Abstract
The postrhinal cortex (POR), which is the rodent homologue of the primate parahippocampal cortex (PHC), has been implicated in contextual and spatial processing. For instance, prior studies have demonstrated that permanent lesions of POR impair contextual fear conditioning. In contrast, permanent lesions of POR prior to training do not impact auditory fear conditioning. In the current experiments, we examined the role of POR in the expression of auditory fear conditioning by using chemogenetics to silence neural activity in POR at the time of retrieval testing. Considering that extinction is context-dependent, and POR contributes to contextual memory, we hypothesized that POR would be necessary for expression of auditory fear conditioning following extinction. We found that POR inactivation during retrieval impaired freezing to an auditory cue that was tested in the conditioning context (A) after it had been extinguished in a different context (B). However, the involvement of POR was not specific to extinction. POR inactivation also impaired freezing to an auditory fear cue that had not undergone extinction. Thus, while prior studies have identified a role for POR in contextual fear conditioning, the current findings extend the functional role of POR to include the expression of auditory fear conditioning.
Keywords: postrhinal, chemogenetics, extinction, fear conditioning, renewal
Postrhinal cortex contributions to the retrieval of extinguished and non-extinguished auditory fear conditioning The parahippocampal cortex (PHC) is a component of the medial temporal lobe (MTL), a brain system essential for human memory (Ranganath & Ritchey, 2012; Squire et al., 2004). The rodent homologue of PHC is the postrhinal cortex (POR; Burwell, Witter, & Amaral, 1995). POR/PHC has extensive connections with other parahippocampal regions, as well as the hippocampal formation, and is often considered part of the “where” processing stream. Indeed, both lesion and electrophysiological recording studies in rodents confirm that POR is important for spatial and contextual processing (e.g., Bucci, Phillips, & Burwell, 2000; LaChance, Todd, & Taube, 2021).
The first experiments to examine the contributions of POR to associative learning and memory for contexts were conducted by Dr. David Bucci as a postdoctoral fellow in Dr. Rebecca Burwell’s laboratory. In the first published study examining the role of POR in fear conditioning, Bucci et al., (2000) reported that neurotoxic damage to the POR produced both anterograde and retrograde deficits of contextual fear memories. Further studies from the same laboratory demonstrated that the role of POR in contextual memory is protracted (Burwell et al., 2004); POR lesions made 1, 28, or 100 days after initial conditioning reduced freezing when rats were returned to the original conditioning context. Overall, this early work demonstrated a critical role of POR in conditioning to contextual cues.
Although damage to POR has consistently been found to impair the expression of contextual fear memories, it often has no impact on the expression of Pavlovian auditory fear conditioning, in which a discrete tone is paired with mild footshock (Bucci et al., 2000; Peck & Taube, 2017; Taylor-Yeremeeva et al., 2021). However, these prior studies have utilized pre-training lesion methods, which leave open the possibility of compensation by other brain structures (Fanselow, 2010). Therefore, one purpose of the current studies was to examine the contribution of POR to the retrieval of auditory fear conditioning by utilizing chemogenetics to temporarily inactivate neural activity in POR at the time of test, thereby precluding the possibility of compensation that may occur with pre-training lesions.
A second purpose of the current experiments was to examine the conditions under which POR might contribute to the retrieval of auditory fear conditioning. Our primary focus was on retrieval following Pavlovian fear extinction, considering that retrieval following extinction is typically more context dependent than initial conditioning (Bouton, 2004). During fear extinction, a tone conditioned stimulus (CS) that was previously paired with an aversive unconditioned stimulus (US) is repeatedly presented alone, and the conditioned response declines (Bouton, Maren, McNally, 2021). However, what is learned during extinction is critically dependent upon the context for its expression. When the tone CS is presented in the extinction context, fear to the tone remains low. But when the tone CS is presented in an alternative context, fear to the tone renews (Bouton & King, 1983). Thus, the expression of fear to an extinguished CS is intimately tied to the context in which the CS is extinguished. Given the important role of POR in contextual memory, we hypothesized that POR would contribute to the contextual retrieval of auditory fear conditioning following extinction.
Experiments 1a and 1b
All rats first underwent surgery in which the POR was bilaterally infused with a virus containing either an inhibitory DREADD (designer receptor exclusively activated by designer drugs) receptor (hM4Di) or a control virus expressing green-fluorescent protein (GFP). In Experiment 1a, after recovery from surgery, all rats received tone-shock pairings in Context A, followed by tone extinction in Context B. Then, all rats were tested for fear to the extinguished tone in both the extinction context (Context B) and the conditioning context (Context A; test order counterbalanced). All rats received injections of the DREADD agonist clozapine-n-oxide (CNO) 30 minutes before the start of both test sessions. In control rats injected with GFP-expressing virus, it was expected that fear to the tone would be low when testing occurred in the extinction context (B) but would be high when testing occurred in the original conditioning context (A). Such a pattern would confirm that conditioned responding to the tone was guided by the context. We hypothesized that POR inactivation in hM4Di-injected rats would disrupt the context-specific expression of extinction, perhaps resulting in low levels of tone fear in both Contexts B and A. To test whether any effects of POR inactivation were specific to the extinguished CS, in Experiment 1b we examined the role of POR in the retrieval of fear to a CS that had not undergone extinction.
Methods
Subjects
The subjects were 24 (Experiment 1a) and 24 (Experiment 1b) naïve male Long-Evans rats (Envigo Laboratories, Indianapolis, IN) that were ~53 days old upon arrival. Rats were allowed one week to acclimate to the vivarium while pair-housed. Following surgery, rats were singly housed in plastic tubs for the remainder of the experiment. Food and water were available ad libitum (Purina standard rat chow, Nestle Purina, St. Louis, MO) in a climate-controlled colony room on a 14:10 light-dark cycle. Throughout the experiment, rats were monitored and cared for in compliance with the Association for the Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Surgery
Coordinates for POR injections were derived from Paxinos & Watson (2007), anatomically defined boundaries, and previously published coordinates (Bucci et al., 2000; Burwell, 2001; Ramos, 2013). Subjects were anesthetized with isoflurane gas (1–3% in oxygen) and placed in a Kopf stereotaxic apparatus. The skin was retracted, and holes were drilled in the skull bilaterally at anterior/ posterior (A/P) 0 and medial/ lateral (M/L) ± 4.4 from lambda. Bilateral infusions were made at the following coordinates as measured from lambda: A/P 0.0, M/L ± 3.5, and dorsal/ ventral (D/V) −4.3 from the dura. A 10 μl Hamilton syringe equipped with a 26-gauge beveled needle connected to an infusion pump (Stoelting) was used to infuse the viral construct (0.8 μl; 0.2 μl/min). The needle was inserted, pointing 26 degrees laterally, with the opening facing posteriorly. The needle was left at the appropriate D/V level for 2 min prior to, and 4 min after, each infusion. Following infusions, the wound was closed using sterile staples, an analgesic was administered, and rats were allowed to recover for 28 days before behavior.
In Experiment 1a, 12 rats (Group “hM4Di”) received infusions of a synapsin-promotor-driven adeno-associated viral vector, pAAV8-hSyn-hM4D(Gi)-mCherry (Addgene, Inc., Watertown, MA). This virus contained the DNA for the inhibitory DREADD receptor, hM4Di. The remaining 12 rats (Group “GFP”) received infusions of a second construct that lacked the hM4Di-mCherry gene but instead expressed GFP (pAAV8-hSyn-EGFP; Addgene, Inc., Watertown MA). In Experiment 1b, 12 rats received infusions of the hM4Di-mCherry virus and 12 rats received infusions of the GFP virus.
Behavioral Apparatus
Behavioral procedures occurred in two sets of four conditioning chambers (Med Associates, Inc., St. Albans, VT, ENV-007; 24 cm W × 30.5 cm L × 29 cm H), which served as the two contexts (Context A and Context B, counterbalanced). Each chamber was housed in a sound-attenuating chamber (Med Associates, ENV-017M; 66 cm W × 56 cm L × 56 cm H) outfitted with an exhaust fan to provide airflow and background noise (68 dB). All chambers were outfitted with a food cup, recessed in the center of the front wall, and a retracted lever (Med Associates, ENV112CM) located on the right of the front wall. Each chamber had two lights: a panel light (Med Associates, ENV-221M) centered on the front wall 16 cm above the grid floor, and a house light (Med Associates, ENV-215M) centered on the back wall 24 cm above the grid floor. Each chamber also had a 2.8 W bulb (with a red cover) mounted to the ceiling of the sound-attenuating chamber to provide background illumination. All chambers had a speaker (Med Associates, ENV-224AM) located 20 cm above and to the right of the food cup.
In one set of four of the chambers, the ceiling and side walls were clear acrylic plastic, the front and back walls were brushed aluminum, and the grid floor was stainless steel rods (5 mm in diameter) spaced 1.5 cm apart (center-to-center). In these chambers, the 2.8 W bulb with the red cover provided background illumination. In the other set of four chambers, the ceiling, door, and side walls were covered with laminated black and white checkerboard paper with 1 cm black and white squares to provide a distinct visual feature. The grid floor was staggered, such that every other bar was on a different plane offset by 0.5 cm. The house light and panel light provided illumination. Because these two sets of four chambers were located in the same room of the laboratory, one olfactory cue was used for Context A, and another was used for Context B to reduce diffusion of the olfactory cues. For Context A sessions, approximately 3 mL of Pine-Sol (Clorox, Co., Oakland, CA) was placed in the back-left part of the chamber tray below the grid floor. For Context B sessions, approximately 0.5g of Vicks Vaporub (Proctor & Gamble, Cincinnati, OH) was placed in the front-right corner of the chamber tray below the grid floor. For both sets of chambers, the speaker was used to deliver a 1500 Hz tone for 10 sec CS and the grid floor was used to deliver a 1.0-mA, 1.0- sec shock US. Surveillance cameras located in the sound attenuating chambers were used to record the subjects’ behavior.
Behavioral Procedures
The two experiments were identical, except that in Experiment 1a rats received extinction of the tone in Context B, whereas in Experiment 1b rats were simply exposed to Context B with no tone presentations.
For both experiments, rats received two days of auditory fear conditioning in Context A. Each session consisted of 3 presentations of the CS, a 10-s tone, which terminated with the onset of the US, a 1-mA, 1-s shock. The first trial began three minutes after rats were placed in the chambers. The time between shock and the next CS presentation was 64 sec. Subjects remained in the chambers for 90-s after the last trial before being returned to their home-cages.
On Day 3, rats were returned to the conditioning chamber (Context A) for a 20-min context exposure session with no tones or shocks presented and freezing to the context was measured. Beginning the following day, rats received one daily session of tone extinction training (Experiment 1a) for 6 consecutive days. Extinction occurred in Context B and consisted of 20 tone presentations (30-s ITIs). No shocks were presented during extinction. The first trial began 3 min after placing the rat in the chamber. In Experiment 1b, rats received the same treatment, with the exception that no tones were ever presented. Thus, rats were simply exposed to Context B alone for 6 consecutive days. The day after the last extinction (Exp 1a) or exposure (Exp 1b) session, rats were re-exposed to Context A for 20 minutes to ensure low levels of freezing to Context A prior to renewal testing. The following two days, freezing to the tone was tested in Context A and Context B (with test order counterbalanced). During each test session, the tone was presented five times (10-s each, 30-s ITI) beginning 3 min after the rat was placed in the chamber. Subjects were injected with CNO 30 min prior to being placed in the chambers on each test day.
To ensure that administration of CNO did not interfere with the ability to perform the freezing response, rats in Experiment 1b received an additional test session 24 hours later. All rats received injections of CNO and were returned to Context A where they received 3 unsignaled foot shocks. The shocks were scheduled in the same manner as initial conditioning, with the exception that no tones were delivered.
Drug preparation and administration
CNO was prepared fresh immediately before injections. Clozapine-n-oxide (CNO) was dissolved in dimethyl sulfoxide (1% DMSO) followed by 0.9% sterile saline to obtain a final concentration of 2mg/ml. After being weighed each day for appropriate dosing, rats received intraperitoneal injections CNO (2 ml/kg; 4 mg/kg) prior to the retrieval tests sessions in Experiments 1a and 1b, and prior to the re-conditioning session that occurred at the end of Experiment 1b.
Behavioral Observations and Data Analysis
The dependent variable was freezing, defined as total motor immobility except for breathing (Blanchard & Blanchard, 1969; Fanselow, 1980). For the conditioning session, behavior was assessed (freezing or not) for each rat every 8 seconds during the 64-s period before the first trial (baseline freezing) and the 64-s periods following each trial (post-shock freezing). During Context A sessions, behavior was assessed every 8 seconds, for the first 8 min and 32 s of the session, yielding 64 observations for each rat. During extinction sessions, freezing behavior was assessed during the 64 s prior to the first tone in the same manner as the conditioning session (baseline freezing). Each rat’s behavior was also assessed every 2 seconds during each presentation of the 10 s tone. Finally, in the test sessions, behavior was assessed during the baseline period prior to the first tone, and during each presentation of the tone in both contexts. In all cases, the frequency of freezing indices was converted to a percentage of time spent freezing.
Data Analysis
Analyses of freezing behavior were conducted using analysis of variance (ANOVA) with virus type (GFP & hM4Di) as the between-subjects variable. An alpha level of 0.05 was used for all analyses. SPSS (Version 28) was used to complete all statistical testing.
Virus verification and analysis
After the conclusion of the behavioral procedures, rats were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline, followed by 10% buffered formalin. The brains were stored in the 10% buffered formalin solution overnight and then placed in a 30% sucrose solution for ~72 hours before being stored at −80 C until tissue preparation. Brain sections (40 μm) were collected using a freezing microtome and coverslipped using Vectashield with DAPI (Vector Laboratories, Inc., Burlingame, CA). The presence and location of fluorescent neurons were determined using an Axioskop I compound microscope (Zeiss) with Stereo Investigator software (version 9, MicroBrightField). For each section containing POR, virus expression was assessed. Subjects were removed from analysis if there were: 1) fewer than 20 labeled cells in POR for each POR containing section, 2) only unilateral expression in POR, or 3) labeled cells present bilaterally in non-POR regions. For all sections, POR virus expression was scored on scale from 0 to 5, with 0 indicating no fluorescence observed and 5 indicating fluorescence present on most neurons.
Results and Discussion
Histology
Representative virus expression for Experiment 1a/b is illustrated in Figure 1. In Experiment 1a, one GFP subject died during surgery, and one hM4Di subject was removed from analysis due to absence of bilateral DREADD expression in POR. Further, one hM4Di rat had adverse reactions (e.g., minor convulsions) to the CNO injection and was removed from the experiment. Thus, the final analysis of Experiment 1a included 11 GFP and 10 hM4Di rats.
Figure 1.
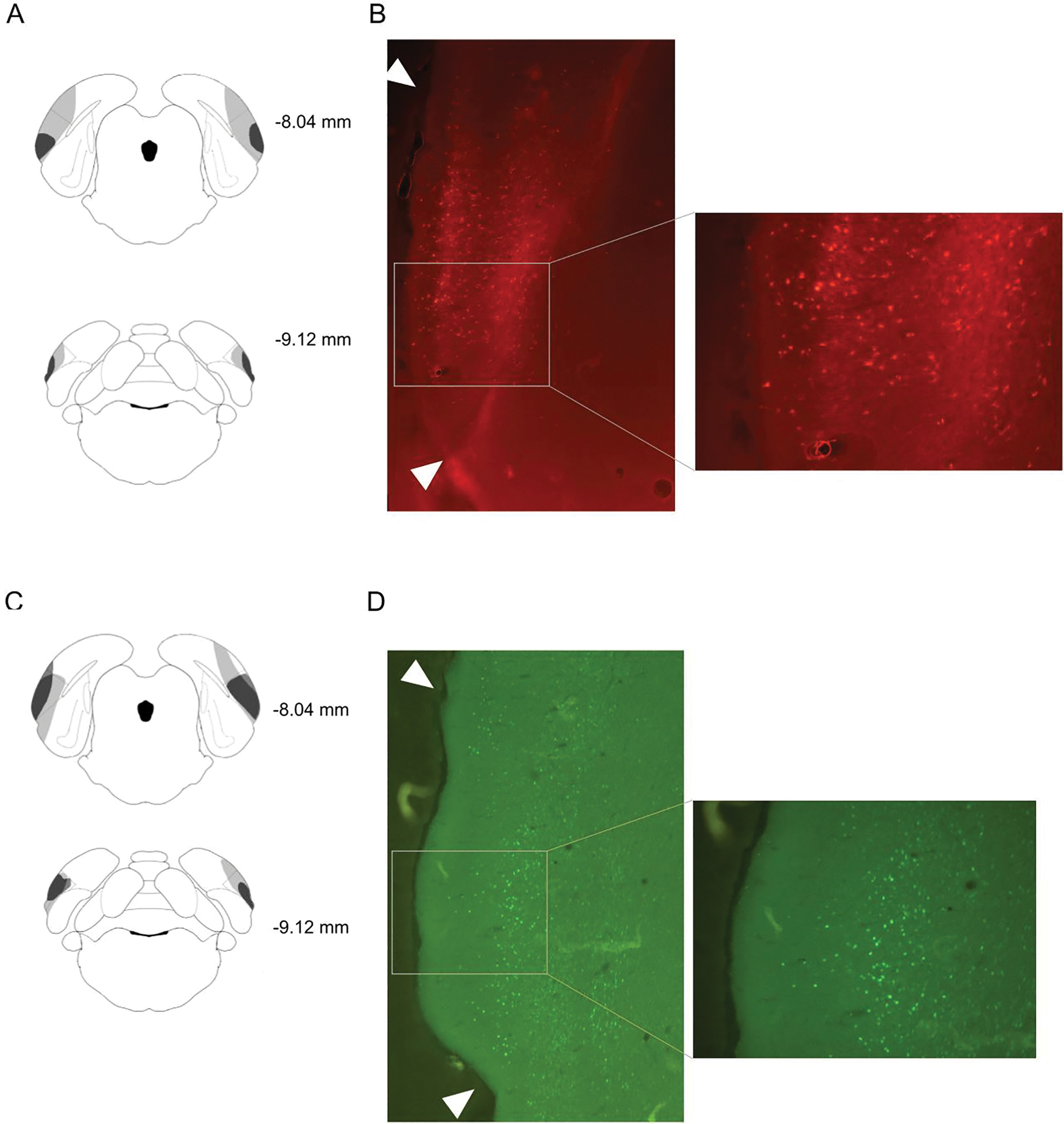
A: Schematic diagram of representative minimum (dark gray) and maximum (light gray) expression of hM4Di. Lateral dashed lines indicate the boundary of POR. Measurements indicate distance from bregma. B: Representative photomicrographs of fluorescent labeling of POR neurons expressing hM4Di at approximately −8.28mm. Arrowheads indicate the boundary of POR. C: Schematic diagram of representative minimum (dark gray) and maximum (light gray) expression of GFP. D: Representative photomicrographs of fluorescent labeling of POR neurons expressing GFP at approximately −8.28 mm. Arrowheads indicate the boundary of POR.
For Group hM4Di, the average percentage of POR-containing sections with virus expression was 51%, ranging from 31–75%, and the average virus expression rating was 2.5, ranging from 1–5. For Group GFP, the average percentage of POR-containing sections with virus expression was 58%, ranging from 26–86%, and the average virus expression rating was 2.9, ranging from 1–5. In most subjects, virus expression was found dorsal to POR along the injection tract where the needle was inserted. These areas include the ventral temporal cortex (TEv) and the visual cortex lateral area (V2L). However, the expression along the tract was generally limited to a small rostral-caudal extent and limited in expression (typically fewer than 20 fluorescing cells). Rarely, expression extended ventrally into the lateral entorhinal cortex (LEC), but this was always unilateral and limited to fewer than 20 fluorescing cells.
For Experiment 1b, one hM4Di subject died post-surgery, and two hM4Di rats had adverse reactions (e.g., minor convulsions) to the CNO injection and were removed from the experiment. The final analysis for this experiment included 12 GFP and 9 hM4Di rats. For Group hM4Di, the average percentage of POR-containing sections with virus expression was 58%, ranging from 23–87%, and the average virus expression rating was 2.9, ranging from 1–5. For Group GFP, the average percentage of POR-containing sections with virus expression was 77%, ranging from 51–98%, and the average virus expression rating was 3.9, ranging from 1–5. As in Experiment 1a, expression in POR adjacent cortical regions was noted in TEv and V2L and ventrally in LEC. The expression outside of POR was generally limited to a small rostral-caudal extent and limited expression (typically fewer than 20 fluorescing cells).
Behavior
The mean percentage of freezing during conditioning, Context A exposure, tone extinction, and renewal for Experiment 1a is presented in Figure 2. Freezing during the baseline periods of all phases is presented in Table 1 (M and SEMs). The average post-shock freezing during conditioning sessions 1 and 2 is presented in the left portion of the Figure 2b. A one way ANOVA revealed an unexpected group difference on Day 1 of conditioning, F(1, 19) = 7.5, p = .013. However, rats in Group hM4Di and GFP froze at similar levels during conditioning Day 2, F(1, 19) < 1. We suspect the difference on Day 1 is a Type 1 error. In a recent study, we found no impact of hM4Di expression (in the absence of CNO) on post-shock freezing when expressed in a related cortical region (Fournier et al., 2021). In addition, this difference was not observed in Experiment 1b. There were no differences in baseline freezing during either Day 1 or Day 2, (both Fs < 1; see Table 1 for M and SEM) and groups did not differ in freezing when re-exposed to Context A after conditioning, (F < 1).
Figure 2.
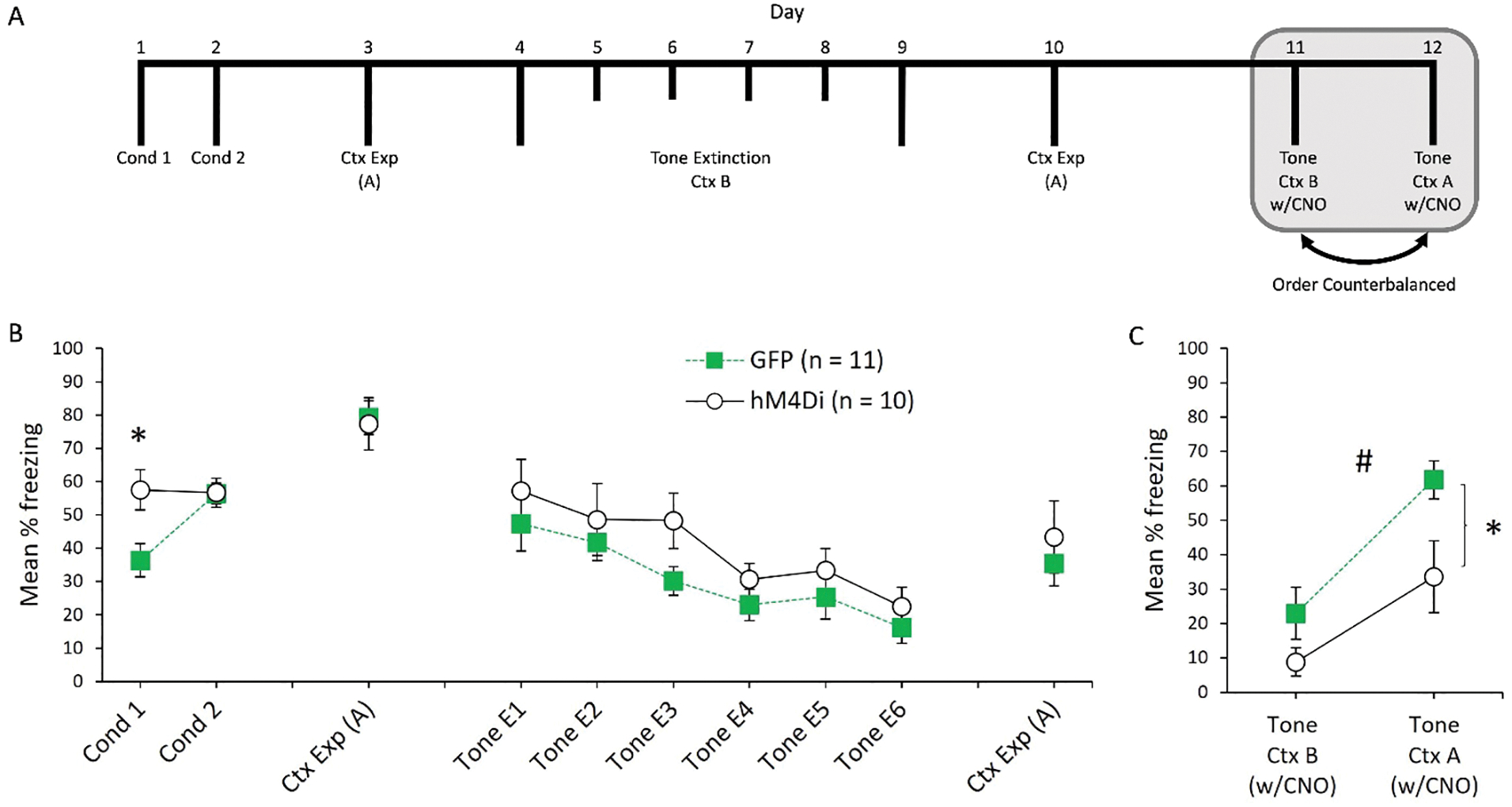
A: Timeline of experimental procedures for Experiment 1a. Rats first received two sessions of auditory fear conditioning in Context A, followed by a single exposure session to Context A. Then, rats underwent 6 sessions of either conditioned stimulus (tone) extinction in Context B, followed by an additional exposure session to Context A. Rats were then tested for fear to the tone in Context A and Context B. Grey shaded region represents CNO injections prior to behavioral testing. B: Post-shock freezing during the two conditioning sessions, followed by freezing during the first session of Context A exposure. Freezing to the tone during the 6 extinction sessions, followed by freezing during the second Context A exposure session. C: freezing to the tone in both Context B and Context A. CNO was injected 30 minutes prior to these sessions. “*” = significant difference (p < .05) between GFP and hM4Di in Context A. “#” = significant main effect of group collapsed across contexts (p < .05).
Table 1.
| Exp | Group | Con 1 | Con 2 | Ext 1 | Ext 2 | Ext 3 | Ext 4 | Ext 5 | Ext 6 | Ext 7 | Ext 8 | Test B | Test A | Ctx A+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp 1a | hM4Di | 0 (0) |
61.25 (12.28) |
33.75 (11.49) |
30.00 (12.28) |
23.75 (9.58) |
16.25 (7.69) |
13.75 (3.93) |
11.25 (5.08) |
n/a | n/a | 7.50 (5.33) |
18.75 (7.73) |
n/a |
| GFP | 0 (0) |
60.22 (11.16) |
27.27 (10.50) |
14.77 (5.79) |
11.36 (5.18) |
2.27 (1.52) |
11.36 (3.55) |
6.82 (3.90) |
n/a | n/a | 5.68 (3.52) |
17.04 (5.90) |
n/a | |
| Exp 1b | hM4Di | 0 (0) |
63.88 (14.35) |
37.50 (13.18) |
25.00 (11.79) |
1.39 (1.39) |
0 (0) |
0 (0) |
0 (0) |
n/a | n/a | 0 (0) |
6.94 (5.5) |
0 (0) |
| GFP | 0 (0) |
73.95 (7.61) |
27.80 (8.95) |
1.04 (1.04) |
1.04 (1.04) |
0 (0) |
0 (0) |
0 (0) |
n/a | n/a | 4.16 (3.2) |
11.45 (4.97) |
12.50 (8.29) |
Note. Mean percent freezing during the baseline period for all sessions of Experiments 1a and 1b. SEMs are presented below each session mean in parentheses. See text for analysis and further details.
For tone extinction, a 2 (Group: GFP vs. hM4Di) × 6 (Sessions) ANOVA revealed a main effect of session, F(5, 95) = 12.85, p < .001. The main effect of group was not significant, F(1, 19) = 1.62, p = .22, and the interaction between group and session was also not significant, F < 1. An identical analysis of baseline freezing during each session revealed a main effect of session F(5, 95) = 3.30, p = .009. Neither the main effect of group nor the group by session interaction were significant, largest F(1, 19) = 2.2, p = .15. When rats were re-exposed to Context A prior to renewal testing, there were no differences in freezing levels between groups, F < 1.
The results of the final retrieval test sessions are presented in Figure 2c. Over two consecutive days, freezing to the tone was tested in Context B and Context A (test order counterbalanced). On both days, rats received injections of CNO 30 minutes prior to being placed in the chambers. A 2 (Group: GFP vs. hM4Di) × 2 (Context) ANOVA revealed a main effect of context, F(1, 19) = 24.42, p < .001, indicating that freezing to the tone was higher in Context A than Context B. In addition, there was a main effect of group, F(1, 19) = 6.95, p = .016 indicating that rats in Group hM4Di froze less overall than rats in Group GFP. Although the interaction between group and context was not significant, F(1, 19) = 1.20, p = .29, planned comparisons revealed that Group hM4Di froze less than Group GFP to the tone in Context A, F(1, 19) = 5.98, p = .024, but the group difference was not significant in Context B, F(1, 19) = 2.52, p = .13. Although freezing was reduced in Context A for Group hM4Di, these rats still froze more to the tone in Context A than Context B, t(9) = 2.49, p = .035. Likewise, Group GFP froze more to the tone in Context A than Context B, t(10) = 4.69, p < .001. Baseline freezing responses during testing was analyzed in the same way. A 2 (Group: GFP vs. hM4Di) × 2 (Context) ANOVA revealed a main effect of context, F(1, 19) = 4.41, p = .049, indicating that baseline freezing to the tone was higher in Context A than Context B (see Table 1). Neither the main effect of group nor the interaction between group and session was significant, both Fs < 1.
The results of Experiment 1a indicate that POR inactivation reduces renewal after extinction. However, it is possible that the reduction in freezing is not specific to cues that have undergone extinction, and instead POR inactivation might have a more general role in the expression of auditory fear conditioning. To test this possibility, Experiment 1b examined the impact of POR inactivation on the expression of auditory fear conditioning in rats that did not receive any prior extinction. The results of Experiment 1b are presented in Figure 3. Freezing during the post-shock period did not differ between groups for conditioning Day 1, F(1, 19) = 2.85, p = .11 or conditioning Day 2, F(1, 19) = 1.91, p = .18. There were no differences in baseline freezing on either day, both Fs < 1 (see Table 1 for Ms and SEMs). Likewise, freezing did not differ between groups when rats were re-exposed to Context A one day following conditioning, F(1, 19) = 1.03, p = .32. Rats were then exposed to Context B for 6 consecutive days, with no tones presented. For these days, baseline freezing was assessed during the same period as in Experiment 1a (see Table 1). A 2 (Group: GFP vs. hM4Di) × 6 (Session) ANOVA revealed a main effect of session, F(5, 95) = 13.03, p < .001. The main effect of group was not significant, F(1, 19) = 2.48, p = .13, nor was the group by session interaction, F(5, 95) = 1.84, p = .11. Finally, groups did not differ when they were re-exposed to A prior to tone retrieval testing, F < 1.
Figure 3.
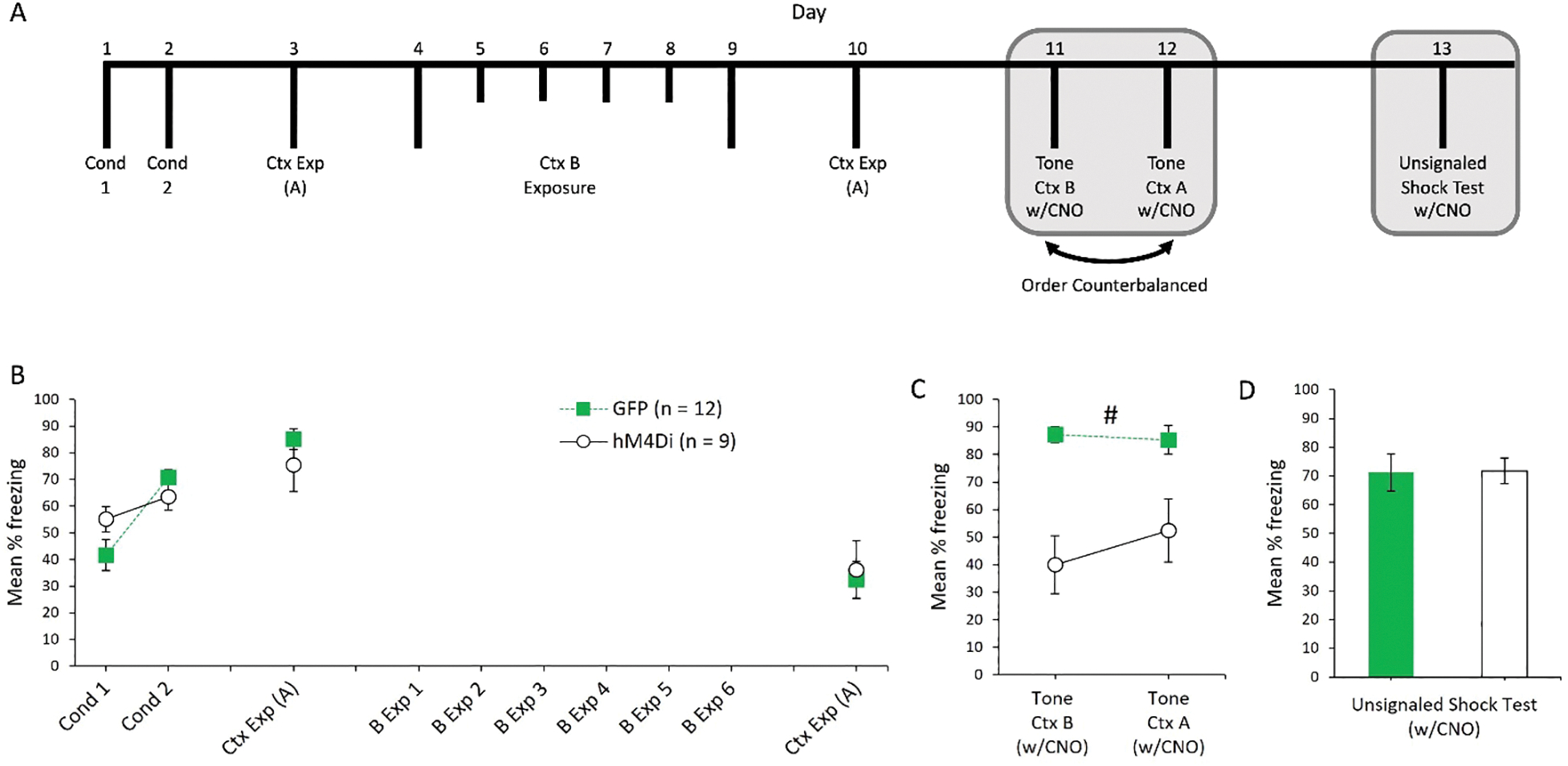
A: Timeline of experimental procedures for Experiment 1b. Rats first received two sessions of auditory fear conditioning in Context A, followed by a single exposure session to Context A. Then, rats underwent 6 sessions of Context B exposure, followed by an additional exposure session to Context A. Rats were then tested for fear to the tone in Context A and Context B, and a final unsignaled shock tests in Context A. Grey shaded region represents CNO injections prior to behavioral testing. B: Post-shock freezing during the two conditioning sessions, followed by freezing during the first and second sessions of Context A exposure. Freezing during exposure to Context B is not depicted (see Table 1). C: Freezing to the tone in both Context B and Context A. CNO was injected 30 minutes prior to these sessions. D: Average post-shock freezing during the unsignaled shock test. “#” = significant main effect of group collapsed across contexts (p < .05).
The results of the two auditory fear retrieval tests are presented in Figure 3C. Prior to these two sessions, all rats were injected with CNO. A 2 (Group: GFP vs. hM4Di) × 2 (Context) ANOVA revealed a main effect of group, F(1, 19) = 36.71, p < .001. Neither the main effect of context nor the interaction between context and group were significant, both Fs < 1. Thus, fear to the tone CS was reduced by POR inactivation, irrespective of the test context. For baseline freezing, neither the main effect of group nor the interaction between group and context was significant, both Fs < 1. The main effect of context approached, but did not reach significance, F(1, 19) = 3.41, p = .08.
In a final test, all rats were injected with CNO, returned to Context A and exposed to three unsignaled shocks (see Figure 3 panel “D”). Freezing did not differ across groups during the post-shock period, F < 1, or during the baseline period prior to the first shock, F(1, 19) = 1.69, p = .21.
Experiment 2
To verify the efficacy of our chemogenetic approach, we measured electrical responses of POR GFP+ (control) and mCherry+ (hM4Di) neurons in vitro in response to depolarizing current injections before and after exposure to bath-applied CNO (5 μM). Given that CNO acts only at M4Di receptors, we predicted that CNO should selectively inhibit the excitability of mCherry+ neurons and would not change the excitability of control cells expressing GFP.
Methods
Subjects
The subjects were four behaviorally naïve adult male Long Evans rats, obtained from Envigo Laboratories (Indianapolis, IN, USA) and were ~60 days old upon arrival. All rats were pair-housed and allowed 4–9 days to acclimate to the vivarium before undergoing surgical procedures. Rats were otherwise maintained as in Experiment 1.
Surgery
Surgical procedures were identical to those in Experiment 1, with two rats injected with the control GFP virus and two rats injected with the hM4Di-expressing virus. Rats were monitored after surgery and were allowed 38–44 days to recover prior to experiments.
Slice preparation.
Rats were anesthetized with vaporized isoflurane, decapitated, and brains rapidly removed into artificial cerebral spinal fluid (aCSF) composed of (in mM): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 6 MgCl2, and 25 glucose (saturated with 95% O2/5% CO2). Coronal brain slices (250 μm thick) of the POR were cut using a Leica VT 1200 slicer and stored in a holding chamber containing aCSF adjusted to 2 mM CaCl2 and 1 mM MgCl2. Slices were maintained in the holding chamber for ~1 hour at 35 °C and then at room temperature (~26 °C) until use in experiments.
Electrophysiology.
Slices were placed in a recording chamber beneath an upright microscope and perfused continuously with oxygenated aCSF heated to 35–36 °C. Pyramidal neurons expressing hM4Di-mCherry or GFP were identified using epifluorescence (470 or 530 nm excitation) and patched under visual control using a 60x water-immersion objective paired with a sCMOS camera. Patch-pipettes (5–7 MΩ) contained a solution consisting of (in mM): 135 K-gluconate, 2 NaCl, 2 MgCl2, 10 HEPES, 3 Na2ATP, and 0.3 NaGTP, pH 7.2 with KOH. Data were acquired using a BVC-700 amplifier (Dagan) interfacing with an ITC-18 digitizer (HEKA) controlled by Axograph software (Axograph). Membrane potentials were sampled at 25 kHz, filtered at 10 kHz, and corrected for the liquid junction potential of +12 mV. Depolarizing current steps sufficient to evoke ~10 action potentials were delivered at 10-second intervals. For each neuron, measurements were made of the resting membrane potential (RMP), input resistance (RN), and the number of action potentials generated by equal-amplitude current injections in baseline conditions and after bath application of 5 μM CNO. In all cases, brain slices were exposed to a single application of CNO.
Results
To confirm the efficacy of hM4Di-mediated inhibition of neurons from control and hM4Di-injected rats, we measured the effects of CNO (5 μM) on RMP, RN, and action potential generation in control (GFP+; n = 10) and hM4Di+ (mCherry+; n = 10) pyramidal neurons in slices of POR (Figure 4A). In baseline conditions, GFP+ neurons had a mean RMP of −79 ± 8 mV, a mean RN of 112 ± 39 MΩ, and generated a mean of 10.3 ± 0.7 action potentials in response to 1.5 s current injections applied at 0.1 Hz (Figure 4B,C). After bath-application of 5 μM CNO for 5 minutes, RMP (−79 ± 8; p = 0.63), RN (114 ± 38 MΩ; p = 0.75) and the number of action potentials (10.1 ± 1.5; p = 0.75) were similar to those observed in baseline conditions. On the other hand, CNO hyperpolarized hM4Di+ neurons by a mean of 4.2 ± 2.4 mV (from −75 ± 5 mV to −80 ± 4 mV; p < 0.001), reduced RN by 32 ± 20% (from 138 ± 75 MΩ to 99 ± 65 MΩ; p < 0.001), and decreased the number of current-evoked action potentials by 8.3 ± 3.7 spikes (from 9.7 ± 2.0 to 1.5 ± 2.5 action potentials; p < 0.001; Figure 4B,C). The effects of CNO were long-lasting, but when given sufficient time (>30 min after washout of CNO), inhibition by CNO was reversible (in 6 of 10 neurons; e.g. Figure 4A). These data confirm that CNO selectively inhibits hM4Di+ neurons in the POR.
Figure 4.
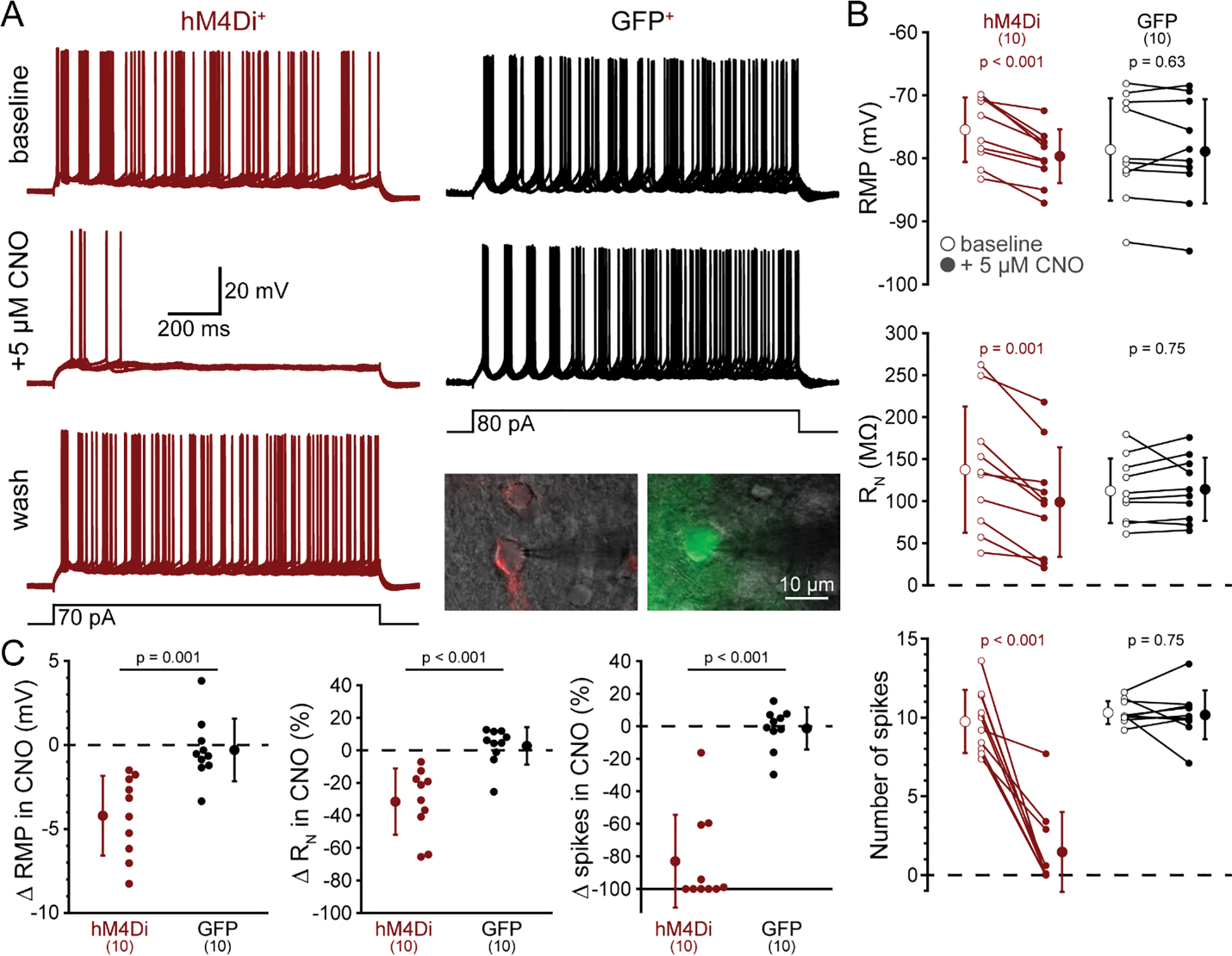
CNO selectively reduces the excitability of hM4Di-expressing cortical neurons in the postrhinal cortex. A, Ten consecutive voltage traces (superimposed) recorded in response to current injections (black traces at bottom) in a neuron expressing mCherry-tagged hM4Di receptors (red, left) or from a GFP-expressing neuron (black, right) in baseline conditions (top traces) or after 5 minutes exposure to CNO (5 μM). A lower trace for the hM4Di-expressing neuron shows reversal of CNO-induced inhibition following removal of CNO (“wash”). Inset are fluorescence images (artificially colored) of punctate mCherry (left) and diffuse GFP (right) superimposed on oblique images of the two neurons from which traces were taken. B, Measurements of resting membrane potential (RMP, top), input resistance (RN, middle), and number of action potentials (bottom) in baseline conditions (open circles) and after application of 5 μM CNO (filled circles) for ten hM4Di-expressing (red) and ten GFP-expressing (black) neurons. Means (± standard deviations; SD) for each condition indicated by enlarged symbols; p-values from paired Student’s t-tests. C, Comparisons of CNO-induced changes in RMP (left), RN (middle), and action potential number (right) in hM4Di-expressing (red) and GFP-expressing (black) neurons. Means (± SD) for each group indicated by enlarged symbols; p-values from Student’s t-tests comparing results across hM4Di+ and GFP+ neurons.
General Discussion
The results of Experiments 1a and 1b indicate that POR contributes to the retrieval / expression of auditory fear conditioning. In Experiment 1a, rats first received tone-shock pairings in Context A prior to extinction of tone fear in Context B. During testing, control rats continued to show little freezing to the tone in the extinction context (B) but freezing to the tone increased in the conditioning context (A). This confirmed that the reduction of learned fear during extinction is specific to the extinction context. However, for rats with neural activity in POR temporarily inactivated during testing, freezing to the tone in Context A was reduced, although renewal was still observed. One interpretation of these findings is that POR inactivation disrupts the normal context-specificity of extinction, resulting in partial generalization of extinction to other contexts. A role for POR in the context-specificity of extinction is consistent with its role in contextual learning and memory, as well as findings that damage to brain structures reciprocally connected with POR, such as the hippocampal formation and entorhinal cortex, reduce fear renewal (Ji & Maren, 2008a, b; for a review see Maren et al., 2013).
However, the results of Experiments 1b demonstrate that the contribution of POR to retrieval of auditory fear conditioning is not specific to extinguished CSs. In this experiment, inactivation of POR reduced freezing to the tone, even though tone fear had not been extinguished. While POR inactivation reduced tone freezing overall, fear to the tone was similar in Context A and B for both groups indicating that the context was not guiding successful retrieval at the time of test. Taken together, a parsimonious interpretation of these findings suggests that POR contributes generally to the retrieval of auditory fear conditioning and is not uniquely related to the context-specificity of extinction learning.
The results of the current experiments extend the functional contribution of POR beyond contextual fear conditioning to include the expression of auditory fear conditioning. While these findings are novel, they add to a growing body of evidence suggesting that areas within the parahippocampal region have a role in auditory fear conditioning. For instance, POR is strongly interconnected with the perirhinal cortex (PER), and in vivo electrophysiological recording studies have demonstrated conditioning-induced changes in tone-elicited firing of PER neurons (Furtak, Allen, & Brown, 2007). More recently, Bartley and Furtak (2021) demonstrated that pre-training PER lesions impair the expression of auditory fear conditioning. POR is also strongly and reciprocally interconnected with dorsal presubiculum (Agster & Burwell, 2013), lesions of which impair the expression of auditory fear conditioning (Robinson & Bucci, 2012). While future studies are required to determine if these regions differ in their contributions to auditory fear conditioning, one possibility is that the parahippocampal region works in concert with the hippocampus to form and retrieve a representation of the entire conditioning episode, including contextual, spatial, and temporal information (see Quinn et al., 2008).
As previously noted, several prior studies have found no impact of POR lesions on auditory fear conditioning (Bucci et al., 2000; Peck & Taube, 2017; Taylor-Yeremeeva et al., 2021). However, because these prior studies used pre-training lesions, it is possible that other brain regions were able to compensate for POR damage. Indeed, there is some evidence that pre-training lesions of the hippocampus are less effective at disrupting retrieval of auditory fear conditioning than post-training lesions (Quinn et al., 2008). Apart from the timing of POR manipulations (pre-training vs. retrieval), an additional difference between the current studies and prior studies is that, by the time of testing, the initial conditioning could be considered “remotely” acquired. Specifically, in Experiments 1a and 1b, there was an 8-day retention interval between the end of conditioning and the start of testing. In contrast, testing occurred 2 days (or less) after initial conditioning in prior lesion studies (Bucci et al., 2000; Peck & Taube, 2017; Taylor-Yeremeeva et al., 2021). Thus, the current findings might reflect a role for POR in the retrieval of remote fear memory. This is consistent with recent findings demonstrating a role for the retrosplenial cortex, a region that is interconnected with POR, in the retrieval of remotely acquired tone fear (Fournier et al., 2021).
In the present experiments, CNO was used to activate the hM4Di receptor, and there is some evidence that CNO itself can alter behavior in rats (MacLaren et al., 2016). However, it seems unlikely that CNO alone produced the deficits in auditory fear retrieval observed in the current experiments for several reasons. First, in these experiments, although all rats received injections of CNO prior to testing, only rats expressing the hM4Di receptor showed a reduction in freezing. Second, two recent experiments from our lab reported no differences in freezing during retrieval of auditory fear conditioning between groups that received injections of CNO or vehicle prior to testing (Fournier et al., 2021; Experiments 3a and 3b). Third, the results of the additional test at the conclusion of Experiment 1b demonstrate that Groups hM4di and GFP were equally capable of performing the conditioned response following injections of CNO. Finally, a recent study of appetitive sensory preconditioning in rats found that chemogenetic inactivation of POR impaired encoding of an association between an auditory cue and a neutral light cue, but inactivation at the time of test did not impair retrieval testing with the auditory cue (Taylor-Yeremeeva et al., 2021). Although the procedures differ from the current experiments in many ways, the intact retrieval observed by Taylor-Yeremeeva et al. (2021) indicates that POR inactivation does not produce generalized deficits in responding to auditory cues.
In summary, chemogenetic inactivation of POR was found to impair the expression of auditory fear conditioning. This occurred when the tone CS had undergone extinction (Experiment 1a) and when it had not (Experiment 1b). These results contrast with prior research, in which pre-training damage to POR was found to have no impact on the expression of auditory fear conditioning (Bucci et al., 2000; Peck & Taube, 2017; Taylor-Yeremeeva et al., 2021) but add to a growing body of research demonstrating that under some circumstances, structures otherwise associated with spatial and contextual learning may also contribute to the retrieval of Pavlovian auditory fear conditioning (Fournier et al., 2021; Quinn et al., 2008).
Highlights.
Examined the role of the postrhinal cortex in the retrieval of auditory fear conditioning.
Inactivation of the postrhinal cortex impaired retrieval of auditory fear conditioning following extinction.
Inactivation of the postrhinal cortex also impaired retrieval of auditory fear conditioning that had not been extinguished.
Funding:
NSF: IOS1353137 (D.J.B), DGE-1313911 (N.E.D)
NIDA: T32DA037202 (D.I.F)
NIMH: K01MH116158 (T.P.T), R01MH118734 (A.T. G., T.P.T)
Author Note
This work was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers K01MH116158 and R01MH118734. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. We dedicate this manuscript to Dr. David Bucci for his endless support, mentorship, and friendship. The current work was originally inspired by conversation between the authors and Dave, and we hope to honor his memory with our continued pursuit of understanding the brain and behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, & Burwell RD (2013). Hippocampal and subicular efferents and afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Behavioral Brain Research, 254, 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley TD, & Furtak SC (2021). Perirhinal damage produces modality-dependent deficits in fear learning. Neurobiology of Learning and Memory, 181, 107427. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2004). Context and behavioral processes in extinction. Learning & Memory, 11, 485–494. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & King DA (1983). Contextual control of the extinction of conditioned fear: tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes, 9, 248–265. [PubMed] [Google Scholar]
- Bouton ME, Maren S, & McNally GP (2021). Behavioral and neurobiological mechanisms of Pavlovian and instrumental extinction learning. Physiological Reviews, 101, 611–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, & Burwell RD (2000). Contributions of the postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience, 114, 882–894. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, & Jutras MJ (2004). Perirhinal and postrhinal contributions to remote memory for context. The Journal of Neuroscience, 24, 11023–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, & Amaral DG (1995). Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomicl literature and comparison with findings from the monkey brain. Hippocampus, 5, 390–408. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2000). A cortical-hippopcampal system for declarative memory. Nature Reviews Neuroscience, 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2010). From contextual fear to a dynamic view of memory systems. Trends in Cognitive Science, 14, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier DI, Cheng HY, Tavakkoli A, Gulledge AT, Bucci DJ, & Todd TP (2021). Retrosplenial cortex inactivation during retrieval, but not encoding, impairs remotely acquired auditory fear conditioning in male rats. Neurobiology of Learning and Memory, 185, 107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Allen TA, & Brown TH (2007). Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. The Journal of Neuroscience, 27, 12277–12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, & Maren S (2008a). Lesions of the entorhinal cortex or fornix disrupt the context-dependence of fear extinction in rats. Behavioral Brain Research, 2, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, & Maren S (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & Memory, 15, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science, 256, 675–677. [DOI] [PubMed] [Google Scholar]
- LaChance PA, Todd TP, & Taube JS (2021). A sense of space in postrhinal cortex. Science, 356, eaax4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DAA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, & Clark SD (2016). Clozapine N-Oxide Administration Produces Behavioral Effects in Long–Evans Rats: Implications for Designing DREADD Experiments. Eneuro, 3(5), ENEURO.0219–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JR, & Taube JS (2017). The postrhinal cortex is not necessary for landmark control in in rat had direction cells. Hippocampus, 27, 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, & Shaham Y (2009). Long-lasting incubation of conditioned fear in rats. Biological Psychiatry, 65, 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, & Fanselow MS (2008). Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus, 18, 640–654. [DOI] [PubMed] [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behavior. Nature Reviews Neuroscience, 13, 713–726. [DOI] [PubMed] [Google Scholar]
- Robinson SR, & Bucci DJ (2012). Fear conditioning is disrupted by damage to the postsubiculum. Hippocampus, 22, 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Poorman CE, Marder TJ, & Bucci DJ (2012). Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. The Journal of Neuroscience, 32, 12076–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, & Clark RE (2004). The medial temporal lobe. Annual Review of Neuroscience, 27, 279–306. [DOI] [PubMed] [Google Scholar]
- Taylor-Yeremeeva EM, et al. (2021). Appetitive and aversive sensory preconditioning in rats is impaired by disruption of the postrhinal cortex. Neurobiology of Learning and Memory, 183, 107461. [DOI] [PubMed] [Google Scholar]


