Abstract
Ten years since the immune checkpoint inhibitor ipilimumab was approved for advanced melanoma, it is time to reflect on the lessons learned regarding modulation of the immune system to treat cancer and on novel approaches to further extend the efficacy of current and emerging immunotherapies. Here, we review the studies that led to our current understanding of the melanoma immune microenvironment in humans and the mechanistic work supporting these observations. We discuss how this information is guiding more precise analyses of the mechanisms of action of immune checkpoint blockade and novel immunotherapeutic approaches. Lastly, we review emerging evidence supporting the negative impact of melanoma metabolic adaptation on anti-tumor immunity and discuss how to counteract such mechanisms for more successful use of immunotherapy.
Introduction
Melanoma is the most aggressive and deadly of skin cancers. Cutaneous melanomas are the most frequent, and are typically associated with UV exposure and elevated tumor mutational burden (TMB), which contribute to high immunogenicity. Several historical observations point to melanoma as an immune responsive tumor. First, 5% of melanoma patients present with metastatic disease that genotypically resembles cutaneous melanoma, but without an identifiable primary melanoma, suggesting that the primary tumor may have spontaneously regressed1. Second, melanoma is often associated with vitiligo – the manifestation of an autoimmune reaction against melanocytes, indicating cross-reactive immune responses targeting melanoma and normal melanocytes. Vitiligo was shown to be a favorable prognostic indicator in patients2, suggesting that anti-melanocytic immune responses help control melanoma growth. Third, melanoma can be infiltrated by reactive lymphocytes3, with dense infiltration of peri-tumoral lymphocytes being associated with better prognosis, and melanoma classification based on tumor-infiltrating lymphocyte (TIL) distribution (brisk, non-brisk and absent) is still used today4–7. However, melanoma disseminates and metastasizes very easily, indicating that active immune suppression or dysfunction must offset its immunogenicity. Reliance on immune evasion mechanisms for disease progression may underscore the specific vulnerability of melanoma to immunotherapy, thus explaining its unique responsiveness to these treatments.
The concept of immune checkpoint blockade (ICB) for the treatment of cancer was pioneered by Jim Allison and colleagues showing that antibodies blocking the T cell co-inhibitory receptor CTLA-4 can regress tumors in mice8. Human CTLA-4 blocking antibodies were then developed and tested in patients, with ipilimumab becoming the first therapy to extend survival in metastatic melanoma9,10, which led to its approval in this disease in 2011. PD-1 was recognized as another key T cell immune checkpoint11. PD-1 or PD-L1 blocking antibodies were found to enhance tumor control in mice12,13 and CD8+ T cell functionality in a chronic viral infection model14. Promising results in early clinical trials with PD-1 blocking antibodies in refractory solid tumors were confirmed in phase-3 studies in melanoma, where the PD-1 inhibitors pembrolizumab and nivolumab were found to extend survival compared to ipilimumab or chemotherapy15–19. These agents were then approved for the treatment of metastatic melanoma in 2014.
Overall, the clinical success with ICB in melanoma has confirmed the therapeutic impact of re-invigorating the immune system to effectively target this disease. However, even in the optimal scenarios with combination ICB, approximately half of patients fail to achieve long-lasting benefit20. This indicates the need for better predictive biomarkers of response and new rational targets for more effective combination treatments to overcome immune resistance. While elevated tumor PD-L1 expression and TMB have been found to correlate with clinical responses to ICB in melanoma21, these biomarkers cannot accurately predict outcome in all cases. Because the longest and most consolidated clinical experience with ICB is in melanoma, this information can be now leveraged to achieve a more precise understanding of the molecular determinants of activity of these therapies in patients.
Here, we provide an updated overview of the immune landscape of human melanoma and how it is modulated by ICB, focusing on studies in patients. Moreover, we highlight current limitations of immunotherapy and delineate the next potential avenues to improve the use of these treatments.
Melanoma-specific T cells and their therapeutic potential
Melanoma TILs are enriched for specificity to melanoma-associated antigens, indicating that anti-melanoma T cells can undergo priming, expansion, and recruitment to the tumor (Fig. 1a). Endogeneous T cell responses to melanoma have been exploited for multiple objectives, including (1) identification of the cognate antigens that can then be used for vaccine development; (2) expansion and/or engineerization of tumor-specific T cells for adoptive cell therapy (ACT).
Figure 1. The immune microenvironment of melanoma.
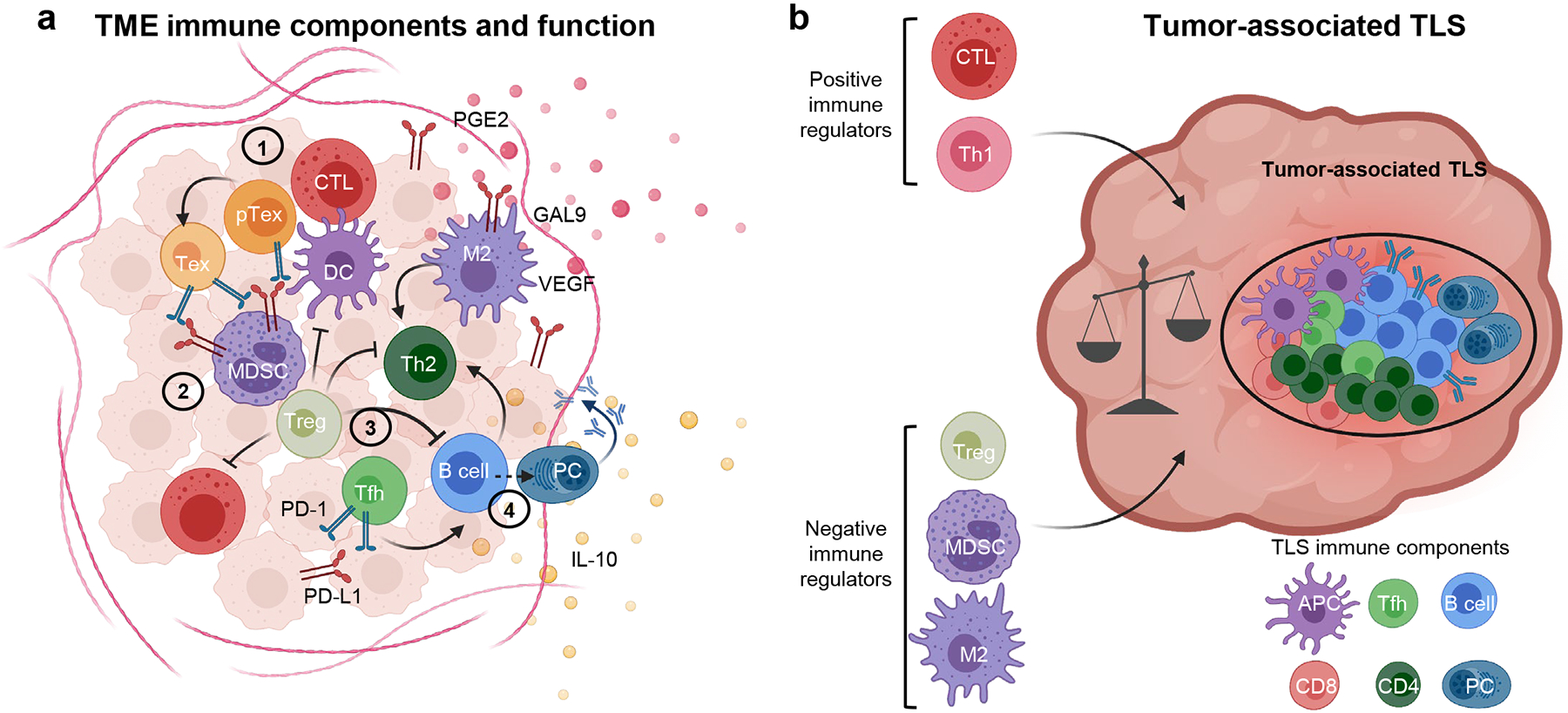
a, Schematic representation of the principal immune components in the melanoma microenvironment. Four main functional modules can be distinguished: (1) a CD8 module (red/orange), including cytotoxic T cells (CTLs) and a spectrum of dysfunctional cells including progenitor Tex (pTEX) and terminal TEX cells; (2) an innate module (violet/purple), which impact on the recruitment and activation of T cells depending on the tolerogenic (tolerogenic DCs; myeloid derived suppressor cells, MDSC), pro- (M2) or anti-tumor (DCs) inflammatory potential; (3) a CD4 module (green), which is highly heterogeneous and can be shaped by the immune cells in the other modules and include immunosuppressive Tregs, Th2 cells with pro-tumor inflammatory potential and Tfh-like cells which can promote B cell function; and (4) a B cell module (blue), including B cells in various stages of differentiation up to plasma cells (PCs), with pro- or anti-tumor function depending on the profile of immunoglobulins produced (IgA, IgG2, IgG4 vs. IgG1 respectively) and expression of co-inhibitory molecules (e.g. IL-10 and PD-L1). Each of these immune modules has a counter-regulatory program to dampen immune responses, thus explaining the coexistence of tumor cells and anti-tumor lymphocytes in the same environment. b, The immune infiltrate in melanoma can organize in cellular aggregates defined as a tertiary lymphoid structure (TLS), with B cells at the core surrounded by T cells and APCs, which generate a germinal-center like pattern. The impact of these structures on anti-tumor immunity is likely determined by the potential to recruit or expand CTLs and Th1 cells vs. immunosuppressive or TEX cells. Created with BioRender.com.
The easy access to cutaneous melanoma lesions prompted the study of melanoma TILs, which was further facilitated by the discovery of IL-222 in 1977 enabling expansion of T cells in vitro for their characterization. T cells recognizing melanocyte differentiation antigens (e.g. MelanA and gp100), cancer-germline (cancer-testis) antigens, and tumor-overexpressed antigens in human melanoma were then identified (Box 1) and comprehensively profiled using peptide-MHC multimers23,24. More recently, high-throughput antigen screening coupled with next-generation sequencing of both T cell receptor (TCR) repertories and tumor genomes have substantially expanded our horizons regarding anti-tumor T cell specificity and their dynamics25. Indeed, advances in tumor sequencing and associated computational approaches have allowed the identification of tumor mutation-derived neoantigens (Box 1) that contribute to tumor immunogenicity and T cell recognition25. Neoantigens are particularly abundant in melanoma, given the elevated frequency of somatic mutations in this disease26. Notably, many anti-melanoma TILs recognize neoantigens derived from tumor somatic mutations27–29, which are attractive for tumor-specific targeting and reduced autoimmune toxicity. To boost T cell responses in vivo against tumor neoantigens, vaccines with tumor-associated neoepitopes have been demonstrated to be safe and to elicit potent tumor-specific T cell responses in melanoma patients30–32. These responses included both CD4+ and CD8+ T cells, pointing towards dual CD4+ and CD8+ immunogenicity of strong neoepitopes and possibly to the ability of CD4+ T cells to directly control melanoma tumors. This mechanism may be particularly relevant in the setting of melanoma, considering its unique MHC-II expression.
Box1: Relevant tumor antigens in melanoma.
Cancer germline antigens:
Cancer-germline genes (e.g Mage-A1, NY-ESO-1) are methylated and silenced in human adult tissue, except male germ cells and trophoblastic cells, which lack MHC-I molecules. Tumors often have aberrant patterns of DNA methylation, resulting in the demethylation, ectopic expression and presentation of cancer-germline genes to T cells, leading to immune recognition147,148.
Melanocyte differentiation antigens:
Genes encoding melanocyte differentiation antigens (e.g. MART-1, gp100, tyrosinase) play a role in the normal differentiation of melanocytes, and are thus shared between the tumor and melanocytes. Normal melanocytes are present in the skin, inner ear, and eye. Immune responses to these antigens target both melanoma and normal melanocytes. A manifestation of this cross-reacting response is vitiligo.
Overexpressed antigens:
Overexpressed antigens (e.g. PRAME) are antigens expressed at low levels in normal host tissue, but overexpressed in tumors, thus creating a therapeutic window for immune intervention.
Neoantigens:
Neoantigens are derived from tumor-specific somatic mutations that are absent in the normal human genome and are exclusively expressed in cancer cells. 95% of somatic mutations are single nucleotide variants (SNV), resulting in proteins (and peptides) with single amino acid substitions149. Neopeptides also arise from nucleotide insertions or deletions (indels), which generate frameshift or non-frame shifted sequences, depending on the number of nucleotides added and deleted, leading to aberrant protein expression. While the minority of mutations are indels (<5% for melanoma)147,150, frameshift mutations can generate a number of immunogenic neoepitopes that are highly distinct from self.
Additional sources of immunogenic antigens:
Immunogenic epitopes can also derive from mutations associated with gene fusion, aberrant mRNA splicing resulting in retained introns, or aberrant translation resulting in cryptic antigens147. In addition, endogenous retroviruses, which are integrated genomic sequences of viral origin inherited as remnants from previous retroviral infections, are normally epigenetically silenced in normal host tissue, but can be re-expressed in tumors147, similar to cancer germline antigens.
Overall, the existence of melanoma-specific TILs indicated that in many cases (1) these cells are numerically or functionally insufficient to completely eradicate the tumor, and (2) they could be boosted to achieve the numbers required for complete tumor eradication in vivo. Towards this goal, ACT using ex vivo expanded TILs was shown to contribute to tumor regressions, especially in melanoma. The Rosenberg group at the Surgery Branch of the National Cancer Institute and other teams demonstrated that ACT of TILs can be clinically effective, further confirming the tumor reactivity of melanoma-derived T cells33–36. Interestingly, ACT using TIL products enriched for tumor-neoantigen specificities were found to produce durable clinical responses with no significant toxicity37–42, suggesting a potential role for T cells recognizing tumor-associated neoantigens in the activity of ACT.
From the early to the most advanced characterization of melanoma TIL specificities and the use of these cells as anti-cancer therapy, these studies have extensively corroborated the biologic and therapeutic relevance of T cell responses in melanoma patients. Whether the recent developments with neoantigen-specific immunotherapy will lead the next chapter of ACT and/or vaccines in melanoma remains to be determined, especially considering that neoantigens arise from unique tumor-specific mutations rarely shared between patients, requiring patient-specific identification and product manufacturing25.
B cell responses in melanoma
Melanoma antigens can also elicit B-cell responses, further supporting the immunogenicity of this disease. Serological analyses from melanoma patients contributed to the definition of several melanoma-associated antigens43–45. Autoantibodies against melanoma-associated antigens were reported to develop in melanoma patients and to correlate with improved prognosis in certain cases46–48. However, whether anti-melanoma antibody responses play a causal role in tumor protection remains to be fully elucidated. Mature B cells can be found in melanoma lesions at higher frequencies compared to normal skin and have been described to localize in aggregates with T cells and dendritic cells (DCs), defined as tertiary lymphoid structures (TLS)49,50 (Fig. 1b). TLS are ectopic lymphoid structures that typically form in response to chronic inflammation and evolve dynamically to adapt to the local tissue injury51. Mature TLS comprising T and B cells in germinal-center-like zones are found more frequently than immature, B-cell-depleted TLS in metastatic melanoma lesions49. Key mediators of TLS formation, including early (CXCL13), intermediate (lymphotoxin beta receptor) and later lympho-angiogenic factors (CCL21, LIGHT), can be overexpressed in metastatic melanoma52,53. It is possible that chronic immunogenic stimuli elicited by melanoma-associated antigens trigger TLS-supporting signals that recruit and expand tumor-specific B cells. In TLS, B cells can theoretically undergo maturation into antibody-secreting cells in the presence of proper T cell-mediated helper signals and/or can serve as antigen presenting cells (APCs) contributing to local tumor-specific T cell priming. The relative impact of these two B cell functions in TLS as well as the role of B cell antigen-specificity and functionality in tumors remain to be precisely and comprehensively elucidated. B cells and their antibody products may be highly heterogeneous, with profiles ranging from pro-inflammatory (IgG1+) to immunosuppressive (e.g. CD1d+IL-10+PD-L1+, IgA+, IgG2+, or IgG4+) (Fig. 1b). This heterogeneity may explain the apparently conflicting results from independent studies reporting associations between B cell infiltrates and either favorable50,54,55 or negative56 prognosis in melanoma patients. These discrepancies may be also explained by the variety of microenvironmental immune signals and their effects on intra-tumor B cell polarization toward pro- or anti-inflammatory profiles. TLS are highly dynamic and can also attract immunosuppressive cells, such as regulatory T cells (Tregs), immature tolerogenic DCs and/or myeloid derived suppressor cells (MDSCs), in response to excessive inflammation57–59 (Fig. 1b). It is still not clear whether bona fide T follicular helper cells (Tfh) with germinal center B cell-selective features can differentiate in tumor-associated TLS. However, T cells with a similar phenotype have been found to overlap with dysfunctional and/or non-conventional suppressive T cells in chronic viral infection and tumors60,61, suggesting their potential negative impact on anti-tumor immunity. The possibility of precisely measuring the immune stimulatory vs. immunosuppressive potential of tumor-associated TLS, and to predict their fate based on composition and inflammatory signals would offer a potentially valuable biomarker for response to immunotherapy. Indeed, despite the many unknowns in TLS and intra-tumor B cell biology, these factors have been recently reported to associate with improved responses to ICB in patients62–64. These initial findings open new avenues to potentially improve immunotherapy by dissecting the biology, heterogeneity, and mechanistic role of TLS in tumors and find ways to properly manipulate these structures for potentiating local anti-tumor immune responses. Mechanistic studies in this space are limited by the lack of TLS formation in mouse tumor models, restricting these analyses to correlative observations in patients. Engineering animal models to induce spontaneous TLS in tumors could help add causal inference to these correlative observations and allow to understand how the manipulation of these structures alters B cell vs. T cell responses and the outcome to different immunotherapies.
Immunosuppressive mechanisms in melanoma
Despite the immunogenicity of melanoma, metastatic melanoma is generally not eliminated spontaneously. The strong immune selective pressure in response to melanoma immunogenicity may induce the tumor to adapt and suppress anti-tumor immunity. In addition, local inflammation can activate homeostatic immunologic feedback, which contributes to this adaptive resistance. As an example, intratumoral CD8+ T cells, by producing CCL22 and IFN-γ, induce intra-tumor Treg accumulation and PD-L1 on tumor cells, respectively65,66. In turn, melanoma-specific TILs are functionally hampered in human melanoma lesions but can regain functionality after ex vivo culture with the proper cytokine growth factors67.
By binding PD-1 expressed on tumor-specific T cells, PD-L1 and PD-L2 induce a negative signaling cascade downstream of PD-1, which dampens T cell activation and tumoricidal function11. Therefore, in melanoma cells, expression of PD-L1 or PD-L2 can offset the positive T cell signals delivered by both MHC-I and MHC-II antigen presentation routes (Box 2).
Box 2: Immune resistance mechanisms in melanoma.
Tolerance:
Tolerance: the mechanisms by which lack of immune responses to an antigen – generally a self-antigen – is maintained. This is achieved through two main mechanisms: central and peripheral tolerance. Central tolerance is established via thymic deletion of high-affinity auto-reactive T cells. Peripheral tolerance is maintained by several mechanisms, including suppression by Tregs and anergy, which is generally induced via suboptimal T cell co-stimulation, deletion via apoptosis or conversion into Tregs. Dose of antigen and TCR affinity are considered major drivers of these mechanisms.
Exhaustion:
Exhaustion: a specific T cell differentiation process driven by chronic antigen stimulation, which leads to high expression of co-inhibitory immune receptors that are meant to dampen chronic TCR signaling and limit activation-induced cell death. In this state of strong contrasting stimuli (through simultaneous TCR stimulation and co-inhibitory pathways), TEX have decreased effector functions, including cytokine production and proliferative potential, but can survive in the hostile TME. Exhaustion appears to be a stepwise process encompassing intermediate reversible states more susceptible to re-invigoration by ICB.
Cell-mediated immunosuppression:
Cell-mediated immunosuppression: these mechanisms involve immunosuppressive cell types, including Tregs, MDSCs and tolerogenic DCs. These are distinct cell types that instruct effector T and B cells not to react to positive immune stimuli.
Expression of immune checkpoint ligands:
PD-L1 and PD-L2 are prototype immune checkpoint ligands often overexpressed in melanoma cells in response to strong inflammatory signals, as a homeostatic mechanism co-opted by tumor cells to protect themselves from an immune attack.
Tumor escape:
Evasion from anti-tumor immunity and immune surveillance. In addition to tumor extrinsic mechanisms in the TME that can contribute to this effect, the tumor itself can evolve to prevent its recognition by the immune system. A strong immune selective pressure applied to highly heterogenous tumor cells induces the enrichment of clones that can evade immune recognition, for example via inactivating genetic mutations of the antigen presentation machinery (B2M, HLA, TAP, etc) and/or IFN-γ-response genes (JAK1, JAK2).
Melanoma can also directly attract immune inhibitory cells. Especially through MHC-II expression, melanoma cells have a unique capacity to interact with and attract immunosuppressive CD4+ T cell subsets. Tregs are increased in peripheral blood (PB), lymph-nodes, and tumor microenvironment (TME) of melanoma patients, and were found to inhibit TIL function61,68,69. The typical Treg increase in metastatic lymph-nodes suggests that melanoma may directly modulate its microenvironment to evade immune surveillance and grow (Fig. 1a). In early studies, melanoma-associated Tregs were found to recognize melanoma antigens70,71. This has been recently confirmed by initial single-cell omics analyses showing that melanoma-infiltrating Tregs are highly clonal and can recognize tumor cells via TCR:pMHC-II interactions, suggesting that melanoma cells can directly activate and expand Tregs, thus controlling local immunosuppression72. Notably, clonal expansion of tumor-specific Tregs was found to be associated with neoantigen burden, which in turn correlated with tumor expression of MHC-II, further pointing to a mechanism whereby melanoma controls Treg expansion via MHC-II expression depending on its immunogenicity72.
Moreover, in melanoma patients a skew toward Th2-polarized CD4+ T cells and related cytokines was reported, once again highlighting an altered CD4+ T cell compartment in the presence of melanoma73–75. A Th2 immune bias is an indicator of chronic and counterproductive inflammatory responses. Over-production of VEGF and galectin-9 by human melanoma cells can support such Th2 bias via M2 macrophage differentiation, thus contributing to tumor-promoting inflammation73,75 (Fig. 1a).
Suboptimal co-stimulation due to insufficient mature DCs may also contribute to a hostile melanoma microenvironment for T cells. DCs play a key role in controlling local inflammation, T cell recruitment, and activation in melanoma76–79 (Fig. 1a). In mouse melanoma models with a constitutively active beta-catenin signaling pathway, lack of infiltrating antigen cross-presenting CD103+ DCs contributed to T cell exclusion from the TME80,81. These observations underscore two important points: (1) active priming in the TME is key to maintain the local T cell pool and to preserve TIL tumoricidal function; (2) the tumor pro-oncogenic program can directly affect DC recruitment, priming capacity, and co-stimulatory potential in the TME. Similarly, abnormal production of prostaglandin E2 (PGE2) by melanoma cells was shown to limit intratumoral recruitment of cross-presenting DC subsets, possibly via downregulation of the DC chemoattractants XCL1 and CCL5 in intra-tumor NK cells82. Importantly, in cutaneous melanoma, UV exposure has been shown to promote the tolerogenic profile of a specialized subset of epidermal DCs called Langerhans cells, which sense the skin barrier surface through langerin – a C-type lectin that functions as a pattern recognition receptor83,84. These cells have high migratory capacity to the skin-draining lymph-nodes. Upon UV exposure, suboptimally activated immature Langerhans cells can migrate into the draining lymph-nodes tolerizing T cells in an antigen-specific way (Box 2). However, some controversy has emerged with respect to the specific type of langerin-expressing DCs primarily contributing to this tolerogenic phenotype84,85. A homeostatic/tolerogenic program was also found to be induced by IFN-γ in DCs infiltrating human tumors, including melanoma86. Overall, these observations indicate that melanoma cells and/or local inflammation, via multiple mechanisms, can negatively affect intra-tumor DC abundance and co-stimulatory capacity, thus limiting the generation of potent T cell responses. Considering the plasticity of DCs, the same findings would point to new potential therapeutic opportunities by re-polarizing DC function and restoration of sufficient local anti-tumor T cell priming and activation87.
Recent progress with ICB therapy in melanoma
As detailed above, the immunogenicity of melanoma – demonstrated by the presence of specific adaptive immune responses – can be offset by activation of regulatory programs that would normally serve to prevent immune pathology. As part of this regulation, immune checkpoint molecules are expressed on activated antigen-experienced TILs and were found to represent effective immunotherapeutic targets in melanoma. CTLA-4 was the first immune checkpoint to be identified. CTLA-4 on T cells competes with the costimulatory molecule CD28 for the same ligands, CD80 and CD86. However, CTLA-4 binds CD80 and CD86 with greater affinity and avidity compared to CD2888, depriving T cells of costimulatory signals. These discoveries led to the seminal finding that CTLA-4 blocking antibodies can regress tumors in mice8, followed by the clinical development of these reagents, with ipilimumab obtaining first indication in metastatic melanoma in 20119,10(Table 1). PD-1 was the second immune checkpoint to be discovered11. CD8+ exhausted T cells (TEX) progressively lose effector functions upon chronic antigen stimulation during infection and cancer and over-express PD-1. PD-1 or PD-L1 blockade can reinvigorate these cells, resulting in their numeric expansion and restoration of effector functions in mice14,89 and humans90–92. These effects were associated with enhanced tumor control in preclinical models12,13,93, which translated into substantial therapeutic activity in patients, with the PD-1 inhibitors pembrolizumab and nivolumab being approved in metastatic melanoma in 201415-18 (Table 1). Considering the different and potentially complementary effects of CTLA-4 and PD-1 blockade94, these therapies were subsequently tested in combination, demonstrating greater long-term efficacy than either agent alone in metastatic melanoma, with 49% of patients remaining alive after 6.5 years (Table 1), albeit at the cost of greater toxicity20. Alternative regimens and/or dosages of anti-PD-1+anti-CTLA-4 are currently investigated to reduce toxicity95.
Table 1.
Pivotal and most advanced clinical trials of ICB in melanoma.
| Summary | Study Name |
|---|---|
| Phase-3 clinical trial comparing ipilimumab +/− gp100 peptide vaccine with gp100 alone, demonstrating that the ipilimumab arms had improved PFS and OS compared to gp100. | NCT00094653 |
| Phase-3 clinical trial demonstrating PFS and OS benefit of ipilimumab+dacarbazine compared to dacarbazine alone. | NCT00324155 |
| Phase-3 clinical trial comparing pembrolizumab Q2 weeks, pembrolizumab Q3 weeks, and ipilimumab, demonstrating improved PFS and OS in the pembrolizumab arms. | KEYNOTE-006 |
| Phase-3 clinical trial demonstrating PFS and OS benefit of nivolumab, as compared to dacarbazine. | CHECKMATE-066 |
| Phase-3 clinical trial demonstrating that both ipilimumab+nivolumab, and nivolumab had improved PFS and OS, compared to ipilimumab. Although this trial was not powered to compare the combination arm with nivolumab, ipilimumab+nivolumab had a numerically greater survival rate. | CHECKMATE-067 |
| Phase-3 clinical trial demonstrating a PFS benefit with relatlimab+nivolumab combination compared to relatlimab. | RELATIVITY-047 |
| Phase-3 clinical trial demonstrating survival benefit with ipilimumab in the adjuvant setting for the first time. | NCT00636168 |
| Phase-3 clinical trial comparing adjuvant nivolumab vs. ipilimumab after complete resection of stage-IIIb/c-IV high risk melanoma patients, which shows improved RFS with nivolumab vs. ipilimumab. | CHECKMATE-238 |
| First randomized phase-3 clinical trial of adjuvant pembrolizumab compared to placebo in stage-II melanoma, which shows initial evidence of reduced risk of recurrence in the adjuvant immunotherapy arm. | KEYNOTE-716 |
| Randomized phase-Ib trial comparing for the first time neoadjuvant vs. adjuvant combination ICB with nivolumab+ipilimumab in high-risk stage-III melanoma patients, demonstrating the feasibility of these treatment regimens in early disease settings, the clinical and immune activity of the neoadjuvant treatment, and the need to optimize treatment regimens for reduced toxicity. | OpACIN trial |
| Phase-2 clinical trial of neoadjuvant nivolumab vs. ipilimumab+nivolumab in high-risk resectable melanoma patients, showing the feasibility of neoadjuvant ICB in melanoma and the need to optimize treatment regimens for reduced toxicity. | NCT02519322 |
| Phase-1b clinical trial investigating neoadjuvant followed by adjuvant anti-PD-1 with pembrolizumab in stage-III/IV resectable melanoma patients, demonstrating the clinical feasibility of neoadjuvant/adjuvant anti-PD-1 therapy in melanoma and rapid pathological and immunologic responses in tumors. | NCT02434354 |
| 3-arm phase-2 clinical trial investigating 3 regimens of combined neoadjuvant ICB with ipilimumab+nivolumab for reduced toxicity, identifying ipilimumab 1 mg/kg plus nivolumab 3 mg/kg as the most favorable schedule (expansion cohort with this regimen is ongoing). | OpACIN-neo trial |
| Extension study of OpACIN-neo, testing whether therapeutic lymph node dissection (TLND) could be omitted in patients that achieve a complete or near-complete pathologic response to neoadjuvant ipilimumab+nivolumab. | PRADO |
| Phase-2 randomized clinical trial testing adjuvant nivolumab vs. nivolumab+ipilimumab based on early pathological responses to neoadjuvant nivolumab in resectable stage-III melanoma patients (ongoing). | NCT04013854 |
| Phase-2 randomized clinical trial comparing adjuvant vs. neoadjuvant anti-PD-1 with pembrolizumab for resectable stage-III-IV high-risk melanoma patients (ongoing). | NCT03698019 |
| First biomarker-driven neoadjuvant immunotherapy phase-1b clinical trial in stage-III melanoma, allocating IFN-γ signature low and high patients to neoadjuvant ICB ± histone deacetylase (HDAC) inhibition, with the aim to induce the IFN-γ pathway through HDAC inhibition in IFN-γ signature low patients. | NCT04133948 |
The incremental discovery of immune co-inhibitory receptors and their co-expression in TEX89 has inspired further development of antibody therapeutics targeting novel immune checkpoint molecules. The most promising emerging ICB target is LAG-3. LAG-3 is a surface inhibitory receptor with structural similarity to CD4 that competitively binds to MHC-II and other ligands, including galectin-3. Among other cell types, LAG-3 is up-regulated on antigen-stimulated T cells, including TEX. Similar to CTLA-4, LAG-3 is also constitutively over-expressed on Tregs, contributing to their suppressive function96. While LAG-3 blockade as monotherapy has modest anti-tumor efficacy, combined anti-LAG-3+anti-PD-1 exhibited substantially enhanced therapeutic activity in several mouse tumor models, including melanoma96. Several antibody therapeutics targeting LAG-3 by mainly blocking the MHC-II:LAG3 interactions are being tested in cancer patients96. The most advanced anti-LAG3 antibody is relatlimab, which has been tested in combination with nivolumab in a phase 2–3 clinical trial in patients with previously untreated metastatic or unresectable melanoma. Results from this trial indicate superior progression-free survival (PFS) of relatlimab+nivolumab vs. nivolumab monotherapy and similar to ipilimumab+nivolumab historically97 (Table 1). Increases in circulating LAG-3+ T cells correlate with shorter survival and/or disease progression after PD-1 blockade in melanoma patients98,99, supporting the rationale for combined PD-1 and LAG-3 inhibition. Whether anti-PD-1+anti-LAG-3 has comparable efficacy to anti-CTLA-4+anti-PD-1 and decreased toxicity, and whether circulating LAG-3+ T cells can serve as a biomarker to allocate patients to anti-LAG-3-containing therapies remain to be directly tested in prospective clinical trials. Similarly, it will be important to understand whether MHC-II expression levels and localization play a role in the activity of LAG-3 blockade.
Additional advancements with ICB in melanoma are coming from its investigation in earlier-stage disease, when administered after (adjuvant therapy) or before (neoadjuvant therapy) surgical resection. Ipilimumab was the first ICB therapy to show durable survival benefit in melanoma in the adjuvant setting100–102 (Table 1), followed by PD-1 blockade with either nivolumab or pembrolizumab, which showed improved relapse-free survival (RFS) compared to placebo or even ipilimumab in high-risk stage-III patients, although with no overall survival (OS) benefit observed to date103. Given the improved toxicity profile compared to ipilimumab, PD-1 blockade has become a standard of care for adjuvant therapy. Recently, adjuvant pembrolizumab obtained FDA approval for stage-II/C melanoma, based on its improved RFS compared to placebo104.
Neoadjuvant ICB has also gained momentum, with five studies being completed to date in melanoma105,106. Neoadjuvant ipilimumab+nivolumab or PD-1 blockade alone demonstrated 33–57% and 19–25% complete pathologic response (pCR) rates respectively. As the increased pCR rate with neoadjuvant ipilimumab+nivolumab comes at the cost of greater toxicity, studies to optimize doses and/or regimens for better safety profile are underway (Table 1). Alternative combination regimens are also being investigated, with neoadjuvant nivolumab+relatlimab showing impressive pCR rates (59%)107.
Notably, early pathologic responses to neoadjuvant ICB strongly predict RFS. Pathologic responses (<50% viable tumor) within 6 weeks of anti-PD-1±anti-CTLA-4 were associated with >94% RFS at 2 years in a recent pooled analysis of stage-III melanoma patients108. The ability to predict long-term outcomes based on early pathological responses to neoadjuvant therapy provides unique ability to tailor the type and duration of adjuvant therapy in a personalized manner. Moreover, initial biomarker analyses have reported elevated baseline IFN-γ signature scores and TMB as well as post-treatment tumor fibrosis to be associated with pathologic responses and survival after neoadjuvant ICB109,110, offering biological parameters that can further improve patients’ allocation to the right treatment. Trials are now underway testing whether these potential biomarkers can be used to inform clinical management. For example, ongoing trials are investigating the advantage of modifying adjuvant therapy (NCT04013854) or surgical management111 based on pathologic response to neoadjuvant therapy, or baseline tumor IFN-γ scores (NCT4133948). Finally, the clinical efficacy of neoadjuvant vs. adjuvant ICB with pembrolizumab is being directly tested in prospective studies (NCT03698019, Table 1).
Overall, these studies point to the feasibility of ICB in earlier-stage disease. In particular, neoadjuvant ICB offers the advantage of rapid activity evaluation, which facilitates early treatment modification if necessary. Moreover, neoadjuvant treatment serves as an efficient platform for analysis of immune correlates and resistance mechanisms in patients, which is key to guide the most rational treatment combinations, as discussed below.
Assessment of pharmacodynamic ICB activity for refined biomarker discovery
The precise tracking and interrogation of the immunologic responses to ICB is critical for understanding the mechanism(s) of action and identifying early predictive biomarkers. The direct pharmacodynamic effect of immunotherapy is on the immune compartment, with an indirect effect on the tumor. Neoadjuvant studies offer an optimal setting to investigate response predictors and tumor resistance mechanisms in humans because of the availability of paired pre-treatment and on-treatment samples. Early neoadjuvant trials in melanoma identified pharmacodynamic immune responses of CD4+ and CD8+ TILs to high-dose IFN alpha-2b112 and anti-CTLA-4106 at 4–6 weeks post therapy, demonstrating that the immunologic activity of immunotherapies occurs early in humans.
Subsequent studies interrogated changes in cellular states in response to ICB more in-depth. Through these analyses, CTLA-4 blockade was found to primarily expand ICOS+Tbet+CD4+ effector T cells in PB and tumors, including melanoma113–115. Tregs have been extensively studied as an another potentially relevant pharmacodynamic target of CTLA-4 blockade, because CTLA-4 is maximally expressed in Tregs, especially intratumoral Tregs116. While in murine tumor models, including melanoma, intratumoral Tregs selectively decrease after anti-CTLA-4 and this contributes to the anti-tumor activity (Fig. 2), similar effects have not been definitely proven in human patients. Ipilimumab is an IgG1 antibody and can mediate antibody-dependent-cellular-cytotoxicity (ADCC) of human Tregs ex vivo117. However, Foxp3+ Tregs were not found to be reduced in melanoma biopsies after ipilimumab treatment118. In addition, in mice and humans, peripheral Tregs are overall expanded after CTLA-4 blockade independent of the therapeutic activity106,119,120. This indicates that CTLA-4 can limit Treg expansion and that when this pathway is blocked more Tregs are generated in the periphery, despite potentially being less suppressive during CTLA-4 inhibition (Fig. 2)61,121,122. This peripheral Treg expansion can contribute to replenish the intra-tumor Treg pool, thus explaining our inability to detect substantial intratumoral Treg loss even upon treatment with a depleting anti-CTLA-4 antibody. Deeper Treg profiling will help understand whether certain Treg subsets (e.g. CTLA-4hi Tregs) may be specifically modulated and/or reduced by CTLA-4 blockade and can be monitored as robust biomarkers. There may also be distinct mechanisms of action of anti-CTLA-4 against peripheral and intratumoral Tregs, which can be determined by the different composition and function of the microenvironment. These observations led to the development of optimized versions of the anti-CTLA-4 ipilimumab for increased ADCC of Tregs or preferential activation in the TME to limit the effect on peripheral Tregs123. These agents are currently in clinical testing for advanced solid cancers, including melanoma.
Figure 2. Main cellular targets of ICB in humans.
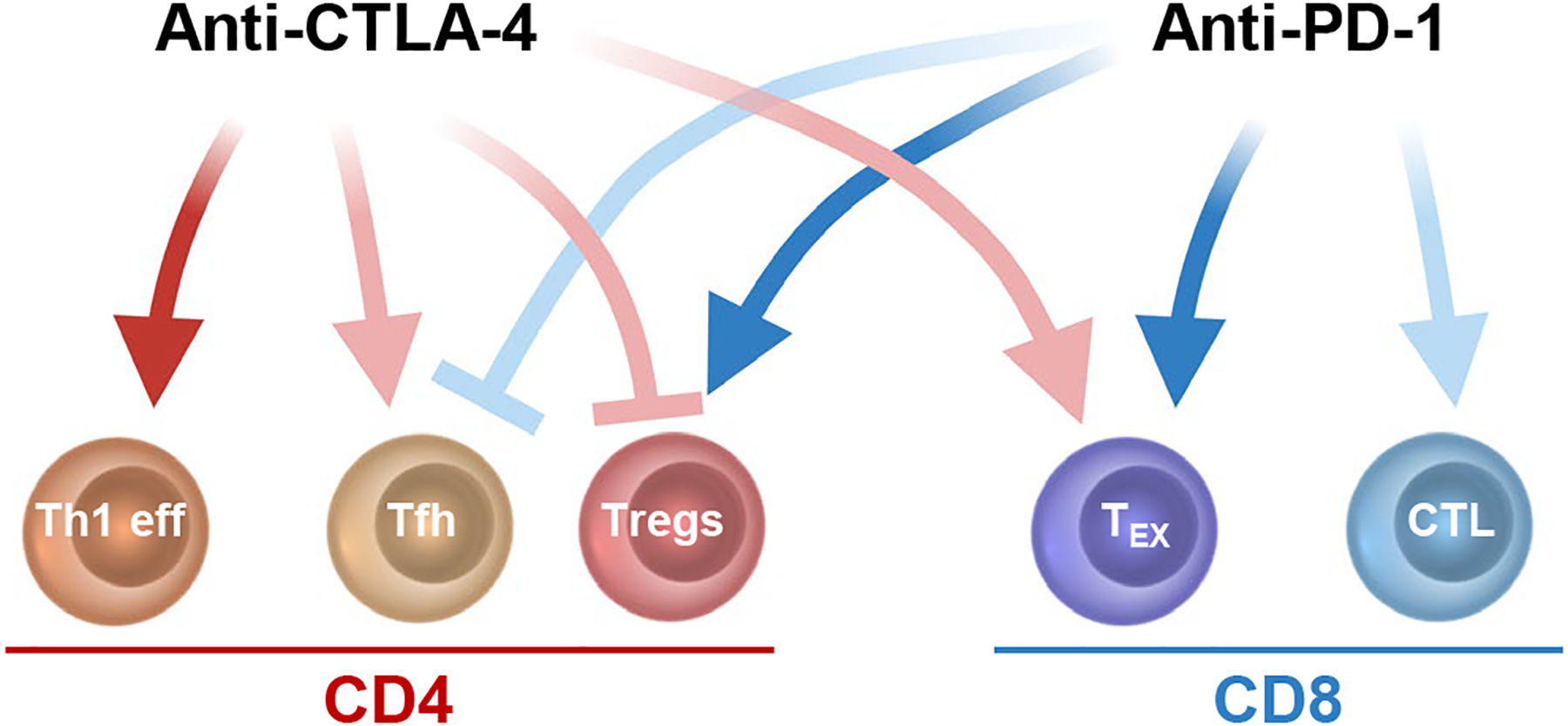
Schematic representation of the immune cell types primarily affected by anti-CTLA-4 (red arrows) and ani-PD-1 (blue arrows) as reported in humans. Notably, most of these cell types overexpress the targets of ICB, potentially explaining their preferential modulation after ICB. CTLA-4 blockade induces Th1 effector cells (eff) and Tfh cells, while counteracting Treg function and possibly expanding Tex. PD-1 blockade reinvigorates TEX and possibly potentiates effector CD8+ CTLs but expands functionally suppressive Tregs and can decrease Tfh. Combined assessment of these immune cell subsets during ICB will likely help select relevant pharmacodynamic changes that can be then taken as reliable activity biomarkers for these treatments. Dark color, definitive evidence; light color, weaker evidence. Created with BioRender.com.
Recent studies have also carefully profiled the kinetics and composition of the pharmacodynamic immune responses of human cancers – including melanoma – to PD-1 blockade. PD-1 blockade results in early activation and proliferation of T cells and Tregs. The CD8 response is largely composed of TEX over-expressing PD-1, CTLA-4, and Eomes90,91. Early CD8+ TEX reinvigoration was associated with clinical and pathologic responses after a single dose of PD-1 blockade, with TEX proliferation peaking by 7 days after the initial treatment dose90. The heterogeneity of CD8+ TEX is now being deconvoluted, with specific TEX subsets shown to preferentially respond to anti-PD-1, such as progenitor CD8+ TEX124. However, the immunologic response to PD-1 blockade in humans is heterogenous and may extend to other CD8+ T cell subsets, such as effector-memory CD8+ T cells125 (Fig. 2). Increases in Treg proliferation and activation after PD-1 blockade was associated with poor clinical outcomes90,91. This data is consistent with recent studies showing that PD-1 blockade potentiates Treg immunosuppression126. Therefore, the relative effects of anti-PD-1 on CD8+ T cells vs. Tregs may play an important role in dictating clinical outcomes127. Other PD-1hi T cells that can be preferentially impacted by PD-1 blockade and respond to this treatment are Tfh and T follicular regulatory cells (Tfr). These are the CD4+ T cells displaying the highest PD-1 expression; however, their dynamic changes and role in the response to immunotherapy are not fully understood. A population of circulating PD-1hiFoxp3−CD4+ T cells resembling Tfh was described to be decreased during PD-1 blockade in melanoma patients, but expanded upon anti-CTLA-4 (Fig. 2)61, according to observations in CTLA-4-deficient mice121,128. Intriguingly, these cells were found to suppress T cell effector functions in mice and humans and to accumulate in progressing melanoma tumors in mice61. Notably, in a retrospective analysis, melanoma patients with lower frequencies of these Tfh-like cells in PB showed improved responses to PD-1 blockade61. The precise role and predictive/prognostic significance of bona-fide Tfh cells in the response to immunotherapy need to be confirmed in prospective studies in patients.
Advances in sequencing technology are expected to tremendously accelerate the interrogation of these pharmacodynamic immune responses, through single-cell transcriptional characterization of T cell states129 and paired RNA-TCR sequencing analyses of clonally expanded T cells. Initial studies have shown that CD8+ TEX preferentially responding to ICB are more clonal than other T cell states and are enriched for tumor-reactive cells across solid cancer types29,129,130. These initial results provide proof-of-principle that we will soon be able to monitor and interrogate the antigen-specific pharmacodynamic immune responses to immunotherapy at unparalleled depth.
Overcoming immunotherapy resistance by targeting melanoma metabolism
Despite the success of ICB, the efficacy of these therapies, even in combination, has reached a plateau, and novel classes of drugs are urgently needed. Tumor metabolic dependencies are emerging as key tumor vulnerabilies that may be amenable for targeting in combination with immunotherapy. Tumor cells generally adapt to undergo aerobic glycolysis, which is energetically less efficient than oxidative phosphorylation, but can feed metabolic intermediates into anabolic processes to sustain rapid cell division. Aerobic glycolysis is normally used by rapidly dividing cells, such as activated lymphocytes. However, in the TME, cancer cells are selected to acquire a metabolic advantage over normal immune cells, thus tipping the balance in favor of tumor progression and immune evasion.
Metabolic competition is particularly relevant in melanoma, as its oncogenic program unavoidably converges into activation of MAPK and PI3K-AKT signaling pathways – most commonly via activating BRAF(V600E) mutation or PTEN loss/inactivation – which support a highly glycolytic profile131–135. Elevated glycolysis in human melanoma negatively correlates with T cell infiltration and activation122,134,136, and response to ACT or ICB134,137. Supporting these correlative observations in patients, pharmacologic or genetic targeting of glycolysis in tumor cells improves the activity of either ACT or ICB in mice122,134.
Intriguingly, progressing melanoma can acquire a hypermetabolic phenotype sustaining oxidative metabolism. These traits have been found to specifically distinguish brain melanoma metastases in patients138 and to contribute to ICB therapy resistance in mice139,140. To counteract this hypermetabolic phenotype and enhance immunotherapy, the antidiabetic biguanides are being investigated in melanoma, with promising results for phenformin in combination with anti-PD-1 in melanoma-bearing mice141 and initial retrospective analyses showing reduced occurrence of new brain metastases and trends toward favorable outcomes in patients receiving metformin during ICB142.
Decreased oxygen tension in the microenvironment of hyper-oxidative tumors can promote T cell exhaustion143 and T cell resistance to anti-PD-1-mediated reinvigoration140 (Fig. 3). Conversely, tumor glycolysis and glucose deprivation in the microenvironment pose preferential resistance to CTLA-4 blockade122 (Fig. 3). The preferential barriers posed by oxidative vs. glycolytic tumor metabolism to anti-PD-1 and anti-CTLA-4 blockade, respectively, may be at least partially explained by the distinct cellular localization of the direct targets of these immunotherapies. While PD-1 blockade primarily serves to reinvigorate dysfunctional PD-1+ T cells which are pushed to terminal irreversible exhaustion states in low oxygen tension, CTLA-4 blockade has a role in counteracting Tregs, whose stability is potentiated in glucose-deprived environments122,144 (Fig. 3). To counteract Treg function independent of tumor glycolysis, recent studies have shown that targeting lactate144 or fatty acid metabolism145 in Tregs enhances the response to ICB in mouse melanoma models.
Figure 3. Impact of melanoma metabolism on the immune microenvironment and response to ICB.
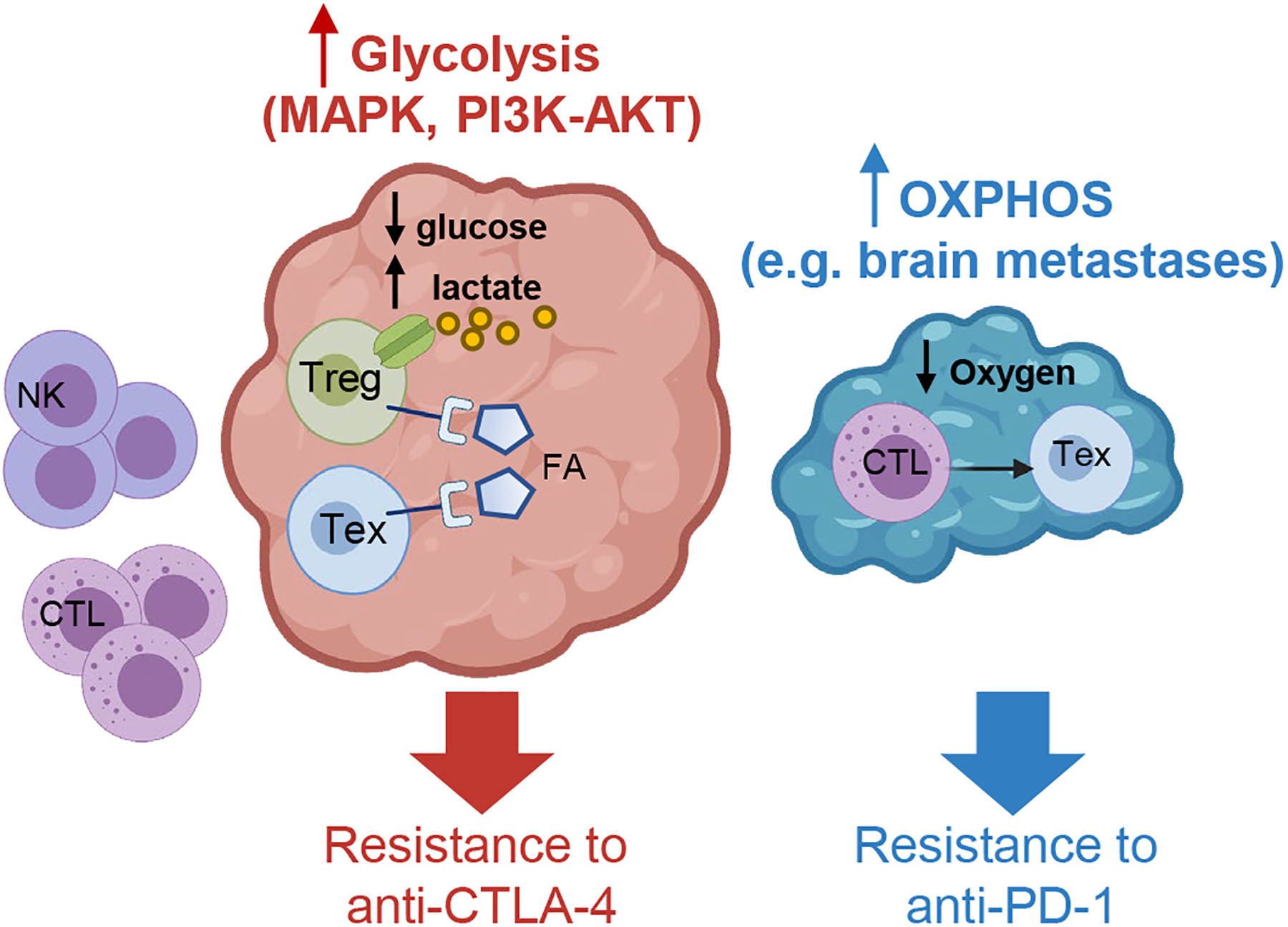
Schematic representation of possible metabolic scenarios in melanoma. Left, the pro-oncogenic program of melanoma, converging on MAPK and/or PI3K-AKT activation, supports a highly glycolytic profile, which results in a glucose-deprived and lactate-enriched environment that creates a metabolic barrier to glycolytic effector immune cells (e.g. NK cells and CTLs) and preferentially retains Tregs and TEX due to their reliance on alternative sources of fuels (e.g. lactate and fatty acids, FA). This TME potentiates Treg stability and suppressive profiles and poses specific obstacles to the activity of CTLA-4 blockade. Right, the metabolic state of melanoma can evolve with tumor progression and metastasis dissemination through the acquisition of a hypermetabolic phenotype, including the ability to sustain oxidative metabolism, such as in brain metastases. Low oxygen tension fosters the development of terminal TEX, generating an environment that is particularly refractory to the activity of PD-1 blockade. Created with BioRender.com.
Together, these findings highlight three major concepts: (1) the existence of symbiotic metabolic interactions between tumor and dysfunctional/suppressive immune cells that use complementary sources of fuel in the TME; (2) the possibility to interfere with these vicious interactions as a useful strategy to combine with immunotherapy; (3) the relevance of profiling tumor metabolic states as an additional key information to guide therapeutic decisions for assigning patients to the right immunotherapy.
Conclusions and future directions
Despite its immunogenicity, metastatic melanoma grows and disseminates, due to immunosuppression and escape mechanisms. CTLA-4 and PD-1 blockade can efficiently target some of these mechanisms, by improving T cell priming, counteracting Treg suppression and re-invigorating PD-1hi TEX. However, many patients still do not derive long-lasting benefit even upon combined ICB.
In the era of combination immunotherapy, with more agents becoming available, the mechanism of single-agent therapies needs to be clearly delineated for guiding their rational combination. As the immunologic effects of immunotherapy occur early, we need to focus on these early events to identify (1) robust biomarkers, (2) resistance mechanisms, and (3) if necessary, add proper therapies in a timely manner, in order to capitalize on the prior pharmacodynamic response. Moving forward, the neoadjuvant treatment setting appears to be an optimal platform to efficiently explore all these aspects. Moreover, toxicity from current and new immunotherapy combinations remains a critical point to address146. Understanding the molecular mediators of immune toxicity (e.g. antibody- vs. T cell mediated) will substantially help control these side effects and improve patients’ management.
The successful history of immunotherapy – especially with ICB – in melanoma has paved the way for its development in other cancers. Here, we have summarized the main lessons learned in the setting of melanoma immunobiology and immunotherapy, which can inform and accelerate further development of these treatments in other cancer types (Box 3). Comparing and contrasting efficacy and toxicity of immunotherapy across tumor types will tremendously aid in delineating the key parameters to assess for predicting response, limiting toxicity, and guiding therapeutic decisions to overcome treatment resistance.
Box 3: Main lessons learned with ICB in melanoma.
Expression of melanosomal antigens, elevated TMB and neoantigen load, and the ability to present antigens through both MHC-I and MHC-II routes contribute to melanoma immunogenicity;
Tumor antigen-specific T cells are important in melanoma control, response to ICB and can be efficiently manipulated for ACT;
Re-invigoration of TEX is a major pharmacodynamic response to PD-1 blockade, which can be detected as early as one week post dosing;
Melanoma-specific antibodies are detected in patients and intratumoral B cells associate with improved outcomes to ICB, providing the rationale for investigating the role of B cell responses in the anti-tumor activity of ICB;
Immunosuppressive mechanisms in melanoma are multiple and complex, and require further analysis to precisely reconstruct their coordination and dynamics during tumor progression and in immunotherapy resistance;
Tregs are emerging as an important mechanism of resistance to PD-1 blockade but not necessarily CTLA-4 blockade;
ICB appears relatively safe and effective in early disease settings, such as neoadjuvant and adjuvant therapy, supporting further investigation of these approaches for regimen and indication optimization;
Emerging consensus in neoadjuvant immunotherapy trial design is expected to dramatically advance our understanding of relevant pharmacodynamic immune responses in patients and accelerate the development of rational combination regimens, while substantially improving patients’ management;
Longitudinal assessment of pre- vs. on-treatment samples is needed to appropriately define the prognostic vs. predictive potential of immune parameters correlating with outcome and infer their biologic role in the response to immunotherapy based on their modulation during treatment;
The characteristic oncogenic program in melanoma supporting metabolic plasticity and fitness, together with preclinical evidence of differential activity of ICB depending on the tumor metabolic state, open the way to systematically investigate these relationships as potential biomarkers for patients’ stratification and treatment allocation as well as to devise novel precision-medicine combinations based on metabolic- and immune-therapies.
Acknowledgements
We would like to thank Drs. G. Linette, B. Carreno, R. Amaravadi, T. Mitchell, and J.D. Wolchok for their insightful feedback on this manuscript. A.C.H. is funded by grant K08 CA230157 from the NIH, the Doris Duke CSDA, Damon Runyon CIA, and funding from the Tara Miller Melanoma Foundation. R.Z. is supported by the Parker Institute for Cancer Immunotherapy Bridge Fellows Award. R.Z. acknowledges funding from the NCI SPORE (P50-CA192937).
Conflicts of interest
A.C.H. is a consultant for Immunai and receives research support from Bristol Myers Squibb. R.Z. is inventor on patent applications related to work on GITR, PD-1 and CTLA-4. R.Z. is scientific advisory board member of iTEOS Therapeutics and receives grant support from AstraZeneca and Bristol Myers Squibb.
References
- 1.Jakob JA et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 118, 4014–4023, doi: 10.1002/cncr.26724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen BE, Manga P, Lin K & Elbuluk N Vitiligo and Melanoma-Associated Vitiligo: Understanding Their Similarities and Differences. American Journal of Clinical Dermatology 21, 669–680, doi: 10.1007/s40257-020-00524-0 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Clark WH Jr., From L, Bernardino EA & Mihm MC The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 29, 705–727 (1969). [PubMed] [Google Scholar]
- 4.Clark WH Jr. et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 81, 1893–1904, doi: 10.1093/jnci/81.24.1893 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Clemente CG et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77, 1303–1310, doi: (1996). [DOI] [PubMed] [Google Scholar]
- 6.Azimi F et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30, 2678–2683, doi: 10.1200/JCO.2011.37.8539 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Thomas NE et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 31, 4252–4259, doi: 10.1200/JCO.2013.51.3002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leach DR, Krummel MF & Allison JP Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736, doi: 10.1126/science.271.5256.1734 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711–723, doi: 10.1056/NEJMoa1003466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364, 2517–2526, doi: 10.1056/NEJMoa1104621 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Pauken KE, Torchia JA, Chaudhri A, Sharpe AH & Freeman GJ Emerging concepts in PD-1 checkpoint biology. Semin Immunol, 101480, doi: 10.1016/j.smim.2021.101480 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strome SE et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 63, 6501–6505 (2003). [PubMed] [Google Scholar]
- 13.Iwai Y et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 99, 12293–12297, doi: 10.1073/pnas.192461099192461099 [pii] (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber DL et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687, doi:nature04444 [pii] 10.1038/nature04444 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Robert C et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372, 2521–2532, doi: 10.1056/NEJMoa1503093 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Robert C et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20, 1239–1251, doi: 10.1016/S1470-2045(19)30388-2 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Ascierto PA et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol 5, 187–194, doi: 10.1001/jamaoncol.2018.4514 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372, 320–330, doi: 10.1056/NEJMoa1412082 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Brahmer JR et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28, 3167–3175, doi: 10.1200/JCO.2009.26.7609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol, JCO2102229, doi: 10.1200/JCO.21.02229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zappasodi R, Wolchok JD & Merghoub T Strategies for Predicting Response to Checkpoint Inhibitors. Curr Hematol Malig Rep, doi: 10.1007/s11899-018-0471-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruscetti FW, Morgan DA & Gallo RC Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol 119, 131–138 (1977). [PubMed] [Google Scholar]
- 23.Kvistborg P et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology 1, 409–418, doi: 10.4161/onci.18851 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen RS et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res 72, 1642–1650, doi: 10.1158/0008-5472.CAN-11-2614 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Tran E, Robbins PF & Rosenberg SA ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nature immunology 18, 255–262, doi: 10.1038/ni.3682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421, doi: 10.1038/nature12477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Eynde BJ & van der Bruggen P T cell defined tumor antigens. Curr Opin Immunol 9, 684–693, doi: 10.1016/s0952-7915(97)80050-7 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Lennerz V et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci U S A 102, 16013–16018, doi: 10.1073/pnas.0500090102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira G et al. Phenotype, specificity and avidity of antitumour CD8(+) T cells in melanoma. Nature 596, 119–125, doi: 10.1038/s41586-021-03704-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ott PA et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221, doi: 10.1038/nature22991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin U et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226, doi: 10.1038/nature23003 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Carreno BM et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348, 803–808, doi: 10.1126/science.aaa3828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itzhaki O et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother 34, 212–220, doi: 10.1097/CJI.0b013e318209c94c (2011). [DOI] [PubMed] [Google Scholar]
- 34.Radvanyi LG et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res 18, 6758–6770, doi: 10.1158/1078-0432.CCR-12-1177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg SA et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17, 4550–4557, doi: 10.1158/1078-0432.CCR-11-0116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goff SL et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol 34, 2389–2397, doi: 10.1200/JCO.2016.66.7220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linnemann C et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med 21, 81–85, doi: 10.1038/nm.3773 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Lu YC et al. Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression. J Immunol 190, 6034–6042, doi: 10.4049/jimmunol.1202830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prickett TD et al. Durable Complete Response from Metastatic Melanoma after Transfer of Autologous T Cells Recognizing 10 Mutated Tumor Antigens. Cancer immunology research 4, 669–678, doi: 10.1158/2326-6066.CIR-15-0215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins PF et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 19, 747–752, doi: 10.1038/nm.3161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran E et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–1390, doi: 10.1126/science.aad1253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishna S et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334, doi: 10.1126/science.abb9847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert AE et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS One 6, e19330, doi: 10.1371/journal.pone.0019330 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfreundschuh M The genealogy of SEREX. Cancer Immun 12, 7 (2012). [PMC free article] [PubMed] [Google Scholar]
- 45.Sahin U et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A 92, 11810–11813, doi: 10.1073/pnas.92.25.11810 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vijayasaradhi S, Bouchard B & Houghton AN The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med 171, 1375–1380, doi: 10.1084/jem.171.4.1375 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang RF, Robbins PF, Kawakami Y, Kang XQ & Rosenberg SA Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med 181, 799–804, doi: 10.1084/jem.181.2.799 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livingston PO et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol 12, 1036–1044, doi: 10.1200/JCO.1994.12.5.1036 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Cipponi A et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 72, 3997–4007, doi: 10.1158/0008-5472.CAN-12-1377 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Ladanyi A et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother 60, 1729–1738, doi: 10.1007/s00262-011-1071-x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sautes-Fridman C, Petitprez F, Calderaro J & Fridman WH Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 19, 307–325, doi: 10.1038/s41568-019-0144-6 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Peng Y & Tobin DJ A new 12-gene diagnostic biomarker signature of melanoma revealed by integrated microarray analysis. PeerJ 1, e49, doi: 10.7717/peerj.49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mortarini R et al. Constitutive expression and costimulatory function of LIGHT/TNFSF14 on human melanoma cells and melanoma-derived microvesicles. Cancer Res 65, 3428–3436, doi: 10.1158/0008-5472.CAN-04-3239 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Erdag G et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 72, 1070–1080, doi: 10.1158/0008-5472.CAN-11-3218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg K et al. Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum Pathol 54, 157–164, doi: 10.1016/j.humpath.2016.03.022 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Rodriguez M, Thompson AK & Monteagudo C A significant percentage of CD20-positive TILs correlates with poor prognosis in patients with primary cutaneous malignant melanoma. Histopathology 65, 726–728, doi: 10.1111/his.12437 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Eschweiler S et al. Intratumoral follicular regulatory T cells curtail anti-PD-1 treatment efficacy. Nature immunology 22, 1052–1063, doi: 10.1038/s41590-021-00958-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi NS et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity 43, 579–590, doi: 10.1016/j.immuni.2015.08.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zlotnik A, Burkhardt AM & Homey B Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11, 597–606, doi: 10.1038/nri3049 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Crawford A et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 40, 289–302, doi: 10.1016/j.immuni.2014.01.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zappasodi R et al. Non-conventional Inhibitory CD4(+)Foxp3(−)PD-1(hi) T Cells as a Biomarker of Immune Checkpoint Blockade Activity. Cancer Cell 33, 1017–1032 e1017, doi: 10.1016/j.ccell.2018.05.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cabrita R et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565, doi: 10.1038/s41586-019-1914-8 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Helmink BA et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555, doi: 10.1038/s41586-019-1922-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petitprez F et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560, doi: 10.1038/s41586-019-1906-8 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Spranger S et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5, 200ra116, doi: 10.1126/scitranslmed.3006504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taube JM et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4, 127ra137, doi: 10.1126/scitranslmed.3003689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zippelius A et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res 64, 2865–2873, doi: 10.1158/0008-5472.can-03-3066 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Ahmadzadeh M et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood 112, 4953–4960, doi: 10.1182/blood-2008-06-163048 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viguier M et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 173, 1444–1453, doi: 10.4049/jimmunol.173.2.1444 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Mukherji B et al. Clonal analysis of cytotoxic and regulatory T cell responses against human melanoma. J Exp Med 169, 1961–1976, doi: 10.1084/jem.169.6.1961 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang HY, Peng G, Guo Z, Shevach EM & Wang RF Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol 174, 2661–2670, doi: 10.4049/jimmunol.174.5.2661 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Oliveira G et al. 655 Landscape of helper and regulatory CD4+ T cells in melanoma. Journal for ImmunoTherapy of Cancer 9, A684, doi: 10.1136/jitc-2021-SITC2021.655 (2021). [DOI] [Google Scholar]
- 73.Enninga EA, Nevala WK, Holtan SG, Leontovich AA & Markovic SN Galectin-9 modulates immunity by promoting Th2/M2 differentiation and impacts survival in patients with metastatic melanoma. Melanoma Res 26, 429–441, doi: 10.1097/CMR.0000000000000281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauerova L et al. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma 49, 159–166 (2002). [PubMed] [Google Scholar]
- 75.Nevala WK et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 15, 1931–1939, doi: 10.1158/1078-0432.CCR-08-1980 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klarquist JS & Janssen EM Melanoma-infiltrating dendritic cells: Limitations and opportunities of mouse models. Oncoimmunology 1, 1584–1593, doi: 10.4161/onci.22660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marzagalli M, Ebelt ND & Manuel ER Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol 59, 236–250, doi: 10.1016/j.semcancer.2019.08.002 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Pieniazek M, Matkowski R & Donizy P Macrophages in skin melanoma-the key element in melanomagenesis. Oncol Lett 15, 5399–5404, doi: 10.3892/ol.2018.8021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tucci M et al. Immune System Evasion as Hallmark of Melanoma Progression: The Role of Dendritic Cells. Front Oncol 9, 1148, doi: 10.3389/fonc.2019.01148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spranger S, Bao R & Gajewski TF Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235, doi: 10.1038/nature14404 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Spranger S, Dai D, Horton B & Gajewski TF Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 31, 711–723 e714, doi: 10.1016/j.ccell.2017.04.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bottcher JP et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 172, 1022–1037 e1014, doi: 10.1016/j.cell.2018.01.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun X, Zhang N, Yin C, Zhu B & Li X Ultraviolet Radiation and Melanomagenesis: From Mechanism to Immunotherapy. Front Oncol 10, 951, doi: 10.3389/fonc.2020.00951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwarz A et al. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol 130, 1419–1427, doi: 10.1038/jid.2009.429 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Wang L, Jameson SC & Hogquist KA Epidermal Langerhans cells are not required for UV-induced immunosuppression. J Immunol 183, 5548–5553, doi: 10.4049/jimmunol.0900235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nirschl CJ et al. IFNγ-Dependent Tissue-Immune Homeostasis Is Co-opted in the Tumor Microenvironment. Cell 170, 127–141.e115, doi: 10.1016/j.cell.2017.06.016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khalil DN et al. In situ vaccination with defined factors overcomes T cell exhaustion in distant tumors. J Clin Invest 129, 3435–3447, doi: 10.1172/JCI128562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esensten JH, Helou YA, Chopra G, Weiss A & Bluestone JA CD28 Costimulation: From Mechanism to Therapy. Immunity 44, 973–988, doi: 10.1016/j.immuni.2016.04.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blackburn SD et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology 10, 29–37, doi:ni.1679 [pii] 10.1038/ni.1679 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang AC et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 25, 454–461, doi: 10.1038/s41591-019-0357-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang AC et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65, doi: 10.1038/nature22079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kamphorst AO et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 114, 4993–4998, doi: 10.1073/pnas.1705327114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curiel TJ et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9, 562–567, doi: 10.1038/nm863 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Zappasodi R, Merghoub T & Wolchok JD Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell 33, 581–598, doi: 10.1016/j.ccell.2018.03.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Long GV et al. Standard-Dose Pembrolizumab Plus Alternate-Dose Ipilimumab in Advanced Melanoma: KEYNOTE-029 Cohort 1C, a Phase 2 Randomized Study of Two Dosing Schedules. Clin Cancer Res, doi: 10.1158/1078-0432.Ccr-21-0793 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maruhashi T, Sugiura D, Okazaki IM & Okazaki T LAG-3: from molecular functions to clinical applications. J Immunother Cancer 8, doi: 10.1136/jitc-2020-001014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tawbi HA et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. New England Journal of Medicine 386, 24–34, doi: 10.1056/NEJMoa2109970 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen R et al. LAG-3 expression on peripheral blood cells identifies patients with poorer outcomes after immune checkpoint blockade. Sci Transl Med 13, eabf5107, doi: 10.1126/scitranslmed.abf5107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrews LP et al. Resistance to PD1 blockade in the absence of metalloprotease-mediated LAG3 shedding. Sci Immunol 5, doi: 10.1126/sciimmunol.abc2728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eggermont AM et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 375, 1845–1855, doi: 10.1056/NEJMoa1611299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eggermont AMM et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer 119, 1–10, doi: 10.1016/j.ejca.2019.07.001 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Curti BD & Faries MB Recent Advances in the Treatment of Melanoma. N Engl J Med 384, 2229–2240, doi: 10.1056/NEJMra2034861 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Eggermont AMM et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 22, 643–654, doi: 10.1016/S1470-2045(21)00065-6 (2021). [DOI] [PubMed] [Google Scholar]
- 104.Luke JJ Pembrolizumab versus placebo after complete resection of high-risk stage II melanoma: Efficacy and safety results from the KEYNOTE-716 double-blind phase III trial. Annals of Oncology 32, S1283–S1346, doi: 10.1016/annonc/annonc741 (2021). [DOI] [Google Scholar]
- 105.Amaria RN et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol 20, e378–e389, doi: 10.1016/S1470-2045(19)30332-8 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Tarhini AA et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 9, e87705, doi: 10.1371/journal.pone.0087705 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amaria RN et al. Neoadjuvant and adjuvant nivolumab (nivo) with anti-LAG3 antibody relatlimab (rela) for patients (pts) with resectable clinical stage III melanoma. Journal of Clinical Oncology 39, 9502–9502, doi: 10.1200/JCO.2021.39.15_suppl.9502 (2021). [DOI] [Google Scholar]
- 108.Menzies AM et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 27, 301–309, doi: 10.1038/s41591-020-01188-3 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Rawson RV et al. Pathological response and tumour bed histopathological features correlate with survival following neoadjuvant immunotherapy in stage III melanoma. Ann Oncol 32, 766–777, doi: 10.1016/j.annonc.2021.03.006 (2021). [DOI] [PubMed] [Google Scholar]
- 110.Rozeman EA et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nature Medicine 27, 256–263, doi: 10.1038/s41591-020-01211-7 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Blank CU et al. First safety and efficacy results of PRADO: A phase II study of personalized response-driven surgery and adjuvant therapy after neoadjuvant ipilimumab (IPI) and nivolumab (NIVO) in resectable stage III melanoma. Journal of Clinical Oncology 38, 10002–10002, doi: 10.1200/JCO.2020.38.15_suppl.10002 (2020). [DOI] [Google Scholar]
- 112.Moschos SJ et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol 24, 3164–3171, doi: 10.1200/JCO.2005.05.2498 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Carthon BC et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 16, 2861–2871, doi: 10.1158/1078-0432.CCR-10-0569 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ng Tang D et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer immunology research 1, 229–234, doi: 10.1158/2326-6066.CIR-13-0020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei SC, Duffy CR & Allison JP Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 8, 1069–1086, doi: 10.1158/2159-8290.CD-18-0367 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Arce Vargas F et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 33, 649–663 e644, doi: 10.1016/j.ccell.2018.02.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romano E et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A 112, 6140–6145, doi: 10.1073/pnas.1417320112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharma A et al. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3(+) Regulatory T Cells (Tregs) in Human Cancers. Clin Cancer Res 25, 1233–1238, doi: 10.1158/1078-0432.Ccr-18-0762 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kavanagh B et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood 112, 1175–1183, doi: 10.1182/blood-2007-11-125435 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Selby MJ et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer immunology research 1, 32–42, doi: 10.1158/2326-6066.CIR-13-0013 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Wing JB, Ise W, Kurosaki T & Sakaguchi S Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41, 1013–1025, doi: 10.1016/j.immuni.2014.12.006 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Zappasodi R et al. CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours. Nature 591, 652–658, doi: 10.1038/s41586-021-03326-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Korman AJ et al. Abstract SY09–01: Next-generation anti-CTLA-4 antibodies. Cancer Res 77, SY09–01, doi: 10.1158/1538-7445.AM2017-SY09-01 (2017). [DOI] [Google Scholar]
- 124.Hashimoto M et al. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu Rev Med 69, 301–318, doi: 10.1146/annurev-med-012017-043208 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Valpione S et al. Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer 1, 210–221, doi: 10.1038/s43018-019-0022-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kamada T et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 116, 9999–10008, doi: 10.1073/pnas.1822001116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumagai S et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 21, 1346–1358, doi: 10.1038/s41590-020-0769-3 (2020). [DOI] [PubMed] [Google Scholar]
- 128.Sage PT, Paterson AM, Lovitch SB & Sharpe AH The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41, 1026–1039, doi: 10.1016/j.immuni.2014.12.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van der Leun AM, Thommen DS & Schumacher TN CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 20, 218–232, doi: 10.1038/s41568-019-0235-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li H et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 176, 775–789 e718, doi: 10.1016/j.cell.2018.11.043 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Falck Miniotis M et al. MEK1/2 Inhibition Decreases Lactate in BRAF-Driven Human Cancer Cells. Cancer Res 73, 4039, doi: 10.1158/0008-5472.CAN-12-1969 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Hall A et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget 4, 584–599, doi: 10.18632/oncotarget.965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haq R et al. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell 23, 302–315, doi: 10.1016/j.ccr.2013.02.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cascone T et al. Increased Tumor Glycolysis Characterizes Immune Resistance to Adoptive T Cell Therapy. Cell Metab 27, 977–987 e974, doi: 10.1016/j.cmet.2018.02.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peng W et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov 6, 202–216, doi: 10.1158/2159-8290.CD-15-0283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brand A et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab 24, 657–671, doi: 10.1016/j.cmet.2016.08.011 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Hugo W et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165, 35–44, doi: 10.1016/j.cell.2016.02.065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fischer GM et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov 9, 628–645, doi: 10.1158/2159-8290.CD-18-1489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jaiswal AR et al. Melanoma Evolves Complete Immunotherapy Resistance through the Acquisition of a Hypermetabolic Phenotype. Cancer immunology research 8, 1365–1380, doi: 10.1158/2326-6066.CIR-19-0005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Najjar YG et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight 4, doi: 10.1172/jci.insight.124989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim SH et al. Phenformin Inhibits Myeloid-Derived Suppressor Cells and Enhances the Anti-Tumor Activity of PD-1 Blockade in Melanoma. J Invest Dermatol 137, 1740–1748, doi: 10.1016/j.jid.2017.03.033 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Afzal MZ, Mercado RR & Shirai K Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. Journal for ImmunoTherapy of Cancer 6, 64, doi: 10.1186/s40425-018-0375-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scharping NE et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nature immunology 22, 205–215, doi: 10.1038/s41590-020-00834-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Watson MJ et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651, doi: 10.1038/s41586-020-03045-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang H et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nature immunology 21, 298–308, doi: 10.1038/s41590-019-0589-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brahmer JR et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. Journal for ImmunoTherapy of Cancer 9, e002435, doi: 10.1136/jitc-2021-002435 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leko V & Rosenberg SA Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 38, 454–472, doi: 10.1016/j.ccell.2020.07.013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coulie PG, Van den Eynde BJ, van der Bruggen P & Boon T Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 14, 135–146, doi: 10.1038/nrc3670 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Vogelstein B et al. Cancer genome landscapes. Science 339, 1546–1558, doi: 10.1126/science.1235122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Turajlic S et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol 18, 1009–1021, doi: 10.1016/S1470-2045(17)30516-8 (2017). [DOI] [PubMed] [Google Scholar]


