Abstract
Existing phenylalanine hydroxylase (PAH)-deficient mice strains are useful models of untreated or late-treated human phenylketonuria (PKU), as most contemporary therapies can only be initiated after weaning and the pups have already suffered irreversible consequences of chronic hyperphenylalaninemia (HPA) during early brain development. Therefore, we sought to evaluate whether enzyme substitution therapy with pegvaliase initiated near birth and administered repetitively to C57Bl/6-Pahenu2/enu2 mice would prevent HPA-related behavioral and cognitive deficits and form a model for early-treated PKU. The main results of three reported experiments are: 1) lifelong weekly pegvaliase treatment prevented the cognitive deficits associated with HPA in contrast to persisting deficits in mice treated with pegvaliase only as adults. 2) Cognitive deficits reappear in mice treated with weekly pegvaliase from birth but in which pegvaliase is discontinued at 3 months age. 3) Twice weekly pegvaliase injection also prevented cognitive deficits but again cognitive deficits emerged in early-treated animals following discontinuation of pegvaliase treatment during adulthood, particularly in females. In all studies, pegvaliase treatment was associated with complete correction of brain monoamine neurotransmitter content and with improved overall growth of the mice as measured by body weight. Mean total brain weight however remained low in all PAH deficient mice regardless of treatment. Application of enzyme substitution therapy with pegvaliase, initiated near birth and continued into adulthood, to PAH-deficient Pahenu2/enu2 mice models contemporary early-treated human PKU. This model will be useful for exploring the differential pathophysiologic effects of HPA at different developmental stages of the murine brain.
Keywords: Hyperphenylalaninemia, Phenylketonuria, Phenylalanine hydroxylase, Pegvaliase, Tyrosine, Tryptophan, Dopamine, Serotonin, Tyrosine hydroxylase, Tryptophan hydroxylase, Cognition, Behavior
1. Introduction
Phenylketonuria (PKU), due to recessively inherited phenylalanine hydroxylase (PAH) deficiency (Fig. 1) (OMIM # 261600), is among the most common inborn errors of metabolism in humans. Hyperphenylalaninemia (HPA), if left untreated, is associated with severely impaired brain growth and development yielding profound cognitive disability. Newborn screening in medically-developed countries and early initiation of dietary phenylalanine (Phe) restriction prevents the major manifestations of the disease [1]. However, reemergence of chronically elevated blood Phe due to loss of dietary control in adolescents and adults with PKU is frequently associated with disturbed executive functioning [2,3], psychiatric symptoms [4], and in extreme cases with adult onset white matter degeneration, ataxia and seizures [5]. The proximate pathologic mechanisms that mediate the neurobehavioral and neurocognitive effects of HPA are likely multifactorial and still incompletely understood, but central nervous system (CNS) dopamine and serotonin deficiencies at least play major roles in the symptoms of anxiety and impaired executive functioning associated with HPA [3,6,7]. Current knowledge regarding the biology and pathophysiology of PAH deficiency has recently been systematically reviewed [8].
Fig. 1.
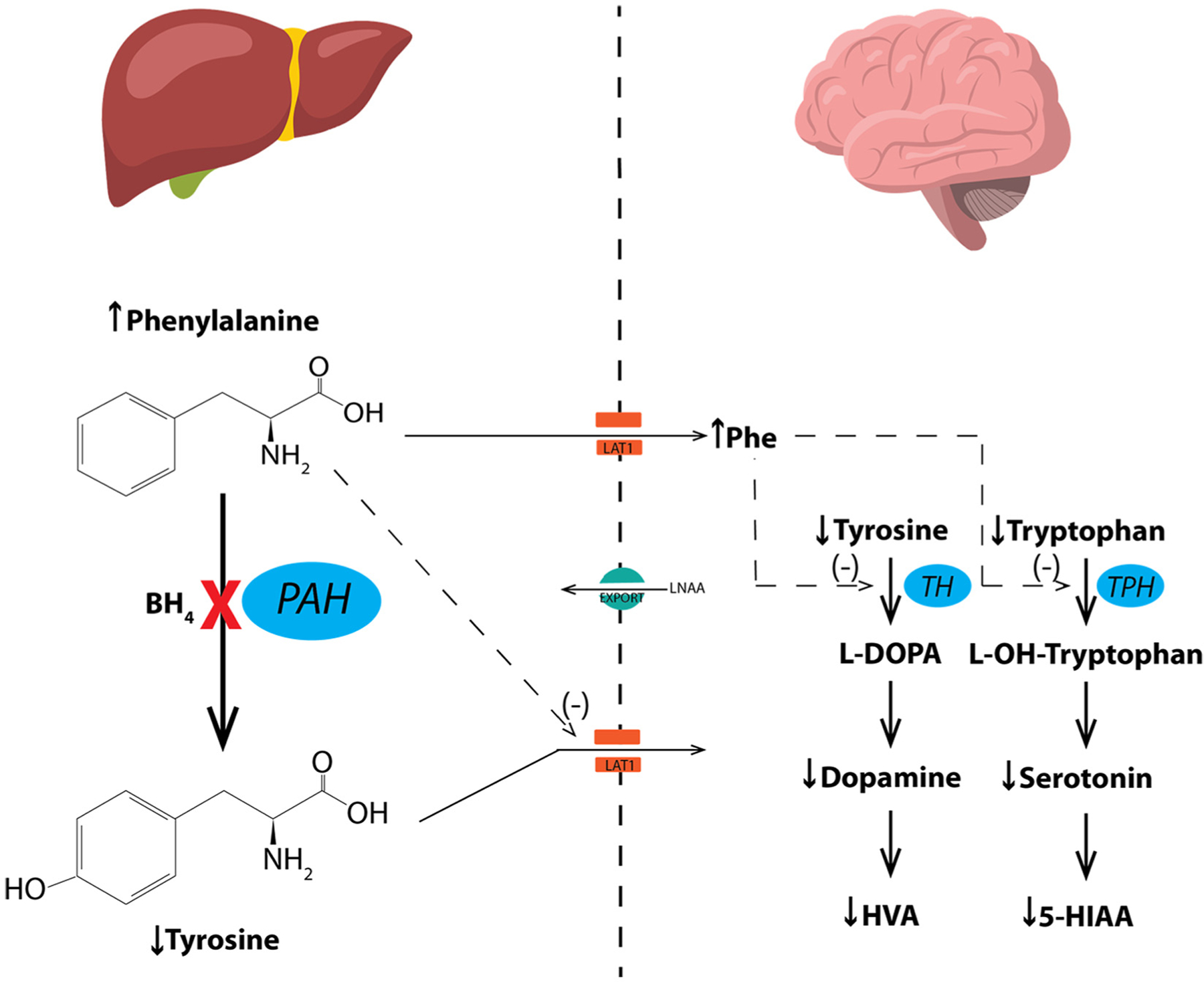
Phenylalanine metabolism in liver and the effects of hyperphenylalaninemia (HPA) upon monoamine neurotransmitter metabolism in brain.
Phenylalanine hydroxylase (PAH) catalyzes the hydroxylation of L-phenylalanine (Phe) to L-tyrosine (Tyr), a reaction that occurs predominantly in liver but also in proximal renal tubules. The reaction requires the reduced pterin, tetrahydrobiopterin (BH4), along with ferrous iron and molecular oxygen (not shown) as cofactors. In individuals with recessively-inherited mutations in the PAH gene, PAH enzymatic activity is either entirely lacking or severely diminished. PAH deficiency causes hyperphenylalaninemia (HPA) and accumulation of Phe in brain. Blood tyrosine concentration is also diminished relative to PAH-sufficient individuals, but hypotyrosinemia is not severe typically because of dietary tyrosine intake. In the setting of hyperphenylalaninemia, deamination of Phe forms phenylpyruvate and other phenylketones which are readily excreted in urine and are the source of the colloquial name phenylketonuria (PKU).
The pathophysiologic mechanisms that underlie the cognitive and behavioral deficits associated with HPA are multifactorial, but monoamine neurotransmitter deficiencies in brain have been implicated by many investigators particularly in the etiology of anxiety, mood disorders, and impaired executive function. The postulated causes of monoamine neurotransmitter deficiency are threefold. First, HPA may cause relative deficiency of Tyr and L-tryptophan (Trp), the substrates of dopamine and serotonin synthesis respectively. The large neutral amino acids (LNAA) including Phe, Tyr and Trp cross the blood brain barrier (BBB) from the circulation to brain via the LAT1/SLCA7A5 transporter. Elevated blood Phe competitively inhibits transport of the other LNAA into brain reducing their concentrations. However, the compensatory activity of energy-requiring amino acid exporters (EXPORT) may be able to mitigate amino acid imbalance to some degree and restore homeostasis. Secondly, constitutive expression of tyrosine hydroxylase (TH) and neuronal tryptophan hydroxylase 2 (TPH2), the rate limiting enzymatic steps in the synthesis of dopamine and serotonin respectively, is decreased in brains exposed to elevated Phe from birth. Thirdly, and perhaps more importantly, elevated brain Phe competitively inhibits the activities of TH and TPH2.
Prior investigations into disease pathophysiology in a PAH-deficient mouse model, the Pahenu2 mouse, have revealed severe behavioral alterations and cognitive deficits that are only partly ameliorated by application of phenylalanine (Phe)-lowering therapy during adulthood [9]. This animal model of untreated human PKU [10] harbors a missense mutation in the seventh exon of the Pah gene [11] that yields complete PAH deficiency in homozygous mice. Untreated Pahenu2/enu2 mice exhibit HPA, growth failure [12], hypopigmentation, maternal effects upon fetal development [13] and cognitive deficits, including impaired memory [14,15] and spatial recognition [16]. Significant CNS dopamine and serotonin deficiencies have been reported in hyperphenylalaninemic Pahenu2/enu2 mice [17–21], and have been implicated in the pathogenesis of the memory deficits exhibited by these animals [14,15]. The causes of monoamine neurotransmitter deficiency [9] are likely a combination of relative deficiency of L-tyrosine (Tyr) and L-tryptophan (Trp), the substrates for dopamine and serotonin synthesis respectively, in the brain due to Phe-mediated competition for Tyr and Trp uptake across the blood-brain barrier [22], decreased constitutive expression of tyrosine hydrolase (TH) and neuronal tryptophan hydroxylase (TPH2) activities [9], the rate-limiting steps in dopamine and serotonin synthesis, and perhaps most importantly, Phe-mediated competitive inhibition of TH and TPH2 activities [23]. The central elements of monoamine neurotransmitter synthesis and the turnover of these neurotransmitters are depicted in Fig. 1. Treatment of hyperphenylalaninemic adult Pahenu2/enu2 mice with either a Phe-restricted diet or liver-directed gene therapy resulted in correction of monoamine neurotransmitter deficiencies and associated improvement in nesting behaviors and reduced anxiety levels in the open field [9]. However, the severe cognitive deficits exhibited in the Morris water maze testing remained unaltered suggesting that cognitive impairments are not related to monoamine neurotransmitter deficiency but rather to some other pathologic mechanism associated with chronic HPA during early development.
In this study, we developed a model analogous to contemporary dietary therapy for humans with PAH deficiency in which blood Phe concentrations are well controlled during infancy and childhood but then metabolic control is lost during adulthood. As it is impossible to initiate dietary therapy prior to weaning, we turned to enzyme substitution therapy with recombinant PEGylated Anabaena variabilis phenylalanine ammonia lyase (pegvaliase) initiated during the first week of life to treat HPA from near birth. Pegvaliase is a recently FDA-approved novel treatment for adults with PAH deficiency [24,25]. In our first experiment, we examined whether weekly pegvaliase treatment from birth could prevent HPA, monoamine neurotransmitter deficiency and associated behavioral alterations and cognitive deficits in Pahenu2/enu2 mice. In our second experiment, we learned that cognitive impairment could recur if pegvaliase therapy was discontinued during adulthood and confirmed that weekly therapy was insufficient to fully correct brain growth in Pahenu2/enu2 mice. In our final and penultimate experiment, we compared behavioral and cognitive outcomes in adult Pahenu2/enu2 mice that had been continuously treated with twice weekly pegvaliase from birth with that from mice in which pegvaliase had been discontinued at 4 months age and allowed to suffer the reemergence of HPA effects. Here, we report on this novel model of contemporary PKU treatment.
2. Materials and methods
2.1. Animal husbandry
Animal care and experimentation were performed in accordance with the guidelines of the Dept. of Comparative Medicine, Oregon Health & Science University and the National Institutes of Health Guide for the Care and Use of Laboratory animals. C57Bl/6-Pahenu2/enu2 mice (hereafter referred to as PAH−/−), which are homozygous for a missense p.F263S mutation in exon 7 of the murine Pah gene, are completely deficient in liver PAH activity, exhibit HPA on an unrestricted diet, and are a representative animal model of human PKU [10]. Genotyping for the presence of the Pahenu2 mutation was performed by a Taqman quantitative PCR assay. Control animals were wild type C57Bl/6 mice (PAH+/+), which were co-reared littermates of PAH−/− mice resulting from Pahenu2/+ X Pahenu2/+ breedings in our mouse colony. All mice had access to tap water and standard mouse chow (Lab Diet Picolab Rodent diet 20, PMI Nutrition International, St. Louis, MO) ad libitum, providing approximately 24% protein and 1.04% L-phenylalanine by weight. Given that adult mice consume approximately 5 g chow per day, daily L-phenylalanine intake is estimated to be approximately 50 mg per day. The animals were housed under a standard 12 h on, 12 h off light cycle. All surgical procedures were carried out with inhaled isoflurane general anesthesia.
2.2. Pegvaliase treatment
Pegvaliase (PEGylated recombinant A. variabilis phenylalanine ammonia lyase, kindly provided by BioMarin Pharmaceutical Corp, San Rafael, CA), 20 mg pegvaliase protein/ml, was diluted to 10 mg/ml final concentration using a diluent provided by the manufacturer. PAH−/− pups were injected intraperitoneally with 100 μg pegvaliase/g body weight, beginning in the first week of life. Typical injection volumes were 10–20 μl in pups increasing to 120–150 μl in adult mice. In the first two experiments, pegvaliase was administered weekly, typically on Monday mornings. In the final experiment, pegvaliase was administered twice per week, typically on Monday and Thursday mornings.
2.3. Behavioral and cognitive testing
All mice were tested during the light period in the following behavioral and cognitive tests in the following order: open field, rotarod, and nest building (week 1), water maze (week 2), passive avoidance and contextual and cued fear conditioning (week 3). The open field and water maze tests are described below. The rotarod, contextual and cued fear conditioning were performed as previously reported [26]. The passive avoidance test was performed as described [27]. The referenced methods are not further described here because we found no effects of either HPA or treatment upon these measures.
2.4. Open field
Mice were placed for ten minutes in a 40.64 cm × 40.64 cm brightly lit open arena. The movement and location of the mice was recorded and analyzed with Noldus Ethovision video tracking software (version 7.1 XT, Wageningen, The Netherlands) to assess exploratory behavior and measures of anxiety. The center zone (20.32 × 20.34 cm) and peripheral zone (remaining part of the open field) were also analyzed separately to assess measurements of anxiety. More anxious mice spend less time in the more anxiety-provoking center area of the open field [28].
2.5. Nest building
Nest building was analyzed as described [29].
2.6. Water maze
First, ability to navigate to a visible platform (task learning) was evaluated using the water maze and Ethovision video tracking software. The maze (circular tub, 122 cm diameter) contained a platform marked by a beacon during visible platform training. The location of the platform was changed between sessions. There was a total of 10 visible platform sessions in Experiment 1 and four sessions in Experiments 2 and 3. Longer visible platform training was included in Experiment 1 to assess whether PAH−/− mice would catch up to PAH+/+ mice with prolonged visible platform training. As that was not the case but PAH−/− mice did demonstrate some improvement over the first 4 sessions, we decided to include only 4 sessions for visible platform training in Experiments 2 and 3. Each session consisted of two trials. Each test day consisted of 2 sessions, one in the morning and one in the afternoon. Each trial was up to 60 s with a 10–15 min inter-trial interval. If the mouse did not find the platform in 60 s then it was led to the platform by the experimenter and allowed to remain there for 3 s before being removed from the maze. Mice were placed in the water maze facing the wall of the pool at nine different drop locations around the pool. The drop location was alternated for each trial. Time to reach the platform (latency), cumulative distance to the platform, and swim speeds were analyzed as performance measures.
2.7. Euthanasia and tissue harvest
Animals were sedated using inhaled isoflurane anesthesia. Whole blood was collected by cardiac puncture, allowed to clot in an Eppendorf tube, and serum was separated by centrifugation. The mice were then euthanized by exsanguination and perfused with 20 ml normal saline via the left cardiac ventricle to clear blood from the cerebral circulation. Following decapitation, whole brain was rapidly excised from the cranium, split sagitally, and immediately submerged in liquid nitrogen. Half brains and serum samples were stored at −80 °C until processing for amino acid or neurotransmitter analysis.
2.8. Amino acid analysis
Amino acid concentrations in sera or brain tissue were measured by pre-column derivatization with 6-aminoquinolyl-N-hydroxy-succinimidyl carbamate (AQC, Waters AccQ Tag™ derivitization system) and separation by ultra-high performance liquid chromatography and UV absorbance detection (Waters Acquity™ UPLC, Milford, MA) using the Waters Masstrak Amino Acid Analysis method. Serum samples were de-proteinized by adding an equal volume of 10% sulfosalicylic acid containing the non-physiological amino acid norvaline (250 μM) as an internal recovery standard. For analysis of amino acid concentrations in brain, frozen half-brains were mechanically pulverized to powder using a Covaris tissue pulverizer (Covaris, Woburn, MA). Frozen brain powder was then suspended in 5 volumes/weight ice cold phosphate-buffered saline and sonicated at 10 watts power for 10 s × 2 while on ice. Following clarification of the supernatant by spinning at 14,000 RPM for 5 min in an Eppendorf centrifuge, the supernatant was treated similarly to a serum sample for measurement of amino acid concentrations using the MassTrak system. Serum amino acid concentrations were reported as μM and corrected for dilution to reflect the actual concentrations in serum. The measured amino acid concentrations in the brain homogenates were corrected for the tissue wet weight and reported as nmol/g wet brain weight.
2.9. Brain monoamine neurotransmitter analysis
Mouse half-brains were pulverized to powder as above. Brain powder was mechanically homogenized in ice-cold homogenizing buffer (50 mM Tris-HCl, pH 7.5, 0.1 M KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, and 1 μM pepstatin), 4 μl/mg tissue and further processed according to a previously published method [30]. Monoamine neurotransmitter concentrations (L-DOPA, dopamine, HVA, 5-HTP, serotonin, and 5-HIAA) were measured in brain tissue by HPLC and electrochemical detection [31]. Measured brain homogenate monoamine neurotransmitter concentrations were corrected for the protein content of the homogenate and expressed as pmol/mg protein.
2.10. Statistical analysis
Biochemical, behavioral, and cognitive, data were analyzed using two-way analyses of variance (ANOVA) with genotype or treatment group, and sex as between-subjects factors. The water maze learning curves were analyzed using repeated-measures ANOVA with genotype, treatment, and sex as between group variables. Inter-group comparisons were performed post analysis using Tukey’s multiple comparison tests when appropriate. We considered p < 0.05 as statistically significant. Statistical analyses of biochemical data were performed using GraphPad Prism™ (version 9.2.0, San Diego, CA) software. Statistical analyses of behavioral and cognitive data were performed using SPSS software (vs22, Chicago, IL).
3. Results
3.1. Experiment 1: weekly pegvaliase treatment initiated neonatally prevented behavioral alterations and cognitive disabilities associated with untreated murine PAH deficiency, but mice treated only during adulthood continued to exhibit severe behavioral alterations and cognitive deficits
As we have previously reported [9], untreated PAH−/− mice exhibit significant behavioral alterations and cognitive disabilities specifically with increased measures of anxiety in the open field, poor nest building, and impaired performance in the Morris water maze test. Blood Phe-lowering treatments initiated only after weaning at 3–4 weeks age have been associated with improved nest building and performance in the open field but have not improved performance in the Morris water maze test. We hypothesized that initiation of pegvaliase treatment shortly after birth to prevent HPA throughout juvenile life would prevent all behavioral alterations and cognitive deficits associated with murine PKU but that cognitive deficits specifically would remain unaltered by pegvaliase treatment initiated during adulthood. To test this hypothesis, we treated eight PAH−/− mice (5 males and 3 females) with weekly pegvaliase by intraperitoneal injection beginning at P2–3 and continued treatment until six months age. A separate cohort of PAH−/− mice (4 males and 4 females) received weekly pegvaliase injections initiated only during adulthood (11 weeks age for six animals; due to difficulty achieving consistent pregnancy timing to synchronize litters, two females were 13 weeks old at initiation of therapy) and continued through the end of the experiment. Behavioral and cognitive assessments began at age 5 months and were completed by 6 months at which time the animals were euthanized for tissue harvest. Behavioral and cognitive performance were compared to that of co-reared PAH+/+ littermates (5 males and 4 females) that had received weekly saline IP injections from P2–3. The results of Water Maze testing are displayed in Fig. 2 with supportive statistics provided in Supplementary Table 1. Cumulative distance to the target (cm) for the visible platform sessions and subsequent hidden platform sessions are displayed in Fig. 2b. PAH−/− mice treated with pegvaliase only as adults exhibit severely impaired ability to locate the visible platform (task learning) and essentially never learn the placement of the hidden platform (spatial learning or acquisition) while the learning ability of neonatally-treated PAH−/− mice was similar to that of PAH+/+ animals. Two-way ANOVAs of both the visible and hidden platform data demonstrate significant effects of treatment group but no effect of sex. Post hoc multiple intergroup comparisons using Tukey’s correction demonstrated significant differences between PAH+/+ vs. adult-treated PAH −/− and neonatal-treated PAH−/− vs. adult-treated PAH−/−. Identical outcomes were found when latency to the platform (sec) was used as performance measure (Fig. 2c). In the probe trial (no platform), adult-treated PAH−/− mice exhibited a significantly greater cumulative distance to target (cm) (p < 0.0001) (Fig. 2d) and significantly less time exploring the target quadrant (p < 0.001) (Fig. 2e) in comparison to PAH+/+ or neonatal-treated PAH−/− mice, demonstrating significantly impaired memory retention. The inability of PAH−/− mice to locate the visible platform is not due to any vision impairment; we have previously performed passive avoidance trials in PAH−/− mice (data not shown) and found no deficit in the ability of PAH−/− mice to sense and run from light to a dark compartment.
Fig. 2.
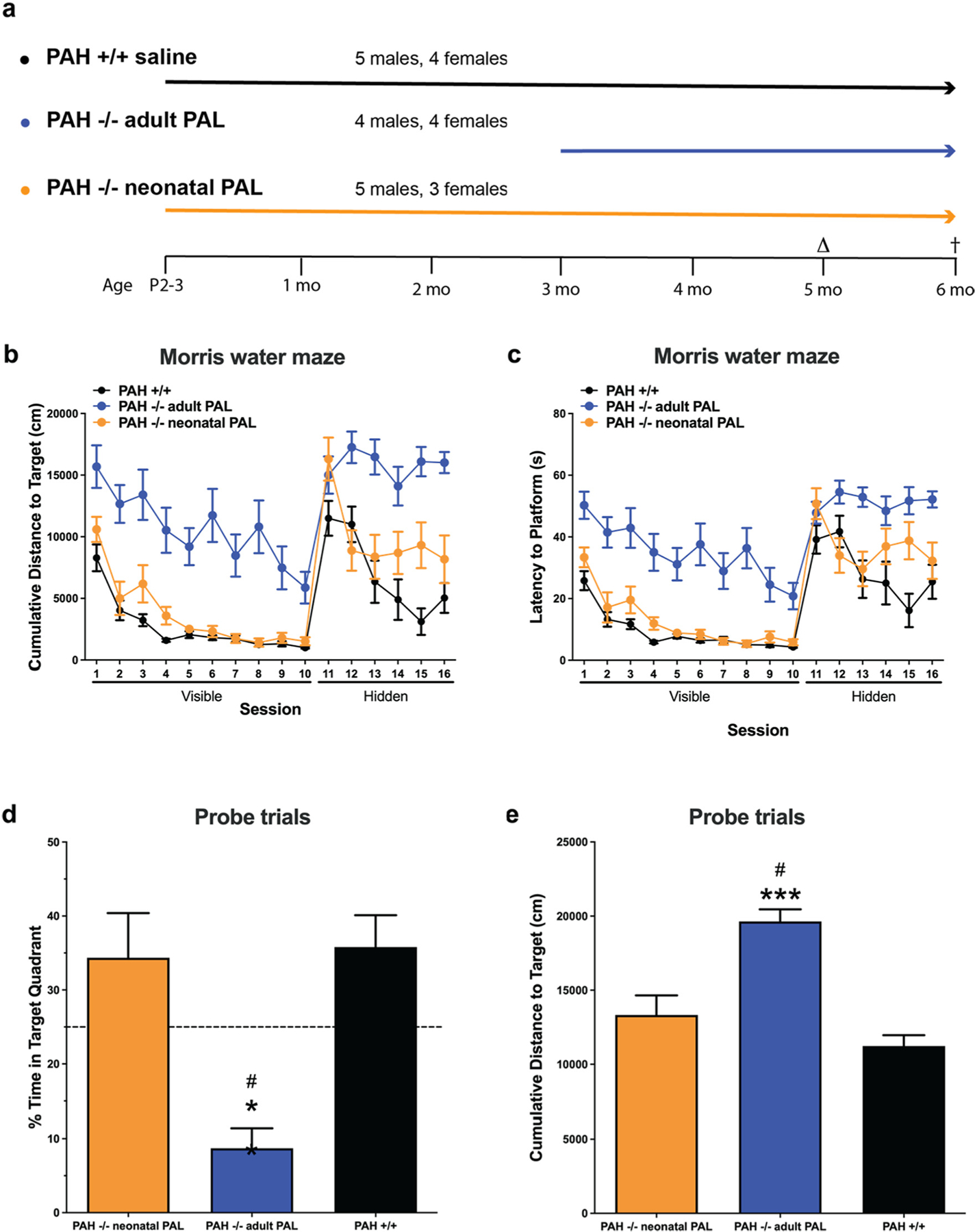
Morris water maze testing in Experiment 1. Results of Morris water maze testing in saline-treated PAH+/+ mice in comparison to PAH−/− mice treated with weekly pegvaliase from birth (PAH−/− neonatal PAL) or with weekly pegvaliase initiated during adulthood (PAH−/− adult PAL). The experimental groups and timeline of the experiment are presented (2a). Behavioral and cognitive assessments were initiated at age 5 months (Δ) with euthanasia at 6 months (†). 2b) Cumulative distance to target in cm for training sessions to the visible platform followed by test sessions to a hidden platform. 2c) Results of the same sessions expressed as latency to platform in seconds. 2d) Results of subsequent probe trials when the platform was completely removed expressed as % time spent swimming in the target quadrant (mean ± SEM). ** - p < 0.01 by Tukey’s post hoc comparison. 2e) Results of probe trials expressed as cumulative distance to target in cm (mean ± SEM). *** - p < 0.001 by Tukey’s post hoc comparison.
In the open field, mice treated with pegvaliase as neonates tended to be less active than wild-type mice (Supplementary Fig. 1a and b) although this difference did not reach statistical significance. This seemed related to the treatment as the activity levels in wild-type and PAH−/− mice were similar. PAH−/− mice, with or without treatment, spent less time in the center of the open field (Supplementary Fig. 1c), indicating that the treatment did not reduce anxiety levels in PAH−/− mice.
The results of Novel Object Exploration testing are displayed in Supplementary Fig. 1 with statistics presented in Supplementary Table 1. There were no significant differences in the exploration times among the three experimental groups, but both the wild type mice and PAH−/− mice treated with pegvaliase as neonates spent a significantly greater percentage of time exploring the novel object over the familiar object (p < 0.0001) (Supplementary Fig. 1d). In contrast, adult treated PAH−/− mice did not. A similar pattern was seen when the discrimination index was analyzed (Supplementary Fig. 1e) but there were no significant group differences. To summarize the results of the water maze and novel object recognition tests, lifelong pegvaliase treatment largely prevented the memory deficits associated with untreated HPA in PAH−/− mice.
Untreated HPA adversely affects both overall growth and growth of the brain in mice and humans. In this experiment, final body weight at 5 months age is presented in Fig. 3a with the related statistical analyses presented in Supplementary Table 2. Two-way ANOVA revealed only a significant effect of sex but not of treatment group upon final body weight. The mean (± SEM) body weight of adult-treated female PAH−/− mice (19.7 ± 0.6 g) remained slightly lower than either wild type (21.1 ± 1.2 g) or neonatal-treated female PAH −/− (21.3 ± 2.1 g) mice, but males, the weights of all experimental mice were similar, wild type − 27.8 ± 1.1 g, neonatal-treated − 24.6 ± 0.3 g adult-treated − 26.1 ± 0.6 g, although the body weights of wild type mice were larger than that of neonatal-treated PAH −/− mice (p = 0.0485).
Fig. 3.
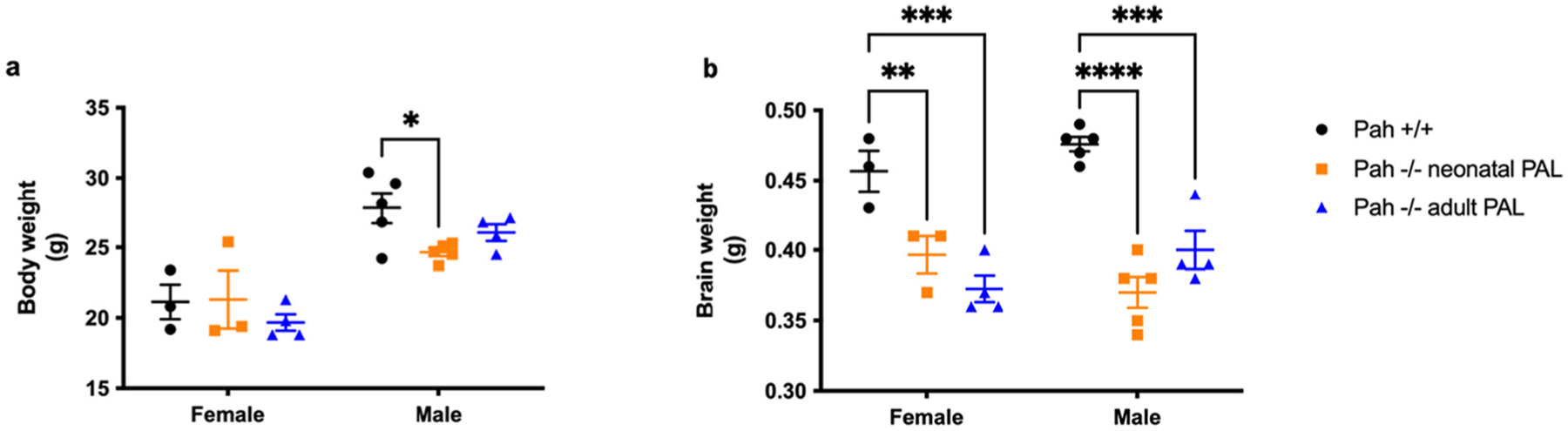
Body and brain weights in Experiment 1. Final body weight and brain weight (mean ± SEM) in PAH+/+ mice in comparison to PAH−/− mice treated with weekly pegvaliase from birth (PAH−/− neonatal PAL) or with weekly pegvaliase initiated during adulthood (PAH−/− adult PAL). Intergroup comparison by post hoc Tukey’s multiple comparisons test: * - p < 0.05, ** - p < 0.01, *** - p < 0.001, **** - p < 0.0001.
Pegvaliase therapy interestingly had no effect upon brain growth in PAH−/− mice. Mean brain weight is presented in Fig. 3b with the related statistical analyses in Supplementary Table 2. There was an effect of treatment group (Two-way ANOVA p < 0.0001) but no effect of sex upon brain weight, with lower brain weights in PAH−/− mice than sex-matched wild-type mice (females: 0.457 ± 0.015 g; males: 0.476 ± 0.005 g), regardless of treatment of PAH−/− mice with pegvaliase as neonates (females: 0.397 ± 0.013 g, p = 0.009; males: 0.370 ± 0.011 g, p < 0.0001) or as adults (females: 0.372 ± 0.009 g, p = 0.0002; males: 0.400 ± 0.014 g, p = 0.0002).
Plasma amino acid concentrations and brain amino acid contents at euthanasia are presented in Fig. 4 with the related statistical analyses in Supplementary Table 3. Euthanasia was carried out with variable delays after a final pegvaliase injection in individual mice. Serum Phe concentrations in mice euthanized at 5–6 days after the final pegvaliase dose had risen substantially in comparison to mice euthanized earlier in the week. For that reason, the standard errors of the serum Phe concentrations in pegvaliase-treated PAH−/− mice (Fig. 4a) are high, and differences between groups did not reach statistical significance. The mean serum Phe of male PAH−/− mice treated with pegvaliase as adults (mean ± SEM = 733 ± 427 μM, range 2.1–2307 μM;) was greater than that of male PAH−/− treated as neonates (mean ± SEM = 269± 230 μM, range 0–1418 μM), but this trend was not statistically significant (p = 0.2786). There was no such difference in pegvaliase-treated female mice (adult treated mean ± SEM = 18.3 ± 12.9 μM, range 0–56.1 μM; neonatal mean ± SEM = 11.6 ± 10.4 μM, range 0–42.7 μM, p = 0.9998). The mean serum Phe in male PAH+/+ was 69.0 ± 8.3 μM (range 40.0–101.1 μm) and 68.8 ± 8.1 μM (range 59.6–85.0 μM) in female PAH+/+ mice. There were no significant effects of either treatment group or sex upon serum Phe by two-way ANOVA. Slight variability was seen in brain Phe content (Fig. 4b). Two-way ANOVA revealed a slightly significant effect of sex (p = 0.039), with higher levels in males than females, but no effect of group upon brain Phe. The mean brain Phe of male PAH−/− mice was similar in both neonatal and adult pegvaliase-treated groups (neonatal mean ± SEM = 322 ± 150 nmol/g, range 105–890; adult mean ± SEM = 371 ± 147 nmol/g, range 73–697). These values are greater than in male PAH+/+ mice (mean ± SEM = 115 ± 33 nmol/g, range 51–232). The brain Phe content in female PAH−/− mice was somewhat better controlled than in male mice (neonatal female PAH −/− mice mean ± SEM = 138 ± 102 nmol/g, range 33–341; adult female PAH−/− mice mean ± SEM = 54 ± 9 nmol/g, range 36–72). This compared to mean ± SEM = 63.7 ± 7.3 nmol/g (range 51.4–83.4) in female PAH+/+ mice.
Fig. 4.
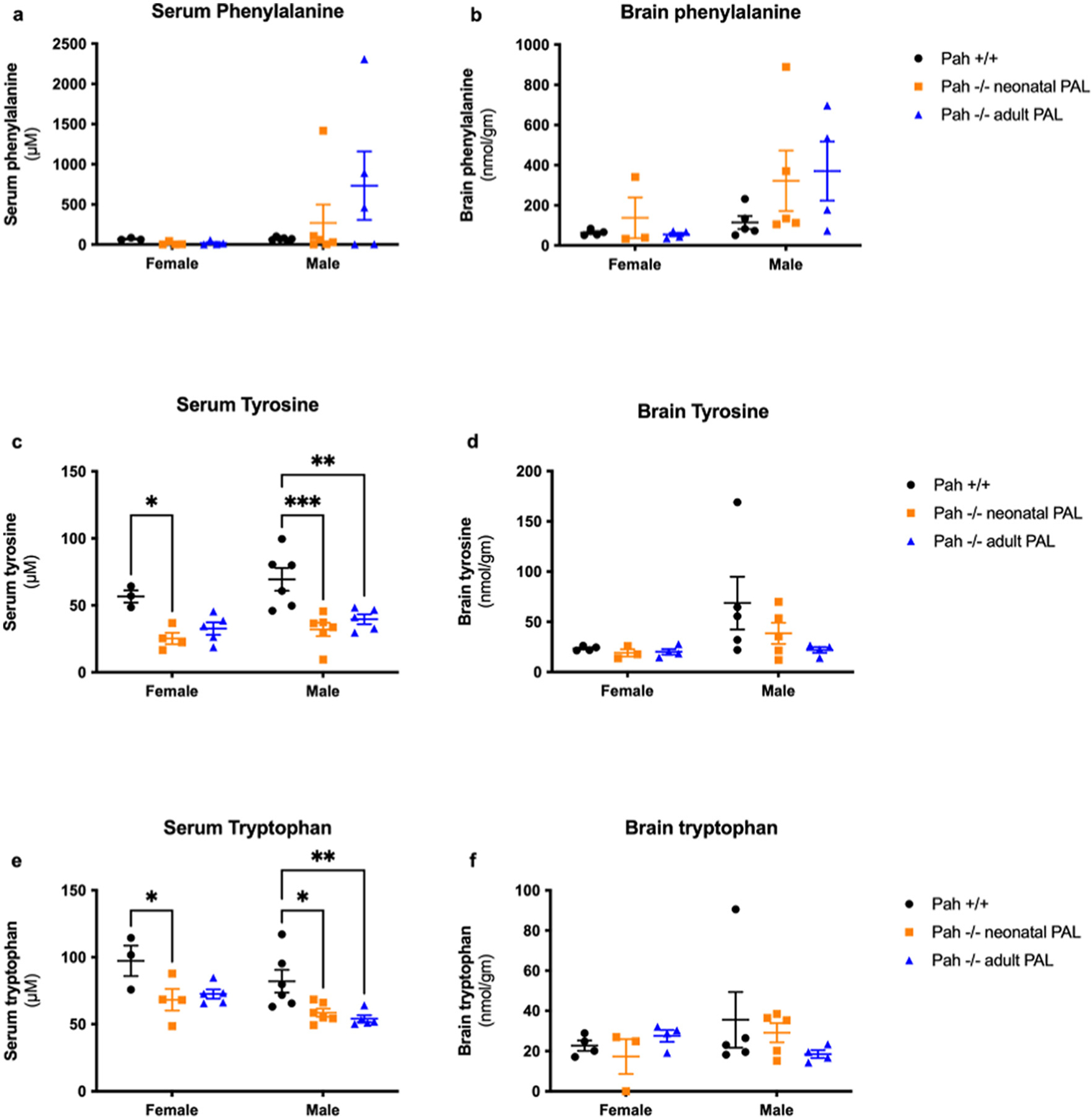
Serum and brain aromatic amino acid content in Experiment 1. Serum (μM) and brain (nmol/g) phenylalanine, tyrosine, and tryptophan contents (mean ± SEM) in PAH+/+ mice in comparison to PAH−/− mice treated with weekly pegvaliase from birth (PAH−/− neonatal PAL) or with weekly pegvaliase initiated during adulthood (PAH−/− adult PAL). Intergroup comparison by post hoc Tukey’s multiple comparisons test: * - p < 0.05, ** - p < 0.01, *** - p < 0.001, **** - p < 0.0001.
Pegvaliase activity does not result in tyrosine (Tyr) production, so continued relative Tyr deficiency in pegvaliase-treated PAH−/− mice displayed in Fig. 4c was expected. Two-way ANOVA revealed a significant effect (p < 0.0001) of treatment group upon serum Tyr but no effect of sex. Mean serum Tyr in male PAH−/− mice treated with pegvaliase as neonates (32.1 ± 5.0 μM, p = 0.0002)) or as adults (39.7 ± 3.7 μM, p = 0.0029) were significantly lower than serum Tyr in male PAH+/+ mice (69.4 ± 8.5 μM). In the females, similar differences were noted; mean serum Tyr in female PAH+/+ mice (56.7 ± 4.5 μM) was greater than that of either neonatal treated (25.4 ± 4.2 μM, p = 0.0125) or adult-treated (32.7 ± 4.6 μM, p = 0.0501) PAH−/− mice. Brain Tyr content however was not significantly affected by either experimental group or sex (Fig. 4d). In contrast to serum Tyr levels, brain Tyr of male neonatal treated (38.5 ± 10.5 nmol/g) or adult-treated (22.0 ± 2.8 nmol/g) PAH−/− mice were not different from PAH+/+ mice (68.7 ± 26.2 nmol/g). Similarly, female neonatal treated (19.2 ± 3.6 nmol/g) and adult treated (20.2 ± 2.8 nmol/g) PAH−/− mice exhibited approximately the same brain Tyr content as PAH+/+ mice (23.6 ± 1.1 nmol/g).
Serum tryptophan (Trp) concentrations were surprisingly lower in both neonatal and adult-treated PAH−/− mice (Fig. 4e). Two-way ANOVA revealed a significant effect of group (p = 0.0006) and of sex (p = 0.0113) upon serum Trp. Mean serum Trp in both neonatal-treated male PAH−/− mice (58.6 ± 3.0 μM, p = 0.0185) and adult-treated male PAH−/− mice (54.0 ± 2.5 μM), p = 0.0072) was significantly lower than that of male PAH+/+ mice (82.1 ± 8.5 μM). In females, only the difference between neonatally-treated PAH−/− mice (68.2 ± 8.0 μM, p = 0.0279) and PAH+/+ mice (97.3 ± 11.3 μM) was significant while the difference with adult-treated PAH−/− mice (72.5 ± 3.4 μM, p = 0.0527) just trended towards significance. In the brain (Fig. 4f), there was no significant effect of group or sex upon Trp content. Brain Trp contents of male PAH+/+ (35.6 ± 13.8 nmol/g), neonatal-treated PAH−/− (29.1 ± 4.8 nmol/g), adult-treated PAH−/− (18.5 ± 2.0 nmol/g) and female PAH+/+ (22.7 ± 2.6 nmol/g), neonatal-treated PAH−/− (17.3 ± 8.7 nmol/g), adult-treated PAH−/− (27.5 ± 2.9 nmol/g) mice were similar.
Monoamine neurotransmitter deficiency in brain is a known consequence of HPA. This can be caused by relative deficiency of brain Tyr or Trp content, the precursors for dopamine and serotonin synthesis respectively, due to competitive inhibition of their transport into the brain at the blood-brain barrier due to elevated blood Phe. As measured here and as we have noted previously [9], the brain content of Tyr and Trp is not substantially decreased in PAH−/− mice despite low blood Tyr and Trp concentrations. More likely mechanisms causing monoamine neurotransmitter deficiency include constitutively decreased expression of TH and TPH2 activities, the rate limiting steps in dopamine and serotonin synthesis respectively, or Phe-mediated competitive inhibition of TH and neuronal TPH2 [9]. We measured monoamine neurotransmitters in homogenates of half-brains collected at euthanasia in all three experimental groups. The data are displayed in Fig. 5 with the related statistical analysis provided in Supplementary Table 4. Because pegvaliase treatment had substantially corrected blood and brain Phe concentrations in PAH−/− mice, monoamine neurotransmitter content in brains of pegvaliase treated PAH−/− mice, although not normal, was substantially greater than that previously reported in untreated PAH−/− mice. For brain dopamine (Fig. 5a), there was no significant effect of group or sex upon brain dopamine. The brain content of dopamine was very similar for all groups: male PAH+/+ = 214.3 ± 29.2 nmol/g, male neonatal treated PAH−/− = 190.6 ± 22.8 nmol/g, male adult treated PAH−/− = 206.1 ± 19.7 nmol/g, female PAH+/+ = 247.9 ± 36.2 nmol/g, female neonatal treated PAH−/− = 203.1 ± 10.7 nmol/g, and female adult treated PAH−/− = 188.4 ± 35.1 nmol/g. Brain homovanillic acid (HVA) content (Fig. 5b), a marker of dopamine turnover, remained slightly depressed in PAH−/− mice despite pegvaliase treatment. Two-way ANOVA revealed a moderate effect of group (p = 0.0094) but not sex upon brain HVA content but the only intergroup difference to reach statistical significance was between female PAH+/+ (33.2 ± 2.7 nmol/g) and female adult treated PAH−/− mice (17.4 ± 1.8 nmol/g, p = 0.035), even though HVA was also low in other PAH−/− groups (female neonatal PAH−/− = 23.7 ± 1.2 nmol/g, male neonatal PAH−/− = 23.9 ± 1.9 nmol/g, male adult PAH−/− = 24.6 ± 2.4 nmol/g) in comparison to male PAH+/+ mice (29.6 ± 2.7 nmol/g).
Fig. 5.
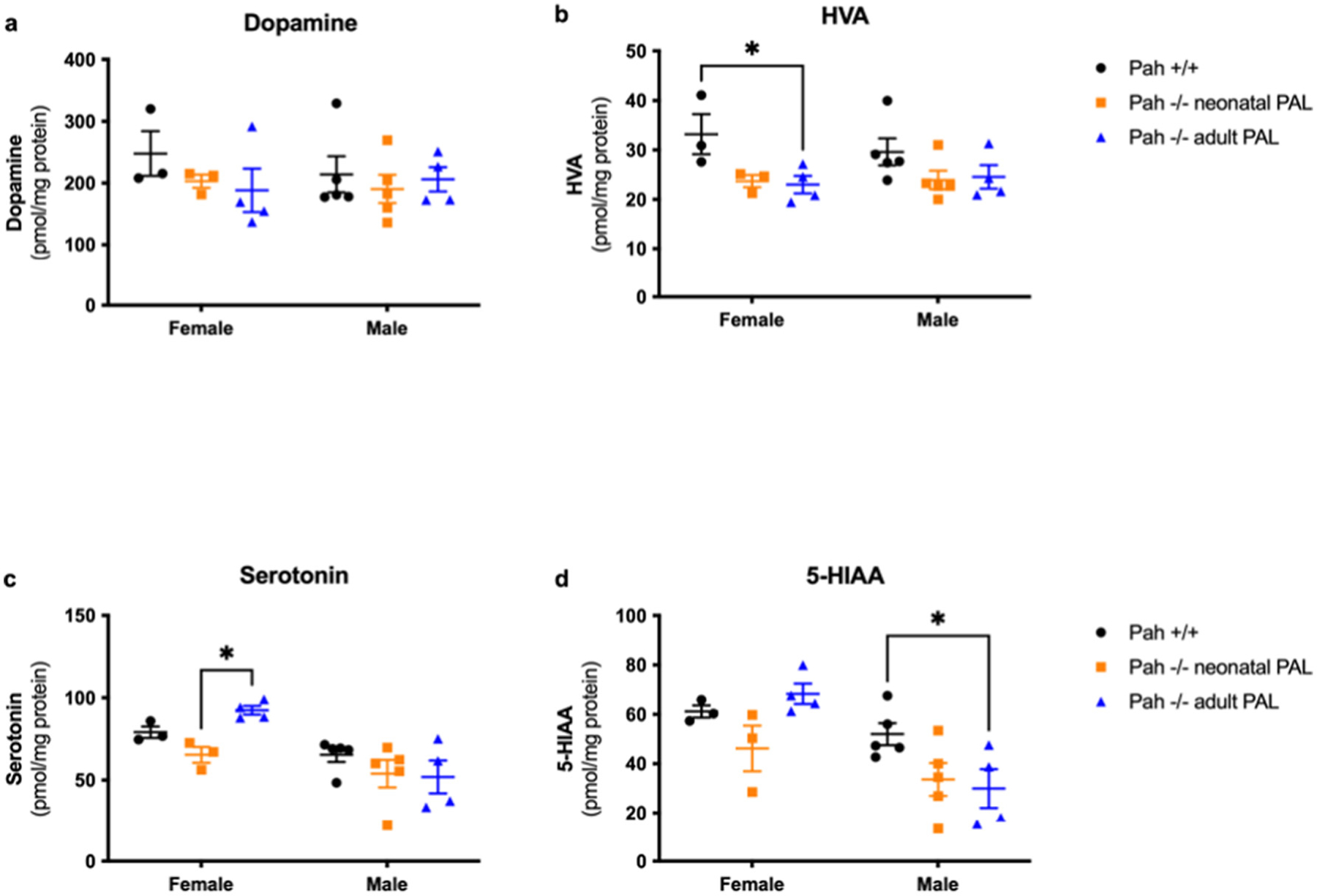
Brain monoamine neurotransmitter contents in Experiment 1. Brain dopamine, homovanillic acid (HVA), serotonin, and 5-hydroxyindoleacetic acid (5-HIAA) content (nmol/g, mean ± SEM) in PAH+/+ mice in comparison to PAH−/− mice treated with weekly pegvaliase from birth (PAH−/− neonatal PAL) or with weekly pegvaliase initiated during adulthood (PAH−/− adult PAL). Intergroup comparison by post hoc Tukey’s multiple comparisons test: * - p < 0.05, ** - p < 0.01, *** - p < 0.001, **** - p < 0.0001.
Pegvaliase treatment corrected brain serotonin deficiency we had previously observed in untreated PAH−/− mice (Fig. 5c). Two-way ANOVA revealed an effect of sex (p = 0.001) but not of group (p = 0.1387) upon brain serotonin content. Mean brain serotonin was somewhat greater in female mice than in males; the reason for this difference is unknown. Within sex groups, brain serotonin content was similar (male PAH+/+ = 65.4 ± 4.3 nmol/g, male neonatal PAH−/− = 54.0 ± 8.3 nmol/g, and male adult PAH−/− = 52.0 ± 10.0 nmol/g) with only a slightly significant intergroup difference between female neonatal PAH−/− (65.4 ± 4.8 nmol/g) and female adult PAH−/− (92.4 ± 2.6 nmol/g, p = 0.0424) with serotonin in female PAH+/+ mice = 79.1 ± 3.4 nmol/g. Similar results were found for the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA)(Fig. 5d). Two-way ANOVA again revealed a significant effect of sex (p = 0.0011) but not of treatment group (p = 0.0569), and again females exhibit slightly higher 5-HIAA content in brain than males. There were no significant intergroup differences among female mice (PAH+/+ = 61.2 ± 4.4 nmol/g, neonatal PAH−/− = 46.2 ± 9.2 nmol/g, adult PAH−/− = 68.3 ± 4.1 nmol/g). Among male mice (PAH+/+ = 52.0 ± 4.4 nmol/g, neonatal PAH−/− = 33.6 ± 6.7 nmol/g, adult PAH−/− = 29.9 ± 7.9 nmol/g), only the difference between male PAH+/+ and adult-treated PAH−/− mice reached statistical significance (p = 0.0414).
3.2. Experiment 2: cognitive deficits reappeared after discontinuation of weekly pegvaliase therapy in early-treated female PAH-deficient mice
Our goal was to model contemporary human PKU therapy in which children enjoy good metabolic control due to dietary Phe restriction but then suffer the consequences of HPA during adolescence and adulthood. Weekly pegvaliase injections were initiated in neonatal PAH−/− mice (4 males, 6 females) and continued until 3 months age, then replaced with weekly saline injections through behavioral and cognitive testing commencing at 4 months and continued until euthanasia at approximately 5 months age. Results were compared to those of saline-treated PAH+/+ mice (5 males, 5 females). As expected, serum Phe concentrations at the end of the experiment were extremely elevated in PAH−/− mice (males, mean ± SEM = 2134 ± 83.4 μM; females, 2107 ± 117 μM) in comparison to PAH+/+ mice (males, mean ± SEM = 65.7 ± 1.4 μM; females, 58.5 ± 5.3 μM) as pegvaliase treatment had been discontinued prior to behavioral and cognitive testing. The results of Water Maze testing are displayed in Fig. 6 with supporting statistics presented in the Supplementary Table 5. Although male PAH−/− mice performed as well as PAH+/+ animals in the water maze, female hyperphenylalaninemic PAH−/− mice exhibited impaired performance during both the visible and hidden platform trials compared to PAH+/+, as measured by increased cumulative distance to target and increased latency to reach the target. These data suggest that reemergence of hyperphenylalaninemia during adulthood can adversely affect task learning and spatial learning in females despite early treatment. In males, hyperphenylalaninemic PAH−/− mice showed slightly improved performance during the hidden platform trials.
Fig. 6.
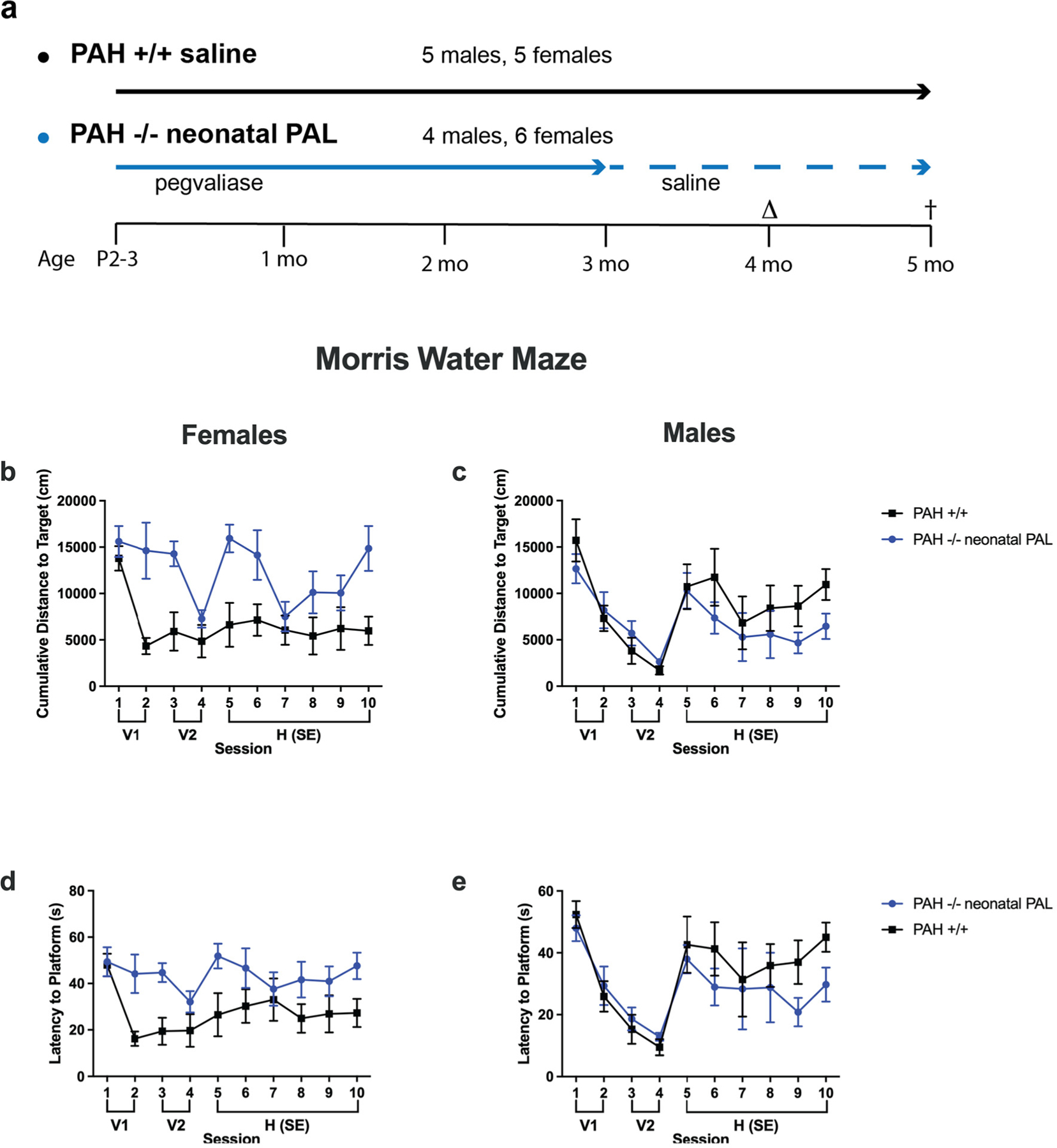
Morris water maze evaluation in Experiment 2. Results of Morris water maze evaluation in PAH+/+ mice in comparison to PAH−/− mice treated weekly from birth with pegvaliase (PAH−/− neonatal PAL) and then switched to saline during adulthood. The experimental groups and timeline of the experiment are presented (6a). Behavioral and cognitive assessments were initiated at age 4 months (Δ) with euthanasia at 5 months (†). Data are displayed as mean ± SEM. V1 and V2 are separate training sessions with the visible platform. Cumulative Distance to Target in cm for female mice (6b) and male mice (6c) and Latency to Platform in sec for females (6d) and males (6e).
Unlike our first trial described above of weekly pegvaliase treatment continuing into adulthood, weekly pegvaliase therapy begun neonatally but discontinued at 3 months age in this cohort did not correct the growth of body weight in PAH−/− mice (Fig. 7a). Two-way ANOVA of final body weight demonstrated a significant effect of both genotype and sex. The mean final body weight of neonatal pegvaliase-treated male PAH−/− mice was 22.2 ± 0.1 g in comparison to 27.2 ± 0.4 g in PAH+/+ mice (p < 0.001). In females, the final body weight in pegvaliase treated PAH−/− mice (19.2 ± 0.8 g) was also lower in comparison to PAH+/+ mice (22.1 ± 0.3 g, p = 0.0083). Similar to results from our initial trial, weekly pegvaliase treatment in this cohort also did not correct brain growth in PAH−/− mice (Fig. 7b). Two-way ANOVA revealed a significant effect of only genotype but not sex upon brain weight. The mean brain weight in male pegvaliase treated PAH−/− mice (0.368 ± 0.005 g) was significantly lower than that in male PAH+/+ mice (0.438 ± 0.003 g, p < 0.0001). In females, brain weight in pegvaliase treated PAH−/− mice (0.341 ± 0.008 g) was also significantly lower than in PAH+/+ mice (0.451 ± 0.004 g, p < 0.0001).
Fig. 7.
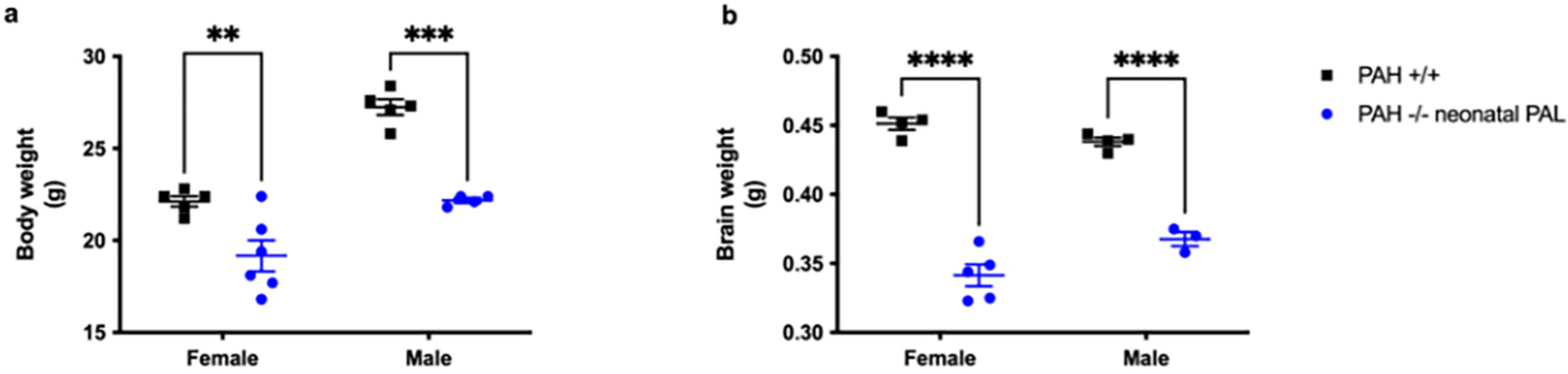
Body and brain weight in Experiment 2. Mean (± SEM) body weight (7a) and brain weight (7b) of PAH+/+ mice in comparison to PAH−/− mice treated from birth with weekly pegvaliase (PAH−/− neonatal PAL). ** - p < 0.01, *** - p < 0.001, **** - p < 0.0001.
3.3. Experiment 3: comparison of continuous pegvaliase treatment to treatment discontinued during adulthood
To model common status of contemporary dietary therapy in adult humans with PKU, we sought to compare the effects of discontinuing pegvaliase therapy in adult mice and allowing reemergence of hyperphenylalaninemia to continuation of pegvaliase therapy and maintenance of low blood Phe concentrations. In this definitive experiment, because weekly pegvaliase treatment had not fully corrected deficits in brain growth nor maintained low blood phenylalanine through the duration of a week, pegvaliase dosing was doubled to twice per week injections on Mondays and Thursdays in order to maintain consistently lower blood Phe concentrations. Four cohorts of mice were studied: 1) PAH−/− treated with twice weekly pegvaliase through 4 months age then saline injections were substituted for pegvaliase until euthanasia at 6 months age (5 males, 5 females)(designated pegvaliase-treated high Phe or PHP), 2) PAH−/− treated with twice weekly pegvaliase throughout the experiment (5 males, 5 females)(designated pegvaliase-treated low Phe or PLP), 3) PAH−/− treated with saline only (6 males and 6 females)(PAH−/−), and 4) PAH+/+ treated with saline (10 males and 10 females)(PAH+/+). Behavioral and cognitive testing commenced at 5 months age, was completed by six months age, and euthanasia for tissue collection occurred at 6 months age. Behavioral and cognitive evaluation included assessment of nest building, of performance in an elevated zero maze which assesses anxiety-like behavior, performance in a Y maze which assesses hippocampus-dependent spontaneous alteration, activity levels and measures of anxiety in the open field, object recognition, task learning and spatial learning and memory in the Morris water maze, and fear learning and memory. Results of assessments in the elevated zero maze, Y maze and fear conditioning are not reported as we found no statistically significant differences between groups.
Results of nest building assessments are displayed in Fig. 8 with the related statistical analysis presented in Supplementary Table 6. Saline-treated PAH−/− mice had lower nest building scores than wild-type mice. In pegvaliase-treated PAH−/− mice, PLP, but not PHP improved nest building although it did not fully restore the levels to those seen in wild-type mice. As there was both an effect of sex and an interaction between sex and experimental group, we also analyzed the nest building in females and males separately. All PAH−/− mice had lower nest building scores in both females and males; neither treatment improved the nest scores of PAH−/− females but both treatments rescued the nest scores of PAH−/− males.
Fig. 8.
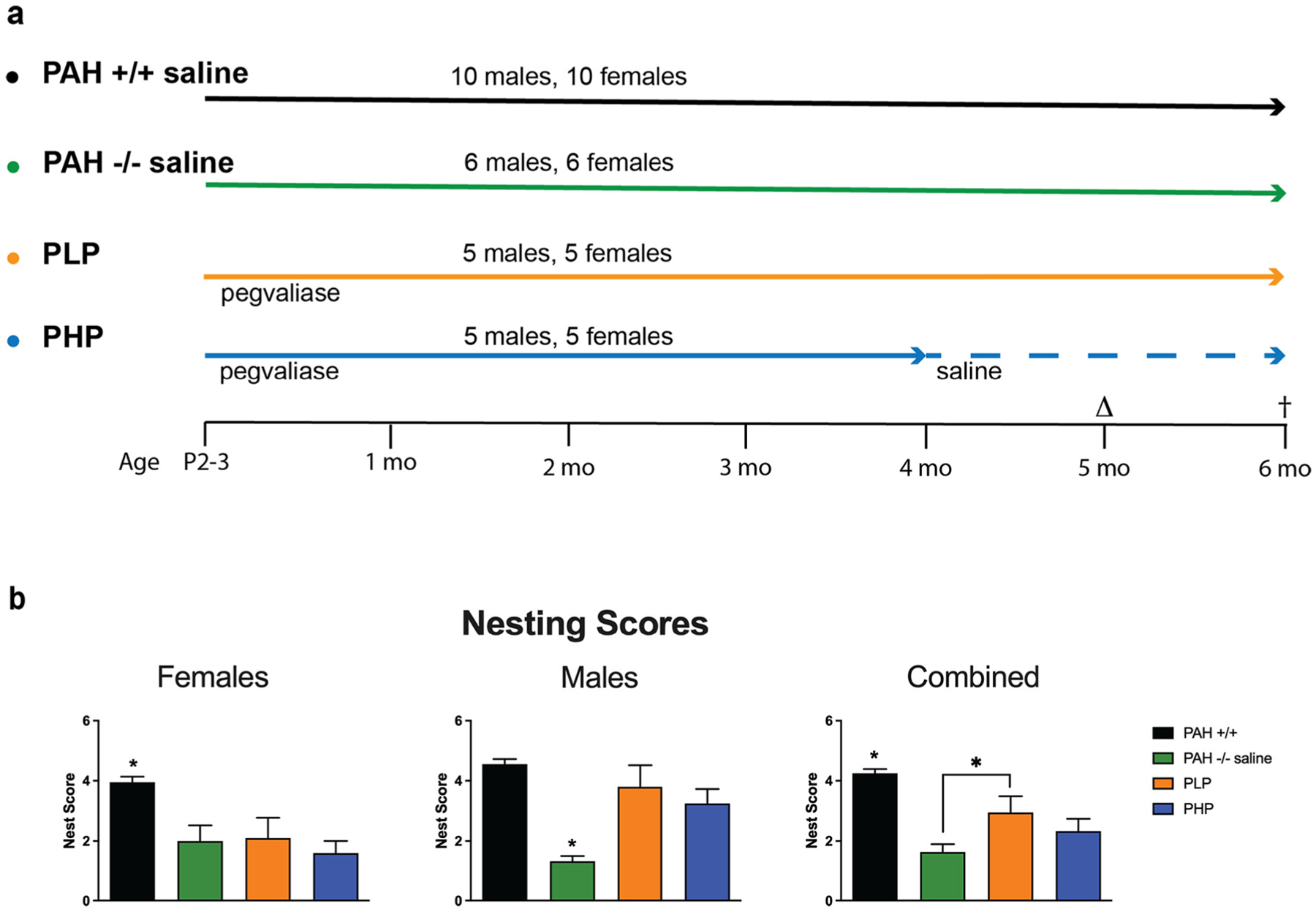
Experimental timeline and nesting scores in Experiment 3. The experimental groups and timeline of Experiment 3 are presented (8a). In the first two groups, PAH+/+ and PAH−/− mice receive twice weekly saline injections from P2–3 until 6 months age. In the third group, PAH−/− mice received twice weekly pegvaliase injections throughout the experiment (pegvaliase low Phe = PLP). In the final group, PAH−/− mice received twice weekly pegvaliase from P2–3 until 4 months age and then were switched to saline injections for the remainder of the experiment (pegvaliase high Phe = PHP). Behavioral and cognitive assessments were initiated at age 5 months (Δ) with euthanasia at 6 months (†). 8b) Evaluation of qualitative nesting quality in saline-treated PAH+/+ and PAH−/− mice in comparison to PAH−/− mice treated with twice weekly pegvaliase from birth and either continued throughout the experiment (PLP) or discontinued at 4 months age (PHP). Data are expressed as mean ± SEM. * - p < 0.05.
The results of testing in the Open Field are displayed in Fig. 9 with the related statistical analysis presented in Supplementary Table 6. Compared to wild-type mice, PAH−/− mice showed increased activity levels. This was rescued by either treatment. Hippocampus-dependent spatial habituation learning is indicated by lower activity levels on day 2 than day 1 in the open field. While spatial habituation learning was seen in wild-type mice, untreated and PLP PAH−/− mice, it was not seen in PHP PAH−/− mice. As there was an effect of sex and typically females are more active than males in the open field, we also analyzed the females and males separately. In females, PAH−/− mice were more active than wild-type mice. In addition, PAH−/− mice did not show spatial habituation learning and showed similar activity levels on day 1 and 2 of the open field. In PAH−/− mice, PHP treatment reduced activity levels while PLP treatment restored spatial habituation learning. In males, saline-treated PAH−/− mice did not show impaired spatial habituation learning, and PLP treatment reduced activity levels. When time spent in the center of the open field was assessed, wild-type mice spent less time in the center of the open field on day 2 than day 1. This was not seen in saline-treated or pegvaliase-treated PAH−/− mice.
Fig. 9.
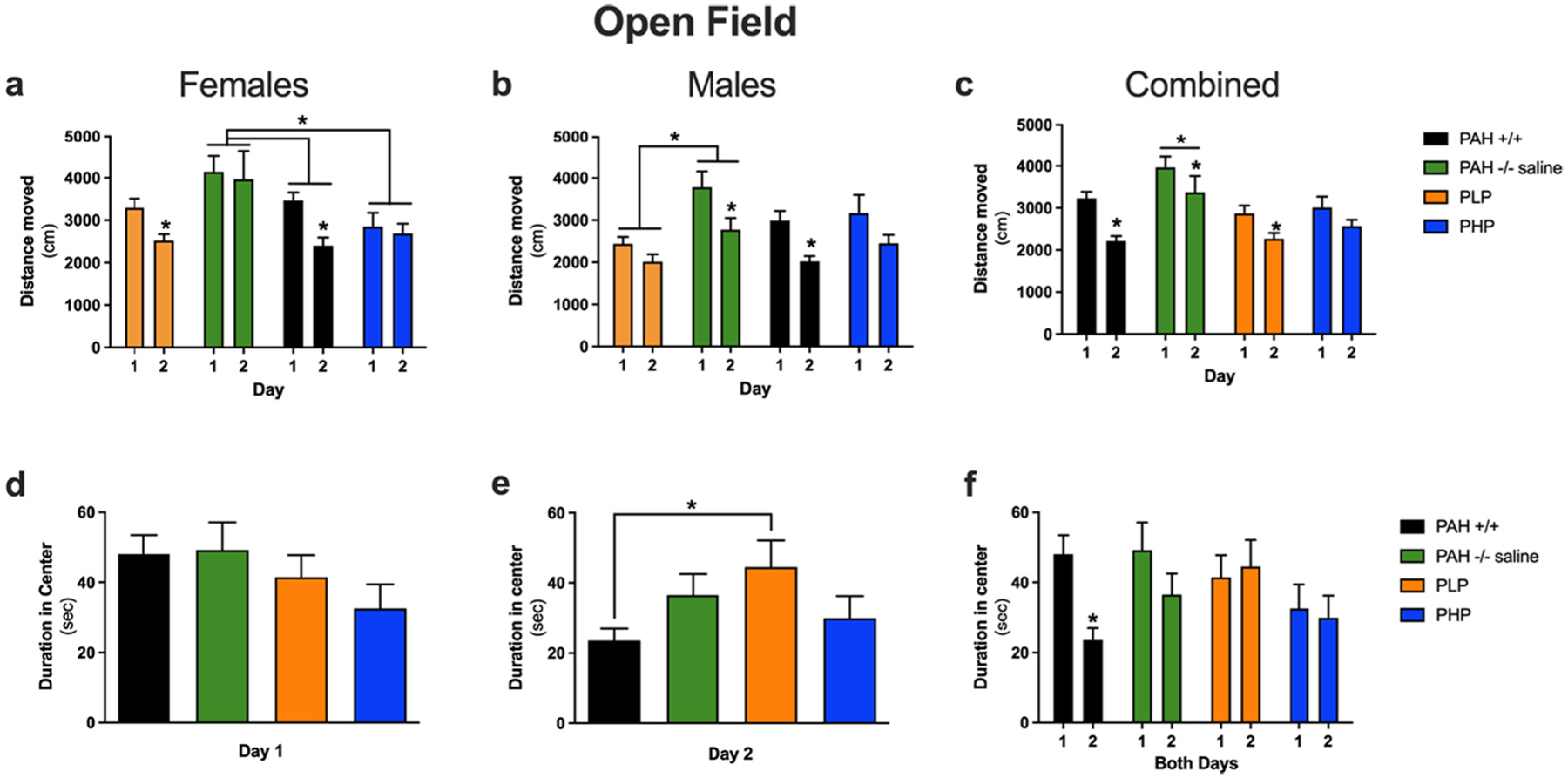
Assessments in the open field for Experiment 3. Evaluation of behavior in the Open Field in saline-treated PAH+/+ and PAH−/− mice in comparison to PAH−/− mice treated with twice weekly pegvaliase from birth and either continued throughout the experiment (PLP) or discontinued at 4 months age (PHP). Panels a, b, and c display the cumulative distance moved (cm) in the open field on two consecutive days of testing for female, male, or both sexes combined respectively. The duration (sec) spent in the center of the open field on days 1 and 2 of testing are displayed in panels d and e with both days displayed together in panel f. Data are expressed as mean ± SEM. Asterisks above a single group depict a significant difference between two days of testing within the group. Brackets spanning groups depict significant differences between groups by post hoc Tukey’s comparison. * - p < 0.05.
The results of Novel Object Recognition testing are displayed in Fig. 10 with the related statistical analysis presented in Supplementary Table 6. Wild-type mice showed object recognition and spent significantly more time exploring the novel than the familiar object. In contrast, no significant preference was seen in saline-treated PAH−/− mice. Both pegvaliase treatment regimens rescued object recognition.
Fig. 10. –
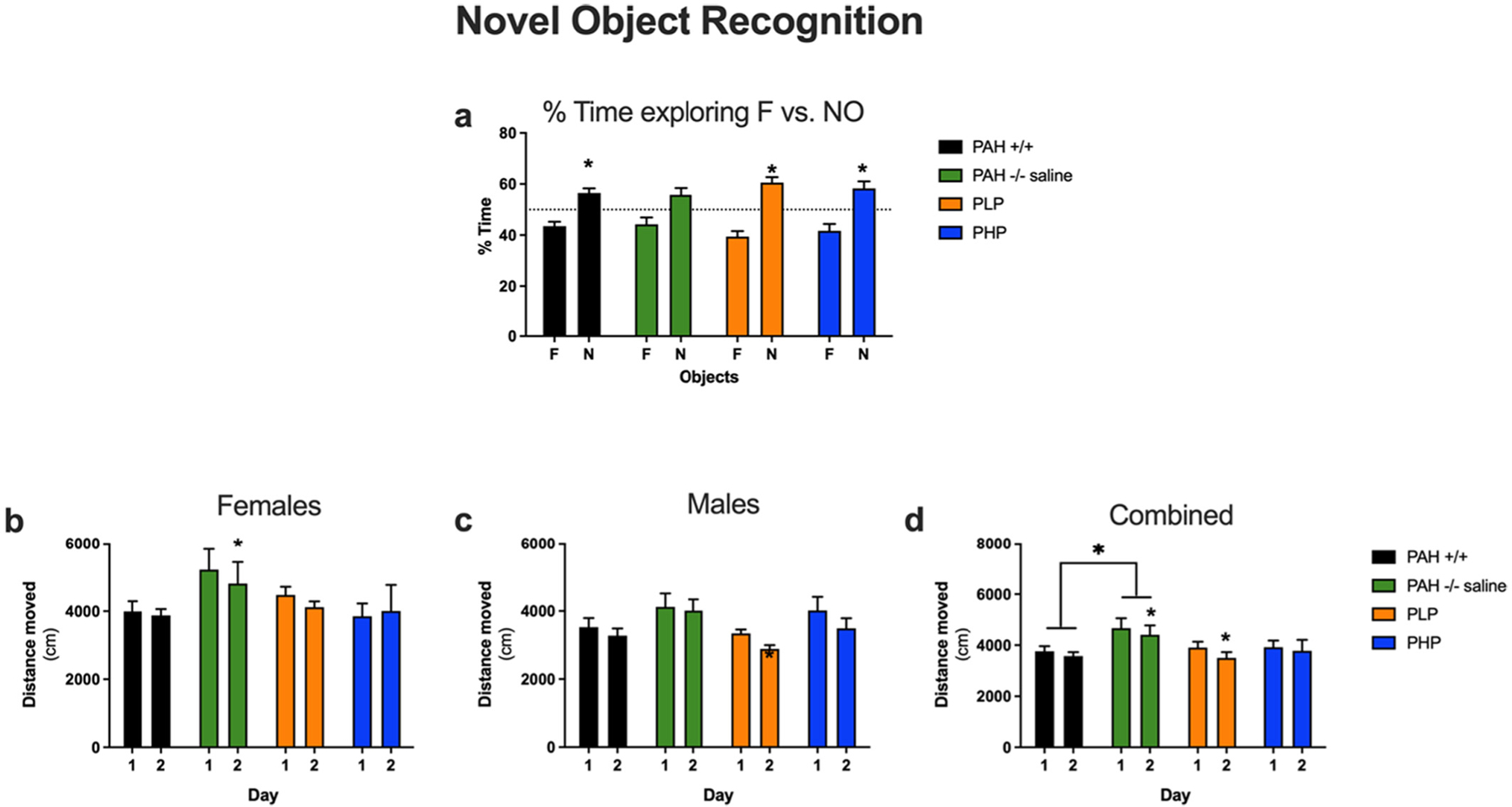
Results of novel object recognition testing in Experiment 3. Evaluation of novel object recognition in saline-treated PAH+/+ and PAH−/− mice in comparison to PAH−/− mice treated with twice weekly pegvaliase from birth and either continued throughout the experiment (PLP) or discontinued at 4 months age (PHP). Data are presented as mean ± SEM. Panel a – Comparison of time spent exploring the familiar (F) vs the novel (N) object. Panels b, c, and d - Distance traveled exploring the familiar and novel objects on Days 1 and 2 in females, males, and both sexes combined respectively. *– p < 0.05.
The results of the Morris water maze test are displayed in Figs. 11, 12 and 13 with statistical analysis presented in Supplementary Table 6. During visible platform training as measured by latency to the target (Fig. 11a), saline-treated PAH−/− mice showed impaired performance as compared to wild-type mice. In PAH−/− mice, both pegvaliase treatment regimens improved performance. During hidden platform training, saline-treated PAH−/− mice also showed impaired performance as compared to wild-type mice. In pegvaliase-treated PAH−/− mice, there was a trend towards a beneficial effect in PLP, but not PHP mice, on performance during hidden platform training. As there was an effect of sex and an interaction with sex during visible platform training and a trend towards an interaction with sex during hidden platform training, we also analyzed the performance of females and males separately (Fig. 11b, c). During visible platform training, performance was impaired in saline-treated PAH−/− female mice as compared to sex-matched wild-type mice. Performance in visible platform training improved in both groups of pegvaliase-treated female PAH−/− mice but not equal to that of PAH+/+ females. During hidden platform training, saline-treated PAH−/− female mice also showed impaired performance as compared to wild-type mice and only PHP females showed significant treatment effects. During visible platform training, performance was also impaired in saline-treated PAH−/− male mice as compared to sex-matched wild-type mice. In male pegvaliase-treated PAH−/− mice, PLP male mice exhibited similar performance with the visible platform as PAH+/+ mice but PHP male mice remain impaired. During hidden platform training, PAH−/− male mice also showed impaired performance as compared to sex-matched wild-type mice. In PAH−/− male mice, there was a trend towards a beneficial treatment effect in both PLP and PHP mice. A similar pattern was seen when cumulative distance to the platform location (Fig. 12a, b, c) was used as a performance measure. As group differences in swim speeds can affect latency and cumulative distance to the platform measures, we analyzed swim speeds as well. There were no group differences in swim speeds during the visible platform training (data not shown). There was no effect of treatment when total distance swum during the hidden platform training was analyzed (Fig. 13a, b, c). This indicates that the treatment effect seen for latency and cumulative distance to the platform are cognitive performance related and not due to possible differences in swimming ability during the hidden platform training.
Fig. 11.
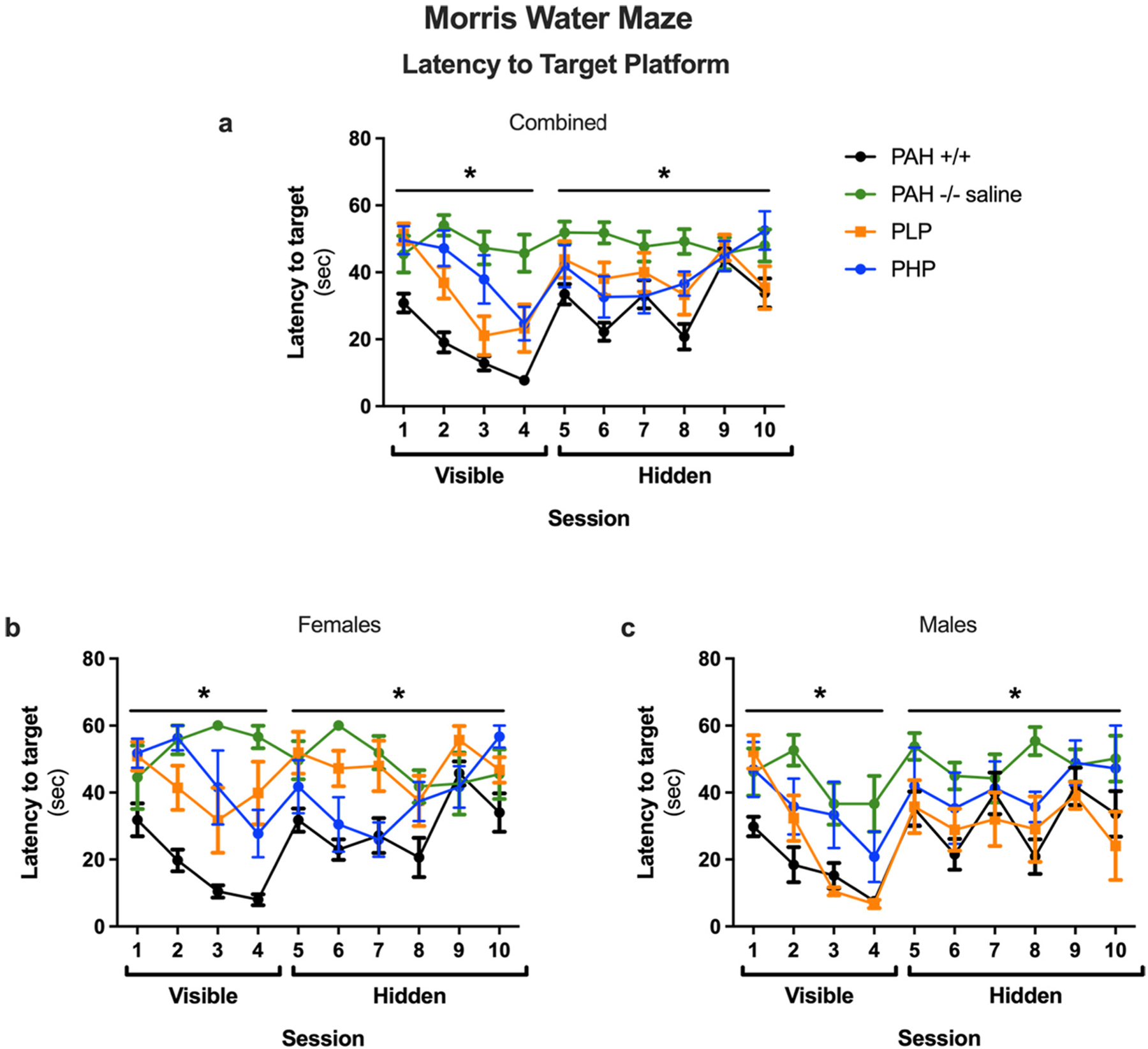
Latency to target platform in Morris water maze of Experiment 3. Results of Morris water maze testing as measured by latency to the target platform (sec) in saline-treated PAH+/+ and PAH−/− mice in comparison to PAH−/− mice treated with twice weekly pegvaliase from birth and either continued throughout the experiment (PLP) or discontinued at 4 months age (PHP). Data displayed are mean ± SEM. Panels a, b, and c – both sexes combined, females, or males respectively. *– Significant effect of group detected by ANOVA (p < 0.05).
Fig. 12.
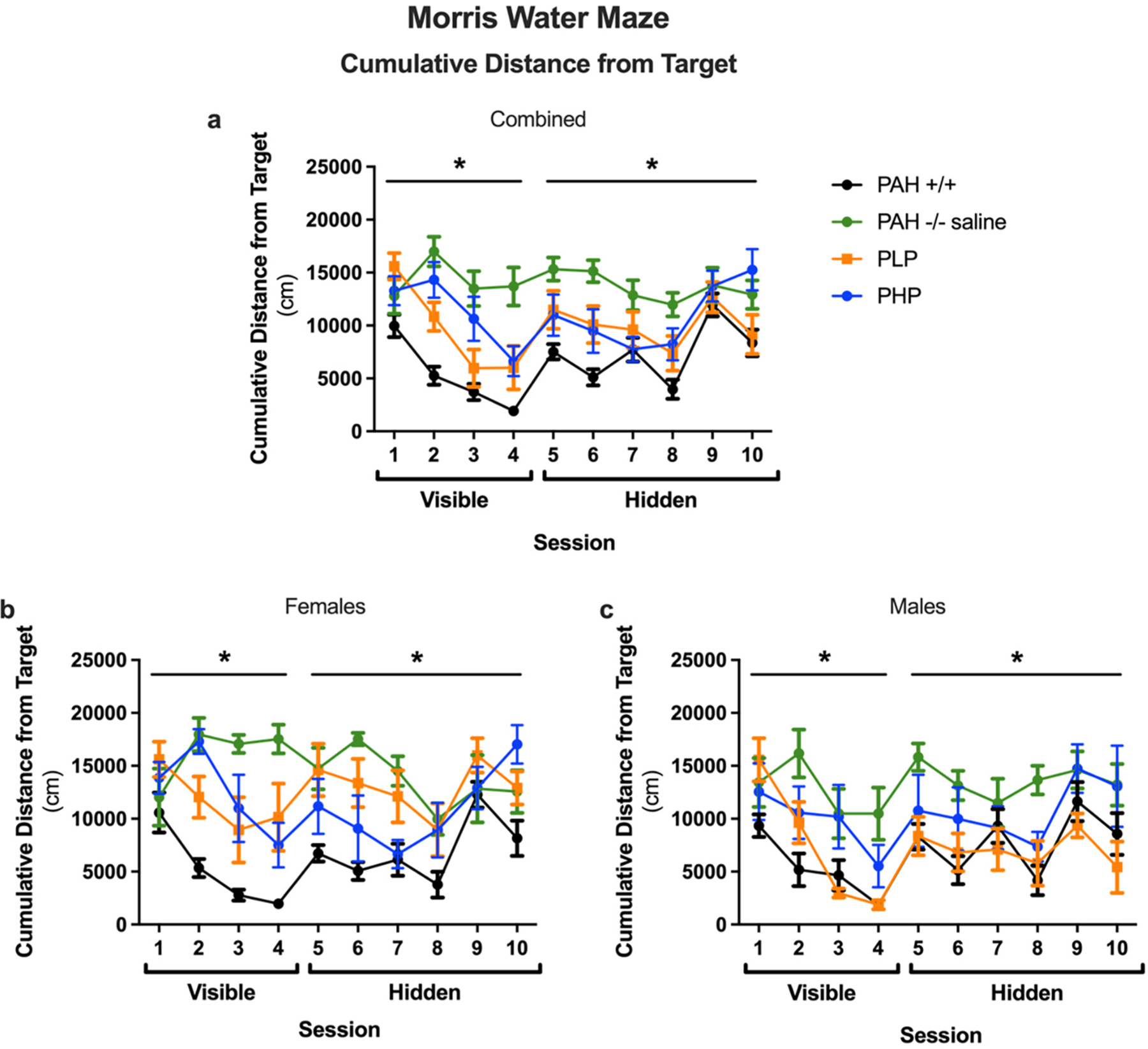
Cumulative distance from the platform location in Morris water maze of Experiment 3. Results of Morris water maze testing as measured by cumulative distance from the target platform (cm) in saline-treated PAH+/+ and PAH−/− mice in comparison to PAH−/− mice treated with twice weekly pegvaliase from birth and either continued throughout the experiment (PLP) or discontinued at 4 months age (PHP). Data displayed are mean ± SEM. Panels a, b, and c – both sexes combined, females, or males respectively. *– Significant effect of group detected by ANOVA (p < 0.05).
Fig. 13.
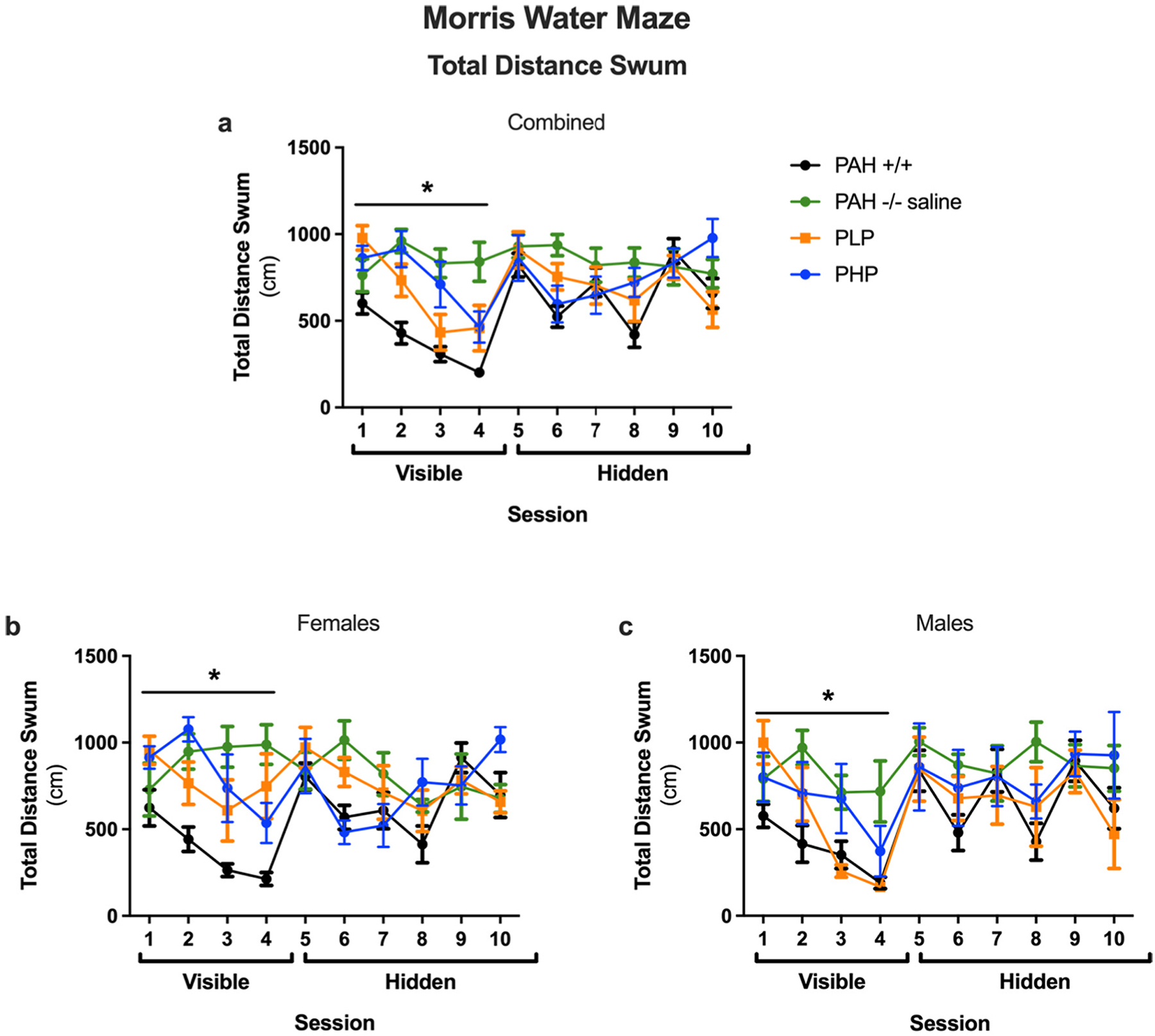
Total distance swum in Morris water maze of Experiment 3. Results of Morris water maze testing as measured by total distance swum (cm) in saline-treated PAH+/+ and PAH−/− mice in comparison to PAH−/− mice treated with twice weekly pegvaliase from birth and either continued throughout the experiment (PLP) or discontinued at 4 months age (PHP). Data displayed are mean ± SEM. Panels a, b, and c – both sexes combined, females, or males respectively. *– Significant effect of group detected by ANOVA (p < 0.05).
During the probe trials (no platform) (Supplementary Fig. 2), PAH−/− mice showed impaired spatial memory retention and swam further away from the platform location. In PAH−/− mice, PLP, but not PHP, rescued this impairment. As there was an effect of sex and an interaction with sex, we also analyzed the performance of the females and males separately. In females, spatial memory retention was worse in saline-treated and PLP-treated PAH−/− mice. There was also a trend towards worse performance in PHP-treated PAH−/− mice than wild-type female mice but it did not reach significance. In males, spatial memory retention was worse in saline-treated PAH−/− than wild-type mice. In PAH−/− male mice, PLP, but not PHP, rescued this impairment.
Pegvaliase treatment was associated with improved growth in PAH−/− mice (Fig. 14, statistics in Supplementary Table 7). Two-way ANOVA revealed significant effects of both group and sex upon final body weight. The final body weight of saline-treated PAH−/− mice (mean ± SEM for females = 19.3 ± 0.2 g, p = 0.0007; males = 24.6 ± 1.0 g, p < 0.0001) remained significantly below that of wild type animals (females = 23.0 ± 0.6 g, males = 28.9 ± 0.7 g). The weights of PHP mice (females = 21.8 ± 0.8 g, males = 28.1 ± 0.6 g) and of female PLP mice (22.3 ± 0.3 g) were not significantly different from wild type, but male PLP mice remained slightly underweight (26.4 ± 0.4 g, p = 0.0464) in comparison to PAH+/+ animals. Brain weights again however remained low in nearly all PAH−/− groups regardless of pegvaliase treatment. Two-way ANOVA demonstrated significant effects of both group and sex upon brain weight. Brain weight was significantly lower than wild type mice (females = 0.456 ± 0.005 g, males = 0.450 ± 0.003 g) in saline-treated PAH−/− mice (females = 0.378 ± 0.011 g, p < 0.0001; males = 0.407 ± 0.014 g, p = 0.0087), PLP mice (females = 0.388 ± 0.014 g, p = 0.0002; males = 0.398 ± 0.011 g, p = 0.0044), and female PHP mice (0.381 ± 0.017 g, p < 0.0001). Brain weight of male PHP mice (0.424 ± 0.008 g) was not significantly different (p = 0.2732) from wild type animals.
Fig. 14.
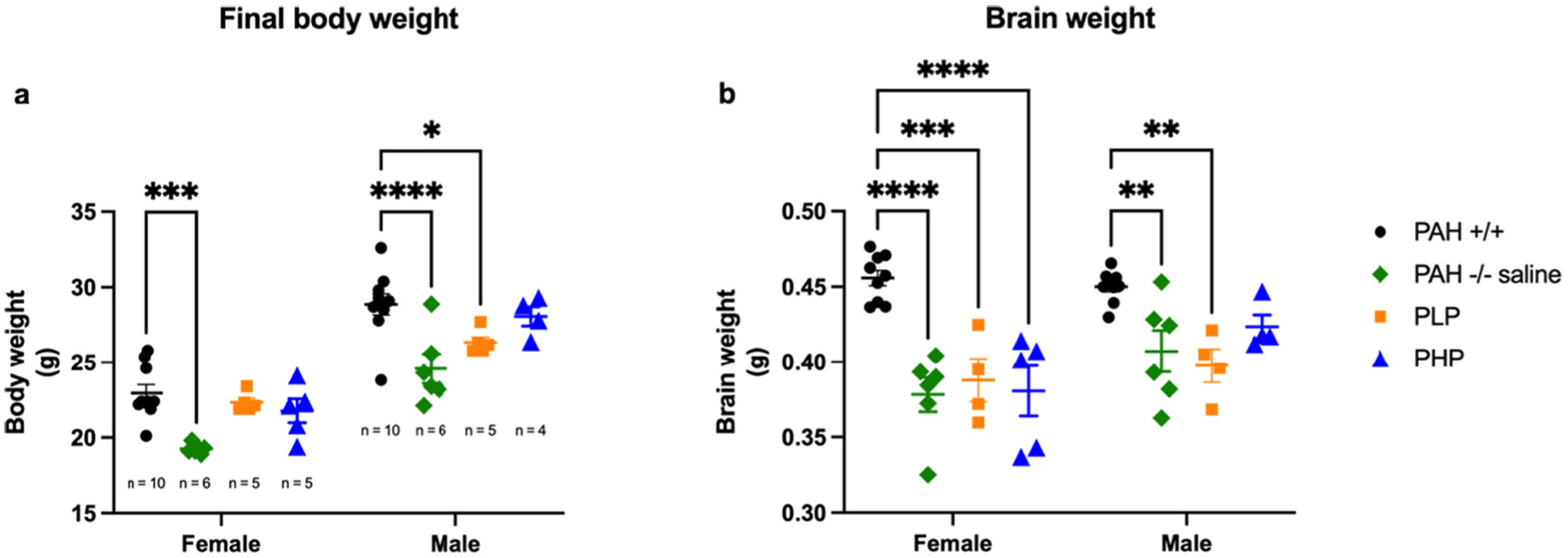
Body and brain weight in Experiment 3. Data are mean ± SEM. Statistical comparison by post hoc Tukey’s multi-group comparison. * - p < 0.05, ** - p < 0.01, ***- p < 0.001, **** - p < 0.0001.
After euthanasia, blood and brain were collected for amino acid and monoamine neurotransmitter analyses. Serum and brain amino acid analyses are presented in Fig. 15 with the related statistical analyses presented in Supplementary Table 8. Two-way ANOVA of serum Phe revealed a significant effect of group and sex with a minor interaction between group and sex. As expected, serum Phe concentrations were significantly greater in PAH−/− treated with saline from birth (female mean ± SEM serum Phe = 2544 ± 134 μM, males = 1996 ± 194 μM) or in PHP mice (females = 2859 ± 157 μM, males = 2523 ± 79 μM) in comparison to PLP mice (females = 120 ± 119 μM, males = 13 ± 8 μM) or to PAH+/+ mice (females = 108 ± 24 μM, males = 119 ± 19 μM). Similar results were found for brain Phe content. Two-way ANOVA of brain Phe content revealed a significant effect of group and sex with no interaction between group and sex. Brain Phe concentrations were significantly greater in PAH−/− treated with saline from birth (female mean ± SEM brain Phe = 967 ± 51 nmol/g, males = 762 ± 33 nmol/g) or in PHP mice (females = 789 ± 79 nmol/g, males = 784 ± 38 nmol/g) in comparison to PLP mice (females = 362 ± 125 nmol/g, males = 190 ± 25 nmol/g) or to PAH+/+ mice (females = 192 ± 14 nmol/g, males = 240 ± 37 nmol/g). Plotting brain Phe content vs serum Phe reveals a strong linear correlation (R2 = 0.82) between the two parameters (Supplementary Fig. 3a).
Fig. 15.
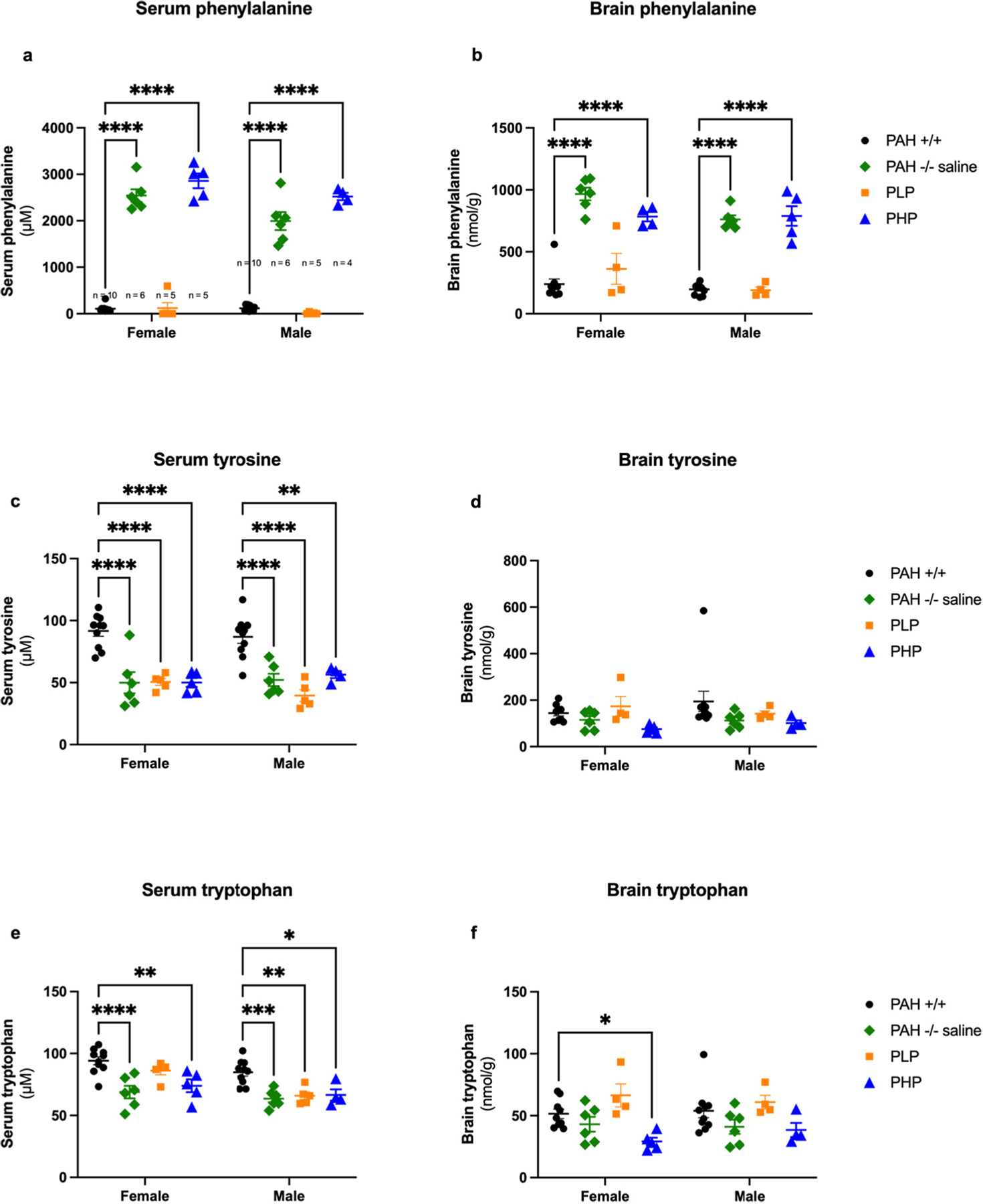
Serum and brain amino acid content in Experiment 3. Data are displayed as mean ± SEM. Statistical comparison by post hoc Tukey’s multi-group comparison. * - p < 0.05, ** - p < 0.01, *** - p < 0.001, **** - p < 0.0001.
Serum Tyr concentrations are on average lower in PAH−/− mice than in PAH+/+ mice and remain so in pegvaliase-treated mice. Two-way ANOVA revealed only a significant effect of group but no effect of sex upon serum Tyr and no interaction between group and sex. Mean serum Tyr was greater in PAH+/+ mice (females = 92 ± 4 μM, males = 87 ± 5 μM) than in saline-treated PAH−/− (females = 50 ± 9 μM, males = 52 ± 5 μM), PLP (females = 51 ± 3 μM, males = 40 ± 5 μM), or PHP mice (females = 50 ± 4 μM, males = 56 ± 3 μM). Two-way ANOVA revealed only a slightly significant effect of group but no effect of sex upon brain Tyr content. Brain Tyr content of PAH+/+ mice (females = 144 ± 12 nmol/g, males = 195 ± 44 nmol/g) and PLP mice (females = 174 ± 42 nmol/g, males = 142 ± 12 nmol/g) was slightly higher than that of saline-treated PAH−/− mice (females = 115 ± 17 nmol/g, males = 113 ± 14 nmol/g) or PHP mice (females = 76 ± 16 nmol/g, males = 101 ± 23 nmol/g), but these differences did not reach statistical significance by post hoc intergroup comparison. Brain Tyr content correlated poorly with either serum Tyr (R2 = 0.11) or Phe (R2 = 0.19) concentrations (Supplementary Fig. 3b and c).
Serum Trp concentrations were also on average decreased in all PAH−/− mice in comparison to wild type littermates. Two-way ANOVA revealed significant effects of both group and sex without any interaction between group and sex. Trp concentrations were greater in females than males in all groups and greater in PAH+/+ mice than in PAH−/− mice. In female mice, serum Trp was greatest in saline-treated PAH+/+ mice (94 ± 3 μM) in comparison to saline-treated PAH−/− (69 ± 5 μM), PLP (86 ± 3 μM), and PHP mice (74 ± 15 μM). In male mice, again the highest serum Trp concentrations were seen in PAH+/+ mice (85 ± 3 μM) in comparison to saline-treated PAH−/− (64 ± 3 μM), PLP (66 ± 3 μM), or PHP mice (67 ± 5 μM). In the brain, hyperphenylalaninemic mice tended to exhibit slightly decreased brain Trp content in comparison to mice with lower blood Phe concentrations. Two-way ANOVA revealed a significant effect of group but no effect of sex upon brain Trp. Both PAH+/+ (females = 52 ± 4 nmol/g, males = 54 ± 6 nmol/g) and PLP mice (females = 66 ± 9 nmol/g, males = 61 ± 6 nmol/g) exhibited greater Trp content than saline-treated PAH−/− (females = 43 ± 6 nmol/g, males = 41 ± 6 nmol/g) or PHP mice (females = 29 ± 3 nmol/g, males = 39 ± 16 nmol/g). Again, correlations between brain Trp content and serum Trp (R2 = 0.05, Supplementary Fig. 3d) or serum Phe (R2 = 0.34, Supplementary Fig. 3e) were poor.
We measured brain monoamine neurotransmitters in whole brain homogenate of the mice in all experimental groups. Because of some variability in outcomes of cohorts measured at different times, the sex-specific data were normalized to the mean of PAH+/+ mice on a given date. The data are displayed relative to PAH+/+ mice in Fig. 16 with statistical data in Supplementary Table 9. Hyperphenylalaninemic PAH−/− mice exhibited a mild brain dopamine deficiency that is slightly worse in female than in male mice. Two-way ANOVA revealed significant effects of both group and sex with a significant interaction between group and sex upon brain dopamine content. Relative brain dopamine content was lower in saline-treated female PAH−/− mice (59 ± 5%) and pegvaliase-discontinued female PHP mice (61 ± 9%) in comparison to either female PLP (101 ± 11%) or female PAH+/+ mice (100 ± 1%). The differences in male mice were not as great and did not reach statistical significance by post hoc intergroup comparison: PAH+/+ (100 ± 5%), saline-treated PAH−/− (72 ± 13%), PLP (92 ± 10%), and PHP mice (84 ± 13%). Two-way ANOVA revealed significant effects of both group and sex upon brain HVA content without any interaction between group and sex. As with dopamine, brain HVA content was generally lower in hyperphenylalaninemic mice than in PAH+/+ mice. Mean brain HVA content in female PAH−/− mice measured only 43 ± 3% of female PAH+/+ mice, 49 ± 12% in female PHP mice and surprisingly only 38 ± 10% in female PLP mice. The differences in brain HVA content among male animals did not reach statistical significance: PAH+/+ = 100 ± 5%, PAH−/− = 74 ± 8%, PHP = 91 ± 17%, PLP = 86 ± 9%.
Fig. 16.
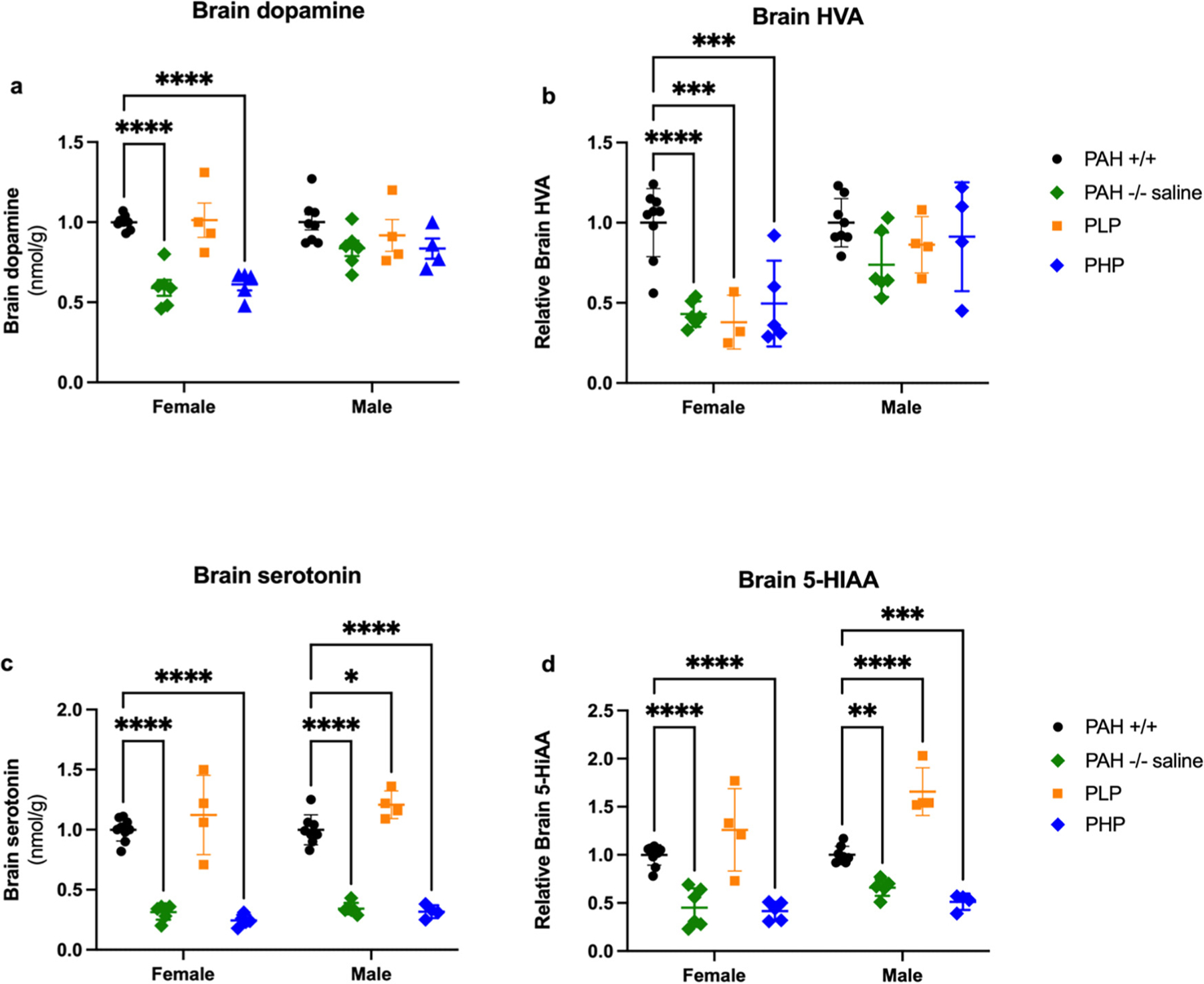
Relative brain neurotransmitters in Experiment 3. Monoamine neurotransmitters (mean ± SEM) are displayed relative to the sex specific PAH+/+ mean. Statistical comparison by post hoc Tukey’s multi-group comparison. * - p < 0.05, ** - p < 0.01, *** - p < 0.001, **** - p < 0.0001.
Serotonin deficiency is more severe in hyperphenylalaninemic mice than the relatively minor effects seen upon the dopamine axis, and continuous pegvaliase treatment had a significant positive effect upon serotonin status in Experiment 3. Two-way ANOVA demonstrated a significant effect of group and a lesser effect of sex but no interaction between group and sex. Brain serotonin content was severely decreased in all mice that were hyperphenylalaninemic at euthanasia but completely normal in continuously pegvaliase-treated mice. In female mice, serotonin content in saline-treated PAH−/− mice (31 ± 3%) and pegvaliase-discontinued PHP mice (24 ± 2%) were significantly lower than that of PAH+/+ mice (100 ± 3%) or continuously pegvaliase-treated PLP mice (112 ± 19%). Similar differences were seen in male mice: saline-treated PAH−/− mice (34 ± 2%), PHP mice (32 ± 3%) compared to PAH+/+ (100 ± 4%) or PLP mice (121 ± 6%). Similar results were found for brain content of 5-HIAA, the metabolic product of serotonin breakdown. Two-way ANOVA demonstrated significant effects of both group and sex with a slight interaction between group and sex. The differences between groups were more exaggerated in female mice in comparison to male mice; this may be because female hyperphenylalaninemic mice exhibit higher blood Phe concentrations than males and therefore experience more severe depression of monoamine neurotransmitter synthesis. In females, brain 5-HIAA content in saline-treated PAH−/− mice (45 ± 8%) and PHP mice (41 ± 4%) was significantly decreased in comparison to either PAH+/+ (100 ± 4%) or continuously pegvaliase-treated PLP mice (126 ± 25%). Similar differences were seen in male mice: saline treated PAH−/− mice (66 ± 4%) and PHP mice (51 ± 4%) vs. PAH+/+ (100 ± 3%) and PLP mice (166 ± 12%).
Plots of brain dopamine or serotonin content vs. serum or brain aromatic amino acid concentrations are presented in Supplementary Fig. 4. Although Tyr and Trp are the substrates for dopamine and serotonin synthesis respectively, correlations between brain monoamine neurotransmitters and the concentrations of their substrate precursors in either serum or brain were not strong. However, correlations with Phe were much stronger particularly for brain serotonin. The correlations (R2) between serotonin and serum or brain Phe were 0.88 and 0.80 respectively, supporting the hypothesis that Phe-mediated competitive inhibition of TPH2 activity is largely responsible for serotonin deficiency in HPA mice.
4. Conclusions
Prior investigations into the behavioral and cognitive phenotypes of hyperphenylalaninemic Pahenu2/enu2 mice only model late-treated human PKU as most available therapies, including restriction of dietary phenylalanine intake, the mainstay of contemporary PKU treatment, could not be initiated until after weaning and therefore after the brain of the Pahenu2/enu2 pup had already suffered the neuropathologic consequences of HPA for at least 3 weeks since birth. In our prior studies, we discovered that impaired nesting behaviors and activity in the open field could be rescued by Phe-lowering treatments initiated after weaning in association with complete correction of CNS monoamine neurotransmitter deficiencies [9], but the severe memory deficit exhibited in the Morris water maze was unaffected by treatments begun after weaning. We reasoned that prevention of these cognitive deficits would require maintenance of low blood Phe from birth. The advent of injectable pegvaliase enzyme substitution therapy for PKU [24,25,32,33] allowed for the possibility of treating HPA mice from birth; repetitive pegvaliase treatment of adult HPA mice has been shown previously to partially correct maternal PKU syndrome [34] and importantly to partially restore TH expression in Pahenu2 mouse brain [35]. Our goal then was to explore the use of pegvaliase treatment initiated from near birth to develop a murine model of early-treated PKU.
In Experiment 1, we sought to determine whether weekly administration of pegvaliase begun during infancy would prevent the behavioral and cognitive deficits associated with HPA in PAH−/− mice in comparison to initiating treatment during adulthood. Serum Phe concentrations were not monitored during the experiment as repetitive phlebotomy can alter performance in subsequent behavioral and cognitive assessments. At the end of the experiment, mean serum and brain Phe in both pegvaliase treated cohorts was reduced from that of historical untreated PAH−/− data but somewhat variable due to different timings of euthanasia relative to the final pegvaliase injection. This variability is not unexpected as the pharmacodynamics of pegvaliase treatment in mice likely would require multiple injections throughout the week to maintain consistently low blood Phe with minimal fluctuation [34]. Despite the variability in blood and brain Phe, both neonatal onset and adult onset pegvaliase treatments were associated with normal body weight in female PAH−/− mice and nearly complete correction of body weight in male mice with near normalization of CNS monoamine neurotransmitters, parameters that are typically severely impaired in HPA mice. Remarkably, brain weight remained low in all PAH−/− animals despite treatment. Importantly, early treated PAH−/− mice exhibited normal cognitive function similar to that of wild type mice while animals treated only during adulthood exhibited severe memory deficits, as we have observed previously with other Phe-lowering treatments begun after weaning [9].
In contemporary dietary therapy for PKU, many adolescents and adults struggle to adhere to the diet and consequently suffer the effects of increasing blood and brain Phe after years of maintaining appropriate metabolic control. In Experiment 2, we explored whether HPA-related cognitive deficits would appear in PAH−/− mice pegvaliase treated from birth but in which pegvaliase was discontinued at 3 months age. In contrast to Experiment 1, discontinuation of pegvaliase treatment was associated with impaired physical growth of both body and brain in both male and female PAH−/− mice. Memory deficits as assessed in the water maze reemerged after pegvaliase treatment had been discontinued but here, there was a dichotomy related to sex. Male PAH−/− mice performed well in locating the visible platform but exhibited impaired ability to locate the platform when it was hidden. Female PAH−/− mice however exhibited severe cognitive deficits in both visible and hidden platform trials. Female Pahenu2/enu2 mice commonly display higher mean blood Phe concentrations than males [9,34], and chronic exposure to greater brain Phe content could have been an explanation for poorer performance in cognitive assessments. However, we did not measure an actual significant difference in the mean blood Phe concentrations of female and male PAH−/− mice at euthanasia in this specific experiment. Alternately, these data suggest that females might be more susceptible than males to detrimental effects of HPA on cognitive performance. It is also conceivable that female PAH−/− mice might be more susceptible than males to impairments in spatial learning and memory in the water maze, as is seen in aged C57Bl/6 J wild-type mice [36].
We designed Experiment 3 as the penultimate evaluation of early pegvaliase treatment with comparisons to wild type PAH+/+ and saline-treated PAH−/− mice and to PAH−/− mice in which pegvaliase was discontinued during adulthood. We also treated them twice per week with pegvaliase reasoning that improved blood Phe stability would lead to improved outcomes. At the termination of the experiment, blood and brain amino acid contents including Phe contents were as expected for the genotype of the mice and the ongoing treatment in place at the time of euthanasia. CNS monoamine neurotransmitter concentrations were also as expected with complete correction of dopamine and serotonin deficiencies in PAH−/− who remained on pegvaliase treatment but with persistent abnormalities in those for which pegvaliase had been discontinued or in saline-treated PAH−/− mice. Body weight was again fully corrected in female pegvaliase-treated animals and nearly completely corrected in pegvaliase-treated males in comparison to saline-treated mice but brain weight remained low in all PAH−/− mice. Behavioral performance in the open field demonstrated increased activity levels in saline-treated and PHP PAH−/− mice, with normal activity in continuously pegvaliase treated PAH−/− mice. In the Morris water maze, pegvaliase treatment, either continuously or discontinued, enhanced cognitive performance during visible platform training and preserved cognitive performance during hidden platform training in male PAH−/− mice in comparison to saline-treated PAH−/− mice but mice in which pegvaliase had been discontinued performed worse in hidden platform trials. Female PAH−/− mice exhibited consistent cognitive deficits relative to PAH+/+ animals but with pegvaliase-treated mice performing better than saline-treated animals. Comparing the ability of PAH+/+ mice to locate the hidden platform in Experiment 1 with that in Experiments 2 and 3, the hidden platform learning curves of PAH+/+ mice were much better in Experiment 1. In Experiment 1, the mice received ten visible platform training sessions while in Experiments 2 and 3, the mice received only four visible platform training sessions. This indicates that the additional task learning PAH+/+ mice received in Experiment 1 might have contributed to their improved cognitive performance during hidden platform training. We recognize that PAH+/+ mice did not perform in any trial at a level expected from age-matched C57BL/6 J wild-type mice [36]. As PAH+/+ mice were co-reared littermates of PAH−/− mice resulting from Pahenu2/+ X Pahenu2/+ matings, it is conceivable that parental effects of the non-wild-type parent might have contributed to the relative poorer performance of PAH+/+ mice during hidden platform training in Experiments 2 and 3.
The pathophysiologic mechanisms that underlie the effects of HPA upon the brain and specifically behavioral and cognitive performance are incompletely understood but are likely multifactorial [8,37]. Monoamine neurotransmitter deficiencies, particularly of the serotonin axis, have been repeatedly demonstrated in both humans with PKU and in HPA animal models [8], as we have reported in our previous work [9, 20,21] and confirmed again in this study. Impairment of dopaminergic neural circuits in the prefrontal cortex, the so-called dopamine hypothesis, is a popular explanation for the deficits in working memory and inhibitory control that are displayed by some children with PKU treated with dietary Phe restriction but with blood Phe concentrations 3–5 times above normal [38]. However, our current results along with our prior efforts clearly demonstrate that Phe reducing treatments (dietary Phe restriction, liver-directed gene therapy, and now weekly pegvaliase treatment) begun during adulthood effectively correct CNS monoamine neurotransmitter deficiencies yet severe memory deficits persist in PAH−/− mice. Importantly, prior experiments with PAH−/− mice differ from contemporary human PKU treatment in that these animals are late-treated and their memory deficit could relate to irreversible neurologic damage suffered during a period of HPA and brain development prior to weaning. Now, in Experiment 3, we demonstrate a model of early treated PKU that nearly completely ameliorates the HPA phenotype associated with Pahenu2 mice. Discontinuation of pegvaliase treatment during adult allows reemergence of monoamine neurotransmitter deficiency, with the serotonin axis more severely affected than the dopamine axis. Cognitive deficits also recur but not as severely as in saline-treated PAH−/− mice. Male PHP mice continued to perform relatively well in visible platform trials although faltering somewhat with the hidden platform. Taken together, these results do not strongly support a clear pathophysiologic link between dopamine deficiency and learning and memory impairments in this early-treated mouse model.
We have suggested in a prior publication [9] that the most likely mechanisms underlying monoamine neurotransmitter deficiency in Pahenu2 mice involve a combination of constitutively low expression of TH and neuronal TPH2 in brain and Phe-mediated competitive inhibition of TH and TPH2 activities rather than brain Tyr and Trp deficiency. Our results here again demonstrate little correlation between the blood and brain concentrations of Tyr or Trp, and little correlation between serum or brain Tyr and dopamine or serum or brain Trp and serotonin. There is a modest correlation between serum or brain Phe and brain Tyr or Trp suggesting that Phe does slightly influence the brain content of Tyr and Trp. We suggest that compensatory adjustments to the activity of amino acid exporters at the blood-brain barrier are at least partly able to counter the effects of Phe-mediated competitive inhibition of Tyr and Trp import and work to maintain CNS amino acid homeostasis.
Brain dopamine and serotonin content was completely corrected in all mice receiving pegvaliase treatment at the time of euthanasia and consequently exhibiting low blood and brain Phe. Mild deficiency of HVA or 5-HIAA content persisted in some pegvaliase-treated mice, most often in female mice, suggesting continued mild impairment of flux through the monoamine neurotransmitter metabolic pathways. Constitutive TH and TPH2 expression may yet be decreased; we did not assess this possibility directly. Brain dopamine and serotonin contents most strongly correlated with both serum and brain Phe concentrations suggesting again that Phe-mediated competitive inhibition of TH and TPH2 activities are the greatest contributors to neurotransmitter deficiencies in HPA. If this hypothesis is true, then reduction of brain Phe, rather than manipulation of brain Tyr or Trp content, is necessary to substantially improve brain monoamine neurotransmitter content in HPA.
Pegvaliase treatment initiated during early infancy ameliorate most but not all manifestations of chronic HPA in Pahenu2/enu2 mice. The physical growth of female mice was completely corrected through continuous treatment with pegvaliase, but male mice remained slightly growth impaired. The reason for this sex difference is unknown. The growth of the brain remained conspicuously impaired in all PAH−/− mice regardless of treatment. Despite small brain size, behavioral and cognitive deficits were yet largely corrected in mice treated with pegvaliase from birth. Why precisely is the Pahenu2 mouse brain small? Limited neuropathologic examinations by other groups have documented normal neuron and microglial cell numbers. Increased phenylalanine in primary neuronal cell cultures has been shown to reduce synaptic density [39], but the opposite has actually been found in hyperphenylalaninemic Pahenu2/enu2 mice [40] with higher hippocampal synaptic density documented between 3 and 12 weeks of life. In our experiments here, we had planned to examine the frequency of dendritic branching and synaptic density within the hippocampus of select mice from each of the experimental groups in Experiment 3 through stereo-optic analysis of Golgi-Cox stained thick sections; however, in preliminary data (not shown), we did not replicate the findings of Horling, et al. [40] and detected no significant differences between PAH+/+ and PAH−/− brains. The most consistent neuropathologic abnormality in brains of hyperphenylalaninemic mice [41,42] and of untreated humans with PKU born prior to newborn screening [43–46] is impaired myelination. There is evidence that oligodendrocytes assume a non-myelinating phenotype under HPA conditions [41] and furthermore that phenylalanine may inhibit cerebral lipid synthesis through inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase activity [47]. We hypothesize that myelination may have continued to be impaired in PAH−/− mice in our experiments despite pegvaliase treatment, causing brain growth to remain reduced in comparison to PAH+/+ animals. We however did not histologically prepare brain tissue from our experimental groups with an appropriate method that would have allowed assessment of myelin status. The validity of our myelin hypothesis, and if it is true, why early pegvaliase treatment was insufficient to fully correct myelination will require further investigation.
In these experiments, we have for the first time demonstrated that the behavioral and cognitive phenotypes associated with HPA in Pah-enu2/enu2 mice can be prevented through use of a Phe-lowering treatment initiated shortly after birth and propose that repetitive application of pegvaliase enzyme substitution therapy to PAH−/− mice is a facile model of early-treated PKU in humans. The model will be useful to evaluate the effects of HPA at different time points in the course of murine brain development and to separate phenotypic outcomes related to the effects of HPA on early brain development vs. late effects on a previously well-treated brain. The model will also be useful in the continued study of the pathophysiologic connections between HPA and aberrant neuropathology. The most important observation from this project has been that significant cognitive deficits, particularly memory impairments, emerge in early-treated adult mice in whom pegvaliase therapy has been discontinued and chronic HPA has reoccurred. These results corroborate the abundant evidence of neuropsychiatric dysfunction in adults with chronic HPA. ‘Treatment for life’ to maintain low blood and brain Phe concentrations is absolutely necessary to achieve ideal neurocognitive and psychiatric outcomes in mice and humans with PKU.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 NS080866. COH would like to acknowledge the ongoing financial support of the National PKU Alliance (NPKUA). The authors thank BioMarin Pharmaceutical Inc. for the very generous contribution of pegvaliase for this project.
Abbreviations:
- HPA
hyperphenylalaninemia
- PKU
phenylketonuria
- PAH
phenylalanine hydroxylase
- Phe
L-phenylalanine
- Tyr
L-tyrosine
- Trp
L-tryptophan
- DA
dopamine
- HVA
homovanillic acid
- 5-HIAA
5-hydroxyindoleacetic acid
- TH
tyrosine hydroxylase
- TPH2
neuronal tryptophan hydroxylase
- PHP
pegvaliase-treated high Phe
- PLP
pegvaliase-treated low Phe
- CNS
central nervous system
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgme.2022.03.008.
References
- [1].Azen CG, Koch R, Friedman EG, Berlow S, Coldwell J, Krause W, Matalon R, McCabe E, O’Flynn M, Peterson R, Intellectual development in 12-year-old children treated for phenylketonuria, Am. J. Dis. Child 145 (1991) 35–39. [DOI] [PubMed] [Google Scholar]
- [2].VanZutphen KH, Packman W, Sporri L, Needham MC, Morgan C, Weisiger K, Packman S, Executive functioning in children and adolescents with phenylketonuria, Clin. Genet 72 (2007) 13–18. [DOI] [PubMed] [Google Scholar]
- [3].Christ S, Huijbregts S, de Sonneville L, White D, Executive function in early-treated phenylketonuria: profile and underlying mechanisms, Mol. Genet. Metab 99 (2010) S22–S32. [DOI] [PubMed] [Google Scholar]
- [4].Bilder DA, Burton BK, Coon H, Leviton L, Ashworth J, Lundy BD, Vespa H, Bakian AV, Longo N, Psychiatric symptoms in adults with phenylketonuria, Mol. Genet. Metab 108 (2013) 155–160. [DOI] [PubMed] [Google Scholar]
- [5].Thompson AJ, Smith I, Brenton D, Youl BD, Rylance G, Davidson DC, Kendall B, Lees AJ, Neurological deterioration in young adults with phenylketonuria, Lancet 336 (1990) 602–605. [DOI] [PubMed] [Google Scholar]
- [6].de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ, Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses, Mol. Genet. Metab 99 (2010) S86–S89. [DOI] [PubMed] [Google Scholar]
- [7].Feillet F, van Spronsen FJ, MacDonald A, Trefz FK, Demirkol M, Giovannini M, Belanger-Quintana A, Blau N, Challenges and pitfalls in the management of phenylketonuria, Pediatrics 126 (2010) 333–341. [DOI] [PubMed] [Google Scholar]
- [8].van Spronsen FJ, Blau N, Harding C, Burlina A, Longo N, Bosch AM, Phenylketonuria, Nat. Rev. Dis. Prim 7 (2021) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Winn SR, Scherer T, Thony B, Ying M, Martinez A, Weber S, Raber J, Harding CO, Blood phenylalanine reduction corrects CNS dopamine and serotonin deficiencies and partially improves behavioral performance in adult phenylketonuric mice, Mol. Genet. Metab 123 (2018) 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McDonald JD, Bode VC, Dove WF, Shedlovsky A, Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase, Proc. Natl. Acad. Sci. U. S. A 87 (1990) 1965–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McDonald JD, Charlton CK, Characterization of mutations at the mouse phenylalanine hydroxylase locus, Genomics 39 (1997) 402–405. [DOI] [PubMed] [Google Scholar]
- [12].McDonald JD, Postnatal growth in a mouse genetic model of classical phenylketonuria, Contemp. Top. Lab. Anim. Sci 39 (2000) 54–56. [PubMed] [Google Scholar]
- [13].McDonald JD, Dyer CA, Gailis L, Kirby ML, Cardiovascular defects among the progeny of mouse phenylketonuria females, Pediatr. Res 42 (1997) 103–107. [DOI] [PubMed] [Google Scholar]
- [14].Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A, Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation, J. Neurosci 19 (1999) 6175–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bruinenberg VM, van der Goot E, van Vliet D, de Groot MJ, Mazzola PN, Heiner-Fokkema MR, van Faassen M, van Spronsen FJ, van der Zee EA, The behavioral consequence of phenylketonuria in mice depends on the genetic background, Front. Behav. Neurosci 10 (2016) 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cabib S, Pascucci T, Ventura R, Romano V, Puglisi-Allegra S, The behavioral profile of severe mental retardation in a genetic mouse model of phenylketonuria, Behav. Genet 33 (2003) 301–310. [DOI] [PubMed] [Google Scholar]
- [17].Puglisi-Allegra S, Cabib S, Pascucci T, Ventura R, Cali F, Romano V, Dramatic brain aminergic deficit in a genetic mouse model of phenylketonuria, Neuroreport 11 (2000) 1361–1364. [DOI] [PubMed] [Google Scholar]
- [18].Pascucci T, Ventura R, Puglisi-Allegra S, Cabib S, Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria, Neuroreport 13 (2002) 2561–2564. [DOI] [PubMed] [Google Scholar]
- [19].Pascucci T, Andolina D, Ventura R, Puglisi-Allegra S, Cabib S, Reduced availability of brain amines during critical phases of postnatal development in a genetic mouse model of cognitive delay, Brain Res 1217 (2008) 232–238. [DOI] [PubMed] [Google Scholar]
- [20].Harding CO, Winn SR, Gibson KM, Arning E, Bottiglieri T, Grompe M, Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU), J. Inherit. Metab. Dis 37 (2014) 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Winn SR, Scherer T, Thony B, Harding CO, High dose sapropterin dihydrochloride therapy improves monoamine neurotransmitter turnover in murine phenylketonuria (PKU), Mol. Genet. Metab 117 (2016) 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ, Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria, J. Clin. Invest 103 (1999) 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ogawa S, Ichinose H, Effect of metals and phenylalanine on the activity of human tryptophan hydroxylase-2: comparison with that on tyrosine hydroxylase activity, Neurosci. Lett 401 (2006) 261–265. [DOI] [PubMed] [Google Scholar]
- [24].Harding CO, Amato RS, Stuy M, Longo N, Burton BK, Posner J, Weng HH, Merilainen M, Gu Z, Jiang J, Vockley J, Investigators P, Pegvaliase for the treatment of phenylketonuria: A pivotal, double-blind randomized discontinuation Phase 3 clinical trial, Mol. Genet. Metabol 124 (2018) 20–26. [DOI] [PubMed] [Google Scholar]
- [25].Thomas J, Levy H, Amato S, Vockley J, Zori R, Dimmock D, Harding CO, Bilder DA, Weng HH, Olbertz J, Merilainen M, Jiang J, Larimore K, Gupta S, Gu Z, Northrup H, Investigators P, Pegvaliase for the treatment of phenylketonuria: results of a long-term phase 3 clinical trial program (PRISM), Mol. Genet. Metabol 124 (2018) 27–38. [DOI] [PubMed] [Google Scholar]
- [26].Olsen RH, Johnson LA, Zuloaga DG, Limoli CL, Raber J, Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria, J. Neurochem 125 (2013) 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Benice TS, Raber J, Testosterone and dihydrotestosterone differentially improve cognition in aged female mice, Learn. Mem 16 (2009) 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Choleris E, Thomas AW, Kavaliers M, Prato FS, A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field, Neurosci. Biobehav. Rev 25 (2001) 235–260. [DOI] [PubMed] [Google Scholar]
- [29].Johnson LA, Zuloaga DG, Bidiman E, Marzulla T, Weber S, Wahbeh H, Raber J, ApoE2 exaggerates PTSD-related behavioral, cognitive, and neuroendocrine alterations, Neuropsychopharmacology 40 (2015) 2443–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elzaouk L, Leimbacher W, Turri M, Ledermann B, Burki K, Blau N, Thony B, Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in pts−/− mice rescued by feeding neurotransmitter precursors and H4-biopterin, J. Biol. Chem 278 (2003) 28303–28311. [DOI] [PubMed] [Google Scholar]
- [31].Blau N, Thony B, Renneberg A, Penzien JM, Hyland K, Hoffmann GF, Variant of dihydropteridine reductase deficiency without hyperphenylalaninaemia: effect of oral phenylalanine loading, J. Inherit. Metab. Dis 22 (1999) 216–220. [DOI] [PubMed] [Google Scholar]
- [32].Bell SM, Wendt DJ, Zhang Y, Taylor TW, Long S, Tsuruda L, Zhao B, Laipis P, Fitzpatrick PA, Formulation and PEGylation optimization of the therapeutic PEGylated phenylalanine ammonia lyase for the treatment of phenylketonuria, PLoS One 12 (2017), e0173269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sarkissian CN, Gamez A, Wang L, Charbonneau M, Fitzpatrick P, Lemontt JF, Zhao B, Vellard M, Bell SM, Henschell C, Lambert A, Tsuruda L, Stevens RC, Scriver CR, Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 20894–20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zeile WL, McCune HC, Musson DG, O’Donnell B, O’Neill CA, Tsuruda LS, Zori RT, Laipis PJ, Maternal phenylketonuria syndrome: studies in mice suggest a potential approach to a continuing problem, Pediatr. Res 83 (2018) 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Goldfinger M, Zeile WL, Corado CR, O’Neill CA, Tsuruda LS, Laipis PJ, Cooper JD, Partial rescue of neuropathology in the murine model of PKU following administration of recombinant phenylalanine ammonia lyase (pegvaliase), Mol. Genet. Metab 122 (2017) 33–35. [DOI] [PubMed] [Google Scholar]
- [36].Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J, Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity, Neuroscience 137 (2006) 413–423. [DOI] [PubMed] [Google Scholar]
- [37].Schuck PF, Malgarin F, Cararo JH, Cardoso F, Streck EL, Ferreira GC, Phenylketonuria pathophysiology: on the role of metabolic alterations, Aging Dis 6 (2015) 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Diamond A, Evidence for the importance of dopamine for prefrontal cortex functions early in life, Philos. Trans. R. Soc. Lond. B Biol. Sci 351 (1996) 1483–1493 discussion 1494. [DOI] [PubMed] [Google Scholar]
- [39].Horster F, Schwab MA, Sauer SW, Pietz J, Hoffmann GF, Okun JG, Kolker S, Kins S, Phenylalanine reduces synaptic density in mixed cortical cultures from mice, Pediatr. Res 59 (2006) 544–548. [DOI] [PubMed] [Google Scholar]
- [40].Horling K, Schlegel G, Schulz S, Vierk R, Ullrich K, Santer R, Rune GM, Hippocampal synaptic connectivity in phenylketonuria, Hum. Mol. Genet 24 (2015) 1007–1018. [DOI] [PubMed] [Google Scholar]
- [41].Dyer CA, Kendler A, Philibotte T, Gardiner P, Cruz J, Levy HL, Evidence for central nervous system glial cell plasticity in phenylketonuria, J. Neuropathol. Exp. Neurol 55 (1996) 795–814. [DOI] [PubMed] [Google Scholar]
- [42].Joseph B, Dyer CA, Relationship between myelin production and dopamine synthesis in the PKU mouse brain, J. Neurochem 86 (2003) 615–626. [DOI] [PubMed] [Google Scholar]
- [43].Alvord EC Jr., Stevenson LD, Vogel FS, Engle RL Jr., Neuropathological findings in phenyl-pyruvic oligophrenia (phenyl-ketonuria), J. Neuropathol. Exp. Neurol 9 (1950) 298–310. [DOI] [PubMed] [Google Scholar]
- [44].Malamud N, Neuropathology of phenylketonuria, J. Neuropathol. Exp. Neurol 25 (1966) 254–268. [DOI] [PubMed] [Google Scholar]
- [45].Bauman ML, Kemper TL, Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria, Acta Neuropathol 58 (1982) 55–63. [DOI] [PubMed] [Google Scholar]
- [46].Huttenlocher PR, The neuropathology of phenylketonuria: human and animal studies, Eur. J. Pediatr 159 (Suppl 2) (2000) S102–S106. [DOI] [PubMed] [Google Scholar]
- [47].Shefer S, Tint GS, Jean-Guillaume D, Daikhin E, Kendler A, Nguyen LB, Yudkoff M, Dyer CA, Is there a relationship between 3-hydroxy-3-methylglutaryl coenzyme a reductase activity and forebrain pathology in the PKU mouse? J. Neurosci. Res 61 (2000) 549–563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


