Abstract
Prokaryotes employ clustered regularly interspaced short palindromic repeat (CRISPR) and CRISPR-associated (Cas) proteins as an adaptive immune system. CRISPR-Cas systems preserve molecular memories of infections by integrating short fragments of foreign nucleic acids as spacers into the host CRISPR array, in a process termed adaptation. Functional spacers ensure a robust immune response by Cas effectors, which neutralize subsequent infection through RNA-guided interference pathways. In this review, we summarize recent discoveries that have advanced our understanding of adaptation, with a focus on how functional spacers are generated and incorporated through many widespread but type-specific mechanisms. Finally we highlight future directions and remaining questions for a thorough understanding of CRISPR adaptation.
Keywords: CRISPR-Cas, spacers, Cas1-Cas2, Cas9, Cas4, PAM
The discovery of a bacterial adaptive defense system
Viruses are the most abundant biological entities in the biosphere, and the pressure of virus infections has driven the evolution of numerous prokaryotic defense systems, including CRISPR-Cas (see Glossary) systems [1]. CRISPR arrays were first discovered in the genome of Escherichia coli, noted as unusual repetitive sequences located downstream of the iap gene [2]. These repeats were separated by variable “spacer” sequences that originated from viruses and plasmids [3]-[5]. The first experimental evidence demonstrating that CRISPRs provide adaptive immunity was observed in the lactic acid bacterium Streptococcus thermophilus. Indeed, upon challenge with virulent phages, the bacterial population gained resistance by acquiring new spacers against the infecting phages into the CRISPR array [6]. Shortly thereafter, cas genes that are often found in the neighborhood of the CRISPR array were shown to use the immunological memory by expressing a CRISPR RNA (crRNA) that confers resistance in a sequence-specific manner [7], [8]. Since these pioneering discoveries, the biological function of each Cas component during the immunization and neutralization phases of CRISPR immunity have been largely elucidated. Cas proteins are required for integration of spacers in the CRISPR array, biogenesis of the crRNA, and RNA-guided interference against the invader upon subsequent reinfection (Figure 1) [9]. Thus, spacer acquisition underlies the subsequent expression and interference phases that neutralize invaders upon re-infection.
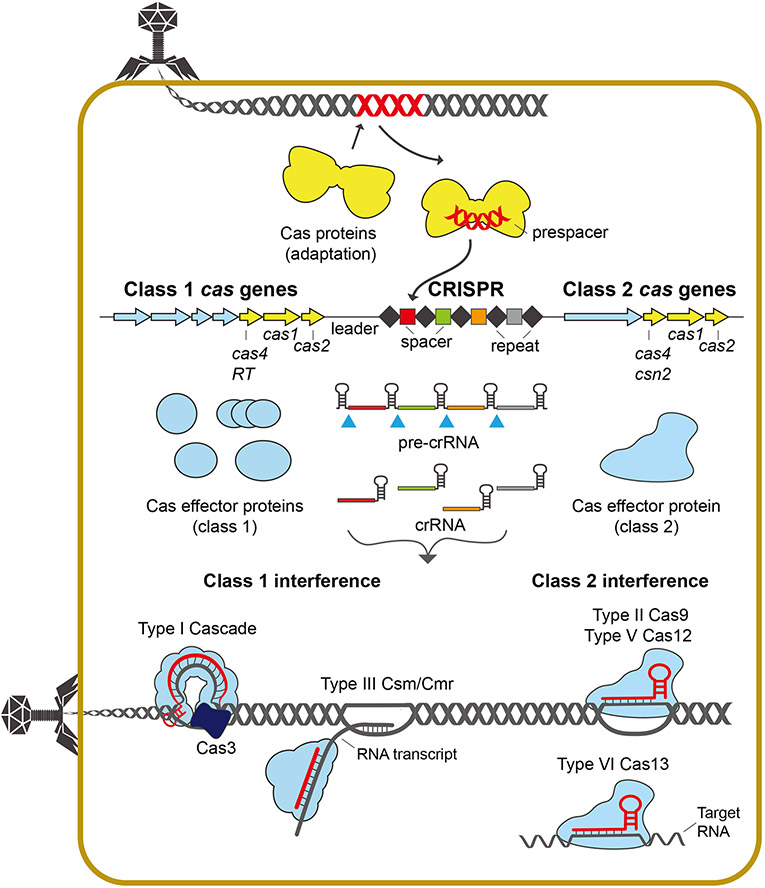
Figure 1. Overview of CRISPR-Cas systems.
CRISPR-Cas operons contain a CRISPR, made up of repeats (black diamonds) and spacers (colored boxes) and cas genes expressing Cas proteins involved in adaptation (yellow) or interference (blue). The adaptation Cas proteins capture and integrate short fragments from foreign genomes into the CRISPR array as new spacers. The array is transcribed into a pre-crRNA and processed into crRNAs, allowing formation of an effector complex with multiple Cas proteins (class 1) or a single Cas protein (class 2). Class 1 type I systems use CRISPR-associated complex for antiviral defense (Cascade) complexes and type III systems use Csm-(III-A)/Cmr-(III-B) effector complexes. Cascade recognizes DNA targets and recruits Cas3 nuclease for degradation. In type III systems, the effector complex provides immunity in transcription-dependent manner, exhibiting both target RNA and ssDNA cleavage activities. Class 2 systems encompass a single effector protein, Cas9 in type II, Cas12 in type V, and Cas13 in type VI. The crRNA-guided effector complexes target the foreign nucleic acids that are complementary to the crRNA sequences. Type II Cas9 and type V Cas12 cleaves target DNA, resulting in dsDNA breaks. Type VI uses Cas13 for degradation of target RNAs.
CRISPR-Cas systems are present in approximately 40% of bacteria and 90% of archaea and categorized into two classes, six types, and many subtypes based on the composition of cas gene content (Figure 1) [10]. Although class 1 and 2 systems vary in the composition and stoichiometry of their effector complexes that carry out RNA-guided interference, most systems share common genes, cas1 and cas2, that are involved in adaptation.
Adaptation is a dynamic process of acquiring new spacers from the invading genomes by selecting, processing, and integrating short fragments from the invaders into CRISPR arrays. An early study in E. coli showed that spacer acquisition requires the cas1 and cas2 genes along with the AT-rich leader and first repeat of the CRISPR array [11]. Structural and biochemical studies demonstrated that Cas1 and Cas2 proteins form a heterohexameric complex [12]-[14]. The Cas1-Cas2 complex integrates spacers at the first repeat sequence within the CRISPR array through concurrent cleavage-ligation via transesterification [15]. Phylogenetic analysis suggests that cas1 likely have originated from casposons, a distinct family of self-replicating transposons, [16], [17].
The conservation of the cas1 and cas2 genes suggests that the Cas1-Cas2 complex and mechanism of integration is universal to most CRISPR-Cas systems. However, the presence of sub-type-specific adaptation genes and the potential role of the interference machinery and other host factors in spacer acquisition point to different adaptation mechanisms between diverse CRISPR-Cas systems. Recent studies have shed light on numerous long-standing questions related to these additional factors. Here, we summarize the current models of CRISPR adaptation and highlight the remaining key questions.
Generation of prespacers upon naïve infection
New spacers are acquired within a CRISPR arrays through integration of a prespacer substrate at the first repeat in the array by the Cas1-Cas2 complex. In the absence of a previously acquired spacer, host cells are naïve to infection by an invader, requiring de novo generation of prespacers from the invading genome. During naïve acquisition, spacers can be acquired either from foreign nucleic acids or from the host genome, as observed in type I, II and III systems [18]-[20]. Self-acquisition results in auto-immunity and programmed cell death [21]. Indeed, mutants with the increased rate of spacer acquisition showed high levels of autoimmunity events [22], suggesting that the rate of spacer acquisition needs to be balanced to minimize fitness costs of host cells. To fully benefit from CRISPR-Cas immunity, spacers must be preferentially acquired from foreign DNA.
Such a preference has been observed in some sub-types [11], [23], [24], and is thought to be due to a connection between free DNA ends and spacer acquisition [18], [23], [25]-[27]. In one current model, the host DNA repair machinery, RecBCD in Gram-negative strains [23] and AddAB in Gram-positive strains [27], generates prespacer substrates (Figure 2 A-B). These complexes recognize free DNA ends and degrade the DNA processively until arriving at a chi site. Host chromosomes are enriched with chi sites compared to the genomes of phages or plasmids, resulting in biased DNA pools of foreign nucleic acids based on abundance of RecBCD or AddAB degradation products. Degradation of linear dsDNA during phage DNA injection enables prespacer generation at the very early stage of the infection, resulting in more effective immune response [27]. Origins of replication are also “hot spots” for spacer acquisition [23], [25], [26], [28], suggesting another potential link between replication and adaptation.
Figure 2. Model of CRISPR adaptation mechanisms.
(A-B) Prespacers may be generated through the activity of RecBCD or AddAB, in Gram negative and positive bacteria, respectively, or through interference activity by type I (A) or type II (B) effector complexes. (C-E) Models for prespacer selection and processing in (C) E. coli type I-E, (D) G. sulfurreducens type I-G, and (E) S. thermophilus type II-A systems. Type I systems acquire spacers from RecBCD or Cascade-Cas3 activities. RecBCD or Cascade-Cas3 activities provide ssDNA substrates for naïve or primed adaptation. In type I systems, Cas1-Cas2 (C) or Cas4-Cas1-Cas2 (D) complex captures ssDNA with PAM sequences and facilitates annealing of complimentary ssDNAs. Non-PAM overhangs are cleaved by DnaQ exonuclease while PAM end overhangs are protected by Cas1 (C) or Cas4 (D). IHF may induce bending of the leader (green), allowing recognition of upstream leader motifs by Cas1-Cas2. The adaptation complex integrates the processed non-PAM end first at the junction of the leader (green) and the first repeat (black) (L-site integration) followed by the cleavage of PAM-end by DnaQ in Cas1-Cas2 (C) or Cas4 in Cas4-Cas1-Cas2 (D) complex. DnaQ or Cas4 dissociates from the complex after the cleavage and the processed PAM-ends are integrated at the junction of the repeat and spacer (blue) (S-site integration). (E) Type II-A systems acquire spacers from AddAB and Cas9 activities. Cas9 is required for both naïve and primed adaptation for selecting prespacers flanking PAM sequences. Csn2-Cas1-Cas2 multisubunit complex binds dsDNA substrates. The exact roles of Cas9 and Csn2 in this process remain unknown. Cas1-Cas2 is sufficient for integration at L-site and S-sites. After integration, gaps are filled through transcription-coupled repair mechanism.
Notably, spacers can be integrated independent of RecBCD [23] or AddAB [27]. Thus, diverse mechanisms must exist for spacer generation during naïve host-pathogen encounters. The observed preference for free DNA ends may suggest synergistic coordination between CRISPR-Cas and restriction-modification (RM) systems. RM systems are available in 90% of prokaryotes and cleave any foreign DNA with a specific recognition site that has not undergone methylation. While it has been demonstrated that RM systems and CRISPR systems act together to increase phage resistance [29], whether RM systems provide DNA pools or create signals to CRISPR systems remains to be determined. This CRISPR-RM synergy also suggests that other co-existing immune mechanisms act together, an important research frontier as new immune mechanisms continue to be discovered [30], [31].
Primed generation of prespacers
Following immunization with an initial spacer, bacterial populations can rapidly diversify their CRISPR arrays through a process called primed adaptation or priming. In priming, pre-existing spacers within a host CRISPR drive interference against a foreign DNA, which significantly enhances the acquisition of additional spacers from that foreign DNA [19], [32]-[34]. Priming was originally discovered in the E. coli type I-E system and has since been observed in several type I and type II subtypes [9]. Because the crRNA-guided effector complex drives spacer acquisition, priming requires both adaptation and interference cas genes (Figure 2 A-B). In type I systems, this includes genes expressing the immune effector complex, Cascade, and the nuclease-helicase Cas3 (Figure 2A) [32]. In type II-A systems, the effector endonuclease Cas9 is required for adaptation in general, as well as for primed adaptation (Figure 2B) [19], [34], [35].
Priming has been best characterized in the type I-E and I-F systems. During interference, Cas3 degrades Cascade-bound target DNA into ssDNA fragments, generating potential substrates for the adaptation machinery (Figure 2A) [36]. Cas3 reels ssDNA within this complex, allowing translocation via DNA looping [37]-[39]. Primed acquisition has been observed to occur from each strand upstream or downstream of the target in type I-F, and more recently in type I-E [40], [41]. While Cas3 nicking and degradation may account for these acquisition events from sequences downstream of the target [38], it remains unclear whether or how bidirectional degradation may facilitate acquisition of spacers from upstream of the Cascade target.
Several lines of evidence suggest that Cascade recruits Cas3 with Cas1-Cas2, forming a primed acquisition complex (PAC) that enables direct handoff of Cas3 degradation products to Cas1-Cas2 [37], [39]. Indeed, recent evidence suggests the same prespacer DNA fragments are associated with both Cas1 and Cas3 in E. coli [42]. Moreover, in type I-F systems, Cas3 is fused to Cas2, forming an obligate Cas1-Cas2/3 complex in which Cas1 regulates Cas3 degradation activity [43], [44]. Despite this mounting evidence for a higher-order adaptation/interference complex, the exact mechanism of prespacer searching and handoff from Cas3 to Cas1-Cas2 remains unclear and requires further study.
Type II priming is less mechanistically characterized, but follows similar principles to type I systems. Priming requires the nuclease activity of Cas9 [34]. Cas9 is thought to physically associate with Cas1-Cas2 and the additional adaptation protein, Csn2 [35], which may enable efficient Cas1-Cas2 recruitment to Cas9 cleavage sites (Figure 2B) [45]. Thus, it is likely that both type I and II systems form higher-order complexes that combine interference and adaptation machinery for efficient spacer acquisition.
Primed adaptation occurs at both perfect and imperfect targets that impair interference [28], [34], [46]. Target mutations can decrease the rate of interference either by slowing target binding or inducing conformational changes in Cascade that reduce Cas3 recruitment [47]-[49]. In type I systems, persistent exposure to the target DNA enables more priming [46], either through multiple rounds of Cas3-dependent fragment generation or through prolonged PAC activity on the longer-lived target [37], [39]. Thus, priming enables uptake of multiple spacers during fast-paced co-evolution, in which mutated phages can drive the emergence of new populations of bacteria bearing new spacers [50]. In addition, imperfect acquisition of non-immunogenic spacers can drive priming in type I systems [51]. Together, these mechanisms diversify the spacer content in a bacterial population, which limits the effectiveness of mutations at individual target sites to drive phage escape [52].
Selection of spacers
Successful adaptation requires the selection of functional spacers that can generate robust interference by the crRNA-effector complex. Some CRISPR systems target RNA rather than DNA, and require spacer acquisition from regions of the genome that are actively transcribed [53]-[55] (see BOX 1). DNA-targeting CRISPR systems require spacer acquisition preferentially from the template strand of foreign DNA containing protospacer-adjacent motifs (PAM) [56]. These 2-to 5-bp sequences are located directly upstream (type I and V) or downstream (type II) of Cas effector targets [57], and are required for dsDNA unwinding and target binding [58]-[61]. Requirement of a PAM at both the adaptation and interference stages suggests co-evolution of spacer acquisition and effector complexes to recognize the same PAM sequences, as has been observed in type I systems [12]. Alternatively, the effector complex may be directly involved in PAM recognition during spacer acquisition [26], [35].
Box 1. Spacer acquisition in RNA-targeting CRISPR systems.
In type III and VI CRISPR systems, the interference machinery targets RNA transcripts [80], and thus display hot-spots for spacer acquisition from highly expressed genes [81] or preferentially from coding strands to directly target RNA [56]. Some type III systems feature Cas1 fused to a reverse transcriptase (RT) (Figure 1), enabling spacer acquisition directly from RNA transcripts through RT-directed prespacer synthesis [82], [83]. Type III systems that lack RT preferentially uptake DNA substrates containing predicted secondary structures [20]. In addition to the RT domain, these fusion proteins may also include the Cas6 endoribonuclease that is required for maturation of the guide CRISPR RNA during the expression stage [84]. Recent structures of Cas6-RT-Cas1-Cas2 revealed close contact between the three active sites, suggesting coordination between the different activities associated with this complex [84]. However, although spacers can be acquired from RNA templates, RT-Cas1 prefers a DNA substrate for spacer integration [84]. Thus, it is possible that RT-Cas1-containing systems may diversify spacer selection from multiple nucleic acid sources.
Interestingly, some type III and VI systems lack adaptation machinery and co-exist with multiple CRISPR systems, suggesting that adaptation mechanisms may be shared across different systems within the same organism. Indeed, crosstalk was observed in a recent study where the adaptation machinery from a type II-C system provides spacers for an RNA-targeting type VI-B system [85]. Most commonly co-occurring type I systems acquire spacers preferentially from the coding strand, similar to strand bias observed in RNA-targeting type III systems [56]. In these cases, it remains unclear whether and how spacers are specifically acquired with a strand bias to enable sharing between multiple CRISPR systems. It is possible that transcripts may increase the rate of acquisition by the adaptation machinery, producing a non-self-acquisition bias when phages reprogram host cells for viral proliferation. Alternatively, spacers may be selected based on the level of anti-phage activity that they provide. Further work will be necessary to distinguish these possible mechanisms for biased spacer acquisition.
Adaptation complexes in type I systems use either Cas1 or an additional adaptation factor, Cas4, for PAM recognition (Figure 3). Cas4 is found in all type I systems with the exception of the well-studied type I-E and I-F systems. Structural studies of the E. coli type I-E Cas1-Cas2 complex revealed that a loop at the C-terminus of Cas1 is responsible for PAM recognition (Figure 3A-B) [12]. The Cas1-Cas2 complex preferentially integrates duplexes containing single-stranded 3’ overhangs in vitro [12], [13]. Similarly, prespacers associated with Cas1 isolated from E. coli cells are double-stranded, containing a short 3’ overhang on the PAM-end and a blunt end on the PAM-distal end [41]. However, degradation products of RecBCD and Cas3-mediated interference are single-stranded [36], [62], raising the question of how Cas1-Cas2 captures the required double-stranded substrates. A recent single molecule study showed that E. coli Cas1-Cas2 preferentially binds to a single strand of DNA containing a PAM sequence and facilitates annealing of complementary ssDNA, generating a suitable substrate comprising a duplex with 3’end overhangs (Figure 2C) [63]. Thus, PAM-recognition during prespacer capture may play a dual role in facilitating formation of a double-stranded substrate and in ensuring that functional spacers are integrated.
Figure 3: PAM-recognition in type I adaptation complexes.
(A) Type I-E adaptation complexes are composed of two Cas1 dimers (yellow/beige) sandwiching a Cas2 dimer (salmon/pink) and lacks a Cas4 subunit. The prespacer (white/gray) spans the length of the Cas2 subunits, allowing threading of 3’ overhangs into the Cas1 active sites. (B) The PAM in one overhang is recognized by Cas1 and protected from degradation by exonucleases by a loop in the C-terminal tail of Cas1. The CTT PAM-complementary sequence is recognized through a combination of specific hydrogen-bonding interactions and stacking interactions with the two Cas1 subunits. (C) Other type I systems (excluding type I-E) contain Cas4 subunits that assemble with the core Cas1-Cas2 complex. The type I-G system contains a Cas4-1 fusion protein, in which one Cas4 domain (blue) recognizes the PAM-end of the prespacer. (D) Specific recognition of the GAA PAM-complementary sequence is recognized by several residues adjacent to an Fe4S4 cluster, mainly through stacking and van der Waals interactions that recognize the shape of the sequence and a few specific hydrogen-bonding interactions. (A-B) PDB ID: 5DQZ; (C-D) PDB ID: 7MI5).
In contrast to type I-E and I-F, Cas1 from type I systems containing Cas4 lack the C-terminal extension required for PAM recognition [63]. In these systems, Cas4 is required for PAM recognition (Figures 2 and 3C-D) [64]-[67]. Cas4 is a RecB-like nuclease widespread in type I, II and V DNA-targeting CRISPR systems [10]. Stand-alone cas4 genes are also found at locations separate from the cas operon and even in some phage genomes [10]. Cas4 forms a complex with Cas1-Cas2 [68], [69] and is required for quality-control during spacer acquisition by specifying both the PAM sequence and the orientation of integration [64]-[68]. A recent structural study of the type I-G adaptation complex, containing a Cas4-Cas1 fusion protein, showed that the Cas4 domain reads PAM sequences mainly through shape recognition using its iron-sulfur cluster binding domain (Figure 3C-D) [69].
Type II systems rely on the Cas effector Cas9 for PAM selectivity while acquiring spacers [34], [35]. In type II-A systems, all four Cas proteins, Cas1, Cas2, Csn2, and Cas9, are required for spacer acquisition in vivo, and are thought to form a supercomplex (Figure 2E) [19], [35], [70]. Cas9 employs the same PAM-interacting domain used for detecting the PAM during target cleavage for PAM-specific spacer acquisition [19], [35]. Notably, the PAM interacting domains of Cas9 orthologs show relatively low conservation, resulting in large diversities of PAM sequences in type II systems and suggesting a variety of PAM-recognition mechanisms during spacer acquisition [56], [71].
The exact function of Csn2 remains mysterious. Csn2 inhibits in vitro integration activities of Cas1-Cas2 [72] and forms a multi-subunit complex with Cas1-Cas2 where two Csn2 tetramers form a channel that threads dsDNA while Cas1-Cas2 has no contacts with the DNA [45]. Given that Cas9 is not part of Csn2-Cas1-Cas2 multi-subunit complex in the presence of DNase [45], it is possible that dsDNA cleavage by Cas9 generates free DNA ends for the recruitment of the multi-subunit complex. Consistently, spacers are preferentially acquired from regions close to Cas9 cleavage sites during priming [34]. Moreover, Cas9 tightly associates to its cleavage products [61], possibly protecting free DNA ends from the host DNA repair machinery. This may enable handoff of free DNA ends to the Csn2-Cas1-Cas2 multi-subunit complex. Further studies are required to resolve exactly how recognition of DNA ends by the Csn2-Cas1-Cas2 complex is coordinated with PAM recognition by Cas9 during spacer capture.
Asymmetrical prespacer trimming controls directional integration
Once bound by the adaptation complex, prespacers must be processed and integrated at the first repeat in the CRISPR array to ensure timely immune response (see BOX 2). High-fidelity spacer acquisition requires orchestration of several events. Prespacers must be processed precisely to produce a template for a functional crRNA. Because the PAM is not part of the spacer, the PAM sequences in the 3’ overhangs must be removed from the prespacer substrate prior to integration. Prespacer processing must also occur in a manner that allows the spacer to be oriented properly during integration into the CRISPR array. Additionally, in type I systems, the PAM-end of the prespacer must be integrated at the leader-distal end of the repeat for proper orientation of the crRNA template (Figure 2A-B). Recent work has revealed that asymmetrical processing of prespacers enables PAM-dependent directional integration [63], [69], [73].
Box 2. Polarized spacer integration at the 5’-end of the CRISPR.
New spacers are predominantly integrated at the leader end of the CRISPR array, which directs transcription of the downstream repeat and spacer sequences (Figure 1) [14], [15], [72], [76]. This polarization provides a chronology of infections, where the newest spacers are closer to the leader while the oldest are at the distal end [11]. Indeed, the most abundant crRNA species are generated from the spacers at the leader end of the CRISPR array [86] and erroneous insertions of spacers in the middle of the array results in selective pressure against infections due to lowered relative expression of these spacers corresponding with low immunity [87]. Thus, integration at the leader end ensures a rapid and robust immune response against the most recent invaders.
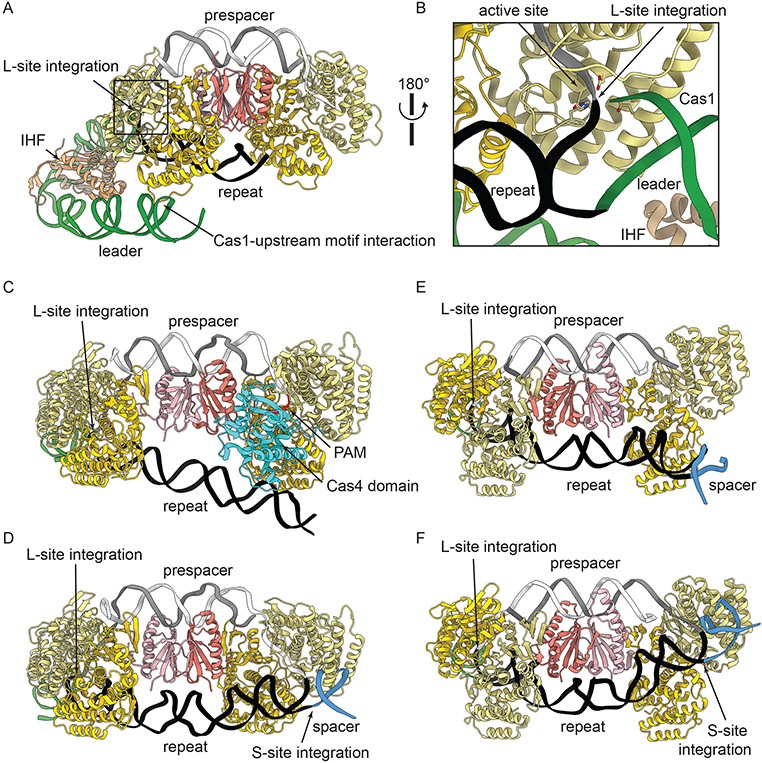
Polarized integration requires recognition of the leader-repeat junction by Cas1 (Figure 4) [11], [72], [88]. Off target integration events threaten genome integrity, and are expected to be very rare [72]. The specificity of Cas1 for this sequence enables rare non-canonical spacer integration outside of the CRISPR array at sites with similarity to the CRISPR leader-repeat junction, resulting in the potential spontaneous generation of CRISPR loci with new types of repeats [89]. Specificity for leader-end integration is also assisted by bacterial nucleoid associated proteins such as IHF (integration host factor) in some systems [43], [76], [90], [91]. In type I-E, IHF induces bending of the leader by ~120° [91] bringing upstream leader motifs into closer proximity to Cas1-Cas2 (Figure 4 A, B) [76]. These leader-Cas1-Cas2 interactions induced by IHF substantially increase integration at the leader-proximal repeat [90]. In addition to conserved leader motifs, the phase between IHF binding sites and the upstream sequences are critical for polarized adaptation [92]. Phasing between leader motifs is sub-type dependent, suggesting differences in interactions between the adaptation machinery, host factors, and motifs in varied CRISPR systems. Notably, IHF is present only in gram negative bacteria, suggesting that many gram positive bacteria or archaea use other DNA bending factors or depend solely on intrinsic sequence specificities of Cas1-Cas2 to the leader-repeat junction.
Within the Cas1-Cas2 complex, the length of the prespacer is constrained by the distance between the two Cas1 homodimers (Figure 3A, C) [12]-[14]. Cas2 is a dimeric structural protein that bridges two dimers of Cas1. The prespacer spans the length of Cas2, allowing threading of single-strand 3’ overhangs into the Cas1 integrase active sites [12]. The adaptation complex protects the prespacer ends from cellular exonucleases, contributing to determination of spacer length [63], [69]. Indeed, non-specific trimming by exonucleases is thought to be a requirement for maturation of the prespacer following binding by the adaptation complex. Some systems contain a fusion of Cas2 and a DnaQ-like domain that is involved in prespacer maturation [74]. However, most type I systems lack a Cas2-DnaQ fusion and rely instead on the non-specific trimming activities of host exonucleases [63], [73].
In the E. coli type I-E system, DnaQ exonucleases trim both ends of the prespacer. The C-terminal tail of Cas1 recognizes and protects the PAM-containing end of the prespacer (Figures 2C and 3A-B), while the PAM-distal strand is trimmed to the optimal length needed for the integration [63]. This asymmetrical prespacer maturation is also observed in vivo during priming [41]. Mutations of the Cas1 C-terminal tail reduces PAM protection from exonuclease activity, generating symmetrically trimmed prespacers [63].
The PAM-dependence of asymmetric trimming suggests a mechanism for biased orientation of integration. As a result of asymmetric trimming by DnaQ, Cas1-Cas2 integrates the non-PAM end at the leader-repeat junction, resulting in a half-site integration intermediate (Figures 2 and 4) [63]. The second nucleophilic attack takes place at the junction of the repeat and the first spacer. In order to maintain the constant length of repeats, Cas1-Cas2 likely makes nonspecific interactions with an inverted repeat motif, which acts as an anchor to specify the second site integration [75]. Then, Cas1-Cas2 induces bending of both the prespacer and repeat for placing the Cas1 active site adjacent to the second integration site [14], [76]. Once loaded on the second site, the C-terminal tail of Cas1 exposes the PAM end for maturation by DnaQ, allowing processing to the optimal length. Cas1 subsequently integrates the PAM end at the junction of repeat-spacer, generating the full-site integration product.
Figure 4: Integration of prespacers at first repeat of CRISPR array.
(A) Integration may be directed to the first repeat in the CRISPR array through interactions between Cas1 and upstream regions of the leader (green). IHF (tan) induces DNA bending in the leader, enabling this interaction. (B) Integration at the leader-repeat junction (L-site integration) occurs within the Cas1 active site. Proteins are colored as in Figure 3, DNA as in Figure 2. (C-E) Structures capturing half-site (C, E) and full-site (D, F) integration products for type I-G (C, D) and II-A (E, F). (C) L-site integration triggers cleavage of the PAM (red) by Cas4, likely through activation based on interactions between the repeat and the Cas1 domain. (D) PAM cleavage leads to dissociation of the Cas4 domain and integration of the PAM end at the repeat-spacer junction (S-site integration). (E-F) Type II-A Cas1-Cas2 performs half-site integration first at the L-site €, followed by S-site integration (F). (A-B) PDB ID: 5WFE; (C) PDB ID: 7MIB; (D) PDB ID: 7MI9; (E) PDB ID: 5XVO; (F) PDB ID: 5XVP.
Systems containing Cas4 employ a similar but distinct mechanism for asymmetric processing and directional integration. While some type I systems contain two Cas4 proteins, each of which recognizes one end of the prespacer, defining the orientation [67], most systems contain only a single Cas4 protein. In these systems, stable binding of the PAM-end of the prespacer by Cas4 prevents trimming by cellular exonucleases, analogous to the protection provided by the Cas1 C-terminal tail in type I-E (Figures 2D and 3C-D) [69]. Cas4 is required not only for PAM selection, but also for endonucleolytic cleavage just upstream of the PAM [65], [67], [68]. This cleavage event is essential for precise definition of a crRNA spacer sequence that can base pair with a target region just adjacent to the PAM. In systems containing a single Cas4 protein, the timing of PAM-end cleavage must be controlled to ensure orientational integration. Cas4 delays processing PAM sequences on prespacers until correctly oriented for integration [69]. Interaction between the repeat and a Cas1 subunit adjacent to the endonucleolytic Cas4 domain triggers cleavage activity, enabling PAM trimming and completion of full-site integration (Figures 2D and 4 C-D) [69].
The role of Cas4 in type II and V systems is unreported to date, but may be similar to type I systems, as has been observed for Cas1-Cas2 function. However, Cas4 is only found in a limited number of sub-types within these systems, suggesting that these systems may use alternative factors for biasing spacer processing and orientation, similar to type I-E. Indeed, a recent study showed that Cas9 can process the PAM-end of the prespacer [70], similar to Cas4 (Figure 2E). However, although the Cas9 PAM-interaction domain is required for PAM selection during adaptation [35], Cas9 cleavage activity is not required for either PAM selection or prespacer processing. This suggests that cellular exonucleases are sufficient for prespacer processing in the type II-A system, which lacks a Cas4 protein. Further studies are needed to fully understand the interplay between trimming and orientation by the class 2 adaptation machinery.
Once prespacers are fully integrated, the spacer is flanked on either side by a single stranded repeat. These ssDNA gaps must be filled to complete integration [77]. A recent single molecule study demonstrated that resolution of the post-integration complex is driven by a transcription coupled repair mechanism to rapidly repair these gaps during transcription of the CRISPR array by RNA polymerase (Figure 2) [78]. To prevent a double-strand break induced by simultaneous unwinding and repair of the newly integrated spacer, RNA polymerase transcribes a single-stranded repeat, exposing the strand for gap filling by DNA polymerase. Cas1-Cas2 protects the spacer region, defining the boundary for repeat generation. Alternatively, in type III systems, CRISPR-associated primase-polymerases (CAPPs) are often found within the same operon with Cas1-Cas2 and act as a DNA-dependent primase and polymerase, implying that CAPPs are involved in CRISPR-specific strand displacement synthesis [79]. Together, these studies suggest that DNA repair following spacer integration may be facilitated through a variety of mechanism, depending on the host- or type-specific machinery.
Concluding remarks
Since the discovery of adaptive immunity in bacteria and archaea, our understanding of the underlying molecular mechanisms of CRISPR spacer acquisition has grown substantially. Through genetic, biochemical, and structural approaches, we now have clear understanding of how spacers are selected, processed, and integrated to ensure the fidelity of the CRISPR array [63], [69]. The functional roles of several sub-type specific Cas proteins and other host factors have also been revealed [63], [69], [78]. Together with recent studies, a plausible model has developed to explain the timing between processing and integrating spacers, which enables spacer orientation correctly in the CRISPR array for proper interference by Cas effector complexes.
Yet, many questions still remain (see Outstanding questions). Alternative mechanisms of spacer biogenesis during naïve adaptation likely exist, but remain unknown. Although it is increasingly clear that the adaptation and interference complexes coordinate prespacer handoff during primed adaptation, the exact mechanism remains to be determined. The role of auxiliary adaptation factors also continues to be explored. How is Cas4 allosterically activated for PAM cleavage following half-site integration? What are the mechanistic roles of multiple or standalone Cas4 proteins? What are the exact roles for Cas9 and Csn2 in type II adaptation? Do Cas12 proteins participate in adaptation in type V systems? How do RNA-targeting CRISPR-Cas systems ensure selection of spacers from highly transcribed regions or coding strands? The answers to these questions will enhance our understanding of CRISPR immunity in the microbial world. It is an exciting time for research on CRISPR adaptation, and the next few years will undoubtedly reveal many more of its secrets.
Acknowledgments
H.L. acknowledges support from Dongyang Mirae University. D.G.S. acknowledges support from NIH R35GM140876 and NSF 1652661.
Glossary
- DnaQ
3’−5’ DNA exonucleases homologous to the DNA directed proofreading exonuclease (epsilon subunit) of DNA polymerase III in E. coli
- Cas
CRISPR associated proteins. Genes expressing these proteins are usually found adjacent to a CRISPR array. Cas proteins are involved in spacer acquisition, biogenesis of CRISPR RNA, and interference
- Cas1-Cas2
A core Cas protein complex involved in integration of new spacers into CRISPR arrays. Cas1 functions as an integrase and Cas2 functions as a structural scaffold
- Cas4
a RecB like nuclease involved in PAM recognition and processing during adaptation. Cas4 contains four conserved cysteine residues that coordinate an iron-sulfur cluster
- Chi site
homologous recombinational hotspot octamer sequences, which change function of RecBCD from an exonuclease to a helicase
- CRISPR
loci found in the genomes of bacteria and archaea, composed of short DNA fragments that originate from viruses or plasmids called spacers that are separated by identical repeating DNA sequences, called repeats
- CRISPR RNA (crRNA)
Short RNAs derived upon transcription of the CRISPR and subsequent processing, used by effector complexes as guides to target foreign nucleic acids
- Effector complex
Cas protein complexes that mediate CRISPR RNA guided interference activity
- Leader
AT-rich non-coding DNA sequences that are located at the 5’ end of CRISPR arrays, upstream of the first CRISPR repeat and often downstream of the last cas genes. Often contains the promoter elements for expression of the CRISPR array and sequence elements that guide spacer integration to the leader proximal repeat
- PAM
2-5 bp DNA sequences that are found adjacent to target DNA, but not in the host CRISPR array. Cas effectors use the PAM for establishing integration orientation and locating and unwinding target DNA
- Prespacers
Variable DNA sequences that act as substrates for adaptation complexes prior to integration into CRISPR arrays
- Priming
Adaptation mechanism that allows rapid acquisition spacers in the presence of pre-existing spacers
- Repeats
30-50 bp DNA sequences found within CRISPR arrays that are interspersed with variable DNA sequences (spacers) and often contain heptameric palindromic sequences
- Spacers
variable DNA sequences in CRISPR arrays, originating from viruses or plasmids, that specify immunity against these foreign elements
References
- [1].Hampton HG, Watson BNJ, and Fineran PC, “The arms race between bacteria and their phage foes,” Nature, vol. 577, no. 7790, pp. 327–336, 2020, doi: 10.1038/s41586-019-1894-8. [DOI] [PubMed] [Google Scholar]
- [2].Ishino Y, Shinagawa H, Makino K, Amemura M, and Nakatura A, “Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isoenzyme conversion in Escherichia coli, and identification of the gene product,” J. Bacteriol, vol. 169, no. 12, pp. 5429–5433, 1987, doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pourcel C, Salvignol G, and Vergnaud G, “CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies,” Microbiology, vol. 151, no. 3, pp. 653–663, 2005, doi: 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- [4].Mojica FJM, Díez-Villaseñor C, García-Martínez J, and Soria E, “Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements,” J. Mol. Evol, vol. 60, no. 2, pp. 174–182, 2005, doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- [5].Bolotin A, Quinquis B, Sorokin A, and Dusko Ehrlich S, “Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin,” Microbiology, vol. 151, no. 8, pp. 2551–2561, 2005, doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- [6].Barrangou R et al. , “CRISPR provides acquired resistance against viruses in prokaryotes,” Science (80-. )., vol. 315, no. March, pp. 1709–1712, 2007, doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- [7].Brouns SJJ et al. , “Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes Stan,” Science (80-. )., vol. 321, no. August, pp. 960–964, 2008, doi: 10.1126/science.1229223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jansen R, Van Embden JDA, Gaastra W, and Schouls LM, “Identification of genes that are associated with DNA repeats in prokaryotes,” Mol. Microbiol, vol. 43, no. 6, pp. 1565–1575, 2002, doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- [9].Nussenzweig PM and Marraffini LA, “Molecular Mechanisms of CRISPR-Cas Immunity in Bacteria,” Annu. Rev. Genet, vol. 54, pp. 93–120, 2020, doi: 10.1146/annurev-genet-022120-112523. [DOI] [PubMed] [Google Scholar]
- [10].Makarova KS et al. , “Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants,” Nat. Rev. Microbiol, vol. 18, no. 2, pp. 67–83, 2020, doi: 10.1038/s41579-019-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yosef I, Goren MG, and Qimron U, “Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli,” Nucleic Acids Res., vol. 40, no. 12, pp. 5569–5576, 2012, doi: 10.1093/nar/gks216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang J et al. , “Structural and Mechanistic Basis of PAM-Dependent Spacer Acquisition in CRISPR-Cas Systems,” Cell, vol. 163, no. 4, pp. 840–853, 2015, doi: 10.1016/j.cell.2015.10.008. [DOI] [PubMed] [Google Scholar]
- [13].Nuñez JK, Harrington LB, Kranzusch PJ, Engelman AN, and Doudna JA, “Foreign DNA capture during CRISPR–Cas adaptive immunity,” Nature, vol. 527, no. 7579, pp. 535–538, 2015, doi: 10.1038/nature15760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xiao Y, Ng S, Nam KH, and Ke A, “How type II CRISPR–Cas establish immunity through Cas1–Cas2-mediated spacer integration,” Nature, pp. 1–23, 2017, doi: 10.1038/nature24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nuñez JK, Lee ASY, Engelman A, and Doudna J. a., “Integrase-mediated spacer acquisition during CRISPR–Cas adaptive immunity,” Nature, 2015, doi: 10.1038/nature14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krupovic M, Makarova KS, Forterre P, Prangishvili D, and V Koonin E, “Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prokaryotic CRISPR-Cas immunity,” BMC Biol., vol. 12, no. 1, p. 36, 2014, doi: 10.1186/1741-7007-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koonin EV and Makarova KS, “Mobile genetic elements and evolution of crispr-cas systems: All theway there and back,” Genome Biol. Evol, vol. 9, no. 10, pp. 2812–2825, 2017, doi: 10.1093/gbe/evx192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shiimori M, Garrett SC, Chambers DP, Glover CVC, Graveley BR, and Terns MP, “Role of free DNA ends and protospacer adjacent motifs for CRISPR DNA uptake in Pyrococcus furiosus,” Nucleic Acids Res., no. October, pp. 1–14, 2017, doi: 10.1093/nar/gkx839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wei Y, Terns RM, Terns MP, Terns MP, and Terns MP, “Cas9 function and host genome sampling in type II-A CRISPR–cas adaptation,” Genes Dev., vol. 29, no. 4, pp. 356–361, 2015, doi: 10.1101/gad.257550.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang X, Garrett S, Graveley BR, and Terns MP, “Unique properties of spacer acquisition by the type III-A CRISPR-Cas system,” pp. 1–21, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wimmer F and Beisel CL, “CRISPR-Cas Systems and the Paradox of Self-Targeting Spacers,” Front. Microbiol, vol. 10, no. January, pp. 1–17, 2020, doi: 10.3389/fmicb.2019.03078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heler R, Wright AV, Vucelja M, Bikard D, Doudna JA, and Marraffini LA, “Mutations in Cas9 Enhance the Rate of Acquisition of Viral Spacer Sequences during the CRISPR-Cas Immune Response,” Mol. Cell, vol. 65, no. 1, pp. 168–175, 2017, doi: 10.1016/j.molcel.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levy A et al. , “CRISPR adaptation biases explain preference for acquisition of foreign DNA,” Nature, vol. 520, no. 7548, pp. 505–510, 2015, doi: 10.1038/nature14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nuñez JK, Kranzusch PJ, Noeske J, V Wright A, Davies CW, and a Doudna J, “Cas1-Cas2 complex formation mediates spacer acquisition during CRISPR-Cas adaptive immunity.,” Nat. Struct. Mol. Biol, vol. 21, no. 6, pp. 528–34, 2014, doi: 10.1038/nsmb.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vorontsova D et al. , “Foreign DNA acquisition by the I-F CRISPR – Cas system requires all components of the interference machinery,” pp. 1–13, 2015, doi: 10.1093/nar/gkv1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wiegand T et al. , “Reproducible Antigen Recognition by the Type I-F CRISPR-Cas System,” Cris. J, vol. 3, no. 5, pp. 378–387, 2020, doi: 10.1089/crispr.2020.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Modell JW, Jiang W, and Marraffini LA, “CRISPR–Cas systems exploit viral DNA injection to establish and maintain adaptive immunity,” Nature, vol. 10, 2017, doi: 10.1038/nature21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].[] Staals RHJ, Jackson SA, Biswas A, Brouns SJJ, Brown CM, and Fineran PC, “Interference-driven spacer acquisition is dominant over naive and primed adaptation in a native CRISPR–Cas system,” Nat. Commun, vol. 7, pp. 1–13, 2016, doi: 10.1038/ncomms12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dupuis MÈ, Villion M, Magadán AH, and Moineau S, “CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance,” Nat. Commun, vol. 4, no. May, pp. 1–7, 2013, doi: 10.1038/ncomms3087. [DOI] [PubMed] [Google Scholar]
- [30].Doron S et al. , “Systematic discovery of anti-phage defense systems in the microbial pan-genome,” Science (80-. )., vol. 359, no. 6379, 2018, doi: 10.1126/science.aar4120.Systematic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gao L et al. , “Diverse Enzymatic Activities Mediate Antiviral Immunity in Prokaryotes Linyi,” Science (80-. )., vol. 369, no. 6507, pp. 1077–1084, 2020, doi: 10.1126/science.aba0372.Diverse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Datsenko KA, Pougach K, Tikhonov A, Wanner BL, Severinov K, and Semenova E, “Molecular memory of prior infections activates the CRISPR / Cas adaptive bacterial immunity system,” Nat. Commun, vol. 3, no. May, pp. 945–947, 2012, doi: 10.1038/ncomms1937. [DOI] [PubMed] [Google Scholar]
- [33].Swarts DC, Mosterd C, van Passel MWJ, and Brouns SJJ, “CRISPR interference directs strand specific spacer acquisition,” PLoS One, vol. 7, no. 4, pp. 1–7, 2012, doi: 10.1371/journal.pone.0035888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nussenzweig PM, McGinn J, and Marraffini LA, “Cas9 Cleavage of Viral Genomes Primes the Acquisition of New Immunological Memories,” Cell Host Microbe, vol. 26, no. 4, pp. 515–526.e6, 2019, doi: 10.1016/j.chom.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Heler R et al. , “Cas9 specifies functional viral targets during CRISPR-Cas adaptation,” Nature, vol. 519, no. 7542, pp. 1–16, 2015, doi: 10.1038/nature14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kunne T et al. , “Cas3-Derived Target DNA Degradation Fragments Fuel Primed CRISPR Adaptation Article Cas3-Derived Target DNA Degradation Fragments Fuel Primed CRISPR Adaptation,” pp. 1–13, 2016, doi: 10.1016/j.molcel.2016.07.011. [DOI] [PubMed] [Google Scholar]
- [37].Dillard KE et al. , “Assembly and Translocation of a CRISPR-Cas Primed Acquisition Complex,” Cell, vol. 175, no. 4, pp. 934–946.e15, 2018, doi: 10.1016/j.cell.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loeff L, Brouns SJJ, and Joo C, “Repetitive DNA Reeling by the Cascade-Cas3 Complex in Nucleotide Unwinding Steps,” Mol. Cell, vol. 70, no. 3, pp. 385–394.e3, 2018, doi: 10.1016/j.molcel.2018.03.031. [DOI] [PubMed] [Google Scholar]
- [39].Redding S et al. , “Surveillance and Processing of Foreign DNA by the Escherichia coli CRISPR-Cas System Article Surveillance and Processing of Foreign DNA by the Escherichia coli CRISPR-Cas System,” Cell, pp. 1–12, 2015, doi: 10.1016/j.cell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Richter C et al. , “Priming in the Type I-F CRISPR-Cas system triggers strand-independent spacer acquisition, bi-directionally from the primed protospacer,” Nucleic Acids Res., vol. 42, no. 13, pp. 8516–8526, 2014, doi: 10.1093/nar/gku527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shiriaeva AA et al. , “Detection of spacer precursors formed in vivo during primed CRISPR adaptation,” Nat. Commun, vol. 10, no. 1, pp. 1–9, 2019, doi: 10.1038/s41467-019-12417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Musharova O et al. , “Prespacers formed during primed adaptation associate with the Cas1–Cas2 adaptation complex and the Cas3 interference nuclease–helicase,” Proc. Natl. Acad. Sci. U. S. A, vol. 118, no. 22, 2021, doi: 10.1073/pnas.2021291118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fagerlund RD et al. , “Spacer capture and integration by a type I-F Cas1–Cas2-3 CRISPR adaptation complex,” Proc. Natl. Acad. Sci, p. 201618421, 2017, doi: 10.1073/pnas.1618421114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rollins MF et al. , “Cas1 and the Csy complex are opposing regulators of Cas2/3 nuclease activity,” Proc. Natl. Acad. Sci, vol. 1, p. 201616395, 2017, doi: 10.1073/pnas.1616395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wilkinson M, Drabavicius G, Silanskas A, Gasiunas G, Siksnys V, and Wigley DB, “Structure of the DNA-Bound Spacer Capture Complex of a Type II CRISPR-Cas System,” Mol. Cell, vol. 75, no. 1, pp. 90–101.e5, 2019, doi: 10.1016/j.molcel.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Semenova E, Savitskaya E, Musharova O, Strotskaya A, and Vorontsova D, “Highly efficient primed spacer acquisition from targets destroyed by the Escherichia coli type I-E CRISPR-Cas interfering complex,” 2016, doi: 10.1073/pnas.1602639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xue C, Whitis NR, Sashital DG, Xue C, Whitis NR, and Sashital DG, “Conformational Control of Cascade Interference and Priming Activities in CRISPR Immunity Short Article Conformational Control of Cascade Interference and Priming Activities in CRISPR Immunity,” Mol. Cell, pp. 1–9, 2016, doi: 10.1016/j.molcel.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Krivoy A et al. , “Primed CRISPR adaptation in Escherichia coli cells does not depend on conformational changes in the Cascade effector complex detected in Vitro,” Nucleic Acids Res., vol. 46, no. 8, pp. 4087–4098, 2018, doi: 10.1093/nar/gky219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xiao Y, Xiao Y, Luo M, Dolan AE, Liao M, and Ke A, “Structure basis for RNA-guided DNA degradation by Cascade and Cas3,” vol. 0839, no. June, pp. 1–12, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pyenson NC and Marraffini LA, “Co-evolution within structured bacterial communities results in multiple expansion of CRISPR loci and enhanced immunity,” Elife, vol. 9, pp. 9–11, 2020, doi: 10.7554/eLife.53078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jackson SA, Birkholz N, Malone LM, and Fineran PC, “Imprecise Spacer Acquisition Generates CRISPR-Cas Immune Diversity through Primed Adaptation,” Cell Host Microbe, vol. 25, no. 2, pp. 250–260.e4, 2019, doi: 10.1016/j.chom.2018.12.014. [DOI] [PubMed] [Google Scholar]
- [52].Van Houte S et al. , “The diversity-generating benefits of a prokaryotic adaptive immune system,” Nature, vol. 532, no. 7599, pp. 385–388, 2016, doi: 10.1038/nature17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Samai P, Pyenson N, Jiang W, Goldberg GW, Hatoum-Aslan A, and Marraffini LA, “Co-transcriptional DNA and RNA Cleavage during Type III CRISPR-Cas Immunity,” Cell, vol. 161, no. 5, pp. 1164–1174, 2015, doi: 10.1016/j.cell.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Abudayyeh OO et al. , “C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector,” vol. 5573, no. June, 2016, doi: 10.1101/054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hale CR et al. , “RNA-Guided RNA Cleavage by a CRISPR RNA-Cas Protein Complex,” Cell, vol. 139, no. 5, pp. 945–956, 2009, doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vink JNA, Baijens JHL, and Brouns SJJ, “PAM-repeat associations and spacer selection preferences in single and co-occurring CRISPR-Cas systems,” Genome Biol., vol. 22, no. 1, pp. 1–25, 2021, doi: 10.1186/s13059-021-02495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mojica FJM, Díez-Villaseñor C, García-Martínez J, and Almendros C, “Short motif sequences determine the targets of the prokaryotic CRISPR defence system,” Microbiology, vol. 155, no. 3, pp. 733–740, 2009, doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- [58].Hayes RP et al. , “Structural basis for promiscuous PAM recognition in type I–E Cascade from E. coli,” Nature, pp. 1–16, 2016, doi: 10.1038/nature16995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Anders C, Niewoehner O, Duerst A, and Jinek M, “Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease.,” Nature, 2014, doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sashital DG, Jinek M, and a Doudna J, “An RNA-induced conformational change required for CRISPR RNA cleavage by the endoribonuclease Cse3.,” Nat. Struct. Mol. Biol, vol. 18, no. 6, pp. 680–687, 2011, doi: 10.1038/nsmb.2043. [DOI] [PubMed] [Google Scholar]
- [61].Sternberg SH, Redding S, Jinek M, Greene EC, and Doudna JA, “DNA interrogation by the CRISPR RNA-guided endonuclease Cas9,” Nature, vol. 507, no. 7490, pp. 62–67, 2014, doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dillingham MS and Kowalczykowski SC, “RecBCD Enzyme and the Repair of Double-Stranded DNA Breaks,” Microbiol. Mol. Biol. Rev, vol. 72, no. 4, pp. 642–671, 2008, doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim S, Loeff L, Colombo S, Jergic S, Brouns SJJ, and Joo C, “Selective loading and processing of prespacers for precise CRISPR adaptation,” Nature, vol. 579, no. 7797, pp. 141–145, 2020, doi: 10.1038/s41586-020-2018-1. [DOI] [PubMed] [Google Scholar]
- [64].Rollie C, Graham S, Rouillon C, and White MF, “NAR breakthrough article: Prespacer processing and specific integration in a type I-A CRISPR system,” Nucleic Acids Res., vol. 46, no. 3, pp. 1007–1020, 2018, doi: 10.1093/nar/gkx1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee H, Zhou Y, Taylor DW, and Sashital DG, “Cas4-Dependent Prespacer Processing Ensures High-Fidelity Programming of CRISPR Arrays,” Mol. Cell, pp. 1–12, 2018, doi: 10.1016/j.molcel.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kieper SN et al. , “Cas4 Facilitates PAM-Compatible Spacer Selection during CRISPR Adaptation,” Cell Rep., vol. 22, no. 13, pp. 3377–3384, 2018, doi: 10.1016/j.celrep.2018.02.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shiimori M, Garrett SC, Graveley BR, and Terns MP, “Cas4 Nucleases Define the PAM, Length, and Orientation of DNA Fragments Integrated at CRISPR Loci,” Mol. Cell, vol. 70, no. 5, pp. 814–824.e6, 2018, doi: 10.1016/j.molcel.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lee H, Dhingra Y, and Sashital DG, “The Cas4-Cas1-Cas2 complex mediates precise prespacer processing during CRISPR adaptation,” Elife, vol. 8, pp. 1–26, 2019, doi: 10.7554/eLife.44248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hu C et al. , “Mechanism for Cas4-assisted directional spacer acquisition in CRISPR–Cas,” Nature, vol. 598, no. 7881, pp. 515–520, 2021, doi: 10.1038/s41586-021-03951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jakhanwal S, Cress BF, Maguin P, Lobba MJ, Marraffini LA, and Doudna JA, “A CRISPR-Cas9-integrase complex generates precise DNA fragments for genome integration,” Nucleic Acids Res., vol. 49, no. 6, pp. 3546–3556, 2021, doi: 10.1093/nar/gkab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gasiunas G et al. , “A catalogue of biochemically diverse CRISPR-Cas9 orthologs,” Nat. Commun, vol. 11, no. 1, 2020, doi: 10.1038/s41467-020-19344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wright AV and Doudna JA, “Protecting genome integrity during CRISPR immune adaptation,” Nat. Struct. Mol. Biol, vol. 23, no. 10, pp. 876–883, 2016, doi: 10.1038/nsmb.3289. [DOI] [PubMed] [Google Scholar]
- [73].Ramachandran A, Summerville L, Learn BA, DeBell L, and Bailey S, “Processing and integration of functionally oriented prespacers in the Escherichia coli CRISPR system depends on bacterial host exonucleases,” J. Biol. Chem, vol. 295, no. 11, pp. 3403–3414, 2020, doi: 10.1074/jbc.RA119.012196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Drabavicius G, Sinkunas T, Silanskas A, Gasiunas G, Venclovas Č, and Siksnys V, “DnaQ exonuclease-like domain of Cas2 promotes spacer integration in a type I-E CRISPR-Cas system,” EMBO Rep., vol. 19, no. 7, p. e45543, 2018, doi: 10.15252/embr.201745543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Goren MG, Doron S, Globus R, Amitai G, Sorek R, and Qimron U, “Repeat Size Determination by Two Molecular Rulers in the Type I-E CRISPR Array,” Cell Rep., vol. 16, no. 11, pp. 2811–2818, 2016, doi: 10.1016/j.celrep.2016.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wright AV, Liu J-J, Knott GJ, Doxzen KW, Nogales E, and Doudna JA, “Structures of the CRISPR genome integration complex.,” Science, vol. 0679, p. eaao0679, 2017, doi: 10.1126/science.aao0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ivančić-Baće I, Cass SD, Wearne SJ, and Bolt EL, “Different genome stability proteins underpin primed and naïve adaptation in E. coli CRISPR-Cas immunity.,” Nucleic Acids Res., vol. 43, no. 22, pp. 10821–30, 2015, doi: 10.1093/nar/gkv1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Budhathoki JB et al. , “Real-time Observation of CRISPR spacer acquisition by Cas1– Cas2 integrase,” Nat. Struct. Mol. Biol, vol. 27, no. 5, pp. 489–499, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zabrady K, Zabrady M, Kolesar P, Li AWH, and Doherty AJ, “CRISPR-Associated Primase-Polymerases are implicated in prokaryotic CRISPR-Cas adaptation,” Nat. Commun, vol. 12, no. 1, pp. 1–18, 2021, doi: 10.1038/s41467-021-23535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu TY, Liu JJ, Aditham AJ, Nogales E, and Doudna JA, “Target preference of Type III-A CRISPR-Cas complexes at the transcription bubble,” Nat. Commun, vol. 10, no. 1, 2019, doi: 10.1038/s41467-019-10780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Artamonova D et al. , “Spacer acquisition by Type III CRISPR–Cas system during bacteriophage infection of Thermus thermophilus,” Nucleic Acids Res., vol. 48, no. 17, pp. 9787–9803, 2020, doi: 10.1093/nar/gkaa685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Silas S et al. , “Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase-Cas1 fusion protein,” Science (80-. )., vol. 351, no. 6276, pp. aad4234–aad4234, 2016, doi: 10.1126/science.aad4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].González-Delgado A, Mestre MR, Martínez-Abarca F, and Toro N, “Spacer acquisition from RNA mediated by a natural reverse transcriptase-Cas1 fusion protein associated with a type III-D CRISPR-Cas system in Vibrio vulnificus,” Nucleic Acids Res., vol. 47, no. 19, pp. 10202–10211, 2019, doi: 10.1093/nar/gkz746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang JY, Hoel CM, Al-Shayeb B, Banfield JF, Brohawn SG, and Doudna JA, “Structural coordination between active sites of a CRISPR reverse transcriptase-integrase complex,” Nat. Commun, vol. 12, no. 1, pp. 1–14, 2021, doi: 10.1038/s41467-021-22900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hoikkala V et al. , “Cooperation between different crispr-cas types enables adaptation in an RNA-targeting system,” MBio, vol. 12, no. 2, 2021, doi: 10.1128/mBio.03338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hale CR et al. , “Essential Features and Rational Design of CRISPR RNAs that Function with the Cas RAMP Module Complex to Cleave RNAs,” Mol. Cell, vol. 45, no. 3, pp. 292–302, 2012, doi: 10.1016/j.molcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].McGinn J and Marraffini LA, “CRISPR-Cas Systems Optimize Their Immune Response by Specifying the Site of Spacer Integration,” Mol. Cell, vol. 64, no. 3, pp. 616–623, 2016, doi: 10.1016/j.molcel.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rollie C, Schneider S, Brinkmann AS, Bolt EL, and White MF, “Intrinsic sequence specificity of the Cas1 integrase directs new spacer acquisition,” Elife, vol. 4, no. AUGUST2015, Aug. 2015, doi: 10.7554/eLife.08716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nivala J, Shipman SL, and Church GM, “Spontaneous CRISPR loci generation in vivo by non-canonical spacer integration,” Nat. Microbiol, vol. 3, no. 3, pp. 310–318, 2018, doi: 10.1038/s41564-017-0097-z.Spontaneous. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nuñez JK, Bai L, Harrington LB, Hinder TL, and Doudna JA, “CRISPR Immunological Memory Requires a Host Factor for Specificity,” Mol. Cell, pp. 1–10, 2016, doi: 10.1016/j.molcel.2016.04.027. [DOI] [PubMed] [Google Scholar]
- [91].Yoganand KNR, Sivathanu R, Nimkar S, Anand B, and Cas C, “Asymmetric positioning of Cas1–2 complex and Integration Host Factor induced DNA bending guide the unidirectional homing of protospacer in CRISPR-Cas type I-E system,” Nucleic Acids Res., pp. 1–15, 2016, doi: 10.1093/gbe/evw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Santiago-Frangos A, Buyukyoruk M, Wiegand T, Krishna P, and Wiedenheft B, “Distribution and phasing of sequence motifs that facilitate CRISPR adaptation,” Curr. Biol, vol. 31, no. 16, pp. 3515–3524.e6, 2021, doi: 10.1016/j.cub.2021.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]