Abstract
Background.
Few prior studies have investigated differences in precipitants leading to hospitalisations for acute heart failure (AHF) in a cohort with global representation.
Methods.
We analysed the prevalence of precipitants and their association with outcomes in 18,553 patients hospitalised for AHF in REPORT-HF (prospective international REgistry to assess medical Practice with lOngitudinal obseRvation for Treatment of Heart Failure) according to left ventricular ejection fraction (LVEF) subtype (reduced [HFrEF] and preserved ejection fraction [HFpEF]) and presentation (new-onset vs. decompensated chronic HF [DCHF]). Patients were enrolled from 358 centres in 44 countries stratified according to Latin America, North America, western Europe, Eastern Europe, Eastern Mediterranean and Africa, Southeast Asia, and Western Pacific. Precipitants were pre-defined as mutually exclusive categories and selected according to the local investigators discretion. Outcomes included in-hospital and 1-year mortality.
Results.
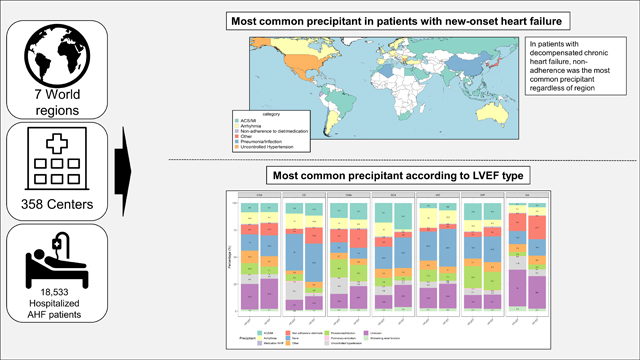
The median age was 67 (IQR 57–77) years, and 39% were women. Acute coronary syndrome (ACS) was the most common precipitant in patients with new-onset HF in all regions except for North America and Western Europe, where uncontrolled hypertension and arrhythmia were the most common precipitants, independent of HF subtype and other confounders. In patients with DCHF, non-adherence to diet/medication was the most common precipitant regardless of region. Uncontrolled hypertension was a more likely precipitant in HFpEF, non-adherence to diet/medication, and ACS were more likely precipitants in HFrEF. Patients admitted due to worsening renal function had the worst in-hospital (4%) and 1-year post-discharge (30%) mortality rates, regardless of region, HF subtype and admission type (Pinteraction >0.05 for all).
Conclusion:
Data on global differences in precipitants for AHF highlight potential regional differences in targets for preventing hospitalisation for AHF and identifying those at highest risk for early mortality.
Keywords: Precipitants, heart failure, HF admission, HF-subtype, global differences
Graphical Abstract
Introduction
Hospitalisation for acute heart failure (AHF) is associated with significant morbidity and mortality.1 Numerous clinical factors can precipitate hospitalisation for AHF, including acute coronary syndromes/myocardial infarction (ACS/MI), infection, uncontrolled hypertension, arrhythmias, worsening renal function (WRF) and non-adherence to medication or diet.2,3,12–14,4–11 Knowledge of the frequency of precipitating factors is essential, as this can inform targets for prevention before hospital admission and treatment during hospitalisation. Further, understanding the association between precipitants and mortality may identify a subset of patients who may require intensive management strategies during their inpatient stay.
Several studies have investigated the frequency of factors precipitating AHF hospitalisation, yet these were almost exclusively from (Western) Europe and North America.2,3,5–8,10,11,13,14 Importantly, geographical differences in precipitants according to HF presentation (decompensated chronic HF vs new-onset HF) and left ventricular ejection fraction type (HFrEF vs. HFmrEF and HFpEF) in patients hospitalised for AHF are poorly described.
REPORT-HF (Prospective international Registry to assess medical practice with longitudinal observation for treatment of Heart Failure) is the largest prospective global AHF registry with inclusion from 44 countries across seven world regions. REPORT-HF is thus uniquely positioned to investigate 1) geographic differences in precipitants leading up to hospitalisation for AHF and 2) the association with HF outcomes. Therefore, this study aims to investigate geographic differences in precipitants of AHF and their association with in-hospital and 1-year all-cause mortality according to left ventricular ejection fraction (LVEF) subtype and HF presentation.
Methods
Study design and population
The study population is derived from REPORT-HF. The rationale and design of the REPORT-HF registry have been previously described.15–17 In short, REPORT-HF was a large, well-characterised global cohort of patients hospitalised for AHF, defined as either new-onset (first diagnosis) HF or decompensated chronic HF (DCHF), as assessed by the clinician/investigator. Patients were excluded if they participated in another clinical trial related to any investigational treatments or did not provide informed consent. Three hundred fifty-eight hospitals from 44 countries in seven world regions participated in the REPORT-HF registry. Enrolment was completed between the 23rd of July 2014 and the 24th of March 2017.
This study was performed in accordance with the Declaration of Helsinki.18 At each participating site, the protocol was approved by either the institutional review board, the ethics committee, or both. Written informed consent was obtained from all patients or a legal representative if permitted.
Definitions and study outcomes
For the current study, the CRF asked the investigators to choose from 12 different answers on precipitating factors: ACS/MI, arrhythmias, uncontrolled hypertension, non-adhering to diet, non-adhering to prescribed medications, prescription of medications likely to worsen HF, pneumonia/respiratory tract infection, pulmonary embolism, WRF, other, none, and unknown. Patients listed as non-adhering to diet (n=621), or medication (n=999) were combined into one non-adherence group. Data on precipitants was missing in 750 (4%). We included these patients together with 2,918 patients, which had precipitant selected as “unknown” in the CRF. The precipitant question in REPORT-HF consisted of predefined precipitant categories, which were selected according to the physician investigator’s clinical judgement. These categories, while predefined and mutually exclusive, did not include specific quantitative measurements e.g. there were no specific creatinine/ GFR measurements to qualify for ‘worsening renal function’, nor specific blood pressure measurements to qualify for ‘uncontrolled blood pressure’.
Patients with an LVEF of less than 40% were classified as HF with reduced ejection fraction (HFrEF). An LVEF of 40–49% was defined as HF with mildly reduced ejection fraction (HFmrEF), whereas an ejection fraction of at least 50% was defined as HF with preserved ejection fraction (HFpEF). Based on a modified version of the World Health Organization classification, participating countries were stratified into 7 regions: (1) Western Europe (n = 3594), (2) Eastern Europe (n = 2802), (3) Western Pacific (n = 3354), (4) Southeast Asia (n = 2329), (5) North America (n = 1592), (6) Central and South America (n = 2641), and (7) Eastern Mediterranean Region and Africa (n = 2241).
Follow-up information was collected at 6 and 12 months after hospital discharge via regular follow-up visits or telephone interviews. Local investigators were asked to ascertain the cause of death and indicate if it either was due to a cardiovascular, non-cardiovascular, or unknown cause. The outcomes of interest were in-hospital and one-year post-discharge mortality.
Statistical analysis
Comparisons of demographic and clinical parameters among HF precipitants or regions using χ2 tests for categorical variables and analysis of variance for continuous variables. Categorical variables were described as numbers and percentages, and continuous variables were expressed as means ± standard deviation or median [25th and 75th percentiles] depending on their distribution. Cox proportional-hazards models were used to calculate unadjusted hazard ratios. The precipitating factor with the lowest hazard ratio for the chosen outcome (risk nadir) was used as a reference. Multivariable models were selected based on expert knowledge. The model for 1-year post-discharge mortality included age, sex, hypertension, atrial fibrillation, chronic obstructive pulmonary disease/asthma, chronic kidney disease, coronary artery disease, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), LVEF category (HFrEF, HFmrEF, HFpEF, missing), HF diagnosis (new-onset vs DCHF), ACEi/ARB at discharge, MRAs at discharge, beta-blockers at discharge, diuretics at discharge and geographic region. The model for in-hospital mortality included age, sex, hypertension, atrial fibrillation, chronic obstructive pulmonary disease/asthma, chronic kidney disease, coronary artery disease, CABG, PCI, LVEF category, HF diagnosis, systolic blood pressure at admission, heart rhythm at admission and geographic region. We tested for interaction between precipitant and LVEF subtype (HFrEF; HFmrEF; HFpEF) and presentation (new-onset HF vs DCHF). A two-sided p-value <0.05 was considered statistically significant. Stata SE16 (StataCorp. 2017. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC) was used for statistical analyses.
Results
Baseline characteristics
The population’s median age was 67 (IQR 57–77) years, and 39% were women. In total, 20% of patients had an unknown precipitant, and 23% had no precipitant. Among known precipitants, ACS was most frequently reported (13%), followed by pneumonia and respiratory tract infection (10%) and uncontrolled hypertension (6%). Medication likely to worsen HF (<1%) and pulmonary embolism (<1%) were least frequently reported as the primary precipitant.
Table 1 shows the baseline characteristics according to the most likely precipitant. Patients with arrhythmia as precipitating factor were the oldest, and those with non-adherence to diet or medications were the youngest. Patients with pulmonary embolism as precipitant were more likely in New York Heart Association class III/IV at discharge than patients with other precipitants. Dyspnea at rest was most common in patients with pulmonary embolism. Orthopnea occurred most frequently in patients with a precipitant factor of non-adherence to diet/medication, peripheral oedema was most common in patients with WRF, and rales were most common in patients with pneumonia/infection.
Table 1.
Baseline characteristics according to most likely precipitant
| ACS/MI | Pneumonia/infection | Arrhythmia | Non-adherence diet/meds | Other | Uncontrolled hypertension | Worsening renal function | Medication WHF | Pulmonary embolism | None | Unknown | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 2346 (13%) | 1800 (10%) | 1776 (10%) | 1620 (9%) | 1283 (7%) | 1112 (6%) | 423 (2%) | 112 (<1%) | 93 (<1%) | 4320 (23%) | 3668 (20%) |
| Median age (IQR) - yr | 66 (58, 75) | 69 (58, 79) | 71 (61, 79) | 63 (54, 72) | 66 (54, 76) | 66 (57, 76) | 67 (58, 75) | 68 (56, 77) | 67 (52, 77) | 67 (57, 77) | 68 (58, 77) |
| Race - no. (%) | |||||||||||
| Caucasian | 902 (38%) | 793 (44%) | 1172 (66%) | 799 (49%) | 618 (48%) | 579 (52%) | 163 (39%) | 60 (54%) | 48 (52%) | 2528 (59%) | 1994 (54%) |
| Black | 38 (2%) | 48 (3%) | 37 (2%) | 189 (12%) | 57 (4%) | 113 (10%) | 34 (8%) | 3 (3%) | 1 (1%) | 100 (2%) | 247 (7%) |
| Asian | 1184 (50%) | 730 (41%) | 360 (20%) | 370 (23%) | 431 (34%) | 256 (23%) | 166 (39%) | 37 (33%) | 36 (39%) | 1338 (31%) | 830 (23%) |
| Native American | 35 (1%) | 27 (2%) | 40 (2%) | 44 (3%) | 30 (2%) | 36 (3%) | 21 (5%) | 1 (1%) | 0 (0%) | 60 (1%) | 81 (2%) |
| Pacific Islander | 2 (<1%) | 0 (0%) | 2 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (<1%) | 0 (0%) |
| Other | 185 (8%) | 202 (11%) | 165 (9%) | 217 (13%) | 147 (11%) | 128 (12%) | 39 (9%) | 11 (10%) | 8 (9%) | 292 (7%) | 516 (14%) |
| DCHF - no. (%) | 1403 (60%) | 715 (40%) | 845 (48%) | 357 (22%) | 490 (38%) | 625 (56%) | 153 (36%) | 30 (27%) | 51 (55%) | 1874 (43%) | 1359 (37%) |
| NYHA class at discharge - no. (%) | |||||||||||
| I | 414 (18%) | 180 (10%) | 203 (12%) | 180 (11%) | 136 (11%) | 212 (19%) | 30 (7%) | 18 (16%) | 7 (8%) | 304 (7%) | 338 (9%) |
| II | 826 (36%) | 725 (42%) | 583 (33%) | 636 (40%) | 382 (31%) | 379 (34%) | 128 (32%) | 36 (33%) | 27 (30%) | 1173 (28%) | 860 (24%) |
| III | 312 (14%) | 314 (18%) | 270 (15%) | 284 (18%) | 201 (16%) | 158 (14%) | 65 (16%) | 21 (19%) | 22 (24%) | 877 (21%) | 462 (13%) |
| IV | 103 (4%) | 47 (3%) | 41 (2%) | 41 (3%) | 37 (3%) | 30 (3%) | 17 (4%) | 4 (4%) | 6 (7%) | 172 (4%) | 127 (4%) |
| Missing/Unknown | 637 (28%) | 467 (27%) | 651 (37%) | 446 (28%) | 487 (39%) | 322 (29%) | 163 (40%) | 31 (28%) | 28 (31%) | 1693 (40%) | 1789 (50%) |
| LVEF Subtype - no. (%) | |||||||||||
| <40 | 1095 (47%) | 800 (44%) | 737 (41%) | 941 (58%) | 661 (52%) | 316 (28%) | 212 (50%) | 61 (54%) | 37 (40%) | 2183 (51%) | 1861 (51%) |
| ≥40 and <50 | 537 (23%) | 273 (15%) | 287 (16%) | 204 (13%) | 166 (13%) | 175 (16%) | 62 (15%) | 17 (15%) | 15 (16%) | 635 (15%) | 500 (14%) |
| ≥50 | 526 (22%) | 579 (32%) | 644 (36%) | 365 (23%) | 370 (29%) | 509 (46%) | 115 (27%) | 27 (24%) | 38 (41%) | 1130 (26%) | 865 (24%) |
| Unknown | 188 (8%) | 148 (8%) | 108 (6%) | 110 (7%) | 86 (7%) | 112 (10%) | 34 (8%) | 7 (6%) | 3 (3%) | 372 (9%) | 442 (12%) |
| Median heart rate (IQR) - bpm | 88 (75, 100) | 88 (76, 102) | 100 (78, 125) | 87 (75, 100) | 84 (71, 100) | 87 (74, 101) | 81 (71, 98) | 82 (72, 95) | 90 (80, 108) | 83 (71, 99) | 85 (72, 100) |
| Median systolic blood pressure (IQR) - mm Hg | 130 (116, 150) | 130 (111, 146) | 130 (112, 145) | 130 (110, 148) | 123 (110, 140) | 168 (144, 190) | 132 (113, 160) | 130 (110, 140) | 120 (110, 140) | 130 (110, 146) | 128 (110, 147) |
| Median diastolic blood pressure (IQR) - mm Hg | 80 (70, 90) | 77 (67, 88) | 80 (70, 90) | 80 (69, 90) | 72 (64, 83) | 92 (80, 106) | 78 (65, 90) | 77 (65, 85) | 76 (70, 90) | 78 (69, 89) | 76 (65, 87) |
| Signs and symptoms - no. (%) | |||||||||||
| Dyspnea at rest | 1789 (83%) | 1464 (86%) | 1287 (80%) | 1264 (87%) | 902 (82%) | 866 (87%) | 327 (86%) | 80 (80%) | 77 (93%) | 3149 (80%) | 2404 (83%) |
| Orthopnea | 1433 (73%) | 1282 (85%) | 1132 (77%) | 1171 (86%) | 744 (75%) | 740 (81%) | 274 (80%) | 74 (80%) | 58 (73%) | 2728 (76%) | 1998 (77%) |
| Peripheral edema | 1114 (52%) | 1170 (70%) | 1170 (70%) | 1207 (78%) | 804 (69%) | 695 (68%) | 309 (78%) | 81 (74%) | 60 (71%) | 2632 (70%) | 2163 (71%) |
| Pulmonary rales | 1333 (68%) | 1312 (82%) | 1072 (70%) | 1008 (70%) | 661 (63%) | 700 (71%) | 249 (71%) | 70 (72%) | 45 (59%) | 2140 (62%) | 1693 (63%) |
| Medical history - no. (%) | |||||||||||
| Hypertension | 1495 (64%) | 1126 (63%) | 1167 (66%) | 1061 (65%) | 714 (56%) | 1016 (91%) | 300 (71%) | 68 (61%) | 55 (59%) | 2572 (60%) | 2234 (61%) |
| Atrial fibrillation/flutter | 304 (13%) | 584 (32%) | 1185 (67%) | 492 (30%) | 361 (28%) | 201 (18%) | 108 (26%) | 43 (38%) | 22 (24%) | 1345 (31%) | 1121 (31%) |
| COPD/Asthma | 237 (10%) | 426 (24%) | 223 (13%) | 258 (16%) | 184 (14%) | 169 (15%) | 58 (14%) | 12 (11%) | 16 (17%) | 560 (13%) | 512 (14%) |
| Anemia | 1141 (49%) | 879 (49%) | 702 (40%) | 831 (51%) | 737 (57%) | 515 (46%) | 336 (79%) | 66 (59%) | 39 (42%) | 1922 (45%) | 1561 (43%) |
| Valvular heart disease | 148 (6%) | 372 (21%) | 502 (28%) | 355 (22%) | 411 (32%) | 145 (13%) | 83 (20%) | 32 (29%) | 11 (12%) | 983 (23%) | 637 (17%) |
| Coronary artery disease | 2346 (100%) | 790 (44%) | 599 (34%) | 721 (45%) | 486 (38%) | 387 (35%) | 202 (48%) | 46 (41%) | 26 (28%) | 1822 (42%) | 1504 (41%) |
| Diabetes | 1103 (47%) | 700 (39%) | 484 (27%) | 639 (39%) | 441 (34%) | 477 (43%) | 250 (59%) | 49 (44%) | 25 (27%) | 1537 (36%) | 1362 (37%) |
| CKD | 362 (15%) | 351 (20%) | 300 (17%) | 325 (20%) | 267 (21%) | 245 (22%) | 276 (65%) | 22 (20%) | 18 (19%) | 845 (20%) | 755 (21%) |
| Medication at discharge - no. (%) | |||||||||||
| ACEi/ARB/ARNi | 1504 (66%) | 1090 (64%) | 1191 (68%) | 1079 (68%) | 768 (62%) | 851 (78%) | 161 (40%) | 75 (68%) | 58 (64%) | 2824 (67%) | 2295 (64%) |
| MRA | 956 (42%) | 884 (52%) | 848 (49%) | 833 (53%) | 636 (52%) | 374 (34%) | 93 (23%) | 49 (45%) | 50 (56%) | 2382 (57%) | 1747 (49%) |
| Beta-blockers | 1573 (69%) | 1131 (66%) | 1343 (77%) | 1202 (76%) | 866 (70%) | 791 (72%) | 252 (63%) | 75 (68%) | 60 (67%) | 3119 (74%) | 2631 (74%) |
| Diuretics | 1600 (70%) | 1440 (84%) | 1453 (83%) | 1432 (90%) | 1064 (86%) | 869 (79%) | 327 (82%) | 99 (90%) | 69 (77%) | 3739 (89%) | 3061 (86%) |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin type II receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; bpm, beats per minute; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DCHF, decompensated chronic heart failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; IQR, interquartile range; LVEF, left ventricular ejection fraction.
New-onset HF vs decompensated chronic HF
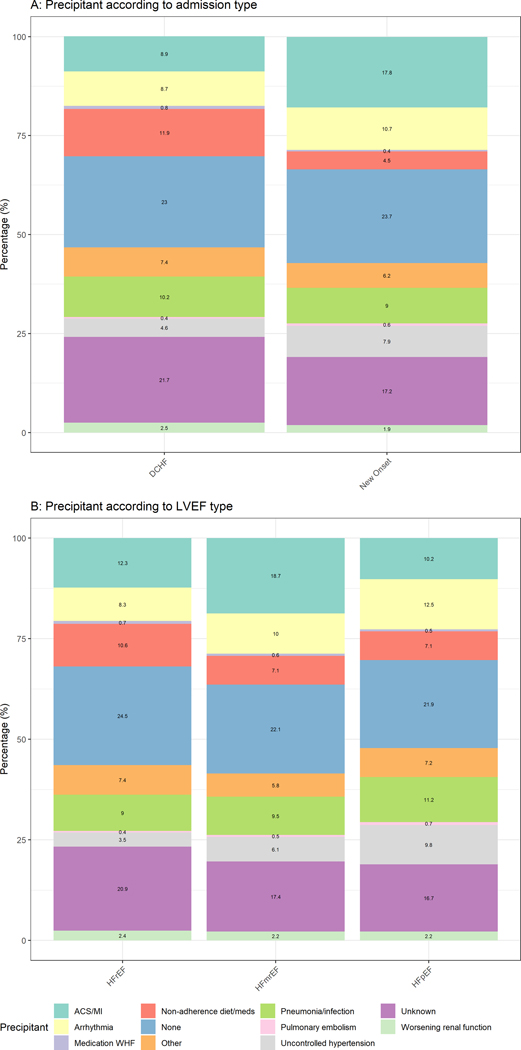
In total, 7,902 (43%) patients were admitted with new-onset HF, and 10,651(57%) patients had DCHF. Figure 1A shows that patients with new-onset HF most frequently presented with ACS/MI (18%), arrhythmias (11%), and uncontrolled hypertension (8%). Patients with DCHF were mostly admitted due to non-adherence to diet/medication (12%) or pneumonia/respiratory tract infection (10%). Multivariable analyses adjusting for age, sex, hypertension, atrial fibrillation, chronic obstructive pulmonary disease/asthma, chronic kidney disease, coronary artery disease, region and LVEF subtype in Supplementary Table 1, show that patients admitted with ACS/MI, arrhythmia, uncontrolled hypertension, pneumonia/infection, or pulmonary embolism had a significantly higher odds ratio for having new-onset HF (P for all <0.05). Patients admitted for non-adherence to diet/medication or medication that can cause or worsen HF had a significantly higher odds ratio for having DCHF (P for all <0.05).
Figure 1.
Stacked bar charts depicting percentages of precipitant stratified by admission type (A) and HF-subgroup (B)
Supplementary Table 2 shows the number and percentages of precipitants stratified by region and presentation (new-onset HF vs DCHF). Having ACS/MI as a precipitant was most common in patients with new-onset HF from the Western Pacific (21%) region, Eastern Mediterranean or African region (22%) and Southeast Asia (27%). Arrhythmia as precipitant was most common in patients with new-onset HF from Eastern (16%) and Western (18%) Europe. Non-adherence to diet or pharmacotherapy was a more common reason for admission in patients with DCHF from Southeast Asia (14%), the Eastern Mediterranean or African region (18%), and North America (23%). Pneumonia or respiratory tract infection was a common precipitant in patients from the Eastern Mediterranean or African region with DHCF (16%) and new-onset HF (13%) and in patients from the Western Pacific with DCHF (20%) and new-onset HF (16%).
Precipitants according to left ventricular ejection fraction subtype
In total, 8,904 (48%) patients were admitted with HFrEF, 2,871 (16%) patients with HFmrEF and 5,168 (28%) patients with HFpEF. Figure 1B suggests uncontrolled hypertension was a more likely precipitant in HFpEF than HFrEF (10% vs 4%, respectively). In multivariable analyses (Supplementary Table 1), patients with HFpEF more commonly had arrhythmia, uncontrolled hypertension, pneumonia or infection and pulmonary embolism as likely precipitants than patients with HFrEF.
Supplementary Table 3 shows differences in precipitants stratified according to geographic region and LVEF subtype. ACS/MI was most common in patients with HFmrEF (22%) from the Eastern Mediterranean or African region and patients with HFrEF (24%) or HFmrEF (42%) from Southeast Asia. Arrhythmia as precipitating factor was most common in patients with HFpEF from Western Europe (18%). Non-adherence to diet or medication as precipitant was most common in patients with HFrEF from North America (22%). Pneumonia and infection were more prevalent in the Western Pacific and Eastern Mediterranean or African region, regardless of LVEF subtype.
In-hospital and post-discharge mortality
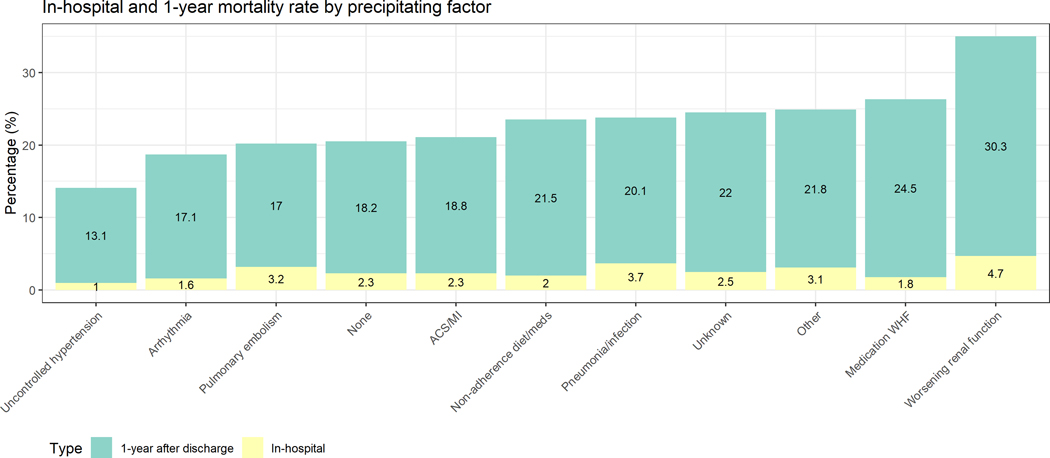
In total, 451 patients (2.4%) died in-hospital. Among 18,102 patients discharged alive, 470 were lost-to-follow-up, and 3,461 (20%) died. Figure 2 depicts the in-hospital mortality and post-discharge mortality according to precipitant. The cumulative mortality at 1-year was lowest in patients with uncontrolled hypertension as precipitant and highest in patients with WRF as precipitant. Table 2 suggests the in-hospital mortality was lowest in patients hospitalised with uncontrolled hypertension (1.0%). Patients hospitalised for WRF had the highest in-hospital mortality (4.7%). Patients hospitalised with pneumonia/infection had an in-hospital mortality rate of 3.7%. These differences remained significant after correcting for confounders. We did not find a significant interaction between precipitant and geographic region for in-hospital and 1-year post-discharge mortality (Pinteraction >0.05), suggesting that the association of precipitants with mortality was similar across geographic regions.
Figure 2.
Bar charts depicting prevalence of in-hospital and 1-year post-discharge mortality, stratified by precipitating factor
Table 2:
Association with in-hospital and 1-year mortality
| In-hospital mortality | |||
|---|---|---|---|
| Univariable | Multivariable | ||
| N (%) | OR (95%CI) p-value | OR (95%CI) p-value | |
| Uncontrolled hypertension | 11 (1.0%) | Ref | Ref |
| ACS/MI | 54 (2.3%) | 2.36 (1.23–4.53) 0.010 | 1.61 (0.82–3.15) 0.165 |
| Arrhythmia | 28 (1.6%) | 1.60 (0.80–3.23) 0.187 | 0.92 (0.44–1.91) 0.828 |
| Non-adherence Med/diet | 33 (2.0%) | 2.08 (1.05–4.14) 0.036 | 1.23 (0.61–2.49) 0.565 |
| Medication worsening HF | 2 (1.8%) | 1.82 (0.40–8.32) 0.440 | 1.07 (0.23–4.95) 0.933 |
| Pneumonia/infection | 67 (3.7%) | 3.87 (2.04–7.35) <0.001 | 2.32 (1.20–4.50) 0.013 |
| Pulmonary embolism | 3 (3.2%) | 3.34 (0.91–12.18) 0.068 | 2.29 (0.62–8.50) 0.217 |
| WRF | 20 (4.7%) | 5.00 (2.36–10.46) <0.001 | 2.50 (1.16–5.41) 0.019 |
| Other | 40 (3.1%) | 3.22 (1.64–6.31) 0.001 | 1.62 (0.81–3.23) 0.175 |
| None | 101 (2.3%) | 2.40 (1.28–4.48) 0.006 | 1.43 (0.75–2.73) 0.280 |
| Unknown | 92 (2.5%) | 2.56 (1.37–4.83) 0.003 | 1.31 (0.68–2.51)0.417 |
| 1-year post-discharge mortality | |||
| Univariable | Multivariable | ||
| Deaths per 100 py (95%) | HR (95%CI) p-value | HR (95%CI) p-value | |
| Uncontrolled hypertension | 14.2 (12.0 to 16.7) | Ref | Ref |
| ACS/MI | 21.3 (19.4 to 23.5) | 1.49 (1.24–1.81) <0.001 | 1.30 (1.07–1.58) 0.009 |
| Arrhythmia | 19.2 (17.1 to 21.5) | 1.35 (1.10–1.65) 0.004 | 1.12 (0.91–1.39) 0.268 |
| Non-adherence Med/diet | 24.6 (22.0 to 27.4) | 1.72 (1.41–2.09) <0.001 | 1.30 (1.07–1.60) 0.010 |
| Medication worsening HF | 28.8 (19.6 to 42.2) | 2.01 (1.32–3.05) 0.001 | 1.52 (0.99–2.32) 0.050 |
| Pneumonia/infection | 22.9 (20.5 to 25.4) | 1.60 (1.32–1.95) <0.001 | 1.21 (0.98–1.48) 0.072 |
| Pulmonary embolism | 19.2 (11.6 to 31.8) | 1.35 (0.79–2.30) 0.272 | 1.29 (0.76–2.21) 0.344 |
| WRF | 37.5 (31.4 to 44.9) | 2.60 (2.04–3.33) <0.001 | 1.62 (1.26–2.09) <0.001 |
| Other | 25.0 (22.2 to 28.3) | 1.75 (1.43–2.15) <0.001 | 1.34 (1.09–1.66) 0.006 |
| None | 20.5 (19.1 to 22.0) | 1.44 (1.20–1.72) <0.001 | 1.20 (0.99–1.44) 0.058 |
| Unknown | 25.4 (23.7 to 27.3) | 1.77 (1.48–2.13) <0.001 | 1.31 (1.09–1.58) 0.004 |
Abbreviations: ACS/MI, acute coronary syndrome/myocardial infarction; HF, Heart failure; WRF, Worsening Renal Function; OR, odds ratio; CI, confidence interval; py, patient years
Multivariable model: age, sex, hypertension, atrial fibrillation, copd/asthma, chronic kidney disease, coronary artery disease, ACEi/ARB at discharge, MRAs at discharge, beta-blockers at discharge and diuretics at discharge.
The 1-year post-discharge mortality incidence in Table 2 ranged from 14.2 (95%CI 12.0–16.7) per 100 patient-years for patients admitted with uncontrolled hypertension to 37.5 (95% 31.4–44.9) per 100 patient-years for patients admitted due to WRF. After correction for confounders, relative differences remained highly significant: compared with patients hospitalised with uncontrolled hypertension, patients hospitalised with ACS/MI, non-adherence to diet/medication and WRF had worse 1-year mortality.
Discussion
In this detailed global analysis on the frequency of precipitants for AHF and their association with post-discharge mortality according to region, LVEF-subtype, and HF-admission type, we found 1) important regional variations in the frequency of precipitants according to LVEF subtype and HF presentation, a significant proportion of which could be mitigated with outpatient surveillance and treatment; and 2) the type of precipitant was significantly associated with in-hospital and post-discharge mortality. Together, our results identify regional specific treatment targets to prevent HF admission and premature death potentially.
Previous reports on the frequency and association with mortality precipitants for patients hospitalised for AHF were predominantly from North America2,3,6–8,12,13,19, and Western Europe4,5,9–12,14. Despite being home to most of the world’s population, limited studies investigated precipitants to AHF according to LVEF subtype and HF presentation in Asia or the Eastern Mediterranean and African region. Our data suggest ACS/MI is the most common precipitant for AHF regardless of geographic region. Close to a quarter of new-onset HF patients from Southeast Asia and the Eastern Mediterranean and African region were admitted with ACS/MI, compared to only 7% in Western Europe or 6% in North America. Differences in risk factors and access to care might explain this finding. Risk factors like smoking and hypertension are increasing in prevalence in southeast Asia and Northern Africa20,21. The PURE registry showed that revascularisation and treatment for coronary artery disease were less common in lower-income regions, especially in Southeast Asia, possibly precipitating new-onset HF due to untreated and unrecognised ACS/MI22. Together, this suggests that prevantative measures targeting artherosclerosis like smoking cessation or statins could also prevent hospitalizations for AHF in these regions. Our data suggest cardiac arrhythmia is a common precipitant for patients being admitted for new-onset HF, especially in regions like Western Europe with a high prevalence of atrial fibrillation17 and elderly patients. This suggests that interventions targeting arrhythmias, like remote monitoring with hand held devices or smart watches might be beneficial. Uncontrolled hypertension was common in patients with new-onset HF from North America. The large proportion of African Americans (~50%) enrolled in North America in REPORT-HF, in whom hypertension is a particularly important issue23, likely explains this observation. Therefore, more aggressive treatment of hypertension in these patients might prevent or delay acute hospitalization for HF. Close to a quarter of patients in North America with DCHF and 18% of patients in the Eastern Mediterranean or African region were admitted due to non-adherence to diet or medication, highlighting significant areas for improving HF self-care care and education. Our previous study highlighted that non-adherence to diet/medication was a substantial issue in African-Americans23. Pneumonia/respiratory tract infection was a common precipitant in the Eastern Mediterranean or North African region and the Western Pacific. This observation might be explained by regional differences in strategies regarding preventive medicine, including flu vaccination.24
To our knowledge, this is the first global report on differences in precipitants according to LVEF subtype. A previous study from the GWTG-HF database found pneumonia was relatively common among patients with HFpEF and was associated with a longer in-hospital stay.8 Consistent with findings from the GWTG-HF19 study, patients with HFrEF frequently were admitted due to non-adherence. Similar to results from the CHARM program, uncontrolled hypertension was a more critical precipitant in patients with HFpEF than HFrEF12.
Differences in precipitants were associated with in-hospital and post-discharge mortality. The cumulative (in-hospital and post-discharge) mortality ranged from 13% in patients with uncontrolled hypertension to 30% in patients with WRF. These results confirm findings from previous studies in North America and Western Europe2,8,9,14,25. In OPTIMIZE-HF, uncontrolled hypertension was associated with the lowest mortality and patients with ACS/MI or WRF had the worst mortality2. In BIOSTAT-CHF, WRF was most strongly associated with a composite of all-cause death or hospitalisation for HF14. We extend these previous results to all global regions included in REPORT-HF: we did not find a significant interaction between precipitants and region, precipitants and LVEF subtype, or precipitants and HF presentation, for in-hospital or one-year mortality. Our results suggest a unifying global message to identify precipitants amenable to outpatient interventions. A considerable proportion of precipitants in REPORT-HF could be addressed with preventative strategies, including ACS/MI, non-adherence to diet/medication and pneumonia/respiratory tract infection. In many lower-income regions in REPORT-HF, like Southeast Asia, the Western Pacific and the Eastern Mediterranean or Africa, these possibly amenable precipitants constituted a significant proportion of cases, highlighting the unmet need for improved treatment and prevention of hospitalisation for AHF.
Study limitations
Our findings describe important global patterns concerning precipitants and their association with in-hospital and post-discharge mortality. However, they should be interpreted considering the following limitations. There is a possible selection bias of the participating sites. Furthermore, patients had to provide informed consent. Sicker patients might not have been included in this registry, which might explain the low in-hospital mortality rates. Patients could be hospitalised due to more than one precipitant. The local investigator determined the main precipitant. Lastly, a significant proportion of precipitants were either classified as ‘unknown’ or ‘none’. This might reflect differences in local documentation or could be a consequence of the study protocol only allowing one precipitant.
Conclusion
Precipitants for AHF hospitalisations vary by region and according to HF presentation (DCHF versus new-onset HF) and LVEF subtype. ACS, pneumonia or respiratory tract infection and uncontrolled hypertension were common precipitants in patients with new-onset HF globally, especially in Southeast Asia and the Eastern Mediterranean or Africa. In HFpEF, uncontrolled hypertension was more common than in HFrEF. Precipitants significantly predicted in-hospital and post-discharge mortality globally, irrespective of HF presentation or LVEF subtype. Knowledge of modifiable risk factors precipitating hospitalisation for AHF highlights possible region-specific targets for preventing AHF hospital admission and prevention of early mortality.
Supplementary Material
Acknowledgments
The REPORT-HF registry steering committee and investigators thank Novartis for their generosity in funding this large observational registry.
Declaration of interests
GF reports research grants from the EU; committee fees from Novartis related to REPORT-HF; and committee member in trials or registries, sponsored by Servier, Boehringer Ingelheim, Medtronic and Vifor. CEA reports grants and personal fees from Novartis; she further acknowledges non-financial support from the University Hospital Würzburg and the Comprehensive Heart Failure Center Würzburg, and grant support from the German Ministry for Education and Research. UD reports research support from AstraZeneca, Pfizer, Boehringer Ingelheim, Vifor, Roche Diagnostics and Boston Scientific and speaker’s honoraria and consultancies from AstraZeneca, Novartis and Amgen MH received honoraria as a lecturer from Novartis, Aventis, Amgen, Merck Sharp & Dohme, AstraZeneca, and Merck. SPC reports research grants from National Institutes of Health, Agency for Healthcare Research and Quality, American Heart Association, Patient-Centered Outcomes Research Institute and consulting fees from Novartis, Medtronic, Vixiar, and Ortho Clinical. MG, AS, and AO are employed by Novartis. JGFC reports grants and personal fees from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Philips, Stealth Biopharmaceuticals, and Torrent Pharmaceuticals; grants, personal fees, and non-financial support from Medtronic, Novartis, and Vifor; personal fees from Myokardia, Sanofi, Servier, and Abbott; and grants and non-financial support from Pharmacosmos and PharmaNord. SVP reports personal fees from Laboratorios Bago, Laboratorios Ferrer, Abbott–St Jude, Novartis, United Therapeutics, Janssen Cilag, and Servier; and grants from Tecnologia Disruptiva San Pablo. CSPL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; has served as consultant or on the advisory board, steering committee, or executive committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global, Radcliffe Group, and Corpus; and serves as cofounder and non-executive director of eKo.ai. All other authors declare no competing interests.
REPORT-HF: was funded by Novartis. CSL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global LLC, Radcliffe Group Ltd and Corpus; and serves as cofounder & non-executive director of eKo.ai. JT has received personal grants and speaker fees from Olink proteomics and Roche diagnostics. GF reports research grants from the European Union. Committee fees from Novartis related to REPORT-HF. Committee member in trials and/or registries sponsored by Servier, BI, Medtronic, Vifor. CEA reports grants, personal fees and other from Novartis. She further acknowledges non-financial support from the University Hospital Würzburg, non-financial support from the Comprehensive Heart Failure Center Würzburg and grant support from the German Ministry for Education and Research (BMBF). UD reports research support from Astra Zeneca, Pfizer, Boehringer Ingelheim, Vifor, Roche Diagnostics, Boston Scientific and speakeŕs honoraria and consultancies from Astra Zeneca, Novartis and Amgen. MH received honoraria as a lecturer from Novartis, Aventis, Amgen, MSD, Astrazeneca and Merck. SPC reports research grants from NIH, AHRQ, AHA, PCORI and consulting fees from Novartis, Medtronic, Vixiar and Ortho Clinical. MG, AS and AO are employed by Novartis. JGFC reports grants and personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Bayer, grants and personal fees from Bristol Myers Squibb, grants, personal fees and non-financial support from Medtronic, personal fees from Myokardia, grants, personal fees and non-financial support from Novartis, grants and personal fees from Philips, grants and non-financial support from Pharmacosmos, grants and non-financial support from PharmaNord, personal fees from Sanofi, personal fees from Servier, grants and personal fees from Stealth Biopharmaceuticals, grants and personal fees from Torrent Pharmaceuticals, grants, personal fees and non-financial support from Vifor, personal fees from Abbott.
List of abbreviations
- ACEi
Angiotensin-Converting Enzyme-Inhibitors
- ACS/MI
Acute Coronary Syndrome / Myocardial Infarction
- AHF
Acute Heart Failure
- ARB
Angiotensin Receptor Blockers
- DCHF
Decompensated Chronic Heart Failure
- eGFR
estimated Glomerular Filtration Rate
- HF
Heart Failure
- HFmrEF
Heart Failure with mid-range Ejection Fraction
- HFpEF
Heart Failure with preserved Ejection Fraction
- HFrEF
Heart Failure with reduced Ejection Fraction
- LVEF
Left Ventricular Ejection Fraction
- MRA
Mineralocorticoid Receptor Antagonist
- NYHA
New York Heart Association
- WRF
Worsening Renal Function
Footnotes
Conflicts of interest. The remaining authors have nothing to disclose.
Bibliography
- 1.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Filippatos G, McMurray JJV, Aboyans V, Achenbach S, Agewall S, Al-Attar N, Atherton JJ, Bauersachs J, Camm AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Pieper K, Sun JL, Yancy CW, Young JB. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: Findings from OPTIMIZE-HF. Arch Intern Med 2008;168:847–854. [DOI] [PubMed] [Google Scholar]
- 3.Chin MH, Goldman L. Factors contributing to the hospitalization of patients with congestive heart failure. Am J Public Health American Public Health Association; 1997;87:643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opasich C, Febo O, Riccardi PG, Traversi E, Forni G, Pinna G, Pozzoli M, Riccardi R, Mortara A, Sanarico M, Cobelli F, Tavazzi L. Concomitant factors of decompensation in chronic heart failure. Am J Cardiol 1996;78:354–357. [DOI] [PubMed] [Google Scholar]
- 5.Michalsen A, König G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart; 1998;80:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med 1988;148:2013–2016. [PubMed] [Google Scholar]
- 7.Tsuyuki RT, McKelvie RS, Arnold JMO, Avezum AJ. Barretto ACP, Carvalho ACC, Isaac DL, Kitching AD, Piegas LS, Teo KK, Yusuf S. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med 2001;161:2337–2342. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Hear Fail; 2016;4:464–472. [DOI] [PubMed] [Google Scholar]
- 9.Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi DJ, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen-Solal A, Iwashyna TJ, Mebazaa A. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail 2017;19:201–208. [DOI] [PubMed] [Google Scholar]
- 10.Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574–1585. [DOI] [PubMed] [Google Scholar]
- 11.Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo-Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez-Fernandez S, Miani D, Filippatos G, Maggioni AP. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail; 2017;19:1242–1254. [DOI] [PubMed] [Google Scholar]
- 12.Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur J Heart Fail; 2018;20:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambardekar AV, Fonarow GC, Hernandez AF, Pan W, Yancy CW, Krantz MJ. Characteristics and in-hospital outcomes for nonadherent patients with heart failure: Findings from Get With The Guidelines-Heart Failure (GWTG-HF). Am Heart J 2009;158:644–652. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Voors AA, Girerd N, Billotte M, Anker SD, Cleland JG, Lang CC, Ng LL, van Veldhuisen DJ, Dickstein K, Metra M, Duarte K, Rossignol P, Zannad F, Ferreira JP. Heart failure etiologies and clinical factors precipitating for worsening heart failure: Findings from BIOSTAT-CHF. Eur J Intern Med; 2020;71:62–69. [DOI] [PubMed] [Google Scholar]
- 15.Filippatos G, Khan SS, Ambrosy AP, Cleland JGF, Collins SP, Lam CSP, Angermann CE, Ertl G, Dahlström U, Hu D, Dickstein K, Perrone SV., Ghadanfar M,Bermann G, Noe A, Schweizer A, Maier T, Gheorghiade M. International REgistry to assess medical Practice with lOngitudinal obseRvation for Treatment of Heart Failure (REPORT-HF): Rationale for and design of a global registry. Eur J Heart Fail 2015;17:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippatos G, Angermann CE, Cleland JGF, Lam CSP, Dahlström U, Dickstein K, Ertl G, Hassanein M, Hart KW, Lindsell CJ, Perrone SV., Guerin T, Ghadanfar M, Schweizer A, Obergfell A, Collins SP. Global Differences in Characteristics, Precipitants, and Initial Management of Patients Presenting with Acute Heart Failure. JAMA Cardiol; 2020;5:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, Tay WT, Dickstein K, Ertl G, Hassanein M, Perrone SV., Ghadanfar M, Schweizer A, Obergfell A, Lam CSP, Filippatos G, Collins SP. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Heal; 2020;8:e411–e422. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA - J. Am. Med. Assoc. 2013. p. 2191–2194. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Hear Fail; 2016;4:464–472. [DOI] [PubMed] [Google Scholar]
- 20.Reitsma MB, Kendrick PJ, Ababneh E, Abbafati C, Abbasi-Kangevari M, Abdoli A, Abedi A, Abhilash ES, Abila DB, Aboyans V, Abu-Rmeileh NM, Adebayo OM, Advani SM, Aghaali M, Ahinkorah BO, Ahmad S, Ahmadi K, Ahmed H, Aji B, Akunna CJ, Al-Aly Z, Alanzi TM, Alhabib KF, Ali L, Alif SM, Alipour V, Aljunid SM, Alla F, Allebeck P, Alvis-Guzman N, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet; 2021;397:2337–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, Sophiea MK, Iurilli ML, Lhoste VP, Cowan MJ, Savin S, Woodward M, Balanova Y, Cifkova R, Damasceno A, Elliott P, Farzadfar F, He J, Ikeda N, Kengne AP, Khang Y-H, Kim HC, Laxmaiah A, Lin H-H, Maira PM, Miranda JJ, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet; 2021;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, Avezum A, Bengtsson Boström K, Chifamba J, Gulec S, Gupta R, Igumbor EU, Iqbal R, Ismail N, Joseph P, Kaur M, Khatib R, Kruger IM, Lamelas P, Lanas F, Lear SA, Li W, Wang C, Quiang D, Wang Y, Lopez-Jaramillo P, Mohammadifard N, Mohan V, Mony PK, Poirier P, et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob Heal; 2019;7:e748–e760. [DOI] [PubMed] [Google Scholar]
- 23.Tromp J, Ouwerkerk W, Cleland JGF, Chandramouli C, Angermann CE, Dahlstrom U, Ertl G, Hassanein M, Perrone SV., Ghadanfar M, Schweizer A, Obergfell A, Dickstein K, Collins SP, Filippatos G, Lam CS. A Global Perspective of Racial Differences and Outcomes in Patients Presenting with Acute Heart Failure. Am Heart J; 2021; [DOI] [PubMed] [Google Scholar]
- 24.Modin D, Jørgensen ME, Gislason G, Jensen JS, Køber L, Claggett B, Hegde SM, Solomon SD, Torp-Pedersen C, Biering-Sørensen T. Influenza Vaccine in Heart Failure: Cumulative Number of Vaccinations, Frequency, Timing, and Survival: A Danish Nationwide Cohort Study. Circulation; 2019;139:575–586. [DOI] [PubMed] [Google Scholar]
- 25.Berkovitch A, Maor E, Sabbag A, Chernomordik F, Elis A, Arbel Y, Goldenberg I, Grossman E, Klempfner R. Precipitating factors for acute heart failure hospitalization and long-term survival. Med (United States); 2015;94:e2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.