | −4.25 | [−4.92, −3.58] | 0.60 | [−0.99, 2.19] |
| YTL | −13.68 | [−18.67, −8.69] | 0.70 | [−1.96, 3.36] | 3.31 | [−2.11, 8.73] | 1.06 | [−1.83, 3.95] | 13.73 | [8.37, 19.09] |
| PTL | 5.78 | [−1.34, 12.90] | 24.10 | [5.84, 42.36] | 18.95 | [2.90, 35.00] | 20.17 | [6.66, 33.68] | 29.16 | [6.60, 51.72] |
| BTL | 5.35 | [−4.95, 15.65] | 10.45 | [−0.07, 20.97] | 23.54 | [4.72, 42.36] | 19.33 | [2.35, 36.31] | 19.53 | [7.93, 31.13] |
| OTL | 12.17 | [10.11, 14.23] | 38.77 | [28.91, 48.63] | 38.76 | [26.28, 51.24] | 27.21 | [20.63, 33.79] | 60.23 | [47.39, 73.07] |
| OBL | 10.15 | [6.80, 13.50] | 42.40 | [34.05, 50.75] | 40.39 | [32.42, 48.36] | 31.59 | [28.13, 35.05] | 61.44 | [52.00, 70.88] |
| RTL | 10.93 | [6.81, 15.05] | 36.77 | [24.12, 49.42] | 37.19 | [26.49, 47.89] | 26.44 | [14.97, 37.91] | 56.53 | [42.52, 70.54] |
Specificity = Percent of non-proficient light (380–454 & 561–780 nm) − Percent of circadian-proficient light (455–560 nm)
Figure 4.
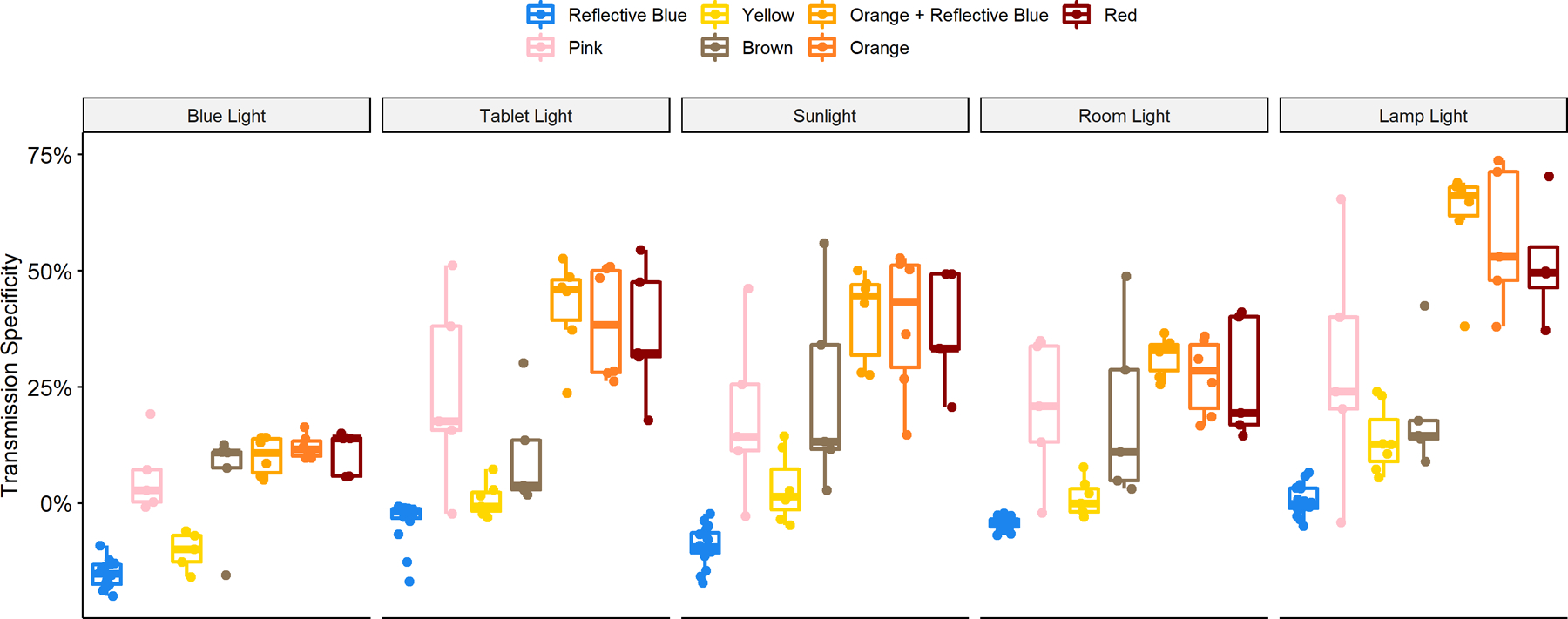
Boxplots of transmission specificity for each lens group by light source, with individual glasses overlaid. Orange- and red-tinted lens groups had the highest transmission specificity, while reflective blue tinted lenses had the lowest specificity.
Measurements for cyanopic, melanopic, rhodopic, chloropic, and erythropic are recorded in α-opic lux, and are reported in Table 3 for each light source and lens tint group. As the human circadian system is influenced by rods and cones (Hattar et al. 2003), as well as ipRGCs (Berson et al. 2002; Zaidi et al. 2007), it is imperative to report data on the projected intensities each cell type would receive (Enezi et al. 2011; Lucas et al. 2014).
Table 3:
Cyanopic, melanopic, rhodopic, chloropic, and erythropic lux reported by lens group and light source, workbook used was from the Lucas group (Lucas et al. 2014).
| Retinal photopigment weighted illuminances(α-opic lux) | |||||||
|---|---|---|---|---|---|---|---|
| Group | Photopic illuminance (lux)1 | Cyanopic2 | Melanopic3 | Rhodopic4 | Chloropic5 | Erythropic6 | |
| Blue Light | Light Source | 206.74 | 795.83 | 1,287.45 | 958.63 | 532.21 | 293.55 |
| RBL | 189.01 | 701.98 | 1,163.98 | 868.35 | 483.64 | 267.07 | |
| YTL | 134.52 | 427.7 | 763.53 | 576.44 | 327.31 | 183.57 | |
| PTL | 86.28 | 349.87 | 541.35 | 401.54 | 222.24 | 123.33 | |
| BTL | 99.42 | 349.1 | 586.04 | 439.2 | 246.93 | 138.13 | |
| OTL | 13.79 | 14.55 | 34.51 | 29.01 | 20.66 | 15.07 | |
| OBL | 10.35 | 11.32 | 27.17 | 22.9 | 16.01 | 11.22 | |
| RTL | 2.76 | 4.28 | 6.95 | 5.43 | 3.81 | 3.38 | |
| Tablet Light | Light Source | 36.92 | 38.18 | 31.59 | 34.18 | 35.53 | 35.06 |
| RBL | 34.57 | 30.76 | 27.93 | 30.91 | 32.87 | 32.69 | |
| YTL | 31.67 | 16.49 | 19.77 | 24.19 | 28.53 | 29.61 | |
| PTL | 17.88 | 18.99 | 13.68 | 14.37 | 15.96 | 17.86 | |
| BTL | 23.02 | 15.79 | 14.91 | 17.32 | 20.39 | 22.16 | |
| OTL | 17.10 | 0.67 | 3.07 | 6.07 | 12.33 | 16.9 | |
| OBL | 17.21 | 0.67 | 2.93 | 5.87 | 12.26 | 17.13 | |
| RTL | 5.63 | 0.58 | 0.52 | 0.9 | 3.05 | 6.45 | |
| Sunlight | Light Source | 2047.21 | 1,756.18 | 1,909.40 | 1,951.42 | 2,016.61 | 2,027.20 |
| RBL | 1804.94 | 1,245.63 | 1,628.18 | 1,682.84 | 1,761.81 | 1,777.45 | |
| YTL | 1613.08 | 710.26 | 1,154.97 | 1,288.29 | 1,485.91 | 1,569.97 | |
| PTL | 900.36 | 716.95 | 718.12 | 728.88 | 816.26 | 922.72 | |
| BTL | 1153.56 | 633.36 | 822.41 | 892.2 | 1,040.44 | 1,148.18 | |
| OTL | 855.33 | 61.84 | 166.47 | 294.02 | 611.03 | 862.74 | |
| OBL | 836.25 | 35.54 | 134.64 | 261.84 | 584.91 | 846.56 | |
| RTL | 353.34 | 89.1 | 98.81 | 119.12 | 225.87 | 392.52 | |
| Room Light | Light Source | 73.03 | 38.03 | 49.69 | 57.21 | 66.66 | 70.48 |
| RBL | 56.37 | 25.11 | 37.08 | 43.23 | 51.06 | 54.28 | |
| YTL | 53.81 | 16.12 | 29.39 | 36.91 | 46.88 | 51.48 | |
| PTL | 31.62 | 14.67 | 17.82 | 20.46 | 26.48 | 31.85 | |
| BTL | 38.41 | 13.49 | 20.28 | 24.78 | 32.43 | 37.73 | |
| OTL | 33.05 | 5.14 | 9.73 | 14.56 | 24.77 | 33.02 | |
| OBL | 31.53 | 4.4 | 8.56 | 13.22 | 23.29 | 31.59 | |
| RTL | 20.26 | 6.35 | 8.54 | 10.28 | 15.27 | 21.12 | |
| Lamp Light | Light Source | 30.60 | 5.26 | 12.62 | 15.86 | 23.65 | 31.15 |
| RBL | 27.32 | 4.5 | 11.12 | 14.04 | 21.05 | 27.82 | |
| YTL | 26.25 | 2.73 | 8.76 | 11.97 | 19.55 | 26.73 | |
| PTL | 16.54 | 2.4 | 5.09 | 6.53 | 11.52 | 17.58 | |
| BTL | 20.15 | 2.28 | 6.16 | 8.32 | 14.41 | 20.97 | |
| OTL | 17.68 | 0.14 | 1.59 | 3.74 | 10.94 | 18.92 | |
| OBL | 17.28 | −0.03 | 1.35 | 3.42 | 10.56 | 18.56 | |
| RTL | 7.94 | −0.02 | 0.28 | 0.74 | 3.76 | 9.41 | |
Photopic illuminance (lux): typical measure of intensity
Cyanopic lux: unit of measurement for short-wavelength (S) cones
Melanopic lux: unit of measurement for ipRGCs
Rhodopic lux: unit of measurement for rods
Chloropic lux: unit of measurement for medium-wavelength (M) cones
Erythropic lux: unit of measurement for long-wavelength (L) cones
The individual performance of each blue blocker by absolute irradiance, percent transmission, and transmission specificity across circadian-proficient, circadian-non-proficient, and total visible spectra for each light source are presented in Table S1. The specific manufacturer’s details for each blue blocker are presented in Table S2.
Discussion
This study demonstrated that blue blocker efficacy can be easily distinguished based on lens tint. Clear lenses with a reflective blue tint consistently had the highest circadian-proficient transmission and the lowest transmission specificity of any blue blocker. By contrast, orange-tinted and red-tinted lens groups had the lowest transmission of circadian-proficient light, and thus the highest transmission specificity. The remaining lens tint groups – yellow, pink, and brown – had wide variability in blocking circadian-proficient light. These data indicate that orange and red tinted lenses have the best potential to protect endogenous circadian rhythmicity from nighttime light exposure, although human studies are needed to confirm this and examine other physiological effects of blue blocker use.
Orange or red-tinted lenses are most likely to benefit melatonin secretion, circadian rhythmicity, and sleep when used to prevent undesirable exposure to circadian-proficient light. These findings agree with prior studies of blue blockers on physiological outcomes associated with the non-image forming system. Sasseville and colleagues reported that following a 60 min bright light pulse between 1AM and 2AM, melatonin secretion did not decrease from baseline among participants wearing orange-tinted lenses but did decrease among participants wearing gray (control) lenses (Sasseville et al. 2006). Thus, orange-tinted lenses may sustain melatonin levels at night when exposed to artificial light. Mobile/portable electronic devices are a common source of such artificial light, which is unfortunate because they are often enriched with circadian-proficient light (Gringras et al. 2015). However, use of brown tinted blue blockers two hours before habitual bedtime limited the melatonin suppression associated with exposure to these devices (Ayaki et al. 2016). Moreover, participants using brown tinted blue blockers reported greater evening sleepiness, shorter sleep onset latencies, and better sleep efficiency compared to gray-tinted control glasses (Ayaki et al. 2016). Blue blockers may also be effective among adolescents, as evening use of orange-tinted blue blockers among 13 adolescent males mitigated the melatonin suppression and alerting effects caused by blue-light exposure (van der Lely et al. 2015). Similarly, a pilot study of nine young adults with delayed sleep phase disorder found that habitual use of orange-tinted blue blockers may have advanced dim light melatonin onset and objectively assessed sleep onset time (Esaki et al. 2016). Adolescents are already prone to a pattern of delayed sleep that conflicts with work and school schedules to create recurring sleep loss (Micic et al. 2016), and nighttime mobile device use may exacerbate delayed sleep-wake rhythms and further disrupt sleep (Levenson et al. 2017). While these pilot findings need to be replicated, they indicate that blue blockers may offer substantial benefits to sleep and circadian health in adolescents and young adults.
Individuals with bipolar disorder may also benefit from blue blockers. Light can influence neural substrates of mood and cognition, either directly mediated by ipRGCs (LeGates et al. 2012; LeGates et al. 2014) or indirectly through behavioral and brain changes (Bedrosian and Nelson 2017). Abrupt changes in lighting conditions can provoke manic episodes (Bauer et al. 2012; Bauer et al. 2015) and symptom severity often tracks alterations in circadian rhythmicity (Dallaspezia and Benedetti 2015). In fact, use of dark therapy, which imposes dark lighting for 14 hours per day, may protect circadian rhythmicity in manic patients, and preliminary data indicate this intervention can decrease symptom severity and ease rapid cycling between mania and depression (Wehr et al. 1998; Wirz-Justice et al. 1999; Barbini et al. 2005). Use of blue blockers may achieve a similar effect, as two small studies reported that blue blockers decreased the intensity of manic symptoms after five days of inpatient use (Henriksen et al. 2014; Henriksen et al. 2020). While these preliminary data are encouraging, more rigorous studies are needed to confirm these effects.
Shift workers experience an inverted sleep-wake rhythm owing to their nighttime employment. This leads to an uncoupling of the biological night from sleep (Wickwire et al. 2017). Those unable to adapt to this schedule are at increased likelihood of being diagnosed with shift work disorder (SWD) (Gumenyuk et al. 2012), which increases risk for many behavioral and health related morbidities (Drake et al. 2004). One way to guide shift workers onto better regularized schedules is to couple their biological/physiological night with the time they are sleeping (daytime) by decreasing circadian-proficient light exposure in the hours before sleep (Knauth and Hornberger 2003). The sunlight panel in Figure 4 indicates that red and orange-tinted lenses could protect night workers from circadian-proficient light exposure while maintaining enough visibility to travel home in the morning. A pilot study of eight permanent shift workers wearing orange-tinted blue blockers after a night shift found a mean nightly increase in subjective total sleep time of 32–34 minutes (Sasseville et al. 2009). Considering these data, future studies should examine variations between lens types in regulating melatonin profiles after a night shift.
Other forms of blue light filtering, such as screen dimmers, are advertised to promote better sleep-wake rhythms. Depending on the device, screen dimmers can decrease the intensity of light emission from a display and shift the color output towards a warmer hue. However, there is (as of yet) no evidence that screen dimmers improve sleep measures (Duraccio et al. 2021) or leptin levels (Driller et al. 2019). This may be because the dimmer itself is ineffective, or because the non-visual effects of other sources of ambient lighting are not addressed by screen dimmers. Ambient light sources, like fluorescent lights, have been shown to significantly decrease melatonin secretion (Rahman et al. 2017). Melanopic lux was calculated from the fluorescent light (31.18) and the circadian-sensitive LED (18.59) and a significant attenuation of melatonin was found when using the circadian-sensitive LED compared to the fluorescent light (Rahman et al. 2017). This group also found that reaction times from the Psychomotor Vigilance Test were slower after exposure to the circadian-sensitive LED compared to when measured during the fluorescent lighting (Rahman et al. 2017). These results indicate that the non-imaging forming system can be influenced by multiple forms of light, and melanopic lux can be used to estimate relative melatonin suppression. In either case, blue blockers are likely a better solution as they cover a greater part of the visual field and likely exert greater reductions in circadian-proficient light exposure than screen dimmers and light boxes.
The strengths of this study include the systematic testing process and the wide range of blue blockers assessed. However, the lack of human measurements relating to subjective arousal, sleep, or physiological assessments of circulating melatonin or cortisol mean that these results cannot yet be extrapolated to people. Future research is needed to confirm how sensitive humans are to changes in transmission of circadian-proficient light and/or transmission specificity before better estimates of blue blocker efficacy can be assessed. Additionally, idealized readings in a laboratory setting do not necessarily translate to individual everyday use. Future work will require evaluation of similar parameters in humans under typical lighting conditions.
Supplementary Material
Individual performance metrics for 50 blue blocking glasses.
Manufacturer, model/SKU, and/or ASIN used of each blue blocker.
Footnotes
No potential competing interest was reported by the authors.
References
- Aarts MPJ, Hartmeyer SL, Morsink K, Kort HSM, de Kort YAW. 2020. Can Special Light Glasses Reduce Sleepiness and Improve Sleep of Nightshift Workers? A Placebo-Controlled Explorative Field Study. Clocks Sleep [Internet]. [accessed 2021 Jun 16] 2(2):225–245. 10.3390/clockssleep2020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Güler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. 2010. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci [Internet]. [accessed 2021 Aug 4] 13(9):1107–1112. 10.1038/nn.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleman K, Figueiro MG, Rea MS. 2013. Controlling light-dark exposure patterns rather than sleep schedules determines circadian phase. Sleep Med. 14(5):456–461. 10.1016/j.sleep.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki M, Hattori A, Maruyama Y, Nakano M, Yoshimura M, Kitazawa M, Negishi K, Tsubota K. 2016. Protective effect of blue-light shield eyewear for adults against light pollution from self-luminous devices used at night. Chronobiology International [Internet]. [accessed 2021 Jul 14] 33(1):134–139. 10.3109/07420528.2015.1119158 [DOI] [PubMed] [Google Scholar]
- Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. 1991. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 50(3):583–588. 10.1016/0031-9384(91)90549-4 [DOI] [PubMed] [Google Scholar]
- Barbini B, Benedetti F, Colombo C, Dotoli D, Bernasconi A, Cigala-Fulgosi M, Florita M, Smeraldi E. 2005. Dark therapy for mania: a pilot study. Bipolar Disorders [Internet]. [accessed 2021 Aug 13] 7(1):98–101. 10.1111/j.1399-5618.2004.00166.x [DOI] [PubMed] [Google Scholar]
- Barger LK, Sullivan JP, Lockley SW, Czeisler CA. 2021. Exposure to Short Wavelength-Enriched White Light and Exercise Improves Alertness and Performance in Operational NASA Flight Controllers Working Overnight Shifts. J Occup Environ Med. 63(2):111–118. 10.1097/JOM.0000000000002054 [DOI] [PubMed] [Google Scholar]
- Bauer M, Glenn T, Alda M, Andreassen OA, Angelopoulos E, Ardau R, Baethge C, Bauer R, Baune BT, Bellivier F, et al. 2015. Influence of light exposure during early life on the age of onset of bipolar disorder. Journal of Psychiatric Research [Internet]. [accessed 2021 Aug 13] 64:1–8. 10.1016/j.jpsychires.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Bauer M, Glenn T, Alda M, Andreassen OA, Ardau R, Bellivier F, Berk M, Bjella TD, Bossini L, Zompo MD, et al. 2012. Impact of sunlight on the age of onset of bipolar disorder. Bipolar Disorders [Internet]. [accessed 2021 Aug 13] 14(6):654–663. 10.1111/j.1399-5618.2012.01025.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ. 2017. Timing of light exposure affects mood and brain circuits. Transl Psychiatry [Internet]. [accessed 2021 Nov 14] 7(1):e1017. 10.1038/tp.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. 2002. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science [Internet]. [accessed 2021 Apr 27] 295(5557):1070–1073. 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- Bhat S, Pinto-Zipp G, Upadhyay H, Polos PG. 2018. “To sleep, perchance to tweet”: in-bed electronic social media use and its associations with insomnia, daytime sleepiness, mood, and sleep duration in adults. Sleep Health [Internet]. [accessed 2021 Aug 4] 4(2):166–173. 10.1016/j.sleh.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. 2001. Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor. J Neurosci [Internet]. [accessed 2021 Mar 29] 21(16):6405–6412. 10.1523/JNEUROSCI.21-16-06405.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni O, Sette S, Fontanesi L, Baiocco R, Laghi F, Baumgartner E. 2015. Technology Use and Sleep Quality in Preadolescence and Adolescence. J Clin Sleep Med [Internet]. [accessed 2021 Mar 29] 11(12):1433–1441. 10.5664/jcsm.5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Frey S, Anders D, Späti J, Bues M, Pross A, Mager R, Wirz-Justice A, Stefani O. 2011. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol (1985). 110(5):1432–1438. 10.1152/japplphysiol.00165.2011 [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. 2000. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 115(1):75–83. 10.1016/s0166-4328(00)00236-9 [DOI] [PubMed] [Google Scholar]
- Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A [Internet]. [accessed 2021 Jan 25] 112(4):1232–1237. 10.1073/pnas.1418490112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A-M, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE, Czeisler CA. 2012. Human responses to bright light of different durations. J Physiol. 590(13):3103–3112. 10.1113/jphysiol.2011.226555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Lasauskaite R, Cajochen C. 2017. In a Heartbeat: Light and Cardiovascular Physiology. Front Neurol [Internet]. [accessed 2021 Jul 18] 8:541. 10.3389/fneur.2017.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Oelhafen P, Lang D, Götz T, Krebs J, Cajochen C. 2013. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 22(5):573–580. 10.1111/jsr.12050 [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Benedetti F. 2015. Chronobiology of Bipolar Disorder: Therapeutic Implication. Curr Psychiatry Rep [Internet]. [accessed 2021 Aug 13] 17(8):68. 10.1007/s11920-015-0606-9 [DOI] [PubMed] [Google Scholar]
- Deacon SJ, Arendt J. 1994. Phase-shifts in melatonin, 6-sulphatoxymelatonin and alertness rhythms after treatment with moderately bright light at night. Clinical Endocrinology [Internet]. [accessed 2021 Nov 15] 40(3):413–420. 10.1111/j.1365-2265.1994.tb03940.x [DOI] [PubMed] [Google Scholar]
- Diepen HC van, Schoonderwoerd RA, Ramkisoensing A, Janse JAM, Hattar S, Meijer JH. 2021. Distinct contribution of cone photoreceptor subtypes to the mammalian biological clock. PNAS [Internet]. [accessed 2021 Jun 18] 118(22). 10.1073/pnas.2024500118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. 2004. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 27(8):1453–1462. 10.1093/sleep/27.8.1453 [DOI] [PubMed] [Google Scholar]
- Driller MW, Jacobson G, Uiga L. 2019. Hunger hormone and sleep responses to the built-in blue-light filter on an electronic device: a pilot study. Sleep Sci [Internet]. [accessed 2021 Jul 14] 12(3):171–177. 10.5935/1984-0063.20190074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraccio KM, Zaugg KK, Blackburn RC, Jensen CD. 2021. Does iPhone night shift mitigate negative effects of smartphone use on sleep outcomes in emerging adults? Sleep Health [Internet]. [accessed 2021 Jul 21]. 10.1016/j.sleh.2021.03.005 [DOI] [PubMed] [Google Scholar]
- al Enezi J, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. 2011. A “Melanopic” Spectral Efficiency Function Predicts the Sensitivity of Melanopsin Photoreceptors to Polychromatic Lights. J Biol Rhythms [Internet]. [accessed 2021 Nov 8] 26(4):314–323. 10.1177/0748730411409719 [DOI] [PubMed] [Google Scholar]
- Esaki Y, Kitajima T, Ito Y, Koike S, Nakao Y, Tsuchiya A, Hirose M, Iwata N. 2016. Wearing blue light-blocking glasses in the evening advances circadian rhythms in the patients with delayed sleep phase disorder: An open-label trial. Chronobiology International [Internet]. [accessed 2021 Jan 25] 33(8):1037–1044. 10.1080/07420528.2016.1194289 [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Sahin L, Wood B, Plitnick B. 2016. Light at Night and Measures of Alertness and Performance: Implications for Shift Workers. Biological Research For Nursing [Internet]. [accessed 2021 Nov 15] 18(1):90–100. 10.1177/1099800415572873 [DOI] [PubMed] [Google Scholar]
- Foret J, Daurat A, Tirilly G. 1998. Effect of bright light at night on core temperature, subjective alertness and performance as a function of exposure time. Scandinavian Journal of Work, Environment & Health [Internet]. [accessed 2021 Nov 14] 24:115–120. https://www.jstor.org/stable/40966847 [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SMW, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. 2011. Exposure to Room Light before Bedtime Suppresses Melatonin Onset and Shortens Melatonin Duration in Humans. J Clin Endocrinol Metab [Internet]. [accessed 2021 Jun 16] 96(3):E463–E472. 10.1210/jc.2010-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med [Internet]. [accessed 2021 Jan 25] 2(31):31ra33. 10.1126/scitranslmed.3000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant LK, Kent BA, Mayer MD, Stickgold R, Lockley SW, Rahman SA. 2021. Daytime Exposure to Short Wavelength-Enriched Light Improves Cognitive Performance in Sleep-Restricted College-Aged Adults. Front Neurol [Internet]. [accessed 2021 Jun 16] 12. 10.3389/fneur.2021.624217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringras P, Middleton B, Skene DJ, Revell VL. 2015. Bigger, Brighter, Bluer-Better? Current Light-Emitting Devices – Adverse Sleep Properties and Preventative Strategies. Front Public Health [Internet]. [accessed 2021 Jan 25] 3. 10.3389/fpubh.2015.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V, Roth T, Drake CL. 2012. Circadian Phase, Sleepiness, and Light Exposure Assessment in Night Workers With and Without Shift Work Disorder. Chronobiology International [Internet]. [accessed 2021 Aug 13] 29(7):928–936. 10.3109/07420528.2012.699356 [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau K-W. 2003. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature [Internet]. [accessed 2021 Jan 13] 424(6944):76–81. 10.1038/nature01761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen TEG, Grønli J, Assmus J, Fasmer OB, Schoeyen H, Leskauskaite I, Bjorke-Bertheussen J, Ytrehus K, Lund A. 2020. Blue-blocking glasses as additive treatment for mania: Effects on actigraphy-derived sleep parameters. J Sleep Res. 29(5):e12984. 10.1111/jsr.12984 [DOI] [PubMed] [Google Scholar]
- Henriksen TEG, Skrede S, Fasmer OB, Hamre B, Grønli J, Lund A. 2014. Blocking blue light during mania - markedly increased regularity of sleep and rapid improvement of symptoms: a case report. Bipolar Disord. 16(8):894–898. 10.1111/bdi.12265 [DOI] [PubMed] [Google Scholar]
- Heo J-Y, Kim K, Fava M, Mischoulon D, Papakostas GI, Kim M-J, Kim DJ, Chang K-AJ, Oh Y, Yu B-H, Jeon HJ. 2017. Effects of smartphone use with and without blue light at night in healthy adults: A randomized, double-blind, cross-over, placebo-controlled comparison. J Psychiatr Res. 87:61–70. 10.1016/j.jpsychires.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Jung CM, Khalsa SBS, Scheer FAJL, Cajochen C, Lockley SW, Czeisler CA, Wright KP. 2010. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 25(3):208–216. 10.1177/0748730410368413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladchibachi S, Negelspach DC, Zeitzer JM, Fernandez F. 2019. Optimization of circadian responses with shorter and shorter millisecond flashes. Biology Letters [Internet]. [accessed 2021 Aug 13] 15(8):20190371. 10.1098/rsbl.2019.0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauth P, Hornberger S. 2003. Preventive and compensatory measures for shift workers. Occupational Medicine [Internet]. [accessed 2021 Aug 10] 53(2):109–116. 10.1093/occmed/kqg049 [DOI] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee H-K, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. 2012. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 491(7425):594–598. 10.1038/nature11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S. 2014. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci [Internet]. [accessed 2021 Aug 13] 15(7):443–454. 10.1038/nrn3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely S, Frey S, Garbazza C, Wirz-Justice A, Jenni OG, Steiner R, Wolf S, Cajochen C, Bromundt V, Schmidt C. 2015. Blue Blocker Glasses as a Countermeasure for Alerting Effects of Evening Light-Emitting Diode Screen Exposure in Male Teenagers. Journal of Adolescent Health [Internet]. [accessed 2021 Apr 27] 56(1):113–119. 10.1016/j.jadohealth.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Levenson JC, Shensa A, Sidani JE, Colditz JB, Primack BA. 2017. Social Media Use Before Bed and Sleep Disturbance Among Young Adults in the United States: A Nationally Representative Study. Sleep [Internet]. [accessed 2021 Aug 13] 40(9). 10.1093/sleep/zsx113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. 2003. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 88(9):4502–4505. 10.1210/jc.2003-030570 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Lall GS, Allen AE, Brown TM. 2012. Chapter 1 - How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. In: Kalsbeek A, Merrow M, Roenneberg T, Foster RG, editors. Progress in Brain Research [Internet]. Vol. 199. [place unknown]: Elsevier; [accessed 2021 Feb 1]; p. 1–18. 10.1016/B978-0-444-59427-3.00001-0 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, et al. 2014. Measuring and using light in the melanopsin age. Trends in Neurosciences [Internet]. [accessed 2021 Nov 8] 37(1):1–9. 10.1016/j.tins.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden WP, Van Someren JL, te Lindert BHW, Bruijel J, van Oosterhout F, Coppens JE, Kalsbeek A, Cajochen C, Bourgin P, Van Someren EJW. 2016. Individual Differences in Sleep Timing Relate to Melanopsin-Based Phototransduction in Healthy Adolescents and Young Adults. Sleep [Internet]. [accessed 2021 Jun 16] 39(6):1305–1310. 10.5665/sleep.5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micic G, Lovato N, Gradisar M, Ferguson SA, Burgess HJ, Lack LC. 2016. The etiology of delayed sleep phase disorder. Sleep Medicine Reviews [Internet]. [accessed 2021 Aug 13] 27:29–38. 10.1016/j.smrv.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Mouland JW, Martial F, Watson A, Lucas RJ, Brown TM. 2019. Cones Support Alignment to an Inconsistent World by Suppressing Mouse Circadian Responses to the Blue Colors Associated with Twilight. Curr Biol [Internet]. [accessed 2021 Jan 25] 29(24):4260–4267.e4. 10.1016/j.cub.2019.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar RP, Zeitzer JM. 2016. Temporal integration of light flashes by the human circadian system. J Clin Invest [Internet]. [accessed 2021 Aug 13] 126(3):938–947. 10.1172/JCI82306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negelspach DC, Kaladchibachi S, Fernandez F. 2018. The circadian activity rhythm is reset by nanowatt pulses of ultraviolet light. Proceedings of the Royal Society B: Biological Sciences [Internet]. [accessed 2021 Aug 13] 285(1884):20181288. 10.1098/rspb.2018.1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. 2002. Melanopsin (Opn4) Requirement for Normal Light-Induced Circadian Phase Shifting. Science [Internet]. [accessed 2021 Apr 27] 298(5601):2213–2216. 10.1126/science.1076848 [DOI] [PubMed] [Google Scholar]
- Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, Lockley SW, Cain SW. 2019. High sensitivity and interindividual variability in the response of the human circadian system to evening light. PNAS [Internet]. [accessed 2021 Jan 5] 116(24):12019–12024. 10.1073/pnas.1901824116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V, Tam SKE, Hughes S, Pothecary CA, Jagannath A, Hankins MW, Bannerman DM, Lightman SL, Vyazovskiy VV, Nolan PM, et al. 2016. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 14(6):e1002482. 10.1371/journal.pbio.1002482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plano SA, Casiraghi LP, García Moro P, Paladino N, Golombek DA, Chiesa JJ. 2017. Circadian and Metabolic Effects of Light: Implications in Weight Homeostasis and Health. Front Neurol [Internet]. [accessed 2021 Aug 3] 8:558. 10.3389/fneur.2017.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitnick B, Figueiro M, Wood B, Rea M. 2010. The effects of red and blue light on alertness and mood at night. Lighting Research & Technology [Internet]. [accessed 2021 Nov 15] 42(4):449–458. 10.1177/1477153509360887 [DOI] [Google Scholar]
- Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RCH, van der Beek AJ, van Steeg H, van Kerkhof LWM. 2016. The Relationship Between Shift Work and Metabolic Risk Factors: A Systematic Review of Longitudinal Studies. American Journal of Preventive Medicine [Internet]. [accessed 2021 Aug 4] 50(5):e147–e157. 10.1016/j.amepre.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Puttonen S, Viitasalo K, Härmä M. 2011. Effect of Shiftwork on Systemic Markers of Inflammation. Chronobiology International [Internet]. [accessed 2021 Aug 4] 28(6):528–535. 10.3109/07420528.2011.580869 [DOI] [PubMed] [Google Scholar]
- Rahman SA, Brainard GC, Czeisler CA, Lockley SW. 2021. Spectral sensitivity of circadian phase resetting, melatonin suppression and acute alerting effects of intermittent light exposure. Biochemical Pharmacology [Internet]. [accessed 2021 Aug 13] 191:114504. 10.1016/j.bcp.2021.114504 [DOI] [PubMed] [Google Scholar]
- Rahman SA, St Hilaire MA, Lockley SW. 2017. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol Behav. 177:221–229. 10.1016/j.physbeh.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rångtell FH, Ekstrand E, Rapp L, Lagermalm A, Liethof L, Búcaro MO, Lingfors D, Broman J-E, Schiöth HB, Benedict C. 2016. Two hours of evening reading on a self-luminous tablet vs. reading a physical book does not alter sleep after daytime bright light exposure. Sleep Med. 23:111–118. 10.1016/j.sleep.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. 2002. Role of melanopsin in circadian responses to light. Science. 298(5601):2211–2213. 10.1126/science.1076701 [DOI] [PubMed] [Google Scholar]
- Sasseville A, Benhaberou-Brun D, Fontaine C, Charon M-C, Hébert M. 2009. Wearing Blue-Blockers in the Morning Could Improve Sleep of Workers on a Permanent Night Schedule: A Pilot Study. Chronobiology International [Internet]. [accessed 2021 Aug 10] 26(5):913–925. 10.1080/07420520903044398 [DOI] [PubMed] [Google Scholar]
- Sasseville A, Hébert M. 2010. Using blue-green light at night and blue-blockers during the day to improves adaptation to night work: A pilot study. Progress in Neuro-Psychopharmacology and Biological Psychiatry [Internet]. [accessed 2021 Jan 25] 34(7):1236–1242. 10.1016/j.pnpbp.2010.06.027 [DOI] [PubMed] [Google Scholar]
- Sasseville A, Paquet N, Sévigny J, Hébert M. 2006. Blue blocker glasses impede the capacity of bright light to suppress melatonin production. Journal of Pineal Research [Internet]. [accessed 2021 Jan 25] 41(1):73–78. 10.1111/j.1600-079X.2006.00332.x [DOI] [PubMed] [Google Scholar]
- Shechter A, Kim EW, St-Onge M-P, Westwood AJ. 2018. Blocking nocturnal blue light for insomnia: A randomized controlled trial. J Psychiatr Res. 96:196–202. 10.1016/j.jpsychires.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Gianotti TF, Burgueño A, Alvarez A, González CD, Pirola CJ. 2007. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. Journal of Internal Medicine [Internet]. [accessed 2021 Jan 13] 261(3):285–292. 10.1111/j.1365-2796.2007.01766.x [DOI] [PubMed] [Google Scholar]
- Spitschan M, Lazar R, Cajochen C. 2019. Visual and non-visual properties of filters manipulating short-wavelength light. Ophthalmic and Physiological Optics [Internet]. [accessed 2021 Jul 15] 39(6):459–468. 10.1111/opo.12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitschan M, Lazar R, Yetik E, Cajochen C. 2019. No evidence for an S cone contribution to acute neuroendocrine and alerting responses to light. Current Biology [Internet]. [accessed 2021 Nov 9] 29(24):R1297–R1298. 10.1016/j.cub.2019.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani O, Freyburger M, Veitz S, Basishvili T, Meyer M, Weibel J, Kobayashi K, Shirakawa Y, Cajochen C. 2021. Changing color and intensity of LED lighting across the day impacts on circadian melatonin rhythms and sleep in healthy men. Journal of Pineal Research [Internet]. [accessed 2021 Jun 16] 70(3):e12714. 10.1111/jpi.12714 [DOI] [PubMed] [Google Scholar]
- Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. 2013. Adverse Health Effects of Nighttime Lighting: Comments on American Medical Association Policy Statement. American Journal of Preventive Medicine [Internet]. [accessed 2021 Jan 25] 45(3):343–346. 10.1016/j.amepre.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. 2001. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. The Journal of Physiology [Internet]. [accessed 2021 Apr 26] 535(1):261–267. 10.1111/j.1469-7793.2001.t01-1-00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Turner EH, Shimada JM, Lowe CH, Barker C, Leibenluft E. 1998. Treatment of rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep. Biol Psychiatry. 43(11):822–828. 10.1016/s0006-3223(97)00542-8 [DOI] [PubMed] [Google Scholar]
- Weinmann T, Vetter C, Karch S, Nowak D, Radon K. 2018. Shift work and cognitive impairment in later life – results of a cross-sectional pilot study testing the feasibility of a large-scale epidemiologic investigation. BMC Public Health [Internet]. [accessed 2021 Aug 4] 18:1256. 10.1186/s12889-018-6171-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickwire EM, Geiger-Brown J, Scharf SM, Drake CL. 2017. Shift Work and Shift Work Sleep Disorder. Chest [Internet]. [accessed 2021 Aug 4] 151(5):1156–1172. 10.1016/j.chest.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Quinto C, Cajochen C, Werth E, Hock C. 1999. A rapid-cycling bipolar patient treated with long nights, bedrest, and light. Biological Psychiatry [Internet]. [accessed 2021 Aug 13] 45(8):1075–1077. 10.1016/S0006-3223(98)00289-3 [DOI] [PubMed] [Google Scholar]
- Wright HR, Lack LC. 2001. Effect of Light Wavelength on Suppression and Phase Delay of the Melatonin Rhythm. Chronobiology International [Internet]. [accessed 2021 Jan 5] 18(5):801–808. 10.1081/CBI-100107515 [DOI] [PubMed] [Google Scholar]
- Yuan X, Zhu C, Wang M, Mo F, Du W, Ma X. 2019. Retraction: Night Shift Work Increases the Risks of Multiple Primary Cancers in Women: A Systematic Review and Meta-analysis of 61 Articles. Cancer Epidemiol Biomarkers Prev [Internet]. [accessed 2021 Aug 4] 28(2):423–423. 10.1158/1055-9965.EPI-18-1085 [DOI] [PubMed] [Google Scholar]
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, Czeisler CA, et al. 2007. Short-Wavelength Light Sensitivity of Circadian, Pupillary, and Visual Awareness in Humans Lacking an Outer Retina. Curr Biol [Internet]. [accessed 2021 Jan 25] 17(24):2122–2128. 10.1016/j.cub.2007.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual performance metrics for 50 blue blocking glasses.
Manufacturer, model/SKU, and/or ASIN used of each blue blocker.


