Abstract
A missense mutation (A391T) in SLC39A8 is strongly associated with schizophrenia in genomic studies, though the molecular connection to the brain is unknown. Human carriers of A391T have reduced serum manganese, altered plasma glycosylation, and brain MRI changes consistent with altered metal transport. Here, using a knock-in mouse model homozygous for A391T, we show that the schizophrenia-associated variant changes protein glycosylation in the brain. Glycosylation of Asn residues in glycoproteins (N-glycosylation) was most significantly impaired, with effects differing between regions. RNAseq analysis showed negligible regional variation, consistent with changes in the activity of glycosylation enzymes rather than gene expression. Finally, nearly one third of detected glycoproteins were differentially N-glycosylated in the cortex, including members of several pathways previously implicated in schizophrenia, such as cell adhesion molecules and neurotransmitter receptors that are expressed across all cell types. These findings provide a mechanistic link between a risk allele and potentially reversible biochemical changes in the brain, furthering our molecular understanding of the pathophysiology of schizophrenia and a novel opportunity for therapeutic development.
Introduction
Psychiatric disorders are heterogeneous in nearly every aspect - symptoms, diagnosis, treatment, and course - but a common feature is the lack of clear disease mechanisms to guide therapeutic development. Schizophrenia is a diagnostic classification for individuals afflicted with a combination of cognitive dysfunction, social withdrawal, functional regression, and psychotic symptoms [1]. Between 0.5 - 1.0% of the population are affected and can experience a lifetime of significant disability, economic and social hardships, increased mortality and decreased life expectancy [2]. Age of onset is often during adolescence and early adulthood, suggesting changes in brain development and maturation play a critical role [3]. Studies of disease mechanisms in schizophrenia were stimulated by the observation that dopamine antagonists provide some level of symptomatic relief, which led to decades of research on neurotransmitter signaling [4-6]. Despite this progress, no treatment with a novel mechanism of action has been approved in nearly 50 years.
Genetic liability is the strongest epidemiologic contributor to overall risk for schizophrenia [7]. Current evidence for schizophrenia and most psychiatric disorders is consistent with a polygenic inheritance pattern, with disease contributions from many common variants of small effect and few rare mutations of large effect [8-10], as originally hypothesized over fifty years ago [11]. A major advance came in 2014 with the publication of over 100 loci associated with schizophrenia through GWAS [12], with the most recent study implicating 270 common loci [13]. Most associated variants are in the non-coding region of the genome, requiring careful follow-up studies to map the affected gene and pathophysiologic consequence. The first successful example of such a study involves the complement component 4 (C4) genes (C4A and C4B) near the MHC locus [14]. Individuals with schizophrenia have increased expression of brain C4A, and the schizophrenia-associated allele increases C4A expression in the brain. Global deletion of the C4 gene in mice, which corresponds to both C4A and C4B genes in humans, leads to decreased complement deposition and synaptic pruning during post-natal development [14], providing a potential link between a locus associated with schizophrenia and a molecular pathway implicated in schizophrenia pathogenesis [15]. A recent study demonstrated that whereas overexpression of human C4A in the C4 null mouse could rescue the deficient pruning, it also resulted in abnormal synaptic structure and behavioral abnormalities [16].
For the many other loci, understanding their connection to schizophrenia is a considerable challenge, particularly for those whose roles in the brain are unclear. One such example is a subset of genes involved in protein glycosylation, the post- and co-translational attachment of carbohydrates to asparagine (N-glycans) or serine, threonine and tyrosine (O-glycans) residues, respectively [17, 18]. In addition to at least 5 genes encoding glycosylation enzymes, a variant in the manganese (Mn) transporter SLC39A8 is strongly associated with schizophrenia [12, 19]. The most recent GWAS data have strengthened the association of SLC39A8 and identified several additional glycosylation enzymes associated with schizophrenia [13]. A large number of glycosyltransferases require Mn as an obligatory co-factor [20-22], and numerous case series have described congenital disorders of glycosylation (CDG) caused by a near total absence of Mn in severe homozygous mutation carriers in SLC39A8 [23-26]. SLC39A8 is expressed at relatively low levels in most tissues of the body including the brain [27, 28], and single-cell sequencing data from mouse brain suggest the transporter is enriched within endothelial cells [29].
The schizophrenia-associated variant in SLC39A8 (rs13107325) is of particular interest as it corresponds to a missense mutation (A391T). A391T was confirmed with high probability (>99%) as the causal variant within SLC39A8, and has the most significant disease-associated p-value of any coding variant (3.50e−21), and the largest effect size among all common variants (Odds Ratio, OR: 1.147) [13]. A391T is present in ~8% of the general population, with increased prevalence in European lineages and near absence in Asian and African lineages [30]. In addition to its role in schizophrenia, rs13107325 is one of the most pleotropic variants in the genome and is associated with dozens of traits through GWAS [31], including several neuropsychiatric phenotypes and a decrease in serum Mn levels [32-36]. Like other common variants, the relative effect of rs13107325 on most phenotypes is small, e.g., reducing the concentration of serum Mn by ~10% [19, 36] and increasing the odds ratio of schizophrenia from 1.0 to 1.15 in heterozygous carriers [12, 13].
The pathophysiologic connection between A391T and most conditions, however, remain conjectural [30, 37-40]. We recently described serum glycosylation changes in homozygous SLC39A8 mutation carriers who presented with symptoms of a glycosylation disorder but who were missed by conventional diagnostic methods. Dysglycosylation differences were observed in heterozygous carriers as well, consistent with a dominant effect of SLC39A8-CDG alleles [41]. Lin and colleagues describe an increase of one precursor N-glycan in plasma from a small number of homozygous A391T carriers, suggestive of hypogalactosylation [42]. We subsequently reported a detailed N-glycomic analysis of plasma glycoproteins from homozygous and heterozygotes A391T carriers, and identified dysglycosylation in both genotypes characterized by reduced complexity and branching of large N-glycans, primarily those synthesized by the liver [43]. In that study, we also described a previously unappreciated sex-dependent effect of the A391T variant on glycosylation, with males showing a larger alteration in plasma N-glycosylation compared to females [43]. Although plasma glycosylation is an important peripheral marker for enzymatic activity and disease state in some scenarios, it is unlikely to adequately reflect changes in the brain glycome. Interestingly, analysis of brain MRI data from the UK Biobank of A391T mutation carriers identified a difference in the T2w:T1w ratio in several regions, consistent with an effect of A391T on brain metal transport [43].
Here, to further understand the molecular role of the schizophrenia-associated variant in the brain, we investigated a murine model that expresses the homologous human mutation A391T in the endogenous Slc39a8 (A393T) [44]. By applying several methods that we optimized for analysis of protein glycosylation in the brain [45], in addition to glycoproteomics and a novel method for glycan quantification, we present a comprehensive profile of dysglycosylation in the adult A391T homozygous mouse brain. These results are evidence of biochemical changes in the brain directly caused by a schizophrenia risk variant and which are potentially reversible.
Results
A391T changes brain N-glycosylation of male mice in a region-specific manner
We conducted studies in a mouse model expressing the schizophrenia-associated human variant A391T within the murine gene (Ala 393 in Slc39a8). The animals do not exhibit an overt neurobehavioral or developmental phenotype and they reproduce at the expected frequencies [44]; a similar mouse line shows minimal behavioral differences [46]. We hypothesized that A391T would decrease Mn concentration and subsequent activity of Mn-dependent glycosyltransferases in the brain. The brain N-glycome is dramatically different from other organs, with an abundance of glycans traditionally assumed to be immature or intermediate structures, e.g. high mannose- and hybrid-type N-glycans, along with a scarcity of large, branched complex-type N-glycans containing the usual terminal modifications of galactose and sialic acid, as found in most other tissues [45].
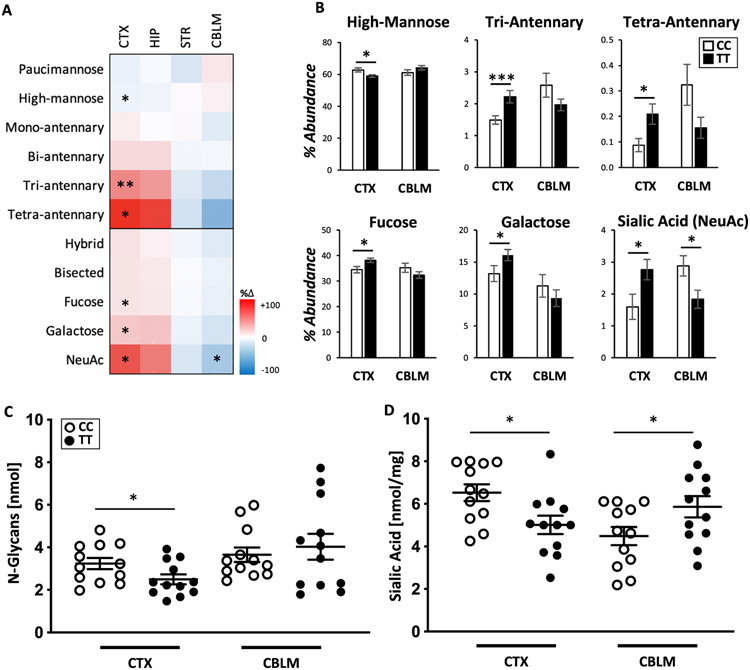
We first analyzed the effect of A391T on the relative abundance of N-glycans in four independent brain regions (frontal cortex, hippocampus, striatum and cerebellum) of male and female adult mice with semi-quantitative MALDI-TOF N-glycomics following Peptide N-Glycosidase (PNGase F) release and permethylation. Homozygous mutant mice (TT) were compared to wild-type littermate controls (CC) at 12-weeks of age (Table 1). We identified bidirectional and region-specific changes in N-glycosylation arising from the A391T mutation, with a larger effect consistently observed in males (Fig. 1A, Fig. S1). High-mannose N-glycans, which make up the majority of N-glycans in the brain, were significantly reduced in the male TT cortex compared to CC controls, with a corresponding increase in the relative abundance of complex-type tri- and tetra-antennary N-glycans, which make up a small fraction of the brain N-glycome (Fig. 1B). In the TT cortex there was also an increased relative abundance of N-glycans containing fucose, galactose, and sialic acid. In the hippocampus, which is evolutionarily most related to the cortex [47], and has the most similar N-glycome profile among brain regions [45], the pattern of N-glycosylation changes in TT mice were similar, though did not reach statistical significance, while N-glycans in the striatum appeared mostly unaffected. In contrast, the cerebellum of TT mice overall displayed an opposite pattern of N-glycome changes compared to cortex, with a significant reduction in sialylated structures (Fig. 1A, 1B). Several individual types of N-glycans differed significantly between genotypes, the majority of which were in the cortex and cerebellum (Table S1). Full descriptions of individual N-glycan characteristics and abundance by region and sex are provided in supplementary material (Table S1, S2). Analyses of N-glycans from cortex and cerebellum at 4-weeks of age in a separate cohort failed to detect any difference between CC and TT mice, suggesting that the changes in glycans due to A391T occur during or after adolescence (Table S3).
Table 1. Categorical analysis of protein N-glycans revealed region-specific changes in male A391T brain.
Data presented as mean percent abundance +/− SEM. Unpaired t-tests performed for each category assuming unequal variance.
| Cortex | Hippocampus | Striatum | Cerebellum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycan Category | CC | TT | p-value | CC | TT | p-value | CC | TT | p-value | CC | TT | p-value |
| Paucimannose | 3.1 ± 0.2 | 2.9 ± 0.2 | 0.5 | 2.6 ± 0.3 | 2.5 ± 0.4 | 0.8 | 2.6 ± 0.3 | 2.2 ± 0.2 | 0.2 | 1.17 ± 0.12 | 1.28 ± 0.08 | 0.5 |
| High-mannose | 63 ± 1 | 59 ± 1 | 0.03* | 61 ± 2 | 58 ± 10 | 0.2 | 61 ± 1 | 62 ± 1 | 0.5 | 61 ± 2 | 64 ± 1 | 0.2 |
| Mono-antennary | 19.0 ± 0.8 | 20.2 ± 0.3 | 0.2 | 19.2 ± 0.7 | 19.3 ± 3.5 | 0.9 | 18.9 ± 0.4 | 19.2 ± 0.5 | 0.6 | 13.0 ± 0.6 | 11 ± 0.5 | 0.06 |
| Bi-antennary | 13.5 ± 0.9 | 15.5 ± 0.7 | 0.1 | 15 ± 1 | 18 ± 3 | 0.2 | 15.4 ± 0.4 | 14.9 ± 0.7 | 0.5 | 21.8 ± 0.9 | 21.0 ± 0.9 | 0.6 |
| Tri-antennary | 1.4 ± 0.1 | 2.2 ± 0.2 | 0.009** | 1.6 ± 0.3 | 2.2 ± 0.4 | 0.08 | 1.7 ± 0.1 | 1.5 ± 2 | 0.4 | 2.6 ± 0.4 | 2.0 ± 0.2 | 0.2 |
| Tetra-antennary | 0.09 ± 0.03 | 0.20 ± 0.04 | 0.03* | 0.12 ± 0.04 | 0.23 ± 0.04 | 0.09 | 0.19 ± 0.05 | 0.16 ± 0.06 | 0.7 | 0.32 ± 0.08 | 0.16 ± 0.04 | 0.1 |
| Hybrid | 4.8 ± 0.2 | 5.4 ± 0.1 | 0.05 | 6.2 ± 0.2 | 6.5 ± 1.2 | 0.3 | 6.6 ± 0.4 | 6.3 ± 0.4 | 0.7 | 8.3 ± 0.4 | 7.3 ± 0.4 | 0.09 |
| Bisected | 30 ± 1 | 33 ± 1 | 0.08 | 32 ± 2 | 35 ± 6 | 0.3 | 32.1 ± 0.6 | 31.7 ± 1.1 | 0.8 | 34 ± 2 | 32 ± 1 | 0.3 |
| Galactose | 13.2 ± 0.6 | 16.1 ± 0.9 | 0.02* | 12.5 ± 1.1 | 15.5 ± 0.9 | 0.06 | 13.6 ± 0.6 | 12.7 ± 1.1 | 0.5 | 11.3 ± 1.1 | 9.3 ± 0.8 | 0.2 |
| Fucose | 35 ± 1 | 38 ± 1 | 0.04* | 36 ± 2 | 39 ± 1 | 0.2 | 36 ± 1 | 35 ± 1 | 0.6 | 35 ± 2 | 32 ± 1 | 0.2 |
| Sialic Acid | 1.6 ± 0.4 | 2.8 ± 0.3 | 0.04* | 2.1 ± 0.5 | 3.2 ± 0.3 | 0.08 | 2.1 ± 0.5 | 1.8 ± 0.6 | 0.7 | 2.9 ± 0.3 | 1.8 ± 0.3 | 0.03* |
Fig. 1. A391T alters brain N-glycans in a region-dependent manner.
A) MALDI-TOF MS analysis of the relative N-glycan abundances from the cortex (CTX), hippocampus (HIP), striatum (STR), and cerebellum (CBLM) of male mice. Data presented as a heat map of percent change in glycan abundance comparing TT mice to CC controls. B) Categorical analysis of MALDI N-glycome data for TT cortex showed a relative decrease of high mannose structures and an increase complex N-glycans (tri- and tetra-antennary), fucosylated, galactosylated, and sialylated N-glycans, while the cerebellum showed an opposite trend with a significant decrease in sialylated N-glycans. CC = 6, TT = 8 for each region in A and B. C) Quantitative analysis of brain N-glycans using F-MAPA derivatization showed a significant decrease in cortex of TT male mice consistent with a global downregulation of N-glycosylation. D) Sialic acid concentration of total glycoproteins (N- and O-), showed a decrease in TT mice, consistent with a decreased absolute abundance of sialic acid. Data presented as mean +/− SEM for B, C, and D. For C and D, N = 4 male mice per group, measured in triplicate. Individual data points shown, with brackets representing mean +/− SEM. p-value *< 0.05, **< 0.01.
O-Mannose glycans are increased in A391T cortex
We are unaware of previous studies of O-glycosylation in relation to SLC39A8, e.g. N-acetylgalactosamine (O-GalNAc) or mannose (O-Man) linked to Ser/Thr residues. After PNGase F treatment, O-glycans were removed from serine and threonine residues through β-elimination, followed by permethylation and MALDI-TOF MS. This generated interpretable O-glycan spectra with 26 unique O-glycans in a smaller subset compared to our analysis of N-glycosylation, as some samples failed to produce a detectable signal above the noise threshold (Table S4). Across the four brain regions, TT cortex showed a significant increase in the relative abundance of O-Man-type glycans to O-GalNAc-type glycans (Fig. S2, Table S5). The majority of O-glycosylation features were unchanged, with only small differences noted in structures of low abundance (cerebellar NeuGc content), or the group with the smallest sample size (hippocampus). A greater proportion of O-Man-type glycans was also present in the small number of female TT cortex samples analyzed, though this difference fell short of significance (Fig. S2, Table S5).
Total N-glycan concentration is decreased in A391T cortex
MALDI-TOF MS provides a semi-quantitative analysis following normalization within a sample. For example, if the absolute concentration of every glycan was uniformly reduced by 50%, but the relative abundance of each glycan remained the same, such a change would be undetectable using standard MALDI-TOF MS techniques. As such, we explored several approaches for directly quantifying N-glycan levels.
Glycoprotein blotting of brain lysate using the lectin Concanavalin A (ConA), which has an affinity for the core α-mannose structure commonly present in many N-glycans, was decreased ~10% in both cortex and cerebellum of A391T mice but fell short of significance (Fig. S3). This was not surprising given the limited sensitivity of quantitative western blotting and the small changes predicted for a common genetic variant. As such, we developed a novel method for quantifying N-glycans based on the fluorescent-linker F-MAPA [48]. Following release with PNGase F, N-glycans were derivatized at their reducing ends with F-MAPA [48], resulting in addition of one fluorescent molecule to each N-glycan (Fig. S3). After purification, derivatized N-glycans from the isolated glycoprotein fetuin, as well as mouse serum and brain, produced linear fluorescent signals across a broad range of protein concentrations, highlighting the quantitative utility of this assay across several tissues (Fig. S3).
Measurement of derivatized N-glycans with F-MAPA showed a decrease in the cortex of TT mice, indicating a reduced absolute concentration of N-glycans (Fig. 2C). To address the discrepancy between the semi-quantitative increase in highly branched/sialylated N-glycans identified by glycomics and the fully quantitative decrease in total N-glycans in TT cortex, we quantified total sialic acid in glycoconjugates in different brain fractions between genotypes. Consistent with prior studies [49], the bulk of sialic acid in both cortex and cerebellum was contained in glycolipids and removed by extraction with chloroform and methanol (Fig. S4). The remaining sialic acid is divided roughly in half between glycoprotein N- and O-glycans based on PNGase F release of total N-glycans. These results, in combination with our striking observation that only a small fraction (~2%) of the N-glycan pool is sialylated, whereas most of the O-glycan pool (~90%) is sialylated [45], indicate that N-glycans are several-fold more abundant in the brain relative to O-glycans. No difference in sialic acid concentration was detected in total brain lysate (glycolipids and glycoproteins) between CC and TT genotypes (Fig. S4). After removal of glycolipids, the amount of sialic acid in the glycoprotein fraction was reduced in TT cortex and increased in TT cerebellum (Fig. 1D). This difference was eliminated with PNGase F treatment (Fig. S4), indicating that the difference in amounts of sialic acid concentration was specific to N-glycans. The differing sialic acid amounts could be explained by either a change in the number of sialic acid monosaccharides per glycan, or a change in the absolute number of glycans present. When normalized for glycan concentration using results from F-MAPA-derivatized N-glycans of the same sample, we detected no difference between region or genotype in the amount of sialic acid per N-glycan (Fig. S4). Taken together, these results demonstrate that the absolute pool of N-glycans was decreased in the cortex of TT mice, and while the relative amount of sialylated N-glycans appears increased via MALDI-TOF glycomics, this minor but key N-glycan modification was also decreased in A391T cortex.
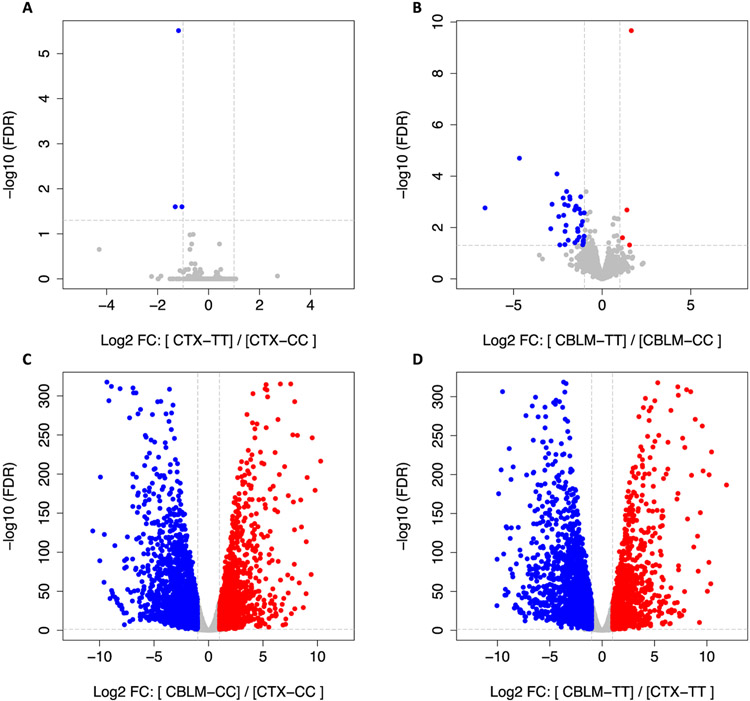
Fig. 2. RNAseq analysis identifies minimal gene expression changes in the cortex and cerebellum of A391T mice.
Volcano plots comparing levels of over 14,000 transcripts in cortex (A) and cerebellum (B) identifies minimal gene expression changes in A391T mice based on genotype. Volcano plots between cortex and cerebellum in both wild-type (C) and A391T (D) mice are consistent with known regional differences in gene expression. Significance thresholds for fold change (log2, 2-fold change) and adjusted p-value (−log10) are shown as grey dotted lines on the on the x- and y-axis, respectively. Increased (red) and decreased (blue) transcripts are shown. N = 4 male mice per group.
Differences in gene expression do not account for glycosylation changes in A391T cortex
We employed global RNAseq to determine if changes in gene expression contributed to glycosylation differences observed in A391T cortex and cerebellum. Minimal differences in gene expression were detected among the ~14,000 transcripts measured between CC and TT cortex (Fig. 2A) and cerebellum (Fig. 2B) by RNAseq, with only 3 and 42 transcripts being differentially expressed on a transcriptome-wide level, respectively (Table S6). Of note, there was no significant difference in expression of glycosyltransferases, glycosylhydrolases, or Slc39a8 in either region between genotypes. Comparison between cortex and cerebellum within each genotype (Fig. 2C, Fig. 2D) showed nearly identical regional expression patterns, consistent with known regional differences (Fig S5).
N-glycoprotein levels are altered in A391T cortex
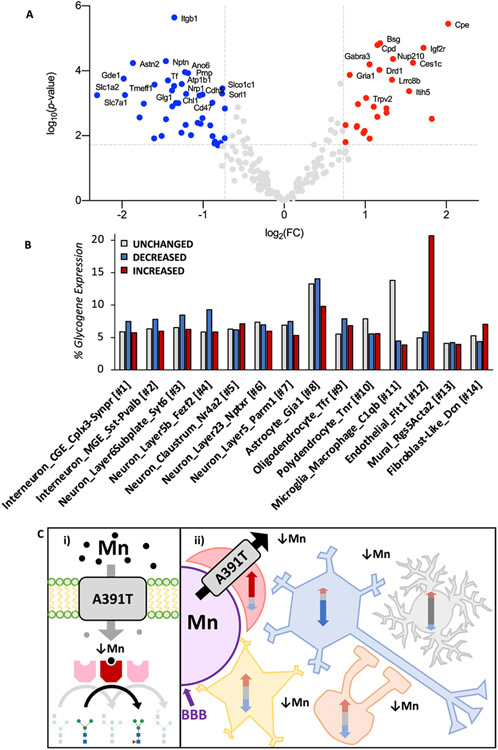
The above glycomic and RNAseq data indicate that there are changes in the enzymatic activity of glycosyltransferases in A391T mice rather than their expression. However, this does not provide information on altered target protein glycosylation or glycoprotein abundance, which could be affected in a broad or target-specific manner. To obtain quantitative data on a protein-specific level, we used hydrophilic interaction liquid chromatography (HILIC) and multi-lectin affinity enrichment to isolate tryptic N-glycopeptides from the cortex of male CC and TT mice, followed by analysis with LC-MS/MS. A total of 517 peptide sequence fragments (PSMs) with 554 unique N-glycosylation consensus sites were detected, originating from 210 distinct glycoproteins, here termed N-glycoproteins (Table S7). All samples produced similar abundance distributions (Fig. S6). Near uniform N-glycoprotein levels were seen in the five CC samples, and TT mice showed a similar pattern in 4 of 5 samples, with one sample appearing as an outlier from both CC and TT groupings (Fig. S6). Repeat genotyping confirmed that both homozygous mutation status and sex were accurate, and the sample was included in subsequent analyses. Of the 210 N-glycoproteins, 66 had significantly different levels in TT cortex, with 41 decreased and 25 increased based on an adjusted p-value < 0.05 and fold change > 2.0 (Fig. 3A).
Fig. 3. A391T mouse cortex has altered N-glycosylation of one third of glycoproteins originating from all cell types.
A) Volcano plot of differentially N-glycosylated proteins in A391T cortex. Increased (red) and decreased (blue) N-glycoproteins are shown, with the names of the top 30 included. Significance thresholds for fold change (log2, 2-fold change) and adjusted p-value (−log10) are shown as grey dotted lines on the on the x- and y-axis, respectively. N = 5 mice per genotype. B) Single-cell mouse brain expression data for genes encoding differentially N-glycosylated proteins in TT mice were downloaded from www.dropviz.org and compared to all detected N-glycoproteins. After normalization, the sum of clustered transcripts per 100,000 for each distinct cell type is illustrated between groups including unchanged (grey), decreased (blue), and increased (red) glycopeptides. Differentially N-glycosylated proteins are expressed across all cell types despite the restricted expression pattern of Slc39a8 in endothelial cells. C) Model – i) A391T impairs Mn transport and its availability as a co-factor for Mn-dependent glycosyltransferases (red), resulting in changes to the protein glycome. ii) In the cortex, Slc39a8 expression is limited to endothelial cells (red), where A391T impairs Mn transport across the blood brain barrier (BBB, purple) resulting in protein glycosylation changes across all cell types (endothelial cells, red; neurons, blue; microglia, gray: astrocytes, yellow: and oligodendrocytes, orange). Glycoprotein levels can be decreased (blue arrow), unchanged (gray arrow), or increased (red arrow) within different cell types.
Differentially N-glycosylated proteins are enriched in unique cellular components and cell clusters of origin, including glycoproteins implicated in schizophrenia
For further insight into the molecular changes of A391T cortex, we analyzed the list of differentially N-glycosylated proteins using the FUMA GENE2FUNC platform [50]. A list of genes from all detected N-glycoproteins in control cortex (210 genes) and those unchanged (144 genes), decreased (41 genes), and increased (25 genes) in A391T cortex were used as input compared to protein coding genes, all of which were enriched in brain tissue as expected. Gene Ontology (GO) cellular component analysis, based on the PANTHER classification system [51], again confirmed significant enrichment of predictable components in all lists including dendrite, synapse, and neuron, known locations of N-glycoproteins in the brain. N-glycoproteins decreased in A391T cortex showed a unique enrichment of several components of the plasma membrane and genes involved in ion transport, while N-glycoproteins increased in A391T cortex included multiple components of the ER and Golgi networks (Fig. S7). Though there was overlap and the significance of enrichment decreased with the size of the list, these findings suggest the A391T variant has different effects on glycosylation of individual proteins within the same tissue.
Single-cell profiling provides detailed information on the cellular specificity of gene expression in the mouse brain [29]. Slc39a8 is expressed at relatively low levels in the mouse brain, with expression concentrated in endothelial cells, trace levels in microglia and fibroblasts, and a near total absence in other cell types (Fig. S8). To determine the cell types of origin for differentially N-glycosylated proteins in A391T cortex, we downloaded single cell expression data from mouse frontal cortex using the DropViz platform [29] for the lists of all, unchanged, decreased, and increased glycoproteins. Total transcript levels were summed within each of the 14 unique cell-type clusters; these data indicated that differentially glycosylated proteins were expressed in all subtypes, with a similar overall pattern observed between all detected glycoproteins in control mice and those unchanged in TT cortex (Fig. S8). Normalization of transcript levels for comparison across lists highlighted several differences in the cell cluster of origin, including an enrichment of glycoproteins with decreased levels in interneurons and neurons, and an enrichment of glycoproteins with increased levels in endothelial cells, where the majority of Slc39a8 is expressed, while the majority of microglia glycoproteins were unchanged (Fig. 3B). Based on these data we propose a potential model where Slc39a8 serves as a gatekeeper for Mn movement in the brain, with the A391T variant impairing its function in endothelial cells, which results in glycosylation changes across the brain and cell types (Fig. 3C).
Among the 66 differentially glycosylated proteins, several are striking given their function in pathways previously implicated in schizophrenia, including glutamate and GABA neurotransmission (Gria1, Slc1a2, Gabbr1), dopamine signaling (Drd1), and cell adhesion/migration (Cdh5, Ncam1, Ncan, Pcdh19, Reln) (Table 2). In addition, Brinp2 and Gria1 were differentially N-glycosylated in A391T cortex and are encoded by genes associated with schizophrenia through GWAS [12].
Table 2. Select N-glycoproteins linked to schizophrenia pathogenesis with altered levels in A391T cortex.
A subset of glycoproteins with altered N-glycosylation in the A391T cortex are highlighted, including cell adhesion molecules, proteins involved in neurotransmission, and two genes associated with schizophrenia by GWAS*. HGNC - HUGO Gene Nomenclature Committee abbreviation. FC - Fold Change. Adj.P.Val - Adjusted p-value.
| HGNC | Protein | FC | adj.P.Val |
|---|---|---|---|
| Slc1a2 | Excitatory amino acid transporter 2 | −4.90 | 0.004 |
| Ncam1 | Neural cell adhesion molecule 1 | −2.51 | 0.007 |
| Reln | Reelin | −2.42 | 0.006 |
| Gabbr1 | Gamma-aminobutyric acid type B receptor subunit 1 | −1.97 | 0.017 |
| Cdh5 | Cadherin-5 | −1.89 | 0.004 |
| Pcdh19 | Protocadherin-19 | −1.58 | 0.044 |
| Gria1* | Glutamate receptor 1 | 1.61 | 0.002 |
| Ncan | Neurocan core protein | 1.86 | 0.025 |
| Brinp2* | BMP/retinoic acid-inducible neural-specific protein 2 | 2.10 | 0.013 |
| Drd1 | D(1A) dopamine receptor | 2.15 | 0.002 |
Discussion
Here we employed several techniques to demonstrate that in a murine model the schizophrenia risk allele in SLC39A8 reduces the products of Mn-dependent glycosyltransferases in the brain, with a relatively small effect size as predicted for common genetic variants. Rodent studies are not a model for schizophrenia but can be useful to understand downstream effects of risk variants. In contrast to previous studies which have primarily knocked out or overexpressed schizophrenia risk genes in mice, these results show that the biologically relevant risk allele in SLC39A8 alters key molecular pathways of protein glycosylation in the brain.
Protein glycosylation changes in A391T mice were most clearly observed in the N-glycosylation pathway, which contains several Mn-binding glycosyltransferases that contain the metal binding DxD motifs (aspartate-any amino acid-aspartate), such as β-1,4-galactosyltransferases and N-acetylglucosaminyltransferases [20, 21]. In addition, oligosaccharyltransferase (OST), which transfers N-glycans from dolichol anchored precursors to asparagine residues of nascently transcribed proteins in the ER lumen, also requires Mn for activity [52, 53]. Though there was an increase in the relative abundance of tri- and tetra-antennary N-glycans in the cortex of A391T mice by MALDI, quantitative studies using F-MAPA confirmed a reduction of total and sialylated N-glycans. These results are complementary to those in humans, in which a reduction in the relative abundance of tri- and tetra-antennary N-glycans in plasma of A391T carriers and SLC39A8-CDG was noted, consistent with decreased activity of N-glycan branching enzymes in the liver [43]. Nearly all N-glycans in plasma glycoproteins are of the complex type with more than two branches (> 95%); by contrast, these species comprise the minority of N-glycans in the brain, ranging from 15% in the cortex and 25% in the cerebellum [45]. We posit that the relative increase in tri- and tetra-antennary N-glycans in cortex by MALDI reflects normalization of results back to 100% total abundance. Thus, in the setting of a total quantitative decrease of N-glycans, low abundance N-glycans would appear to be increased. Nonetheless, the overall pattern of N-glycans in the cortex was altered by the A391T mutation.
A391T mice also exhibited changes in brain O-glycosylation, specifically a decrease in the abundance of O-GalNAc glycans relative to O-Man glycans. The twenty N-acetylgalactosaminyltransferases (GALNTs), which initiate O-GalNAc-type glycosylation, contain a Mn-binding DxH domain and could be affected by changes in Mn levels [54]. Several factors may contribute to the detection of more N-glycan differences compared to O-glycans in the brain, including their greater relative abundance, increased glycan diversity, and the availability of a highly specific and efficient enzyme to remove N-glycans thus facilitating their study. Studies of protein glycosylation in the brain have primarily focused on the role of N-glycosylation in develop and synaptic transmission [55]. O-Mannose glycans have been investigated in the context of congenital muscular dystrophies resulting from mutations in this pathway [56], however, the functional role and carrier proteins of O-GalNAc glycans in the brain are largely unknown.
Transcriptomics from cortex and cerebellum did not identify changes that could account for the observed glycosylation differences in A391T brain, as the RNA levels of glycosyltransferases, glycosylhydrolases, and the differentially glycosylated targets were unchanged. This observation is consistent with human data, as rs13107325 is not an eQTL for any gene in the brain including SLC39A8 [27], and suggests the glycosylation changes caused by A391T result from alterations of enzyme activity and not protein expression levels.
Glycosylation changes associated with the A391T variant support the converging evidence that dysglycosylation is involved in schizophrenia pathogenesis. Numerous glycosylation enzymes are associated with schizophrenia through GWAS [12, 19] and the number continues to expand, with many glycosylation genes included in the most recent list of prioritized schizophrenia risk genes [13], including several specific to N-glycosylation (MAN2A1, ALG12, MANBA) and O-glycosylation (GALNT10, GALNT17/WBSCR17, TMTC1). In addition, the schizophrenia associated variant at 22q13.2 affects the expression of NAGA, an alpha-N-acetylgalactosaminidase which regulates dendritic spine density [57, 58]. Many genes associated with schizophrenia identified through genetic studies encode proteins that are critically regulated by glycosylation [19]; post-mortem studies have consistently documented brain dysglycosylation in individuals with schizophrenia as discussed in recent reviews [59, 60]. Glycoproteomic changes further illustrate the connection between schizophrenia and glycosylation, as several risk genes and proteins involved in critical developmental and functional pathways in the brain are altered in the cortex of A391T mice. Pathway analyses of the differentially glycosylated proteins suggest that proteins with increased or decreased levels are enriched in distinct pathways and cellular components, consistent with the complexity of glycosylation as a non-template-based protein modification. We predict glycosylation enzymes exert altered substrate specificity and binding in the setting of homeostatic changes in Mn levels, increasing the glycosylation of some priority targets while decreasing others.
SLC39A8 is expressed in most tissues at low levels [28], and in the mouse brain Slc39a8 is restricted primarily to endothelial cells [29]. We observed changes in glycoproteins originating from, though not necessarily restricted to, all cell clusters in the brain. Interestingly, glycoproteins whose levels are increased in TT cortex are enriched in endothelial cells, which express the highest level of Slc39a8, while decreased glycoproteins are enriched in several neuronal clusters, with a minimal change in microglia. It is possible that the biological effects of A391T are related to its peripheral expression, but in either circumstance the final consequence is altered brain glycosylation. A recent report suggests that A391T impairs zinc transport in neurons and changes surface expression of glutamate receptors [61]. However, in that study, the A391T mutant protein was observed to be overexpressed in cultured pyramidal neurons, where the transporter is not expressed at significant levels in the adult brain. SLC39A8 deficiency appears most consistent with a disorder of Mn transport, as CDG cases with undetectable Mn have near normal zinc levels [23, 25, 26] as do human A391T carriers [36, 43] and mouse A391T models [44, 62]. Local zinc transport may be affected by A391T, but we predict that altered receptor expression at the membrane and increased schizophrenia risk from A391T is conferred through Mn deficiency and changes in glycosylation.
Human A391T carriers have alterations in paramagnetic ion transport (Fe and Mn) in the brain, including the lateral putamen and internal globus pallidus [43]. These two basal ganglia structures are very close in proximity, highlighting the importance regional differences play in the effect of common variants. Paramagnetic ion changes were less pronounced in the cortex and cerebellum, with homozygous A391T carriers showing a more diffuse differences primarily within white matter tracks. Mn toxicity, commonly through inhalational occupational exposure, results in accumulation within the basal ganglia and neurotoxicity referred to as manganism, which presents with a Parkinsonian phenotype [63]. Interestingly, the A391T variant is protective against Parkinson disease [33], further supporting a functional role of this variant on Mn transport in the brain. Mn deficiency or excess does not appear to relate directly to schizophrenia risk, as other SNPs which affect Mn levels have no effect in either direction. Thus, the increased risk for schizophrenia in A391T carriers is likely related to when and where the variant results in insufficient availability of this cofactor.
We confirmed our prior observation of a sex-dependent effect of A391T on glycosylation, with human and mouse males showing a larger effect compared to females [43]. Recent investigations of gastrointestinal phenotypes associated with A391T confirmed this sex effect, which may in part be explained by lower Mn levels in male mice [44, 62]. Interestingly, sexually dimorphic responses to synaptic spine density have been demonstrated in mouse models of Mn toxicity [64] along with impaired N-glycan branching [65]. It is important to note, however, that the increased risk for schizophrenia in A391T carriers is equal in males and females [13]. We suspect that A391T confers risk through altered Mn transport during brain development; given the complexity of Mn homeostasis and transport in the brain [66], the effects may normalize over time despite vulnerable pathways during critical windows having been already affected. Interestingly, we did not observe glycosylation changes in male A391T mice at 4 weeks of age, which roughly corresponds with early adolescence in humans, raising the possibility that Mn supplementation after 4 weeks of age might prevent these changes.
In sum, our findings provide mechanistic support that the SLC39A8 schizophrenia risk allele alters glycosylation in the brain and provide several nodes of connection between known genetic risk and disease pathways. Mendelian disorders of glycosylation caused by severe hypofunctioning alleles of SLC39A8 are treated with supplementation of glycosylation precursors and manganese [23, 24]. Although the A391T variant is likely to have a much smaller effect on SLC39A8 function, supplementation with similar factors during critical periods of brain development may lessen the risk of neuropsychiatric conditions in carriers, and targeting glycosylation more broadly in schizophrenia could represent a novel pathway for therapeutic development.
Methods
A391T homozygous knock-in mutant mice
were generated by gene targeting in embryonic stem cells derived from C57BL/6J mice and genotyped as previously described [44]. Mice from both sexes were used in this study and were 12 weeks old at the time of tissue harvest unless otherwise noted, with group sizes of n = 4-8, consistent with prior studies on effect of the variant [44]. Mice were fed Basal Diet chow (Cat# 5755) with 65 ppm Mn from TestDiet (St. Louis, MO, USA). Mouse genotype was blinded during the experiments and decoded only for the final analysis. No live animals were used in this study. All mice were housed and maintained in accordance with the guidelines established by the Animal Care and Use Committee at Massachusetts General Hospital under protocol #2003N000158.
Sample Preparation and Glycomic Analysis
were performed as previously described in detail in a related manuscript [45].
F-MAPA derivation and quantification of released N-glycans.
All chemical reagents were HPLC grade purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise noted, and all reagents were freshly prepared on the day of analysis. PNGase F (New England Biolabs, #P0704) released N-glycans from 1 mg of brain protein lysate were isolated as previously described [45]. Purified N-glycans were derivatized using the fluorescent linker fluorenylmethyloxycarbonate-3-(methoxyamino)propylamine (F-MAPA) as previously described [48]. In brief, purified N-glycans (not permethylated) were resuspended in 80 μL of dimethylsulfoxide/acetic acid at 7:3 (v/v) ratio containing 0.25 M F-MAPA, 0.25 M sodium acetate, and 0.5 mM 2-amino-5-methoxybenzoic acid and incubated for 2 hours at 65°C with gentle rotation at 100 RPM. Ten (10) volumes of ethyl acetate were added, vortexed, and incubated at −20°C for 20 minutes to precipitate N-glycan/F-MAPA conjugates and isolated by centrifugation at 10,000 RPM for 15 minutes. After removal of the supernatant, the pellet containing F-MAPA derived N-glycans was dissolved in 300 μL of Milli-Q filtered water and loaded on a C18 Sep-Pak columns (200 mg) preconditioned with acetonitrile (ACN) then water. Bound F-MAPA-linked N-glycans were washed with 5 mL of water, eluted with 2 mL of 30% ACN, and lyophilized. Purified F-MAPA linked N-glycans were then resuspended in 200 μL of water and detected using a fluorescence spectrophotometer (SpectraMax i3x, Molecular Devices) with excitation wavelength of 265 nm and emission wavelength 315 nm and quantified (SoftMax Pro Software, v7.0.3).
Sialic acid quantification.
Absolute quantification of sialic acid in different glycoconjugate fractions was performed using the Sialic Acid (NANA) Assay Kit (Ab83375, Abcam, Cambridge, MA) according to the manufacturer’s instructions. Briefly, 30 μg of protein or 600 pmol of N-glycan/F-MAPA were incubated with the sialidase NeuA for 4 h at 37°C. The reaction was stopped by boiling the samples for 5 minutes at 95°C, followed by centrifugation at 10,000 RPM for 5 minutes, and the supernatant (containing free sialic acid) was transferred to a 96-wells plate. NANA reactions were performed by adding 25 μL reaction mix (22 μL assay buffer, 1 μL sialic acid converting enzyme, 1 μL sialic acid development mix, 1 μL sialic acid probe) to each supernatant, and incubated for 30 minutes at room temperature in the dark. The fluorescence of each well was then quantified by spectrophotometry (Excitation: 535 nm, Emission: 587 nm) as described above.
RNA Sequencing
was performed as described performed as previously described in related manuscript [45]. In brief, RNA from snap frozen cortex and cerebellum was purified using the RNeasy Lipid Tissue Mini Kit (QIAGEN, 74804). RNA-seq libraries were prepared from total RNA using polyA selection followed by the NEBNext Ultra II Directional RNA Library Prep Kit protocol (New England Biolabs, E7760S). Sequencing was performed on Illumina HiSeq 2500 instrument resulting in approximately 30 million of 50 bp reads per sample. Sequencing reads were mapped in a splice-aware fashion to the mouse reference transcriptome (mm9 assembly) using STAR [67]. Read counts over transcripts were calculated using HTSeq based on the Ensembl annotation for GRCm37/mm9 assembly and presented as Transcripts Per Million (TPM) [68].
N-Glycoproteomic analysis.
Brain cortex samples were lysed in 1 mL lysis buffer (20 mM HEPES pH 7.9, 1% SDS, and protease inhibitor) with protein concentrations determined by BCA assay (Thermo Fisher Scientific, Cat # 23225). Reduction and alkylation were performed as previously described50. S-trap digestion was performed according to the manufacturer's instructions (ProtiFi, Cat # C02-mini), resulting in ~0.6 mg tryptic peptides per sample. To one half of tryptic peptides, 15 mg of HILIC beads (PolyLC, Cat # BMHY0502) pre-activated with 0.1% trifluoroacetic acid (TFA) were added to make a 1:50 peptide-to-beads mass ratio. The samples were vortexed in binding buffer (0.1% TFA, 19.9% water, 80% ACN) for 1 h at room temperature to allow N-glycopeptides to bind to beads. The unbound peptides were washed with 150 μL binding buffer 6 times, and N-glycopeptides were eluted by washing the beads with (150 μL) 0.1% TFA for 5 times. Finally, 2 μL PNGase F (500U/μL) (NEB # P0704) was added to the elution buffer and the samples were incubated for 3 h at 37 °C, followed by lyophilization. To the other half of tryptic peptides, multi-lectin enrichment was performed based on a previously reported protocol with some modifications51. Briefly, tryptic peptides were mixed with a mixture of lectins (90 μg ConA, 90 μg WGA, 36 μg RCA120 in 2X binding buffer (Sigma-Aldrich, Cat L7886, Cat L9640, and Cat L7647) and transferred to a 30 kDa filter (Pall Corporation, Cat # OD030C34), incubated at room temperature for 1 h and unbound peptides were eluted by centrifuging at 14,000 g for 10 minutes. N-glycopeptides were washed with 200 μL binding solution four times and 50 μL digest buffer of 50 mM triethylammonium bicarbonate (TAEB) twice. Finally, 2 μL PNGase F (NEB # P0704) was added to the filter and incubated for 3 h at 37 °C. The deglycosylated N-glycopeptides were eluted with 2 χ 50 μL digest buffer. N-glycopeptides from two enrichment methods were combined and desalted by C18 tips (Thermo Fisher Scientific, Cat #87784) following the manufacturer’s instructions and resuspended in 30 μL 100 mM TEAB buffer. For each sample, 5 μL the corresponding amine-based tandem mass tag (TMT) 10-plex reagents (10 μg/μL) (Thermo Fisher Scientific, Cat # 90406) was added and incubated for 1 hour at room temperature. The reactions were quenched with 2 μL 5% hydroxylamine solution (Oakwood Chemical, Cat # 069272), and the combined mixture was lyophilized. High-pH fractionation was done according to the manufacturer's instructions (Thermo Fisher Scientific, Cat # 84868), which resulted in 15 independent fractions.
A Thermo Scientific EASY-nLC 1000 system was coupled to a Thermo Scientific Orbitrap Fusion Tribrid with a nano-electrospray ion source. Mobile phases A and B were water with 0.1% formic acid (v/v) and ACN with 0.1% formic acid (v/v), respectively. For each fraction, peptides were separated with a linear gradient from 4 to 32% B within 45 min, followed by an increase to 50% B within 10 minutes and further to 98% B within 10 minutes, and re-equilibration. The instrument parameters were set as follows: survey scans of peptide precursors were performed at 120K FWHM resolution over a m/z range of 410-1800. HCD fragmentation was performed on the top 10 most abundant precursors exhibiting a charge state from 2 to 5 at a resolving power setting of 50K and fragmentation energy of 37% in the Orbitrap. CID fragmentation was applied with 35% collision energy and resulting fragments detected using the normal scan rate in the ion trap.
The raw data was processed using Proteome Discoverer 2.4 (Thermo Fisher Scientific). Data was searched against the UniProt/SwissProt mouse (Mus musculus) protein database (May 30, 2019, 17,372 total entries) and contaminant proteins using Sequest HT and Byonic algorithms. Searches were performed with the following guidelines: spectra with a signal-to-noise ratio greater than 1.5; trypsin as enzyme, 2 missed cleavages; variable oxidation on methionine residues (15.995 Da) and deamidation on asparagine (0.984 Da); static carboxyamidomethylation of cysteine residues (57.021 Da), static TMT labeling (229.163 Da) at lysine residues and peptide N-termini; 10 ppm mass error tolerance on precursor ions, and 0.02 Da mass error on fragment ions. Data were filtered with a peptide-to-spectrum match (PSM) of 1% FDR using Percolator. The TMT reporter ions were quantified using the Reporter Ions Quantifier with total peptide normalization. For the obtained PSMs, the data was further filtered with the following guidelines: confidence is high; PSM ambiguity is unambiguous; modifications contain deamidated; exclude all contaminant proteins. Consensus sequence filtering was applied using a string match module with filtering the Subcellular location for Cell membrane, Endoplasmic reticulum, Golgi apparatus, Extracellular, and Secreted. Most of the empty Abundances, if any, were filled in with minimum noise level computed by taking the minimum for each channel in CC and TT cortex. Reducing these minimum groups, five channels for CC and five for TT, required taking the absolute maximum in CC and TT and the absolute minimum in CC and TT. Subsequently, 2000 centroids were generated at random from these 4 points and a minimum noise level was generated using a K-means clustering method. If there were few missing values the maximum and minimum of the Abundances for that PSM in the CC and TT groups were used to generate centroid data. If only one Abundance was missing, the instance was filled with the geometric mean of the PSM for CC or TT. If all Abundances were missing for CC and TT, the PSM was removed. Based on the algorithm in this script of an empty abundance missing completely at random, missing not at random, or missing at random, any valid instances were filled with the appropriate method described above. A limitation of the log-ratio of raw MS data is the dependence on variation within each channel for CC and TT. Applied here was a VSN normalization computed on the imputed matrix using a robust variant of the maximum-likelihood estimator for an additive-multiplicative error model and affine calibration. The model incorporates dependence of the variance on the mean intensity and a variance stabilizing data transformation. Fold Change (FC) was presented after generalized log transformation and normalization of the raw ratio between genotypes to fit a linear model, with log2FC values corresponding to a raw fold change > 2 equaling +/− 0.73.
Glycoprotein pathway analyses
was performed using the GENE2FUNC tool of the FUMA platform [50] on October 30th, 2020. Input lists included all detected N-glycoproteins in CC cortex, unchanged N-glycoproteins in TT cortex, decreased N-glycoproteins in TT cortex, and increased N-glycoproteins in TT cortex, based on corrected p-values < 0.05 and fold change >2.0 compared to protein coding genes as background. Output results for gene set enrichment of GO cellular components [51] were presented.
Glycoprotein cell of origin analyses
was performed using publicly available data downloaded from the DropViz database [29] on October 30th, 2020. Single cell expression data from each gene in the above-described lists of N-glycoproteins were downloaded from frontal cortex containing 14 distinct cell clusters and exponentially natural log transformed to approximate transcripts per 100,000 in each cluster. Transcripts levels for each cluster were then summed for the above described lists of detected glycoproteins (all, unchanged, decreased, increased) and normalized within each group to generate the relative abundance of glycogene expression from each cluster.
Statistical Analysis.
For glycomic studies, the abundance of individual glycans and glycan classes were compared between CC and TT mice using unpaired t-tests assuming unequal variance. For RNAseq results, the EdgeR method was used for differential expression analysis with gene cutoffs of 2-fold change in expression value and false discovery rates (FDR) below 0.05 as previously described. For glycoproteomics results, a linear model was fitted to the expression data for CC and TT cortex, and t-statistics were computed by empirical Bayes moderation of standard errors towards a common value. The heatmap clustering method used was based on average linkage.
Supplementary Material
Acknowledgements:
This work was supported by a foundation grant from the Stanley Center for Psychiatric Research at the Broad Institute of Harvard/MIT (awarded to RGM) and NIH grants P41GM103694 and R24GM137763 (awarded to RDC), and P30DK040561 (awarded to R.I.S). RGM is supported by T32MH112485. CMW is supported by NIH NCI U01CA242098.
Footnotes
Competing Interests: R.J.X. is a cofounder and equity holder of Celsius Therapeutics and Jnana Therapeutics and consultant to Novartis. These companies did not provide support for this work. J.W.S. is a member of the Scientific Advisory Board of Sensorium Therapeutics and has received honoraria for an internal seminar at Biogen, Inc and Tempus Labs. These companies did not provide support for this work. The remaining authors declare no competing interests.
Data Availability.
The data generated during this study are included in this published article and its supplementary information files, and available from the corresponding author on reasonable request. Raw MS glycomics data files are available through GlycoPOST [69] with the dataset identifier GPST000213. The RNAseq data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus [70, 71] and are accessible through GEO Series accession number GSE184516. The MS data were deposited at the ProteomeXchange Consortium [72] via the PRIDE partner repository and are available with the identifier PXD021632.
References
- 1.Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381:1753–1761. [DOI] [PubMed] [Google Scholar]
- 2.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet Lond Engl. 2016;388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485–515. [DOI] [PubMed] [Google Scholar]
- 4.Enna SJ, Bennett JP, Burt DR, Creese I, Snyder SH. Stereospecificity of interaction of neuroleptic drugs with neurotransmitters and correlation with clinical potency. Nature. 1976;263:338–341. [DOI] [PubMed] [Google Scholar]
- 5.van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492–494. [PubMed] [Google Scholar]
- 6.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol Oxf Engl. 2015;29:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avramopoulos D Recent Advances in the Genetics of Schizophrenia. Mol Neuropsychiatry. 2018;4:35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan PF, Geschwind DH. Defining the Genetic, Genomic, Cellular, and Diagnostic Architectures of Psychiatric Disorders. Cell. 2019;177:162–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, Kendler KS. Psychiatric genetics and the structure of psychopathology. Mol Psychiatry. 2019;24:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PH, Anttila V, Won H, Feng Y-CA, Rosenthal J, Zhu Z, et al. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell. 2019;179:1469–1482.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci U S A. 1967;58:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schizophrenia Working Group of the Psychiatric Genomics Consortium, Ripke S, Walters JT, O’Donovan MC. Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. Genetic and Genomic Medicine; 2020. [Google Scholar]
- 14.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Presumey J, Bialas AR, Carroll MC . Complement System in Neural Synapse Elimination in Development and Disease. Adv. Immunol, vol. 135, Elsevier; 2017. p. 53–79. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz M, Yalcin E, Presumey J, Aw E, Ma M, Whelan CW, et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci. 2021;24:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. , editors. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 18.Varki A Biological roles of glycans. Glycobiology. 2017;27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mealer RG, Williams SE, Daly MJ, Scolnick EM, Cummings RD, Smoller JW. Glycobiology and schizophrenia: a biological hypothesis emerging from genomic research. Mol Psychiatry. 2020. 6 May 2020. 10.1038/s41380-020-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishnan B, Ramasamy V, Qasba PK. Structural Snapshots of β-1,4-Galactosyltransferase-I Along the Kinetic Pathway. J Mol Biol. 2006;357:1619–1633. [DOI] [PubMed] [Google Scholar]
- 21.Breton C, Šnajdrová L, Jeanneau C, Koča J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16:29R–37R. [DOI] [PubMed] [Google Scholar]
- 22.Chang A, Singh S, Phillips GN, Thorson JS. Glycosyltransferase structural biology and its role in the design of catalysts for glycosylation. Curr Opin Biotechnol. 2011;22:800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Hogrebe M, Grüneberg M, DuChesne I, von der Heiden AL, Reunert J, et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am J Hum Genet. 2015;97:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Hogrebe M, Fobker M, Brackmann R, Fiedler B, Reunert J, et al. SLC39A8 deficiency: biochemical correction and major clinical improvement by manganese therapy. Genet Med Off J Am Coll Med Genet. 2017. 27 July 2017. 10.1038/gim.2017.106. [DOI] [PubMed] [Google Scholar]
- 25.Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am J Hum Genet. 2015;97:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riley LG, Cowley MJ, Gayevskiy V, Roscioli T, Thorburn DR, Prelog K, et al. A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J Inherit Metab Dis. 2017;40:261–269. [DOI] [PubMed] [Google Scholar]
- 27.Aguet F, Barbeira AN, Bonazzola R, Brown A, Castel SE, Jo B, et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Genetics; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell. 2018; 174:1015–1030.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Wu D-D, Yao Y-G, Huo Y-X, Liu J-W, Su B, et al. Recent Positive Selection Drives the Expansion of a Schizophrenia Risk Nonsynonymous Variant at SLC39A8 in Europeans. Schizophr Bull. 2016;42:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill WD, Marioni RE, Maghzian O, Ritchie SJ, Hagenaars SP, McIntosh AM, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 2019;24:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019;22:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Q, Chen Q, Wang W, Desrivières S, Quinlan EB, Jia T, et al. Association of a Schizophrenia-Risk Nonsynonymous Variant With Putamen Volume in Adolescents: A Voxelwise and Genome-Wide Association Study. JAMA Psychiatry. 2019;76:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng E, Lind PM, Lindgren C, Ingelsson E, Mahajan A, Morris A, et al. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum Mol Genet. 2015;24:4739–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zang Z-S, Xu Y-M, Lau ATY. Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8). Toxicol Res. 2016;5:987–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujishiro H, Himeno S. New Insights into the Roles of ZIP8, a Cadmium and Manganese Transporter, and Its Relation to Human Diseases. Biol Pharm Bull. 2019;42:1076–1082. [DOI] [PubMed] [Google Scholar]
- 39.Nebert DW, Liu Z. SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside. Hum Genomics. 2019;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costas J The highly pleiotropic gene SLC39A8 as an opportunity to gain insight into the molecular pathogenesis of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2018;177:274–283. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Mealer RG, Elias AF, Hoffmann S, Grüneberg M, Biskup S, et al. N-glycome analysis detects dysglycosylation missed by conventional methods in SLC39A8 deficiency. J Inherit Metab Dis. 2020. 27 August 2020. 10.1002/jimd.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin W, Vann DR, Doulias P-T, Wang T, Landesberg G, Li X, et al. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J Clin Invest. 2017;127:2407–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mealer RG, Jenkins BG, Chen C-Y, Daly MJ, Ge T, Lehoux S, et al. The schizophrenia risk locus in SLC39A8 alters brain metal transport and plasma glycosylation. Sci Rep. 2020; 10:13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakata T, Creasey EA, Kadoki M, Lin H, Selig MK, Yao J, et al. A missense variant in SLC39A8 confers risk for Crohn’s disease by disrupting manganese homeostasis and intestinal barrier integrity. Proc Natl Acad Sci U S A. 2020. 2 November 2020. 10.1073/pnas.2014742117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams SE, Noel M, Lehoux S, Cetinbas M, Xavier RJ, Sadreyev RI, et al. Mammalian brain glycoproteins exhibit diminished glycan complexity compared to other tissues. Nat Commun. 2022; 13:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terrillion CE, Kang B, Melia JMP. Behavioral phenotyping of Zip8 393T-KI mice for in vivo study of schizophrenia pathogenesis. Genomics; 2021. [Google Scholar]
- 47.Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360:881–888. [DOI] [PubMed] [Google Scholar]
- 48.Wei M, McKitrick TR, Mehta AY, Gao C, Jia N, McQuillan AM, et al. Novel Reversible Fluorescent Glycan Linker for Functional Glycomics. Bioconjug Chem. 2019;30:2897–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das RC, Heath EC. Dolichyldiphosphoryloligosaccharide--protein oligosaccharyltransferase; solubilization, purification, and properties. Proc Natl Acad Sci U S A. 1980;77:3811–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shrimal S, Gilmore R. Oligosaccharyltransferase structures provide novel insight into the mechanism of asparagine-linked glycosylation in prokaryotic and eukaryotic cells. Glycobiology. 2019;29:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott H, Panin VM. The role of protein N-glycosylation in neural transmission. Glycobiology. 2014;24:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Live D, Wells L, Boons G-J. Dissecting the Molecular Basis of the Role of the O-Mannosylation Pathway in Disease: α-Dystroglycan and Forms of Muscular Dystrophy. ChemBioChem. 2013;14:2392–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Ma C, Li W, Yang Y, Li X, Liu J, et al. A missense variant in NDUFA6 confers schizophrenia risk by affecting YY1 binding and NAGA expression. Mol Psychiatry. 2021. 30 April 2021. 10.1038/s41380-021-01125-x. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Li S, Liu J, Huo Y, Luo X-J. The schizophrenia susceptibility gene NAGA regulates dendritic spine density: further evidence for the dendritic spine pathology of schizophrenia. Mol Psychiatry. 2021. 10 August 2021. 10.1038/s41380-021-01261-4. [DOI] [PubMed] [Google Scholar]
- 59.Williams SE, Mealer RG, Scolnick EM, Smoller JW, Cummings RD. Aberrant glycosylation in schizophrenia: a review of 25 years of post-mortem brain studies. Mol Psychiatry. 2020. 13 May 2020. 10.1038/s41380-020-0761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller TM, Meador-Woodruff JH. Post-translational protein modifications in schizophrenia. Npj Schizophr. 2020;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng WC, Reinhart V, Lanz TA, Weber ML, Pang J, Le KXV, et al. Schizophrenia-associated SLC39A8 polymorphism is a loss-of-function allele altering glutamate receptor and innate immune signaling. Transl Psychiatry. 2021;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sunuwar L, Frkatović A, Sharapov S, Wang Q, Neu HM, Wu X, et al. Pleiotropic ZIP8 A391T implicates abnormal manganese homeostasis in complex human disease. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwakye GF, Paoliello MMB, Mukhopadhyay S, Bowman AB, Aschner M. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int J Environ Res Public Health. 2015;12:7519–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feldcamp L, Doucet J-S, Pawling J, Fadel MP, Fletcher PJ, Maunder R, et al. Mgat5 modulates the effect of early life stress on adult behavior and physical health in mice. Behav Brain Res. 2016;312:253–264. [DOI] [PubMed] [Google Scholar]
- 66.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinforma Oxf Engl. 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinforma Oxf Engl. 2015;31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe Y, Aoki-Kinoshita KF, Ishihama Y, Okuda S. GlycoPOST realizes FAIR principles for glycomics mass spectrometry data. Nucleic Acids Res. 2021;49:D1523–D1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vizcaíno JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Ríos D, et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol. 2014;32:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during this study are included in this published article and its supplementary information files, and available from the corresponding author on reasonable request. Raw MS glycomics data files are available through GlycoPOST [69] with the dataset identifier GPST000213. The RNAseq data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus [70, 71] and are accessible through GEO Series accession number GSE184516. The MS data were deposited at the ProteomeXchange Consortium [72] via the PRIDE partner repository and are available with the identifier PXD021632.