Abstract
Advancements in the development of nanomaterials have led to the creation of a plethora of functional constructs as drug delivery vehicles to address many dire medical needs. The emerging prodrug strategy provides an alternative solution to create nanomedicines of extreme simplicity by directly using the therapeutic agents as molecular building blocks. This review outlines different prodrug-based drug delivery systems, highlights the advantages of the prodrug strategy for therapeutic delivery, and demonstrates how combinations of different functionalities—such as stimuli responsiveness, targeting propensity, and multidrug conjugation—can be incorporated into designed prodrug delivery systems. Furthermore, we discuss the opportunities and challenges facing this rapidly growing field.
Keywords: prodrug, drug delivery, nanomedicine
A prodrug pathway to nanomedicines
The clinical application of small molecule drugs such as cancer chemotherapeutics is often limited by their poor solubility in aqueous environments, lack of delivery specificity, low therapeutic index, insufficient therapeutic efficacy, short circulation lifetime, and severe adverse effects [1,2]. Encapsulation of drugs within nanocarriers of well-defined size and shape has been demonstrated to increase the solubility of hydrophobic drugs, improve the pharmacokinetics of the drug with the promise of reducing adverse side effects, and promote targeting efficiency. From the perspective of fundamental design, nanoparticle-based delivery systems should be stable in circulation, can avoid nonspecific interactions with blood components and clearance by the reticuloendothelial system (RES), and selectively accumulate at the disease site[3]. For therapeutic agents with an intracellular target, this movement into surrounding tissues is followed by cellular uptake and consequent intracellular release of the cargo[4]. Once its function has been fulfilled, the carrier is considered waste/useless material that needs to be metabolized and eventually cleared. [3] While decades of extensive research in nanomedicine have led to the emergence of many nanocarrier-based drug delivery strategies, improvements are still needed to fine-tune and control the composition, morphology, and stability of drug-loaded nanoparticles to meet important medical needs. Particularly, ‘burst release’ and premature drug leakage from nanocarriers are critical challenges that need to be addressed as they often lead to increased toxicities and damages to healthy organs[5].
The self-assembling prodrug approach, which integrates the delivery construct and the therapeutic agent within one chemical substance, providing an emerging platform to improve upon the shortcomings associated with traditional nanomaterial-based delivery systems. Prodrugs are pharmacologically inactive (or less active) compounds that undergo a chemical or enzymatic transformation in vivo to restore the active parent drug. The primary purpose of the pro-moiety is to improve the pharmacokinetic properties of the conjugated therapeutic to achieve longer circulation time, reduced systemic toxicity, enhanced targeting specificity and efficiency, and better-controlled drug release profile [6]. For self-assembling prodrug design, the pro-moiety also plays a critical role in promoting its self-assembly into discrete nanostructures. The resultant prodrug nanostructures eliminate the need for extraneous materials without sacrificing the functional benefits of employing a carrier system. Similarly, this self-assembling prodrug approach can increase the solubility of hydrophobic drugs, decrease chemical instability, reduce toxicity, minimize premature degradation, and address issues like leakage of encapsulated therapeutics and the removal of synthetic carrier. Here, we outline basic design approaches in prodrug-based nanomedicines to control the release of therapeutics through nanoscale drug carrier assemblies and offer examples of materials employed. Since there is substantial knowledge of how prodrug strategies contribute to improved pharmacokinetic, targeting properties, and therapeutic efficacy[7–10], we highlight here the design consideration and the influence of morphology and stability of nanostructures, with a focus on some most recent examples including polymeric prodrugs, small molecule prodrugs, and peptide drug conjugates.
Prodrug linkage and release kinetics: design considerations
Prodrugs are typically designed to be able to dynamically respond to their environment; these molecules display low and often negligible pharmacological activity while in their prodrug form but undergo rapid conversion into biologically active molecules once metabolized [6,11]. The parent drug and pro-moiety are covalently joined via chemically or enzymatically labile linkers, which control the release pathway of the pharmacologically active drug from its pro-moiety conjugation state. Therefore, the linker design determines the chemical and enzymatic conditions in which the prodrug undergoes conversion to restore its functions. An ideal linker controls the kinetic release rate of the pro-moiety-drug complex, enhances the parent drug’s solubility, remains chemically stable across a pH range, reduces toxic side effects, and improves drug-targeting propensity[6].
As a synthetic handle between the active drug and the pro-moiety, the linker design is dictated by the parent drug’s available functional groups [12]. To construct a prodrug, the active component must contain a functional group that can form a chemical bond with the chosen auxiliary moiety. Furthermore, the by-products of the linkage should undergo safe degradation and excretion without exerting any potential toxicity. Commonly used functional groups of parent drugs and linkers are listed in Figure 1. Esters are the most frequently used linkers because the functional groups involved (usually hydroxyl and carboxylic acid) are present in most parent drugs and functional pro-moieties [13]. Moreover, ester prodrugs can often be synthesized through straightforward organic reactions such as Fischer esterification, acid chloride alcoholysis with acid anhydrides, or Stegliche esterification. Once administered, the ester bond is readily hydrolyzed by esterases that are ubiquitously expressed throughout the body, causing the parent drug to be released [14]. Different types of ester bonds can exhibit different stabilities and release profiles in vivo. Although ester bonds are a feasible prodrug linkage strategy, a significant challenge that remains for ester prodrugs is accurately predicting their pharmacokinetics in humans, as there are considerable differences in specific carboxylesterase activities between preclinical models [10]. These non-specific linkages allow for some tunability of therapeutic release rate but no specificity for local in vivo drug release because of the ubiquitous nature of esterases.
Figure 1.
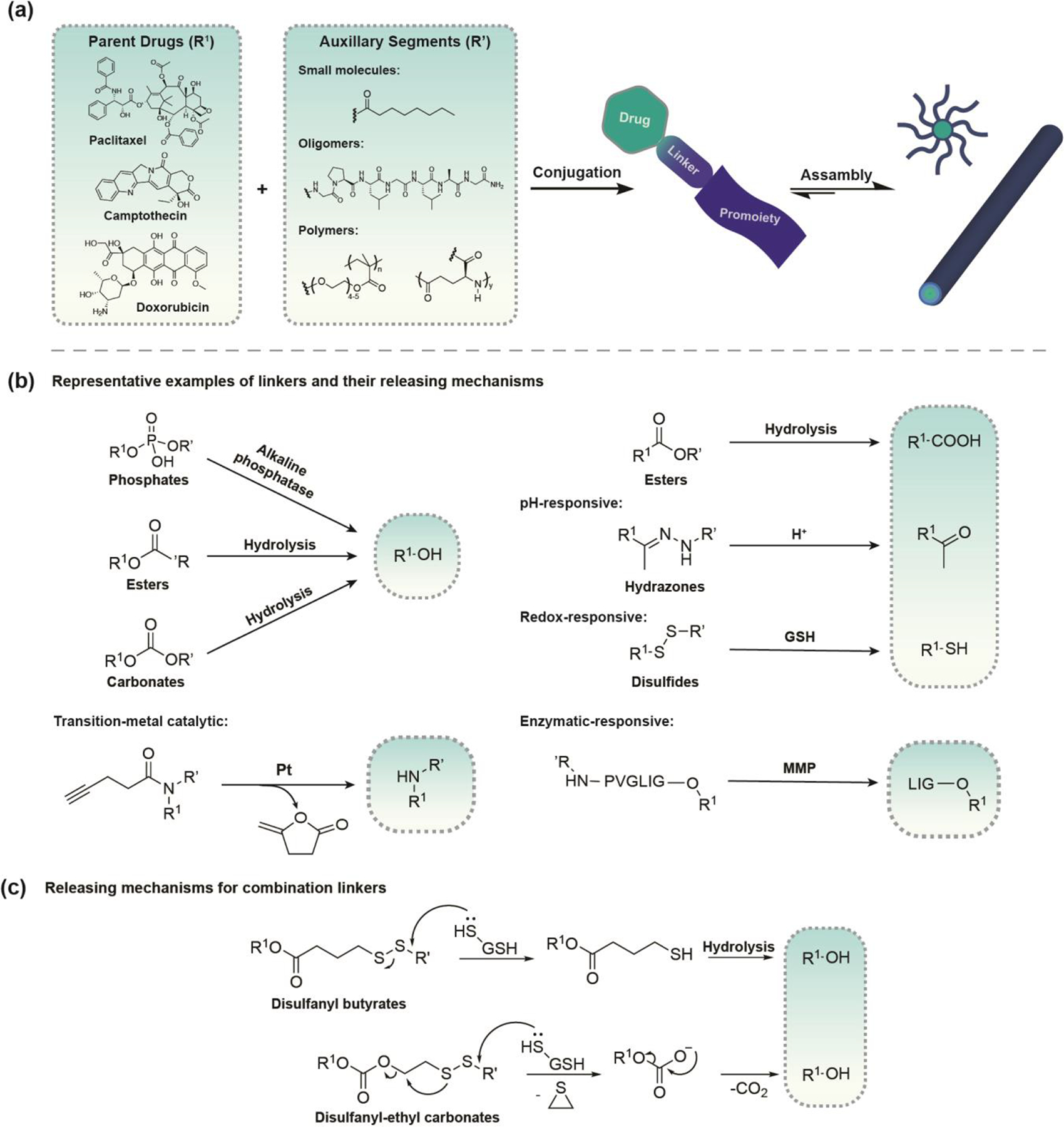
An overview highlighting the design principles for prodrug-based nanomedicine. (a) Schematic of Molecular-graphics illustration of nanostructures formed from the prodrug strategy. Most prodrug strategies require a functional group on the parent drug. The selected prodrug approach is dictated by the liability of the drug that needs to be overcome by the prodrug strategy. (b) Linkage strategies for the most common functional groups found on parent drugs and representative stimuli-responsive linkers. Hydrazone conjugates can be converted to the active drug form in acidic environments. Dithiol linkages are sensitive to redox potential and easily cleaved in the presence of GSH. PVGLIG oligopeptide is selectively cleaved by matrix metalloproteinases (MMPs). (c) Release mechanisms for combination linkers. The choice of degradable linker and release mechanism of free drug from the prodrug can have a significant impact on the drug’s efficacy.
Some linker designs can introduce greater site-selectivity due to their responsiveness to a particular localized trigger/stimulus, thus reducing off-target effects. To improve selectivity, these linkers often require a more involved degradation mechanism[15]. Examples of acid-sensitive and redox-sensitive linkers and their associated release mechanisms are shown in Figure 1(b). Acid-sensitive linkers rely on pH differences between acidic lysosomes/endosomes and the extracellular/cytosolic environment to trigger a faster hydrolysis rate and drug release following cellular uptake, providing a mechanism to selectively direct intracellular therapeutic release and prevent systemic active drug circulation [16]. The imine and hydrazone functionalities are the most common acid-sensitive linkers, which are formed through the conjugation of a carbonyl to an amine or hydrazide, respectively [17–19]. Redox-sensitive linkers provide an effective strategy for the selective release of therapeutics upon exposure to the redox active agents found in different cellular environments. Notably, disulfide bonds utilize substantial differences between intra- and extracellular glutathione (GSH) levels to create prodrug carriers that are stable during circulation but undergo rapid reduction following internalization for intracellular release [17,19–22]. Enzyme labile linkages are also attractive in peptide-based prodrugs because many enzymatic reactions target specific peptide sequences [20,23]. For example, cleavable dipeptide linkers valine-citrulline (Val-Cit) and phenylalanine-lysine (Phe-Lys) are selectively cleaved by lysosomal proteases, like Cathepsin B, which are over-expressed in cancer cells [24]. Numerous peptide-based assemblies that are utilized as drug carriers or probes have been designed to display either direct cleavage of the cargo or disassembly of the material in response to enzymatic triggers [25]. The combination of different drug linkers offers possibilities to expand the scope of conjugation chemistry and provide tunable functionality that can significantly impact the release mechanism of free drugs from assembled nanostructures. For example, reducible disulfanyl butyrate (buSS) linked prodrugs, displayed a retarded release of free drugs due to the nanostructure-promoted formation of inactive disulfide dimers (Figure 1(c)). In contrast, the carbonate analogue undergoes rapid self-immolation upon reduction without dimerization, significantly improving the conjugated drug’s activity [26]. The choice of linkers can have a significant impact on the release mechanism of free drugs from assembled nanostructures, as well as alter the prodrug system’s morphology.
Morphology and stability of drug conjugated nanostructures
In the context of drug conjugate-based nanostructures, the morphology usually refers to the shape and size of the obtained nanostructure; and the nanostructure is influenced by both the parent drug and promoeity [16–18]. The parent drug’s chemical structure affects assembled nanostructure characteristics, particularly by impacting the system’s overall balance between hydrophobicity and hydrophilicity. Changes in the pro-moiety conformation can alter where the drug is located within the structure, leading to changes in protection and/or nanostructure dimensions due to changes in therapeutic location, accessibility, and molecular packing dynamics [27,28].
The shape, particle size, and aspect ratio of nanostructured systems impact pharmacokinetic properties and have been definitively linked to cellular uptake [19,29,30]. Despite the complexity of nanostructure responses in a biological system, there are some general trends for how size and geometry affect the behavior of nanoparticle drug delivery systems. For spherical nanostructures, smaller particles can be internalized through multiple distinct mechanisms and have better tumor penetration than larger nanoparticles, whereas larger nanoparticles have greater accumulation within the tumor site[31,32]. An optimal size is needed to provide cooperative effects of tumor accumulation and penetration, as well as cell internalization and clearance[33]. Shape and size also play an important role in biodistribution as nanoparticles are cleared through the liver and spleen. Small micelles (i.e. 35 nm to 100 nm) and short rods tend to accumulate mainly in the liver; whereas larger particles and long rods are more likely to be trapped in the spleen [34,35]. High aspect ratio rods, discoidal nanoparticles, and nanofibers exhibit slower clearance from the blood than spheres, resulting in prolonged circulation time and improved accumulation in target sites [36,37].
Due to their well-defined supramolecular morphology, the pharmacological outcome of prodrug nanocarriers depends on their nanostructure stability. Stable nanostructures result in reduced clearance, consequently improving drug accumulation within the therapeutic site of action. However, less stable prodrugs display a more rapid renal clearance (as monomers), leading to decreased accumulation in major organs thus reducing off-site toxicity. Studies have shown that critical micelle concentration (CMC), the threshold value at which supramolecular nanostructures can exist in stable assemblies, is an important determinant of the therapeutic efficacy and systemic toxicity of self-assembling prodrugs in vivo. A series of self-assembling prodrugs consisting of two camptothecin-disulfanyl-ethyl carbonate (etcSS) prodrugs conjugated to oligoethylene-glycol (OEG) moieties (Figure 2) demonstrated that prodrug designs with lower CMCs simultaneously elicited improved anti-tumor efficacy but also increased toxicity. [38] The conflicting nature of this stability trend requires that the CMC be optimized to find the appropriate balance between efficacy and toxicity.
Figure 2.
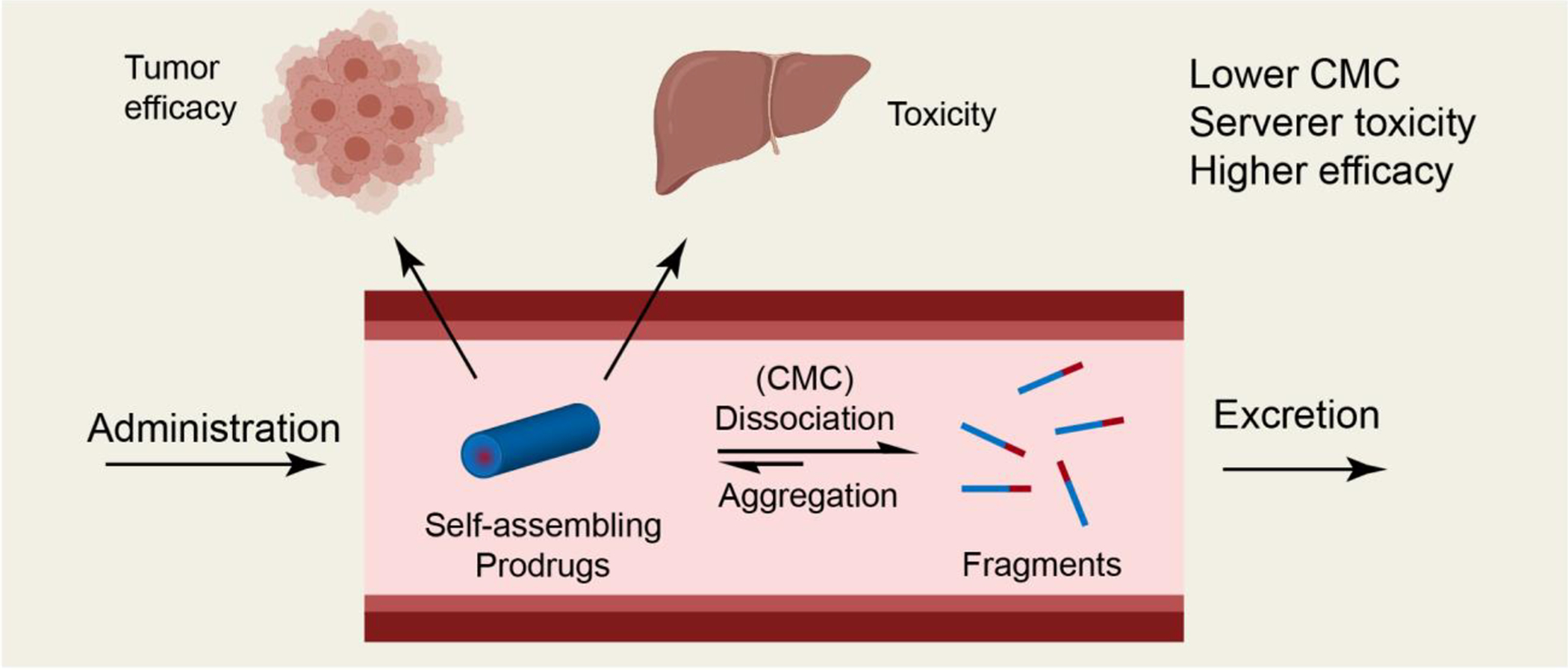
The fundamental role of CMC in the in vivo efficacy and systemic toxicity of self-assembling prodrugs (SAPDs). Illustration of the circulation fate of a SAPD after entering into the circulation, indicates that the lower the CMC of a SP, the lower the percentage of fragments and monomers, leading to reduced excretion and enhanced tumor (improved efficacy) and healthy organ uptake (increased toxicity). Adapted from [38].
Prodrug-based nanomedicine
1. Polymeric prodrugs
Synthetic or natural polymers may be used as the prodrug pro-moiety following chemical modification; and block copolymers are often employed due to their high propensity for self-assembly. Polymeric nanocarriers display significant delivery advantages due to their enhanced aqueous solubility, capacity to protect the therapeutic agents within their core (preventing degradation that could otherwise occur in the surrounding environments), stable circulation in the bloodstream, and improved biodistribution [39,40]. Several polymeric nanocarriers are now in various stages of pre-clinical and clinical development. Despite the rapid progress in this field, several obstacles currently hinder the clinical translation of polymeric prodrug delivery systems, regardless of preclinical validation. Specific challenges include the lack of precise control over carrier structure, batch‐to‐batch variations in drug loading, and poor control over molecular weight and polydispersity [41,42]. Loading drug efficiency is often sacrificed to achieve more specific and effective drug delivery [43]. A systems engineering approach is necessary to address these diverse challenges and create rationally designed prodrug delivery systems that can achieve clinical translation through successful integration of the drug, targeting moiety, and delivery vehicle.
Synergistic programmed release systems
One strategy to improve chemotherapeutic site selectivity is to employ the stimuli responsive property of polymeric conjugates to attain controlled drug release, thereby enhancing bioavailability at the target site and reducing off-target effects. A variety of internal stimuli (temperature, pH, enzyme activities, ROS, host-guest interactions) [14, 29–33] and external stimuli (heat, light, magnetic, and ultrasound) have been used in conjunction with nanoformulated polymeric prodrugs to selectively trigger therapeutic release [39–45]. The ability to incorporate multiple therapeutic functionalities within one drug delivery system is one of the most exciting features of polymeric nanoparticles as a drug delivery platform. Due to the complexity and multiplicity of conjugation sites displayed on polymeric nanoparticles, polymeric nanocarriers allow for the incorporation of various imaging and therapeutic agents in the system, as well as a combination of stimuli responsive and targeting agents [45–48]. By employing stimuli responsive linkages, including MMP-degradable peptides, acid-labile groups, and GSH-cleavable disulfide bonds, tunable and selective control of prodrug activation can be achieved (Figure 3(a)). The interactions between and synergistic effects of the two drugs incorporated within prodrug system can selectively respond to immune modulators through input signal combinations like MMP-2/9, pH of the environment, or glutathione reduction in the tumor microenvironment. By selectively responding to different stimuli, this prodrug nanoparticle system is expected to overcome drug resistance, provide enhanced anti-tumor efficacy, and eradicate cancer stem cells[49].
Figure 3.
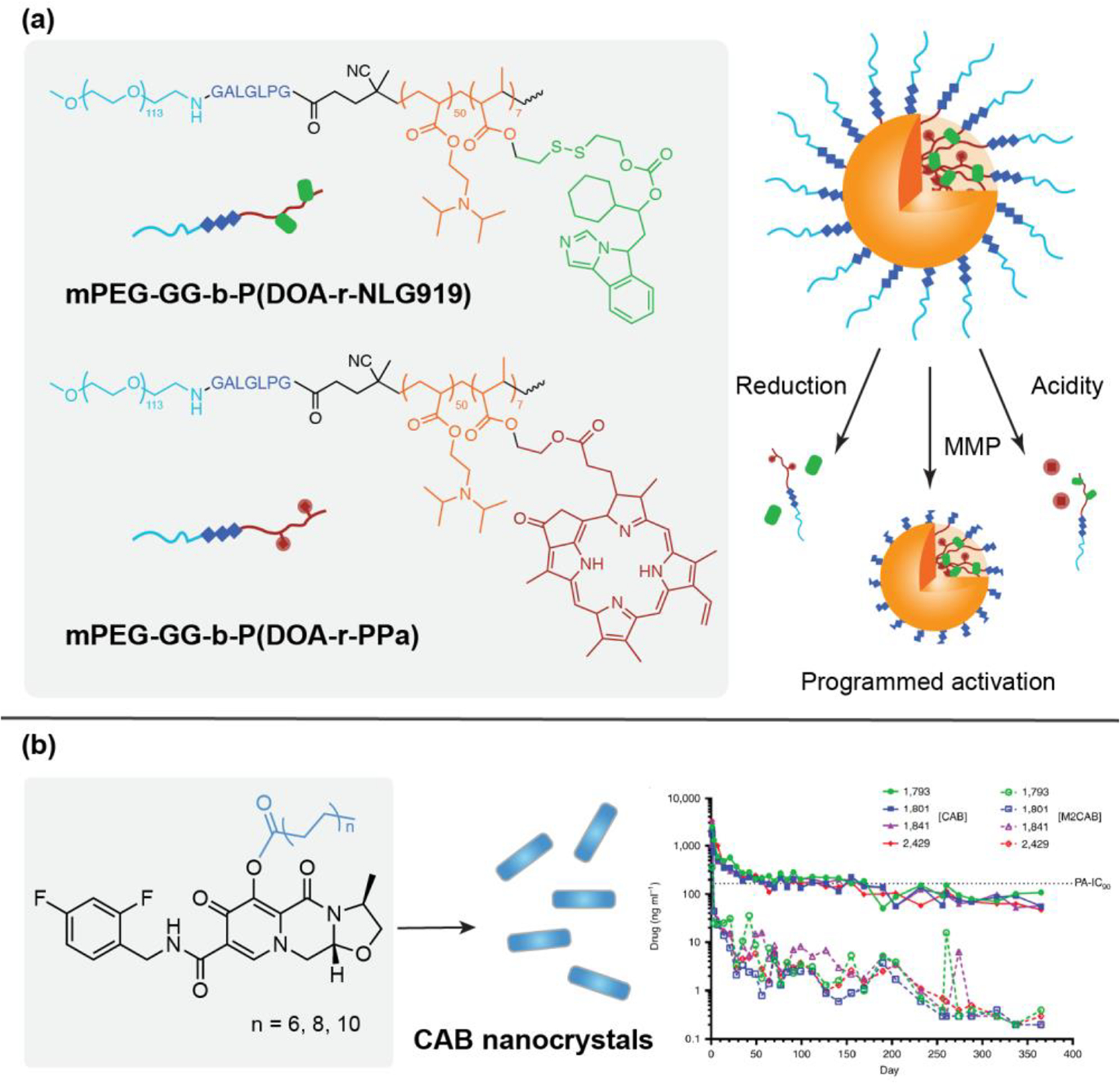
Recent examples of polymeric and small-molecule prodrugs in nanomedicine. (a) Programmed multidrug delivery by supramolecular polymeric drug conjugates. BLPNs prodrugs include an MMP-2/9-degradable GALGLPG peptide sequence, an acid-activatable tertiary amine group, and a GSH-cleavable disulfide linker. With displayed orthogonal response to MMP-2 microenvironment and intracellular acid/reduction microenvironment at the tumor sites, the BLPNs prodrugs enabled codelivery of dual immune modulators and combination immunotherapy. Adapted from [49]; copyright 2020 Wiley. (b) Cabotegravir (CAB) prodrugs modified with fatty acid esters were formulated into nanocrystals. The nanoformulated CAB prodrugs showed a year-long extended release while maintaining plasma CAB concentration above the protein-adjusted 90% inhibitory concentration (PA-IC90). Adapted from [70]; copyright 2020 Springer Nature.
Multidrug loading
Combination drug therapy is used as a first-line treatment for various diseases. It is possible to prevent multidrug resistance (MDR) through the induction of a synergistic effect by using multiple drugs with distinct mechanisms of action, thereby enhancing therapeutic efficacy [50]. The pharmacokinetics of nanocarrier conjugated drugs are determined by the physicochemical properties of the nanostructure. Consequently, it can be simpler to create and optimize a multidrug delivery system than it is to tune the behavior of individual drugs in a combination therapy [51–55]. Furthermore, the cooperative interactions between drugs can stabilize the nanoparticles and facilitate intracellular drug release. In polymeric drug conjugates, the three most common approaches to form a multidrug delivery system are: 1) create a nanocarrier assembly from several different polymeric prodrugs that contain distinct conjugated drugs, 2) conjugation of multiple therapeutics to one polymeric delivery pro-moiety, 3) encapsulation of a second drug, which is usually hydrophobic, within the nanostructure [56,57].
2. Small molecule prodrugs
Despite the successes of polymeric prodrugs, these nanocarriers have several disadvantages like high variability in the composition of their formulated nanostructures, poor batch-to-batch consistency, and low drug loading efficiencies[39]. Small molecule-based prodrugs have several advantages over their polymeric counterparts due to the precisely defined chemical structure of these smaller molecules, including increased drug loading efficiency, improved cell permeability, and less extraneous material to remove following parent drug release. [50] However, to induce the formation of nanostructures from small molecule prodrugs, chemical modifications are often required to moderate their hydrophilic nature [58]. In some cases, surfactants or other stabilizing agents are also necessary for the formation of nanostructures. For small molecule prodrugs, possible pro-moieties include drugs, small peptides, and other functional small molecules [59–62]. For example, squalene conjugated anticancer drugs interact with lipoproteins (LPs) for indirect targeting of cancer cells with high LP receptor expression both in vitro and in vivo [30,63,64].
A variety of nanoprecipitation-formed drug-conjugate nanostructures have been used to deliver both hydrophobic and hydrophilic drugs. A distinctive feature of nanosuspensions is their high drug loading due to their almost pure drug core. Not all therapeutics are suitable for formulation into nanosuspensions because the process requires highly hydrophobic compounds and the nanosuspensions are not easily modifiable with targeting sequences. Thus, it is sometimes necessary to create prodrugs that are capable of nanosuspension formulation through conjugation of hydrophobic functional groups to mask hydrophilicity or to attach targeting moieties for improved efficacy. One application for this prodrug strategy is the long-term delivery of HIV antiretroviral (ARV) agents to improve patient adherence and therapeutic efficacy [65]. Long-acting ARV prodrug libraries often employ a series of hydrophobic fatty ester conjugations for the optimization of prodrug hydrolysis and release rates to attain sustained therapeutic levels in plasma and tissues [66–69]. A nanoformulation of cabotegravir (CAB) conjugated to a carbon chain (NM2CAB) and coated with surfactant produced a drug delivery system with a ‘once-a-year’ injectable profile (Figure 3(b)) and immune cell targeting propensity. In addition to the improved antiviral efficacy of NM2CAB, the long acting formulation could possibly serve as a vaccine mimetic (with respect to its administration profile), in the absence of vaccines against HIV, if dosing intervals are further extended [70].
One of the major disadvantages of delivery systems formed via nanoprecipitation is that most synthesis techniques provide limited control over the size and morphology of the nanostructures. This lack of tunability of morphology and physicochemical properties make it difficult to modify these systems in order to overcome diverse biological barriers. For many nanosuspensions, the initial amphiphilicity of small molecule prodrugs does not play a central role in aggregation. Without the appropriate processing or external inducements, such as nanoprecipitation, the designed small molecule prodrugs may either be too hydrophobic or too hydrophilic for spontaneous assembly into nanostructures in an aqueous phase [71].
3. Biomolecule drug conjugates
Due to the hydrophilicity of the selected peptides and their propensity for beta-sheet hydrogen bonding, peptide drug conjugates that incorporate hydrophobic therapeutics will often exhibit sufficient amphiphilicity for spontaneous assembly into well-defined one-dimensional nanostructures in aqueous environments, without additional surfactants or physical processing techniques[57,72,73]. The integration of peptides in the prodrug approach introduces vast functionality potential, as altering certain amino acids in the peptide sequence can control the physicochemical properties of the prodrug. For example, the covalent attachment of two camptothecin (CPT) molecules to hydrophilic peptides with various surface promoieties (nonionic, cationic, anionic, and zwitterionic) results in a class of CPT prodrugs that undergo tubular assembly in water. The functional nanostructures formed by these prodrugs significantly improved the treatment outcomes in mouse tumor models and reduced systemic toxicity compared to free CPT. Moreover, the peptide design allows for incorporation of biologically active peptide sequences to integrate functionalities such as cell penetration, tumor targeting (through the targeting of a particular receptor on the tumor cell surface), or tissue penetration and the peptide can also act as a conjugation site to expand the number of functionalities[74].
Due to the one-dimensionality of the peptide prodrug nanostructures, prodrug solutions can entangle in response to salt-screening to form hydrogels in situ upon injection. The filament-based hydrogels formed by peptide drug conjugates maintain a linear release profile due to preservation of a constant monomer concentration in the hydrogel (at the CMC), and thereby can act as a sustainable delivery platform for local drug administration [75,76]. As therapeutics are covalently incorporated as components of the conjugate, the hydrogels formed display fixed drug loading with homogeneous distribution of drugs within the gel. One example of this supramolecular hydrogelator strategy is a series of prodrugs consisting of peptide conjugated paclitaxel (PTX), which self-assemble into one-dimensional nanostructures in aqueous conditions and display varying critical gelation concentrations depending on their sequence design [77]. Furthermore, the prodrug-based hydrogels demonstrated responsiveness to the tumor microenvironment for controlled release kinetics and improved therapeutic efficacy [77]. In another example, conjugation of a hydrophilic tissue-penetrating peptide to CPT through a matrix metalloproteinase 2 (MMP-2) responsive linker results in a peptide prodrug that assembles into filamentous structures that can be combined with immune checkpoint blockers such as anti-PD1 antibody (aPD1) (Figure 4(a)). The aPD1 loaded prodrug solution forms an enzyme-responsive hydrogel in situ and serves as a two-component system for enhanced chemoimmuno combinational therapy [75,78].
Figure 4.
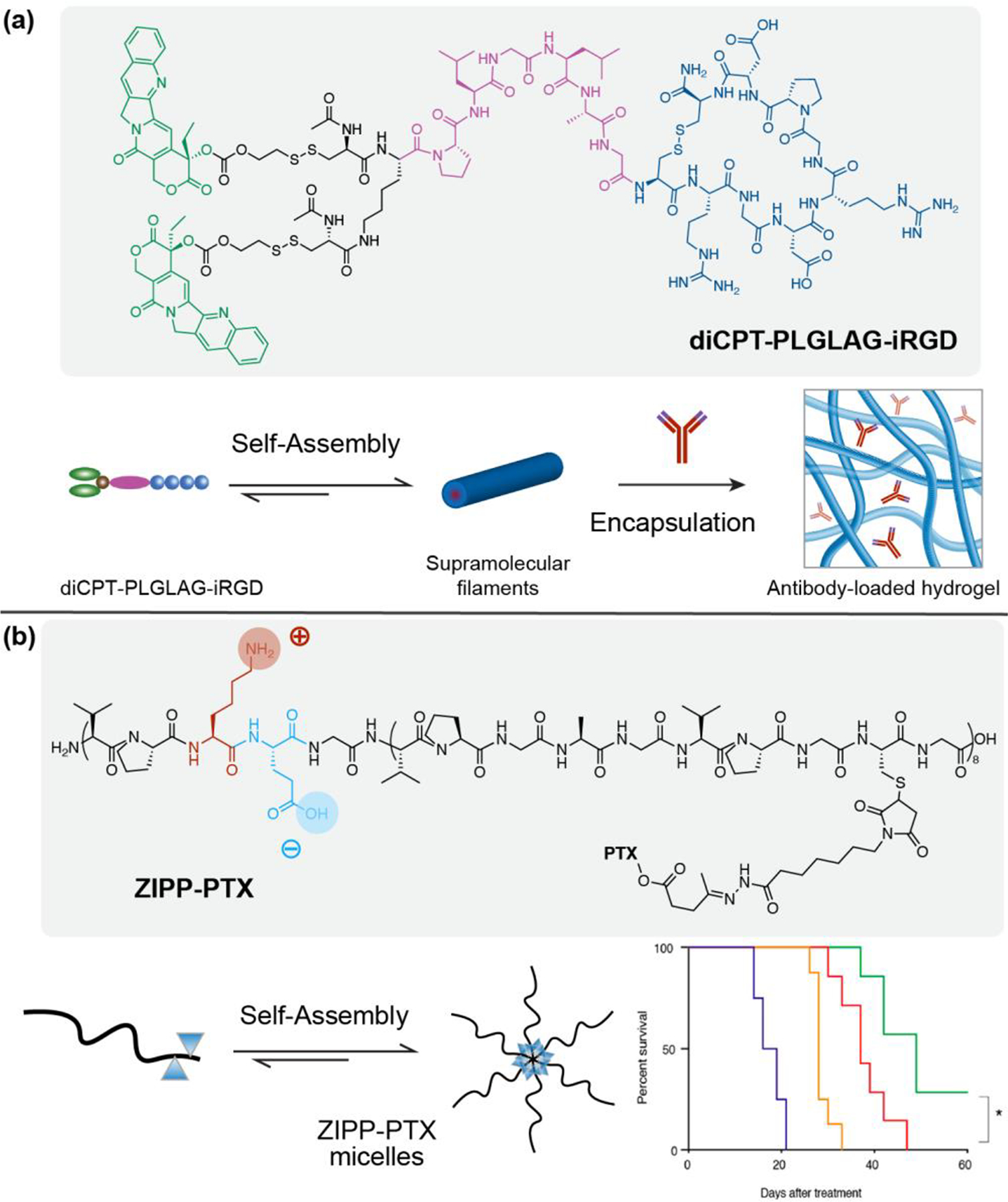
Peptide-based self-assembling prodrugs for enhanced therapeutic. (a) The in situ formed supramolecular hydrogel made of diCPT-PLGLAG-iRGD nanofilaments locally delivered camptothecin and aPD1 as an immune booster for immune checkpoint blockade therapy. Adapted from [78]. (b) Illustration of the spherical nanostructures formed by the self-assembling zwitterionic polypeptide (ZIPP) Paclitaxel conjugates. The ZIPP-PTX nanoparticles increased circulation time and outperformed Abraxane in multiple tumor models. Adapted from [83]; 2020 American Chemical Society.
Artificial biopolymers, such as polypeptides, amino acids, polynucleic acids, and polysaccharides, provide a promising pro-moiety platform for drug delivery systems due to their biocompatibility and customizable architecture [79]. Of these materials, amino acid-based biopolymers have demonstrated the greatest usefulness in drug delivery. For example, polypeptides based on elastin-like polypeptides (ELPs) exhibit unique thermoresponsive behavior associated with a conformational change of the peptide, which results in in situ gelation at body temperature [80,81]. A major advantage of ELP drug delivery systems is their capacity for adaptive variation, which provides a strategy to prolong drug circulation in vivo through modification of the nanostructure surface with stealth coatings [82,83]. Drug conjugated, zwitterionic, ELP derived polypeptides that contain two repeating opposite charges in the polypeptide chain exhibit stealth behavior, increasing the half-life and pharmacological efficacy of the carrier system (Figure 4(b)). In another example of the modified stealth properties that can be imparted to ELP nanoparticles, an albumin-binding ELP peptide-paclitaxel nanoparticle demonstrated a significantly longer half-life in plasma due to its high binding affinity with albumin. The binding with albumin also increased the bioavailability of the ELP nanoparticles in vivo and decreased uptake by the RES system, liver, and spleen to reduce the toxicity [83–85]. Aptamer-prodrug conjugate (ApPdC) micelles that dynamically respond to the tumor microenvironment are another example of a novel artificial biopolymer platform. ApPdCs incorporate hydrophobic prodrugs through a self-assembled, tumor-recognizing aptamer complex and generate toxic free radicals when engulfed by cancer cells [80]. Using AS1411 as a model aptamer loaded with Fe2+-responsive tetraoxane prodrug, the ApPdC micelle was activated via the reduction of loaded hemin, generating toxic free radicals that decreased HepG2 liver cancer cell proliferation through glutathione depletion. The use of aptamer carriers for prodrugs allows for innovative chemodynamic therapies that result in improved targeting of the tumor microenvironment due to the small size, biocompatibility, and high affinity of aptamers for their molecular targets.
Concluding Remarks
This review covered recent progress in the development of prodrug nanotherapeutic systems. The prodrug approach stems from the concept of achieving all the essential features of a nanomedicine by using one chemical compound. As a result of the conjugate design and its subsequent assembly under aqueous conditions, each component of the prodrug, including both the carrier moiety and the therapeutic segment, can potentially impact its pharmacokinetic profile and the eventual therapeutic outcomes. This single-molecular nanomedicine affords advantages in terms of versatility in platform design, simplicity in drug formulation, tunability in size and morphology, and enhancement in chemical stability and release control.
While several prodrug delivery systems are currently being investigated in advanced clinical trials [27, 31, 72], whether this strategy will bear fruit remains to be seen, as several issues may persist for the successful clinical translation of prodrug nanomedicines. For instance, potential risks with regard to the safety of certain pro-moieties, or the prodrug itself, should be carefully considered in the prodrug design and thoroughly explored experimentally (see Outstanding Questions). As a therapeutic construct that benefits from its nanoscale morphology, the toxicity of drug conjugates should be assessed not just by its chemical constituents, but also by its supramolecular constructs that may pose some risks for undesired physical or mechanical interactions with healthy cells. Therefore, toxicology of each prodrug nanomedicine should be carefully evaluated on a case-by-case basis. In addition, since prodrugs need to be converted into their bioactive form, undesired metabolites that could be generated in the conversion process should be identified and their potential pharmaceutical and toxicity profiles must be carefully evaluated. A reliable prodrug manufacturing in a cost-effective manner is also needed to facilitate its clinical translation. We are optimistic for the future application of prodrug nanoparticles in numerous biomedical settings, as new prodrug designs continue to improve over the past few years, and more and more preclinical studies are being conducted to reveal further the promising potential of prodrug nanomedicines.
Outstanding Questions.
What are the unique advantages of utilizing drugs to create its own nanoparticles for self-delivery?
Can we use the prodrug design platform to achieve all the essential features offered by traditional carrier-based nanomedicines such as long-circulation, tumor targeting, and controlled therapeutic release?
How does the prodrug get converted to its parent drug in the disease sites? Will there be a concern for premature release or delayed conversion?
Does the stability of prodrug-based supramolecular nanomedicine contribute positively or negatively to their therapeutic efficacy?
Will the simplicity in supramolecular nanomedicine design accelerate their clinical translation once they prove safe and effective in trials?
Table 1.
Characteristic features of self-assembling prodrugs versus carrier-based nanomedicines.
| Characteristics | Self-Assembling Prodrugs | Carrier-Based Nanomedicines |
|---|---|---|
| Carriers | no carriers/self-delivery | Well-defined nanoparticles |
| Formulation | Molecular self-assembly | Physical encapsulation |
| Drug loading capacity | 100% prodrug loading | Vary from system to system, typically less than 5% |
| Physiochemical properties | Defined by the prodrug molecular design | Defined by the nanoparticle carrier |
| Release mechanism | Linker cleavage through enzymes, hydrolysis or other metabolism pathways | Nanoparticle breakdown and drug diffusion |
| Excretion | Drug metabolism | Nanoparticle breakdown and chemical degradation |
Highlights.
The development of self-assembling prodrugs represents an emerging drug delivery strategy that leverages the assembly potential of therapeutic agents to construct a wide variety of supramolecular nanomedicines.
Therapeutic release can be controlled by the chemical stability of the prodrug through the linker design, and also by the supramolecular stability of the nanomedicine that is determined by its critical assembly concentration.
Biological epitopes for tumor targeting, specific cell recognition, or intracellular trafficking, can be incorporated as part of the prodrug that contributes to its pharmacokinetic and pharmacodynamic profiles.
Prodrug nanoparticles and hydrogels can be used to deliver other therapeutic agents for combination chemotherapy or immunotherapy.
Acknowledgment
We acknowledge financial support from the Johns Hopkins Center for AIDS Research (JHU CFAR) under NIH/NIAID P30AI094189. CF is supported by the Long Acting/ Extended Release Antiretroviral Research Resource Program, R24AI118397 from NIH/NIAID.
Glossary
- Antiretroviral (ARV)
a class of drugs used to suppress retrovirus replication. Combination antiretroviral therapy is the primary treatment for HIV infection
- Critical Micelle Concentration (CMC)
the concentration at which surfactant molecules start to aggregate into micelles. CMC is important to evaluate the stability of a micelle and indicates the propensity of a micellar system to aggregate or dissociate
- Glutathione (GSH)
A tripeptide comprised of glutamic acid, cysteine, and glycine. Glutathione is abundant in mammalian cells and acts as an antioxidant and a free radical scavenger
- Prodrug
a compound with little or no pharmacological activity which can be converted to produce the active parent drug in the body
- Self-assembly
a process in which an ordered structure spontaneously forms from molecular building blocks. Self-assembly is guided by the interactions between individual components in order to minimize the system free energy
- Zwitterionic
a type of molecule that contains an equal number of repeating opposite charges. The net charge of the entire zwitterionic molecule is zero
- Aptamer
a short, single-stranded oligonucleotide that can bind to specific molecular targets including proteins, peptides, small molecules, or cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- 1.Webber MJ and Langer R (2017) Drug delivery by supramolecular design. Chem Soc Rev 46, 6600–6620. 10.1039/c7cs00391a [DOI] [PubMed] [Google Scholar]
- 2.Tran S et al. (2017) Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Medicine 6, 44. 10.1186/s40169-017-0175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lazaro I and Mooney DJ (2021) Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat Mater. 10.1038/s41563-021-01047-7 [DOI] [PubMed] [Google Scholar]
- 4.Blanco E et al. (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33, 941–951. 10.1038/nbt.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller T et al. (2013) Premature drug release of polymeric micelles and its effects on tumor targeting. Int J Pharmaceut 445, 117–124. 10.1016/j.ijpharm.2013.01.059 [DOI] [PubMed] [Google Scholar]
- 6.Rautio J et al. (2008) Prodrugs: design and clinical applications. Nat Rev Drug Discov 7, 255–270. 10.1038/nrd2468 [DOI] [PubMed] [Google Scholar]
- 7.Yang B et al. (2020) Engineering Prodrug Nanomedicine for Cancer Immunotherapy. Adv Sci 7. ARTN 2002365 10.1002/advs.202002365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taresco V et al. (2018) Stimuli-Responsive Prodrug Chemistries for Drug Delivery. Adv Ther-Germany 1. ARTN 1800030 10.1002/adtp.201800030 [DOI] [Google Scholar]
- 9.Walther R et al. (2017) Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv Drug Deliver Rev 118, 65–77. 10.1016/j.addr.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 10.Rautio J et al. (2018) The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov 17, 559–587. 10.1038/nrd.2018.46 [DOI] [PubMed] [Google Scholar]
- 11.Meng Q et al. (2019) Logical design and application of prodrug platforms. Polym Chem-uk 10, 306–324. 10.1039/c8py01160e [DOI] [Google Scholar]
- 12.Hassanzadeh P et al. (2018) Linkers: The key elements for the creation of efficient nanotherapeutics. J Control Release 270, 260–267. 10.1016/j.jconrel.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 13.Beaumont K et al. (2003) Design of Ester Prodrugs to Enhance Oral Absorption of Poorly Permeable Compounds: Challenges to the Discovery Scientist. Curr Drug Metab 4, 461–485. 10.2174/1389200033489253 [DOI] [PubMed] [Google Scholar]
- 14.Neumann W et al. (2018) Esterase-Catalyzed Siderophore Hydrolysis Activates an Enterobactin-Ciprofloxacin Conjugate and Confers Targeted Antibacterial Activity. J Am Chem Soc 140, 5193–5201. 10.1021/jacs.8b01042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie A et al. (2020) Stimuli -responsive prodrug-based cancer nanomedicine. Ebiomedicine 56. ARTN 102821 10.1016/j.ebiom.2020.102821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J et al. (2017) A quantitative study of the intracellular fate of pH-responsive doxorubicin-polypeptide nanoparticles. J Control Release 260, 100–110. 10.1016/j.jconrel.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinoh H et al. (2016) Nanomedicines Eradicating Cancer Stem-like Cells in Vivo by pH-Triggered Intracellular Cooperative Action of Loaded Drugs. Acs Nano 10, 5643–5655. 10.1021/acsnano.6b00900 [DOI] [PubMed] [Google Scholar]
- 18.Wang T et al. (2017) Acidity-Triggered Ligand-Presenting Nanoparticles To Overcome Sequential Drug Delivery Barriers to Tumors. Nano Lett 17, 5429–5436. 10.1021/acs.nanolett.7b02031 [DOI] [PubMed] [Google Scholar]
- 19.Costa SA et al. (2018) Active Targeting of Cancer Cells by Nanobody Decorated Polypeptide Micelle with Bio-orthogonally Conjugated Drug. Nano Lett 19, 247–254. 10.1021/acs.nanolett.8b03837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D et al. (2018) Biodegradable, Hydrogen Peroxide, and Glutathione Dual Responsive Nanoparticles for Potential Programmable Paclitaxel Release. J Am Chem Soc 140, 7373–7376. 10.1021/jacs.7b12025 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y et al. (2020) Alleviating Cellular Oxidative Stress through Treatment with Superoxide-Triggered Persulfide Prodrugs. Angew Chem Int Edit 59, 16698–16704. 10.1002/anie.202006656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling X et al. (2019) Glutathione-Responsive Prodrug Nanoparticles for Effective Drug Delivery and Cancer Therapy. Acs Nano 13, 357–370. 10.1021/acsnano.8b06400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q et al. (2019) Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol 14, 799–809. 10.1038/s41565-019-0485-z [DOI] [PubMed] [Google Scholar]
- 24.Caculitan NG et al. (2017) Cathepsin B Is Dispensable for Cellular Processing of Cathepsin B-Cleavable Antibody-Drug Conjugates. Cancer Res 77, 7027–7037. 10.1158/0008-5472.Can-17-2391 [DOI] [PubMed] [Google Scholar]
- 25.Feng Z et al. (2017) Self-Assembling Ability Determines the Activity of Enzyme-Instructed Self-Assembly for Inhibiting Cancer Cells. J Am Chem Soc 139, 15377–15384. 10.1021/jacs.7b07147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheetham AG et al. (2014) Linker-determined drug release mechanism of free camptothecin from self-assembling drug amphiphiles. Chem Commun 50, 6039–6042. 10.1039/c3cc49453e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su H et al. (2019) Paclitaxel-Promoted Supramolecular Polymerization of Peptide Conjugates. J Am Chem Soc 141, 11997–12004. 10.1021/jacs.9b04730 [DOI] [PubMed] [Google Scholar]
- 28.Wang J et al. (2019) Diglycine Enables Rapid Intrabacterial Hydrolysis for Activating Anbiotics against Gram‐negative Bacteria. Angewandte Chemie Int Ed 58, 10631–10634. 10.1002/anie.201905230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan X et al. (2018) Drug Combination Synergy in Worm-like Polymeric Micelles Improves Treatment Outcome for Small Cell and Non-Small Cell Lung Cancer. Acs Nano 12, 2426–2439. 10.1021/acsnano.7b07878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mougin J et al. (2019) Stacking as a Key Property for Creating Nanoparticles with Tunable Shape: The Case of Squalenoyl-Doxorubicin. Acs Nano 13, 12870–12879. 10.1021/acsnano.9b05303 [DOI] [PubMed] [Google Scholar]
- 31.Behzadi S et al. (2017) Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev 46, 4218–4244. 10.1039/c6cs00636a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu WQ et al. (2020) Intelligent Size-Changeable Nanoparticles for Enhanced Tumor Accumulation and Deep Penetration. Acs Appl Bio Mater 3, 5455–5462. 10.1021/acsabm.0c00917 [DOI] [PubMed] [Google Scholar]
- 33.Mosquera J et al. (2018) Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Accounts Chem Res 51, 2305–2313. 10.1021/acs.accounts.8b00292 [DOI] [PubMed] [Google Scholar]
- 34.Wang J et al. (2015) The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. Acs Nano 9, 7195–7206. 10.1021/acsnano.5b02017 [DOI] [PubMed] [Google Scholar]
- 35.Kaga S et al. (2017) Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly. Biomacromolecules 18, 3963–3970. 10.1021/acs.biomac.7b00995 [DOI] [PubMed] [Google Scholar]
- 36.Kim BJ and Xu B (2020) Enzyme-Instructed Self-Assembly for Cancer Therapy and Imaging. Bioconjugate Chem 31, 492–500. 10.1021/acs.bioconjchem.0c00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabral H et al. (2011) Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol 6, 815–823. 10.1038/nnano.2011.166 [DOI] [PubMed] [Google Scholar]
- 38.Su H et al. (2020) The role of critical micellization concentration in efficacy and toxicity of supramolecular polymers. Proc National Acad Sci 117, 201913655. 10.1073/pnas.1913655117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekladious I et al. (2018) Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov 18, 273–294. 10.1038/s41573-018-0005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabral H et al. (2018) Block Copolymer Micelles in Nanomedicine Applications. Chem Rev 118, 6844–6892. 10.1021/acs.chemrev.8b00199 [DOI] [PubMed] [Google Scholar]
- 41.Ashford MB et al. (2021) Highway to Success-Developing Advanced Polymer Therapeutics. Adv Ther-Germany 4. ARTN 2000285 10.1002/adtp.202000285 [DOI] [Google Scholar]
- 42.Louage B et al. (2016) Well-Defined Polymer-Paclitaxel Prodrugs by a Grafting-from-Drug Approach. Angew Chem Int Edit 55, 11791–11796. 10.1002/anie.201605892 [DOI] [PubMed] [Google Scholar]
- 43.Daglar B et al. (2014) Polymeric nanocarriers for expected nanomedicine: current challenges and future prospects. Rsc Adv 4, 48639–48659. 10.1039/c4ra06406b [DOI] [Google Scholar]
- 44.Pei Q et al. (2018) Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. Acs Nano 12, 1630–1641. 10.1021/acsnano.7b08219 [DOI] [PubMed] [Google Scholar]
- 45.Feng B et al. (2018) Binary Cooperative Prodrug Nanoparticles Improve Immunotherapy by Synergistically Modulating Immune Tumor Microenvironment. Adv Mater 30, 1803001. 10.1002/adma.201803001 [DOI] [PubMed] [Google Scholar]
- 46.Wong XY et al. (2020) Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. Acs Nano 14, 2585–2627. 10.1021/acsnano.9b08133 [DOI] [PubMed] [Google Scholar]
- 47.Guo H et al. (2017) Positively charged polypeptide nanogel enhances mucoadhesion and penetrability of 10-hydroxycamptothecin in orthotopic bladder carcinoma. J Control Release 259, 136–148. 10.1016/j.jconrel.2016.12.041 [DOI] [PubMed] [Google Scholar]
- 48.Liu J et al. (2017) Nanoscale-Coordination-Polymer-Shelled Manganese Dioxide Composite Nanoparticles: A Multistage Redox/pH/H 2 O 2 -Responsive Cancer Theranostic Nanoplatform. Adv Funct Mater 27, 1605926. 10.1002/adfm.201605926 [DOI] [Google Scholar]
- 49.Hou B et al. (2020) Engineering Stimuli-Activatable Boolean Logic Prodrug Nanoparticles for Combination Cancer Immunotherapy. Adv Mater 32. ARTN 1907210 10.1002/adma.201907210 [DOI] [PubMed] [Google Scholar]
- 50.Wang Z et al. (2020) Self-assembling Prodrug Nanotherapeutics for Synergistic Tumor Targeted Drug Delivery. Acta Biomater. 10.1016/j.actbio.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J et al. (2018) Locally Deployable Nanofiber Patch for Sequential Drug Delivery in Treatment of Primary and Advanced Orthotopic Hepatomas. Acs Nano 12, 6685–6699. 10.1021/acsnano.8b01729 [DOI] [PubMed] [Google Scholar]
- 52.Lv Q et al. (2020) Thermosensitive Polypeptide Hydrogels Co‐Loaded with Two Anti‐Tumor Agents to Reduce Multi‐Drug Resistance and Enhance Local Tumor Treatment. Adv Ther-Germany 3, 1900165. 10.1002/adtp.201900165 [DOI] [Google Scholar]
- 53.Xu C et al. (2019) Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J Control Release 295, 153–163. 10.1016/j.jconrel.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 54.Cong Y et al. (2018) Dual Drug Backboned Shattering Polymeric Theranostic Nanomedicine for Synergistic Eradication of Patient-Derived Lung Cancer. Adv Mater 30, 1706220. 10.1002/adma.201706220 [DOI] [PubMed] [Google Scholar]
- 55.Song W et al. (2016) Combining disulfiram and poly(l-glutamic acid)-cisplatin conjugates for combating cisplatin resistance. J Control Release 231, 94–102. 10.1016/j.jconrel.2016.02.039 [DOI] [PubMed] [Google Scholar]
- 56.Wang S et al. (2018) Hierarchical Tumor Microenvironment-Responsive Nanomedicine for Programmed Delivery of Chemotherapeutics. Adv Mater 30, 1803926. 10.1002/adma.201803926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheetham AG et al. (2017) Self-assembling prodrugs. Chem Soc Rev 46, 6638–6663. 10.1039/c7cs00521k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotelevets L et al. (2017) A Squalene-Based Nanomedicine for Oral Treatment of Colon Cancer. Cancer Res 77, 2964–2975. 10.1158/0008-5472.can-16-1741 [DOI] [PubMed] [Google Scholar]
- 59.Cai K et al. (2015) Dimeric Drug Polymeric Nanoparticles with Exceptionally High Drug Loading and Quantitative Loading Efficiency. J Am Chem Soc 137, 3458–3461. 10.1021/ja513034e [DOI] [PubMed] [Google Scholar]
- 60.Wang D et al. (2017) A Molecular Recognition Approach To Synthesize Nucleoside Analogue Based Multifunctional Nanoparticles for Targeted Cancer Therapy. J Am Chem Soc 139, 14021–14024. 10.1021/jacs.7b08303 [DOI] [PubMed] [Google Scholar]
- 61.Zhang F et al. (2017) Transformative Nanomedicine of an Amphiphilic Camptothecin Prodrug for Long Circulation and High Tumor Uptake in Cancer Therapy. Acs Nano 11, 8838–8848. 10.1021/acsnano.7b03003 [DOI] [PubMed] [Google Scholar]
- 62.Wang D et al. (2018) Nucleoside Analogue-Based Supramolecular Nanodrugs Driven by Molecular Recognition for Synergistic Cancer Therapy. J Am Chem Soc 140, 8797–8806. 10.1021/jacs.8b04556 [DOI] [PubMed] [Google Scholar]
- 63.Sobot D et al. (2017) Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat Commun 8, 15678. 10.1038/ncomms15678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng J et al. (2019) A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine. Sci Adv 5, eaau5148. 10.1126/sciadv.aau5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monroe M et al. (2018) Harnessing nanostructured systems for improved treatment and prevention of HIV disease. Bioeng Transl Medicine 3, 102–123. 10.1002/btm2.10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sillman B et al. (2018) Creation of a long-acting nanoformulated dolutegravir. Nat Commun 9, 443. 10.1038/s41467-018-02885-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou T et al. (2017) Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials 151, 53–65. 10.1016/j.biomaterials.2017.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dash PK et al. (2019) Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat Commun 10, 2753. 10.1038/s41467-019-10366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hobson JJ et al. (2019) Semi-solid prodrug nanoparticles for long-acting delivery of water-soluble antiretroviral drugs within combination HIV therapies. Nat Commun 10, 1413. 10.1038/s41467-019-09354-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulkarni TA et al. (2020) A year-long extended release nanoformulated cabotegravir prodrug. Nat Mater, 1–11. 10.1038/s41563-020-0674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang HX et al. (2017) New Generation Nanomedicines Constructed from Self-Assembling Small-Molecule Prodrugs Alleviate Cancer Drug Toxicity. Cancer Res 77, 6963–6974. 10.1158/0008-5472.Can-17-0984 [DOI] [PubMed] [Google Scholar]
- 72.Wang Y et al. (2017) Peptide–drug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliver Rev 110–111, 112–126. 10.1016/j.addr.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiapparelli P et al. (2020) Self-assembling and self-formulating prodrug hydrogelator extends survival in a glioblastoma resection and recurrence model. J Control Release 319, 311–321. 10.1016/j.jconrel.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 74.Wang Y et al. (2019) Supramolecular-based nanofibers. Mater Sci Eng C 101, 650–659. 10.1016/j.msec.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 75.Chakroun RW et al. (2020) Supramolecular Design of Unsymmetric Reverse Bolaamphiphiles for Cell‐Sensitive Hydrogel Degradation and Drug Release. Angewandte Chemie Int Ed 59, 4434–4442. 10.1002/anie.201913087 [DOI] [PubMed] [Google Scholar]
- 76.Wang FH et al. (2020) Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat Biomed Eng 4, 1090.-+. 10.1038/s41551-020-0597-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakroun RW et al. (2019) Fine-Tuning the Linear Release Rate of Paclitaxel-Bearing Supramolecular Filament Hydrogels through Molecular Engineering. Acs Nano 13, 7780–7790. 10.1021/acsnano.9b01689 [DOI] [PubMed] [Google Scholar]
- 78.Wang F et al. (2020) Supramolecular prodrug hydrogelator as an immune booster for checkpoint blocker–based immunotherapy. Sci Adv 6, eaaz8985. 10.1126/sciadv.aaz8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song Z et al. (2017) Synthetic polypeptides: from polymer design to supramolecular assembly and biomedical application. Chem. Soc. Rev 46, 6570–6599. 10.1039/c7cs00460e [DOI] [PubMed] [Google Scholar]
- 80.Saha S et al. (2020) Engineering the Architecture of Elastin‐Like Polypeptides: From Unimers to Hierarchical Self‐Assembly. Adv Ther-Germany 3, 1900164. 10.1002/adtp.201900164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sis MJ and Webber MJ (2019) Drug Delivery with Designed Peptide Assemblies. Trends Pharmacol Sci 40, 747–762. 10.1016/j.tips.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 82.Bhattacharyya J et al. (2015) A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat Commun 6, 7939. 10.1038/ncomms8939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banskota S et al. (2020) Genetically Encoded Stealth Nanoparticles of a Zwitterionic Polypeptide-Paclitaxel Conjugate Have a Wider Therapeutic Window than Abraxane in Multiple Tumor Models. Nano Lett 20, 2396–2409. 10.1021/acs.nanolett.9b05094 [DOI] [PubMed] [Google Scholar]
- 84.Yousefpour P et al. (2019) Conjugate of Doxorubicin to Albumin‐Binding Peptide Outperforms Aldoxorubicin. Small 15, 1804452. 10.1002/smll.201804452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yousefpour P et al. (2018) Genetically Encoding Albumin Binding into Chemotherapeutic-loaded Polypeptide Nanoparticles Enhances Their Antitumor Efficacy. Nano Lett 18, 7784–7793. 10.1021/acs.nanolett.8b03558 [DOI] [PMC free article] [PubMed] [Google Scholar]


