Abstract
Aims
Cardiac implantable electronic device (CIED) therapy is fundamental to the management of LMNA cardiomyopathy due to the high frequency of atrioventricular block and ventricular tachyarrhythmias. We aimed to define the role of cardiac resynchronization therapy (CRT) in impacting heart failure in LMNA cardiomyopathy.
Methods and results
From nine referral centres, LMNA cardiomyopathy patients who underwent CRT with available pre- and post-echocardiograms were identified retrospectively. Factors associated with CRT response were identified (defined as improvement in left ventricular ejection fraction [LVEF] ≥5% 6 months post-implant) and the associated impact on the primary outcome of death, implantation of a left ventricular assist device or cardiac transplantation was assessed. We identified 105 patients (mean age 51 ± 10 years) undergoing CRT, including 70 (67%) who underwent CRT as a CIED upgrade. The mean change in LVEF ~6 months post-CRT was +4 ± 9%. A CRT response occurred in 40 (38%) patients and was associated with lower baseline LVEF or a high percentage of right ventricular pacing prior to CRT in patients with pre-existing CIED. In patients with a European Society of Cardiology class I guideline indication for CRT, response rates were 61%. A CRT response was evident at thresholds of LVEF ≤45% or percent pacing ≥50%. There was a 1.3 year estimated median difference in event-free survival in those who responded to CRT (p = 0.04).
Conclusion
Systolic function improves in patients with LMNA cardiomyopathy who undergo CRT, especially with strong guideline indications for implantation. Post-CRT improvements in LVEF are associated with survival benefits in this population with otherwise limited options.
Keywords: Lamin A/C, Cardiac resynchronization therapy, Dilated cardiomyopathy, Heart failure
Graphical Abstract
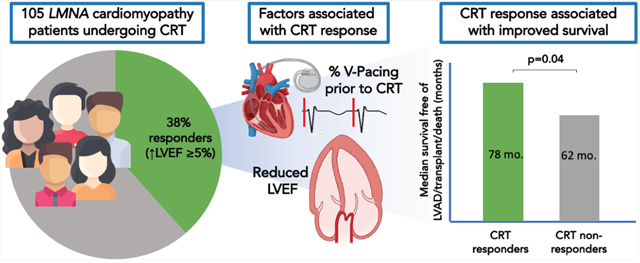
Factors associated with improved systolic function and survival amongst LMNA cardiomyopathy patients undergoing cardiac resynchronization therapy.
Introduction
Dominant mutations in LMNA, present in ~5% of patients with dilated cardiomyopathy (DCM), are associated with age-dependent penetrance of conduction disease and arrhythmia which precede, and are out of proportion to, the severity of systolic dysfunction.1,2 Accordingly, cardiac implantable electronic devices (CIED) are integral to the management of LMNA cardiomyopathy. However, the optimal device selection and timing of implantation is undefined.
The utility of implantable cardioverter defibrillators (ICDs) for the prevention of sudden death in LMNA cardiomyopathy has been informed by observational studies2,3 and validated risk calculators.4 Consensus recommendations influenced by these studies advise criteria for ICD implantation in LMNA cardiomyopathy patients with relatively preserved systolic function.5 However, the impact of CIEDs on progressive heart failure (HF), a major problem in LMNA cardiomyopathy,6,7 has not been well described. Moreover, the mechanism of progressive HF in LMNA cardiomyopathy is not well defined and pacing-induced dyssynchrony represents a potential contributor to disease progression and a remediable therapeutic target.
In this study, we aimed to explore the response to cardiac resynchronization therapy (CRT) in LMNA cardiomyopathy based on improvement in left ventricular systolic function. We hypothesized that patients with LMNA cardiomyopathy would favourably respond to CRT due to their high rates of atrio-ventricular block and frequent right ventricular pacing from pre-existing pacemakers and defibrillators. We identified factors associated with a response to CRT. We then examined the relationship between a response to CRT and the development of end-stage HF which we defined as the composite of death, cardiac transplantation, or implantation of a left ventricular assist device (LVAD). We aimed to identify electrocardiographic and echocardiographic criteria for de novo CRT placement as well as upgrade of an existing CIED to CRT in LMNA cardiomyopathy.
Methods
We retrospectively reviewed data from nine international referral centers with expertise in LMNA cardiomyopathy (online supplementary Methods S1). We included all patients with a pathogenic or likely pathogenic LMNA variant who underwent CRT, either as primary implant or upgrade, regardless of indication, between 2000 and 2019 and with available transthoracic echocardiograms (TTE) before and approximately 6 months after CRT to assess the response to resynchronization. The median time of the requested 6-month echo was 7.5 (range 4.6–11.7) months from baseline. Patients were excluded if they underwent LVAD implant, cardiac transplantation or died prior to the 6-month assessment. Informed consent was obtained from subjects unless a waiver of consent was approved by the local institutional review board.
The respective site investigators collected and de-identified data which were collated centrally. Baseline demographic and clinical characteristics reflected status at the time of CRT implant. Echocardiographic indices, including left ventricular ejection fraction (LVEF) and left ventricular end-diastolic dimension (LVEDD) were obtained from review of the electronic medical record, not primary image review. Analysis of electrocardiograms (ECGs) from the time of CRT implant was performed at each site. Where an ECG was not available at the time of CRT implant, findings of atrio-ventricular block (n =4) or atrial fibrillation (AF)/flutter (n =6) from the time of initial CIED implant were used instead.
For patients who underwent CRT as an upgrade of an existing device, we used review of the CIED interrogation prior to implant for pre-CRT percentage of ventricular pacing. Post-CRT device interrogation was used to obtain the percentage of biventricular pacing at 6 and 24 months. We reviewed CRT implant reports for left ventricular lead position in the coronary sinus in both the left anterior oblique (LAO) and right anterior oblique (RAO) projections. We obtained procedural complications from procedural reports and the electronic medical record.
We obtained New York Heart Association (NYHA) class in follow-up and incident clinical outcomes, including need for LVAD, transplant and death, from the electronic medical record.
We defined CRT response and super-response as an increase in LVEF of ≥5% and ≥10%, respectively, after 6 months.8–10 In six patients, echocardiographic data from 24 months post-CRT were used in place of unavailable 6-month data.
We determined the appropriateness for CRT using the 2021 European Society of Cardiology guidelines on cardiac pacing and CRT.11 Patients not meeting class I or IIa criteria were classified as having a non-classic indication.
The primary outcome was time to LVAD, transplant or death. Other outcomes included change in LVEF and LVEDD at 6 and 24 months, NYHA class at 6 months and QRS duration post-CRT.
Statistical analysis
The cohort was dichotomized based on CRT response and differences in baseline characteristics were compared using t-test for continuous variables, Fisher’s exact/chi-square test for categorical variables and Kruskal–Wallis test for non-normally distributed data. Two tailed p ≤ 0.05 was considered statistically significant. Fisher’s exact test was used to compare NYHA class pre- and 6 months post-CRT. The 6-month change in LVEF, LVEDD and QRS duration was presented as mean ± standard deviation and compared using t-test. Kaplan–Meier plots were used to compare the time from CRT implant to occurrence of the composite primary outcome dichotomized by CRT response at 6 months post-implant. We used logistic regression and Cox proportional hazard regression to identify covariates associated with CRT response and the primary outcome, respectively. Covariates that were found to be significant in a univariable model (p ≤ 0.10) without a significant degree of missingness were then tested in a multivariable model. To determine clinically actionable LVEF and percent ventricular pacing thresholds associated with CRT response, we examined CRT response at LVEF ≤45% and 35% and percent pacing ≥50%, respectively. These thresholds were selected a priori, incorporating thresholds identified in guidelines5 and/or other patient populations.12 The statistical significance of these thresholds for association with CRT response was determined with Fisher’s exact test using a two tailed p-value of ≤0.05 for significance. All analyses were done using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
We identified 117 patients with pathogenic/likely pathogenic LMNA variants and prior CRT. We excluded 12 patients who did not meet inclusion criteria due to lacking echocardiographic data (n =9) or because they experienced an early primary outcome (n =3) precluding assessment of CRT response at 6 months (transplants at 1, 4 and 4 months post-CRT, respectively). The baseline characteristics of the 105 patients who met inclusion criteria, stratified by 6-month response to CRT (responders [n = 40] vs. non-responders [n = 65]), are presented on Table 1. Median follow-up post-CRT implant in all patients was 3.5 (interquartile range 1.7–5.9) years. A complete list of LMNA variants is provided in online supplementary Table 1. Clinical characteristics were typical of a LMNA cardiomyopathy referral population, with a high frequency of primary prevention ICDs and atrial arrhythmias in both groups.
Table 1.
Patient characteristics at the time of cardiac resynchronization therapy
| CRT respondera
(n = 40) |
CRT non-responder (n = 65) |
p-value | |
|---|---|---|---|
| Age, years, mean (± SD) | 52 ± 11 | 51 ± 9 | 0.546 |
| Male sex, n (%) | 21 (53) | 42 (65) | 0.258 |
| Race, n (%) | 0.538 | ||
| White | 37 (93) | 60 (92) | |
| Black | 1 (3) | 4 (6) | |
| Hispanic | 1 (3) | 0 | |
| Asian | 1 (3) | 1 (2) | |
| Missense mutation, n (%) | 17/39 (44) | 27/64 (62) | 0.838 |
| NYHA class, n (%) | n = 34 | n = 47 | 0.482 |
| I | 4 (12) | 6 (13) | |
| II | 15 (44) | 25 (53) | |
| III/IV | 15 (44) | 16 (34) | |
| LVEF %, mean (± SD) | 29 ± 8 | 36 ± 11 | 0.001 |
| LVEF <35%, n (%) | 33/40 (83) | 34/65 (52) | 0.002 |
| LVEDD mm, mean (±SD) | 59 ± 8 | 59 ± 7 | 0.981 |
| Atrio-ventricular block, n (%) | n = 39 | n = 61 | 0.619 |
| None/first degree | 9 (23) | 18 (29) | |
| Mobitz I | 6 (15) | 7 (11) | |
| Mobitz II | 5 (13) | 4 (6) | |
| Complete heart block | 19 (49) | 32 (63) | |
| Atrial fibrillation/flutter, n (%) | 28/39 (72) | 50/65 (77) | 0.559 |
| Primary prevention ICD, n (%) | 24/36 (67) | 36/51 (71) | 0.697 |
| Initial device before upgrade, n (%) | 0.091 | ||
| Single ICD | 3 (8) | 7 (11) | |
| Dual ICD | 8 (20) | 23 (35) | |
| Dual PPM | 15 (38) | 13 (20) | |
| Single PPM (ventricular) | 0 | 1 (2) | |
| None (primary CRT implant) | 14 (35) | 21 (32) | |
| Ventricular pacing pre-CRTb, %, mean (±SD) | 93 ± 13 | 72 ± 38 | 0.015 |
| Medications, n (%) | |||
| ACEi/ARB/ARNI | 29/37 (78) | 38/56 (68) | 0.292 |
| Beta blocker | 28/37 (76) | 39/57 (68) | 0.480 |
| Mineralocorticoid receptor antagonist | 9/35 (26) | 21/56 (38) | 0.204 |
| Furosemide (or equivalent) | 22/35 (63) | 29/54 (54) | 0.344 |
| Furosemide (or equivalent), mg, median (range) | 20 (0–40) | 15 (0–40) | 0.414 |
| NT-proBNP, pg/ml, median (range) | n = 12 1069 (51.5–2297) | n = 23 540 (150–878) | 0.775 |
| QRS duration, mean (±SD), ms | 168 ± 31 | 166 ± 33 | 0.821 |
| QRS duration >150 ms, n (%) | 25/33 (76) | 35/45 (77) | 0.834 |
| QRS morphology, n (%) | n = 35 | n = 57 | 0.226 |
| LBBB | 13 (37) | 15 (26) | |
| IVCD | 1 (3) | 8 (14) | |
| RBBB | 0 | 1 (2) | |
| Paced | 21 (60) | 33 (58) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; PPM, permanent pacemaker; RBBB, right bundle branch block; SD, standard deviation.
CRT responders experienced an improvement in LVEF ≥5%, ~6 months after resynchronization.
Restricted to patients with an existing pacemaker and/or defibrillator prior to CRT implantation.
Eighteen (17%) and 55 (52%) patients had a class I or class IIa indication for CRT, respectively. The remaining 32 patients did not meet the above criteria (n = 24) or lacked data with which to classify the indication for CRT (n =8).
As shown on Table 1, the baseline characteristics which significantly differed between CRT responders and non-responders were reduced LVEF (p = 0.002) and increased frequency of ventricular pacing prior to CRT (p = 0.015). In 67% (n = 70) of the cohort, CRT was performed as an upgrade of an existing CIED. Amongst these patients, the burden of ventricular pacing was 80 ± 33%. In patients with serial pre-CRT echocardiograms available for review, 29 of 52 (56%) patients were upgraded in the setting of a decline in left ventricular function.
Procedural documentation of left ventricular lead implantation was available in 41 patients. In the LAO projection, 91% of leads were in an optimal location (lateral, posterolateral or posterior) in responders versus 78% in non-responders. In the RAO projection, 67% of leads were in an optimal location in responders versus 50% in non-responders. Complications occurred in 9% of patients and included pocket haematoma, infection and lead dislodgement and did not differ between responders and non-responders.
The impact of cardiac resynchronization therapy on cardiac function
As depicted in Figure 1, there was reverse remodelling 6 months after CRT with significant increase in LVEF and decrease in LVEDD that persisted to 24 months. Forty (38%) patients were deemed to be CRT responders based on an improvement in LVEF ≥5% at 6 months. A positive CRT response was highest in patients with a class I indication for implantation (61%) compared those with a class IIa indication (42%), or without a classic indication (19%) for implantation (p = 0.009). The mean increase in LVEF in the CRT responder arm was 13%. Forty-two patients (40%) had no change in LVEF after CRT, in whom LVEF was 34.6 ± 11% at baseline. Eight of these patients maintained their ejection fraction ≥45%. Six months after CRT there was a significant decrease in QRS duration. Amongst patients with right ventricular pacing prior to CRT, QRS duration decreased by 25 ± 25 ms (p < 0.0001) after CRT whereas, amongst patients without a paced QRS prior to CRT, there was no significant change in QRS duration (−4 ± 30 ms, p = 0.86).
Figure 1.
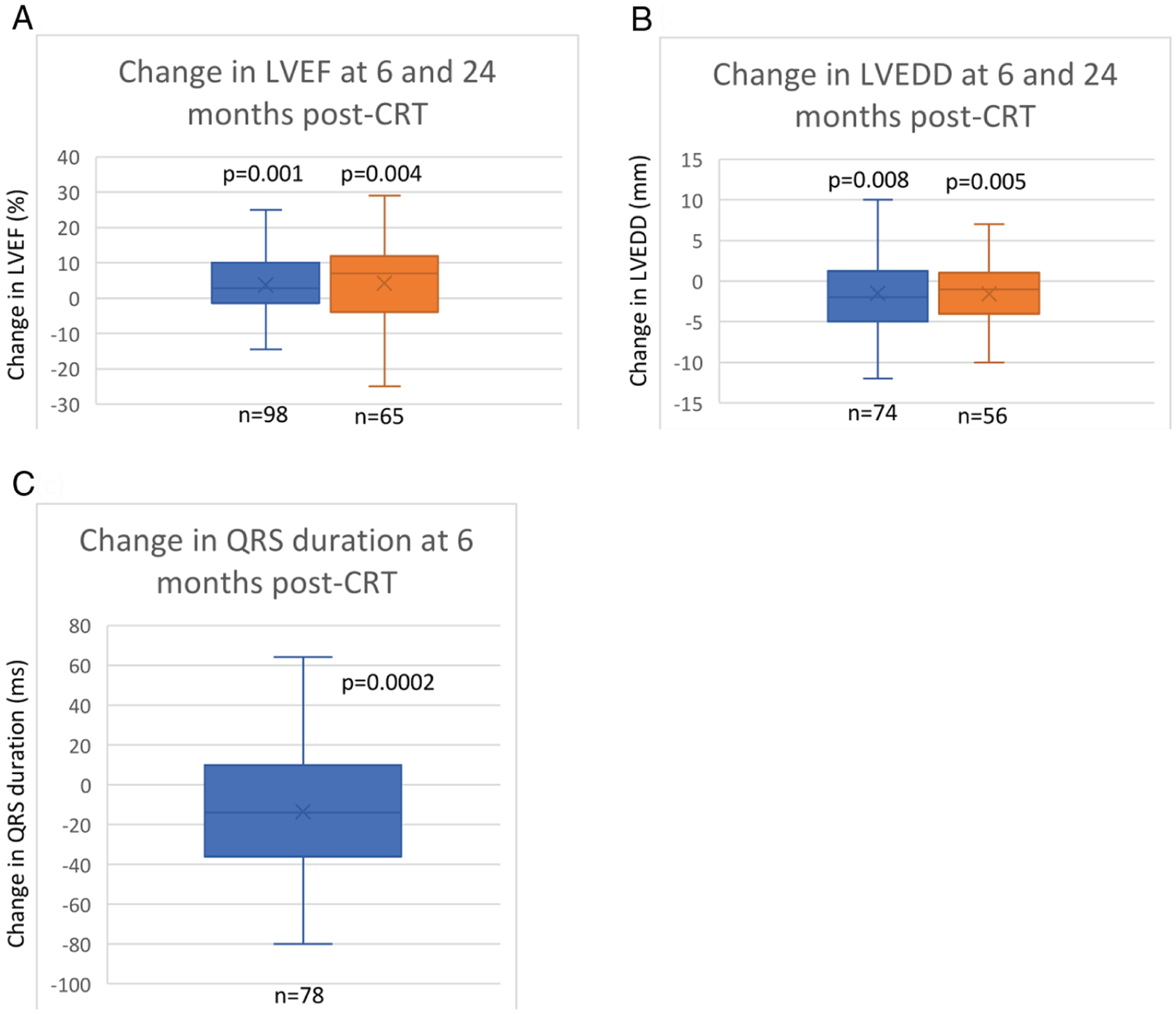
Change in echocardiographic and electrocardiographic parameters post-cardiac resynchronization therapy (CRT). (A) Mean change in left ventricular ejection fraction (LVEF) at 6 (3.7 ± 9.1%) and 24 months from baseline (4.3 ± 12.0%). (B) Mean change in left ventricular end-diastolic dimension (LVEDD) at 6 (−1.5 ± 4.8 mm) and 24 months from baseline (−1.6 ± 4.2 mm). (C) Mean change in QRS duration at 6 months (−13.6 ± 30.2 ms). Six-month data depicted in blue, 24-month data in orange. Whiskers represent minimum and maximum values, horizontal line is median, box represents interquartile range and x is the mean change from baseline (average of patient-level change).
Baseline characteristics associated with a response to CRT in univariable and multivariable models are presented in Table 2. There was an inverse relationship between baseline LVEF and improvement in LVEF with CRT. Patients with LVEF ≤35% were 4.2 times (95% confidence interval [CI] 1.6–10.8, p = 0.003) more likely to experience a CRT response. An increase in the percentage of ventricular pacing pre-CRT was also significantly associated with CRT response. For every 1% increase in pre-CRT ventricular pacing, there was a 3% (p = 0.039) increased odds of CRT response. In a multivariable model, both baseline LVEF and pre-CRT percentage of ventricular pacing remained highly significantly related to CRT response at 6 months whereas neither QRS duration (ms), LBBB, history of AF/flutter, nor strategy of CRT implant (upgrade vs. primary implant) were associated with CRT response.
Table 2.
Baseline characteristics associated with cardiac resynchronization therapy response at 6 months in patients with LMNA cardiomyopathy
| Variable | Univariable | Multivariablea | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Percent ventricular pacing pre-CRT | 1.032 (1.002–1.063) | 0.039 | 1.044 (1.011–1.078) | 0.008 |
| LVEF pre-CRT | 0.933 (0.893–0.975) | 0.002 | 0.881 (0.814–0.954) | 0.002 |
| QRS duration | 1.002 (0.988–1.016) | 0.819 | ||
| LBBB | 1.551 (0.608–3.960) | 0.359 | ||
| AF/flutter pre-CRT | 0.779 (0.315–1.928) | 0.589 | ||
AF, atrial fibrillation; CI, confidence interval; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; OR, odds ratio.
n = 59 for the multivariable model.
We then determined the CRT response rate at a threshold of LVEF being used for sudden cardiac death risk stratification in LMNA cardiomyopathy (LVEF 45%)5 or that used to guide CRT placement in the broader HF population (LVEF 35%). Similarly, we examined the frequency of CRT response amongst patients with a CIED prior to CRT upgrade at a threshold of pacing at 50%. Whether dichotomized at LVEF ≤45% or 35%, the patients with reduced LVEF had significantly greater likelihood of CRT response of 44% (p = 0.0008) and 50%, respectively (p = 0.016). Likewise, patients with a CIED prior to CRT who had ventricular pacing ≥50%, were significantly more likely to have a CRT response than those with <50% ventricular pacing (46 vs. 18%, p = 0.03).
CRT super-response, defined as an increase in LVEF ≥10% at 6 months, was seen in 28 (27%) patients, including 15 (14%) patients with improvement in LVEF to normal (≥55%). The only baseline factor associated with super-response in a univariable model was LVEF ≤35% pre-CRT with an odds ratio (OR) of 4.3 (95% CI 1.36–13.67, p = 0.01).
The relationship between cardiac resynchronization therapy response and clinical outcomes
There was a highly significant improvement in NYHA class with CRT (p < 0.001) in the 81 patients with available pre- and post-CRT NYHA data. At 6 months, an improvement in ≥1 NYHA class occurred in 32 (40%) patients, and an additional 33 (41%) maintained NYHA I or II class post-CRT. The proportion of patients with severe HF symptoms (NYHA class III/IV) decreased from 38% at baseline to 20% at 6 months post-CRT (Figure 2). Patients who responded to CRT were not more likely to experience stable or improved NYHA functional class than non-responders (88% vs. 74%, p = 0.16).
Figure 2.
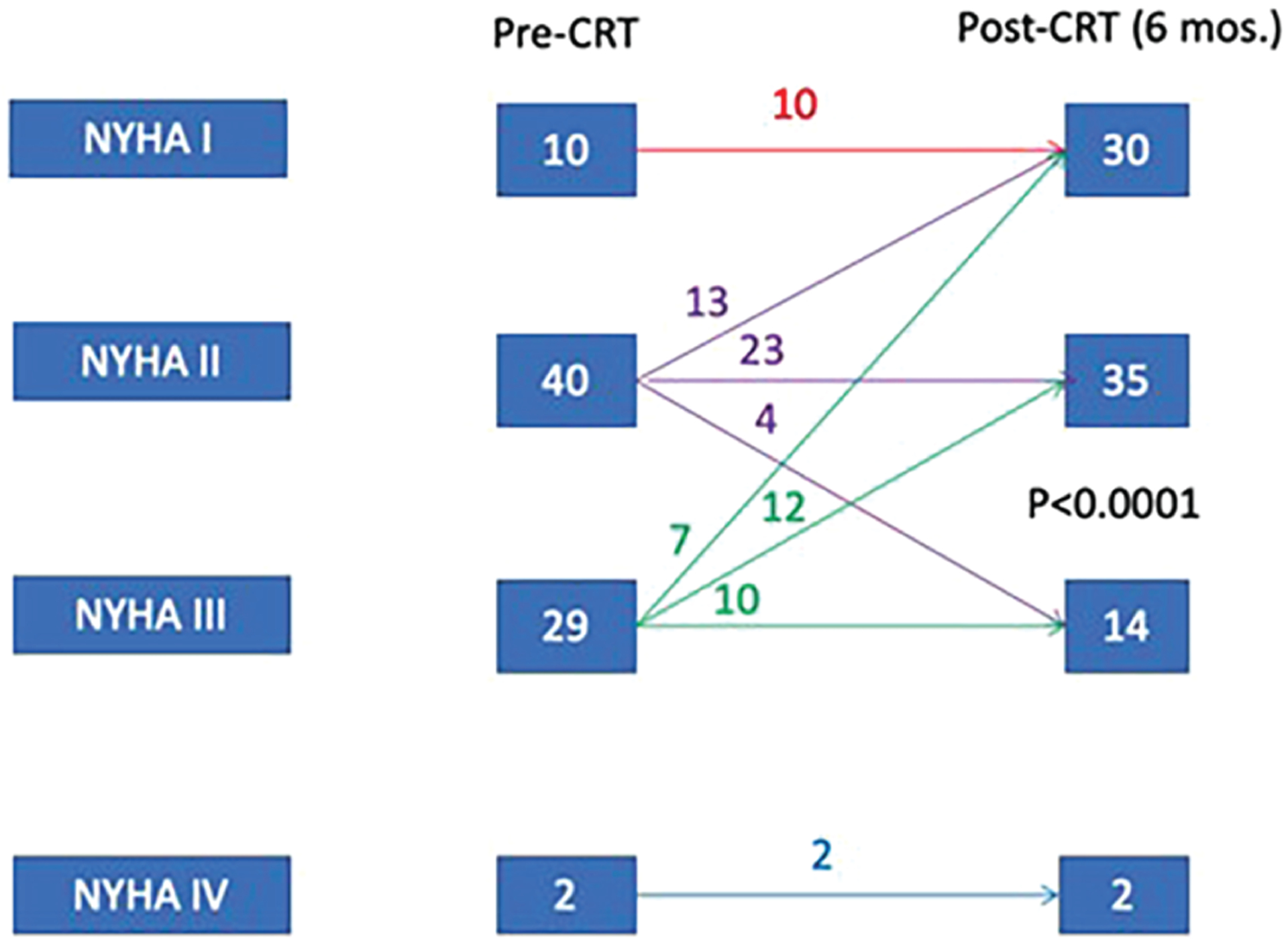
Change in New York Heart Association (NYHA) class after cardiac resynchronization therapy (CRT) in patients with LMNA cardiomyopathy. There was an increase in the percentage of NYHA class I/II patients from baseline (62%) to 6 months post-CRT (80%). p-value calculated with Fisher’s exact test comparing pre and post-CRT NYHA class.
Amongst patients with a CRT response, the median survival free from LVAD implantation or transplant (primary outcome) was 6.5 years (95% CI 3.9–10.3) versus 5.2 years (95% CI 2.8–6.2) in non-responders, indicating a 1.3 year estimated median difference in event-free survival (p = 0.04) (Figure 3). Two years after CRT, freedom from the primary outcome was 90% and 73% in CRT responders and non-responders, respectively.
Figure 3.
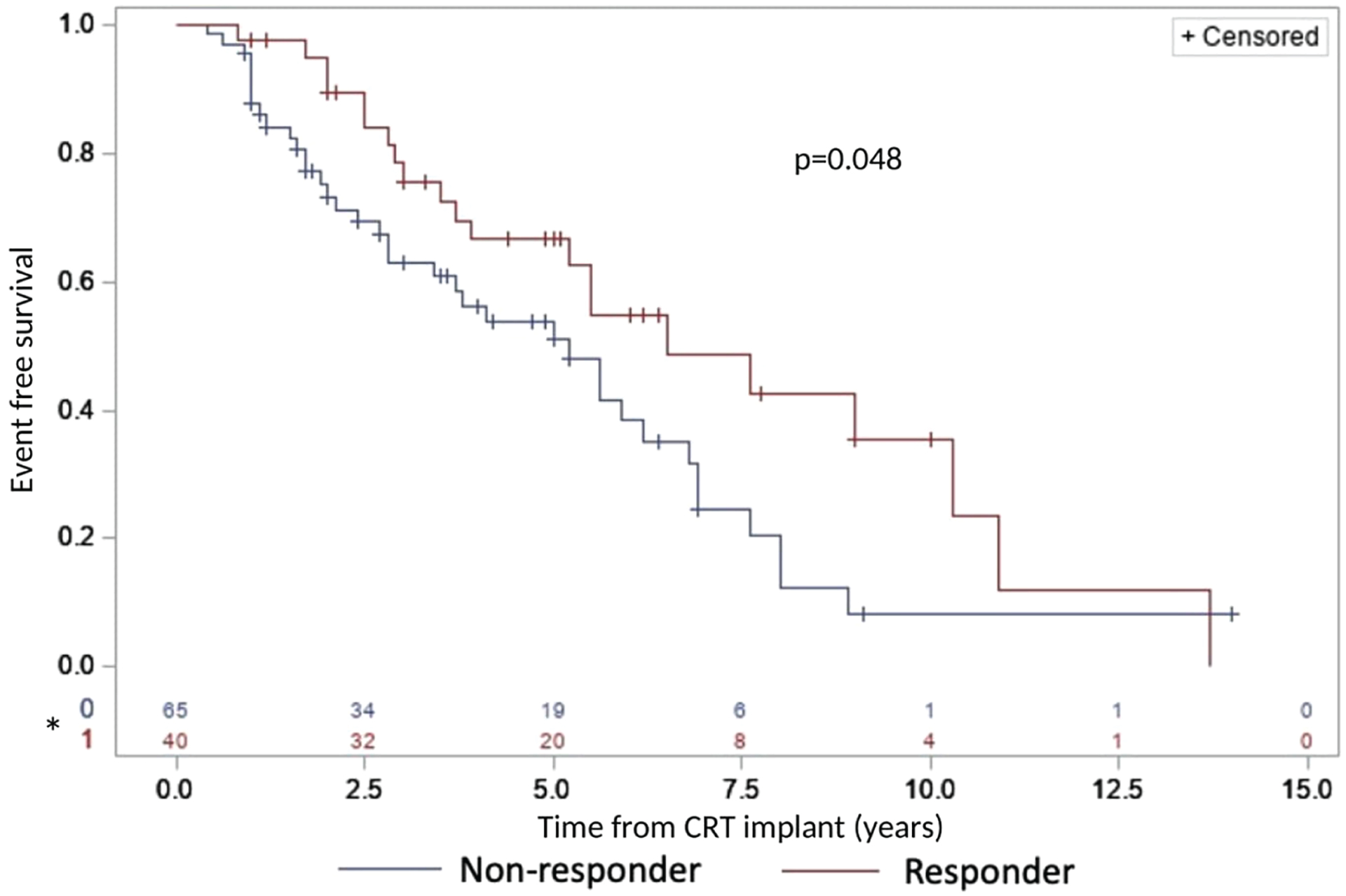
Improved event-free survival in LMNA cardiomyopathy patients with response to cardiac resynchronization therapy (CRT). The primary endpoint was death, transplantation or left ventricular assist device implantation. CRT responder defined as improvement in left ventricular ejection fraction ≥5% after 6 months. *Number at risk.
Baseline characteristics associated with event-free survival in univariable and multivariable analyses are presented in Table 3. Univariable factors associated with the primary outcome included characteristics associated with severe HF at baseline, including severe systolic dysfunction, diuretic use, and HF symptoms. Patients with a positive CRT response were substantially less likely to experience the primary outcome in the univariable (OR 0.580, 95% CI 0.338–0.995, p = 0.048) and multivariable (OR 0.296, 95% CI 0.149–0.585, p = 0.001) analysis.
Table 3.
Factors associated with the composite of death, cardiac transplantation or left ventricular assist device implantation after cardiac resynchronization therapy in patients with LMNA cardiomyopathy
| Variable | Univariable | Multivariablea | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MRA pre-CRT | 2.676 (1.489–4.809) | 0.001 | ||
| NYHA class pre-CRT | 1.930 (1.203–3.098) | 0.007 | 1.821 (1.069–3.101) | 0.027 |
| NT-proBNP pre-CRT | 1.000 (1.000–1.001) | 0.001 | ||
| Loop diuretic pre-CRT | 1.01 (1.005–1.013) | <0.0001 | 1.005 (1.000–1.009) | 0.037 |
| Ventricular pacing % pre-CRT | 0.991 (0.981–1.001) | 0.073 | ||
| LVEF pre-CRT | 0.946 (0.921–0.972) | <0.001 | 0.933 (0.900–0.968) | 0.0002 |
| CRT responder at 6 months | 0.580 (0.338–0.995) | 0.048 | 0.296 (0.149–0.585) | 0.001 |
CI, confidence interval; CRT, cardiac resynchronization therapy; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
n = 75 for the multivariable model.
Outcomes of cardiac resynchronization therapy in patients without a classic indication
Cardiac resynchronization was performed in 32 patients (30%) without class I or IIa indication (n = 24) or where QRS morphology/duration were unavailable (n = 8) to assess indication for CRT. In this group, LVEF increased by ≥5% in 6 patients (including >10% in 3 patients), LVEF was stable (±4%) in 17 patients, and LVEF decreased by ≥5% in 9 patients. In this group, NYHA class was available in 21 patients, in whom 10 patients remained NYHA I or II class and 7 patients improved by at least one NYHA class. The characteristics of patients grouped by indication (class I, IIa and non-classic) for CRT are outlined in online supplementary Table S2.
Discussion
In this study, we present outcomes associated with the primary implant or upgrade to CRT in patients with LMNA cardiomyopathy, a disease for which single-chamber pacemakers and defibrillators are utilized earlier in the course for heart block and ventricular tachyarrhythmias. Utilizing a multicentre LMNA CRT database, we report that the response to CRT, defined as improvement in LVEF by ≥5%, was 38% after 6 months overall, and 61% in patients with a class I indication for CRT, the latter is comparable to the response seen in all-comers with non-ischaemic cardiomyopathy. Factors associated with a CRT response were depressed LVEF pre-CRT and an increased percentage of pre-CRT ventricular pacing amongst patients who underwent CRT as an upgrade of an existing CIED (Graphical Abstract). Clinically actionable thresholds associated with CRT response were noted at LVEF ≤45% and percent pacing ≥50%. Left bundle branch block (LBBB) prior to implant, a conventional predictor of CRT response, was infrequent in this cohort and not significantly associated with an improvement in LVEF. Nevertheless, the clinical impact of successful resynchronization was significant. Amongst patients experiencing a CRT response, there was a median 1.3 year delay in the composite endpoint of LVAD, transplant or death. Our findings emphasize the value of CRT in this population highly vulnerable to end-stage HF.
Definition of cardiac resynchronization therapy response
Although no consensus of successful CRT response has been established, the efficacy of resynchronization has rested upon demonstration of favorable effects on left ventricular size and function with or without changes in functional class and clinical outcomes. In prior studies, CRT response has been defined variably, including improvements in LVEF by ≥5%, reduction in left ventricular end-systolic volume index by ≥15%, or ≥1 NYHA improvement in functional class.8,13,14 LVEF improvement at 12 months ranged from a median of 4.6% (95% CI 3.2–6.4) in the MIRACLE trial15 to as high as 11% in the MADIT-CRT study.16 Improvement in functional status can occur in up to 60%–70% of patients depending on the criteria used.17 Superficially, the 38% CRT response rate reported here is suboptimal. However, this reflects expanded use of CRT in this population, especially to patients with relatively higher LVEF at baseline, and less potential for improvement. Only 13% of the LMNA cardiomyopathy patients presented here would have met the strict inclusion criteria for MADIT-CRT16 and 29% would have met criteria for RAFT18 and despite this, we found an improvement in mean LVEF at 6 months of 4% ± 9% with CRT. Furthermore, 40% of patients improved by ≥1 NYHA class. Although a decrease in QRS duration is not considered a formal criterion for CRT response, it is often seen with successful resynchronization. We found a clinically significant improvement in mean QRS duration of 14 ± 30 ms at 6 months which is comparable to the QRS decrease of between 6 and 37 ms seen in clinical trials of patients undergoing CRT.19
Factors associated with cardiac resynchronization therapy response
Prior studies have elucidated patient factors that portend a good response to CRT. These include non-ischaemic cardiomyopathy, LBBB, wide QRS >150 ms, sinus rhythm and female sex.20 The response to CRT in patients who meet these criteria has been as high as ~70%.20 While the LMNA cardiomyopathy patients we present here shared some features typical of CRT responders (non-ischaemic cardiomyopathy, wide QRS), only a third had LBBB and 74% were in AF/flutter. These are typical characteristics of LMNA cardiomyopathy, and importantly in this cohort we showed that pre-implant LVEF and the burden of pre-CRT right ventricular pacing were significantly associated with a CRT response. While the low frequency of LBBB limited our ability to identify its association with CRT response, this in keeping with the natural history of LMNA cardiomyopathy where atrio-ventricular block and wide QRS morphology from ventricular pacing (not distal conduction system disease) is common.
Getting with the cardiac resynchronization therapy guidelines
Our findings are of particular relevance as left ventricular dysfunction is a late finding in LMNA heart disease that, once present, progresses rapidly to end-stage HF.2,6 Observational studies in LMNA heart disease have shown that the frequency of end-stage HF is highest in those with an ejection fraction <45%.2,6 Moreover, prior studies have documented minimal occurrence of ventricular reverse remodelling with guideline-directed medical therapy in this population, underscoring the paucity of effective therapies for LMNA cardiomyopathy.21 Here however, retrospective application of the 2021 European Society of Cardiology guidelines effectively identified patients likely to benefit from CRT (61%, 42% and 19% for class I, IIa and without indication, respectively). LMNA cardiomyopathy patients with an LVEF ≤35% were most likely to experience a super-response to CRT, suggesting the patients with most to gain from CRT are those with severe remodelling. Moreover, we also noted that patients without any indications for CRT were unlikely to benefit from resynchronization and may be harmed by a decrement in LVEF. These patients may be better served by continued observation in lieu of resynchronization therapy until a guideline indication is present.
Left ventricular systolic dysfunction in LMNA heart disease
These findings inform the pathophysiology of LMNA heart disease. Our findings suggest that a component of adverse remodelling is related to pacing-induced dyssynchrony affecting vulnerable myocardium, but not invariably past the point of recovery, something that has not been examined in model systems. If right ventricular apical pacing is detrimental in LMNA heart disease, what is the optimal timing of CRT implant in these patients? This question is particularly germane given the crucial role played by CIED for heart block and sudden cardiac death prevention at earlier stages of the disease. Amongst patients with pre-existing CIED, we found CRT to be beneficial in patients receiving greater than 50% ventricular pacing, or if there was any associated systolic dysfunction present (LVEF <45%).
Factors underlying cardiac resynchronization therapy non-response
Although CRT was associated with significant improvements in LVEF, over half of the LMNA patients reported here did not meet our criteria for a CRT response. This is partially attributed to the use of CRT beyond conventional indications. Suboptimal position of the left ventricular lead is partially responsible for CRT non-response, as optimal placement could not always be achieved. The burden of ventricular ectopy may also reduce the efficacy of CRT; however, we did not collect adequate data on premature ventricular contraction burden to determine the extent of this mechanism and in turn the value of premature ventricular contraction suppression. Last, our data partially reflect practice prior to the routine use of quadripolar leads and multi-point pacing which can help select additional beneficial vectors in patients who do not initially respond to CRT. Novel technologies such as epicardial or endocardial left ventricular lead placement and conduction system pacing have not been widely studied in patients with LMNA heart disease but are viable alternatives for non-responders that require further investigation.
While AF is associated with suboptimal responses to CRT in the broad HF population, this was not the case in our LMNA cohort. This is especially important given the very high (if not universal) frequency of AF in advanced LMNA cardiomyopathy. We attribute the beneficial effect of CRT in patients with AF due to the high frequency of atrio-ventricular block in this population which contrasts with other forms of non-ischaemic cardiomyopathy and prevents rapid native conduction and enables a high frequency of biventricular pacing.
Clinical implications
We recommend implanting a CRT-D device as the initial device in a patient with LMNA heart disease and an expectation of a high burden of right ventricular pacing (≥50%) or if the LVEF is ≤45% with a wide QRS complex. In those patients who have a dual-chamber ICD or dual-chamber pacemaker, we recommend upgrade to a CRT-D when the burden of right ventricular pacing exceeds 50% and LVEF has declined or is below 45% despite optimal medical therapy. Our findings are compatible with consensus guidelines which advise CRT or conduction system pacing in the broader population of patients with mild systolic dysfunction and an anticipated need for at least 40% ventricular pacing.22 Likewise, based on our findings we would not advise that the absence of LBBB or the presence of AF be used to exclude consideration of CRT in LMNA heart disease.
Limitations
This is an observational study with the inherent limitations which preclude definitive statements of causation with regard to CRT and clinical improvement. Procedural details and premature ventricular contraction burden were not available in a subset of patients which limited our ability to understand CRT non-response in some patients. Lastly, clinical outcomes and imaging findings were reported by individual institutions and not centrally adjudicated.
Supplementary Material
Funding
This work was supported by an unrestricted grant from the O’Hare Family Foundation for research on LMNA heart disease.
Conflict of interest:
A.O. has received consulting income from MyoKardia and Cytokinetics and support from the Winkelman Family Fund for Cardiac Innovation. D.P.J. has received consulting income from 4D Molecular Therapeutics, ADRx Inc, Cytokinetics, Pfizer, and Tenaya Therapeutics unrelated to this work. N.K.L. receives unrestricted support for research on cardiomyopathy from the O’Hare Family and Steggall Family Foundations and has received modest consulting income from MyoKardia, Tenaya, Sarepta, Array BioPharma and Pfizer. All other authors have nothing to disclose.
Footnotes
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Zimmerman RS, Cox S, Lakdawala NK, Cirino A, Mancini-DiNardo D, Clark E, et al. A novel custom resequencing array for dilated cardiomyopathy. Genet Med. 2010;12:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Baldinger SH, Gandjbakhch E, Maury P, Sellal JM, Androulakis AFA, et al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68:2299–307. [DOI] [PubMed] [Google Scholar]
- 3.van Rijsingen IAW, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, et al. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59: 493–500. [DOI] [PubMed] [Google Scholar]
- 4.Wahbi K, Ben Yaou R, Gandjbakhch E, Anselme F, Gossios T, Lakdawala NK, et al. Development and validation of a new risk prediction score for life-threatening ventricular tachyarrhythmias in laminopathies. Circulation. 2019;140: 293–302. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2018;72:e91–220. [DOI] [PubMed] [Google Scholar]
- 6.Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, et al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidhu K, Han L, Picard KCI, Tedrow UB, Lakdawala NK. Ventricular tachycardia in cardiolaminopathy: characteristics and considerations for device programming. Heart Rhythm. 2020;17:1704–10. [DOI] [PubMed] [Google Scholar]
- 8.Park MY, Altman RK, Orencole M, Kumar P, Parks KA, Heist KE, et al. Characteristics of responders to cardiac resynchronization therapy: the impact of echocardiographic left ventricular volume. Clin Cardiol 2012;35:777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinkuniene D, Bucyte S, Ceseviciute K, Abramavicius S, Baronaite-Dudoniene K, Laukaitiene J, et al. Predictors of positive response to cardiac resynchronization therapy. BMC Cardiovasc Disord. 2014;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker GB, Bax JJ, Fung JWH, van der Wall EE, Zhang Q, Schalij MJ, et al. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol. 2006;97:260–3. [DOI] [PubMed] [Google Scholar]
- 11.Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–520. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. ; MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 13.Gorodeski EZ, Magnelli-Reyes C, Moennich LA, Grimaldi A, Rickard J. Cardiac resynchronization therapy-heart failure (CRT-HF) clinic: a novel model of care. PLoS One. 2019;14:e0222610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C; REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–43. [DOI] [PubMed] [Google Scholar]
- 15.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. ; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. [DOI] [PubMed] [Google Scholar]
- 16.Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald AC, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2014;130:2278–86. [DOI] [PubMed] [Google Scholar]
- 17.European Heart Rhythm Association (EHRA); European Society of Cardiology (ESC); Heart Rhythm Society; Heart Failure Society of America (HFSA); American Society of Echocardiography (ASE); American Heart Association (AHA); European Association of Echocardiography (EAE) of ESC; Heart Failure Association of ESC (HFA); Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace 2012;14:1236–86. [DOI] [PubMed] [Google Scholar]
- 18.Tang ASL, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. ; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 19.Korantzopoulos P, Zhang Z, Li G, Fragakis N, Liu T. Meta-analysis of the usefulness of change in QRS width to predict response to cardiac resynchronization therapy. Am J Cardiol. 2016;118:1368–73. [DOI] [PubMed] [Google Scholar]
- 20.Linde C, Ellenbogen K, McAlister FA. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm. 2012;9(8 Suppl):S3–13. [DOI] [PubMed] [Google Scholar]
- 21.Jansweijer JA, Nieuwhof K, Russo F, Hoorntje ET, Jongbloed JD, Lekanne Deprez RH, et al. Truncating titin mutations are associated with a mild and treatable form of dilated cardiomyopathy. Eur J Heart Fail. 2017;19:512–21. [DOI] [PubMed] [Google Scholar]
- 22.Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


