Abstract
Maternal injection of 3H-thymidine ([3H]dT) during gestation in non-human primates (NHPs) has been used to determine the time of neurogenesis for various brain areas, including the lateral geniculate (LGN) and the pulvinar (PUL) nuclei of the caudal thalamus. Here we examine neurogenesis in the rostral thalamus, with focus on the mediodorsal (MD) and the anterior nuclei (ANT), to determine if neurogenesis of rostral and caudal thalamic nuclei is concurrent or instead temporally staggered. The MacBrainResource (MBR) search function identified archived cases (N=10) of [3H]dT labeled specimens, with injection dates ranging from embryonic day 25 (E25)-E50 and postnatal sacrifice dates. Slides were scanned to create digital images for subsequent analysis using Stereo Investigator software. Labeled neurons were mapped within a contour that encompassed the entire rostral thalamus. These maps were superimposed onto closely corresponding sections from the online BrainMaps macaque atlas to facilitate analysis. Our novel approach uncovered a previously undetected spatial-temporal patterning of neurogenesis in the thalamus. At E30, labeled neurons were located in a compact medial band; at E38-E40, labeling was dense ventrolaterally, and at E43, labeling predominated laterally at rostral levels and was widely distributed at caudal levels. Peak neurogenesis occurs earlier in MD (E30-E43) and ANT (E31-E43) than in LGN (E36-E43) and PUL (E36-E45). Birth-dating of neurons in MD and ANT, two higher order relay nuclei implicated in the pathology of schizophrenia, provides further insight into the critical period of vulnerability during which early developmental perturbation may increase incidence of schizophrenia later in life.
Keywords: mediodorsal nucleus, anterior nucleus, neurodevelopment, schizophrenia
Introduction
Neurogenesis in non-human primates (NHPs) is a temporally staggered process that occurs over a discrete and limited span of development, in which cells are born near the ventricular surface and then migrate to their final destinations. The time of neurogenesis of many brain areas in the NHP has been determined by means of maternal injection of 3H-thymidine ([3H]dT) at various gestational ages. In these studies, systemically injected [3H]dT was incorporated into the DNA of dividing cells. Autoradiographic processing of brain tissue resulted in dense silver grain labeling over the newly born neurons, i.e. those cells that had undergone only one final division between time of injection and time of sacrifice. In this manner, neurogenesis in brain regions, including visual cortex (Rakic 1974), neostriatum (Brand and Rakic 1979), septal nuclei (Brand and Rakic 1980), cerebellum (Gould and Rakic 1981), hippocampus (Rakic and Nowakowski 1981), basal forebrain (Kordower and Rakic 1990), amygdala (Kordower et al. 1992), and hypothalamus (van Eerdenburg and Rakic 1994), was examined. The time of origin for neurons in two thalamic nuclei, the lateral geniculate nucleus (LGN; E36-E43) and the pulvinar (PUL; E36-E45), was also determined (Rakic 1977a; Ogren and Rakic 1981). However, neurogenesis of countless brain areas, including the anterior (ANT) and mediodorsal (MD) nuclei of the thalamus, has yet to be investigated. Therefore, it is not known whether these more rostrally situated thalamic nuclei are generated at the same time as their more caudal counterparts, or conversely whether there is a temporal patterning of neurogenesis even within the thalamus. Archived brain slides from previous birth-dating studies, now available to all researchers via MacBrainResource (MBR), provide the unique opportunity to conduct contemporary research while avoiding the difficulties associated with the cost, maintenance, and sacrifice of animals. Thus, these materials are invaluable for addressing unanswered questions regarding brain development and disease.
Early understanding of the function of the ANT and MD thalamic nuclei was inferred from patterns of connectivity with other regions. For example, Papez’s proposed circuit of emotion included interconnections of the ANT with the hypothalamus, cingulate gyrus, and hippocampus (Papez 1937). Re-evaluation of the Papez circuit using modern methods has revealed a more complex pattern of reciprocal and topographic connectivity than originally described, suggesting involvement of the ANT not just in emotional modulation but also in cognitive functioning (Bubb et al. 2017). In fact, new discoveries have highlighted the circuit’s integral role in learning and memory, rather than emotion (Wolff et al. 2015). The ANT, which can be further divided into the anteromedial (AM), anteroventral (AV) and anterodorsal (AD) nuclei, also plays an important role in spatial navigation (Jankowski et al. 2013). For example, numerous “head cells,” i.e., cells that guide spatial orientation by firing when the animal aims its head, have been recorded within both the AV and the AD, and lesion studies have indicated that head cells in the AD may be crucial for proper functioning of hippocampal place cells (Jankowski et al. 2013; Savage et al. 2020). Additionally, a large portion of AV cells fire in synchrony with hippocampal theta rhythms, providing further evidence that the AV is significantly involved in the integration and processing of spatial information (McNaughton et al. 1996). Interestingly, rat studies have suggested that each of the ANT divisions plays a distinct role in learning and memory, as selective inactivation results in specific, task-dependent deficits in acquisition, consolidation, or retrieval (Safari et al. 2020).
The MD is a higher order thalamic relay nucleus with complex, reciprocal connectivity with the prefrontal cortex, as well as input from the medial temporal lobe, striatum, and brain stem (Goldman-Rakic and Porrino 1985; Duque and McCormick 2010; Mitchell 2015; Vertes et al. 2015). The MD has also been identified as an important node in the cortico-striato-thalamo-cortical loop that funnels information from widespread cortical areas to the prefrontal cortex (Alexander and Crutcher 1990). Based on this connectivity, the MD is thought to play a role in executive functions associated with the prefrontal cortex (Mitchell 2015; Vertes et al. 2015; Parnaudeau et al. 2018). Indeed, it has been postulated that thalamo-cortical and cortico-thalamic interconnections reinforce cognitive processes of higher order cortical regions, such that damage to these connections could lead to extensive impairment in the prefrontal cortex processing and ultimately hinder executive functions (Mitchell 2015). However, cognitive deficits after MD damage do not strictly mirror those of interconnected prefrontal cortices but instead manifest as deficits in the integration of multiple cognitive processes in the performance of tasks, many with a working memory component (Mitchell 2015). The MD has been shown to synthesize relevant information in the decision-making process in order to select the appropriate task response (Vertes et al. 2015; Mitchell 2015; DeNicola et al. 2020).
Not surprisingly, diseases that impact the ANT and the MD result in deficits in learning and memory. For example, Korsakoff’s syndrome, which is associated with damage to the ANT and the MD, is characterized by anterograde and retrograde amnesia (Kril and Harper 2012). A cardinal feature of schizophrenia is a deficit in prefrontal-mediated working memory processing (Goldman-Rakic 1990; Driesen et al. 2008). Postmortem studies of patients with schizophrenia have uncovered reductions in the number of neurons in the MD (Pakkenberg 1990; Popken et al. 2000; Young et al. 2000), as well as in the AV and the AM (Young et al. 2000). In a NHP model of schizophrenia, early gestational radiation exposure resulted in a reduction of MD neurons, as well as an adult-onset of cognitive and behavioral deficits resembling those seen in patients with schizophrenia (Selemon et al. 2009; Friedman and Selemon 2010; Selemon and Friedman 2013). As an increased risk of developing schizophrenia has been shown to occur following prenatal insult, these findings suggest that curtailment of thalamic neurogenesis may contribute to the pathogenesis of schizophrenia (Brown 2012; Selemon and Zecevic 2015).
The primary aim of this study was to determine the time of origin of neurons that comprise the ANT and the MD. However, our analysis was not limited to these two nuclei but extended throughout the entire rostral thalamus, revealing a previously undetected topography in the temporal sequence of neurogenesis in the thalamus. Knowing the time of origin of thalamic neurons in non-human primates will provide valuable information for identifying windows of vulnerability to disruption of thalamocortical circuits in neurodevelopmental disorders like schizophrenia and autism.
Materials and Methods
All analyses in this study were conducted using archived brain slides from a new primate brain resource, MacBrainResource (https://medicine.yale.edu/neuroscience/macbrain/). The search function of MBR was used to identify [3H]dT labeled brains of Macaca mulatta monkeys from Collection 1 that were suitable for birth-dating of thalamic neurons. Based on previous studies of neurogenesis in the thalamus (Rakic 1977a; Ogren and Rakic 1981), search parameters included experimental treatment dates, i.e., maternal injection of [3H]dT, ranging from embryonic day 25 (E25) to E50 and postnatal sacrifice dates. It is noteworthy that during the time window investigated in the present study, the vast majority of the newly generated cells destined to populate the thalamus are neurons and not glia. The detection of glia becomes possible weeks later (Rakic 1977a; Ogren and Rakic 1981). Ten cases were found with suitable labeling (Table 1). Autoradiographically processed, Nissl-counterstained sections of the thalamus mounted on slides were located for each of these cases. These slides had been generated using experimental protocols for [3H]dT labeling described in detail previously (Sidman 1970; Rakic 1973; Ogren and Rakic 1981) (Figure 1). Specifically, following systemic injection of [3H]dT at embryonic ages, the brains were fixed via perfusion with Karnovsky’s solution. Blocks of brain tissue were embedded in polyester wax, cut into coronal sections, and mounted onto glass slides. These slides were subsequently coated with a photosensitive emulsion. Following a period of 12– 16 weeks exposure in the dark, the slides were developed and stained with toluidine blue or cresyl violet (Figure 1). Slides spanning the rostral thalamus were selected for scanning on an Aperio ScanScope HR CS2 scanner at 20X magnification. Because some slides had deteriorated over decades of storage, these needed to be restored by recover-slipping (Figure 2) and in some instances re-staining of the Nissl counterstain. Slide images were uploaded to the Aperio eSlide Manager, and Aperio ImageScope was used initially to examine them. Digital files (svs) of slides containing the ANT and the MD were then transferred to a MicroBrightField system (Williston, VT) equipped with Stereo Investigator software (ver.2018.2.2). For each image file, the Trace tool was used to draw a closed contour around the thalamus at a zoom level equivalent to a magnification of ~ 5X. The zoom function at 100% zoom capacity was used in conjunction with the Meander Scan tool to move systematically throughout the closed contour. A cell was considered labeled for [3H]dT, and therefore to have undergone final cell division at the time of injection, if ≥5 silver grains were visible over the cell (Figure 3). When the Meander Scan was complete, the image with the overlaid tracing was exported as a tif file, transferred to Adobe Illustrator, and superimposed onto a closely corresponding section from the online BrainMaps macaque atlas (Mikula et al. 2007; http://brainmaps.org/index.php?p=speciesdata&species=macaca-mulatta) to facilitate anatomic analysis.
Table 1.
Ages of animals at time of 3H-thymidine injection and at postnatal sacrifice
| Case ID | Age at injection Embryonic day (E) | Age at sacrifice Postnatal day (P) |
|---|---|---|
| 071177 | E25 | P74 |
| 092776 | E27 | P67 |
| 101673A | E30 | P76 |
| 052677 | E31 | P56 |
| 101774A | E38 | P72 |
| 120574A | E38 | P60 |
| 031373 | E40 | P62 |
| 120574B | E43 | P70 |
| 080872A | E48 | P110 |
| 051673 | E50 | P61 |
Fig. 1.
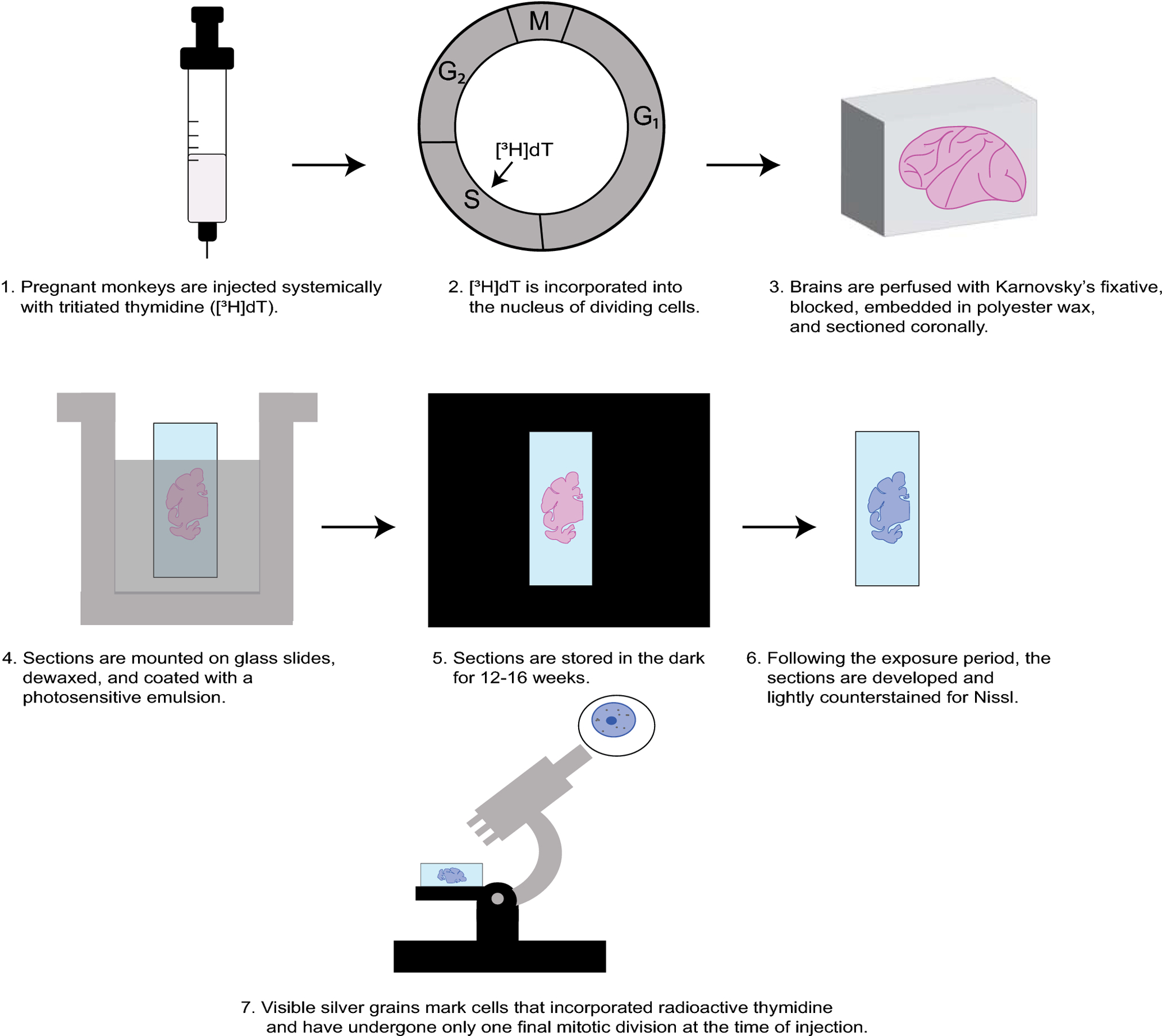
Schematic depiction of the autoradiography methodology carried out years ago in the preparation of specimens now in Collection 1 of MBR. Further technical details can be found in Sidman 1970, Rakic 1973, and Ogren and Rakic 1981. Abbreviations: [3H]dT, tritiated thymidine; S, DNA synthesis; G2, Growth and preparation for mitosis; M, Mitosis; G1, Growth.
Fig. 2.
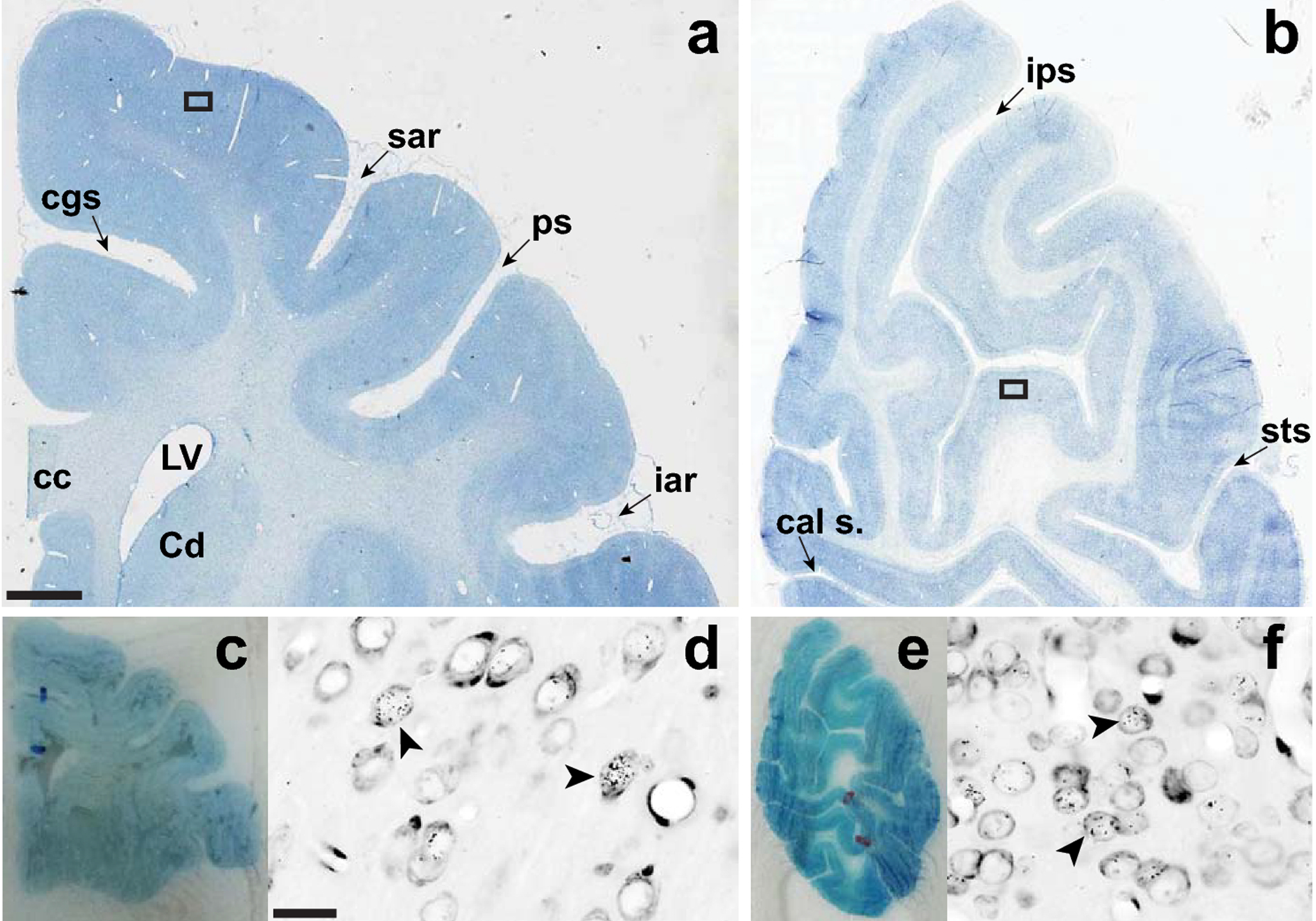
Restoration and scanning of brain sections. Panels a and b illustrate typical, already restored sections through the motor/somatosensory (a) and visual cortices (b) in which the old coverslips and mounting media were removed and replaced. Panels c and e show the corresponding sections before restoration in which the dried crystallized mounting media hinders proper visualization of the tissue. Panels d and f show high magnification views of the boxed areas in a and b. The arrow heads in each panel point to cells labeled with silver grains indicating uptake of tritiated thymidine. Slides were scanned at 20X using a high resolution Aperio ScanScope HR CS2 Digital Scanner. Scale bar in a (0.25 cm) also applies to b (0.33 cm), c (0.75 cm), and e (1.0 cm). Scale bar in d applies to f (20 μm). Abbreviations: cal s., calcarine sulcus; cc, corpus callosum; Cd, head of the caudate nucleus; cgs, cingulate sulcus; iar, inferior arcuate sulcus; ips, intraparietal sulcus; LV, lateral ventricle; ps, principal sulcus; sar, superior arcuate sulcus; sts, superior temporal sulcus.
Fig. 3.
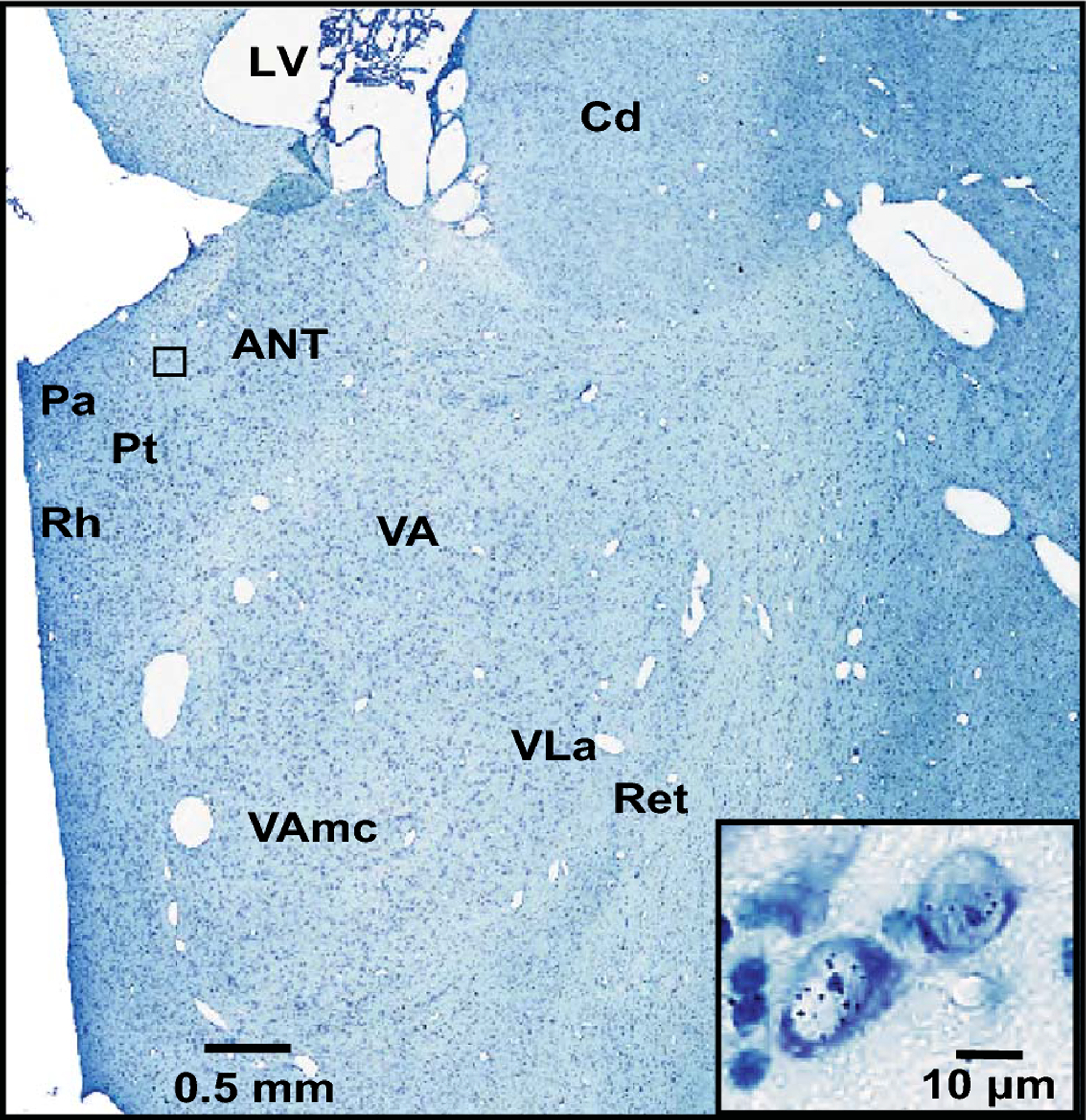
A view of the rostral thalamus including the ANT at E43. The black box indicates the location of the cells depicted in the high-magnification inset in which visible individual silver grains represent positively labeled neurons. Abbreviations: ANT, anterior nucleus; Cd, head of the caudate nucleus; LV, lateral ventricle; Pa, paraventricular nucleus; Pt, parataenial nucleus; Ret, reticular nucleus; Rh, rhomboid nucleus; VA, ventral anterior nucleus; VAmc, ventral anterior nucleus, magnocellular division; VLa, ventral lateral anterior nucleus.
Results
Comparison of [3H]dT labeling across the embryonic ages studied (E25-E50) revealed a distinct spatial-temporal patterning of neurogenesis in the rostral thalamus (Figures 4, 5). The earliest detected cells were located in the medial rostral thalamus (E30-E31), followed by more widespread cellular populations in lateral and ventrally situated nuclei (E38-E40). Later (E43) labeled cells were predominant in lateral nuclei at rostral levels and distributed widely at caudal levels but densest dorsomedially.
Fig. 4.
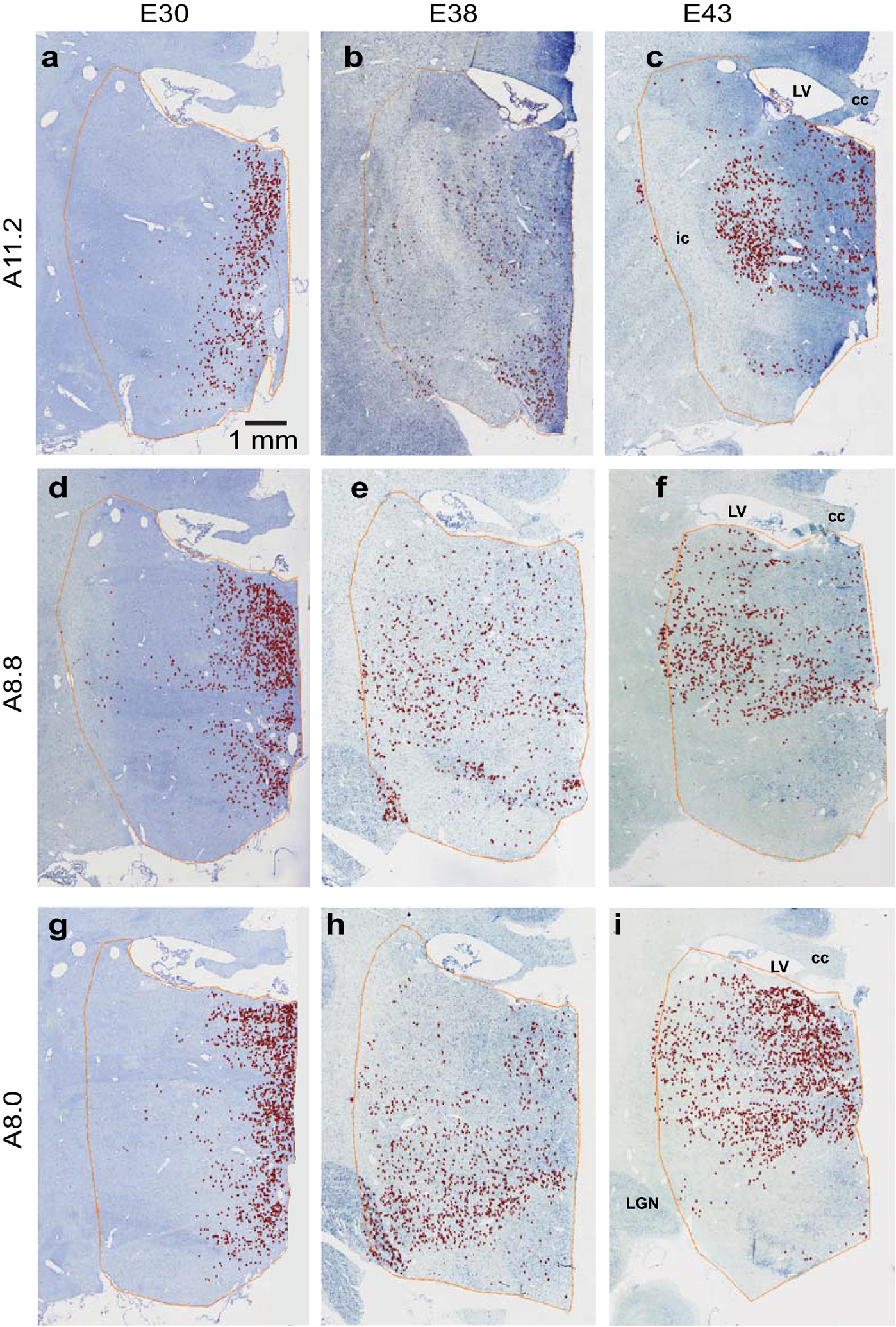
Topographic patterning of [3H]dT labeling in the rostral thalamus. [3H]dT labeled neurons are shown in monkeys injected at gestational ages E30 (a, d, g), E38 (b, e, h), and E43 (c, f, i), and sacrificed postnatally. Labeling at each of these ages is represented at three different rostro-caudal levels of the thalamus corresponding to sections from the online BrainMaps atlas: Horsley-Clarke coordinates A11.2 (a, b, c), A8.8 (d, e, f), and A8.0 (g, h, i). Labeling from E43, originally captured in the right hemisphere, was flipped in order to maintain consistency and facilitate comparison across ages. The scale bar applies to all panels. Abbreviations: cc, corpus callosum; ic, internal capsule; LGN, lateral geniculate nucleus; LV, lateral ventricle.
Fig. 5.
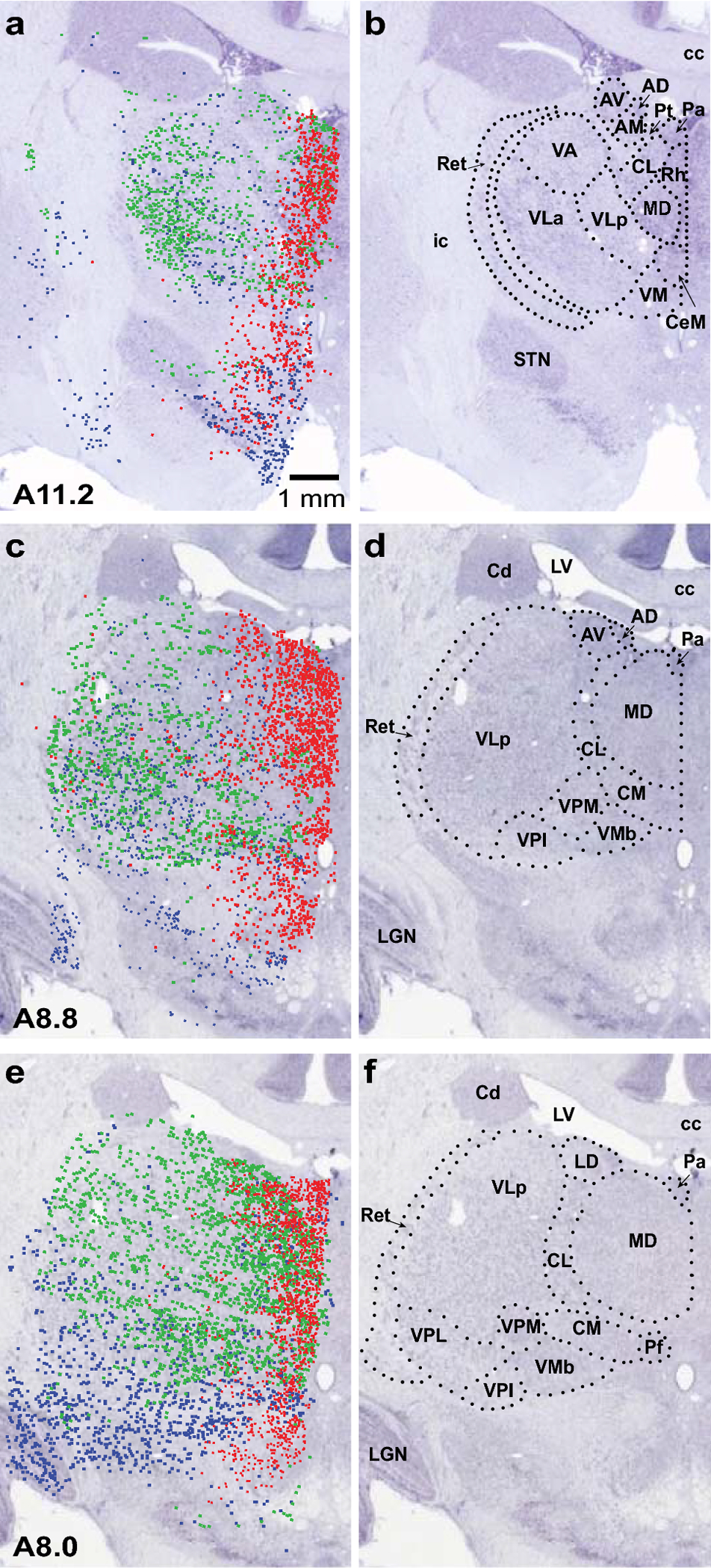
Superimposition of [3H]dT labeling onto corresponding BrainMaps sections with designated nuclear boundaries. In the left column (a, c, e), labeling from three ages (E30, E38 and E43) is superimposed on images of corresponding left hemisphere BrainMaps sections. At E30 (red), labeling was most dense at the medial border of the thalamus; by E38 (blue), labeling became more widespread and laterally distributed and, at caudal levels, it was pronounced ventrolaterally. At E43 (green), labeling was heaviest laterally in the rostral thalamus and widespread at caudal levels but predominating dorsomedially. As in Figure 4, the E43 sections were flipped to facilitate comparison across ages. In the right column (b, d, f), nuclear boundaries and designations are included on the BrainMaps sections to signify delineations between thalamic nuclei. Abbreviations: AD, anterodorsal nucleus; AM, anteromedial nucleus; AV, anteroventral nucleus; cc, corpus callosum; Cd, head of caudate nucleus; CeM, central medial nucleus; CL, central lateral nucleus; CM, centromedian nucleus; ic, internal capsule; LD, lateral dorsal nucleus; LGN, lateral geniculate nucleus; LV, lateral ventricle; MD, mediodorsal nucleus; Pa, paraventricular nucleus; Pf, parafascicular nucleus; Pt, parataenial nucleus; Ret, reticular nucleus; Rh, rhomboid nucleus; STN, subthalamic nucleus; VA, ventral anterior nucleus; VLa, ventral lateral anterior nucleus; VLp, ventral lateral posterior nucleus; VM, ventral medial nucleus; VMb, basal ventral medial nucleus; VPI, ventral posterior inferior nucleus; VPL, ventral posterior lateral nucleus; VPM, ventral posterior medial nucleus. The scale bar applies to all panels.
Neurogenesis of MD and ANT
Dense [3H]dT labeling was observed in the MD during a two-week period (E30-E43) towards the end of what in the macaque (165 days of gestation) corresponds to the first trimester of human gestation, and during a similar time frame for the ANT (E31-E43) (Table 2). A few labeled cells were found in the MD at earlier (E27) and later ages (E48-E50), and in the ANT at (E30). Notably, labeled cells in the MD were more prominent rostrally and medially at early ages and shifted to more caudal and lateral portions of the MD at later ages (Figures 4, 5).
Table 2:
Time of Cell Origin in Thalamic Nuclei
| Age | MD | IL | VA | Midline | ANT | VL | Ret | VP | LGN1 | PUL2 |
|---|---|---|---|---|---|---|---|---|---|---|
| E25 | ||||||||||
| E27 | * | * | * | * | ||||||
| E30 | ** | ** | * | ** | * | * | ||||
| E31 | ** | ** | * | ** | ** | * | ||||
| E38 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| E40 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| E43 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| E48 | * | * | ** | |||||||
| E50 | * | * | * | |||||||
= light label;
= moderate/heavy label;
ANT = anterior nuclei; IL = intralaminar nuclei; LGN = lateral geniculate nucleus; MD = mediodorsal nucleus; PUL = pulvinar; Ret = reticular nucleus VA = ventral anterior nucleus; VL = ventral lateral nucleus; VP = ventral posterior nucleus.
Results from previous studies that examined neurogenesis of the LGN and PUL, respectively (Rakic 1977a; Ogren and Rakic 1981). In addition, positive labeling was observed at E36 in the LGN and at E36 and E45 in the PUL.
Neurogenesis of the rostral thalamus
(E25-E27):
At E25 (Table 2), only a few scattered labeled cells were found in the thalamus. At E27, labeling was still sparse and scattered. A few [3H]dT labeled cells were present in the medial MD, the ventral anterior nucleus (VA), the midline nuclei, and the central lateral nucleus (CL).
(E30-E31):
At E30-E31 (Table 2, Figure 4, 5), thalamic labeling was characterized by a compact medial band that included the medial MD, the midline nuclei, and the intralaminar nuclei (central medial nucleus (CeM), CL, parafascicular nucleus (Pf)). At E30, only light labeling was observed in the ANT; E31 marked the beginning of peak proliferation populating the ANT. Light labeling was also found in the VA and the ventral lateral nucleus (VL).
(E38-E40):
At E38-E40 (Table 2, Figure 4, 5), overall labeling was more evenly and widely distributed throughout the rostral thalamus, extending into more laterally situated nuclei: the lateral MD, the VL, the reticular nucleus (Ret), the ventral posterior nucleus (VP), and the centromedian nucleus (CM). At very rostral levels of the thalamus, moderate labeling was observed in the ANT and the VA. More caudally, labeling was light in these nuclei. At the most caudal levels of the thalamus that were examined, labeling was particularly prominent in the ventral medial nucleus (VM) and the VP.
(E43):
At E43 (Table 2, Figure 4, 5), labeling was predominantly located in the lateral thalamus at rostral levels. More caudally, labeling was widespread throughout the thalamus but most dense dorsomedially. Labeling was dense in both the ANT and the MD, as well as in the midline, the intralaminar, and the ventral nuclei (VA, VL, VP).
(E48-E50):
By E48 (Table 2), moderate labeling was narrowly confined to a single midline nucleus, the paraventricular nucleus (Pa). Only light labeling was present in the medial MD. No labeling was observed in the ANT. At E50, labeling was scarce in the thalamus, with light labeling present in the midline nuclei, the MD, and the Pf.
Discussion
This study establishes that neurogenesis in the thalamus of the macaque brain is not temporally homogeneous but rather exhibits spatial-temporal patterning. Seminal work in this field has found that neurogenesis is temporally staggered throughout the macaque brain, as for example, neurogenesis begins and ends earlier in the hypothalamus than in the striatum (Brand and Rakic 1979; van Eerdenburg and Rakic 1994). Our study indicates that nuclei within the thalamus have non-uniform, albeit overlapping, periods of neurogenesis. Previously, neurogenesis in the neocortex was shown to exhibit inside-out patterning (Rakic 1974). In addition, a prior study established that within one nucleus of the thalamus, the LGN, the gradient for cell population is outside to inside, the opposite of that in the cortex; neurons generated earlier go to the outmost surface of the LGN and those generated later progressively accumulate in more inner positions (Rakic 1977b). The present study examined neurogenesis across multiple nuclei in the rostral thalamus and found a complex, temporally-dependent topography. Early generated cells populate the medial margin of the thalamus, followed by more widespread population concentrated ventrolaterally, and finally by the appearance of labeled cells predominantly in lateral nuclei at rostral levels and more widespread caudally. In keeping with this patterning, we found that the generation of cells in the medially situated ANT and MD begins earlier than that of the previously studied and more laterally located nuclei, the LGN and PUL (Rakic 1977a; Ogren and Rakic 1981). Furthermore, a spatial-temporal gradient of neurogenesis was evident even within the MD. Neurons destined for more caudal levels of the MD, which is exclusively parvocellular (MDpc), are generated later than those for rostral levels that include both the MDpc and the magnocellular MD (MDmc). New insights into the topographic patterning of neurogenesis in the thalamus may contribute to contemporary neuromorphologic models, e.g., the prosomeric model, that are based on genoarchitectonic patterning and data from developmental experimentation (Puelles 2019).
Interspecies Comparisons
Maternal injection of [3H]dT also has been used to examine the development of thalamic nuclei in rodents. Taking into account that the duration of rodent gestation is much shorter (~21 days) in comparison to primates (165 days), the onset of thalamic neurogenesis in rodents (E10 for mice; E13 for rats) occurs later in the gestational period relative to thalamogenesis in primates (Angevine 1970; Altman and Bayer 1988). The findings of the present study shed light on interspecies differences. In contrast to the neurogenetic patterning of the primate thalamus described in this study, neurogenesis of the rodent thalamus exhibits caudo-rostral and latero-medial gradients (McAllister and Das 1977). With regard to specific nuclei, while our results indicate that the generation of the rostrally situated ANT and MD in primates occurs several days before the more caudal LGN and PUL, neurogenesis of the ANT and MD can take place up to two days after the LGN and lateral posterior nucleus (LP) in rats and mice (Angevine 1970; Altman and Bayer 1979, 1988, 1989). In the thalamus as a whole, there exists additional opposing temporal spatial gradients, in which neurogenesis in rodents begins in laterally situated nuclei and spreads medially throughout, whereas neurogenesis of the medial thalamus precedes the lateral portion in primates (Angevine 1970; McAllister and Das 1977).
Technical Considerations
Data were collected from brains at various gestational ages, but not every embryonic age was examined, because the original studies injected animals only at selected ages and because certain cases were excluded from analysis due to insufficient quality of cell labeling. However, we successfully examined cases that spanned the entire interval of neurogenesis for the MD and ANT and included embryonic ages that both preceded and followed this period. While we lacked data for several intervening ages, we were able to observe the trajectory of cell proliferation and identify predominant shifts in patterning of the rostral thalamus over time.
Prior to including cases in this study, we extensively researched all available case records and laboratory notes. In doing so, we found that case 080872A, which previously had been designated as having an injection at E53/E54, in fact had been injected at E48. The resolution of this discrepancy demonstrates the value of having original case notes for reference.
Despite its limitations, this study demonstrates the potential for the application of modern technologies to banked brain tissue to shed new light on neurodevelopmental processes. Today, these experiments, which involve timed pregnancies, systemic injection of [3H]dT in pregnant monkeys, monitoring of fetal health, a functioning infant primate nursery, and long-term survival times, would not only be prohibitively expensive but would also be nearly impossible to replicate given the strict regulatory guidelines for primate research.
Relevance to Disease
Neurogenesis is the most prominent developmental process occurring in the first trimester of gestation (Bystron et al. 2008; Selemon and Zecevic 2015). Therefore, interference with neurogenesis may underly disease development for those neuropsychiatric illnesses associated with environmental insult during early gestation, such as schizophrenia and autism (Brown 2012). Knowledge of the exact dates of proliferation for brain nuclei can help pinpoint the critical period of vulnerability for disease. For example, the pathology of schizophrenia includes reduced number of neurons in the MD and the ANT while sparing the LGN (Pakkenberg 1990; Popken et al. 2000; Young et al. 2000; Selemon and Begovic 2007). The slightly earlier timing of neurogenesis in the MD and the ANT compared with that of the LGN suggests that fetal insult occurring specifically at this earlier window of time may be associated with schizophrenia. An experimental study in the macaque brain that used fetal irradiation at E33-E42 to curtail neurogenesis reaffirms the temporal specificity in neurogenesis, as significant neuron reduction was found in the MD but not in the PUL (Selemon et al. 2009). While this same study failed to detect a significant reduction in neuron number in the ANT, this could have been due to technical limitations related to the small size of the ANT. Interestingly, one postmortem study of subjects with schizophrenia found pathology in the MD that was specific to the parvocellular and densocellular subnuclei (Popken et al. 2000). In the present study, we detected a temporal difference in the rostro-caudal axis of the MD, suggesting that temporal patterning within the MD may explain the selective subnuclear vulnerability. From a broader perspective, the topographic patterning of thalamic neurogenesis suggests that fetal insult at different ages may be associated with distinct clusters of functional disruption. For example, early generated nuclei, such as the ANT and the MD, have been implicated in cognitive behavior (Mitchell 2015; Bubb et al. 2017), whereas some later generated nuclei are sensory relays, e.g., the LGN, and the VP.
Acknowledgments:
This work was supported by grant R01MH113257 (AD and LDS). We thank Mariamma Pappy for technical assistance.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of interest
The authors declare no conflicts of interest.
Availability of data and material
All slides used for analysis in this study are freely available to the public through MacBrainResource (macbrainresource.org), an online macaque brain resource that enables researchers to download digital, high-resolution images of slides.
References
- Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271. 10.1016/0166-2236(90)90107-l [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1979) Development of the diencephalon in the rat. IV. Quantitative study of the time of origin of neurons and the internuclear chronological gradients in the thalamus. J Comp Neurol 188:455–471. 10.1002/cne.901880308 [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1988) Development of the rat thalamus: II. Time and site of origin and settling pattern of neurons derived from the anterior lobule of the thalamic neuroepithelium. J Comp Neurol 275:378–405. 10.1002/cne.902750305 [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1989) Development of the rat thalamus: VI. The posterior lobule of the thalamic neuroepithelium and the time and site of origin and settling pattern of neurons of the lateral geniculate and lateral posterior nuclei. J Comp Neurol 284:581–601. 10.1002/cne.902840407 [DOI] [PubMed] [Google Scholar]
- Angevine JB Jr (1970) Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol 139:129–187. 10.1002/cne.901390202 [DOI] [PubMed] [Google Scholar]
- Brand S, Rakic P (1979) Genesis of the primate neostriatum: [3H]thymidine autoradiographic analysis of the time of neuron origin in the rhesus monkey. Neuroscience 4:767–778. 10.1016/0306-4522(79)90005-8 [DOI] [PubMed] [Google Scholar]
- Brand S, Rakic P (1980) Neurogenesis of the nucleus accumbens septi and neighboring septal nuclei in the rhesus monkey: a combined [3H]thymidine and electron microscopic study. Neuroscience 5:2125–2138. 10.1016/0306-4522(80)90128-1 [DOI] [PubMed] [Google Scholar]
- Brown AS (2012) Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol 72:1272–1276. 10.1002/dneu.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Kinnavane L, Aggleton JP (2017) Hippocampal - diencephalic - cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci Adv 1:2398212817723443. 10.1177/2398212817723443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: Boulder committee revisited. Nat Rev Neurosci 9:110–122. 10.1038/nrn2252 [DOI] [PubMed] [Google Scholar]
- DeNicola AL, Park MY, Crowe DA, MacDonald AW 3rd, Chafee MV (2020) Differential roles of mediodorsal nucleus of the thalamus and prefrontal cortex in decision-making and state representation in a cognitive control task measuring deficits in schizophrenia. J Neurosci 40:1650–1667. 10.1523/JNEUROSCI.1703-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, Skudlarski P, Goldman-Rakic PS, Krystal JH (2008) Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry 64:1026–1034. 10.1016/j.biopsych.2008.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A, McCormick DA (2010) Circuit-based localization of ferret prefrontal cortex. Cereb Cortex 20:1020–1036. 10.1093/cercor/bhp164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HR, Selemon LD (2010) Fetal irradiation interferes with adult cognition in the nonhuman primate. Biol Psychiatry 68:108–111. 10.1016/j.biopsych.2010.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1990) Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res 85:325–336. 10.1016/s0079-6123(08)62688-6 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ (1985) The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 242:535–560. 10.1002/cne.902420406 [DOI] [PubMed] [Google Scholar]
- Gould BB, Rakic P (1981) The total number, time or origin and kinetics of proliferation of neurons comprising the deep cerebellar nuclei in the rhesus monkey. Exp Brain Res 44:195–206. 10.1007/BF00237341 [DOI] [PubMed] [Google Scholar]
- Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, Aggleton JP, O’Mara SM (2013) The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci 7:45. 10.3389/fnsys.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Rakic P (1990) Neurogenesis of the magnocellular basal forebrain nuclei in the rhesus monkey. J Comp Neurol 291:637–653. 10.1002/cne.902910410 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Piecinski P, Rakic P (1992) Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Res Dev Brain Res 68:9–15. 10.1016/0165-3806(92)90242-o [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG (2012) Neuroanatomy and neuropathology associated with Korsakoff’s syndrome. Neuropsychol Rev 22:72–80. 10.1007/s11065-012-9195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JP, Das GD (1977) Neurogenesis in the epithalamus, dorsal thalamus and ventral thalamus of the rat: an autoradiographic and cytological study. J Comp Neurol 172:647–686. 10.1002/cne.901720407 [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL (1996) Deciphering the hippocampal polyglot: The hippocampus as a path integration system. J Exp Biol 199(Pt 1):173–185. [DOI] [PubMed] [Google Scholar]
- Mikula S, Trotts I, Stone J, Jones EG (2007) Internet-Enabled High-Resolution Brain Mapping and Virtual Microscopy. NeuroImage 35:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS (2015) The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev 54:76–88. 10.1016/j.neubiorev.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Ogren MP, Rakic P (1981) The prenatal development of the pulvinar in the monkey: 3H-thymidine autoradiographic and morphometric analyses. Anat Embryol (Berl) 162:1–20. 10.1007/BF00318090 [DOI] [PubMed] [Google Scholar]
- Pakkenberg B (1990) Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 47:1023–1028. 10.1001/archpsyc.1990.01810230039007 [DOI] [PubMed] [Google Scholar]
- Papez JW (1937) A proposed mechanism of emotion. Arch NeurPsych 38:725–743. 10.1001/archneurpsyc.1937.02260220069003 [DOI] [Google Scholar]
- Parnaudeau S, Bolkan SS, Kellendonk C (2018) The mediodorsal thalamus: An essential partner of the prefrontal ccrtex for cognition. Biol Psychiatry 83:648–656. 10.1016/j.biopsych.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE Jr, Potkin SG, Jones EG (2000) Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 97:9276–9280. 10.1073/pnas.150243397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L (2019) Survey of midbrain, diencephalon, and hypothalamus neuroanatomic terms whose prosomeric definition conflicts with columnar tradition. Front Neuroanat 13:20. 10.3389/fnana.2019.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P (1973) Kinetics of proliferation and latency between final cell division and onset of differentiation of cerebellar stellate and basket neurons. J Comp Neurol 147:523–546. 10.1002/cne.901470407 [DOI] [PubMed] [Google Scholar]
- Rakic P (1974) Neurons in rhesus-monkey visual-cortex: Systematic relation between time of origin and eventual disposition. Science 183:425–427. 10.1126/science.183.4123.425 [DOI] [PubMed] [Google Scholar]
- Rakic P (1977a) Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol 176:23–52. 10.1002/cne.901760103 [DOI] [PubMed] [Google Scholar]
- Rakic P (1977b) Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci 278:245–60. 10.1098/rstb.1977.0040 [DOI] [PubMed] [Google Scholar]
- Rakic P, Nowakowski RS (1981) The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol 196:99–128. 10.1002/cne.901960109 [DOI] [PubMed] [Google Scholar]
- Safari V, Nategh M, Dargahi L, Zibaii ME, Khodagholi F, Rafiei S, Khatami L, Motamedi F (2020) Individual subnuclei of the rat anterior thalamic nuclei differently affect spatial memory and passive avoidance tasks. Neuroscience 444:19–32. 10.1016/j.neuroscience.2020.07.046 [DOI] [PubMed] [Google Scholar]
- Savage LM, Nunes PT, Gursky ZH, Milbocker KA, Klintsova AY (2020) Midline thalamic damage associated with alcohol-use disorders: Disruption of distinct thalamocortical pathways and function. Neuropsychol Rev 31:447–471. 10.1007/s11065-020-09450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Begovic A (2007) Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res 151:1–10. 10.1016/j.psychres.2006.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Friedman HR (2013) Motor stereotypies and cognitive perseveration in non-human primates exposed to early gestational irradiation. Neuroscience 248:213–224. 10.1016/j.neuroscience.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N (2015) Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry 5:e623. 10.1038/tp.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Begovic A, Rakic P (2009) Selective reduction of neuron number and volume of the mediodorsal nucleus of the thalamus in macaques following irradiation at early gestational ages. J Comp Neurol 515:454–464. 10.1002/cne.22078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL (1970) Autoradiographic methods and principles for study of the nervous system with thymidine-H3. In: Nauta WJH, Ebbesson SOE (eds) Contemporary research methods in neuroanatomy. Springer, Berlin, Heidelberg, pp 255–270. 10.1007/978-3-642-85986-1_12. [DOI] [Google Scholar]
- van Eerdenburg FJ, Rakic P (1994) Early neurogenesis in the anterior hypothalamus of the rhesus monkey. Brain Res Dev Brain Res 79:290–296. 10.1016/0165-3806(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB (2015) Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev 54:89–107. 10.1016/j.neubiorev.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Alcaraz F, Marchand AR, Coutureau E (2015) Functional heterogeneity of the limbic thalamus: From hippocampal to cortical functions. Neurosci Biobehav Rev 54:120–130. 10.1016/j.neubiorev.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC (2000) Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 47:944–953. 10.1016/s0006-3223(00)00826-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All slides used for analysis in this study are freely available to the public through MacBrainResource (macbrainresource.org), an online macaque brain resource that enables researchers to download digital, high-resolution images of slides.


